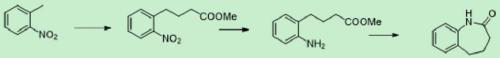
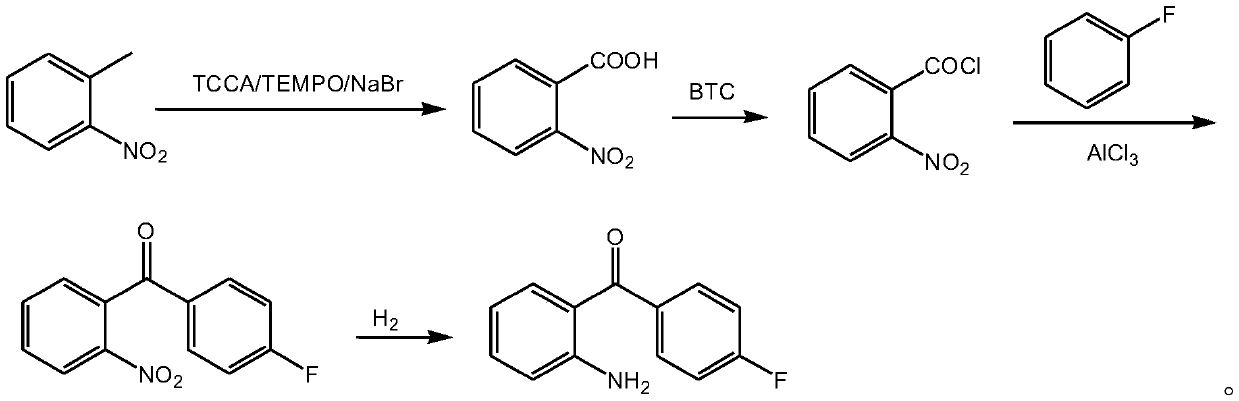
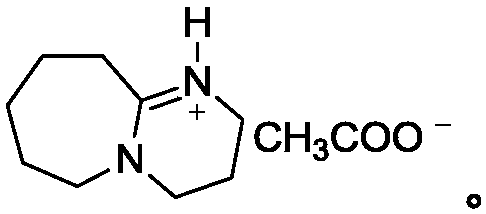
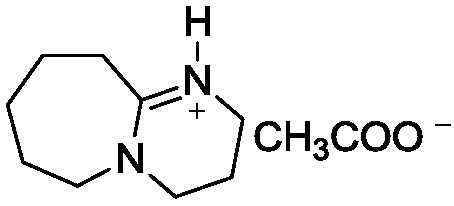
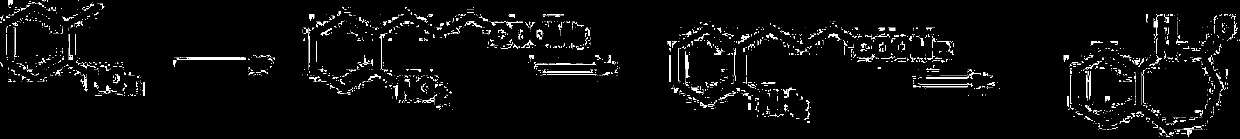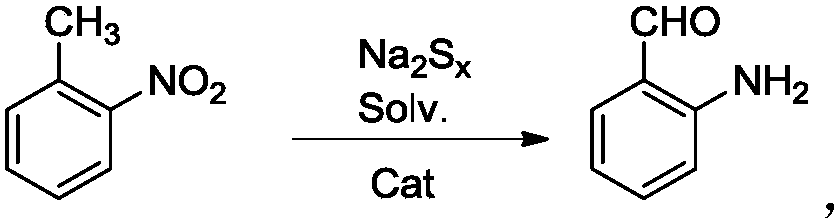Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "O-nitrotoluene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
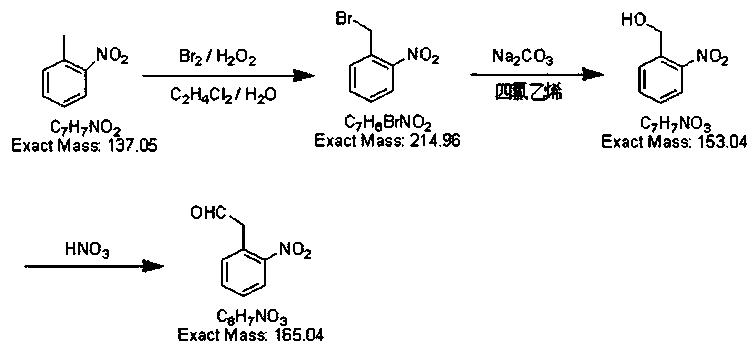
O-NITROTOLUENE is toxic by inhalation, ingestion and skin aborption, targeting the blood, central nervous system, skin, and gastrointestinal tract. Symptoms include, anoxia, weakness or dizziness, nausea and vomiting. If it contacts the eye, the eye should be irrigated immediately. If it contacts the skin, the area should be washed with soap.
Method for preparing age inhibitor 3100
ActiveCN102584596ALow costImprove conversion rateOrganic compound preparationAmino compound preparationOrganic sulfonic acidReaction temperature
The invention discloses a method for preparing an age inhibitor 3100. In the method, paraaminophenol, aniline and o-nitrotoluene are taken as raw material for condensation reaction under the effect of organic sulfonic acid catalyst, the reaction temperature is 110 to 220 DEG C, water and ammonia produced by the reaction can be led out through azeotropic solvent continuously, after the reaction reaches an end point, the temperature is lowered to 100 to 140 DEG C, filtering is performed while hot, and filter liquor is distilled for removing impurities. The method provided by the invention has a high raw material conversion rate, the contents of the obtained effective components are high, the cost is low, the three wastes are less, and the method is suitable for industrial production.
Owner:宜兴市聚金信科技有限公司
Industrialized method for producing 2, 6-dichloro phenyl nitrile
InactiveCN1775747ABiocidePreparation by nitrogen oxide-organic compound reactionTolueneMedicinal chemistry
The invention relates to a method for industrialized producing 2.6-dichlorobenzonitrile, adopting 6-chloro-o-nitrotoluene chloro-o-nitrotoluene as raw material to make chlorination reaction under the action of catalyst, then making cyanogenation reaction with formic acid and hydroxylammonium chloride, and refining again and again to obtain 2.6-dichlorobenzonitrile whose content is 99.8% above. The produced 2.6- dichlorobenzonitrile is a white powdery crystal, purity 99.8%-9995%, melting point 141.0 deg.C -144.0 deg.C, water ratio <=01%, and product yield up to 64%.
