Pharmaceutical formulation of clavulanic acid
A technology of clavulanic acid and clavulanic acid potassium, applied in the field of depression, immediate release composition and sustained release composition, sexual dysfunction and neurological disorders, and treatment of anxiety, which can solve problems such as no CNS effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of clavulanic acid substance tablet
[0065] Example 1A - Preparation of Immediate Release Clavulanic Acid Using Potassium Clavulanate Powder Qualitative tablet
[0066] Illustrative description of tablet manufacturing process: A wet granulation tablet formulation process has been found in which water is included in the granulation step followed by drying to obtain granules with low moisture content (<3%). The dry formulations are non-hygroscopic compared to prior art formulations, but maintain equivalent physical properties (eg, solubility, disintegration, bioavailability, and other physical properties) of tablets made from them. Tablet preparation is carried out by granulating the clavulanic acid with water in the presence of a binder / diluent.
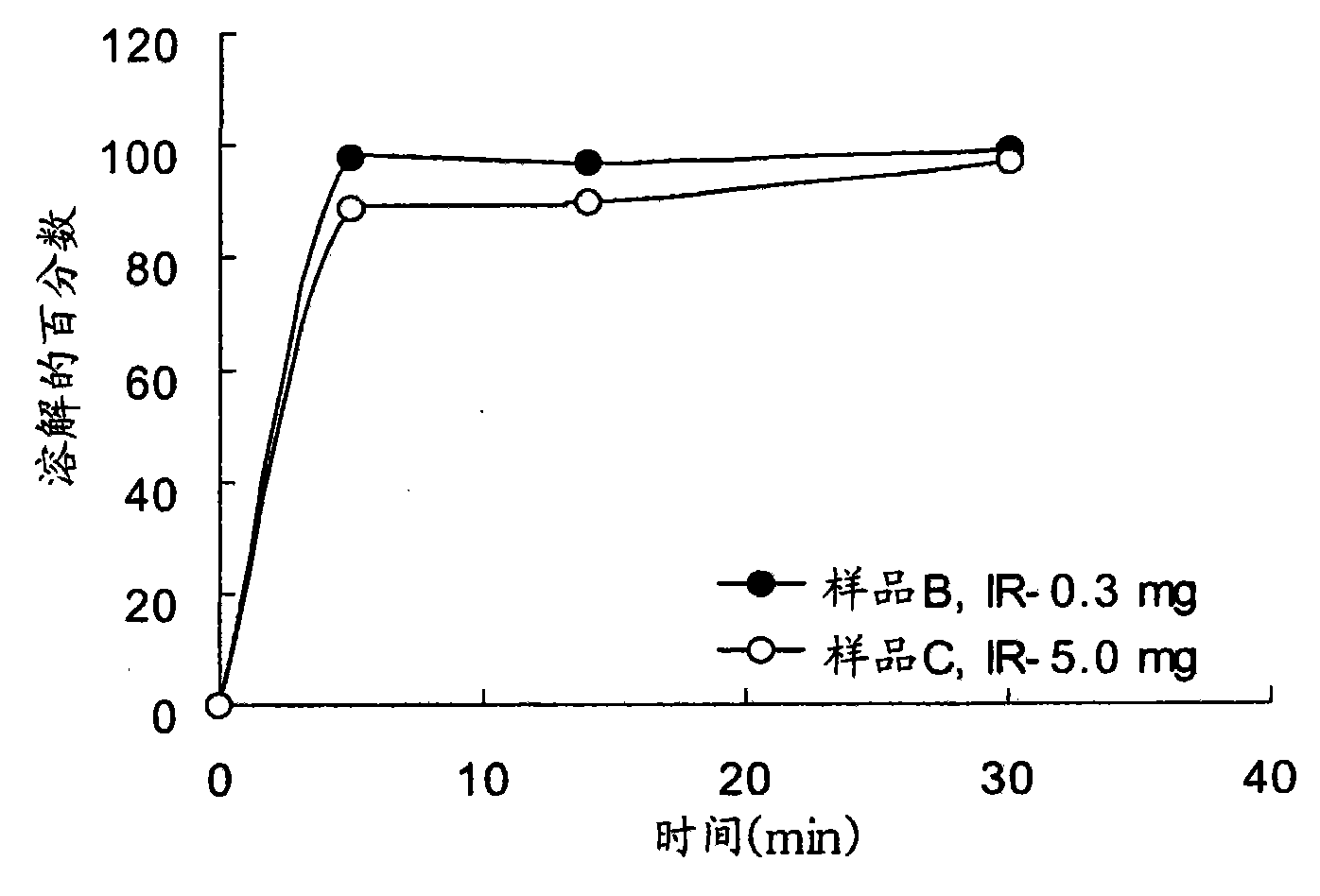
[0067] For the preparation of sample C, Maltrin M150 (130 g) was dissolved in purified water and potassium clavulanate (API; 59.5 g) was added. Prosolve SMCC-50 (490.5g), Pharmaburst (130.0g...
