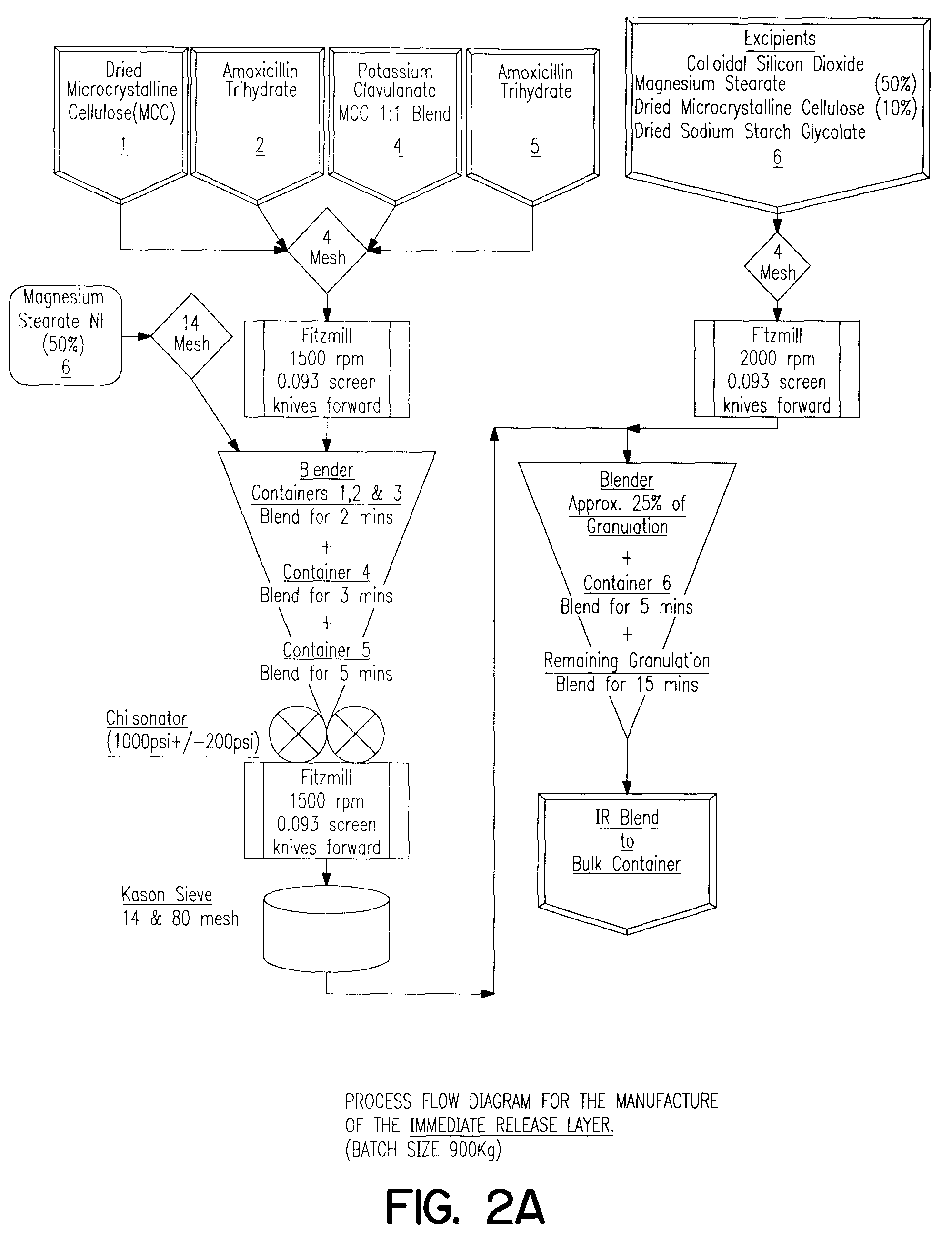
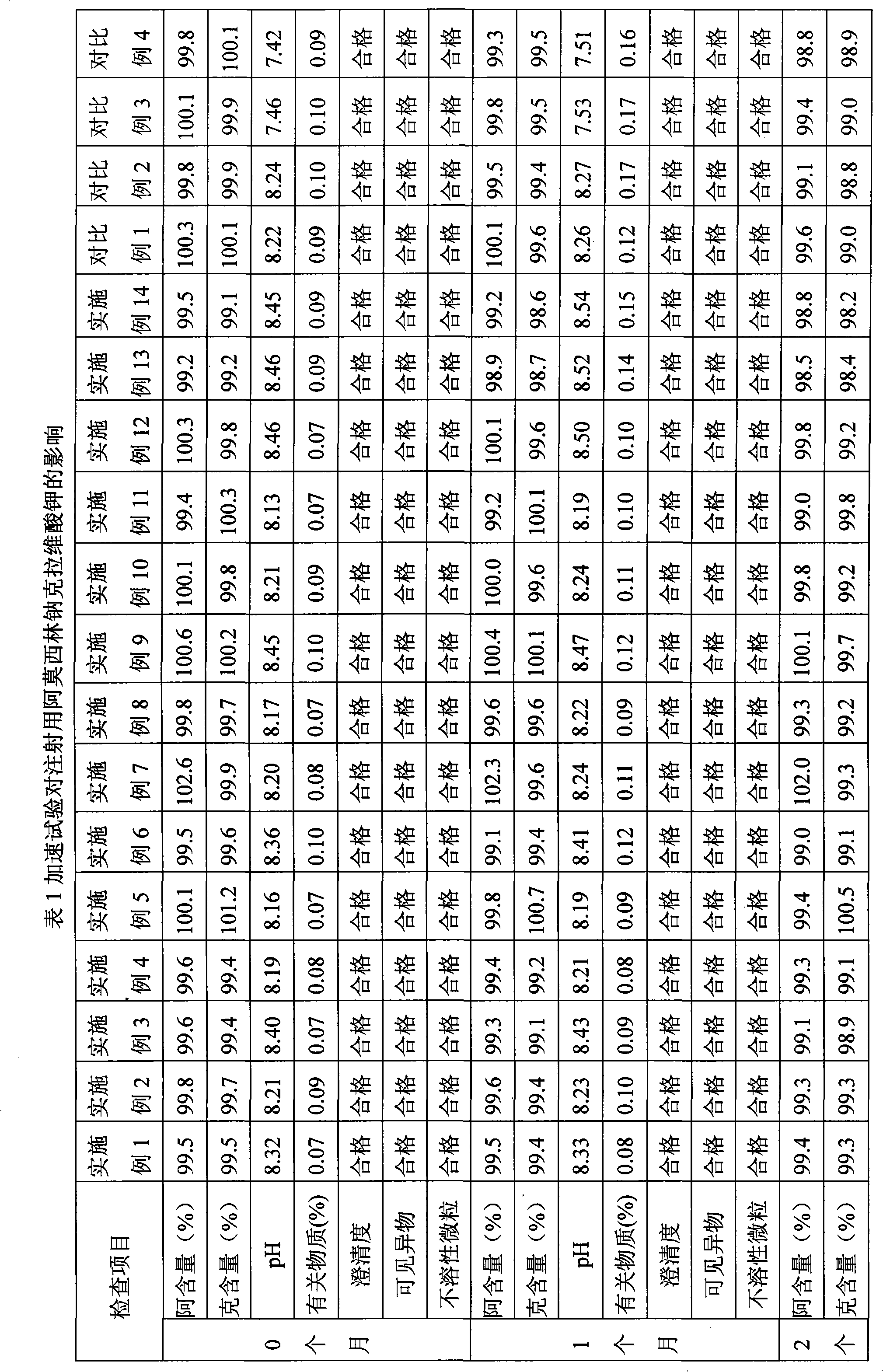
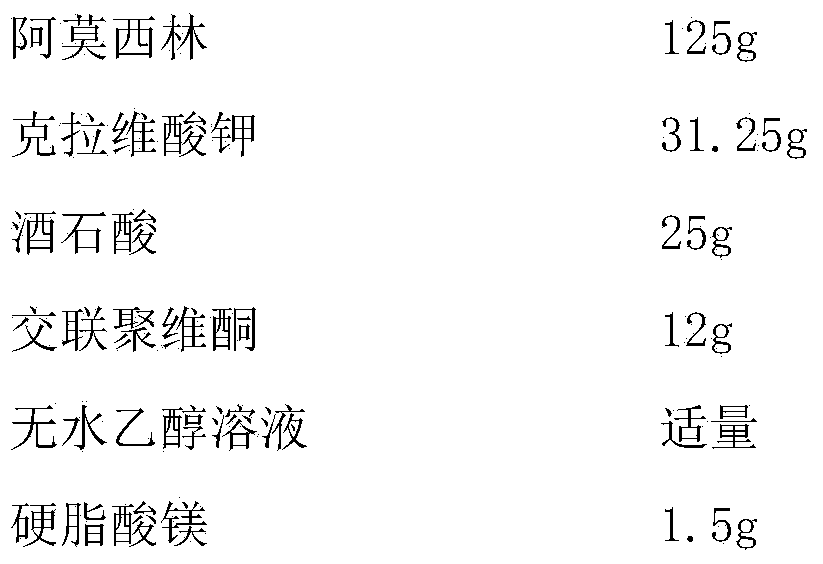
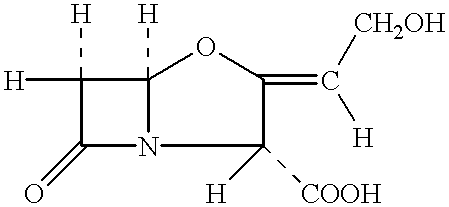
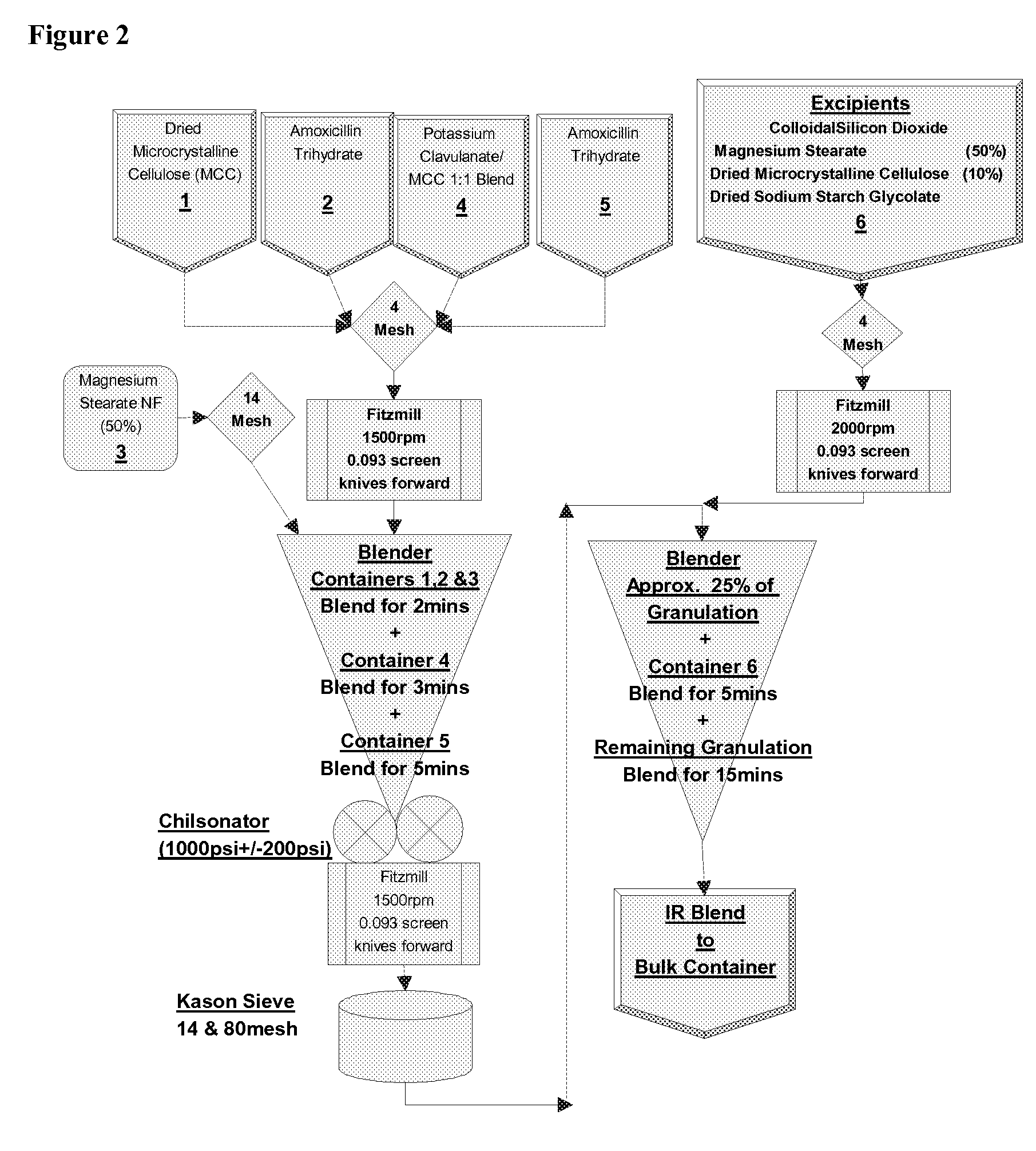
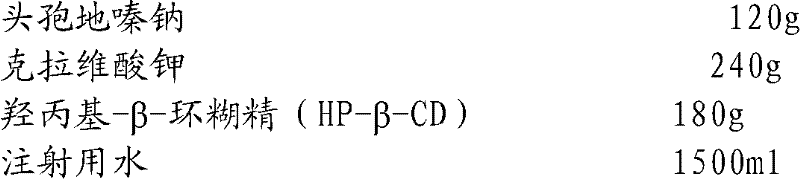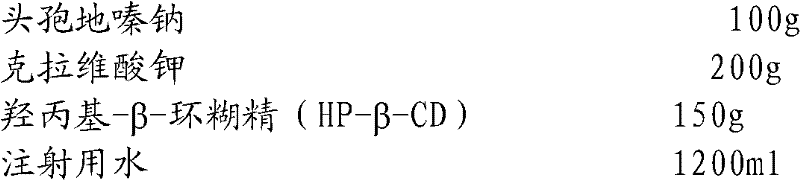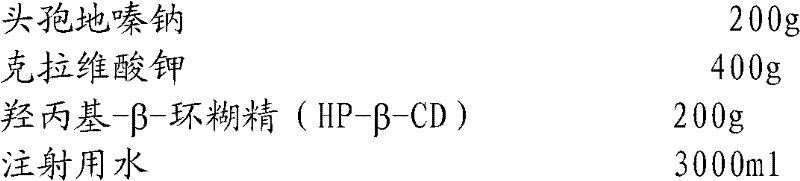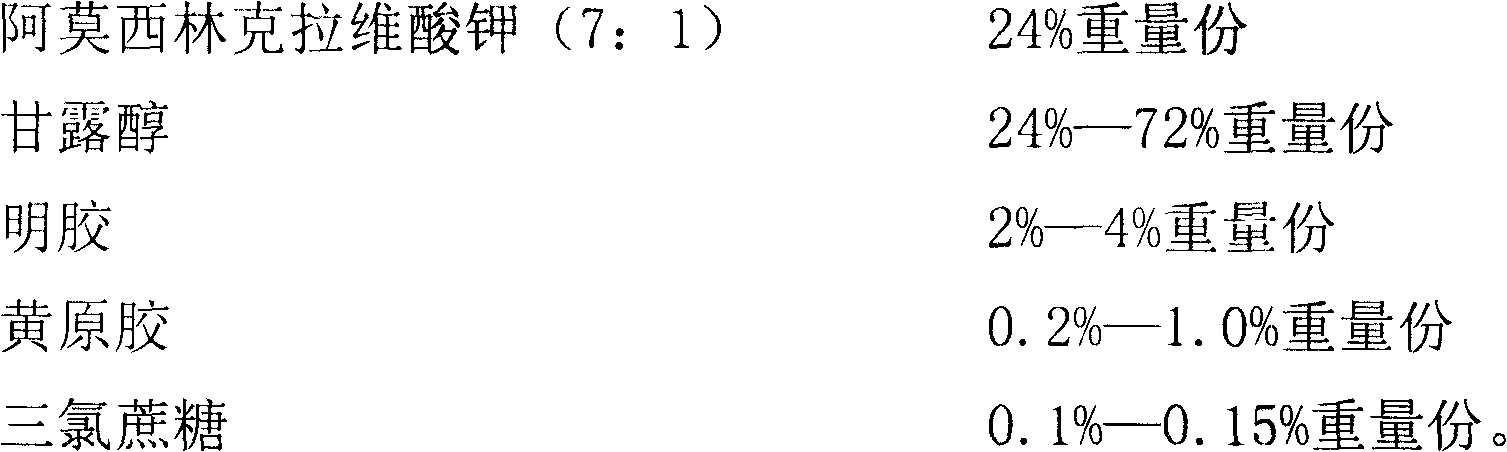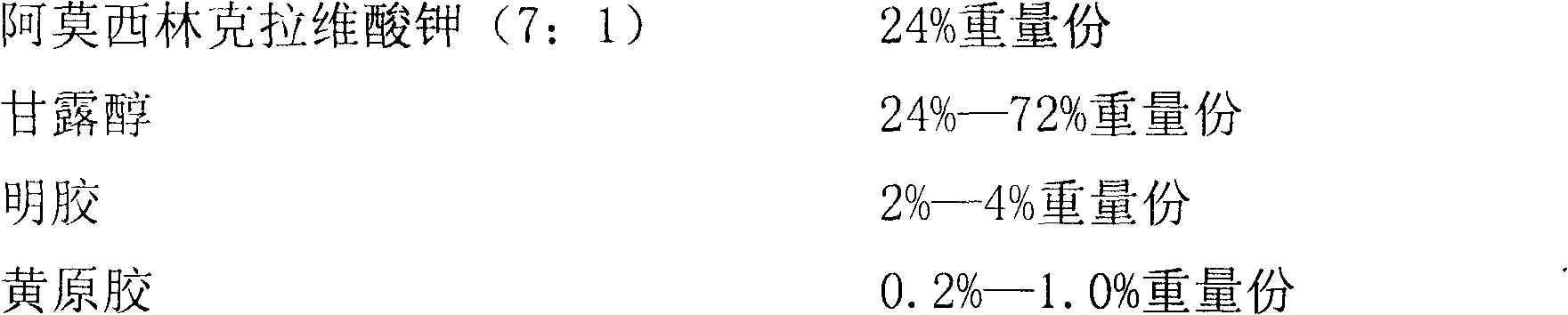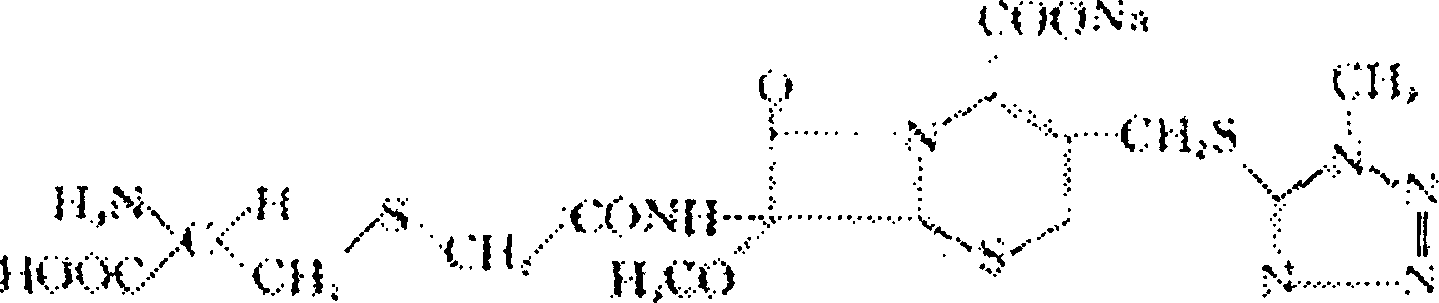Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
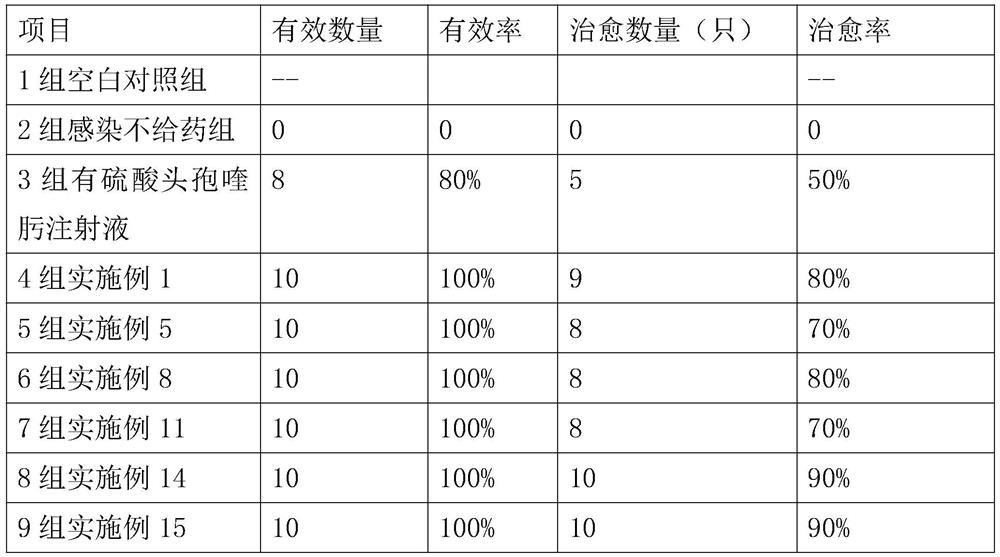
