Amoxicillin potassium clavulanate tablet and method for preparing same
A technology of amoxicillin and clavulanate potassium tablets and clavulanate potassium, which is applied in the field of medicine and can solve problems such as the stability of amoxicillin and clavulanate potassium that are not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
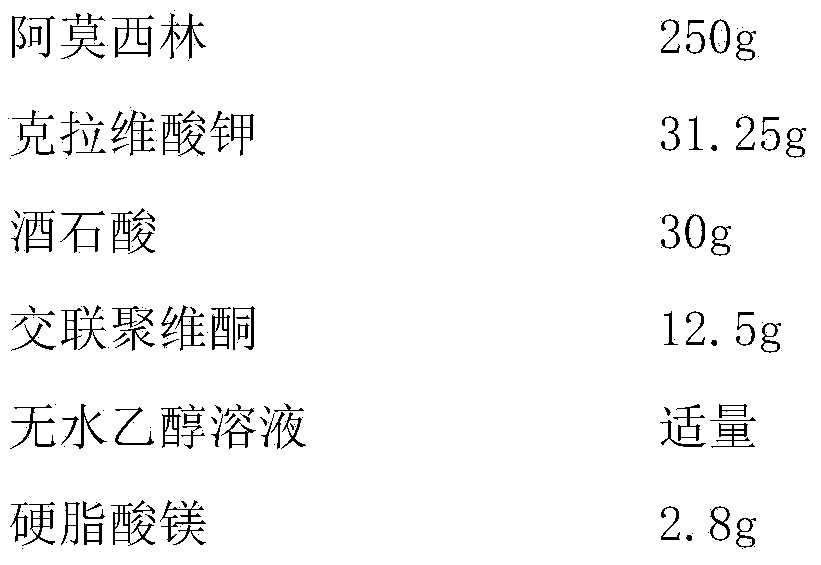
[0027] Embodiment 1 amoxicillin-clavulanate potassium tablet and preparation technology thereof
[0028]
[0029] Preparation Process:
[0030] (1) Pass amoxicillin and tartaric acid through a 100-mesh sieve, mix evenly, add absolute ethanol for wet granulation, granulate with a 16-mesh sieve, and dry at 50°C to obtain drug-containing granules for later use;
[0031] (2) Dry the disintegrant at 105°C until the moisture content is 2.6%, cool to room temperature under the condition of relative humidity less than 45%RH, pass through a 80-mesh sieve, and set aside;
[0032] (3) Under the condition that the relative humidity is less than 45%RH, pass potassium clavulanate through an 80-mesh sieve for later use;
[0033] (4) Mix the drug-containing granules with the sieved crospovidone and potassium clavulanate evenly, add magnesium stearate, mix evenly, and directly compress into tablets.
Embodiment 2
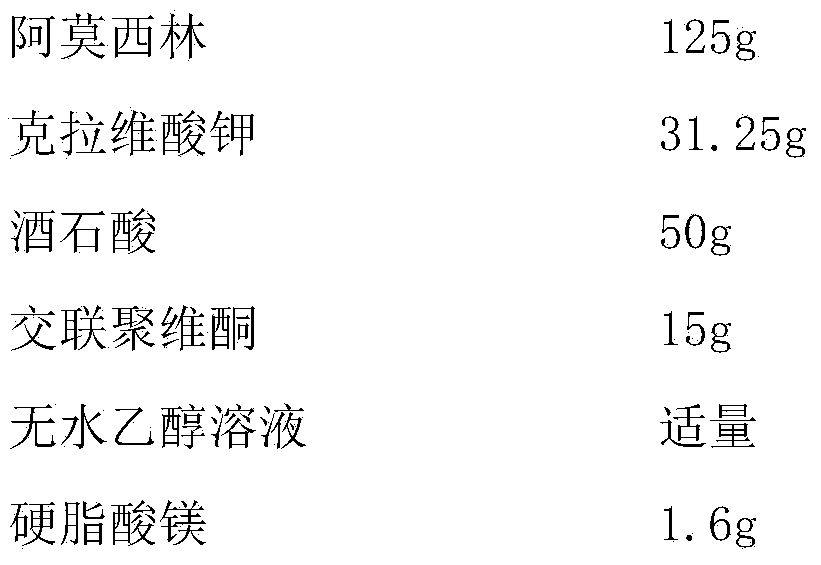
[0034] Embodiment 2 Amoxicillin-clavulanate potassium tablet and preparation technology thereof
[0035]
[0036] Preparation Process:
[0037] (1) Pass amoxicillin and tartaric acid through a 80-mesh sieve, mix evenly, add absolute ethanol to wet granulate, granulate with a 18-mesh sieve, and dry at 45°C to obtain drug-containing granules for later use;
[0038] (2) Dry the disintegrant at 105°C until the moisture content is 2.9%, cool to room temperature under the condition of relative humidity less than 45%RH, pass through a 80-mesh sieve, and set aside;
[0039] (3) Under the condition that the relative humidity is less than 45%RH, pass potassium clavulanate through an 80-mesh sieve for later use;
[0040] (4) Mix the drug-containing granules with the sieved crospovidone and potassium clavulanate evenly, add magnesium stearate, mix evenly, and directly compress into tablets.
Embodiment 3
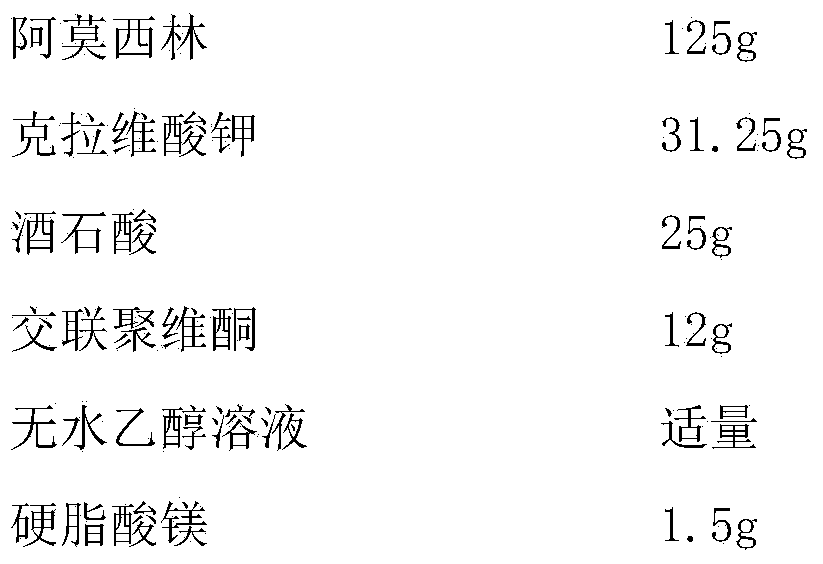
[0041] Embodiment 3 amoxicillin-clavulanate potassium tablet and preparation technology thereof
[0042]
[0043] Preparation Process:
[0044] (1) Pass amoxicillin and tartaric acid through a 100-mesh sieve, mix evenly, add absolute ethanol for wet granulation, granulate with a 18-mesh sieve, and dry at 45°C to obtain drug-containing granules for later use;
[0045] (2) Dry the disintegrant at 105°C until the moisture content is less than 2.8%, cool to room temperature under the condition of relative humidity less than 45%RH, pass through a 80-mesh sieve, and set aside;
[0046] (3) Under the condition that the relative humidity is less than 45%RH, pass potassium clavulanate through an 80-mesh sieve for later use;
[0047] (4) Mix the drug-containing granules with the sieved crospovidone and potassium clavulanate evenly, add magnesium stearate, mix evenly, and directly compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com