Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Malignant Solid Neoplasm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A malignant neoplasm arising from tissues that do not include fluid areas. Representative examples include carcinomas and sarcomas. Hematopoietic and lymphoid tissue malignancies are not considered solid neoplasms.
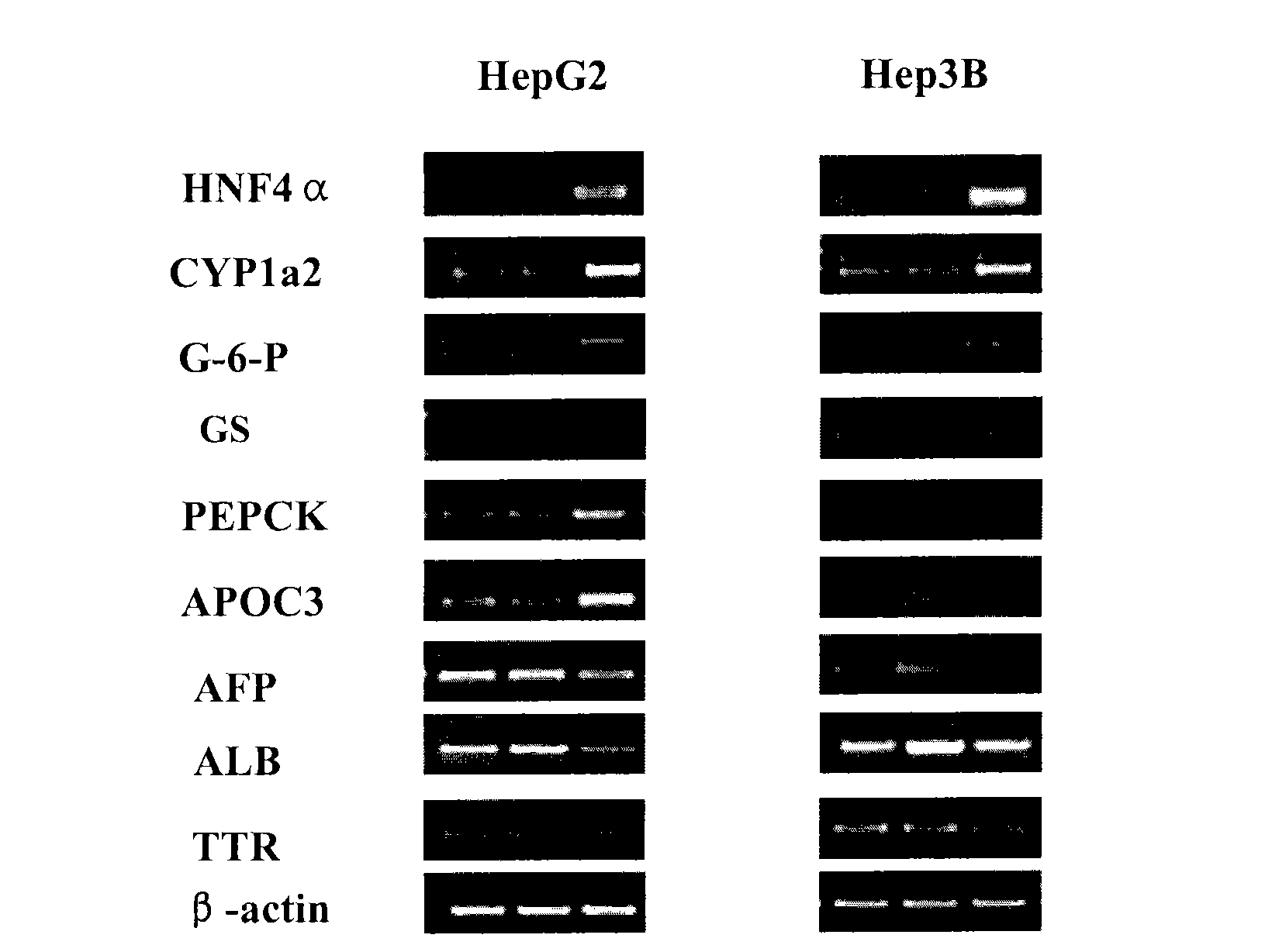
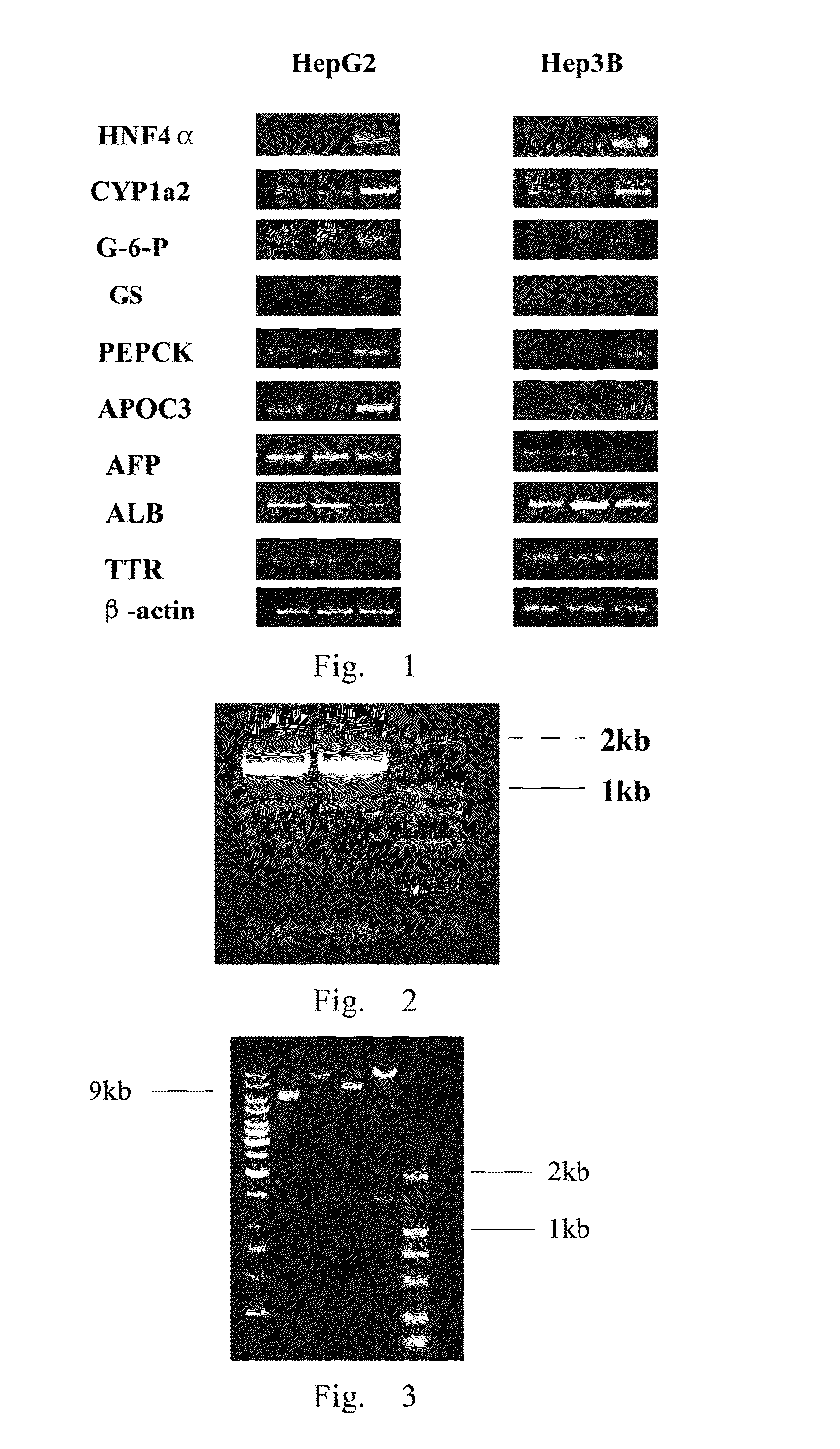
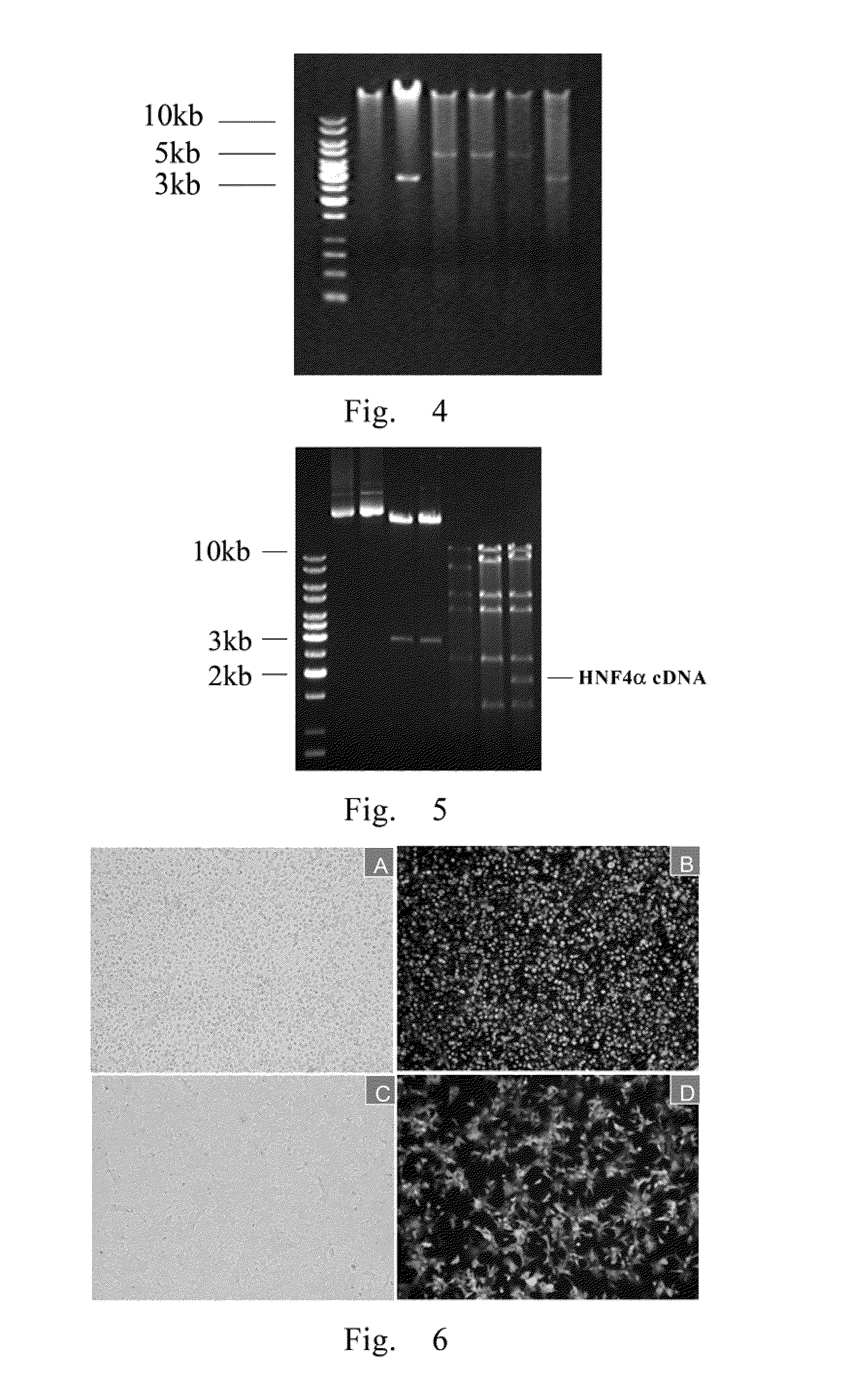
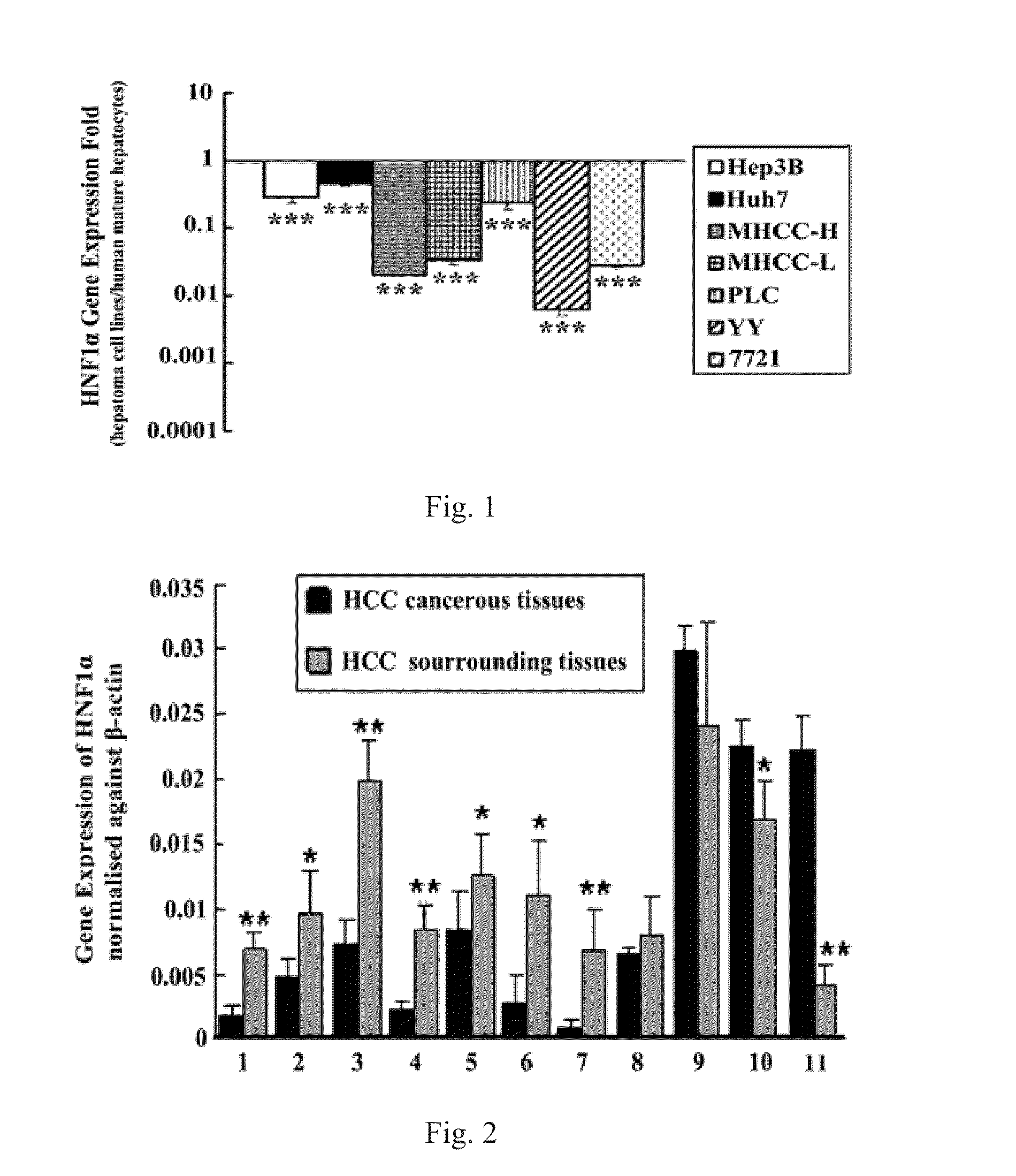
Treatment on human malignant solid tumor by HNF4 alpha inducement and differentiation
ActiveCN101524529AConfirmed differentiation therapyPeptide/protein ingredientsGenetic material ingredientsTumor therapyOncology
The invention relates to treatment on human malignant solid tumor by HNF4 alpha inducement and differentiation, in particularly to a method for utilizing Hepatocyte Neclear Factor-4 alpha (HNF4 alpha) to induce the differentiation of the human malignant solid tumor so as to be applied to the treatment on the malignant solid tumor and application thereof. The research shows that the modulation of gene expression of the HNF4 alpha of malignant solid tumor cells can effectively produce functions of the inducement and the differentiation on the tumor cells so as to provide a new treatment means for inducing and differentiating the tumor.