58 results about "Potassium Clavulanate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of treating a bacterial infection comprising amoxycillin and potassium clavulanate
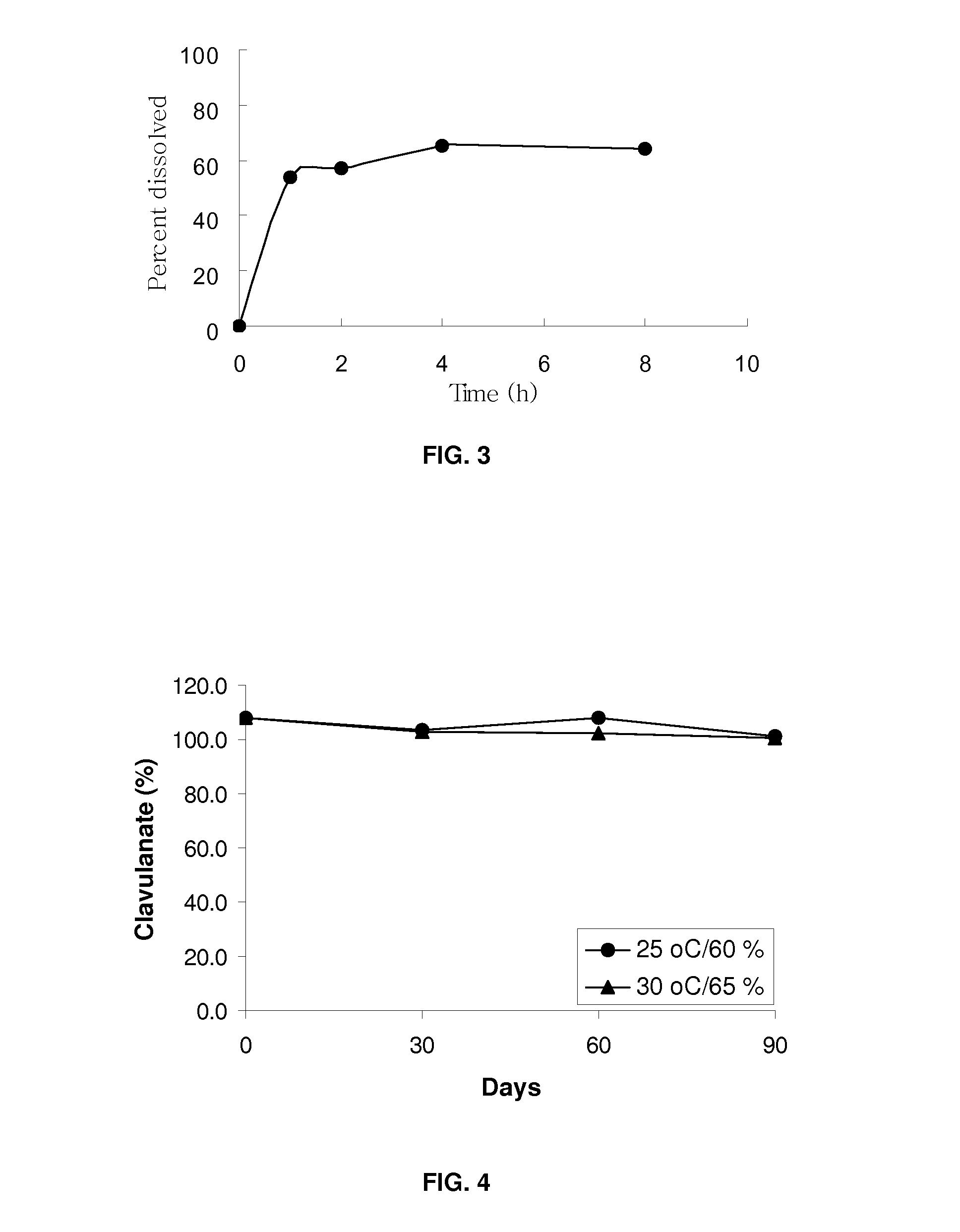
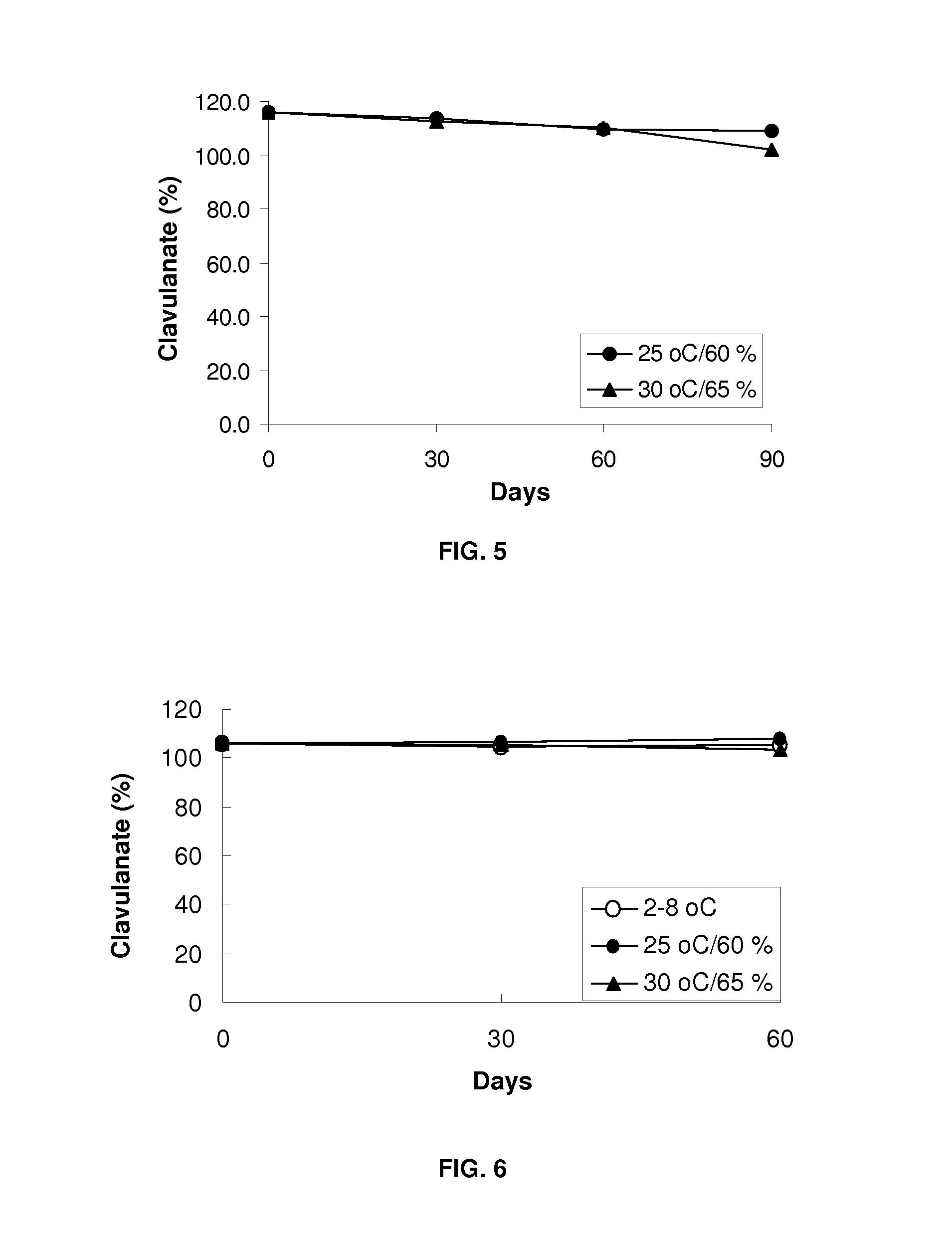
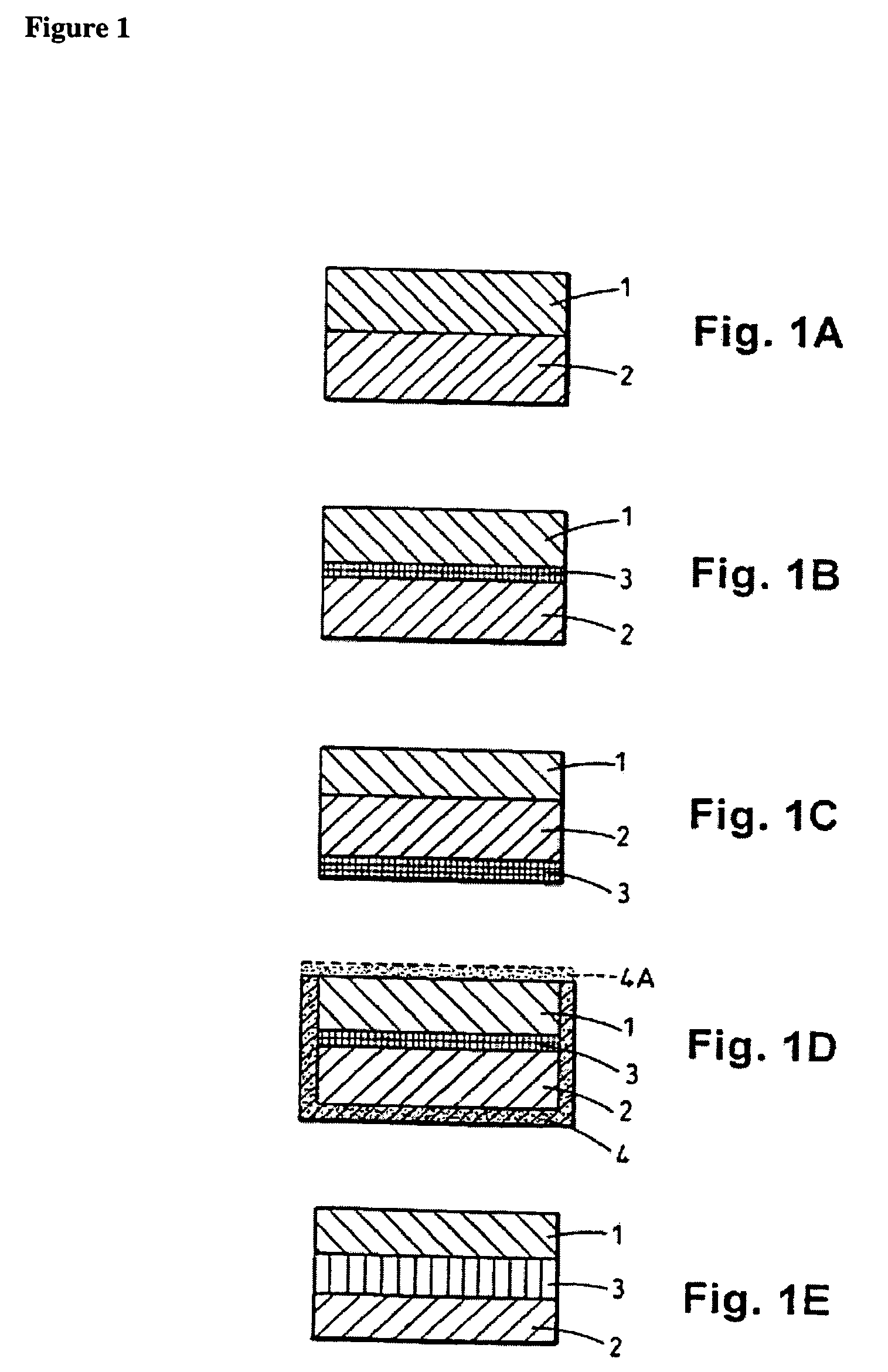
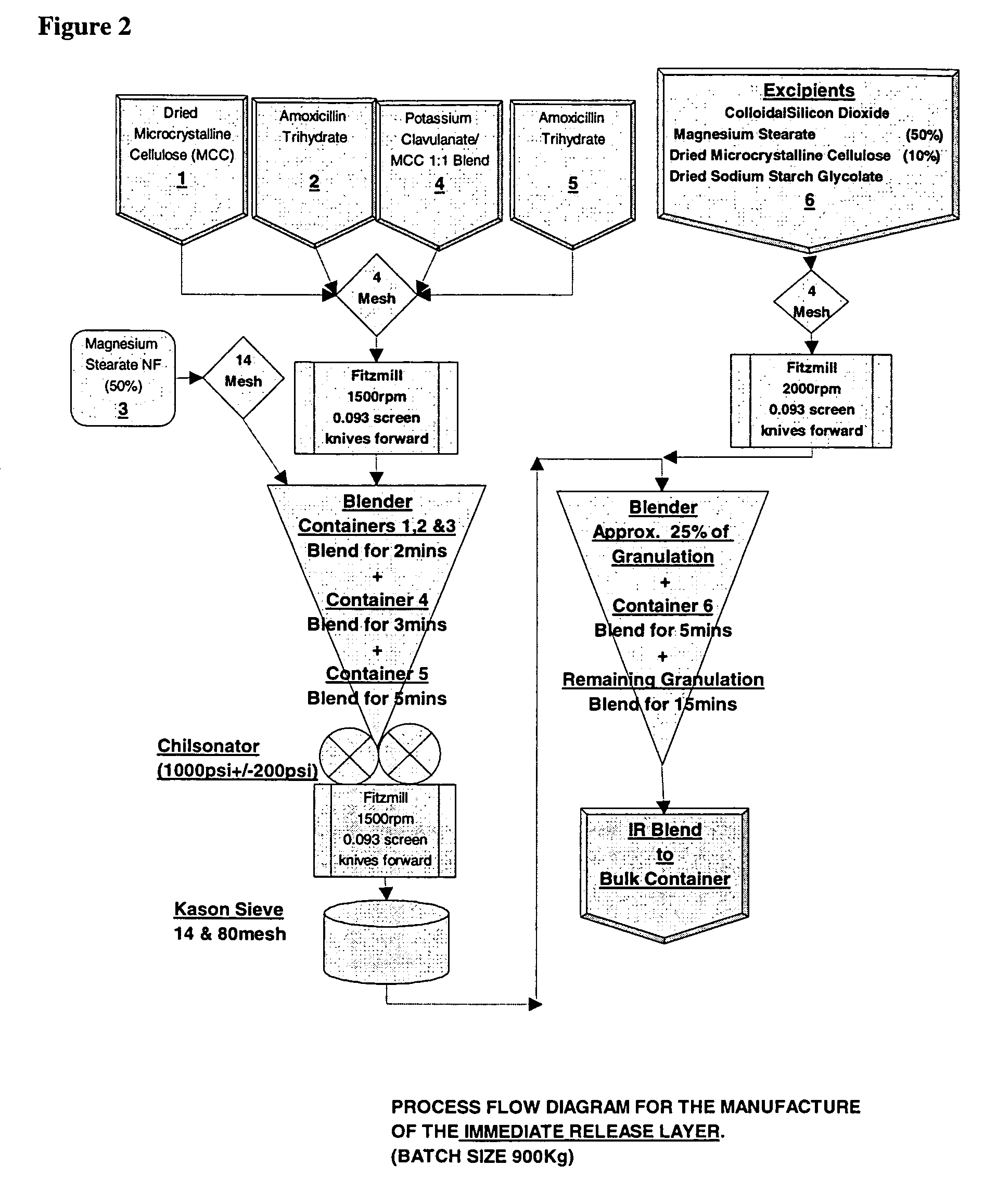
Bacterial infections may be treated using a high dosage regimen of amoxycillin and potassium clavulanate. Preferably, the dosage is provided by a bilayer tablet.