Owner:YANGZHOU TIANCHEN FINE CHEM
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546457AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
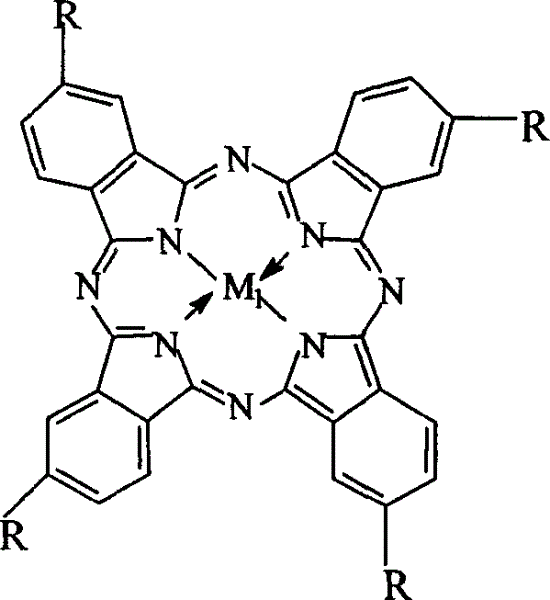
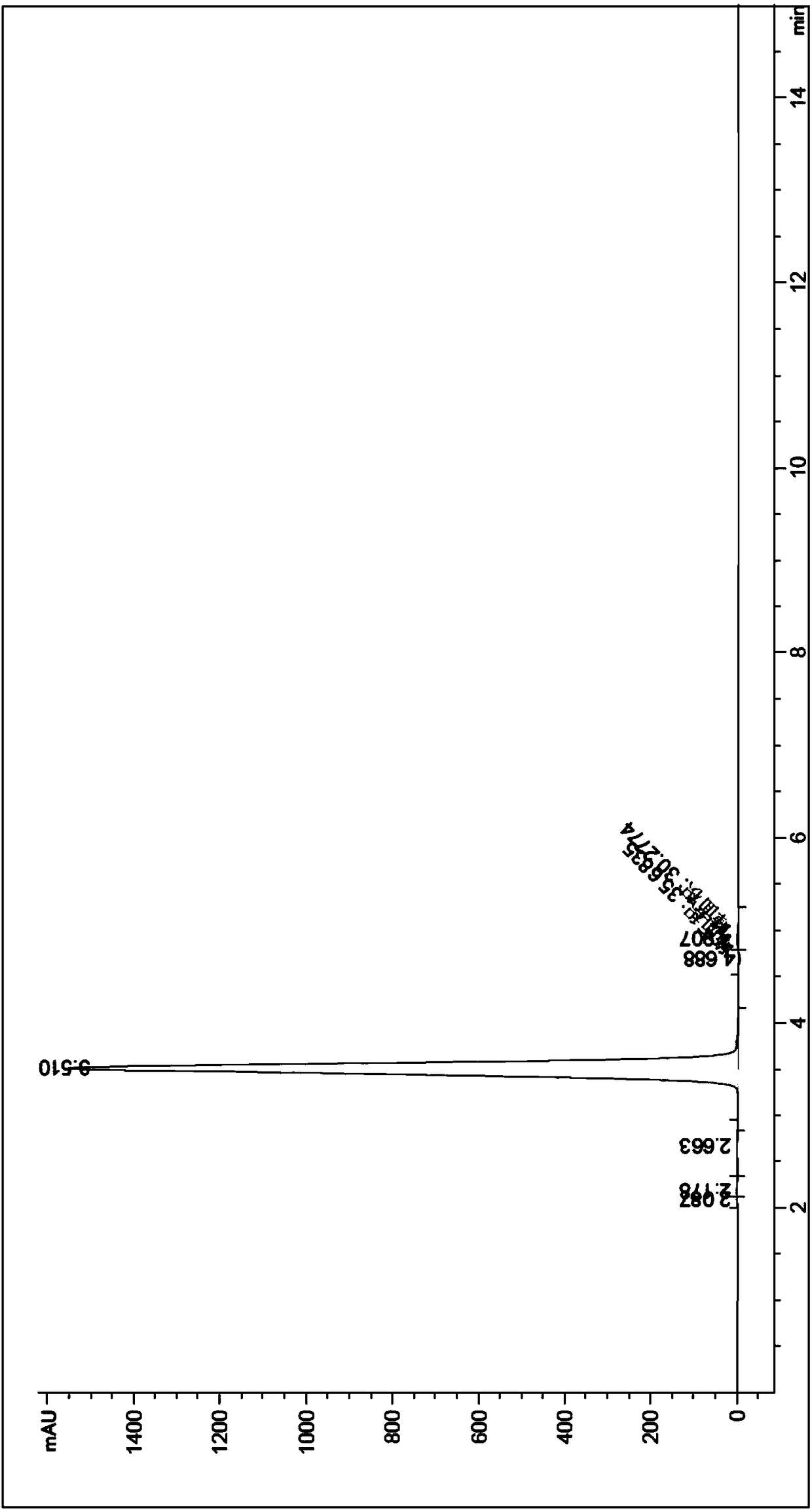
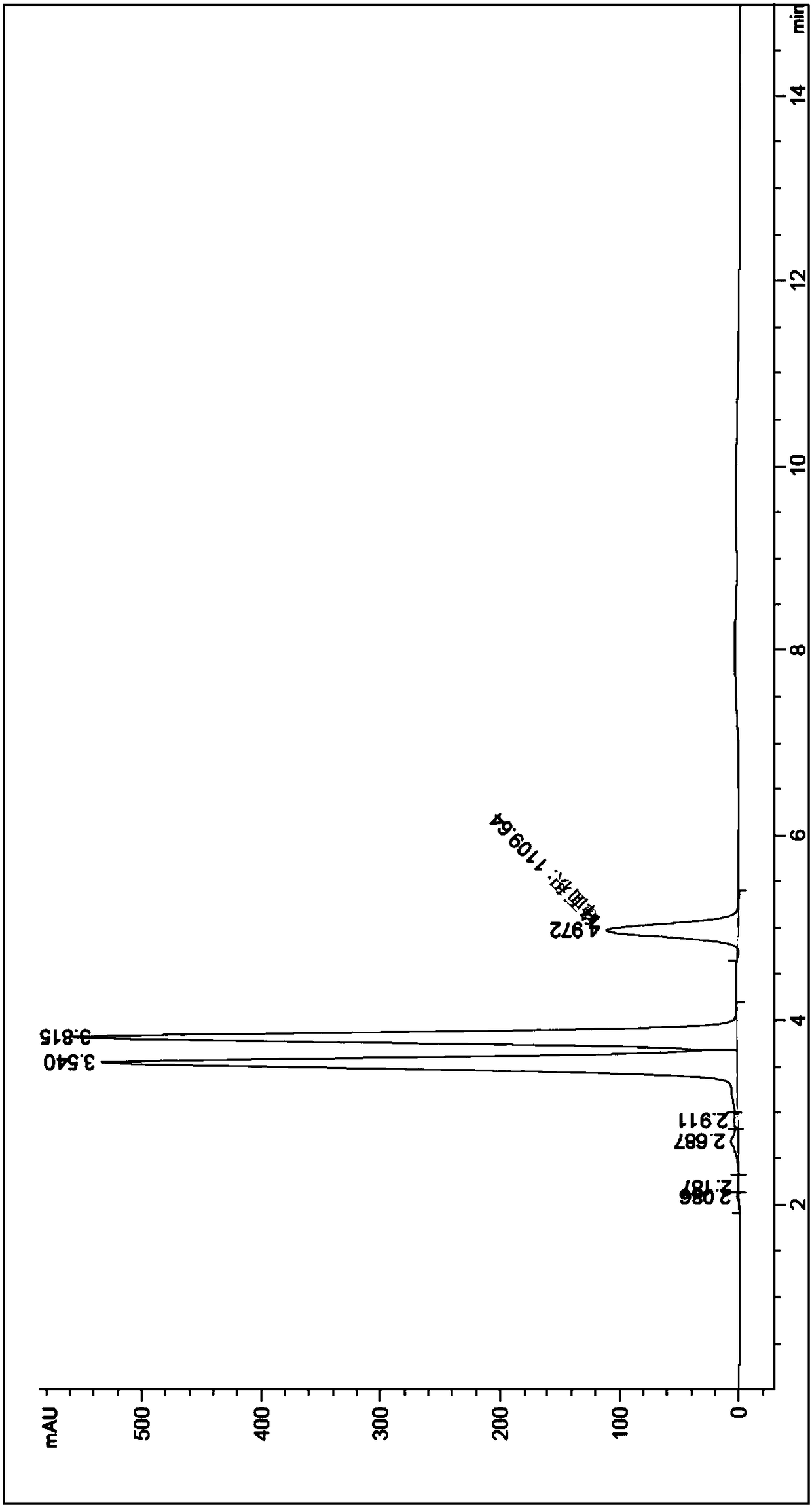
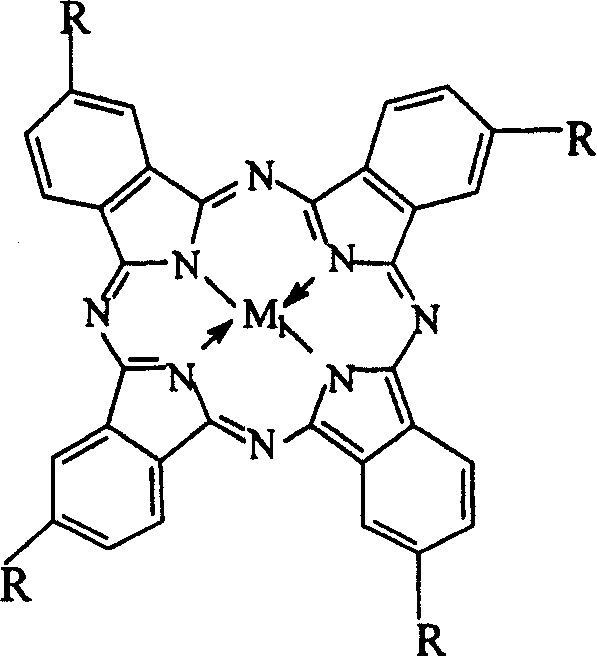
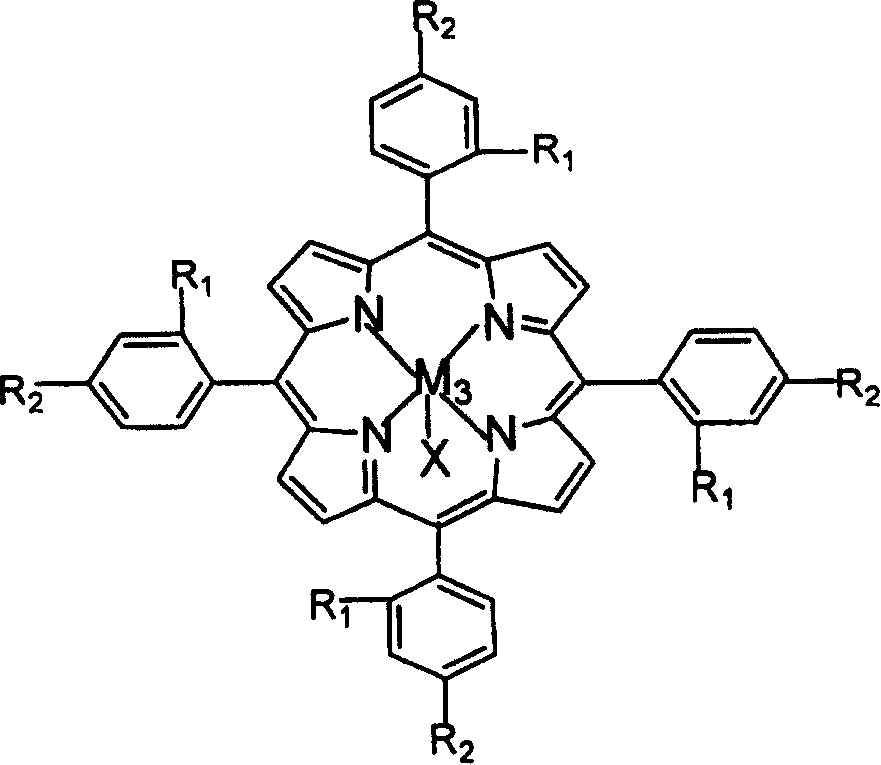
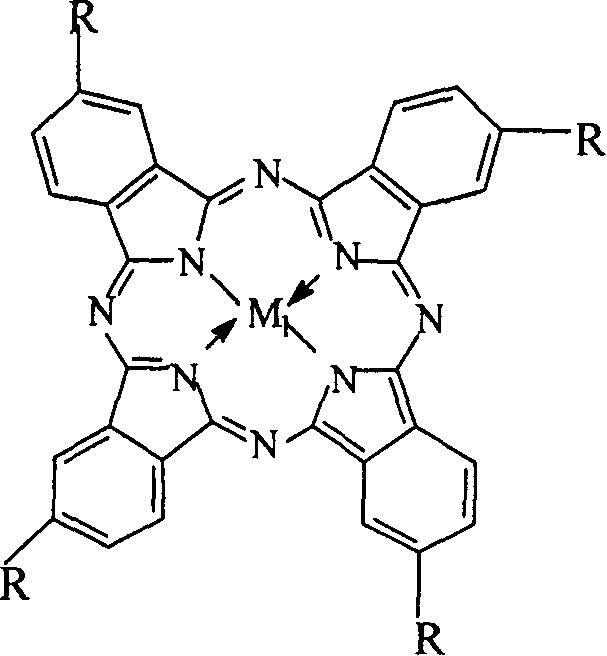
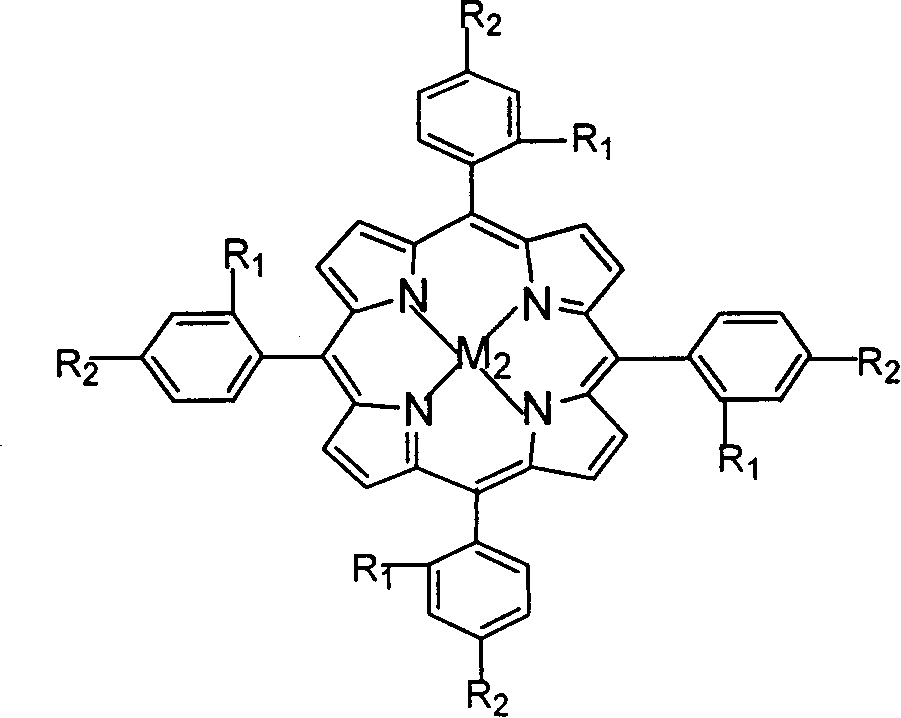
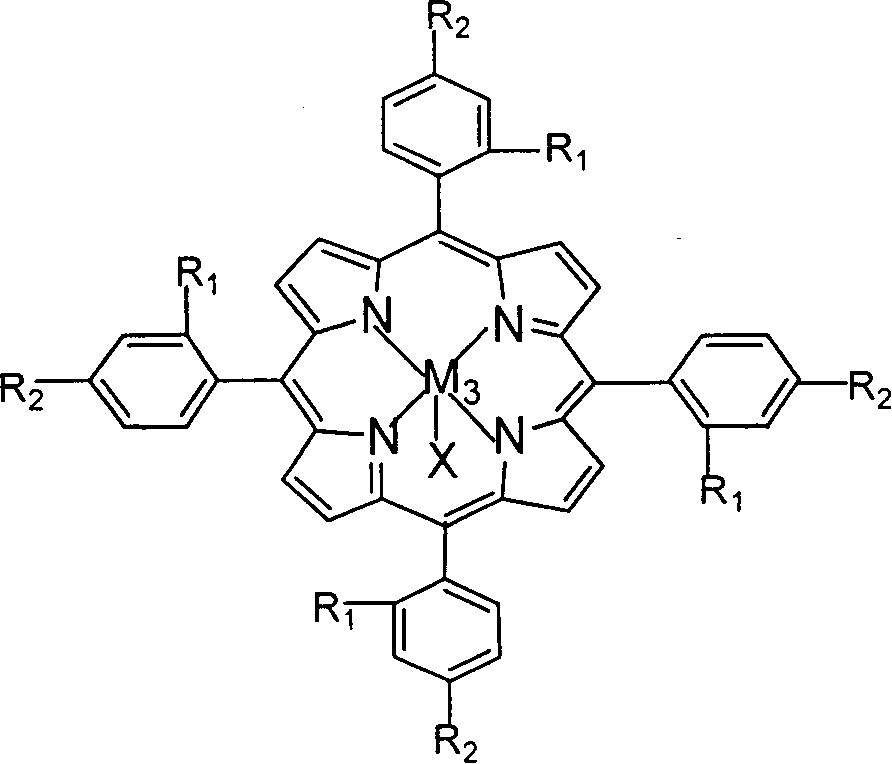
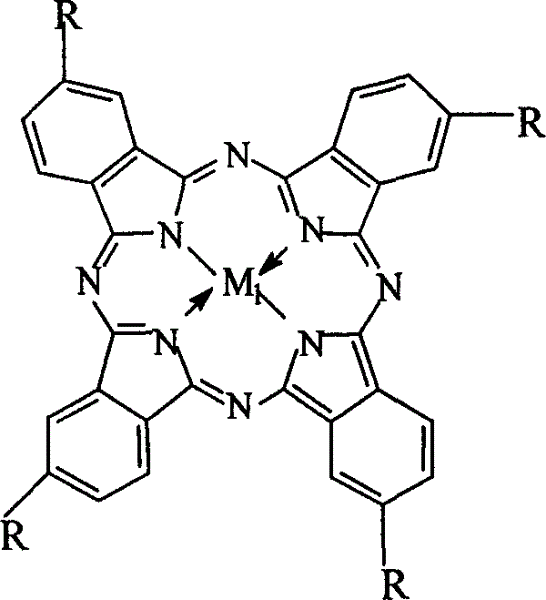
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing o-nitrobenzaldehyde through bionic catalytic oxidation of ortho-methylnitro