Lincomycin Sustained Release Granules
A technology of lincomycin and sustained-release granules, which is applied in the direction of block delivery, digestive system, antibacterial drugs, etc., and can solve the problems of reducing the number of times of taking medicine, low bioavailability, and inconvenient taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of lincomycin inclusion compound includes:
[0025] (1) React lincomycin with γ-cyclodextrin in a water or water-containing ethanol medium in a certain proportion, filter the resulting solution through a microporous membrane until it is clear, and separate the clathrate from the mixture; or
[0026] (2) React lincomycin with γ-cyclodextrin in solid form; or
[0027] (3) Lincomycin reacts with γ-cyclodextrin for high-energy grinding.
[0028] The measurements in the present invention are all weights.
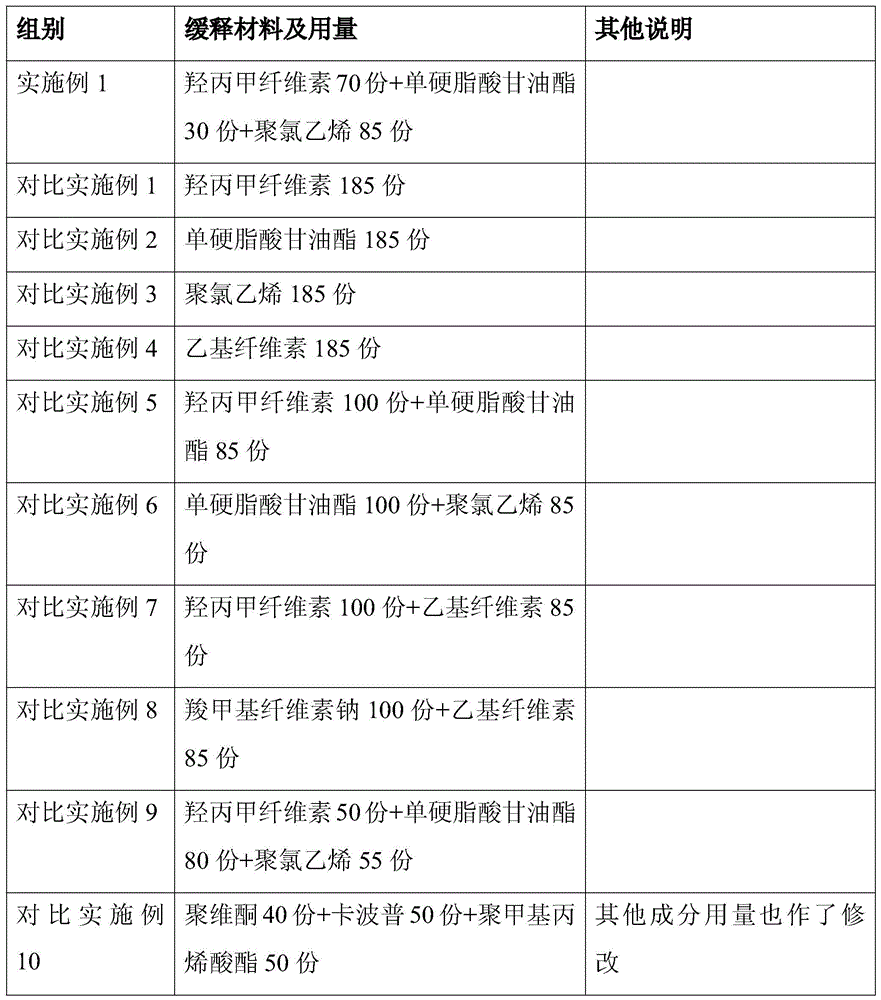
[0029] The prescription of the lincomycin sustained-release granules of the present invention is (by weight):
[0030] Lincomycin Inclusion Compound
Embodiment 1
[0031] Example 1 prescription
[0032] Lincomycin inclusion compound: Lincomycin: γ-cyclodextrin is 2:3.5
[0033] Lincomycin Inclusion Compound
50 servings
190 copies
4 parts
4 parts
70 servings
30 servings
85 servings
[0034] Preparation:
[0035] 1. Prepare the lincomycin inclusion compound according to the following method:
[0036] (1) React lincomycin with γ-cyclodextrin in a water or water-containing ethanol medium in a certain proportion, filter the resulting solution through a microporous membrane until it is clear, and separate the clathrate from the mixture; or
[0037] (2) React lincomycin with γ-cyclodextrin in solid form; or
[0038] (3) Lincomycin reacts with γ-cyclodextrin for high-energy grinding.
[0039] 2. Crush the lincomycin inclusion compound through a 100-mesh sieve, and pulverize the remaining auxiliary materials through an 80-mesh sieve; ac...
Embodiment 2
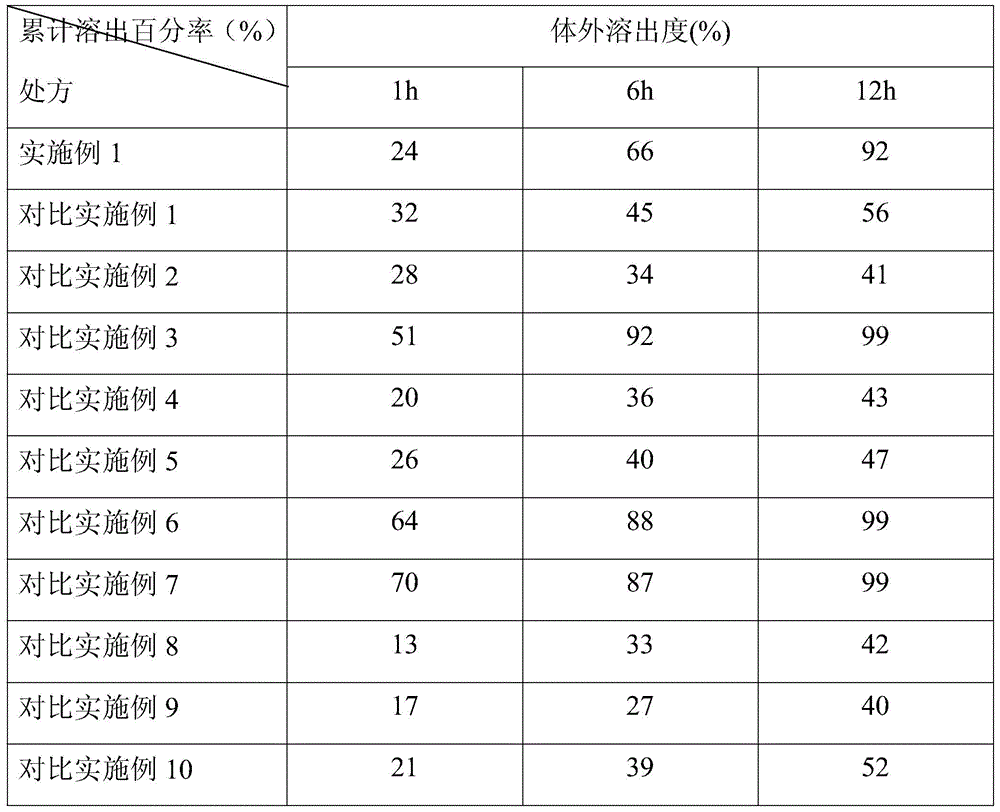
[0041] The lincomycin and γ-cyclodextrin described in Example 1 were fully ground in 50% ethanol to form a uniform paste. After vacuum drying, a white powder was obtained. The solubility of the powder in water is better than that of the unincluded Linco The mycin increased 17 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com