Owner:GLAXO GROUP LTD
Amoxicillin and clavulanate potassium injection and preparation method thereof
The invention belongs to the technical field of medical technology, and particularly relates to an amoxicillin and clavulanate potassium injection and a preparation method of the amoxicillin and clavulanate potassium injection. The amoxicillin and clavulanate potassium injection includes amoxicillin sodium, potassium clavulanate and a pH regulator. The preparation method comprises the steps of crushing and mixing the amoxicillin sodium, the potassium clavulanate and the pH regulator uniformly through air flows according to certain proportion, then respectively packaging the mixture into penicillin bottles, controlling the water content to be 0.5%, filling nitrogen for protection, plugging, rolling a cover and finally packaging. According to the invention, the obtained product is stable in quality, the problems of the color changing and the deterioration caused by the easy oxidization of the similar products can be solved, the product quality is improved, and high economical benefits are obtained.
Owner:LUNAN BETTER PHARMA
Amoxicillin potassium clavulanate powder injection and preparation method thereof
InactiveCN102406614AAvoid the hidden danger of excessive moistureGood curative effectAntibacterial agentsPowder deliverySolubilityCurative effect
The invention discloses an amoxicillin potassium clavulanate powder injection which is prepared from the following raw materials in percentage by weight: 10% of amoxicillin, 2.5% of potassium clavulanate, 4% of cosolvent and the balance of soluble filler. The invention also discloses a preparation method of the amoxicillin potassium clavulanate powder injection. The test proves that the amoxicillin potassium clavulanate powder injection disclosed by the invention has the characteristics of stability in storage, convenience in transportation, good water solubility and the like and greatly improves the curative effect of the amoxicillin and potassium clavulanate, thereby being a novel broad-spectrum and efficient special powder injection for animals.
Owner:上海恒丰强生物技术有限公司
Cefodizime sodium composition and preparation method thereof
InactiveCN102258521AImprove stabilityStable recipe processAntibacterial agentsOrganic active ingredientsCefodizime SodiumFreeze-drying
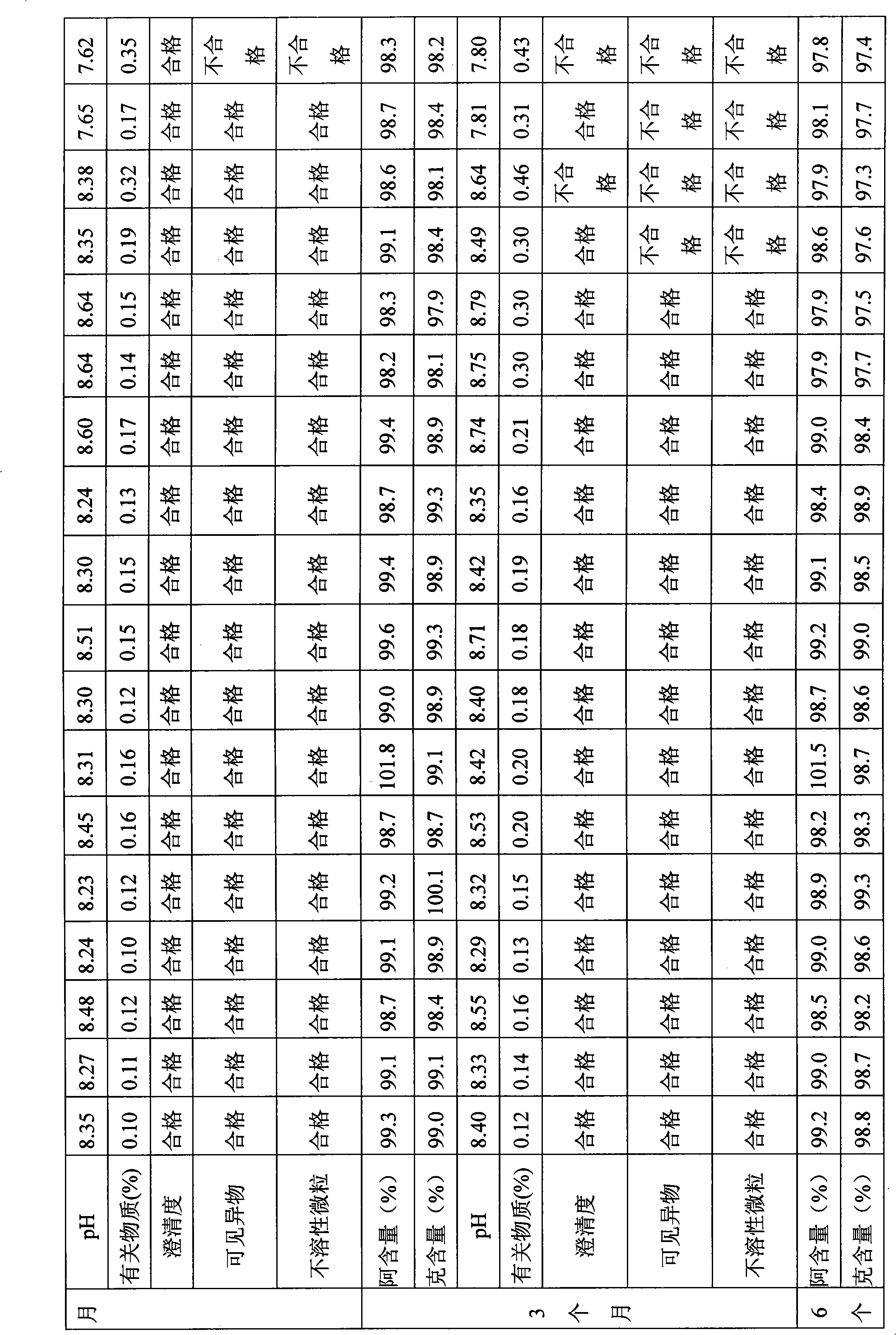
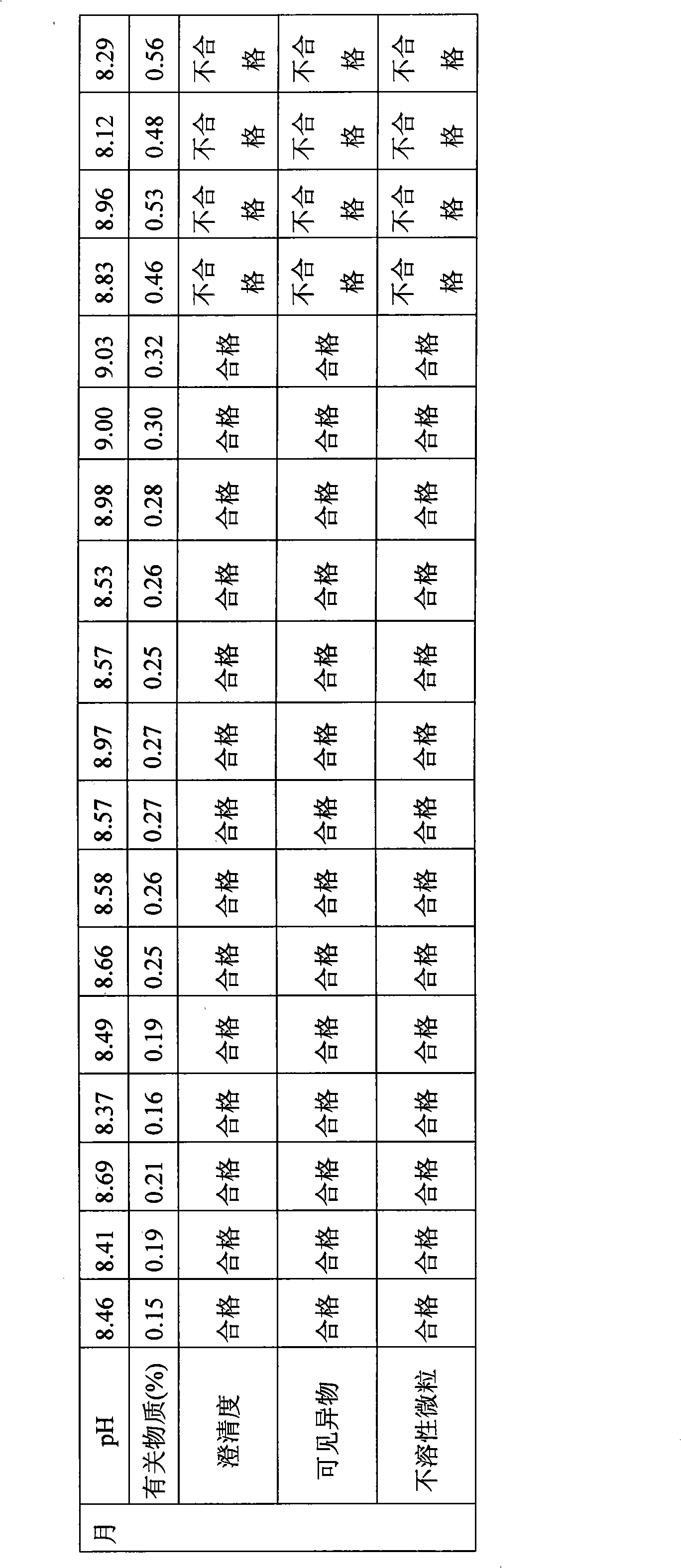
The invention relates to the field of drug synthesis and preparation thereof, relates to a preparation method of an antibacterial drug, in particular to a stable cefodizime sodium composition preparation and a preparation method thereof. The present invention directly dissolves sterile cefodizime sodium in water, adds sterile potassium clavulanate to dissolve it completely, and obtains an aqueous solution of cefodizime sodium / clavulanate potassium, and adds hydroxypropyl-β to the aqueous solution. -Cyclodextrin (HP-β-CD) inclusion, sub-package, and freeze-drying to obtain Cefodizime Sodium / Clavulanate Potassium for Injection. The preparation method provided by the invention is simple, and the cefodizime sodium / clavulanic acid potassium salt is clathrated with hydroxypropyl-β-cyclodextrin, which increases the stability of the sterile cefodizime sodium drug and reduces its toxic and side effects , improve drug availability, and the preparation process is simple, suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD +1
Composition containing amoxicillin and potassium clavulanate, and preparation method thereof
InactiveCN105919941ASolve the disadvantage of being easily destroyed quicklyAntibacterial agentsPowder deliveryInstabilityControllability
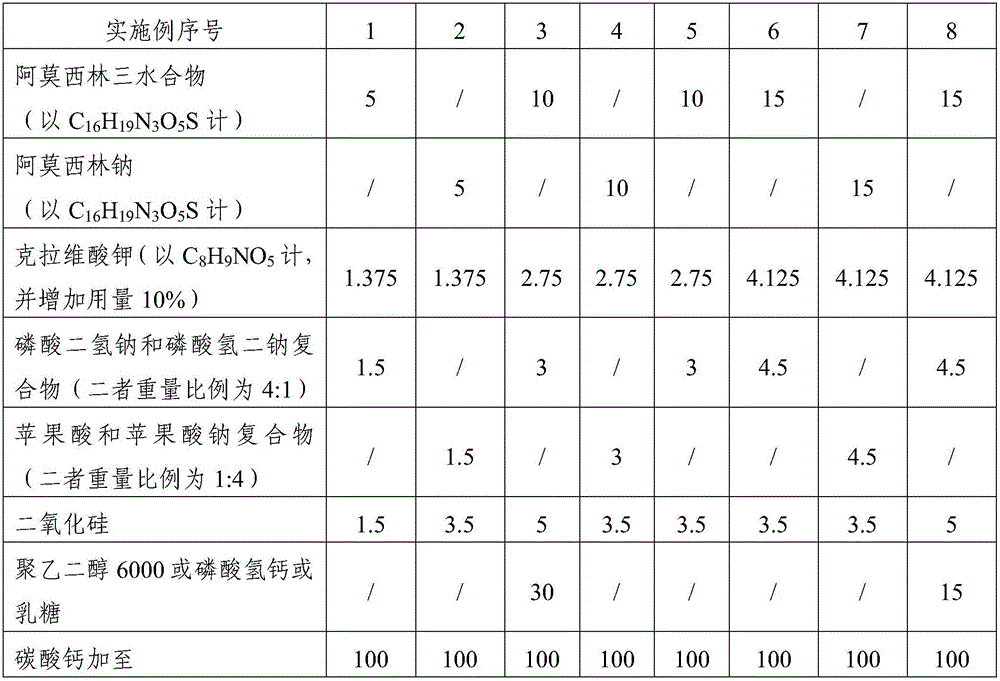
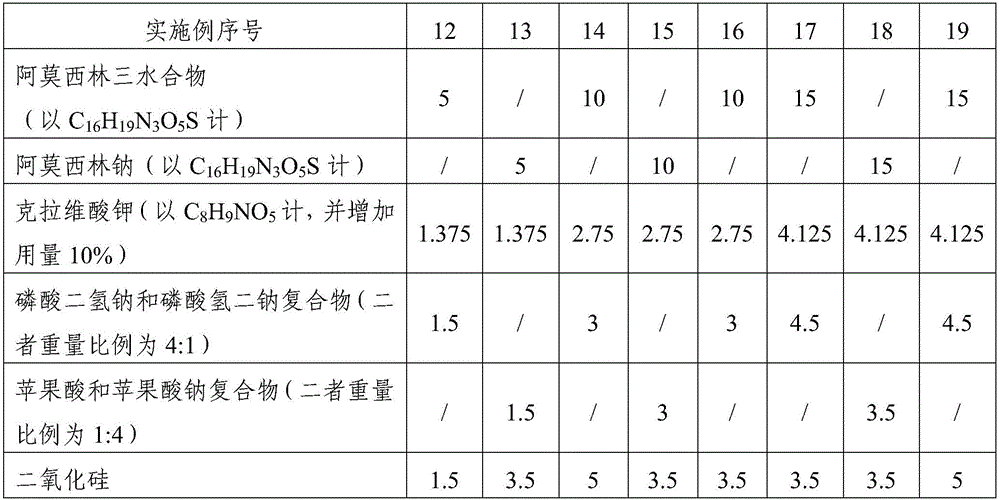
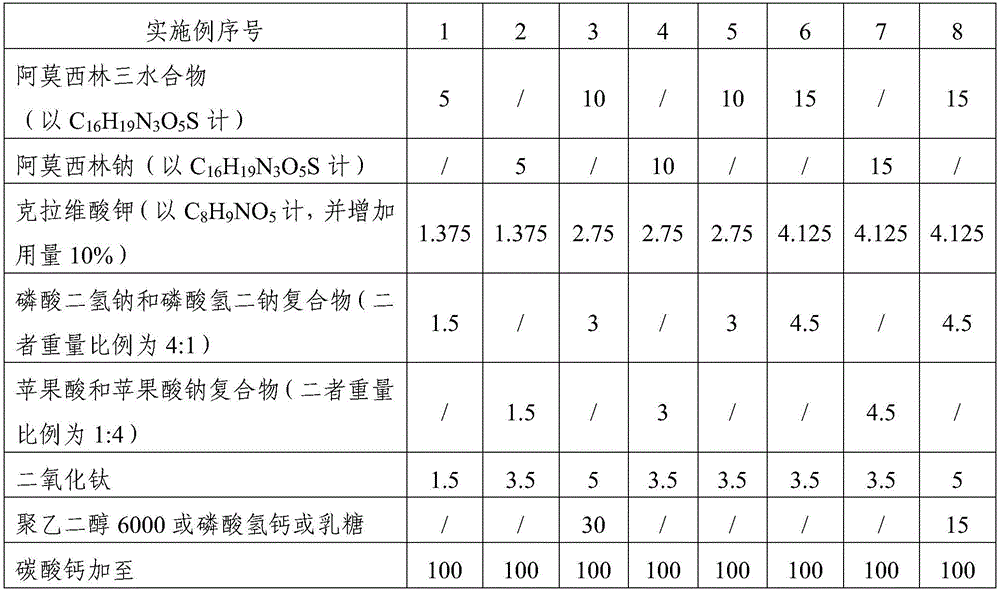
The invention relates to the field of a veterinary medicine preparation, and concretely provides a composition containing amoxicillin and potassium clavulanate, and a preparation method and a use method thereof. The composition is prepared from the following ingredients in parts by weight: 5 to 15 parts of amoxicillin, 1.375 to 4.125 parts of clavulanate, 1 to 5 parts of stabilizing agents and 1 to 5 parts of diluting agents. The problem of instability since the amoxicillin and the potassium clavulanate can easily absorb moisture in air is solved; the preparation process and the use method are simple; the addition of special equipment is not needed; all auxiliary ingredients are medical grade auxiliary materials; the cost is low; the materials can be easily obtained; no toxicity and no residue exist; and the requirements of safety, effectiveness and quality controllability on the veterinary medicine preparation can be met. The stability of the preparation is improved; the formula cost is also reduced; and the large-scale batch production can be realized.