benzene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.2-1.0% weight of ortho-methylnitro benzene, and methyl alcohol is used as solvent, 0.8-3.0 MPa oxygen is let into 3.0-6.0 mol / L strong alkaline methyl alcohol solution, the reaction temperature is controlled between 25 deg. C to 60 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Preparation method of 2-nitrobenzyl bromide
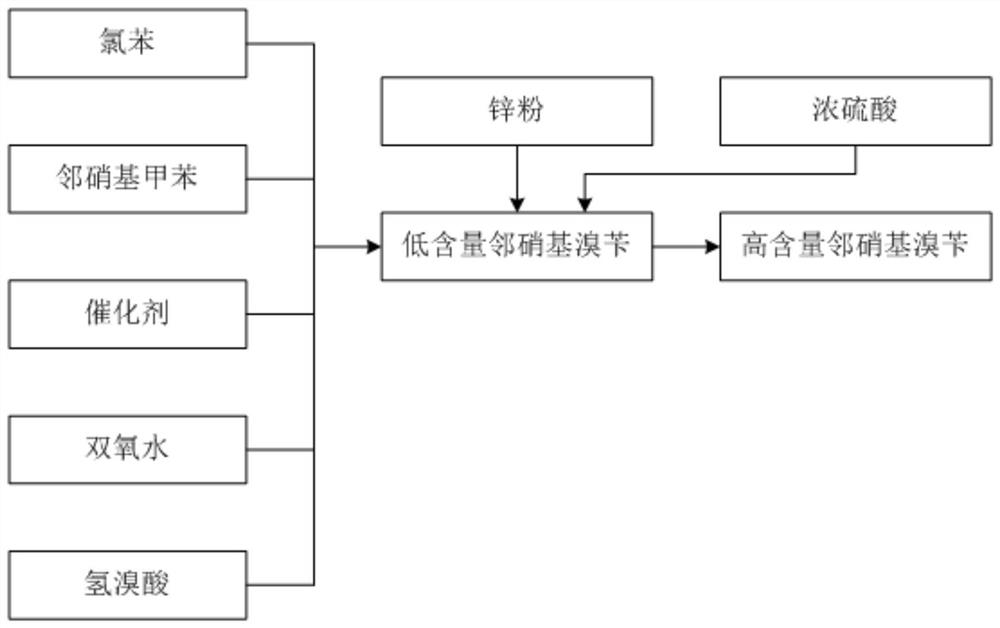
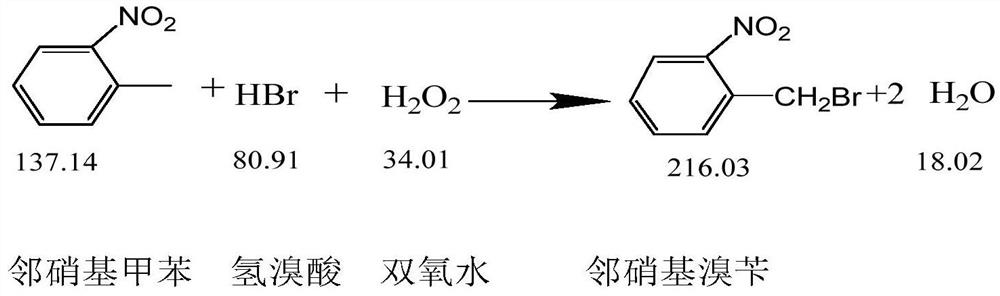
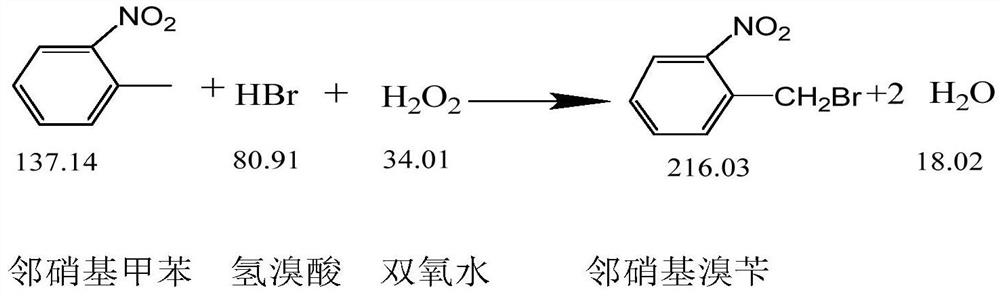
ActiveCN108440301AAdequate responseRaise the reaction temperatureOrganic chemistryOrganic compound preparationChemical synthesisSubstitution reaction
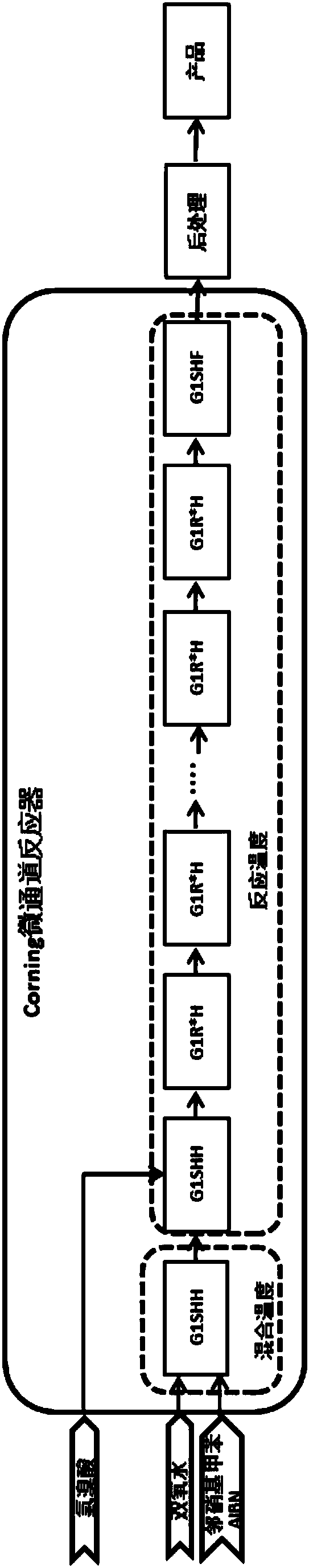