Owner:上海赛迪夫医药科技有限公司
Use of hnf4alpha for treatment of human malignant solid tumors through induction-differentiation therapy
InactiveUS20110077206A1Suppress in vivo formationOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsSolid tumorDifferentiation therapy
Use of hepatocyte nuclear factor 4α (HNF4α) for the treatment of human malignant solid tumors through induction-differentiation therapy is provided.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
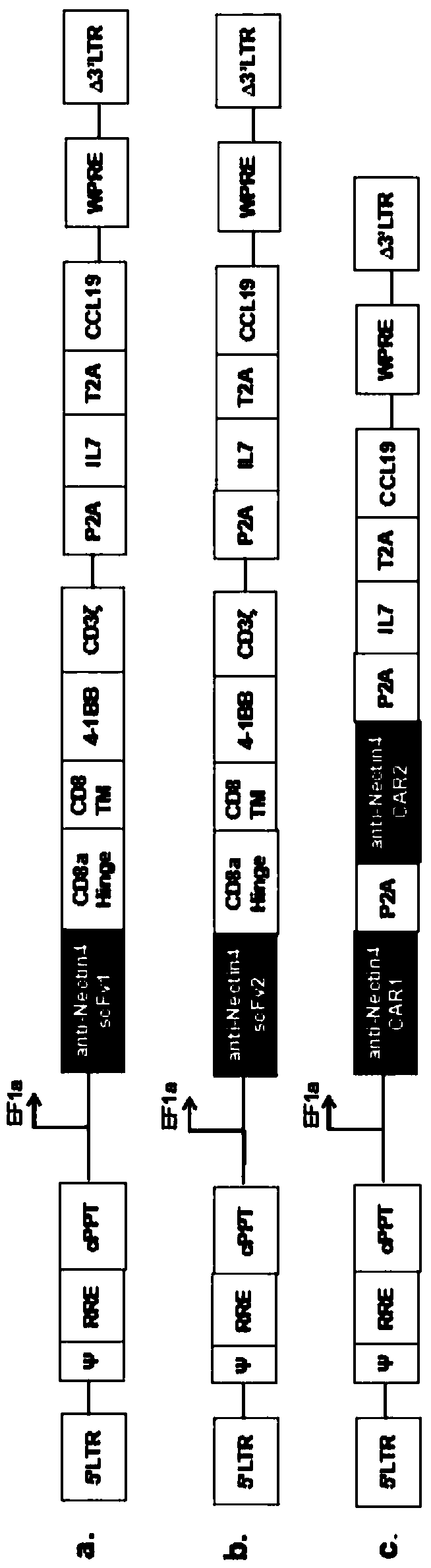
Fourth-generation CAR-T cell as well as construction method and application thereof
ActiveCN109504660AReduce cancer recurrencePromote proliferationAntibody mimetics/scaffoldsMammal material medical ingredientsSurvivabilityLymphatic Spread
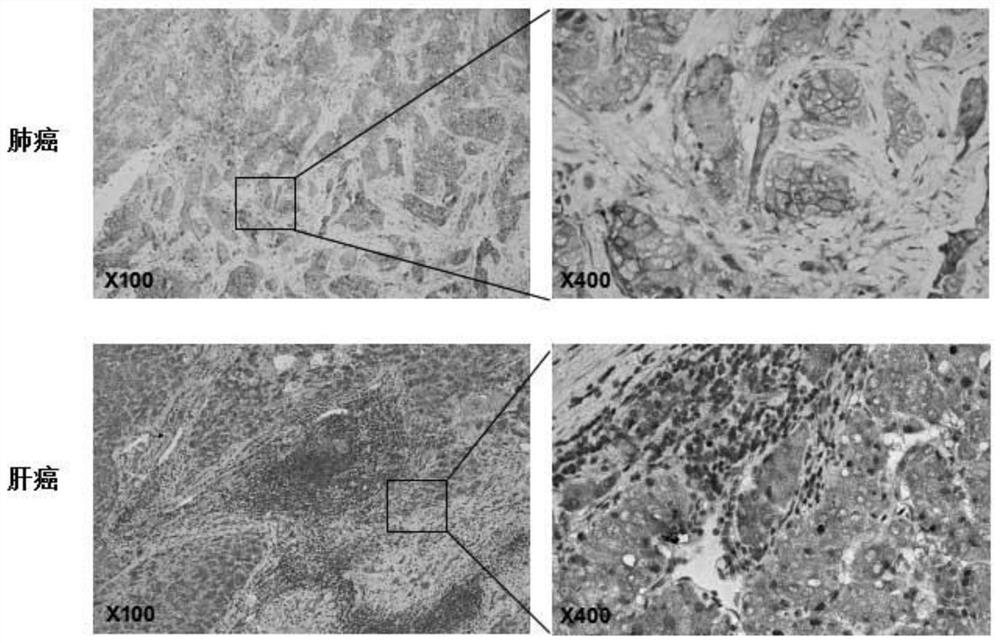
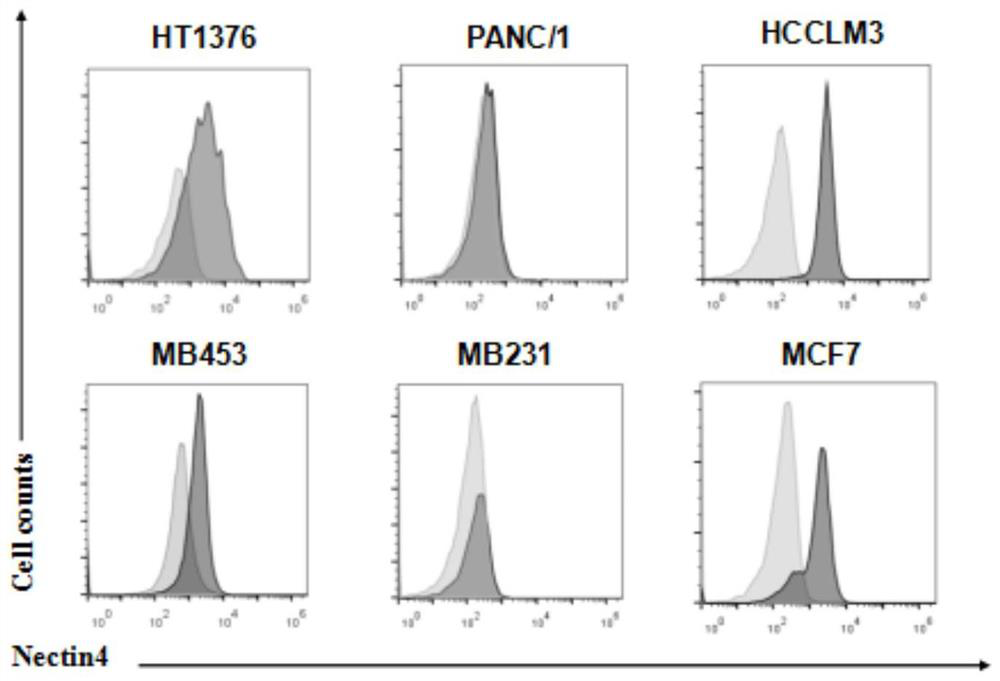
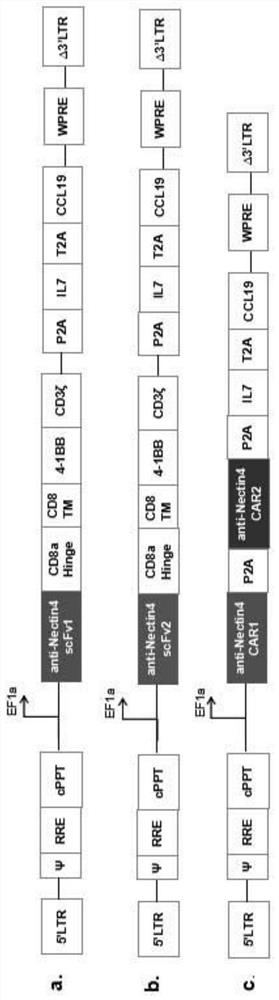
The invention provides construction and application of a fourth-generation chimeric antigen receptor T (CAR-T) cell (expressing IL-7 and CCL19) specific to Nectin-4 dual targets on the surface of a malignant tumor. Two spatial epitopes of a Nectin-4 antigen are taken as target points, and the constructed fourth-generation CAR-T is used for treating and expressing a malignant solid tumor of the Nectin-4 antigen to solve the immune escape problem of the CAR-T during treatment of the solid