Owner:HUNAN TAIGU BIOLOGICAL VETERINARY DRUG CO LTD
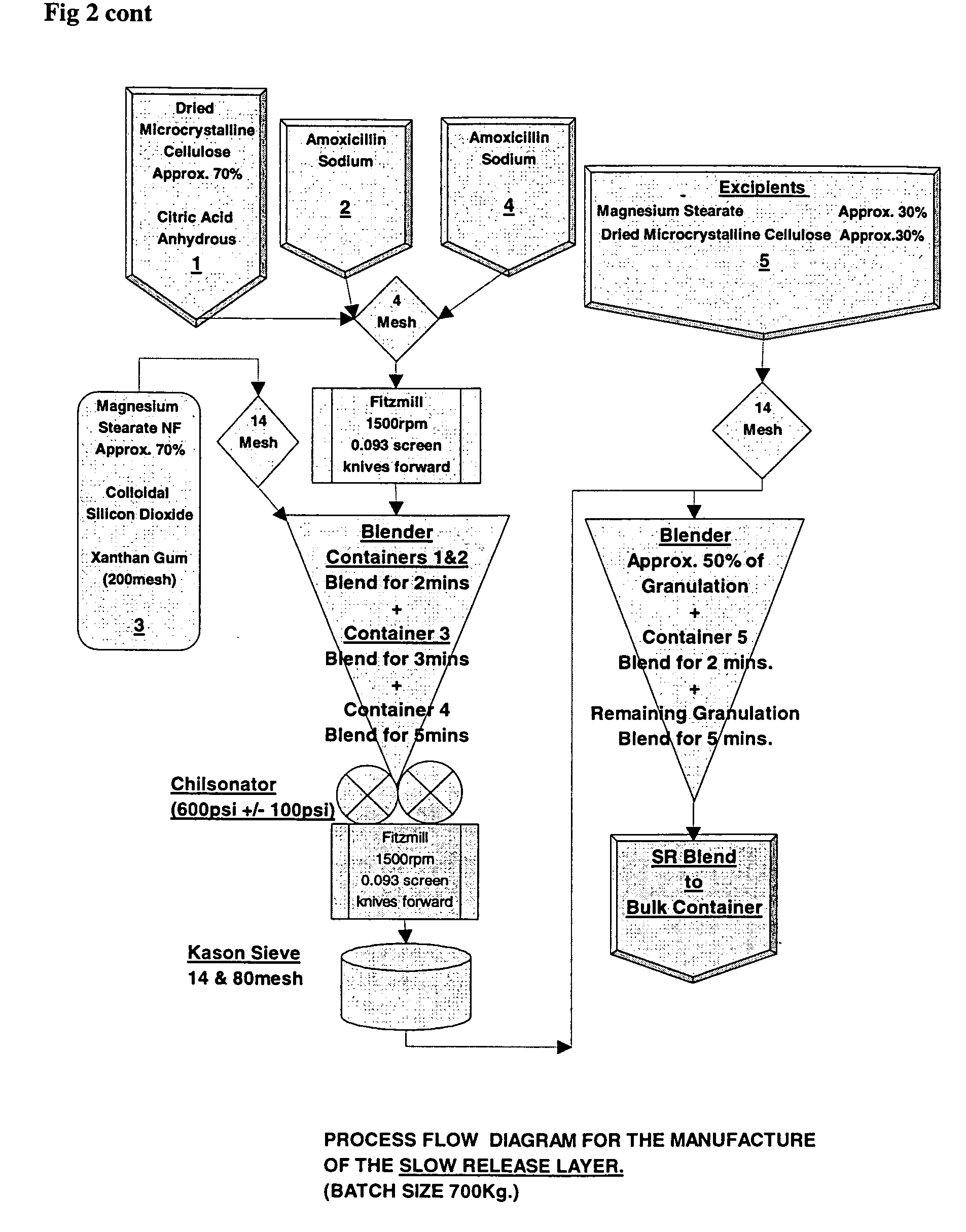
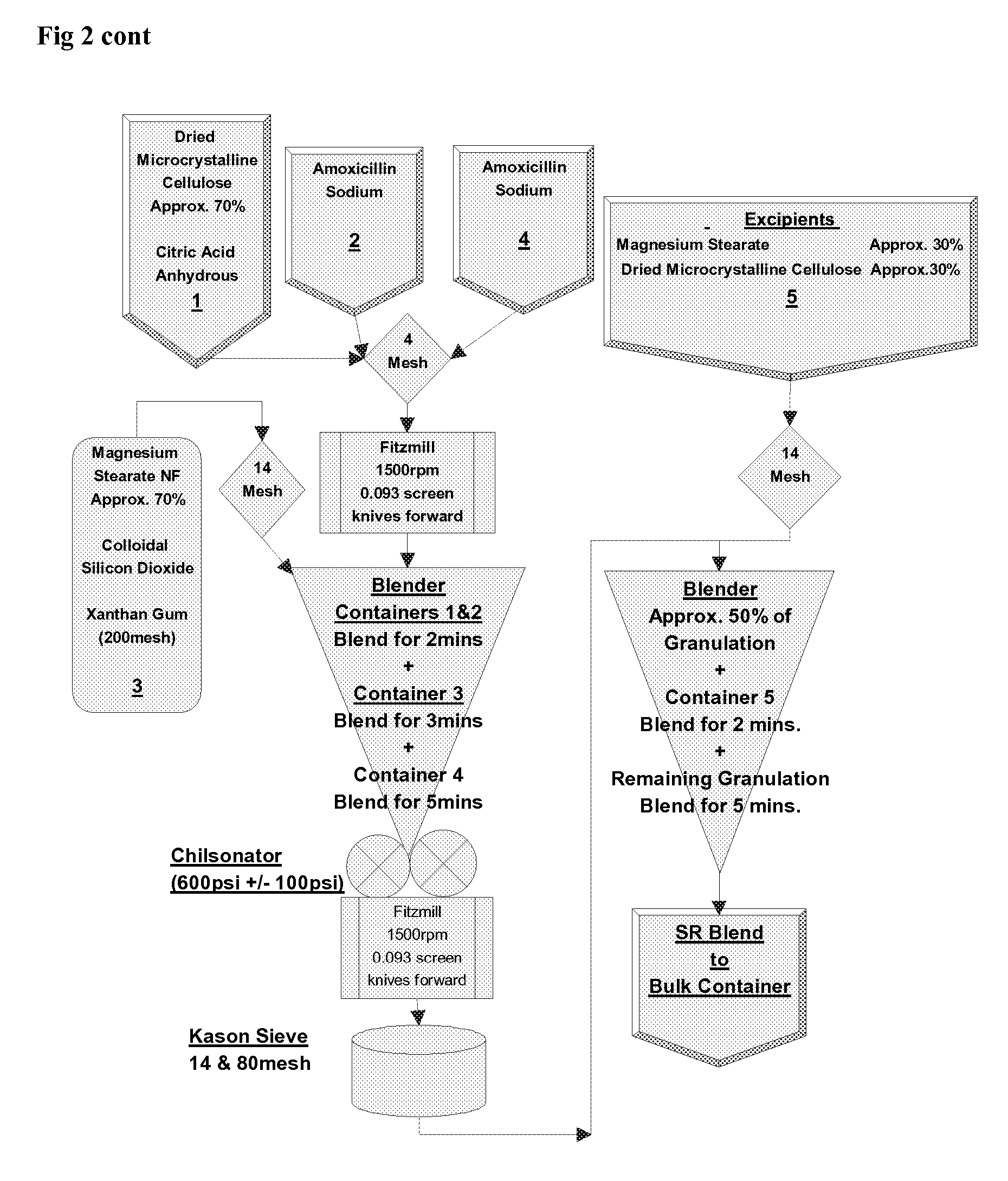
Amoxicillin/potassium clavulanate sustained-release preparation composition and preparation method thereof
The invention discloses an amoxicillin / potassium clavulanate sustained-release preparation composition and a preparation method thereof. The compound is mainly prepared from amoxicillin, potassium clavulanate, sustained-release materials and other appropriate accessories. The amoxicillin / potassium clavulanate sustained-release preparation provided by the invention can delay the release speed of a main drug, reduce the times of taking the medicine and improve the compliance of a patient. The amoxicillin / potassium clavulanate sustained-release preparation composition provided by the invention has quality controllability and quality and good stability of the of preparation process.
Owner:北京瑞伊人科技发展有限公司 +1
Amoxicillin sodium potassium clavulanate composition microballoon injection
InactiveCN101890007AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryGlycerolPhysical chemistry
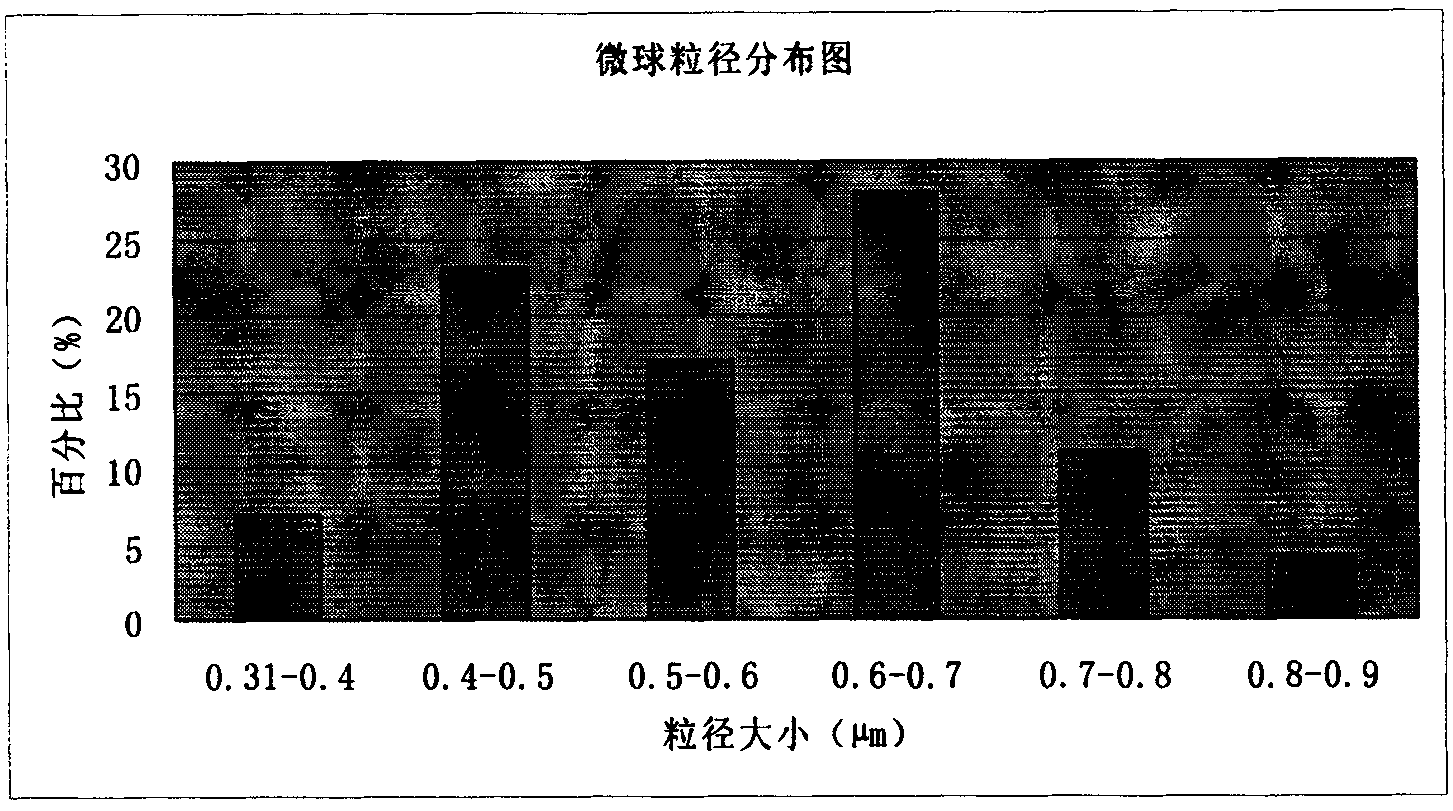
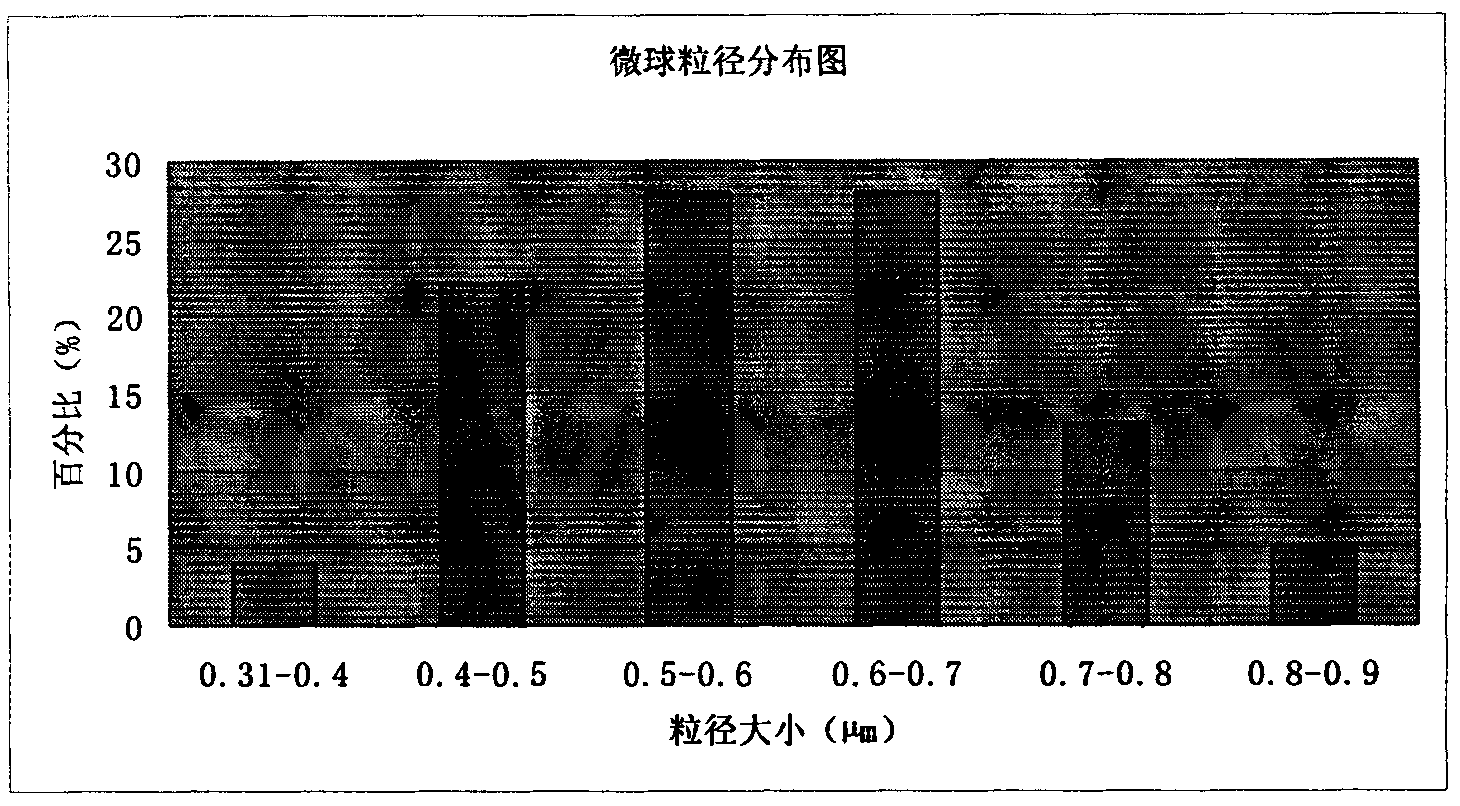
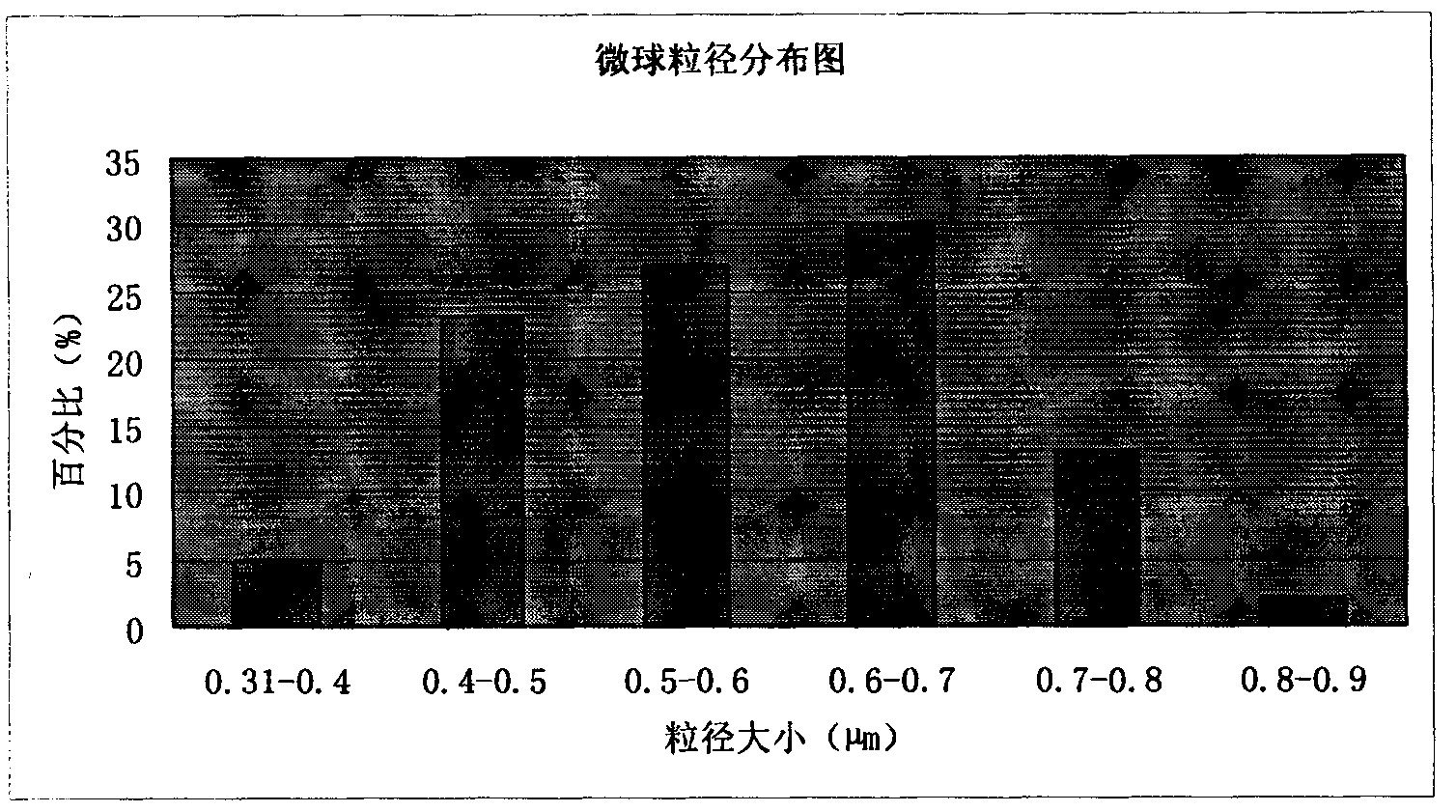
The invention discloses an amoxicillin sodium potassium clavulanate composition microballoon injection, which is characterized by comprising the following components in parts by weight: 5 parts of amoxicillin sodium, 1 part of potassium clavulanate, 4-6 parts of gelatin, 4-6 parts of Arabic gum, 3-6 parts of disodium hydrogen phosphate, 2-4 parts of trehalose and 1-3 parts of glycerol. Compared with the prior art, the amoxicillin sodium potassium clavulanate composition microballoon injection prepared by the invention has good stability, high entrapment rate, good process repeatability, even particle distribution, good injectable property and good slow release character, and is suitable for industrialization production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
