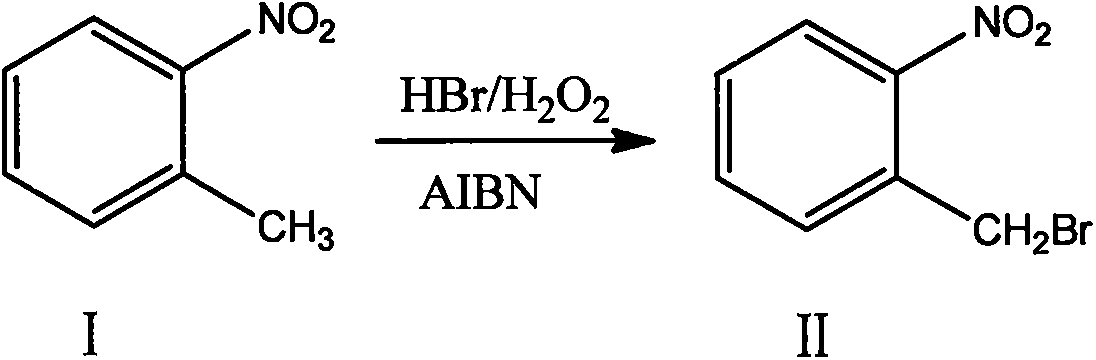
A new synthesis process of o-nitrobenzyl bromide
A kind of ortho-nitrobenzyl bromide and a new synthesis technology, applied in the field of new synthesis technology of ortho-nitrobenzyl bromide, can solve the problems of unsuitable large-scale industrial synthesis technology and high cost, reduce production technology cost and prevent by-products , Environmental pollution-free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 5000L bromination kettle, add 300kg of dichloroethane, 226kg of o-nitrotoluene, and 24kg of azobisisobutyronitrile in sequence, and after stirring for 20 minutes, pump the solution into a high-level tank for use; Add 300kg of dichloroethane and 226kg of o-nitrotoluene, stir evenly, add 740.86kg of 40% hydrobromic acid, and heat up and stir to 72-75°C. The mixed solution and 373.55kg of 30% hydrogen peroxide in the overhead tank were slowly added dropwise to the bromination kettle simultaneously at a rate of 1:4. After the dropwise addition, the insulation reaction was continued for 2 hours. After the reaction was completed, the temperature was lowered to room temperature, and the reaction solution was analyzed by HPLC. The conversion rate of raw materials was greater than 98.7%, and no by-products such as dibromo compounds were produced. The molar ratio of raw materials, 40% hydrobromic acid and 30% hydrogen peroxide in the reaction is 1: 1.0: 1.0. The solution w...
Embodiment 2
[0029] In a 5000L bromination kettle, add 300kg of dichloroethane, 226kg of o-nitrotoluene, and 27kg of azobisisobutyronitrile in sequence, and after stirring for 20 minutes, pump the solution into the high-level tank for use; Add 300kg of dichloroethane and 226kg of o-nitrotoluene, stir evenly, add 889.03kg of 40% hydrobromic acid, and heat up and stir to 72-75°C. The mixed solution and 448.26kg of 30% hydrogen peroxide in the head tank were slowly added dropwise to the bromination kettle simultaneously at a rate of 1:4. After the dropwise addition, the insulation reaction was continued for 2 hours. After the reaction was completed, the temperature was lowered to room temperature, and the reaction liquid was analyzed by HPLC. The conversion rate of raw materials was greater than 99.0%, and no by-products such as dibromo compounds were produced. The mol ratio of raw material, 40% hydrobromic acid and 30% hydrogen peroxide in the reaction is 1: 1.2: 1.2. The solution was allo...
Embodiment 3
[0031] In the 5000L bromination kettle, add 260kg of chlorobenzene, 226kg of o-nitrotoluene, and 24kg of azobisisobutyronitrile in sequence, and after stirring for 20 minutes, pump the solution into the high-level tank for use; then put 260kg into the bromination kettle Chlorobenzene and 226kg of o-nitrotoluene were stirred evenly, then 1481.72kg of 40% hydrobromic acid was added, and the temperature was raised and stirred to 72-75°C. The mixed solution and 744.09kg of 30% hydrogen peroxide in the overhead tank were slowly added dropwise to the bromination kettle simultaneously at a rate of 1:4. After the dropwise addition, the insulation reaction was continued for 2 hours. After the reaction was completed, the temperature was lowered to room temperature, and the reaction solution was analyzed by HPLC. The conversion rate of raw materials was greater than 90.4%, and by-products such as dibromo compounds were formed. The molar ratio of raw materials, 40% hydrobromic acid and 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com