tumor; and the constructed fourth-generation CAR-T can enhance proliferative capacity and sustained survivability, so that the solid tumor is effectively treated, and a new strategy is provided for effective prevention and treatment of postoperative recurrence / metastasis of the solid tumor.
Owner:温州启星生物技术有限公司
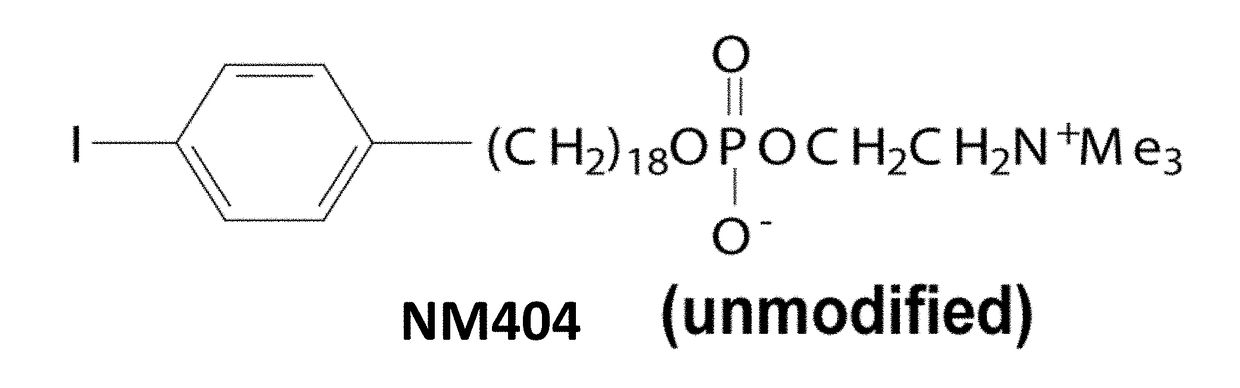
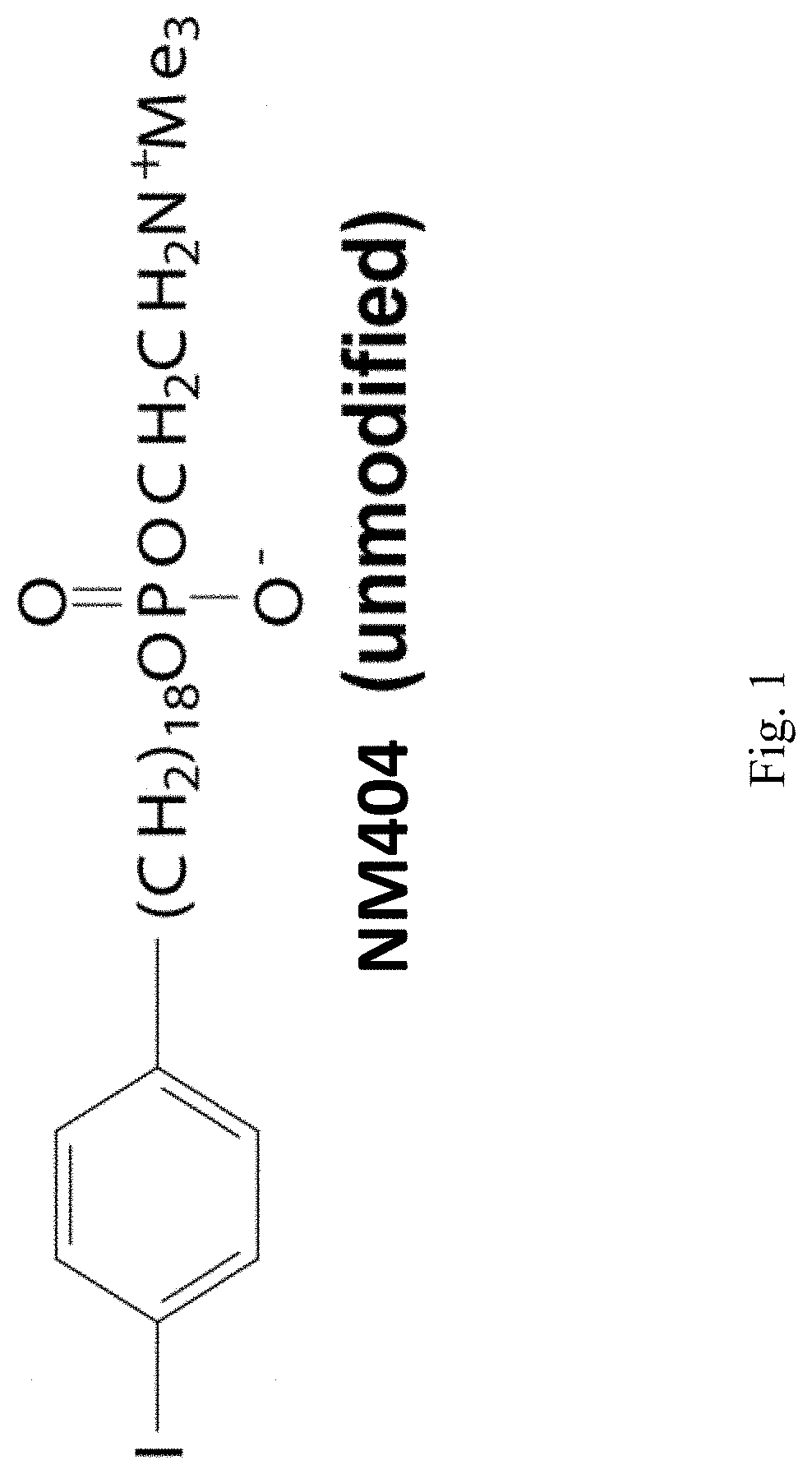
Radiohalogenated Agents for in Situ Immune Modulated Cancer Vaccination
ActiveUS20180015154A1Raise the potentialPeptide/protein ingredientsRadioactive preparation carriersVaccinationSpecific immunity
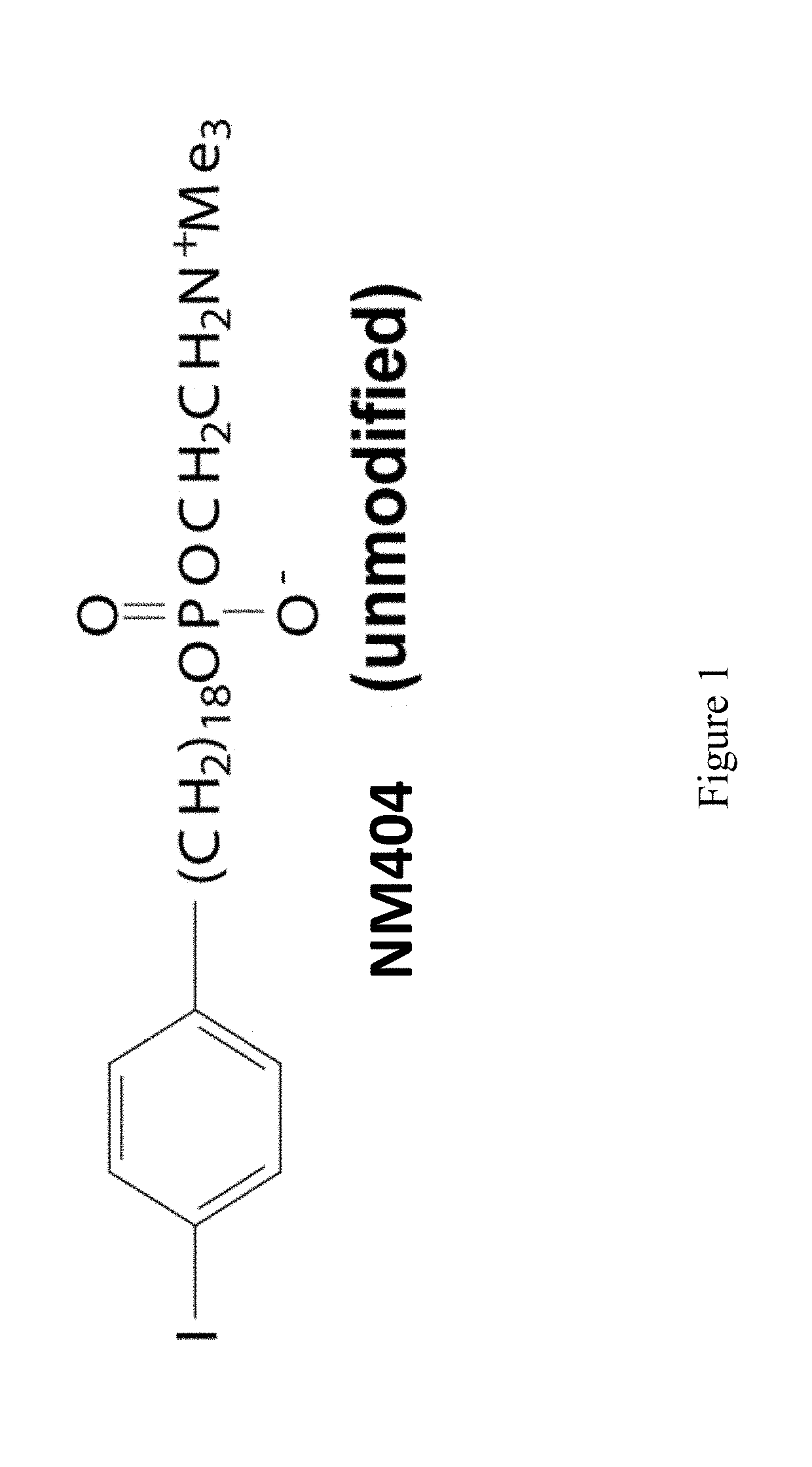
A method of treating a malignant solid tumor in a subject is disclosed herein. The method includes the steps of administering to the subject an immunomodulatory dose of a radiohalogenated compound that is differentially taken up by and retained within malignant solid tumor tissue, and performing in situ tumor vaccination in the subject by intratumorally injecting into (or treating via a separate method) at least one of the malignant solid tumors a composition that includes one or more agents capable of stimulating specific immune cells within the tumor microenvironment. In certain exemplary embodiments, the radiohalogenated compound has the formula:wherein R1 is a radioactive halogen isotope, n is 18 and R2 is —N+(CH3)3.