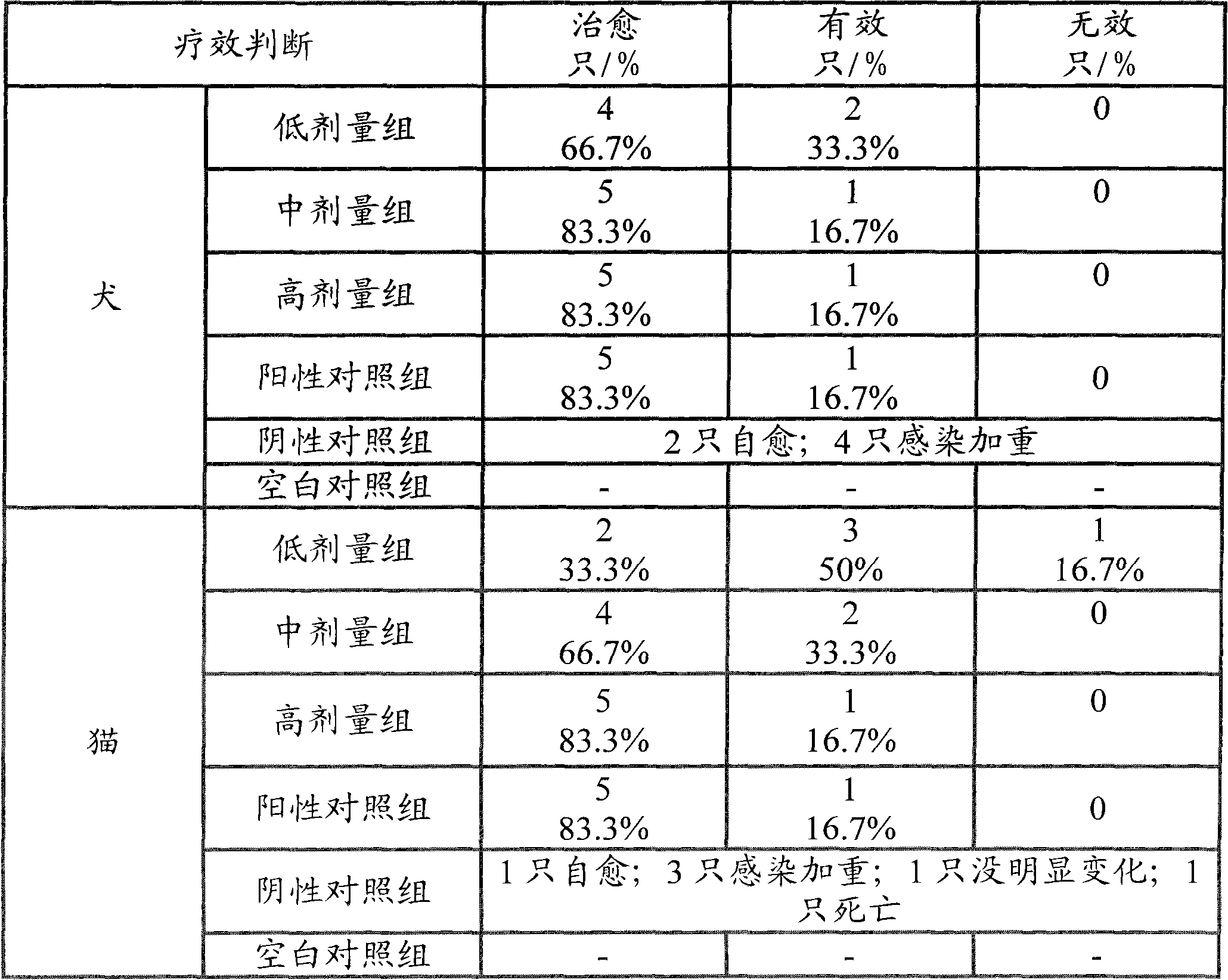
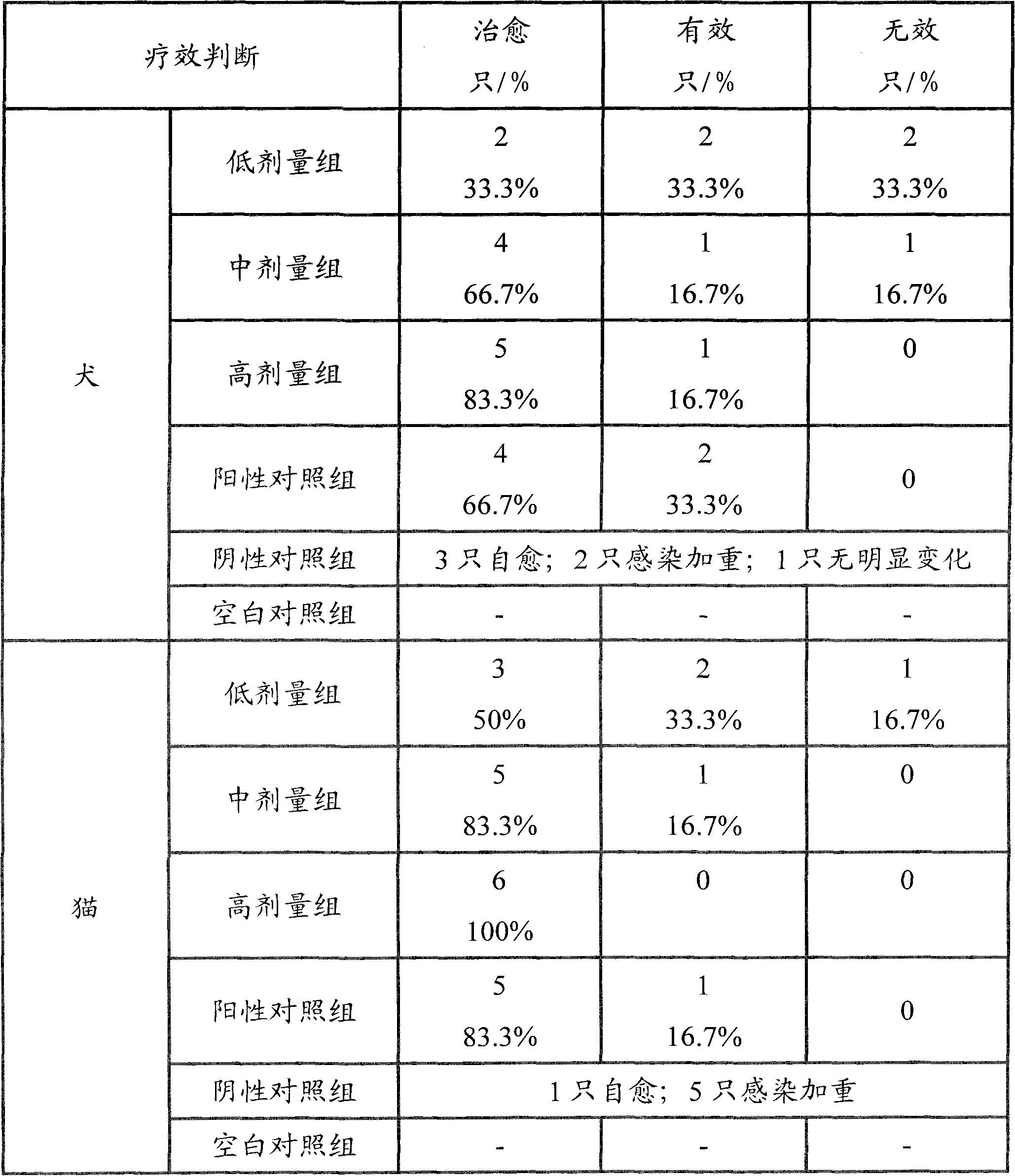
Amoxicillin and potassium clavulanate tablet for dogs and cats, and its preparation method and application
InactiveCN102240285AEasy to useGood treatment effectDigestive systemAntiinfectivesDiluentInfective disorder
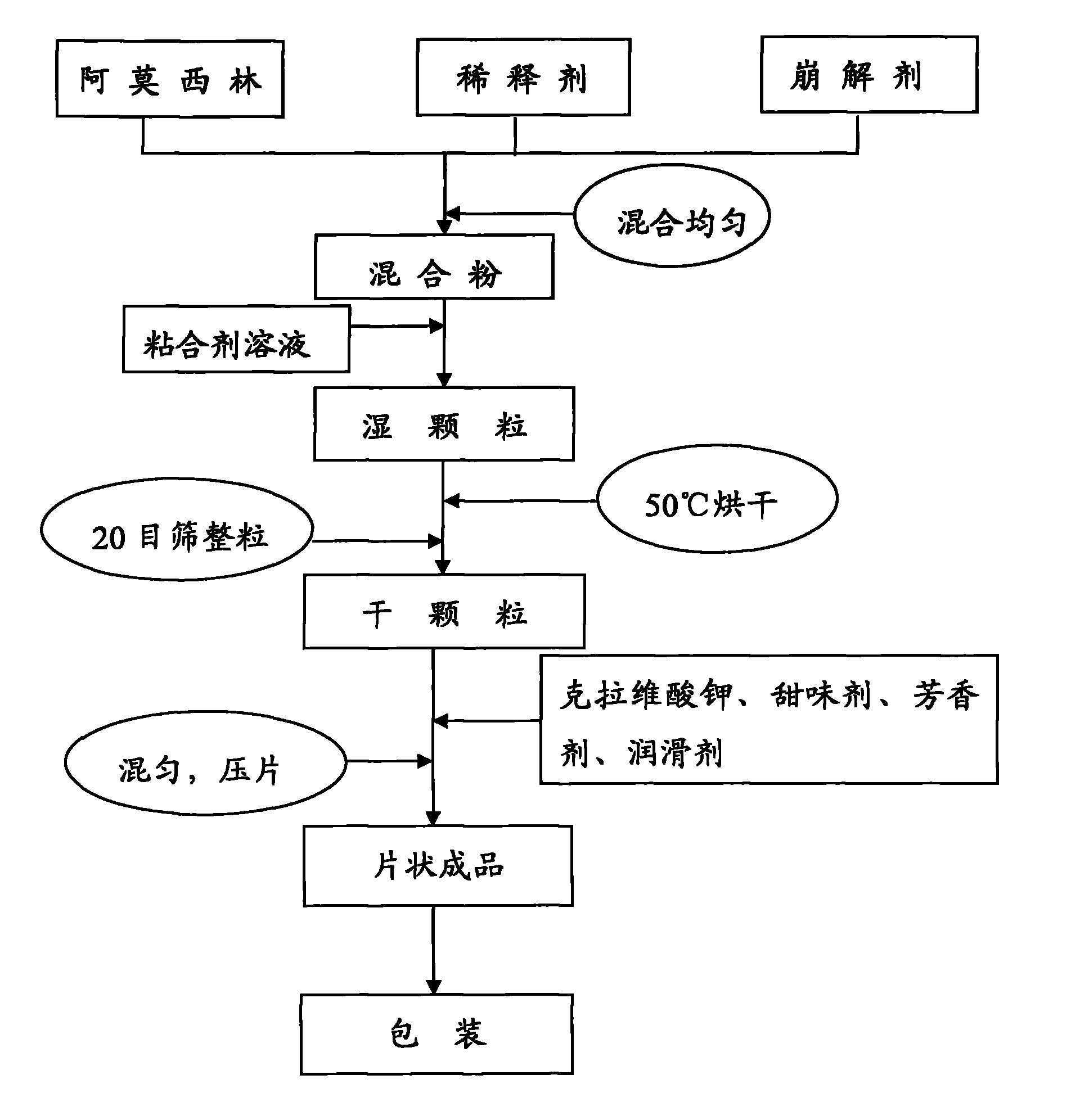
The invention relates to an amoxicillin and potassium clavulanate tablet for dogs and cats, and its preparation method and application; the tablet is mainly used for treating and preventing infectious diseases of dogs and cats. According to the invention, the problem of control scope for environmental temperature and humidity in production of the tablet is solved, accessories that guarantee the stability of the tablet are obtained through screening, and a package form that ensures stabilization of the tablet is employed. The tablet is prepared by granulating amoxicillin in advance and then mixing amoxicillin granules with potassium clavulanate for tabletting, which guarantees the quality of tabletting on one hand and reduces the possibility of decomposition of potassium clavulanate on the other hand. The amoxicillin and potassium clavulanate tablet for dogs and cats comprises, by weight, 5 to 45% of amoxicillin, 1 to 20% of potassium clavulanate, 50 to 90% of a diluent, 1 to 10% of a disintegrating agent, 1 to 5% of an adhesive, 0.1 to 3% of a sweetener, 0.2 to 2% of an aromatic and 0.5 to 3% of a lubricant, wherein the carrier for potassium clavulanate is microcrystalline cellulose. The preparation method mainly comprises the steps of pulverizing, granulating, drying, size grading, granule mixing, tabletting and packaging.
