Synthesis process of pyraclostrobin intermediate o-nitrobenzyl bromide
A technology of pyraclostrobin and synthesis process, which is applied in the field of synthesis process of pyraclostrobin intermediate o-nitrobenzyl bromide, can solve the problem of low content of ortho-nitrobenzyl bromide, and achieve high yield and side effects The effect of less product and high economic benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
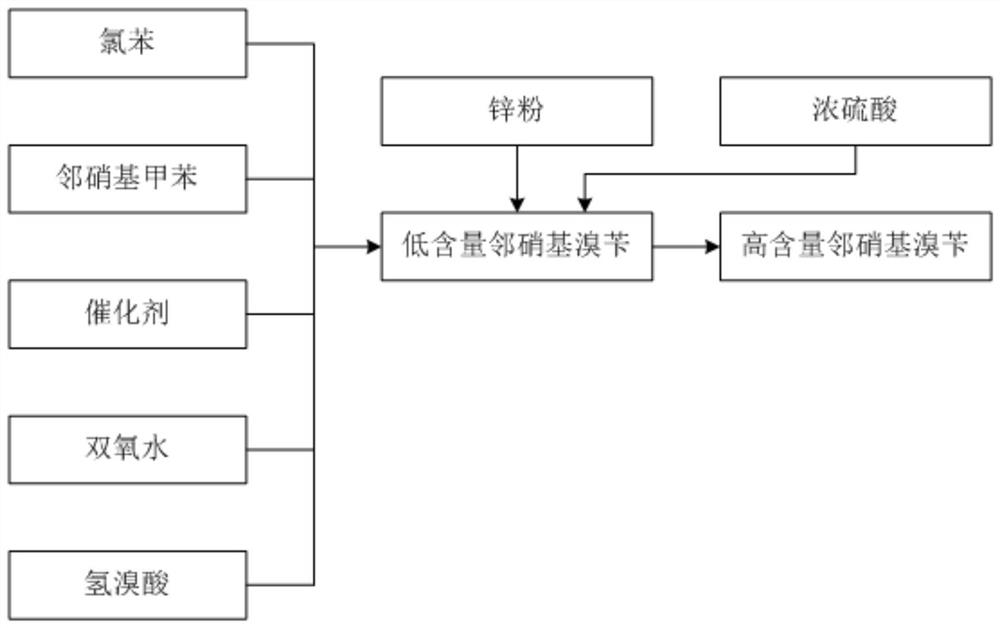
[0027] A kind of synthetic technology of pyraclostrobin intermediate o-nitrobenzyl bromide
[0028] The synthetic technique of pyraclostrobin intermediate o-nitrobenzyl bromide comprises the following steps:
[0029] Step 1, 1000kg of chlorobenzene, 445kg of hydrobromic acid solution with a concentration of 48wt%, and 20kg of catalyst are added to the reaction kettle, and the temperature is raised to 75°C;
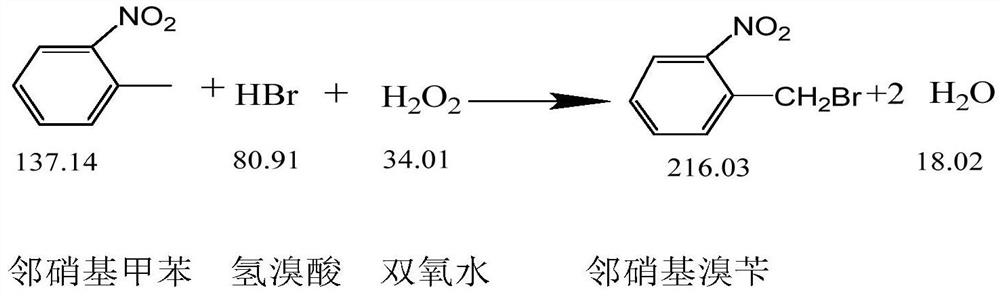
[0030] Step 2: Add 400kg of o-nitrotoluene and 250kg of hydrogen peroxide solution with a concentration of 28% dropwise into the reactor at the same time. After the addition is complete, continue the insulation reaction for 2 hours;
[0031] Step 3, put 25kg of concentrated sulfuric acid and 5kg of zinc powder into the reaction kettle in turn, control the temperature between 85-90°C, and keep it warm for 1 hour;
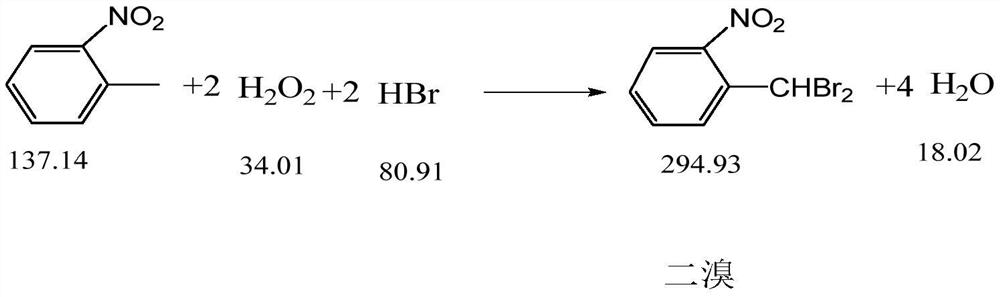
[0032] Step 4, standing for stratification, separating the lower organic phase, sampling and analyzing, the content of o-nitrobenzyl bromide is 78.03%, and the...
Embodiment 2
[0034] A kind of synthetic technology of pyraclostrobin intermediate o-nitrobenzyl bromide
[0035] The synthetic technique of pyraclostrobin intermediate o-nitrobenzyl bromide comprises the following steps:
[0036] Step 1, 800kg of chlorobenzene, 420kg of hydrobromic acid solution with a concentration of 45wt%, and 15kg of catalyst are added to the reactor, and the temperature is raised to 70°C;
[0037] Step 2, add 380kg of o-nitrotoluene and 270kg of hydrogen peroxide solution dropwise at the same time in the reactor, the temperature is controlled at 75°C, and the rate of addition is controlled so that the addition of o-nitrotoluene and hydrogen peroxide solution is completed in 2 hours. Continue the insulation reaction for 1.5 hours;
[0038] Step 3, put 20kg of concentrated sulfuric acid and 2kg of zinc powder into the reaction kettle in turn, control the temperature between 85°C and keep it warm for 1 hour;
[0039] Step 4, standing for stratification, separating the ...
Embodiment 3
[0041] A kind of synthetic technology of pyraclostrobin intermediate o-nitrobenzyl bromide
[0042] The synthetic technique of pyraclostrobin intermediate o-nitrobenzyl bromide comprises the following steps:
[0043] Step 1, 1200kg of chlorobenzene, 460kg of hydrobromic acid solution with a concentration of 50wt%, and 25kg of catalyst are added to the reactor, and the temperature is raised to 80°C;
[0044] Step 2, add 420kg o-nitrotoluene and 240kg concentration of 30% hydrogen peroxide solution to the reactor simultaneously, control the temperature at 85°C, and control the rate of addition so that the o-nitrotoluene and hydrogen peroxide solution are added dropwise in 2 hours, Continue to keep warm for 2 hours;
[0045] Step 3, put 30kg of concentrated sulfuric acid and 8kg of zinc powder into the reaction kettle in turn, control the temperature between 85-90°C, and keep it warm for 1 hour;
[0046] Step 4, standing for stratification, separating the lower organic phase, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com