Owner:WISCONSIN ALUMNI RES FOUND
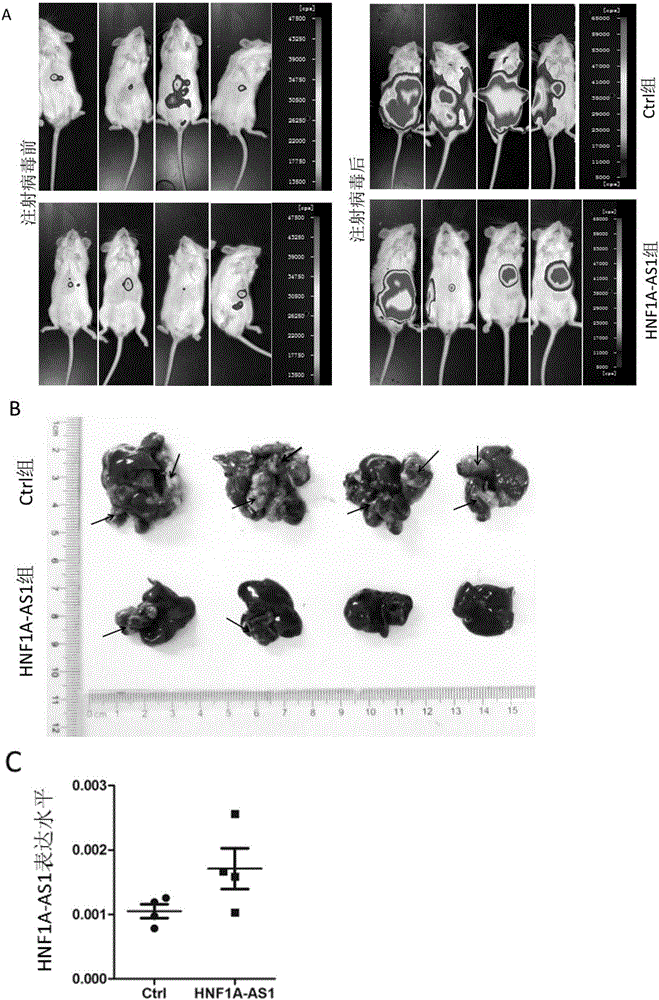
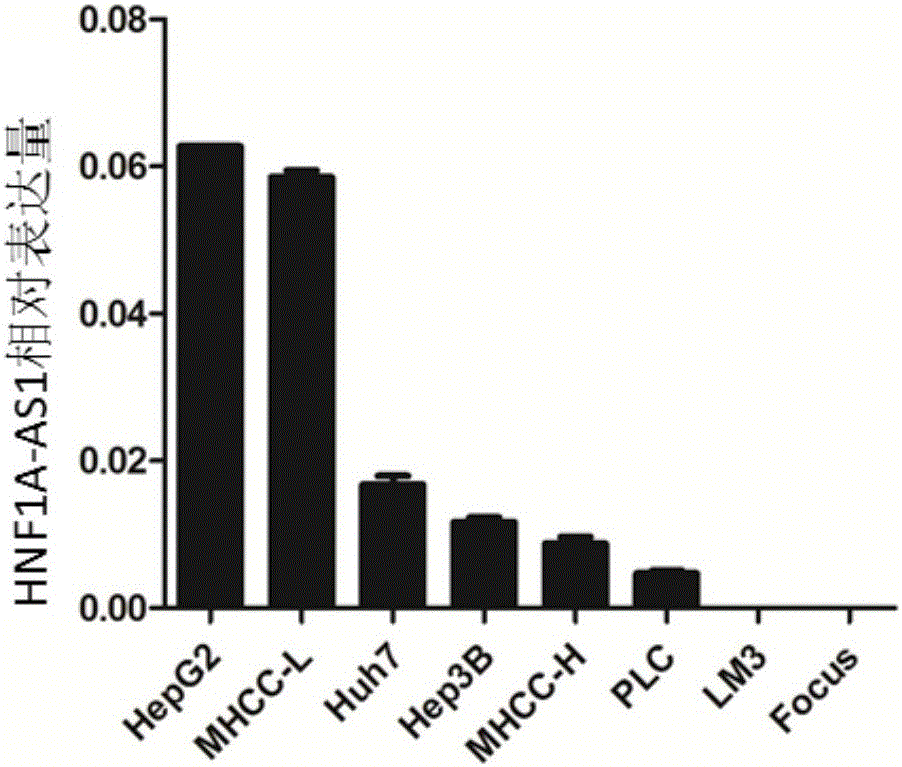
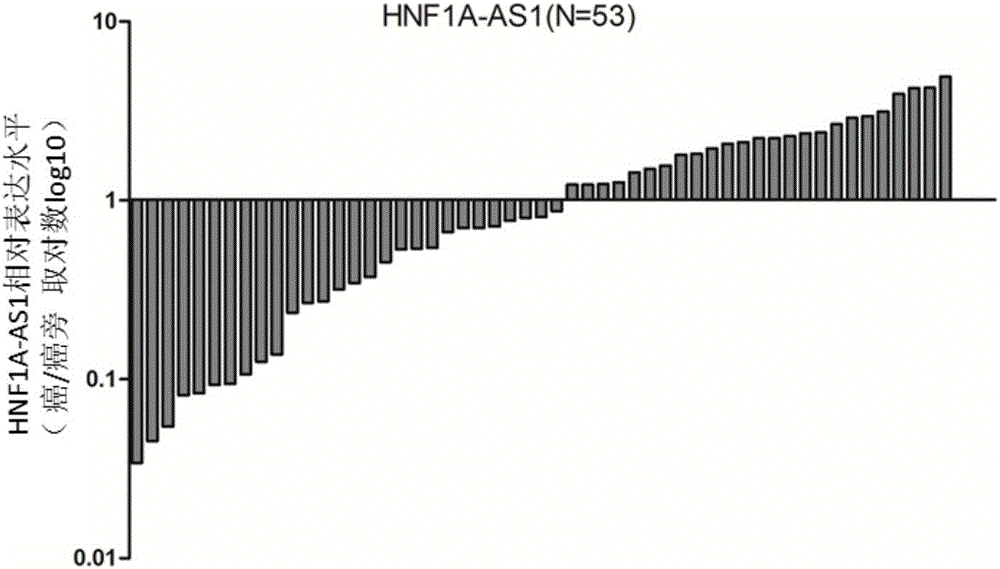
Application of long noncoding RNA HNF1A-AS1 ((hepatocyte nuclear factor-1Alpha Antisense 1) in preparation of drugs for treating human malignant solid tumors
InactiveCN105079821AConfirmed inhibitionOrganic active ingredientsGenetic material ingredientsHepatocyte Nuclear Factor 1alphaMedical diagnosis
The invention relates to the field of gene therapy and medical diagnosis and provides application of long noncoding RNA HNF1A-AS1 (hepatocyte nuclear factor-1Alpha Antisense 1) in the preparation of drugs for treating human malignant solid tumors. Studying shows that by controlling gene expression of malignant solid tumor cells HNF1A-AS1, proliferation of the malignant solid tumors can be effectively inhibited, and thereby a novel target is provided for the clinical treatment of malignant solid tumors.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Use of tumor caryon single-antibody in physical therapeutic tumor as synergist
InactiveCN101028525ADefinite curative effectSmall toxicityOrganic active ingredientsIn-vivo radioactive preparationsAbnormal tissue growthCurative effect
An application of the tumor cell nucleus monoantibody as the synergist in physically treating solid tumor by directionally combining with the necrotic tissue of tumor specifically and killing the tumor cells by the nuclein carried by it is disclosed. It features that the normal tissue can not be damaged.
Owner:SHANGHAI MEDIPHARM BIOTECH
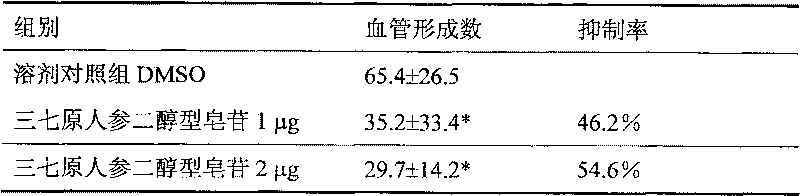
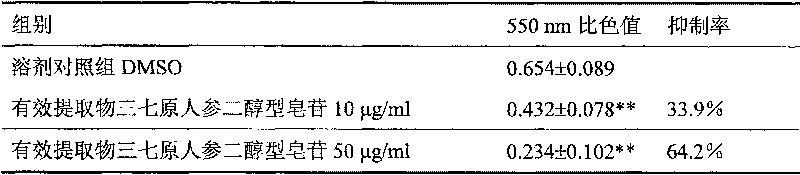
Preparation method of pseudo-ginseng protopanoxadiol saponin and its uasage
InactiveCN101156882AEfficient removalIncrease contentAntineoplastic agentsPlant ingredientsProtopanaxadiolGradient elution
The invention provides a preparation method for extractive originating from an araliaceae plant, notoginseng. The extractive of the category mainly contains extractive of dammarane type, tetracyclic triterpene category, protopanaxadiol type and saponin category, wherein the content of ginsenoside Rb1 and Rd is not lower than 60 percent, and the content of ginsenoside Re, Rg1, and notoginseng saponin R1 is not higher than 5 percent. The notoginseng is extracted by using alcohol containing water as the solvent, chromatography to the extracting solution through a chromatographic column is performed to obtain elutriant, reversed phase and liquid phase gradient elution is performed, the elutriant is collected, and then the target extractive is obtained through concentration. The extraction process of the invention can effectively remove impurities of sugar, protein, amino acid, and the saponin of the protopanaxadiol type of the notoginseng, etc., and improve the content of effective components. The effective components are definite, the process is simple, the quality is controllable, and the invention is suitable for industrialized production, thereby being applied for preparing medicines treating malignant solid tumors.
Owner:ZHEJIANG UNIV
NDA vaccine eucaryon expression carrier and application in preparation of gene vaccine
The invention discloses a eucaryon expression vector of a DNA vaccine and the application. The eucaryon expression vector of DNA vaccine is a recombination pVAX1 eucaryon expression vector which is got by adding the internal ribosome in pVAX1 vector skeleton and entering the site coded sequence. The eucaryon expression vector may show the wide spectrum curing function for breast cancer, pancreatic cancer, restocarcinoma and multi-type malignancy solid tumor, which has good humoral immunity and cell-mediated immunity effect, and inhibits the growth of the tumor.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Anticancer injection of rabdosia diterpene compound or its derivative and its preparing method
InactiveCN1857253AFormulation stabilityPreparation safetyOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectFreeze-drying
The present invention provides a kind of anticancer injection containing rabdosia diterpene or its derivative and beta-cyclodextrin or its derivative. The insoluble rabdosia diterpene is first prepared into cyclodextrin inclusion to raise the dissolubility, and then prepared into injection or freeze dried powder for injection. The anticancer injection has high stability, high treating effect on liver cancer, esophageal cancer, gastric cancer and other malignant solid tumors and leukemia, and patient's compliance.