Owner:NANJING SBEED BIOTECH
Amoxicillin compound and pharmaceutical composition of amoxicillin compound and potassium clavulanate
InactiveCN103145733AImprove stabilityImprove solubilityAntibacterial agentsOrganic chemistrySolubilityStructural formula
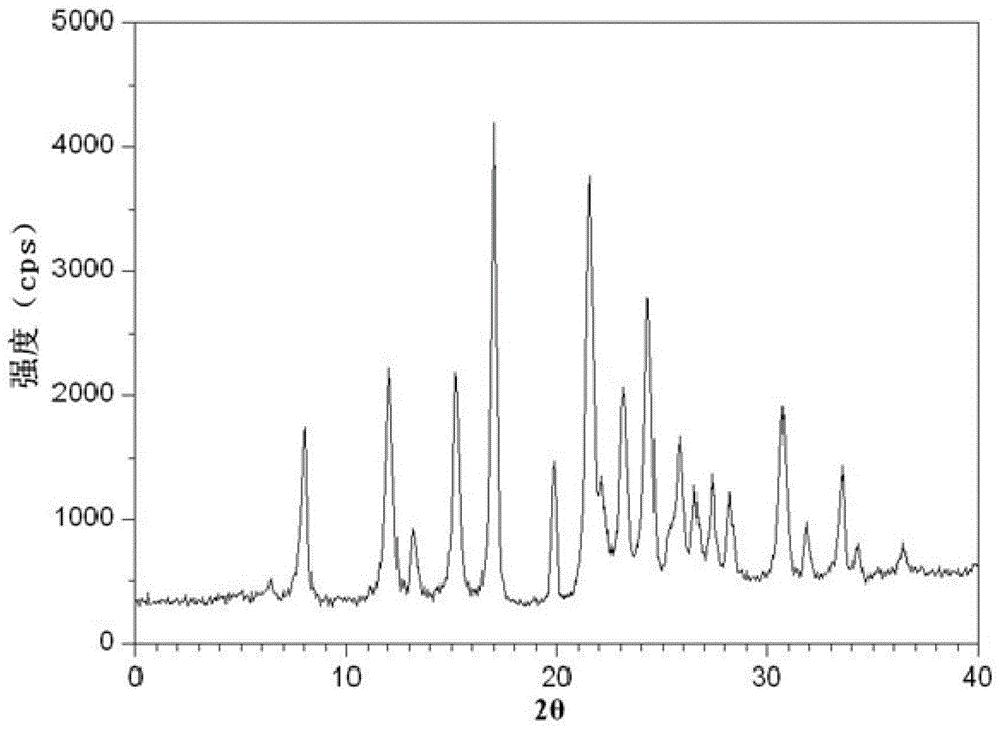
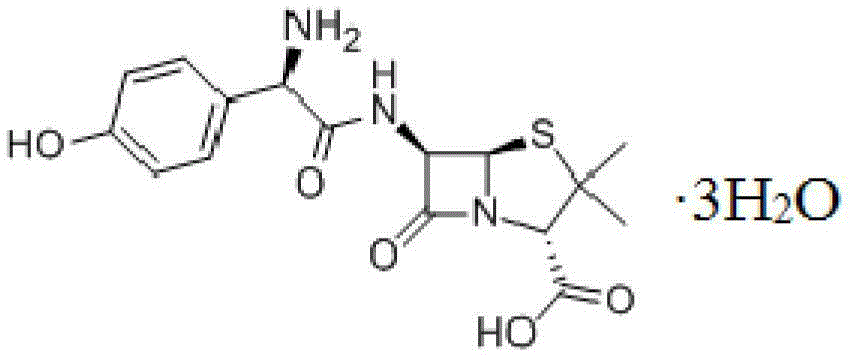
The invention relates to an amoxicillin compound and a pharmaceutical composition of the amoxicillin compound and potassium clavulanate. The pharmaceutical composition is an oral sustained-release preparation. The amoxicillin compound is a crystalline compound which is provided with the following structural formula, wherein the characteristic peaks as shown in an X-ray powder diffraction pattern obtained by measuring through Cu-K alpha rays are displayed in 2 theta of 8.0 degrees, 12.1 degrees, 15.4 degrees, 17.0 degrees, 19.8 degrees, 21.6 degrees, 23.0 degrees, 24.3 degrees, 25.7 degrees, 27.4 degrees, 30.7 degrees and 33.5 degrees. The novel crystalline compound is improved in stability and dissolubility. The amoxicillin and clavulanate potassium prepared from the crystalline compound is higher in stability. The amoxicillin and clavulanate potassium oral sustained-release preparation prepared from the crystalline compound can be synchronously released, and is excellent in in-vitro dissolution and higher in bioavailability.
Owner:四川省惠达药业有限公司
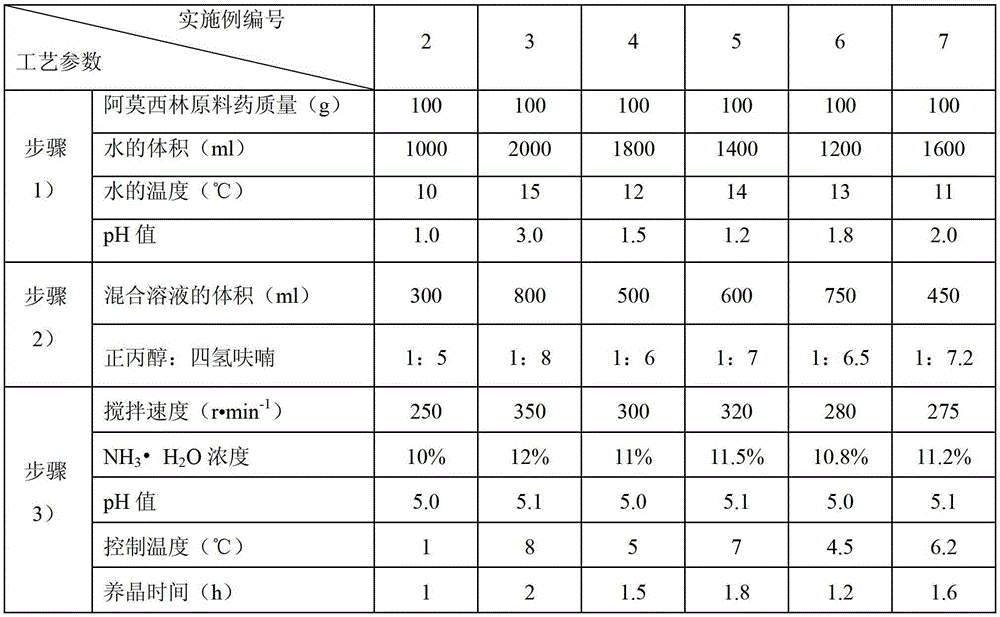
Children amoxicillin-potassium clavulanate composition
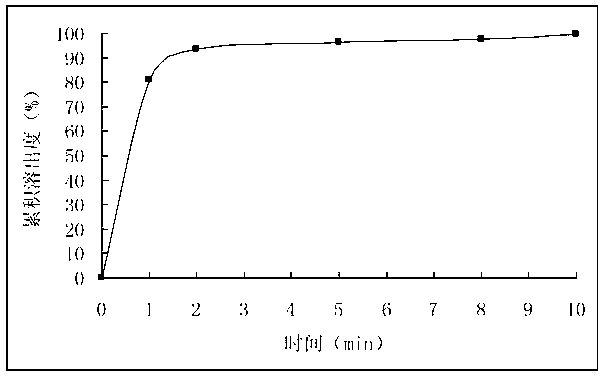
ActiveCN102525978ANon-irritatingNo grittinessAntibacterial agentsPill deliverySide effectOrally disintegrating tablet
The invention discloses a children amoxicillin-potassium clavulanate composition, relating to the technical field of medicinal preparations. The children amoxicillin-potassium clavulanate composition comprises the following components: 24wt% of amoxicillin-potassium clavulanate (7:1), 24-72wt% of mannitol, 2-4wt% of gelatin, 0.2-1.0wt% of xanthan gum and 0.1-0.15wt% of sucralose. The invention further discloses a freeze-dried orally disintegrating tablet of the children paracetamol prepared from the composition, wherein the freeze-dried orally disintegrating tablet comprises simple components, is taken without water and chewing, disintegrates in the oral cavity of a human body within 2 seconds, has the advantages of high effect-taking speed, fewer intestinal tract residue, absorption sufficiency, low side effect and good mouthfeel, and is particularly suitable for being taken by infants and babies. Furthermore, a freeze-drying preparation method is characterized in that a tertiary butanol-water cosolvent is used as a dissolvent, so that the sublimation period of a medicine can be quickened, a freeze-drying period is shortened, the dissolution of amoxicillin and the stability of medicine are enhanced, and the crystallization of the medicine is promoted.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Amoxicillin potassium clavulanate dry suspension and production process thereof
ActiveCN103127099AReduce dosageImprove stabilityAntibacterial agentsPowder deliveryPotassium ClavulanateChemistry
The invention discloses amoxicillin potassium clavulanate dry suspension and a production process thereof. The amoxicillin potassium clavulanate dry suspension is prepared from amoxicillin, potassium clavulanate, hydroxy propyl methylcellulose and the like in a mixing manner. The amoxicillin potassium clavulanate dry suspension has good compliance to a user.
Owner:贝克诺顿(浙江)制药有限公司
Antibiotic medicament containing amoxicillin nano granule and potassium clavulanate
InactiveCN101214244AHigh antibacterial activityAvoid drug resistanceAntibacterial agentsPowder deliveryButyl cyanoacrylateDistilled water
The present invention discloses an antibiotic medicine which contains amoxicillin nano-particles and clavulanate potassium and is made from 0.2 percent to 0.4 percent of amoxicillin, 0.02 percent to 0.25 percent of the clavulanate potassium, 0.5 percent to 2 percent of carrier, 0.5 percent to 2 percent of stabilizing agent, 0.5 percent to 2 percent of surfactant and 93 percent to 98.5 percent of distilled water according to the following method that: carrier monomers and the surfactant are dissolved in the distilled water completely; the amoxicillin is added; pH value is adjusted to be 2 to 3; butyl cyanoacrylate is added while being stirred by magnetic force and is stirred continuously for 5 to 12 hours, the pH value is adjusted to be 6 to 7, flaxen amoxicillin poly butyl cyanoacrylate nano-particle colloid solution is obtained; the clavulanate potassium is added by the proportion of the amoxicillin and the clavulanate potassium of 2 ®U 1 to 10 ®U 1 to obtain the antibiotic medicine which not only has simple preparation method but also has the advantages of wide spectrum, high efficiency, being targeted, slow-released, safe, etc.
Owner:NORTHWEST A & F UNIV
Pharmaceutical formulation of clavulanic acid
InactiveUS20090270358A1Process stabilityImprove stabilityBiocideCarbohydrate active ingredientsImmediate releaseBULK ACTIVE INGREDIENT
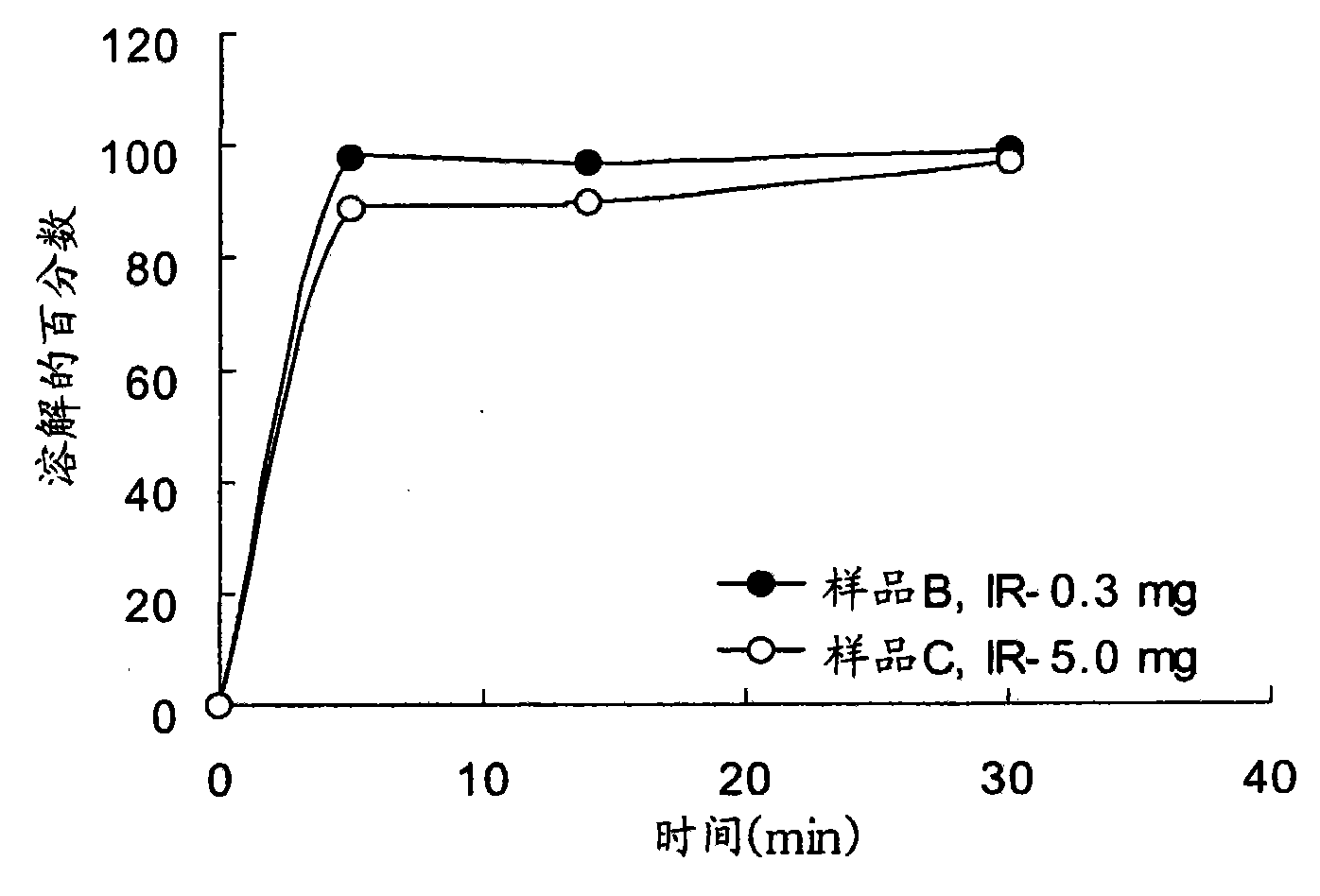
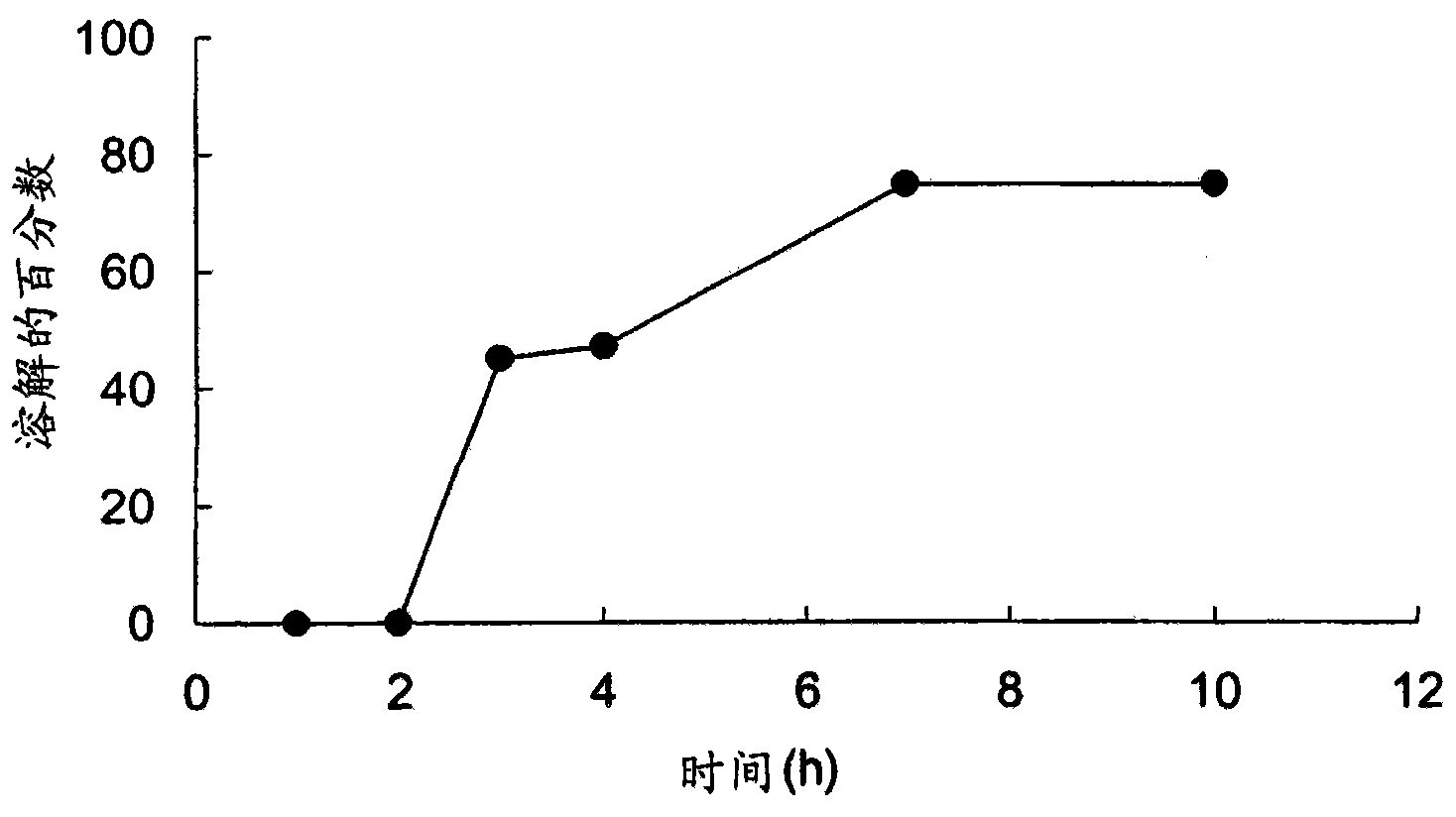
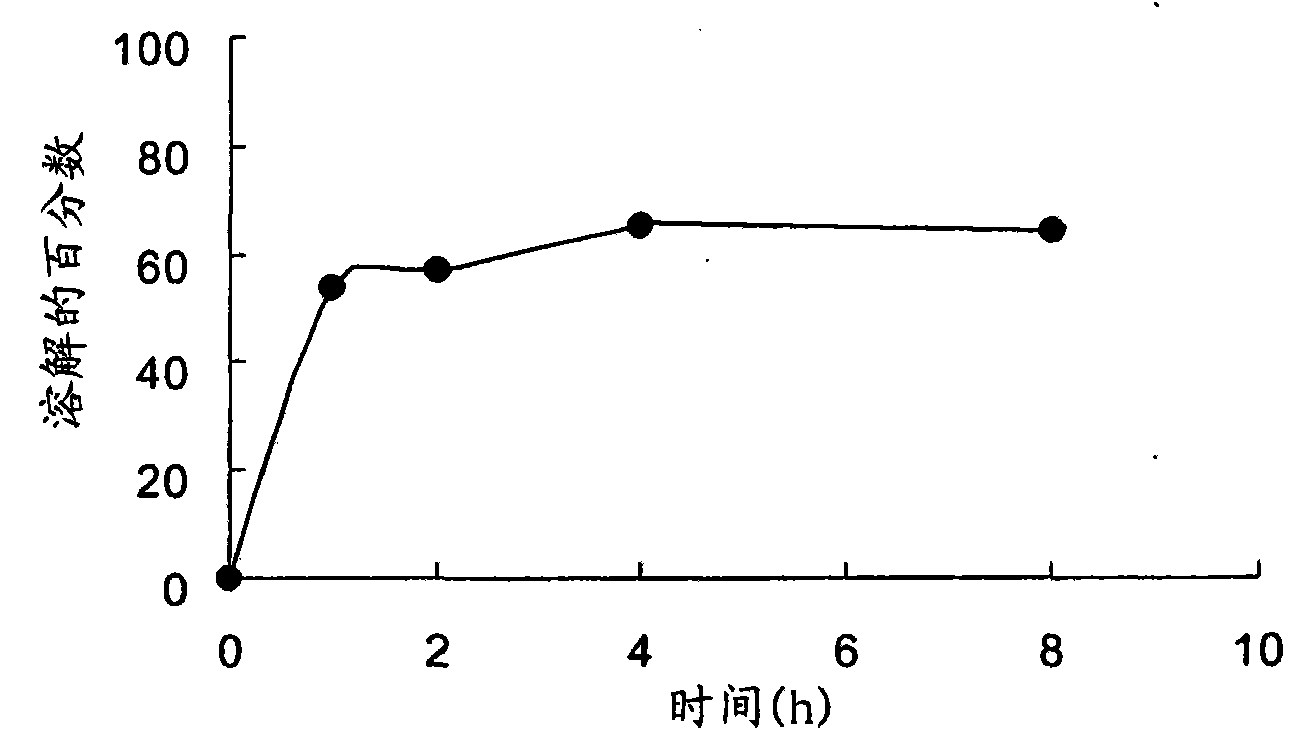
The present invention generally relates to stable pharmaceutical compositions, and methods of making and administering such compositions. In one aspect, the invention features stabilized pharmaceutical compositions that include pharmaceutically active ingredients such as potassium clavulanate or Clavitesse™, preferably in an immediate-release solid dosage form or an extended-release solid dosage form. Also provided are methods for making and using such immediate-release and stabilized compositions or extended-release and stabilized compositions.
