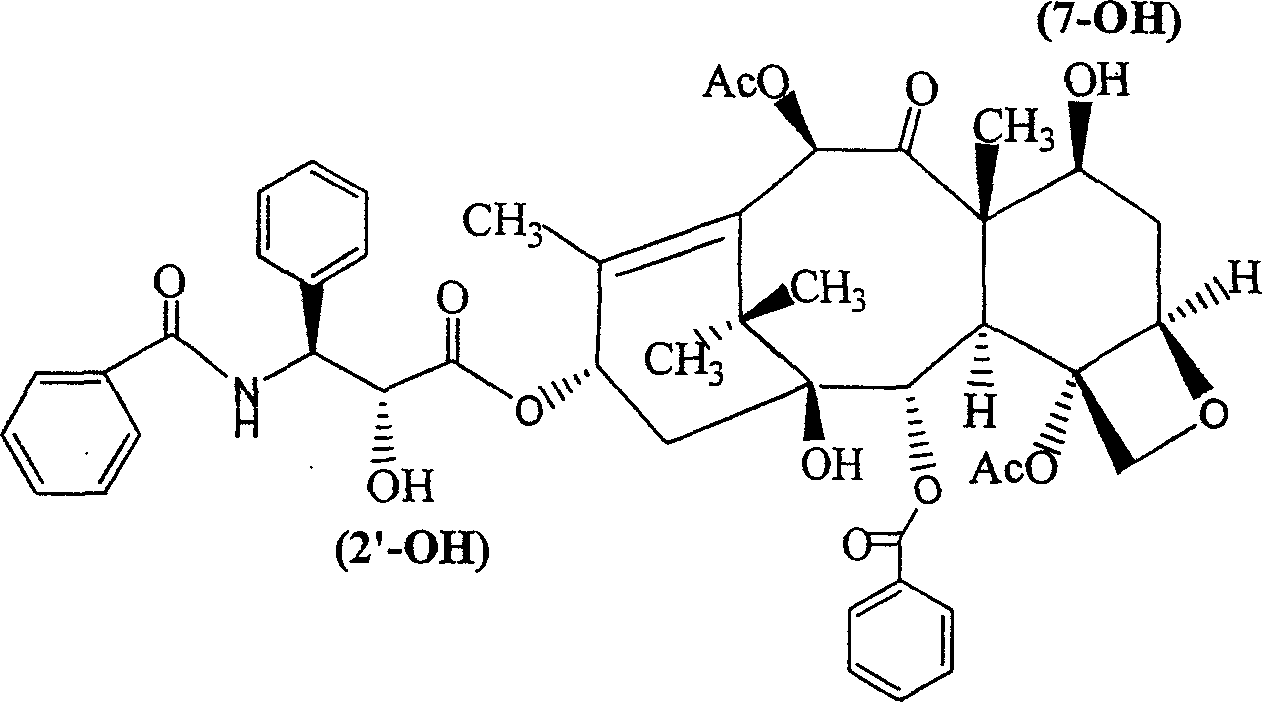
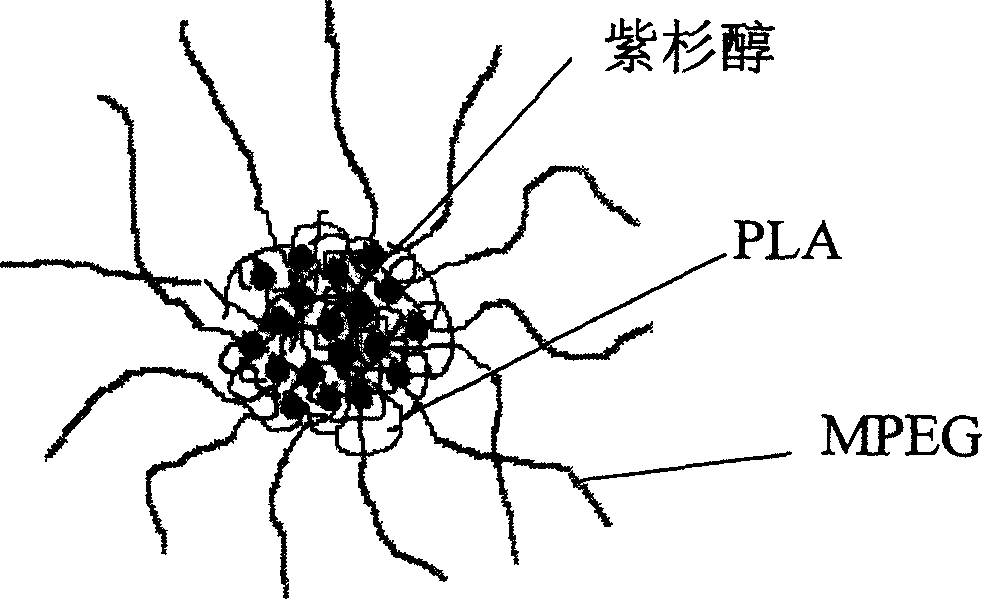
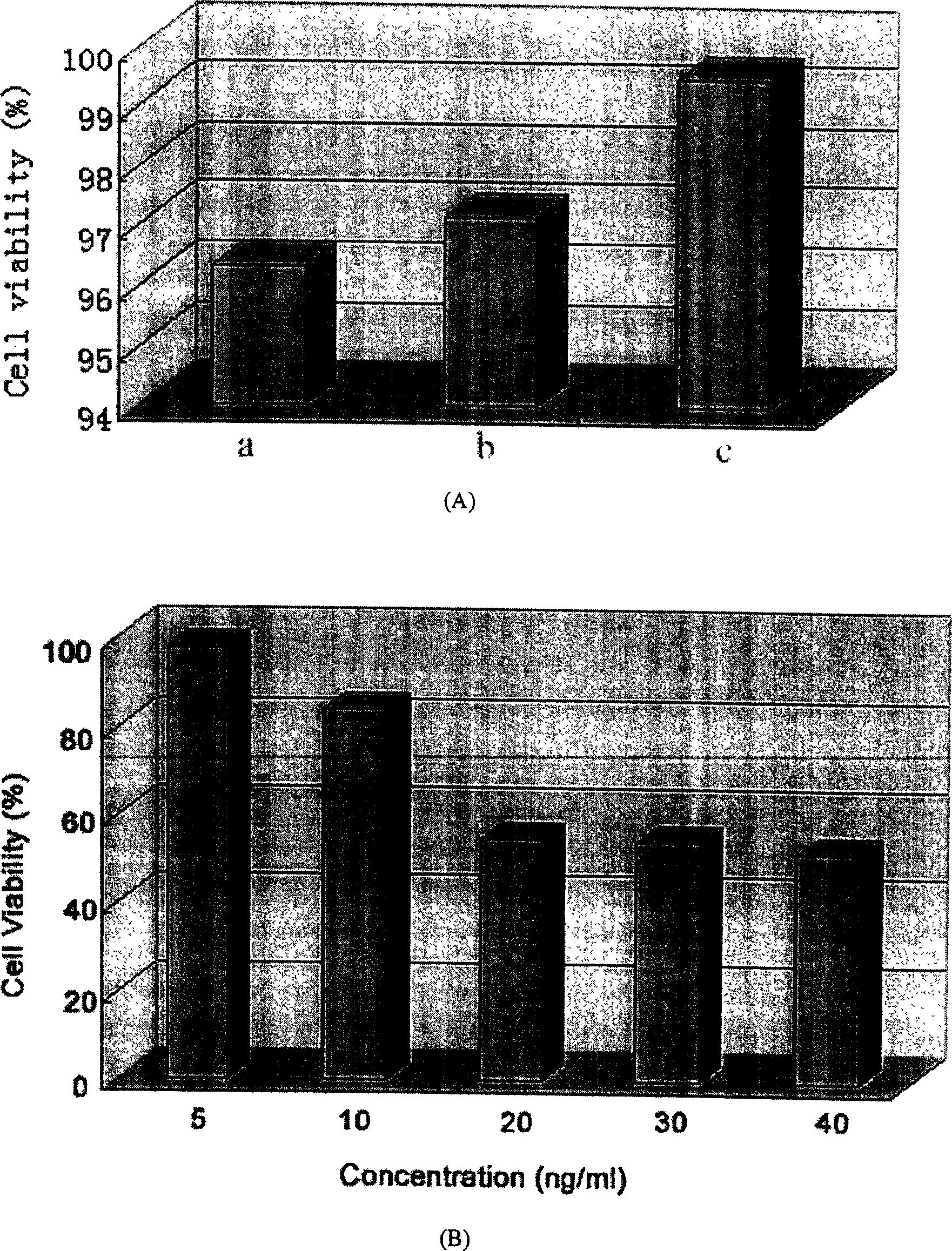
Paclitaxol predrug of biodegradable polymer and its synthesis method
A technology of biodegradation and synthesis method, which is applied in the field of paclitaxel prodrug and its synthesis, which can solve the problems of inability to release the parent drug rapidly, carcinogenicity in rodents, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) 4 g of lactide (LA) monomer recrystallized three times with ethyl acetate and 10 g of polyethylene glycol monomethyl ether (MPEG) with a molecular weight of 4600 were added to a high-purity argon gas exchanged three times with In the dry ampoule of the water separator, reflux condenser and magnetic stirrer, add anhydrous toluene solvent with a total mass ratio of 2:1 to LA and MPEG to azeotropically remove water, then distill off half of the toluene, and add about 0.3 ml molarity is 2×10 -3 mol / l stannous octoate toluene solution. The reaction was stirred at 110°C for 24 hours, and then the product was dissolved in an appropriate amount of dichloromethane, and settled with ether to obtain a white product, which was dried under vacuum at 40°C. (10.2 g, 73% yield).
