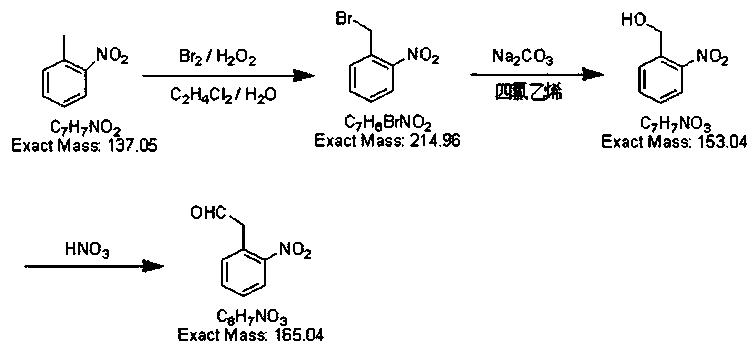
Preparation method of o-nitrobenzaldehyde
A technology of o-nitrobenzaldehyde and o-nitrotoluene, applied in the field of preparation of leading nitrobenzaldehyde, can solve the problems of low selectivity, high production cost, environmental pollution, etc., achieve great application prospects, simple method, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The synthesis of embodiment 1 o-nitrobenzaldehyde
[0014] Before starting the reaction, take a 250ml three-neck flask, add o-nitrotoluene (24ml, 0.2mol), 30ml 1,2-dichloroethane, and 60ml deionized water to it in turn, and stir for 5 minutes with a magnet. Weigh 2 g of azobisisobutyronitrile and add it into the mixed solution of o-nitrotoluene, raise the temperature of the solution to 65° C., and stir for 15 min. Take 6ml of liquid bromine and add it to the dropping funnel, and add about 10ml of deionized water above the liquid bromine to prevent the liquid bromine from volatilizing. Add liquid bromine dropwise to the solution, and the dropwise addition time of liquid bromine is 1.5h. After the liquid bromine was added dropwise, the color of the solution was reddish brown, and the reddish brown in the solution faded to light yellow after continuing to react for about 2 hours. Add 1.5 g of azobisisobutyronitrile to the solution and stir for 15 min, measure 20 ml of 30...
Embodiment 2
[0015] The synthesis of embodiment 2 o-nitrobenzaldehyde
[0016] According to the above-mentioned method for preparing o-nitrobenzyl alcohol, 21 g of crude o-nitrobenzyl alcohol before recrystallization was obtained, which was oxidized according to the ratio of the amount of o-nitrobenzyl alcohol to nitric acid being 1:2.5. Add 23ml of concentrated nitric acid to 22ml of distilled water to prepare dilute nitric acid with a concentration of 40%. Add o-nitrobenzyl alcohol to the dilute nitric acid solution and stir, heat up to 60°C, react for 4 hours, and cool down the solution to 40°C , in the solution, slowly drip the sodium hydroxide solution that concentration is 25%, adjust the pH=8~9 of solution, drop to room temperature and wait for o-nitrobenzaldehyde to separate out, filter and remove filtrate, filter cake is washed to neutrality with distilled water 19 g of light yellow o-nitrobenzaldehyde was obtained, and 25 ml of tetrachloroethylene was added to heat up to 90° C. f...
Embodiment 3
[0017] The synthesis of embodiment 3 o-nitrobenzaldehyde
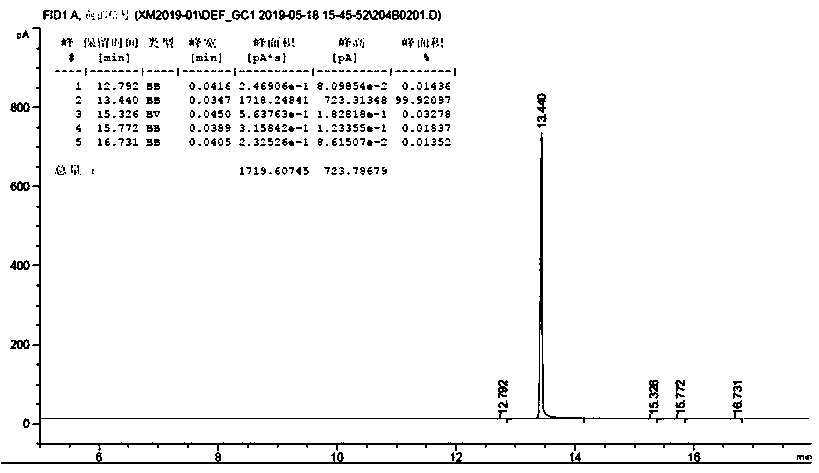
[0018] According to the method described in embodiment 1, obtain the o-nitrobenzaldehyde of 16g light yellow, then carry out the acidification treatment of gac, get the medical grade gac (powder) of 10g, add 50ml dilute sulfuric acid solution (1.5mol / L), in N 2 In the case of protection, heat to 50°C, react for 2 hours, then filter the activated carbon, and wash away excess dilute sulfuric acid with distilled water. Dissolve the light yellow o-nitrobenzaldehyde in 10ml of methanol, add 0.8g of acid-treated activated carbon, heat up to 60°C, stir for 40min, filter to obtain a transparent o-nitrobenzaldehyde solution, and spin evaporate at 30°C. White o-nitrobenzaldehyde (15 g, 50%) was obtained with a purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com