Owner:REXAHN PHARMA INC
Compositions and methods of treatment comprising amoxicillin and potassium clavulanate with xanthan
Owner:GLAXO GRP LTD
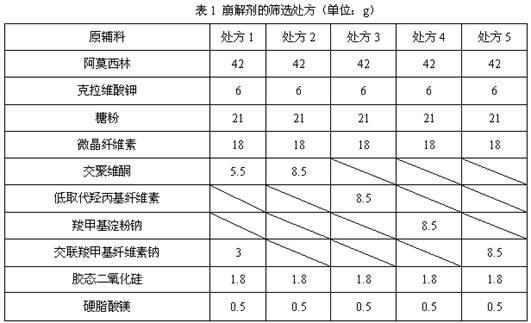
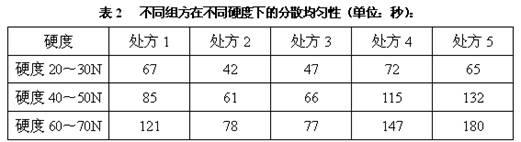
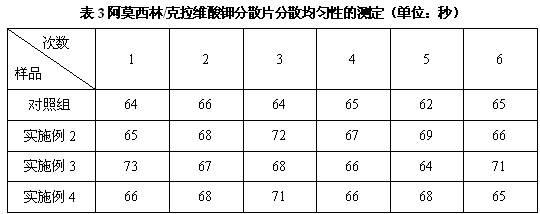
Dispersible tablet containing amoxicillin and potassium clavulanate
ActiveCN102600141AImprove dispersion uniformityHigh dissolution rateAntibacterial agentsPill deliveryWestern medicineLow-substituted hydroxypropylcellulose
The invention belongs to the technical field of western medicine preparations, and particularly relates to a dispersible tablet containing amoxicillin and potassium clavulanate. The dispersible tablet comprises the following components in parts by weight: 420 parts of amoxicillin, 60 parts of potassium clavulanate, 350-400 parts of filler, 80-100 parts of low-substituted hydroxypropyl cellulose, 1-20 parts of colloidal silicon dioxide and 5-8 parts of magnesium stearate. For the dispersible tablet, both the dispersing uniformity and dissolution achieve the technological effects of the existing product, simultaneously, and the hardness of the pressed tablet is within 50-70N, so that the problems of insufficient hardness and difficulty in common packaging existing in products on the market are solved.
Owner:NANJING CHENGONG PHARM CO LTD
Combined antibiotic medicine for inhibiting beta-lactamase
InactiveCN1526397AImprove antibacterial propertiesHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntibiotic effectTAZOBACTAM SODIUM
The combined antibiotic medicine for inhibiting beta-lactamase is prepared by combining Cefminox as one kind of the third generation of cephalosporin and beta-lactamase inhibitor in certain weight ratio. The Cefminox is in the form of its alkali metal salt or its free acid plus co-solvent; and the beta-lactamase inhibitor is Sulbactam, Clavulanic acid, tazobactam or their derivative, such as Sulperazon, tazobactam sodium, potassium Clavulanate, etc. The beta-lactamase inhibitor and Cefminox of the present invention have obvious synergistic antibiotic effect and this makes it possible to solve the increasing resistance of pathogenetic bacteria to Cefminox clinically.
Owner:周宇
Method of recovering effective components from potassium clavulanate mixed powder
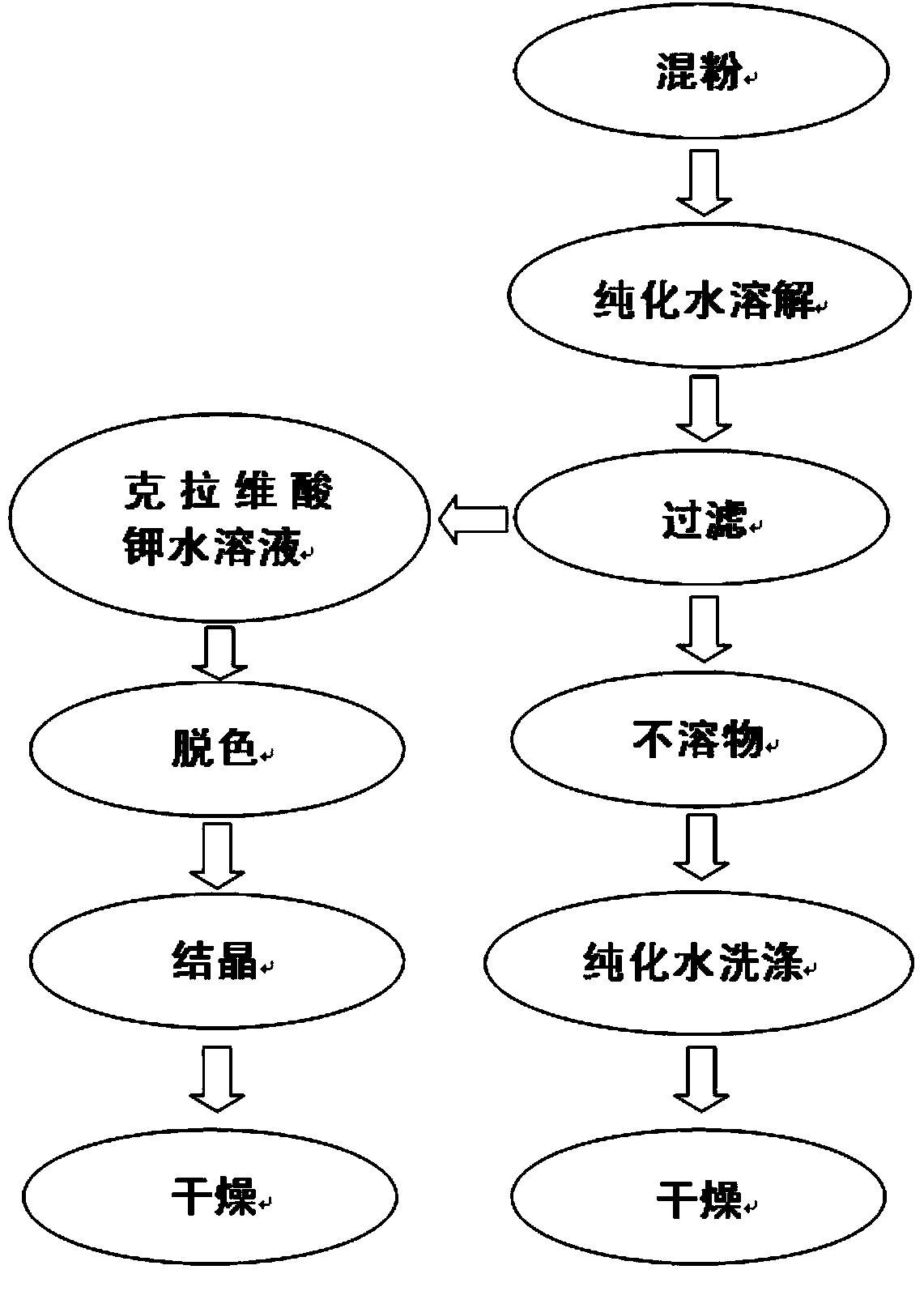
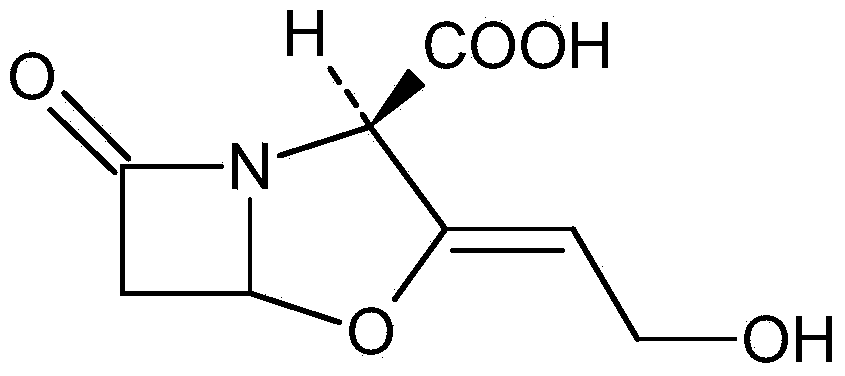
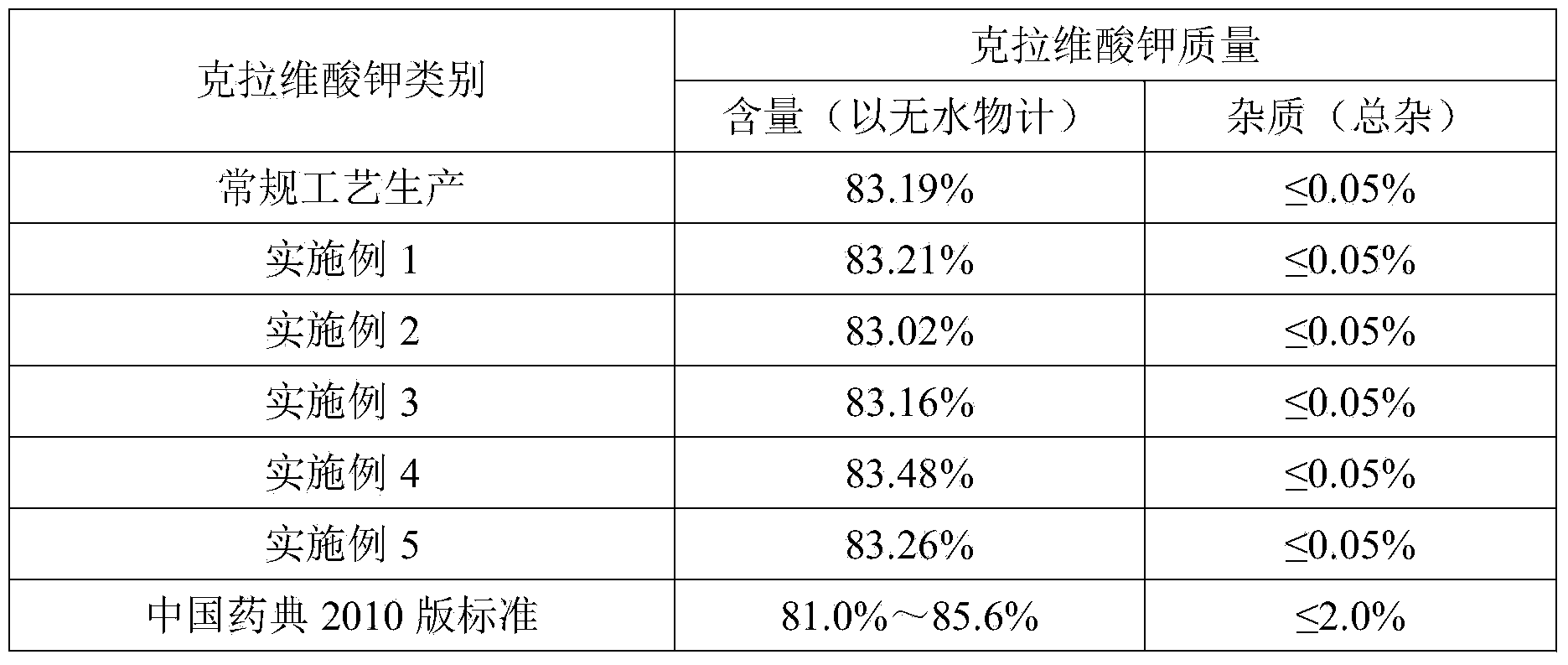
The invention relates to a method of recovering effective components from potassium clavulanate mixed powder. The method comprises the following steps: dissolving potassium clavulanate in the mixed powder by purified water according to different solubilities of various effective components in the mixed powder; initially separating as other components are insoluble; and further purifying to obtain the effective components such as potassium clavulanate which satisfies the requirements of pharmacopeia. The invention creatively develops the novel method of recovering effective components from potassium clavulanate mixed powder and can be used for recovering effective components such as potassium clavulanate from unqualified potassium clavulanate mixed powder so as to turn waste into wealth, thereby satisfying the requirements on green production and circulating economy. Meanwhile, the method is simple in process, high in yield and low in cost, and has high practical and economic values.
