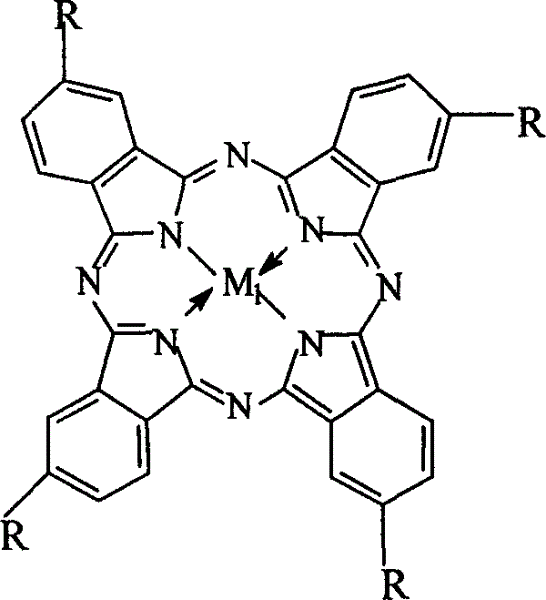
Preparation of o-nitrobenzaldehyde by biomimetic catalysis oxidation of o-nitrotoluene with oxygen
A technology of p-nitrobenzaldehyde and p-nitrotoluene is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, and can solve the problems of difficult separation of by-products, low utilization rate of raw materials, serious environmental pollution and the like, Achieve the effect of easy control of oxidation depth, reduced reaction cost and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 3mg of cobalt phthalocyanine (i.e. R=H in general formula (I), M 1 =Co), 2.8g of p-nitrotoluene and 2.8g of NaOH were charged into a 200ml autoclave, 50ml of methanol was added, oxygen at a pressure of 1.6MPa was introduced, and the reaction was carried out in a water bath at a temperature of 45°C for 12h. The mixed solution after the reaction was first removed the catalyst through suction filtration, then added 50ml of distilled water, added dilute hydrochloric acid to neutralize, filtered, after purification, analyzed and detected by high pressure liquid chromatography, the yield of p-nitrobenzaldehyde was 18.2%. The purity of the final product was 99.0%.
Embodiment 2
[0025] Weigh 10mg of iron phthalocyanine (i.e. R=H in general formula (I), M 1 =Fe), 2.8g of p-nitrotoluene, 3.5g of sodium hydroxide were put into a 200ml autoclave, 50ml of methanol was added, the oxygen pressure was 0.5MPa, the temperature was controlled at 65°C in a water bath, and the reaction was carried out for 6h. The processing steps are the same as in Example 1, and the resulting product is analyzed and detected by high-pressure liquid chromatography. The yield of p-nitrobenzaldehyde is 23.6%, and the purified product has a purity of 98.5%.
Embodiment 3
[0027] Weigh 14mg of copper phthalocyanine (i.e. R=H in general formula (I), M 1 =Cu), 2.8g p-nitrotoluene, 1.4g sodium hydroxide were put into a 200ml autoclave, 50ml methanol was added, the oxygen pressure was 3.0MPa, the temperature was controlled at 20°C in a water bath, and the reaction was carried out for 48h. The processing steps are the same as in Example 1, and the resulting product is detected by high-pressure liquid chromatography, and the yield of p-nitrobenzaldehyde is 16.7%, and the purified product has a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com