Owner:常贵生 +2
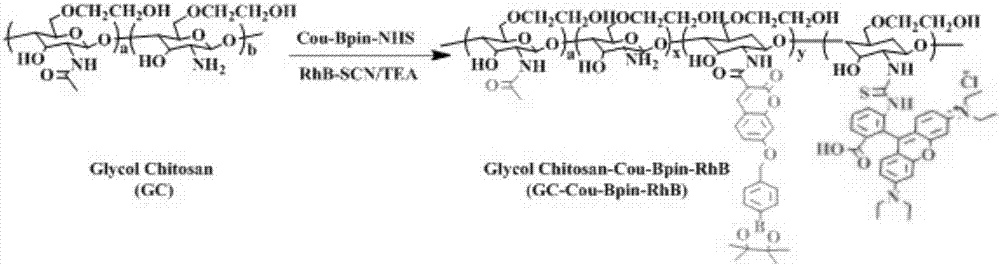
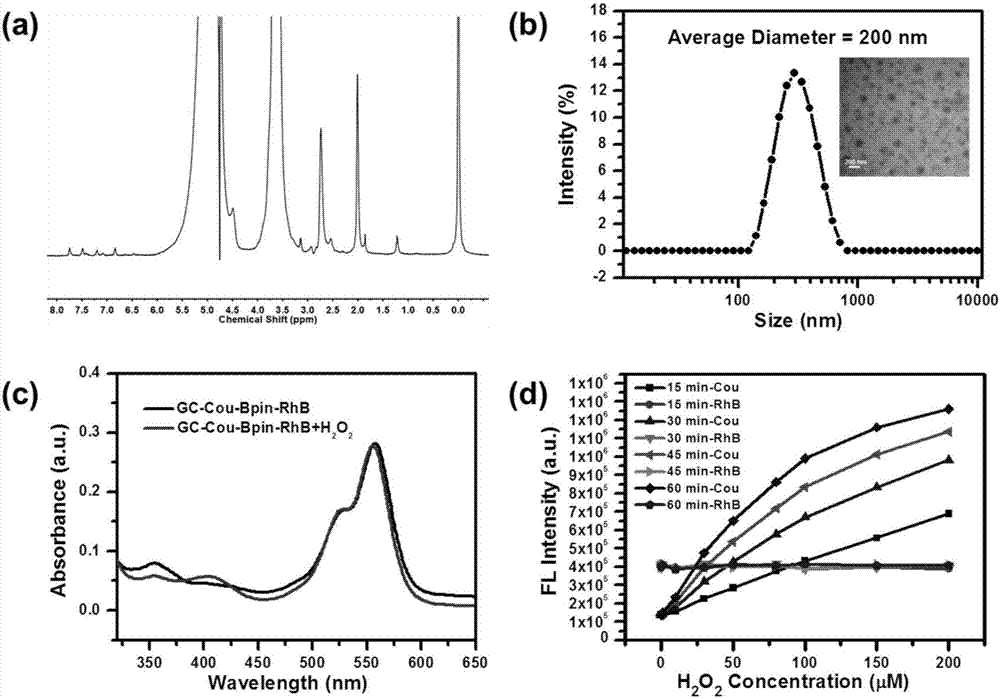
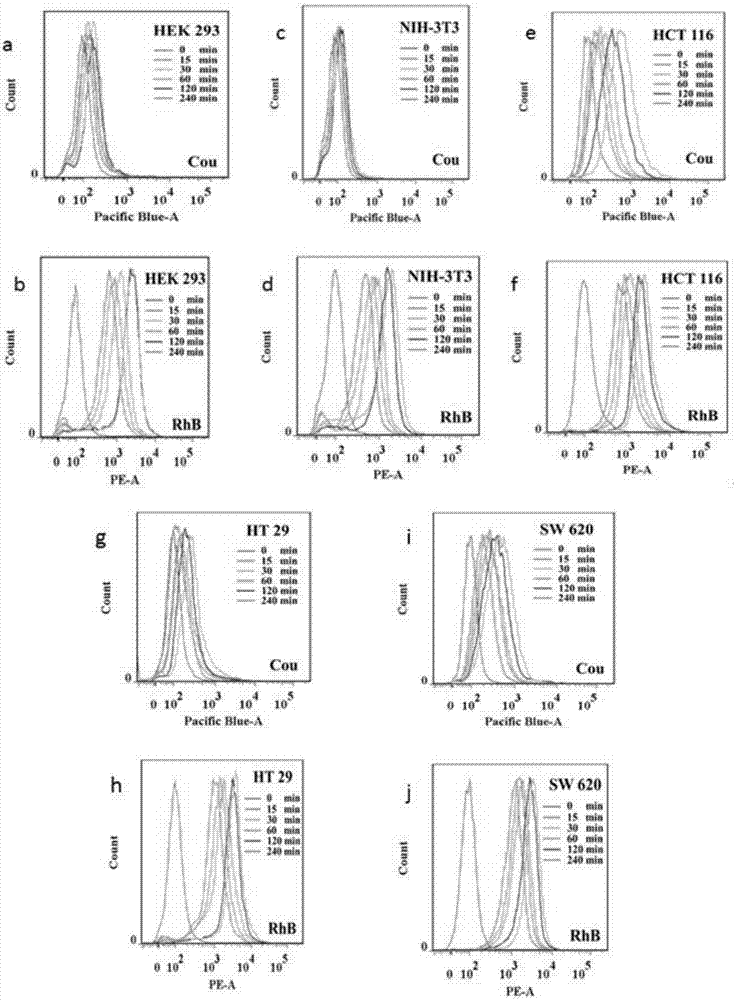
Hydrogen peroxide responsive ratio meter nanoprobe and its use
InactiveCN107543808AGood water solubilityLow cytotoxicityFluorescence/phosphorescenceLuminescent compositionsSolubilityFluorescence
The invention discloses a hydrogen peroxide responsive ratio meter nanoprobe and its use. The nanoprobe comprises a hydrogen peroxide-responsive fluorescent molecule, a hydrogen peroxide-inert fluorescent molecule and a polymer with good biocompatibility. The invention also discloses a use of the hydrogen peroxide responsive ratio meter nanoprobe in preparation of a detection agent of circulatingtumor cells in peripheral blood of malignant solid tumors. The ratio meter nanoprobe has good water solubility, can be easily taken, has low cytotoxicity and can be used for quantitative imaging of hydrogen peroxide in circulating tumor cells. The ratio meter nanoprobe has excellent fluorescence properties, has good sensitivity and specificity to hydrogen peroxide and can detect difference of levels of hydrogen peroxide in malignant solid tumor cells and difference of intracellular intake amounts.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Virulence Attenuated Bacteria For Treatment Of Malignant Solid Tumors
ActiveUS20190015497A1Bacterial antigen ingredientsBacteria material medical ingredientsBacteroidesVirulent characteristics
Owner:UNIVERSITY OF BASEL
Application of Entamoeba histolytica for treating malignant noumenal tumor
InactiveCN1947793AProtozoa antigen ingredientsAntineoplastic agentsAbnormal tissue growthBiologically-Based Therapy
Owner:GUILIN MEDICAL UNIVERSITY
Immune cell with anti-tumor function and application thereof
ActiveCN106754723AGrowth inhibitionAvoid damageGenetically modified cellsMammal material medical ingredientsAntigenSide effect
The invention belongs to the field of medical biotechnology, and particularly relates to a genetically modified immune cell with a malignant solid tumor treating function and application thereof. The immune cell is a specific immune cell which is subjected to gene modification so as to over-express lipid metabolism-related genes. The tumor growth inhibiting capacity of the immune cell is realized by reducing accumulation of lipid droplets of the immune cell itself in a tumor microenvironment, reducing expression of tumor promotion genes / protein, and improving the phagocytotic ability, antigen presentation ability and tumor killing ability of the immune cell. The immune cell is an in-vitro NKT cell, a DC cell, a macrophage, a monocyte, a granulocyte or a T cell. Overexpression of related metabolism regulation genes enables the anti-tumor capacity of the immune cell to be improved remarkably. Compared with a chimeric antigen receptor T cell immunotherapy (CAR-T) which receives much attention currently, toxic and side effects are small, cytokine storm can not be caused, and clinical requirements can be met.
Owner:NANJING UNIV
Method for treating human malignant solid tumor by using hepatocyte nuclear factor-1alpha
ActiveCN102475893AConfirmed differentiation therapyPeptide/protein ingredientsGenetic material ingredientsHepatocyte Nuclear Factor 1alphaTumor therapy
The invention relates to a method for treating human malignant solid tumors by using hepatocyte nuclear factor-1alpha (HNF1alpha). Specifically, the invention relates to a method for inducing differentiation of human malignant solid tumor cells by using HNF1alpha, thereby treating malignant solid tumors. The study of the invention shows that tumor cells can be effectively induced to differentiate through regulation of the gene expression of malignant solid tumor cell HNF1alpha, thus providing a new means for induced differentiation therapy of tumors.
Owner:上海赛迪夫医药科技有限公司
Method applicable to isolation and introduction of infiltrating T lymphocytes in malignant solid tumors
ActiveCN109536444ADoes not affect biological activityEasy to operateCell dissociation methodsMammal material medical ingredientsAbnormal tissue growthT lymphocyte
The invention provides a method applicable to isolation and introduction of infiltrating T lymphocytes in malignant solid tumors, in particular to comprise procedures as follows: 1) processing a tumortissue into a tissue mass with a volume of 0.5-3 mm<3>; 2) spreading a matrix glue in the cell culture plate and transfer the processed tumor tissue mass in step 1 to the matrix glue for coating; 3)transferring the coated tissue mass in step 2 to another cell culture plate and incubating for 10-60 minutes; 4) adding a pre-amplification medium to the cell culture plate in step 3 and carrying on the pre-amplification culture for 10-30 days and using the semi-liquid of the pre-amplification medium as an exchange per 2-3 days. The invention provides an effective method for isolation and introduction of infiltrating T lymphocytes in tumors for the treatment of specific biotheraphy of malignant solid tumors. The tumor infiltrating T lymphocytes isolated by the method have high purity and stronger killing activity of tumor cells.