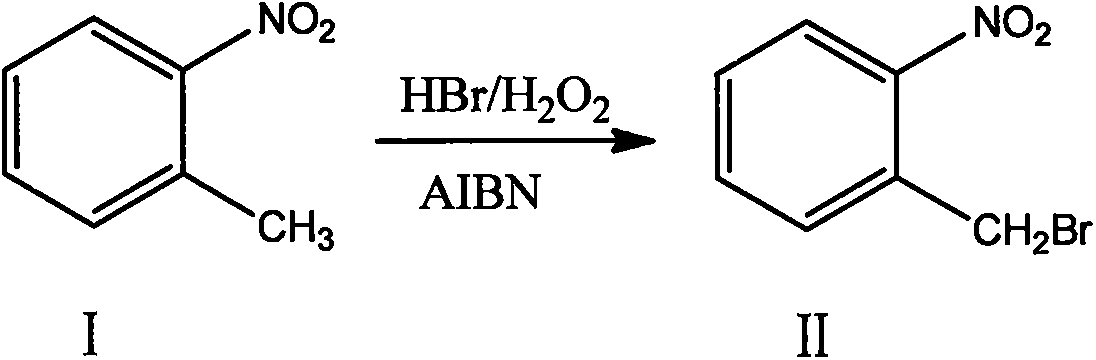
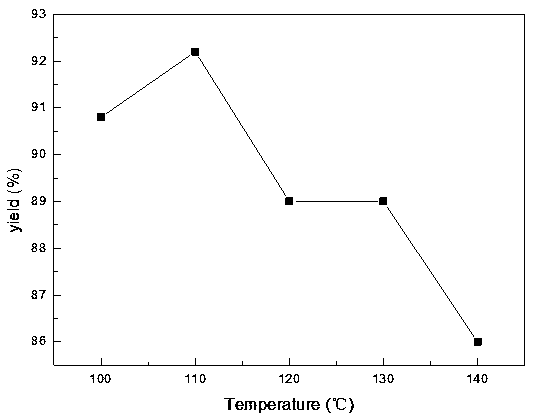
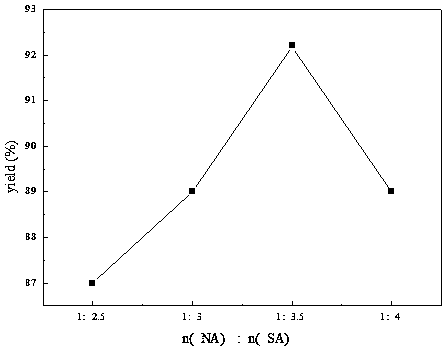
The invention discloses a preparation method of 2-nitrobenzyl bromide, and belongs to the technical field of chemical synthesis. According to the method, o-nitrotoluene, hydrobromic acid and hydrogenperoxide are taken as raw materials, under the initiation of catalysts, the 2-nitrobenzyl bromide is generated by conducting substitution reaction through a micro-channel reactor constantly; the 2-nitrobenzyl bromide is prepared through the micro-channel reactor, the operation is simple, the procedures are simple and the reaction time is greatly shortened.
