Preparation method of 2-nitrobenzyl bromide
A technology of nitrobenzyl bromide and o-nitrotoluene, applied in the field of chemical synthesis, can solve the problems of shortened reaction time, low product yield and purity, long reaction cycle and the like, and achieves shortened reaction time, fast reaction speed and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
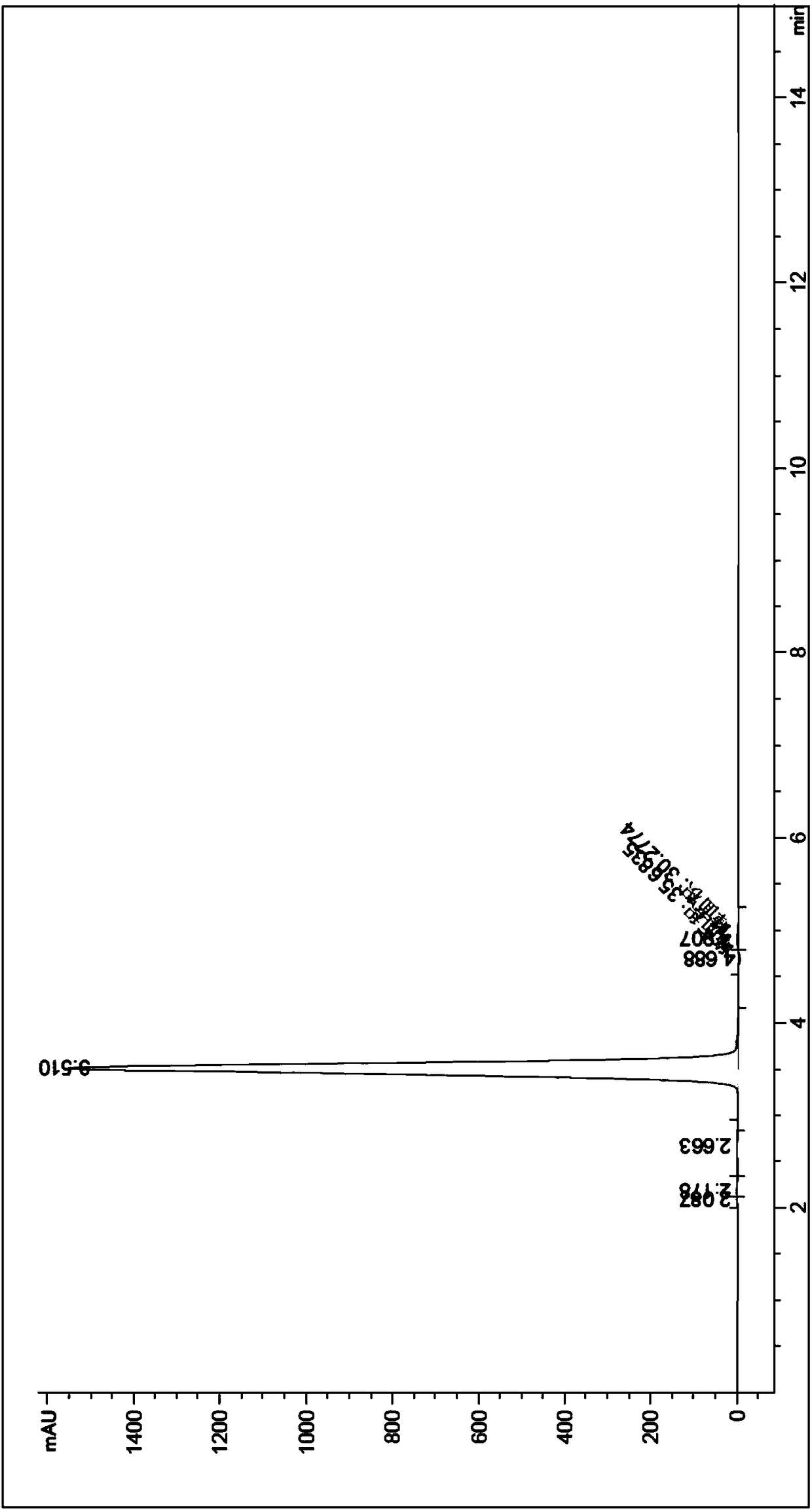
Examples
Embodiment 1
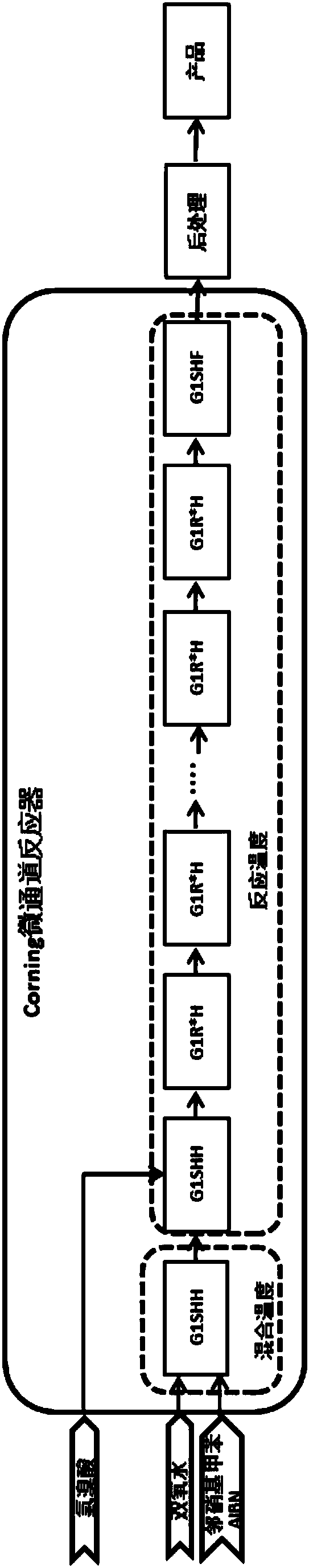
[0039] 1) After the mass percentage of 99.15% o-nitrotoluene and azobisisobutyronitrile are mixed and dissolved, pass into the first module through a metering pump with a mass flow of 7g / min, and azobisisobutyronitrile is 0.03 of the mass of o-nitrotoluene;
[0040] 2) with the mass percentage composition of 5% hydrogen peroxide solution, the mass flow of 24g / min is passed into the first module and mixed with o-nitrotoluene and azobisisobutyronitrile mixed solution, and the o-nitro The molar ratio of toluene and hydrogen peroxide is 1.00:0.7, and the first module controls the reaction temperature to be 60°C;
