Compositions and methods of treatment comprising amoxicillin and potassium clavulante with xanthan
a technology of potassium clavulanate and amoxicillin, which is applied in the field of amoxicillin and potassium clavulanate novel treatment methods and novel formulations, can solve the problems of reducing bioavailability, no advantage, and failing to show any advantage over a conventional capsul
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1000 / 62.5 mg Modified Release Tablet
[0145]
Ingredientmg / tablet% w / wImmediate Release LayerAmoxicillin Trihydrate (ERH 654.1*40.88Potassium Clavulanate76.2#4.76Microcrystalline Cellulose136.48.52Sodium Starch Glycollate18.01.12Colloidal Silicon Dioxide6.30.39Magnesium Stearate9.00.56Total (Immediate Release Layer)900.056.23Slow Release LayerCrystallised Sodium Amoxicillin480.8**30.05Microcrystalline Cellulose113.27.08Xanthan Gum14.00.87Citric Acid78.04.87Colloidal Silicon Dioxide1.500.08Magnesium Stearate14.00.87Total (Sustained Release Layer)700.043.74Film coatOpadry YS-1-7700 - Composition:Hydroxypropylmethylcellulose 2910 6 cp11.6Hydroxypropylmethylcellulose 2910 15 cp3.9Titanium dioxide15.1Polyethylene Glycol 33502.3Polyethylene Glycol 80002.3Total weight of coated tablet1635.2