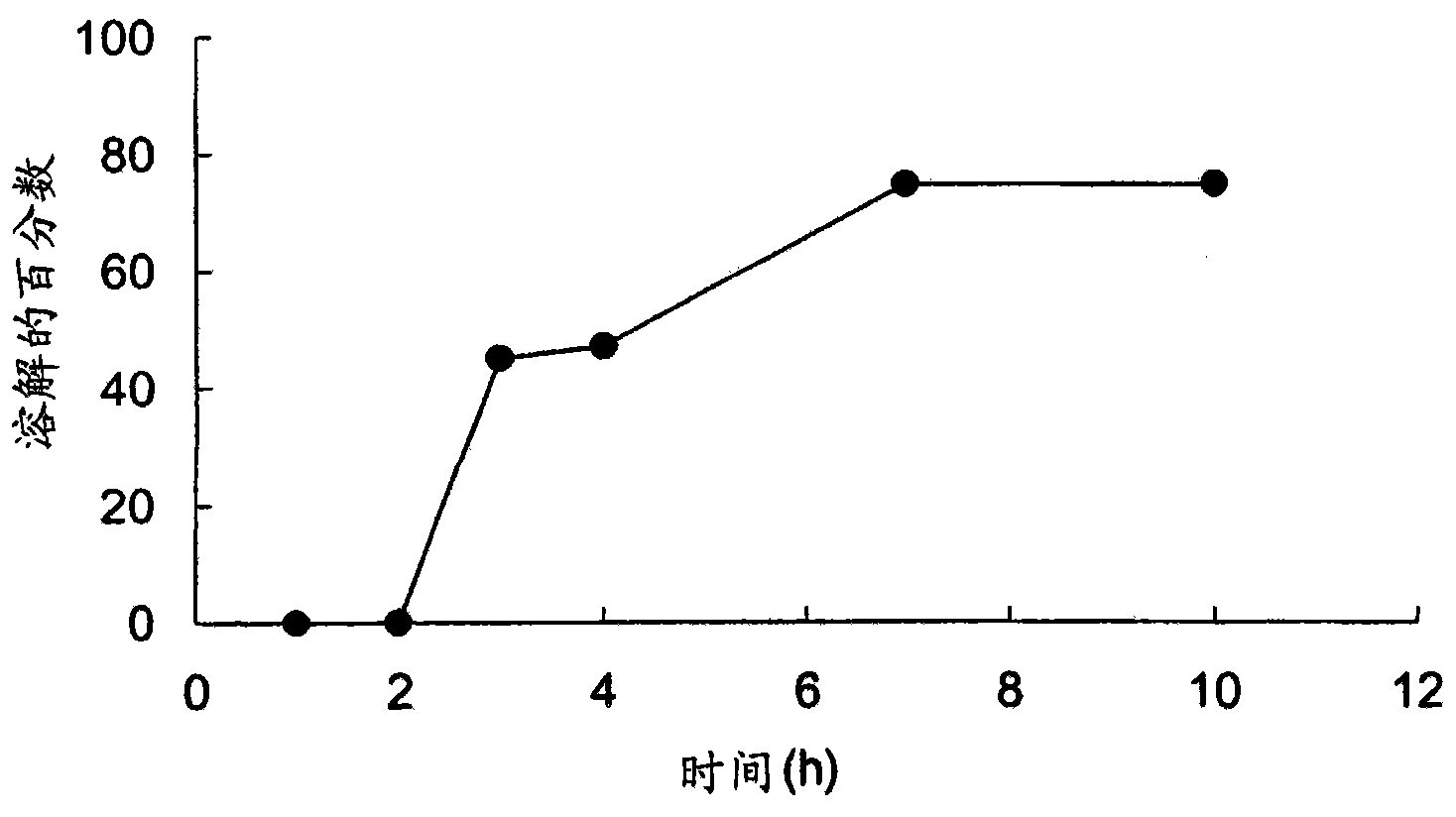
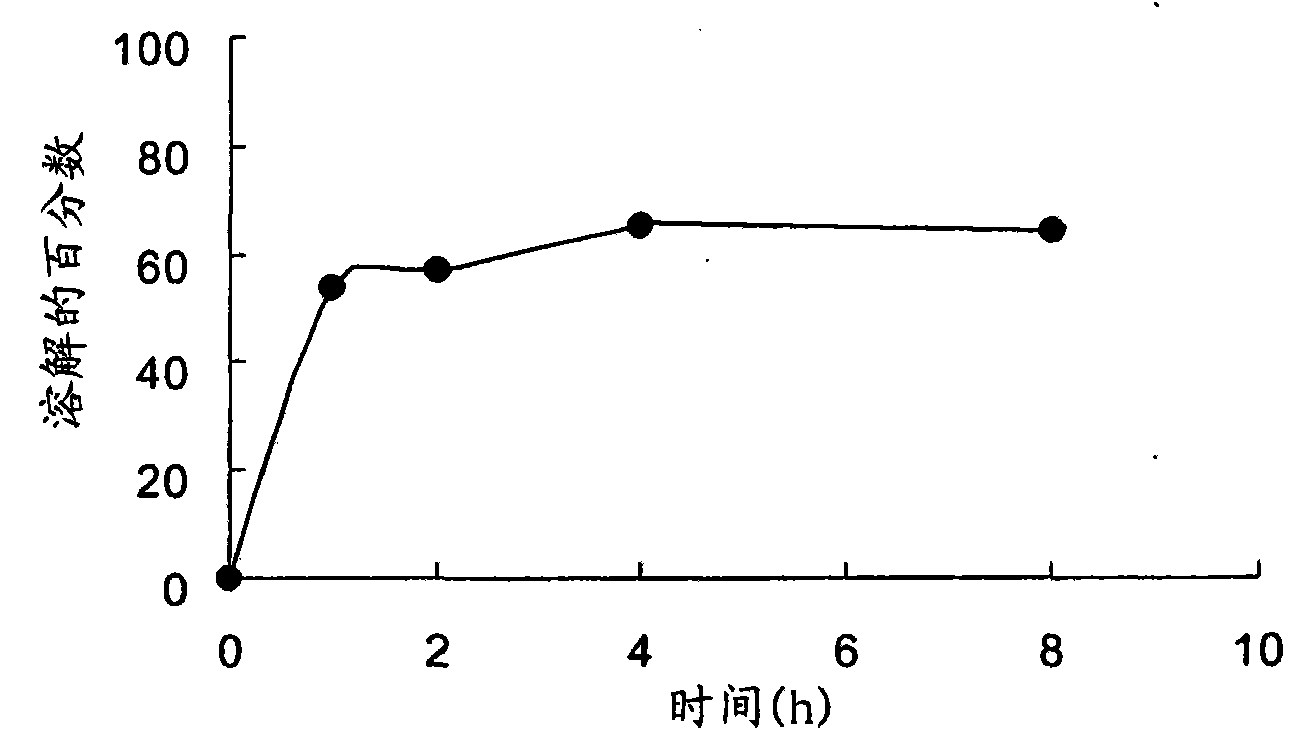
Embodiment 1D
[0072] Example 1D - Preparation of slow-release clavulanates using potassium clavulanate powder Qualitative tablet
[0073] For the preparation of sample G for extended release tablets using potassium clavulanate, potassium clavulanate (API; 20.69g) was sieved through a #60 mesh sieve, while other excipients, Methocel K100LVPrem CR (90.02g), Isomalt (83.56g), Avicel PH-112 (100.41g), Cabosil (1.52g), talc (2.4g) and magnesium stearate (1.5g) were sieved through a #40 mesh sieve. Each component was collected in a separate bag. The API and Methocel K100LV Prem CR were loaded into a V-type mixer and mixed for 5 minutes. The mixture was sieved and mixed for an additional 5 min. Avicel PH-112 and Isomalt were added to the mixture and mixed in a V-blender for 5 minutes. The resulting mixture was sieved and mixed for an additional 5 min. Cabosil and talc were mixed and added to the mixture and the resulting mixture was then mixed for 2 min. Finally, magnesium stearate was bl...
Embodiment 2
[0074] Example 2: Clavulanates thing qualitative analysis
[0075] Clavulanates of the prepared pharmaceutical composition thing The content of the substance was passed through the Waters HPLC (high performance liquid chromatography) system (column: μ Bondapack-NH 2 (10μm)300mm×3.9mm, mobile phase: CH 3 CN: pH 5.2KH 2 PO 4 =65:35, flow rate: 1.0ml / min) The following steps were used for the determination: about 10 tablets were accurately weighed and crushed, 100 mL of water was added and the mixture was sonicated for 20 min. After dilution with water, a portion of the solution was filtered and injected into HPLC. The main peak is determined by the retention time of the sample corresponding to the chromatogram of the HPLC standard preparation. Clavulanates thing The mass % is calculated based on the response factor of the analyte relative to the response factor of the reference standard.
[0076] Clavulanates thing The linearity of the quality standard curve was tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com