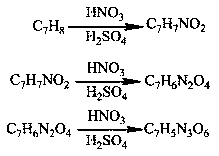
Method for preparing TNT (Trinitrotoluene) by taking nitrotoluene as raw material through one-step method
A technology of nitrotoluene and o-nitrotoluene, applied in the field of one-step preparation of TNT, which can solve the problems of factory equipment corrosion, unstable operation, oxidation side reactions, etc., and achieve the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the method for synthesizing TNT by one-step method
[0046]This embodiment has investigated the influence of reaction time on TNT yield:
[0047] Take 0.05 mol of p-nitrotoluene, 0.15 mol of nitric acid, and 0.5 mol of sulfuric acid, and the reaction temperature is 120°C. The reaction time is 3h, 4h, 5h, 6h, 7h, 8h respectively. The effect of reaction time on the product is shown in Table 1. As can be seen from Table 1, the yield of the product TNT increases first and then remains unchanged with the prolongation of time, and it is the best when the reaction is 4h.
[0048] The influence of table 1 reaction time on TNT yield
[0049]
[0050] Optimization of reaction time: take 0.05mol of p-nitrotoluene, HNO 3 :H 2 SO 4 =3:10, 0.15mol of nitric acid, 0.5mol of sulfuric acid, the reaction temperature is 120 degrees. The reaction time is 3h, 4h, 5h, 6h, 7h, 8h respectively. The product in 3h was a mixture (the reaction was not completed), and the o...
Embodiment 2
[0051] Embodiment 2: the method for synthesizing TNT by one-step method
[0052] This embodiment has investigated the influence of temperature of reaction on TNT yield
[0053] Take 0.05mol of p-nitrotoluene, HNO 3 :H 2 SO 4 =1:3.5, 0.15mol of nitric acid, 0.5mol of sulfuric acid, the reaction time is 4h. The reaction temperatures were 100°C, 110°C, 120°C, 130°C, and 140°C, respectively.
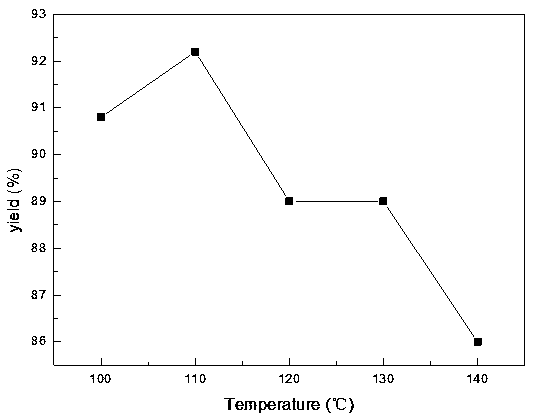
[0054] The influence of reaction temperature on product TNT is as follows figure 1 . Depend on figure 1 It can be seen that the product yield first increases and then decreases, and the product yield is the highest at 110 °C.
[0055] Optimization of reaction temperature: take 0.05mol of p-nitrotoluene, HNO 3 :H 2 SO 4 =1:3.5, 0.15mol of nitric acid, 0.5mol of sulfuric acid, the reaction time is 4h. The reaction temperatures were 100°C, 110°C, 120°C, 130°C, and 140°C, respectively. The yields were 90.8%, 92.2%, 89%, 89%, and 86%, respectively.
Embodiment 3
[0056] Embodiment 3: the method for one-step synthetic TNT
[0057] This embodiment has investigated the influence of nitric acid and the concentrated sulfuric acid mol ratio on TNT yield
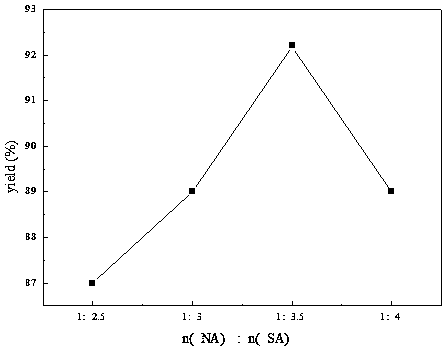
[0058] Take 0.05mol of o-nitrotoluene, the reaction time is 4h, and the reaction temperature is 110°C. The ratio (molar ratio) of nitric acid to sulfuric acid is 1:2.5, 1:3, 1:3.5, 1:4 respectively. Product yield n(NA): n(SA) changes as figure 2 shown. from figure 2 It can be seen that the yield of the target product first increases and then decreases with the increase of concentrated sulfuric acid, and reaches the maximum when n(NA):n(SA)=1:3.5.
[0059] The optimization of the mixed acid ratio of nitric acid and sulfur in the reaction: take 0.05 mol of o-nitrotoluene, the reaction time is 4 hours, and the reaction temperature is 110 degrees. The ratio of nitric acid to sulfuric acid is 1:2.5, 1:3, 1:3.5, 1:4 respectively. The yields were 87%, 89%, 92.2%, and 89%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com