Owner:JILIN TUO HUA BIOTECH
Application of adult stem cells in treating malignant solid tumors
InactiveCN102188446AMammal material medical ingredientsAntineoplastic agentsMesenchymal stem cellNude mouse
The invention relates to application of adult stem cells in treating malignant solid tumors, in particular to application of adult stem cells in preparing a cell preparation for treating malignant solid tumors. The inventor fuses human mesenchymal stem cells and human hemopoietic stem cells respectively with human esophageal carcinoma cells, and injects the fusion cells into SCID (severe combined immune deficiency) / mice and nude mice, and as a result, the emerging rate of tumors of the fusion cells is obviously reduced and the volume thereof is also reduced. The stem cell preparation provided by the invention can obviously inhibit the growth of malignant solid tumors.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
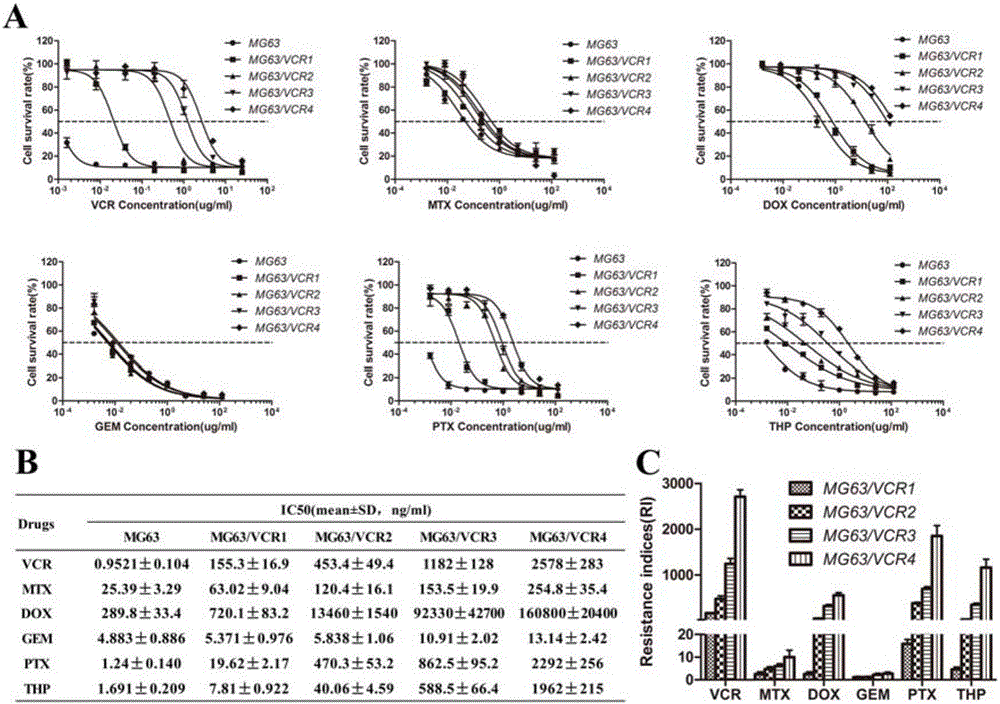
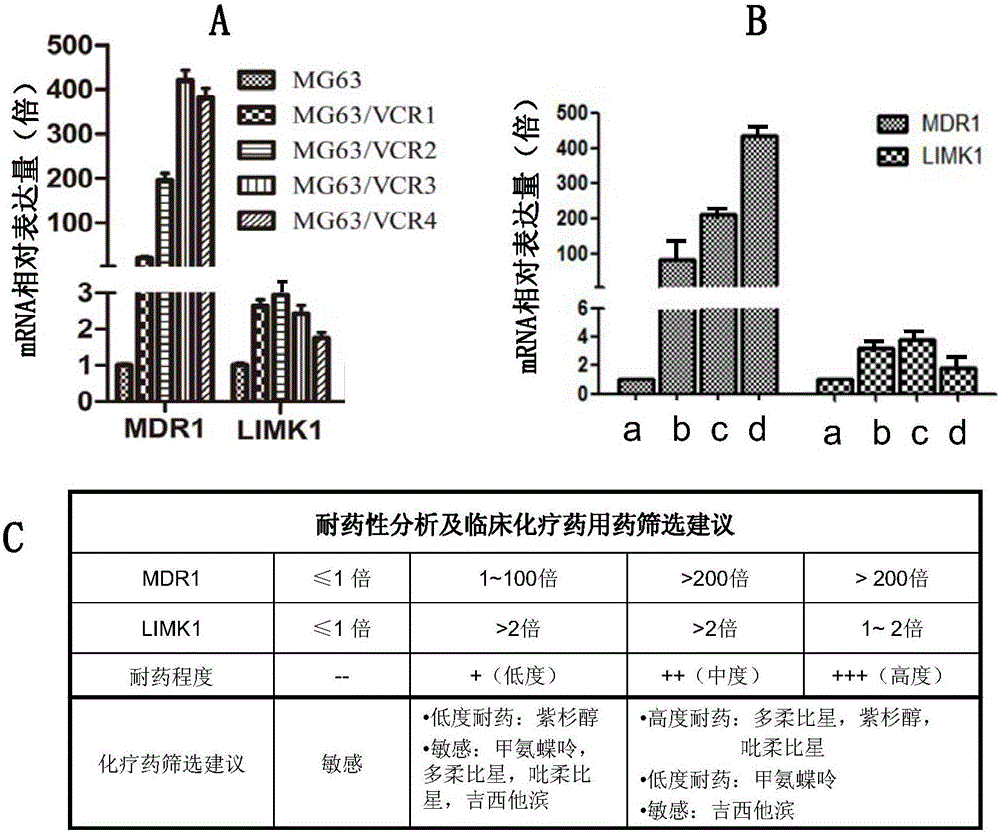
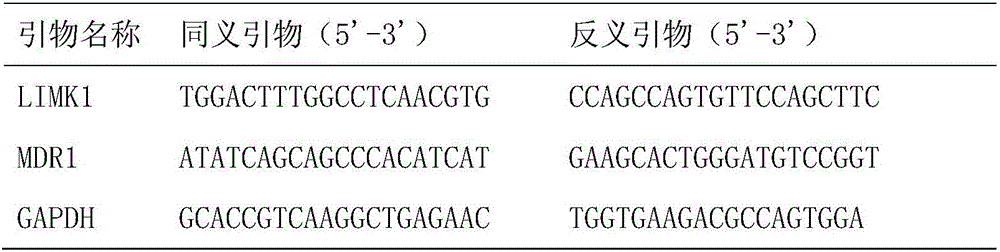
Kit for detecting drug resistance of malignant parenchymatous tumors to chemotherapeutic drugs
ActiveCN106119380AImprove treatment efficiencyReduce waste of resourcesMicrobiological testing/measurementHigh resistanceTumor chemotherapy
The invention discloses a kit for detecting drug resistance of malignant parenchymatous tumors to chemotherapeutic drugs. The kit comprises malignant parenchymatous tumor drug-resistant cell cDNA standard samples, an SYBR Green polymerase chain reaction system, MDR1 and LIMK1 genes for amplification and housekeeping gene primer pairs. When the kit is used, cells of the malignant parenchymatous tumors of a lung cancer and the like are intermittently exposed in vincristine with increasing gradients, and a differential drug-resistant cell substrain is established. It is detected and found through a fluorescent quantitative PCR instrument that expression of the MDR1 gene is enhanced along with increasing of the drug-resistant degree, and expression of the LIMK1 gene is enhanced in cells which have low and moderate resistance to the drugs and retraced in cells which have high resistance to the drugs. Therefore, whether the malignant parenchymatous tumors are resistant to the drugs or not and the drug-resistant degree of the malignant parenchymatous tumors can be diagnosed by jointly detecting expressions of the MDR1 and LIMK1 genes; meanwhile, detection of the expressions of the MDR1 and LIMK1 genes can be used for preparing a chemotherapeutic drug resistance screening preparation, and an accurate therapeutic regimen can be provided for a patient subjected to malignant parenchymatous tumor chemotherapy.