[0046] The NMR spectrum of gained block polymer MPEG-PLA is shown in Figure 4 .
[0047] (2) Ventilate a 100ml three-neck flask with a reflux condenser and a magnetic stirrer three times with high-pu...
Embodiment 2
[0055] (1) 5 g of the refined ε-caprolactone (CL) monomer and 10 g of MPEG with a molecular weight of 2000 are added to a high-purity argon gas exchanged three times with a water separator, a reflux condenser and a magnetic stirrer In the dry ampoule, add anhydrous toluene solvent with a total mass ratio of CL and MPEG of 2:1 to remove water azeotropically, then evaporate half of the toluene, add about 0.2ml molar concentration of 5×10 -4 mol / l stannous octoate toluene solution. The reaction was stirred at 110°C for 24h, then the product was dissolved in an appropriate amount of dichloromethane, and settled with ether to obtain a white product, which was dried under vacuum at 40°C (12.1 g, yield 81%).
[0056] (2) In a 100ml three-necked flask, dissolve 1g of MPEG-PCL diblock polymer and excess mono-tert-butyl diglycolate in 50ml of anhydrous dichloromethane, and add 70mg of DCC and 40mg of DMAP under ice-cooling , The reaction was stirred at 0°C for 24h. The precipitated di...
Embodiment 3
[0059] (1) Add the refined 8g lactide and 2g glycolide mixed monomer and 10g MPEG with a molecular weight of 1000 to a high-purity argon gas exchanged three times with water separator, reflux condenser and magnetic stirrer In a dry ampoule, add anhydrous toluene solvent with a total mass ratio of lactide, glycolide, and MPFG of 2:1 to azeotropically remove water, then distill off half of the toluene, and add about 0.5ml molar concentration of 2 ×10 3 mol / l stannous octoate toluene solution. The reaction was stirred at 110°C for 24 hours, and then the product was dissolved in an appropriate amount of dichloromethane, and settled with ether to obtain a white product, which was dried under vacuum at 40°C. (14.2 g, 71% yield).
[0060](2) In a 100ml there-necked flask, dissolve 1g of polyethylene glycol monomethyl ether-polylactide glycolide block polymer and excess mono-tert-butyl diglycolate in 50ml of anhydrous dichloromethane, and 70mg DCC and 40mg DMAP were added under ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com