Owner:HEBEI CHENGXIN
Method for synthesizing pyraclostrobin intermediate
ActiveCN106008348AReduce dosageReduce generationHydrazine preparationOrganic compound preparationHydrazine compoundBromine
The invention discloses a method for synthesizing a pyraclostrobin intermediate. The synthetic method comprises the following steps: preparing an intermediate 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole from 4-chlorophenylhydrazine hydrochloride prepared with p-bromochlorobenzene and hydrazine hydrate as reaction raw materials; reacting o-nitrotoluene with chlorine in the presence of a catalyst so as to prepare o-nitrobenzyl chloride; and subjecting 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole and o-nitrobenzyl chloride to an etherification reaction so as to obtain the pyraclostrobin intermediate, i.e., an etherification product 2[(N-4-chlorophenyl)-3-pyrazolyl-oxymethyl]nitrobenzene. The synthetic method provided by the invention can greatly reduce the amount of waste water produced in the synthesis process of 4-chlorophenylhydrazine hydrochloride; with 4-chlorophenylhydrazine hydrochloride as a raw material, the produced intermediate 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole has HPLC content of no less than 98% and yield of no less than 90%; since chlorine is used for replacing bromine or hydrobromic acid to prepare o-nitrobenzyl bromide, production cost and environmental protection burden are controlled; and the etherification product with a content of no less than 98% and yield of no less than 90% is obtained.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1243717CEasy to operateSimple stepsOrganic chemistryOrganic compound preparationCatalytic oxidationPorphyrin
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing o-nitrobenzaldehyde through bionic catalytic oxidation of ortho-methylnitro benzene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.2-1.0% weight of ortho-methylnitro benzene, and methyl alcohol is used as solvent, 0.8-3.0 MPa oxygen is let into 3.0-6.0 mol / L strong alkaline methyl alcohol solution, the reaction temperature is controlled between 25 deg. C to 60 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1271040CNo pollution in the processLow costOrganic chemistryOrganic compound preparationReaction temperatureCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
2,2'-dinitrodibenzyl preparation method
ActiveCN105753709AFacilitate the approach to sustainable developmentAchieve sustainable developmentOrganic chemistryOrganic compound preparationBromineSubstitution reaction
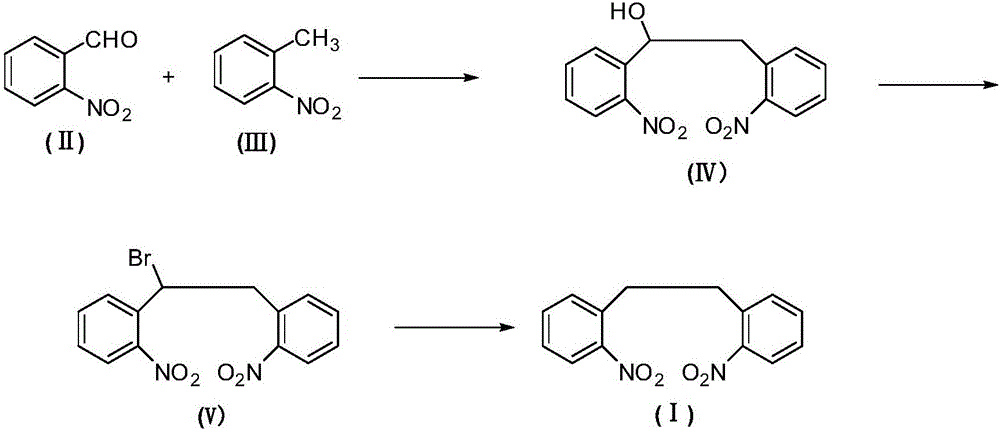
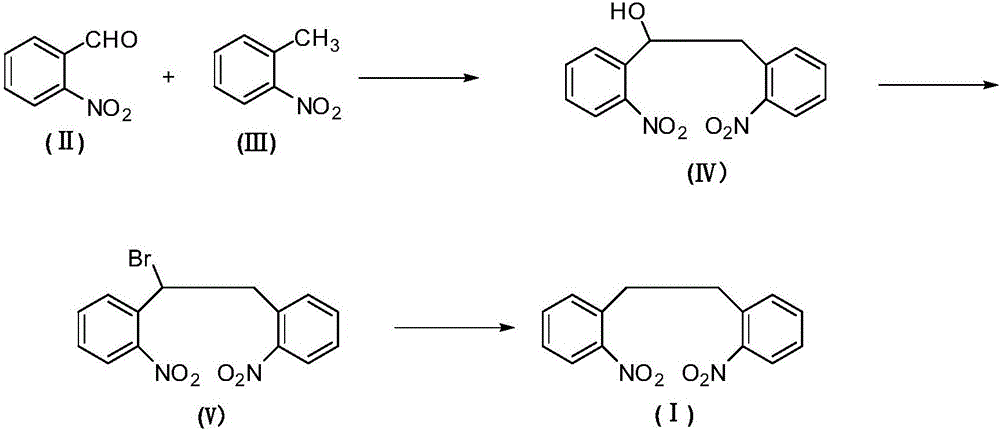
The invention relates to an antiepileptic drug carbamazepine intermediate preparation method, in particular to a 2,2'-dinitrodibenzyl preparation method.The 2,2'-dinitrodibenzyl preparation method includes the steps of subjecting o-nitrobenzaldehyde and o-nitrotoluene serving as raw materials to addition reaction then substitution reaction with a bromination reagent, and debromination under the action of a reducing agent so as to obtain 2,2'-dinitrodibenzyl.The 2,2'-dinitrodibenzyl preparation method has the advantages of a novel synthetic route, conventional reaction steps, simple technologies, high yield, high purity, capability of avoiding high-pollution operating steps and suitability for industrial production.
Owner:ANHUI JINDING PHARMA
Method for preparing 6-chloro-2-nitrotoluene
InactiveCN101985425AIncrease productionReduce manufacturing costOrganic chemistryOrganic compound preparationMature technologyEthyl Chloride
The invention discloses a method for preparing 6-chloro-2-nitrotoluene. The method comprises the following steps of: throwing o-nitrotoluene into a reaction kettle; starting and stirring; adding a catalyst; heating; introducing chlorine gas at the temperature of between 25 and 85 DEG C, wherein tests show that the reaction is ended when the o-nitrotoluene content is 8 to 12 percent; cooling; discharging; and then performing rectification. In the preparation method, by properly selecting the catalyst on the basis of not changing the conventional mature technology, the proportion of the 6-chloro-2-nitrotoluene to 4-chloro-2-nitrotoluene serving as two isomers can be effectively improved, so that the 6-chloro-2-nitrotoluene content is about 65 percent, the production cost is reduced, the energy is saved and the method is suitable for industrial production.
Owner:GUANGZHOU YABANG SHENLIAN CHEM
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
InactiveCN1546458AReduce dosageLow reaction temperatureOrganic chemistryOrganic compound preparationPorphyrinCatalytic oxidation
The invention relates to a process for preparing aromatic aldehydes, in particular a process for preparing p-nitrobenzaldehyde through bionic catalytic oxidation of p-nitrotoluene, wherein metallic phthalocyanine, single nuclear metalloporphyrin or mu-oxy-double nuclear metalloporphyrin having the similar structure as the biological enzymes are selected as the catalyst for the disclosed process, whose dose is 0.1-1.0% weight of p-nitrotoluene, and methyl alcohol is used as solvent, 0.5-3.0 MPa oxygen is let into 0.7-2.8 mol / L strong alkaline methyl alcohol solution, controlling the reaction temperature to be 20-65 deg. C, the reaction time being 6-48 hrs.
Owner:BEIJING UNIV OF TECH
4-fatty sulfoamide-2-nitrobenzyl bromide compounds and synthetic method thereof
InactiveCN105237443ANovel structureMild reaction conditionsSulfonic acid amide preparationFiltrationSolvent
The invention discloses a kind of precursor compounds 4-fatty sulfoamide-2-nitrobenzyl bromide which can form a vesicle and is photodegradable and a synthetic method, and belongs to the field of surfactants. The preparation method comprises adding o-nitrotoluene into chlorosulfonic acid at 0 DEG C, and reacting at 30-80 DEG C to generate 4-chlorosulfonyl-2-nitrotoluene; dissolving the obtained intermediate in a solvent, dropwise adding a long-chain primary amine or secondary amine, adjusting pH to 7-11, and reacting at 30-50 DEG C for obtaining 4-fatty sulfonamide-2-nitrotoluene, performing reduced-pressure concentration on the reaction solution, and performing pumping filtration and drying; and dissolving the product obtained in the above step in a solvent, adding an initiator, adding a brominating agent and reacting at 30-110 DEG C to obtain 4-fatty sulfoamide-2-nitrobenzyl bromide. The synthetic method is simple, the yield of each step of reactions is larger than or equal to 60%, the employed reaction eluant is petroleum ether and ethyl acetate, human body damage is relatively small, and petroleum ether and ethyl acetate are recoverable and reusable, and the synthetic method is friendly to environment.