Owner:SHANDONG NEWTIME PHARMA
Drug composition of amoxicillin potassium clavulanate and application thereof
ActiveCN106474122AElevated serum IgEEnhanced inhibitory effectAntibacterial agentsAntisepticsAir pollutionPotassium Clavulanate
The invention provides a drug composition, wherein the ratio of amoxicillin and potassium clavulanate in the drug composition is 7: 1. The drug composition can significantly lighten the rise of IgE level induced by allergen, and can be used for preventing or treating asthma, in particular to the asthma caused by air pollution such as haze; besides, the drug has good effect for the asthma combined bacterial infection.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
Compound pharmaceutical composition of amoxicillin sodium and potassium clavulanate and preparation method of compound pharmaceutical composition
ActiveCN105362235AImprove stabilitySolve the problem of unstable compatibilityAntibacterial agentsPowder deliveryPotassium ClavulanateSodium carbonate
The invention discloses a compound pharmaceutical composition of amoxicillin sodium and potassium clavulanate. The injection composition consists of amoxicillin sodium, potassium clavulanate and sodium carbonate which serves as a pH value regulator; and the pH value of a mixed solution is regulated to be 8.5-10.0 through the sodium carbonate. In addition, the invention also discloses a preparation method of the compound pharmaceutical composition. The compound pharmaceutical composition disclosed by the invention, through a formula and a process, can solve the problem of poor stability in the prior art, in particular problem of instable compatibility, so that clinical medication is safer.
Owner:FUAN PHARM (GRP) CO LTD +1
Compound sulfamonomethoxine sodium injection as well as preparation method thereof
InactiveCN103550226AHigh cure rateReduce dosageAntibacterial agentsOrganic active ingredientsDiseaseSulfamonomethoxine
The invention discloses a compound sulfamonomethoxine sodium injection, and relates to the field of preparation of veterinary medicines. The compound sulfamonomethoxine sodium injection is prepared from medicines and auxiliary materials such as sulfamonomethoxine sodium, propylene glycol, ethanol, an antioxidant, EDTA (Ethylene Diamine Tetraacetic Acid) and potassium clavulanate. The invention further provides a preparation method of the compound sulfamonomethoxine sodium injection. The compound sulfamonomethoxine sodium injection disclosed by the invention as a novel veterinary compound preparation can be effective by one injection for frequently occurred epidemic mixed influenza. The compound sulfamonomethoxine sodium injection disclosed by the invention has a unique curative effect to porcine contagious pleuropneumonia, cough and enzootic pneumoniae. The compound sulfamonomethoxine sodium injection is superior to a common medicine florfenicol in both curative effect and porcine weight increment.
Owner:南通海门凤成旅游文化发展有限公司
Preparation method of potassium clavulanate/microcrystalline cellulose composition
ActiveCN102058584AAvoid explosionImprove securityAntibacterial agentsOrganic active ingredientsTert-ButylamineFiltration
The invention discloses a preparation method of a potassium clavulanate / microcrystalline cellulose composition, belonging to the preparation field of pharmaceutical raw materials. The preparation method of the invention comprises the following steps of: dissolving clavulanic tert-butylamine powder as a raw material in an isopropyl alcohol solution to prepare a clavulanic tert-butylamine-isopropyl alcohol solution; then, dipping a potassium ethylhexanoate-isopropyl alcohol solution to the clavulanic tert-butylamine-isopropyl alcohol solution until complete crystallization is finished; then, adding microcrystalline cellulose powder in 60-70 percent by weight of the clavulanic tert-butylamine powder; and finally, carrying out suction filtration, washing and drying to obtain the potassium clavulanate / microcrystalline cellulose composition. The adopted preparation method of the invention has low cost, high safety factor and short process flows and is suitable for mass production.
Owner:石药集团中诺药业(石家庄)有限公司
Cefquinome sulfate injection and preparation method thereof
InactiveCN112691108AExpanded antimicrobial spectrumImprove efficacyAntibacterial agentsPharmaceutical product form changeAntiviral drugHypericin
The invention discloses a cefquinome sulfate injection and a preparation method thereof, and belongs to the field of biological medicines. The injection comprises a carrier, a surfactant, a suspending agent and medicinal components, wherein the medicinal components include the following components in percentage by mass of 60-90% of cefquinome sulfate and 1-40% of a synergist; and the synergist is any one or a composition of more of hypericin, sulbactam, trimethoprim and potassium clavulanate in any proportion. According to the invention, the cefquinome sulfate is used as a main bactericidal and antiviral drug, and the hypericin, the sulbactam, the trimethoprim and the potassium clavulanate are used as the synergist, so that the antibacterial spectrum of the whole injection can be enhanced, the medicine effect can be enhanced, and the cure rate can be increased. Low-temperature high shearing is adopted, so that the viscosity of the injection is reduced, and the stability is enhanced.
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
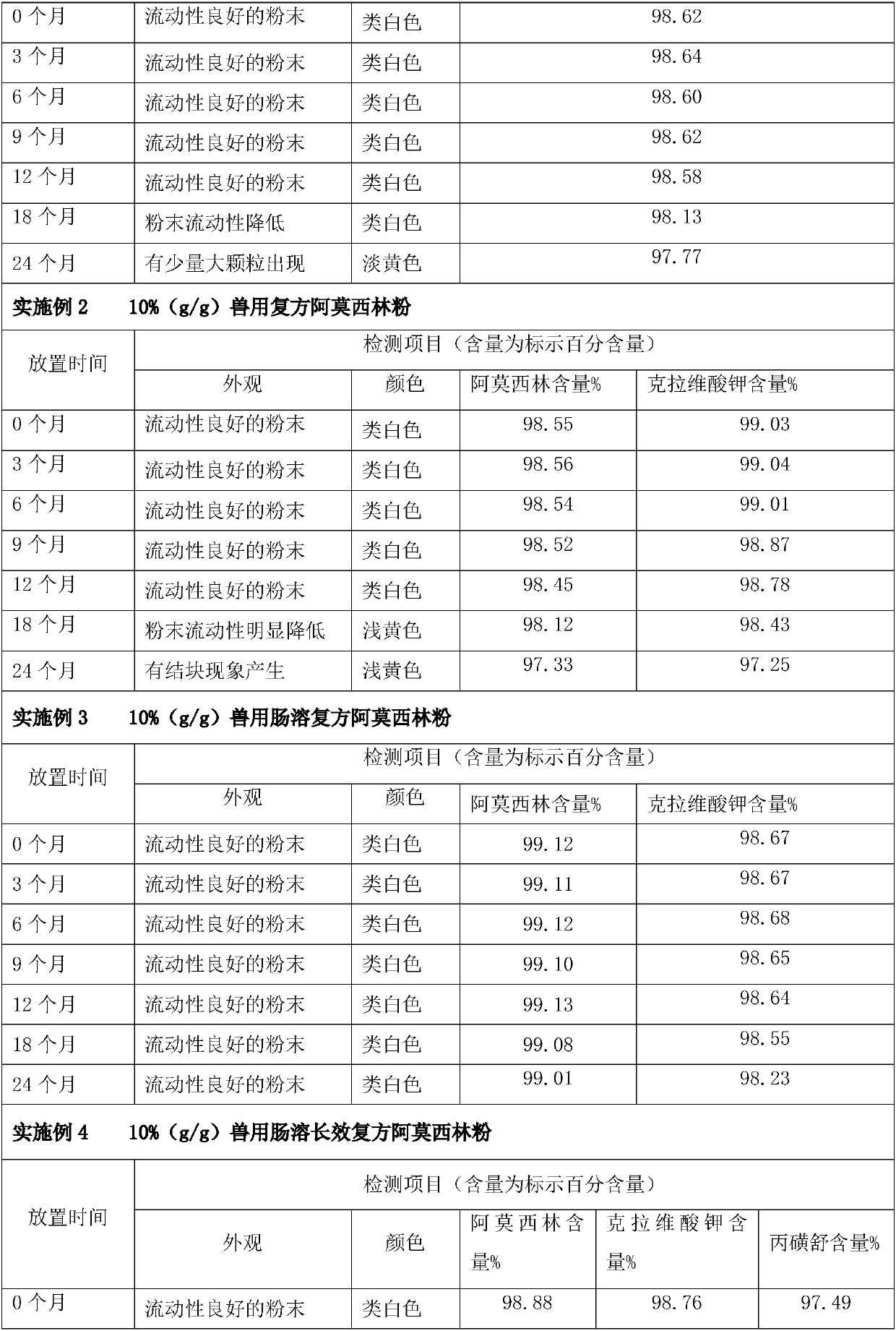
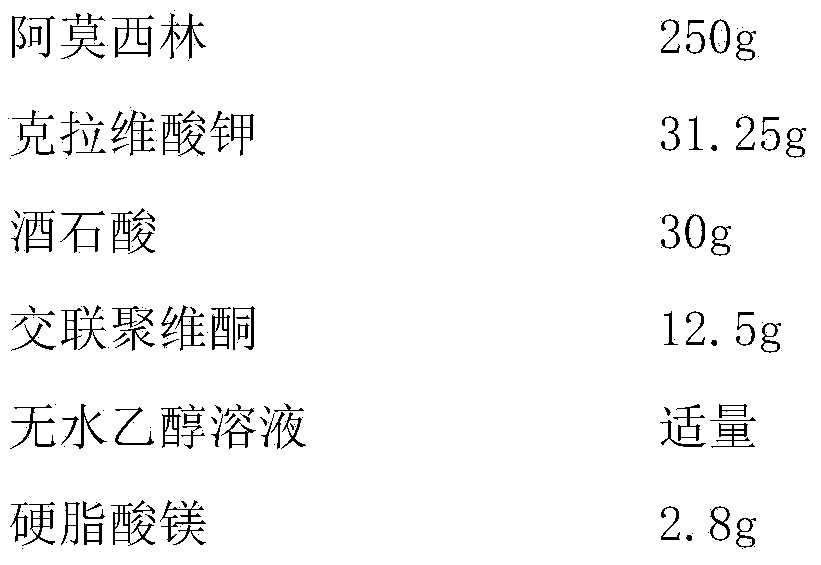
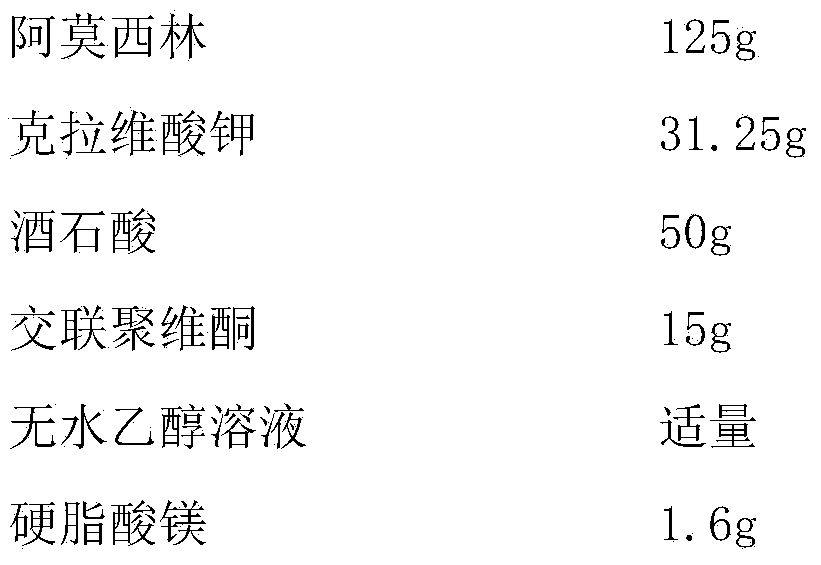
Enteric long-acting compound amoxicillin powder for veterinary use and preparation method thereof
InactiveCN107661507AAvoid decompositionImprove solubilityAntibacterial agentsPowder deliveryDiseaseAcrylic resin
The invention relates to enteric long-acting compound amoxicillin powder for veterinary use and a preparation method thereof. Every 100g of the enteric long-acting compound amoxicillin powder for veterinary use comprises the following components by weight: 8-10g of amoxicillin, 2-3g of potassium clavulanate, 8-10g of probenecid, 20-40g of beta-cyclodextrin, 10-20g of micropowder silica gel, 1-5% of acrylic resin and the balance of glucose. The main drug amoxicillin of the preparation is kept stable in a strong acid environment in the upper portion of the stomach and is not damaged by the strong acid; when the pH of the lower portion of the stomach is close to 6, amoxicillin is released after an enteric material is dissolved; potassium clavulanate can reduce degradation of beta-lactamase generated by sensitive bacteria, and probenecid can prolong discharge of amoxicillin in kidneys, so that the blood concentration is effectively prolonged. According to the product, the bioavailability of amoxicillin is improved greatly, the treatment course of livestock diseases is shortened, the treatment cost of farmers is lowered, and a high quality preventing drug is provided for animal husbandry.
Owner:ANIMAL MEDICINE SICHUAN FEIYANG +1
Powder injection composed of sodium cefoperazone and potassium clavulanate
A powder injection for treating bacterium-infectious diseases is prepared from cefoperazone sodium and potassium clavulanate in weight ratio of (1-16):1.
Owner:苏大阳
Medicine for preventing and treating enterogastritis in bamboo rats and preparation method of medicine
InactiveCN108635468APromote digestionEnhance digestionOrganic active ingredientsDigestive systemIntestinal structureSodium Bentonite
The invention belongs to the technical field of breeding of bamboo rats, and in particular discloses a medicine for preventing and treating enterogastritis in bamboo rats and a preparation method of the medicine. The medicine for preventing and treating enterogastritis in the bamboo rats is mainly prepared by the following materials through baking, extracting, mixing and drying: 10-15 parts of bentonite, 5-9 parts of silicon dioxide, 2-5 parts of sulfaguanidine, 1-5 parts of potassium clavulanate, 1-3 parts of a yeast, 5-10 parts of cherokee rose fruit, 3-8 parts of tea leaves, 3-8 parts of fructus crataegi, 2-8 parts of flos chrysanthemi and 1-5 parts of herba patriniae. The medicine for preventing and treating enterogastritis in bamboo rats provided by the invention, in accordance with principles of cooperative matching and assistant matching, combines chemical medicines and natural Chinese herbal medicines; the medicine mainly functions as diminishing inflammation and killing bacteria, clearing way heat and toxic materials, assisted by effects of clearing intestines and checking diarrhea; the medicine can be used for effectively preventing and treating the enterogastritis in bamboo rats and bring about benefits for healthy growth of the bamboo rats; in addition, the preparation method is simple; and various raw materials can be effectively combined.
Owner:陆川县米场镇华屋竹鼠养殖场
Amoxicillin potassium clavulanate tablet and method for preparing same
ActiveCN103417535AImprove stabilityLess varietyAntibacterial agentsPharmaceutical non-active ingredientsDissolutionMoisture
The invention discloses an amoxicillin potassium clavulanate tablet. In the tablet, the weight ratio of amoxicillin and potassium clavulanate is 4-8:1. The tablet is formed by even mixing of medicated granules, the potassium clavulanate, a disintegrating agent and a lubricating agent in a direct tablet compressing mode, wherein the disintegrating agent is dried to have the moisture content lower than 3%. The medicated granules comprise amoxicillin and tartaric acid, wherein the weight ratio of the amoxicillin and the tartaric acid is 1:0.1-0.5. The tablet is rapid in dissolution, good in stability, few in auxiliary material kind and simple in preparation technology.
Owner:HAINAN ZHONGJI MEDICAL TECH
Novel process for the isolation of clavulanic acid and of pharmaceutically acceptable salts thereof
A process for the isolation of clavulanic acid and pharmaceutically acceptable salts thereof, such as potassium clavulanate, from the aqueous fermentation broth of a clavulanic acid-producing microorganism comprises the microfiltration of the broth without prior treatment.
Owner:LEK PHARMA D D
Compositions and methods of treatment comprising amoxicillin and potassium clavulante with xanthan
Bacterial infections may be treated using a high dosage regimen of amoxicillin and potassium clavulanate. Preferably, the dosage is provided by a bilayer tablet.
Owner:BEECHAM PHARMACEUTICALS PTE LTD
Pharmaceutical formulation of clavulanic acid
InactiveCN101918004AReduce moisture contentCarbohydrate active ingredientsPill deliveryImmediate releaseBULK ACTIVE INGREDIENT
The present invention generally relates to stable pharmaceutical compositions, and methods of making and administering such compositions. In one aspect, the invention features stabilized pharmaceutical compositions that include pharmaceutically active ingredients such as potassium clavulanate or ClavitesseTM, preferably in an immediate-release solid dosage form or an extended-release solid dosage form. Also provided are methods for making and using such immediate-release and stabilized compositions or extended-release and stabilized compositions.
Owner:REXAHN PHARMA INC
Preparing method of cefodizime sodium for injection
InactiveCN106309449AImprove stabilityEasy to prepareAntibacterial agentsPowder deliveryActivated carbonCefodizime Sodium
The invention discloses a preparing method of cefodizime sodium for injection, wherein the method comprises the following steps of adding cefodizime sodium to water to obtain the first solution after stirring and dissolving; adding citric acid and potassium clavulanate to the first solution to obtain a second solution after it is completely stirred and dissolved; Adjusting the pH of the second solution to 6.4-6. 6 to obtain a third solution; adding hydroxypropyl Beta-cyclodextrin to the third solution, heating to 53-58 oC, stirring and mixing for 1-2 hours, and cooling to room temperature to obtain a fourth solution; adding the activated carbon with the total weight of 1-2% to the fourth solution, stirring and mixing for 20-30min, and removing the activated carbon by coarse filtration to obtain a fifth solution; The fifth solution is filtered through a microporous membrane to obtain an injecta; freeze-drying the injecta to the powder; the preparing of cefodizime sodium for injection enjoys good stability, and the preparing method is simple, safe and reliable, suitable for clinical use, and industrial production can be carried out on a large scale, with a wide range of applications.
Owner:南昌立健药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com