[0041] 3) The mixed feed liquid that step 1) and step 2) are processed are pumped into the second module, and synchronously the mass percentage of 5% hydrobromic acid is passed into the second module with a mass flow of 50g / min through a metering pump , carry out mixing and reaction, the second module controls the reaction temperature to be 150°C, and the molar ratio of...
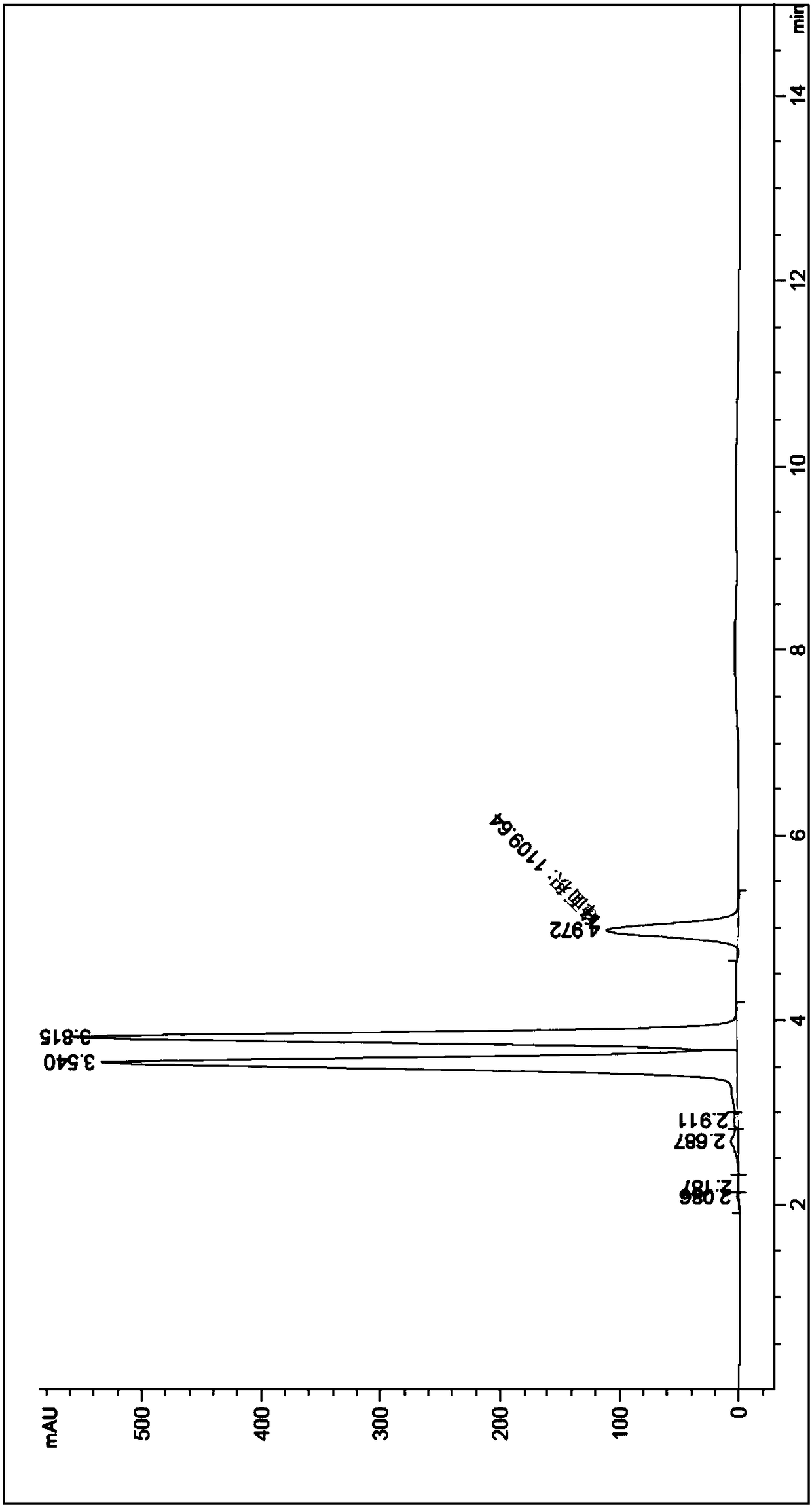
Embodiment 2
[0046] 1) after the mass percentage composition is 99.56% o-nitrotoluene and benzoyl peroxide mixed and dissolved, pass into the first module through a metering pump with the mass flow of 15g / min, and benzoyl peroxide is an o-nitroxide. 0.08 of the mass of toluene;
[0047] 2) with the mass percentage composition of 30% hydrogen peroxide solution, pass into the first module with the mass flow of 15g / min and mix with o-nitrotoluene and benzoyl peroxide mixed solution, o-nitrotoluene The molar ratio with hydrogen peroxide is 1.00:1.2, and the first module controls the reaction temperature to be 70°C;
[0048]3) The mixed feed liquid processed in step 1) and step 2) is pumped into the second module, and the hydrobromic acid that the mass percentage is 48% is passed into the second module with the mass flow of 20g / min through the metering pump synchronously. , carry out mixing and reaction, the second module controls the reaction temperature to be 110°C, and the molar ratio of o-...
Embodiment 3
[0053] 1) after 99.87% o-nitrotoluene and cumene hydrogen peroxide are mixed and dissolved by mass percentage, pass into the first module through a metering pump with the mass flow of 11g / min, and cumene hydrogen peroxide is 0.05 of the mass of o-nitrotoluene;
[0054] 2) be the hydrogen peroxide solution of 20% by mass percentage, pass into the first module with the mass flow rate of 14g / min and mix with o-nitrotoluene and cumene hydrogen peroxide mixed solution, o-nitrotoluene and cumene hydrogen peroxide mixed solution. The molar ratio of toluene and hydrogen peroxide is 1.00:1.0, and the first module controls the reaction temperature to be 65°C;
[0055] 3) The mixed feed liquid processed in step 1) and step 2) is pumped into the second module, and the hydrobromic acid that the mass percentage is 20% is passed into the second module with the mass flow of 26g / min through the metering pump synchronously. , carry out mixing and reaction, the second module controls the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com