Owner:DALIAN UNIV OF TECH
Preparation process of 2-tolylhydrazine hydrochloride
InactiveCN102382009AShorten the reduction reaction timeReduce manufacturing costHydrazine preparationSodium metabisulfiteO-nitrotoluene
The invention relates to a preparation process of 2-tolylhydrazine hydrochloride, which comprises the following steps of: firstly, carrying out a diazo reaction on o-nitrotoluene as an initial raw material to obtain benzenediazonium chloride; secondly, further carrying out a reducing reaction on the benzenediazonium chloride obtained in the first step under the action of a reducing agent to obtain a reduzate; and thirdly, carrying out a hydrolytic reaction on the reduzate obtained in the second step under the action of hydrochloric acid to generate the 2-tolylhydrazine hydrochloride. Particularly, in the second step, sodium metabisulfite is used as the reducing agent in the reducing reaction; and the reducing reaction is carried out under the conditions that the temperature is 15-25DEG C and the pH value is 7-9. According to the method, the production period can be shortened and the production cost is reduced while high yield is obtained.
Owner:袁雪冲
Preparation method of o-nitrobenzaldehyde
InactiveCN110330433AHigh yieldEffective purification methodOrganic chemistryOrganic compound preparationBromineOil water
The invention relates to a preparation method of o-nitrobenzaldehyde, and is mainly applied to the field of medicine synthesis. According to the method disclosed by the invention, o-nitrotoluene is taken as a raw material, an initiator is added and subjected to a bromination reaction with liquid bromine in an oil-water two-phase solvent, hydrolyzed by sodium carbonate, and oxidized by dilute nitric acid to obtain crude o-nitrobenzaldehyde, and finally purification and discoloration are performed to obtain white o-nitrobenzaldehyde with the purity is 99.9%.
Owner:石家庄海象科技有限公司
A kind of method of synthesizing pyraclostrobin intermediate
ActiveCN106008348BReduce dosageReduce generationHydrazine preparationOrganic compound preparationHydrazine compoundBromine
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
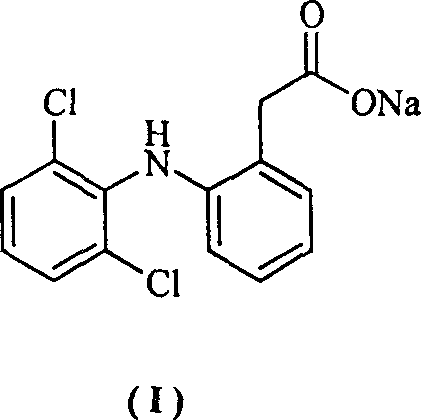
Method for synthesizing dichlofenac sodium
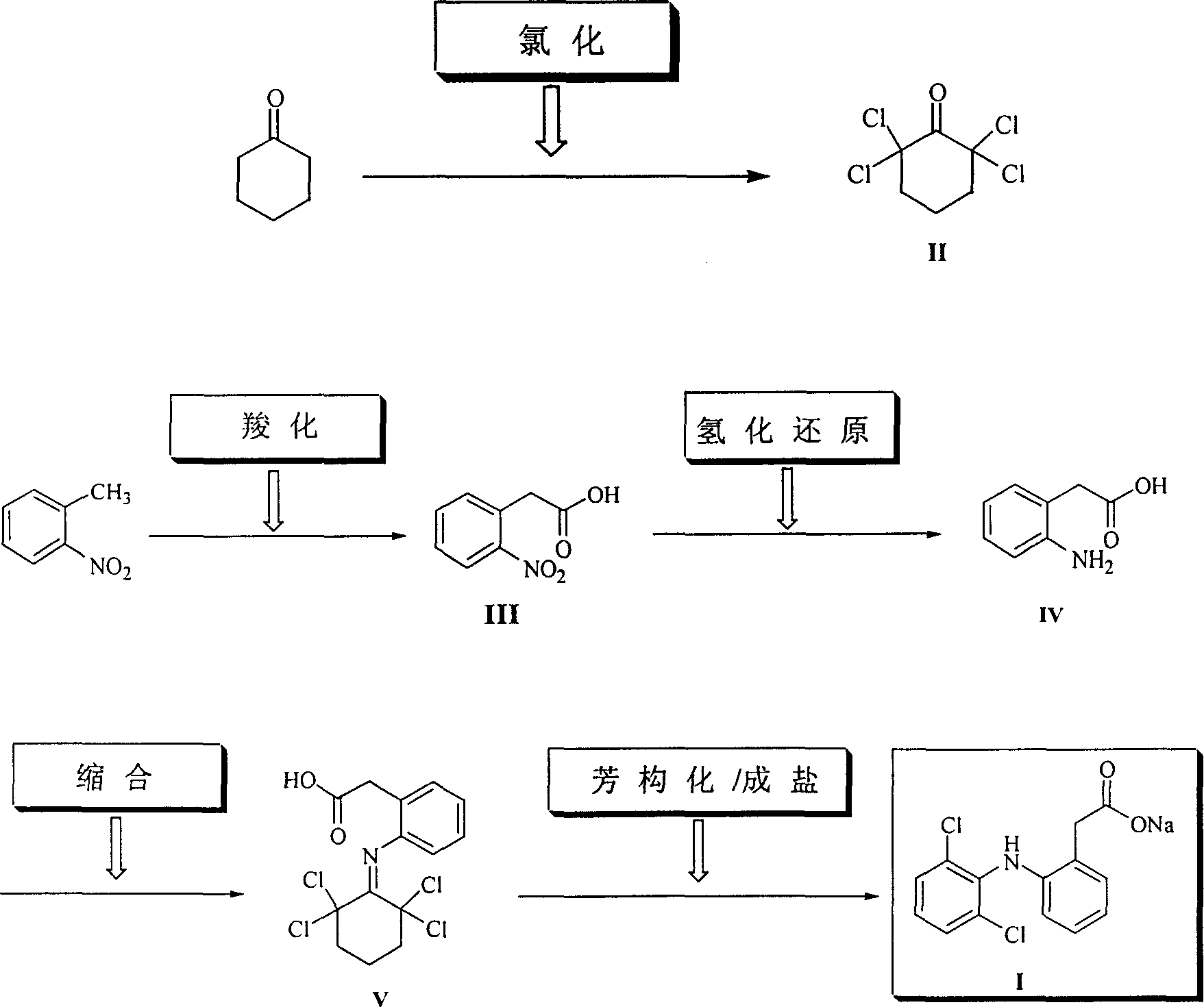
InactiveCN1242984CHigh yieldLow costOrganic compound preparationAmino-carboxyl compound preparationPhenyl acetic acidCyclohexanone
The invention provides a synthetic method of sodium diclofenac. The method includes the following steps: 1. Make the 2, 2, 6, 6-tetrachloride cyclohexanone (II) from the cyclohexanone through chlorination. 2. The carboxylation reaction occurs to the close nitrotoluene and carbon dioxide after the phase-transfer catalysis to get the close nitrophenyl acetic acid (III). 3. The hydrogenation reaction happens to the compound (III) catalysed by the polymer catalyst Pd / D-296 to produce the close aminophenyl acetic acid (IV). 4. The condensation reaction occurs to the compound IV and compound II to produce the compound of N-(2-carboxymerhy1 phenyl)-2,2,6,6-hexamethylene imine (V). 5. After the aromatization reaction and salifying happen to the compound V, we get the sodium diclofenac (I). By the close nitrotoluene, the yield is 85%. The invention is featured by facility of the raw material, simple operation, mild reation condition and easy industrialization.
Owner:FUDAN UNIV
A new synthesis process of o-nitrobenzyl bromide
ActiveCN107778181BHigh yieldHigh purityOrganic chemistryOrganic compound preparationBiochemical engineeringCombinatorial chemistry
The invention relates to a novel nitrobenzyl bromide synthesis process which comprises the following steps: in the presence of 40% hydrobromic acid, 30% hydrogen peroxide and an initiator, namely azodiisobutyronitrile, by taking ortho-nitrotoluene as a raw material, performing a free radical bromination reaction, thereby synthesizing the nitrobenzyl bromide. The 30% hydrogen peroxide is adopted asan oxidant in the process, bromine is provided by oxidizing the hydrobromic acid, the testing conditions are strictly regulated and controlled, then the free radical bromination reaction of the ortho-nitrotoluene has selectivity, the synthesis procedures are controllable, no dibromo-byproduct is generated, meanwhile the yield is far greater than that of the prior art, the production cost is low,the environment is protected, and the process is an ideal method applicable to large-scale industrial production at present.