*Equivalent to 562.5 mg of amoxicillin based on an assay of 86.0%
#Equivalent to 62.5 mg of clavulanic acid based on an assay of 82.0%
**Equivalent to 437.5 mg amoxicillin based on an assay of 91.0%
example 2
[0146] 1000 / 62.5 mg modified release tablet The immediate release layer and film coat are as for the tablet of Example 1
Ingredientmg / tablet% w / wSlow Release LayerCrystallised Sodium Amoxicillin480.8**30.05Microcrystalline Cellulose127.27.95Citric Acid78.04.87Colloidal Silicon Dioxide1.50.09Magnesium Stearate14.00.87Total (Slow Release Layer)700.043.74Total Weight of coated tablet1635.2
**Equivalent to 437.5 mg amoxicillin based on an assay of 91.0%
Preparation of Modified Release Tablets
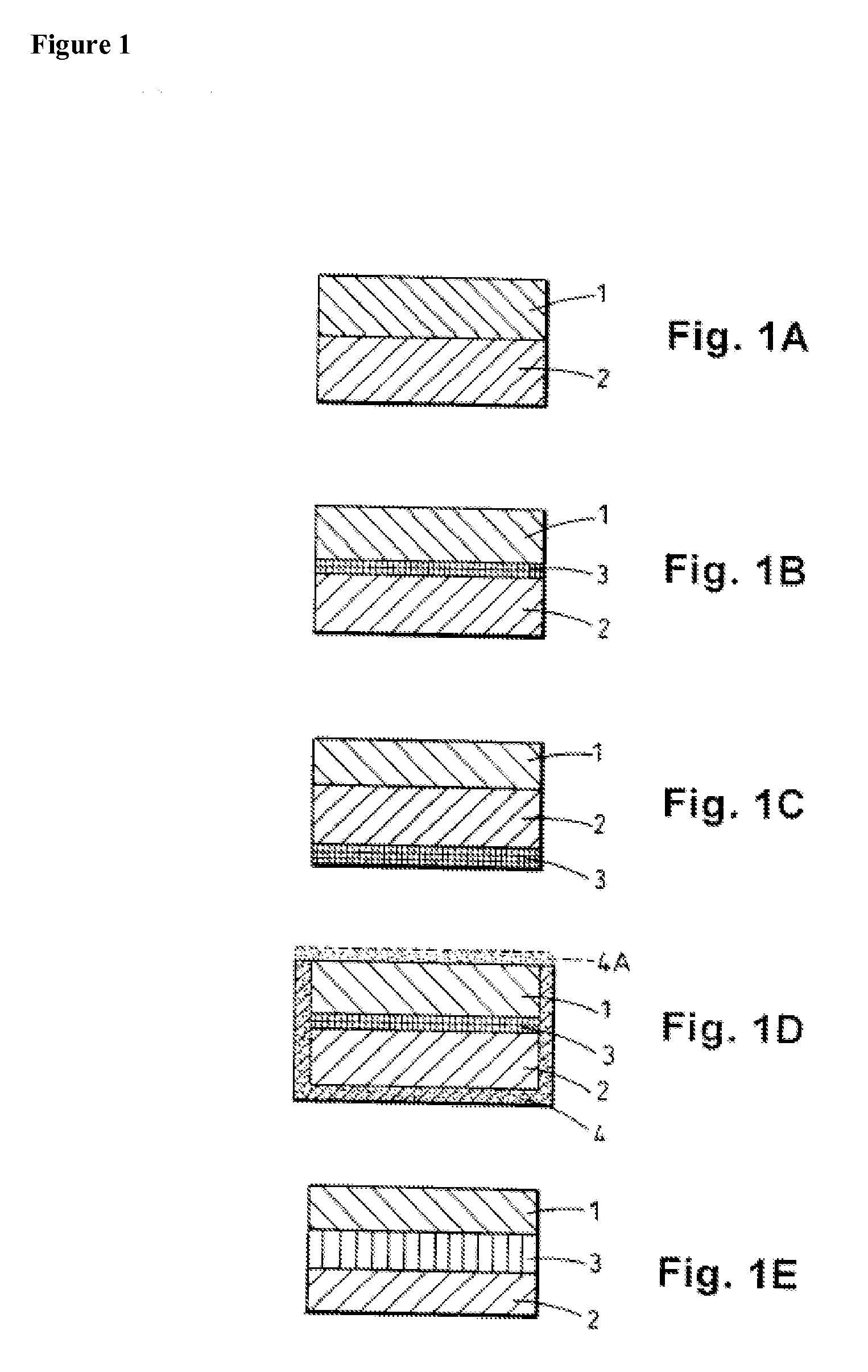
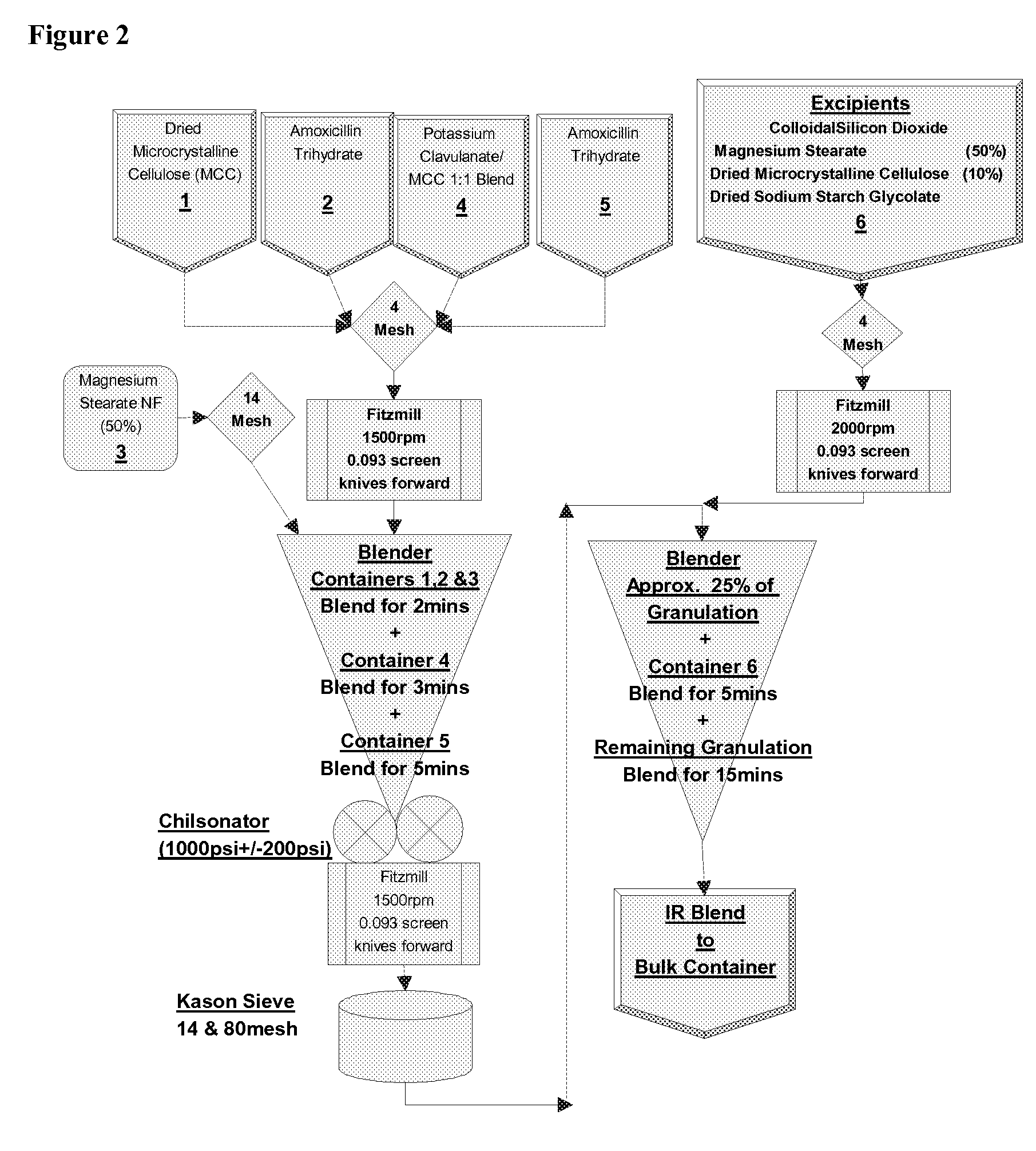
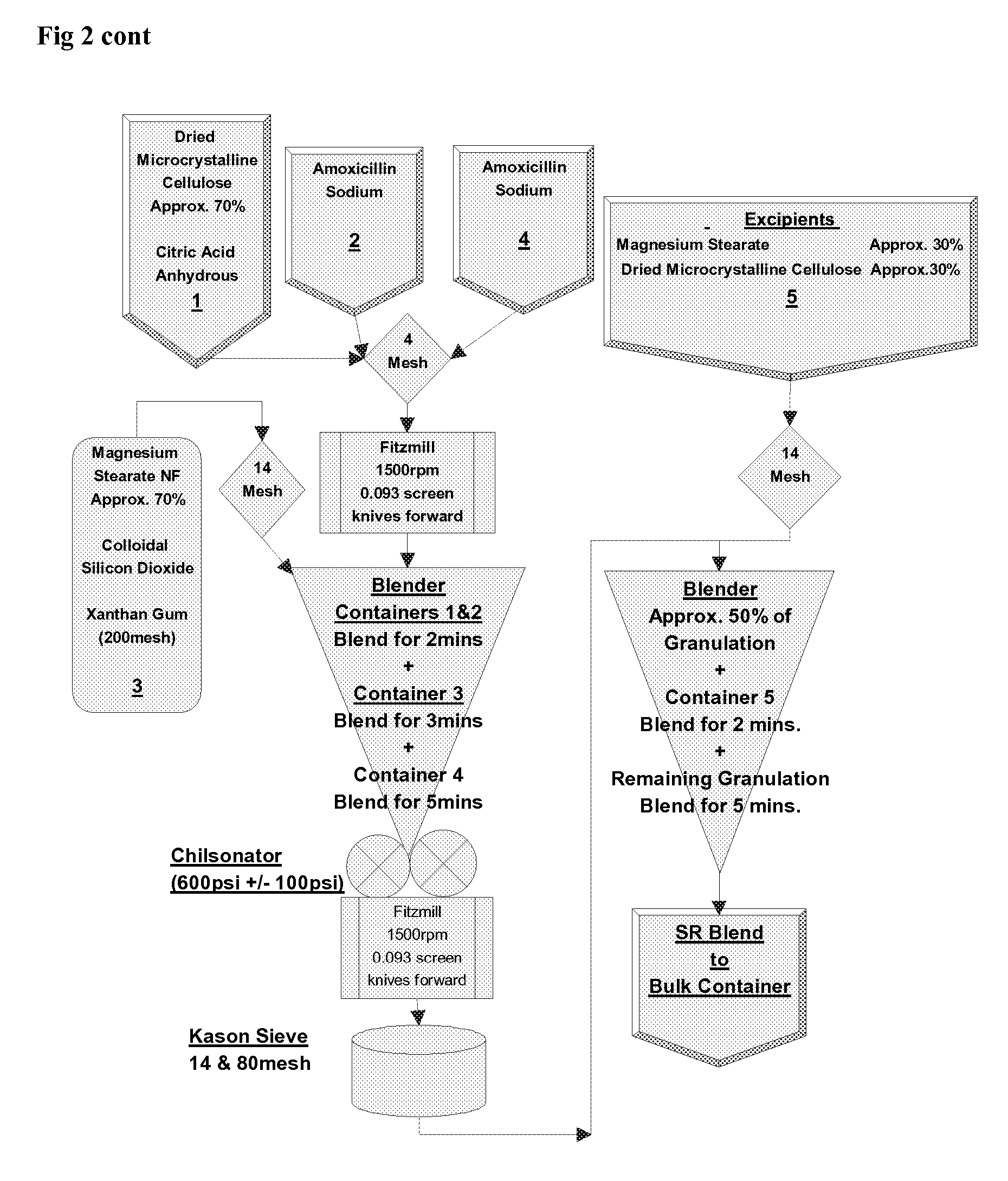
[0147] Modified release tablets were prepared according to the process flow diagram shown in FIG. 2. In brief, immediate and modified release blends are prepared which involve initial sieving and milling, as indicated, before roller compaction in a Chilsonater and further milling, sieving and blending. The two containers comprising amoxicillin trihydrate and the two containers comprising sodium amoxicillin comprise about equal weights of amoxicillin trihydrate and sodium amoxicillin, respectively....
example 3
Slow Release Tablet (875 mg)
[0152]
mg / ta(a) Sodium Amoxicillin TabletCrystallised Sodium Amoxicillin 91%*96173Dried Microcrystalline Cellulose27321Magnesium Stearate11Xanthan gum 200 mesh**54Total1(b) Sodium Amoxicillin Tablet with citric acidCrystallised Sodium Amoxicillin 91%*96166Dried Microcrystalline Cellulose28819Magnesium Stearate141Citric acid10Xanthan gum 200 mesh**22Total1(c) Amoxicillin Trihydrate TabletAmoxicillin Trihydrate 86%*10178Dried Microcrystalline Cellulose2116Magnesium Stearate11Xanthan Gum, 200 mesh**54Total1
*adjusted for the potency of the amoxicillin component and corresponding to 875 mg amoxicillin,
**Xantural 75
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com