Owner:ZHEJIANG ZHONGSHAN CHEM IND GRP
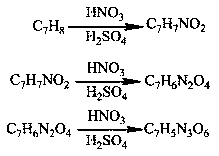
Method for preparing TNT (Trinitrotoluene) by taking nitrotoluene as raw material through one-step method
InactiveCN108707077AAvoid the disadvantages of polluting the environmentSave energyNitro compound preparationP-nitrotolueneNitration
The invention discloses a method for preparing TNT (Trinitrotoluene) by taking nitrotoluene as a raw material through a one-step method. According to the method disclosed by the invention, p-nitrotoluene or o-nitrotoluene is used as the raw material and a mixed system of fuming HNO3 / concentrated H2SO4 is used as a nitrating agent, and 2,4,6-trinitrotoluene TNT is prepared through one-step nitrification reaction; the 2,4,6-trinitrotoluene TNT is subjected to structure characterization by adopting a melting point, thin-layer chromatography, mass spectrometry and liquid chromatography. Influences, caused by a ratio, a feeding manner and a dosage of the mixed system of the fuming HNO3 / concentrated H2SO4, to the yield of a product are explored and reaction conditions are optimized. The TNT is synthesized by taking the nitrotoluene as the raw material through one-step nitrification, so that environment pollution caused by the fact that a lot of waste acid and red water are generated is avoided, and energy source and power consumption caused by reaction steps and nitrification equipment are reduced.
Owner:ZHONGBEI UNIV +1
Synthesis method of DPA
InactiveCN110642725ARaw materials are easy to getShort reaction timeOrganic compound preparationAmino-hyroxy compound preparationMethylanilineCyclohexanone
The invention discloses a synthesis method of DPA. The method comprises the following steps: (1) adding methanol, o-nitrotoluene, a catalyst, concentrated sulfuric acid, distilled water into an autoclave, performing heating with stirring to 50 DEG C, evacuating air and then introducing hydrogen, controlling a pressure in the autoclave to 0.1 MPa, carrying out a reaction for 5 h, then performing suction filtration, distilling a filtrate to remove methanol, diluting a residual solution by adding water and then adjusting pH to be neutral with ammonia water, performing extracting with toluene, washing an organic phase with a dilute sodium hydroxide solution, and performing reduced pressure distillation on the organic phase to obtain 4-methoxy-2-methylaniline; and (2) adding 4-methoxy-2-methylaniline, phenol naphthalene, cyclohexanone, and the catalyst into an autoclave, replacing air with nitrogen, performing heating to 250 DEG C, controlling a pressure in the autoclave to 1 Mpa, performing cooling to room temperature after a reaction is completed, filtering a reaction solution, and performing reduced pressure distillation on a filtrate and collecting various fractions, wherein a latter fraction is DPA. The raw materials are cheap and easy to obtain, the operation is simple, the yield is high, the energy consumption is low, and the method has practical value and economic value, andis suitable for enterprise production.
Owner:胡婷
Method for preparing methylaniline by reducing nitrotoluene through liquid phase continuous catalytic hydrogenation
InactiveCN108285417ALow costReduce lossOrganic compound preparationAmino compound preparationNano catalystMethylaniline
Owner:JIANGSU HUAIHE CHEM
Catalyst for preparing o-toluidine by hydrogenating o-nitrotoluene and preparation method of catalyst
ActiveCN112536033AShape ruleHigh strengthOrganic compound preparationChemical recyclingPtru catalystToluidine
The invention relates to a catalyst for preparing o-toluidine through catalytic hydrogenation of o-nitrotoluene and a preparation method of the catalyst. The catalyst takes metal Cu as an active component and takes modified SiO2 as a carrier. The active component Cu accounts for 15%-25% of the weight of the catalyst. In the preparation process of the catalyst, silica gel is pretreated, so that theprepared catalyst is regular in appearance, high in strength and not easy to crush. The active component copper is impregnated on silica gel in the form of a copper-ammonia complex in an alkaline atmosphere to form a Cu-Si coalition structure, thereby enhancing the thermal stability and carbon deposition resistance of the catalyst, and increasing the one-way service cycle of the catalyst to 8 hours or above. According to the method disclosed by the invention, the pore structure of the catalyst is improved, the proportion of the pore size of 2-15nm reaches more than 80%, the active component is highly dispersed on the carrier, and the conversion rate and selectivity of the catalyst reach more than 99.9%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing o-nitrophenylacetic acid
InactiveCN108752215ARaise the pHChange pHOrganic chemistryOrganic compound preparationAlcoholSteam distillation
The invention discloses a method for synthesizing o-nitrophenylacetic acid. Absolute ethyl alcohol, Pt-MgO-CNTs, metal sodium, o-nitrotoluene, diethyl oxalate, hydrogen peroxide and H2PtCl6 are used as main raw materials, the synthesis process adopts the o-nitrotoluene and diethyl oxalate to be subjected to condensation reaction to obtain the o-nitrophenylacetic acid under the action of the catalyst Pt-MgO-CNTs, the separation method of steam distillation is replaced by vacuum distillation, the reaction time is greatly shortened, and the separation effect is significantly enhanced. The optimummaterial ratio, the temperature of oxidation reactions and the temperature and time of condensation reactions are optimized by a large number of experiments, and the yield is greatly improved.
Owner:杨程飞扬
Synthesis method of benzocaprolactam
The invention discloses a synthesis method of benzocaprolactam. The synthesis method comprises the following steps: S1, using o-nitrotoluene as a raw material, and carrying out a condensation reactionon the o-nitrotoluene and an acrylate under catalysis of an alkali to obtain o-nitrophenylbutyric acid or o-nitrophenyl butyrate; and S2, reducing nitro of the o-nitrophenylbutyric acid or o-nitrophenyl butyrate, and performing ring closing to obtain a target product, wherein nitro of the o-nitrophenylbutyric acid or o-nitrophenyl butyrate is reduced to obtain amino. According to the synthesis method, cheap chemical products are used as initial raw materials, so that cost is low; post-treatment operation is simple, and a qualified product can be obtained through a rectification mode or simplemodes such as extraction, crystallization, filtration and the like, so that industrial production is easy to realize.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Industrialized method for producing 2, 6-dichloro phenyl nitrile
InactiveCN1304367CBiocidePreparation by nitrogen oxide-organic compound reactionMedicinal chemistryHydroxylammonium chloride
The invention relates to a method for industrialized producing 2.6-dichlorobenzonitrile, adopting 6-chloro-o-nitrotoluene chloro-o-nitrotoluene as raw material to make chlorination reaction under the action of catalyst, then making cyanogenation reaction with formic acid and hydroxylammonium chloride, and refining again and again to obtain 2.6-dichlorobenzonitrile whose content is 99.8% above. The produced 2.6- dichlorobenzonitrile is a white powdery crystal, purity 99.8%-9995%, melting point 141.0 deg.C -144.0 deg.C, water ratio <=01%, and product yield up to 64%.
Owner:YANGZHOU TIANCHEN FINE CHEM
A kind of method for preparing 2-amino-4'-fluoro-benzophenone
ActiveCN107652192BHigh purityRaw materials are cheap and easy to getOrganic chemistryOrganic compound preparationBenzoic acidAluminium chloride
The invention discloses a method for preparing 2-amino-4'-fluorobenzophenone. The method comprises the following steps: o-nitrotoluene, trichloroisocyanuric acid, tetramethylpiperidine nitrogen oxideand sodium bromide undergo an oxidation reaction to obtain o-nitrobenzoic acid; the o-nitrobenzoic acid and trichloromethyl carbonate undergo an acylating chlorination to obtain o-nitrobenzoyl chloride; the o-nitrobenzoyl chloride, fluorobenzene and aluminum trichloride undergo a Friedel-Crafts reaction to obtain 2-nitro-4'-fluorobenzophenone; and the 2-nitro-4'-fluorobenzophenone is reduced by hydrogen to the 2-amino-4'-fluorobenzophenone. The method has the advantages of green and environmentally-friendly synthesis route, cheap and easily available initial raw materials, low cost, convenience in operation, suitableness for industrial production, and high yield, and the prepared 2-amino-4'-fluorobenzophenone has a good purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
Synthesis method of O-nitrophenylacetic acid
InactiveCN108997129ARaise the pHChange pHOrganic chemistryOrganic compound preparationAnhydrous ethanolSynthesis methods
The invention discloses a synthesis method of O-nitrophenylacetic acid; anhydrous ethanol, Pt-MgO-CNTs, metallic sodium, O-nitrotoluene, diethyl oxalate, hydrogen peroxide and H2PtCl6 are used as mainraw materials; according to the synthesis process, O-nitrotoluene and diethyl oxalate are subjected to condensation reaction under the action of the catalyst Pt-MgO-CNTs to obtain O-nitrophenylaceticacid; reduced pressure distillation replaces a separation method of water steam distillation, so the reaction time is greatly shortened and the separation effect is enhanced obviously. The optimum material ratio, oxidation reaction temperature and condensation reaction temperature and time are optimized through a large number of experiments, and the yield is greatly improved.
Owner:XUZHOU NORMAL UNIVERSITY
Method for preparation of o-nitrobenzoic acid by catalytic oxidation of o-nitrotoluene
InactiveCN104311425AHigh yieldMild reaction conditionsOrganic chemistryOrganic compound preparationCatalytic oxidationHigh pressure
The invention relates to a method for preparation of o-nitrobenzoic acid by catalytic oxidation, and the method comprises the following steps: adding a solvent, o-nitrotoluene, catalyst MnO2 and RuO4 into a high pressure reactor, introducing oxygen, reacting 5 to 15 hours at 100-150 DEG C under 0.1-2MPa, and after the reaction, filtering the catalyst, extracting, washing with water, and distilling to recover the solvent to obtain the o-nitrobenzoic acid. The filtered catalyst can be recycled.
Owner:王晓伟
A kind of efficient, fast method for synthesizing o-aminobenzaldehyde
Owner:TAIZHOU UNIV +1
Fuel oil additive
InactiveCN106221836AImprove energy savingImprove stabilityLiquid carbonaceous fuelsFuel additivesChlorogenic acidCombustion chamber
The invention relates to a fuel oil additive, and belongs to the technical field of oil-gas additives. The fuel oil additive is prepared from, by mass, 20-30 parts of oxidized petrolatum, 1-4 parts of chlorogenic acid, 2-8 parts of hordenine hydrochloride, 5-12 parts of o-nitrotoluene, 3-6 parts of dialkyl carbonate, 4-8 parts of n-propyl alcohol and 40-60 parts of deionized water. The fuel oil additive has the good energy saving property, can effectively save fuel oil, is good in stability, safe and environmentally friendly, has a good nursing effect on an engine system, can effectively remove carbon deposition and prevent formation of the carbon deposition, has the effective chemical effect of integrally clearing a fuel system, is suitable for a new direct injection fuel oil system, can be used in any types of engines such as a gasoline engine and a diesel engine and remove precipitates from a combustion chamber and an inlet valve, only needs to be poured into an oil tank, effectively prevents pause, improves fuel combustion efficiency and horsepower and reduces emission.
Owner:陈广圣
A kind of synthetic technology of pyraclostrobin intermediate o-nitrobenzyl bromide
ActiveCN113185410BIncrease contentReduce generationOrganic chemistryOrganic compound preparationSulfate zincChlorobenzene
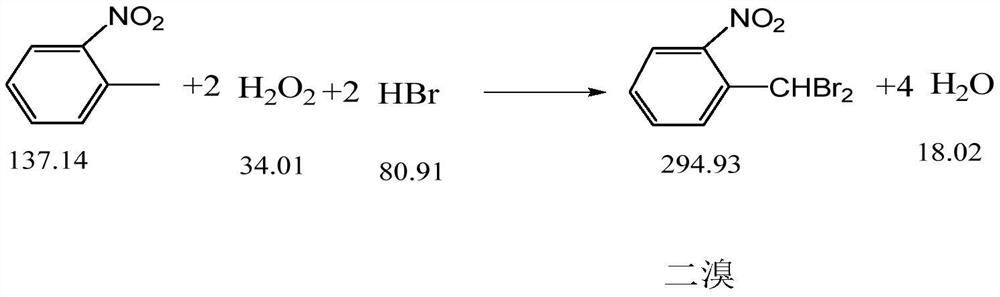
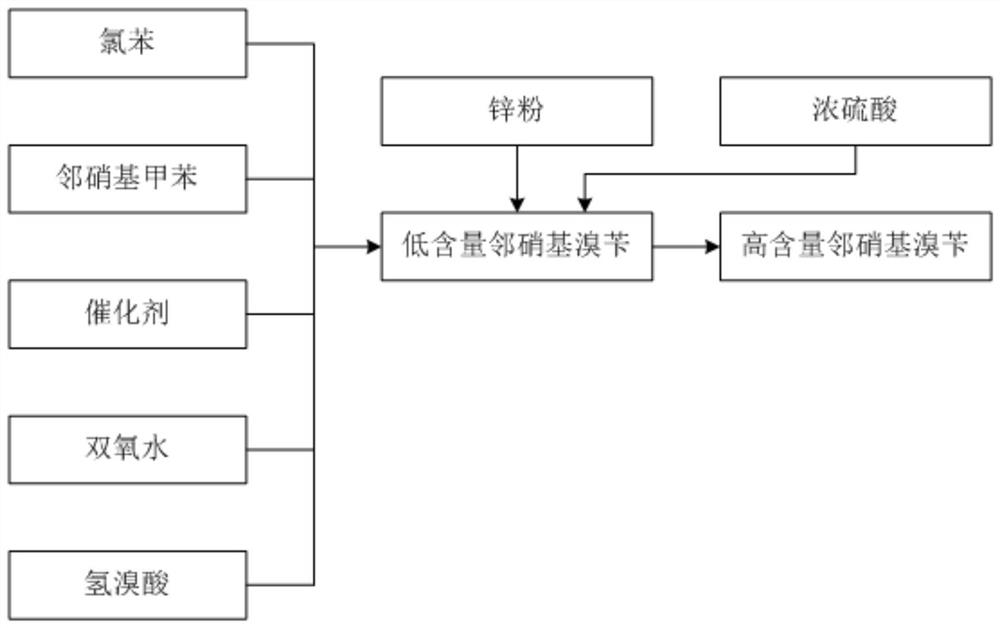
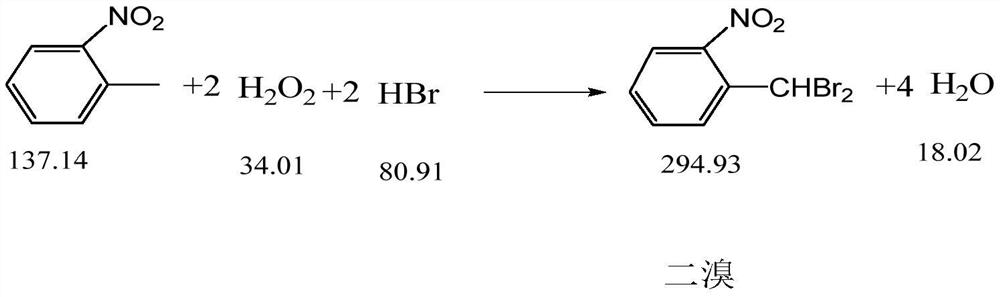
The invention discloses a synthesis process of pyraclostrobin intermediate o-nitrobenzyl bromide, which comprises the following steps: adding chlorobenzene, hydrobromic acid solution and a catalyst into a reaction kettle, raising the temperature; Add o-nitrotoluene and hydrogen peroxide solution, and continue to keep warm for 2 hours; put concentrated sulfuric acid and zinc powder into the reaction kettle in turn, control the temperature between 85-90°C, keep warm for 1 hour; let stand to separate layers, and separate the lower layer The organic phase was sampled and analyzed to obtain o-nitrobenzyl bromide. The content of o-nitrobenzyl bromide obtained by the synthesis process of o-nitrobenzyl bromide of the present invention is more than 75%, and the content of by-product dibromine is below 0.5%. Compared with the old technology, it has high yield and few by-products characteristics, and the synthesis process does not require additional equipment, the process operation is simple, and the economic benefit is high.
Owner:浙江禾本科技股份有限公司 +1
Synthesis process of pyraclostrobin intermediate o-nitrobenzyl bromide
ActiveCN113185410AIncrease contentReduce generationOrganic chemistryOrganic compound preparationChlorobenzenePtru catalyst
The invention discloses a synthesis process of a pyraclostrobin intermediate o-nitrobenzyl bromide. The synthesis process comprises the following steps of: adding chlorobenzene, a hydrobromic acid solution and a catalyst into a reaction kettle, and performing heating; dropwise adding o-nitrotoluene and a hydrogen peroxide solution into the reaction kettle at the same time, and continuously carrying out heat preservation reaction for 2 hours; adding concentrated sulfuric acid and zinc powder into the reaction kettle in sequence, controlling the temperature to be 85-90 DEG C, and keeping the temperature for 1 hour; and standing for layering, separating out a lower organic phase, sampling and analyzing to obtain the o-nitrobenzyl bromide. The content of the o-nitrobenzyl bromide obtained through the synthesis process of the o-nitrobenzyl bromide is 75% or above, the content of the by-product dibromine is 0.5% or below; compared with an old process, the synthesis process has the advantages of being high in yield and few in by-product; and additional equipment is not needed in the synthesis process, the process operation is simple, and economic benefits are high.
Owner:浙江禾本科技股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com