Owner:JILIN UNIV
Method for Poly Signal Activation of Apoptosis of Malignant Solid Tumour Cells
ActiveUS20180250331A1Heavy metal active ingredientsPharmaceutical non-active ingredientsSide effectApoptosis
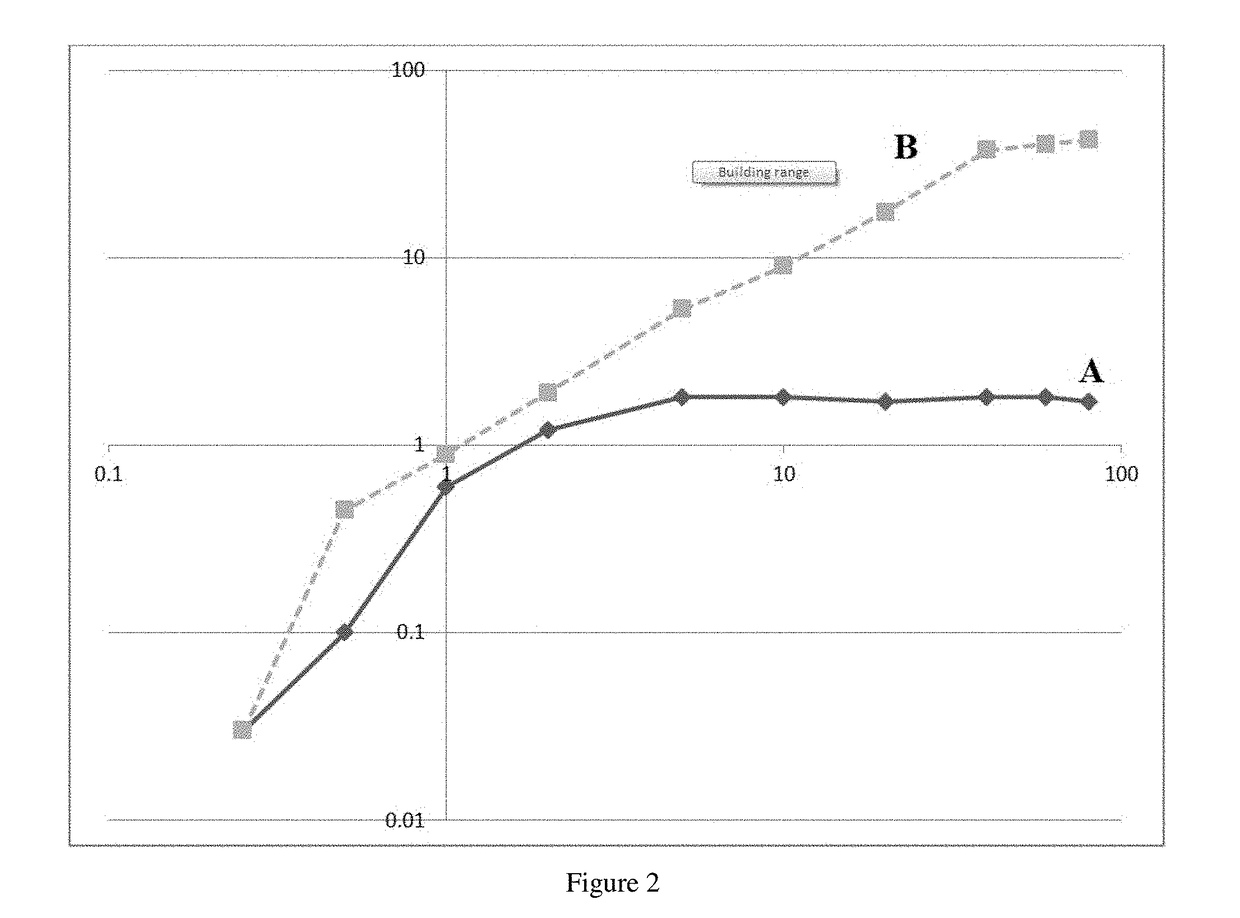
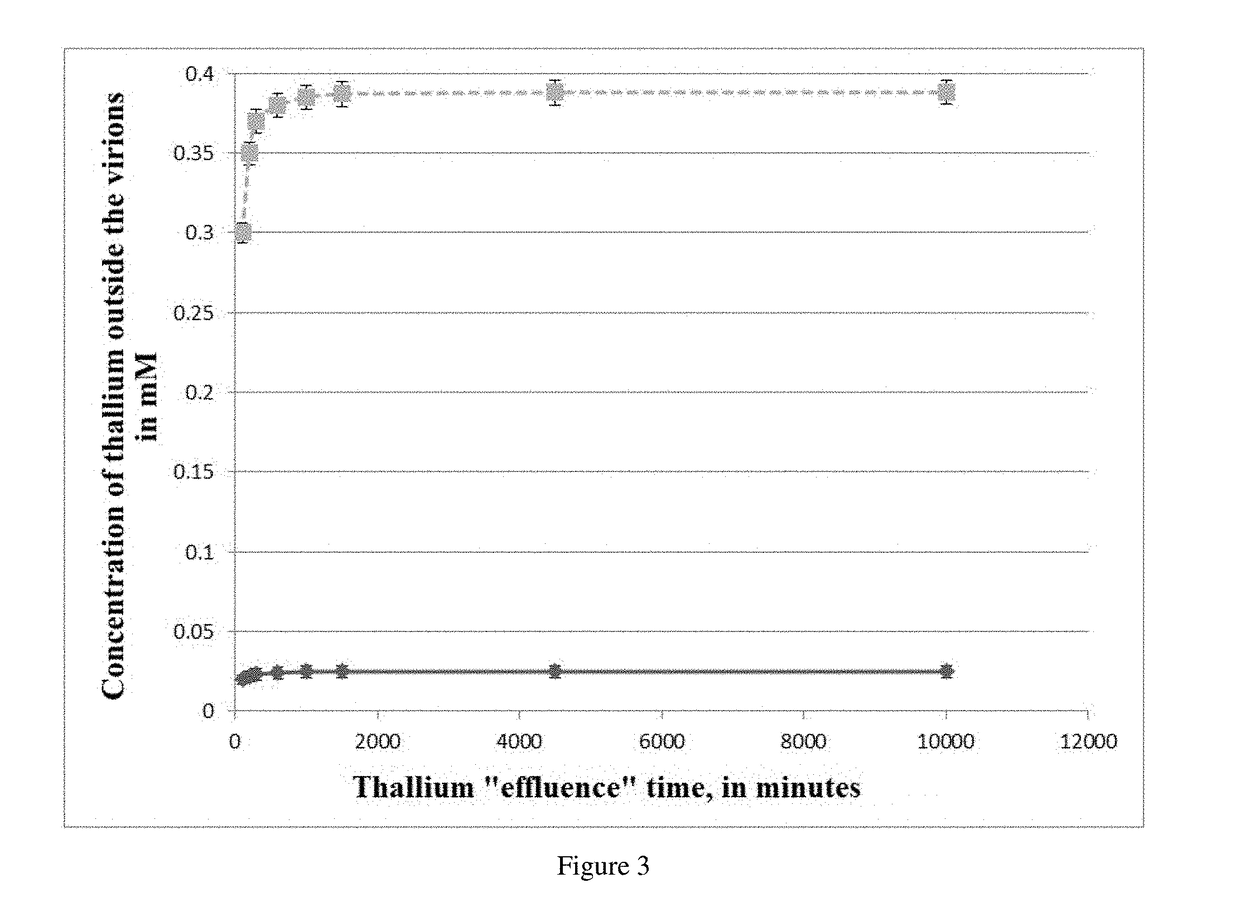
The invention relates to medicine and concerns a method for the poly signal activation of apoptosis of malignant solid tumour cells, carried out by means of the targeted delivery of thallium salts by surface-modified MS2 phage virions which contain a cyclic iRGD ligand that has a high affinity for the integrins avb3 and avb5 and is covalently bound with the shell and with the core, which contains genomic RNA and thallium salts. The invention provides for complex, efficient, prolonged cytotoxic action on focal and metastatic clusters of malignant solid tumour cells, while minimizing undesirable side effects on the healthy cells of an organism.
Owner:OBSHESTVO S OGRANICHENNOI OTVETSTVENNOSTYU BIOTEHNOLOGIYA
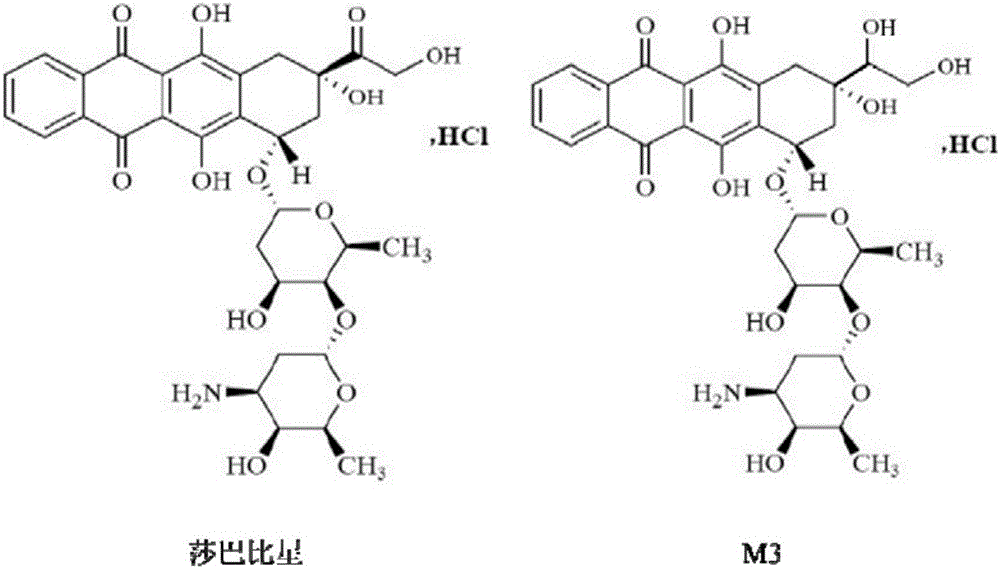
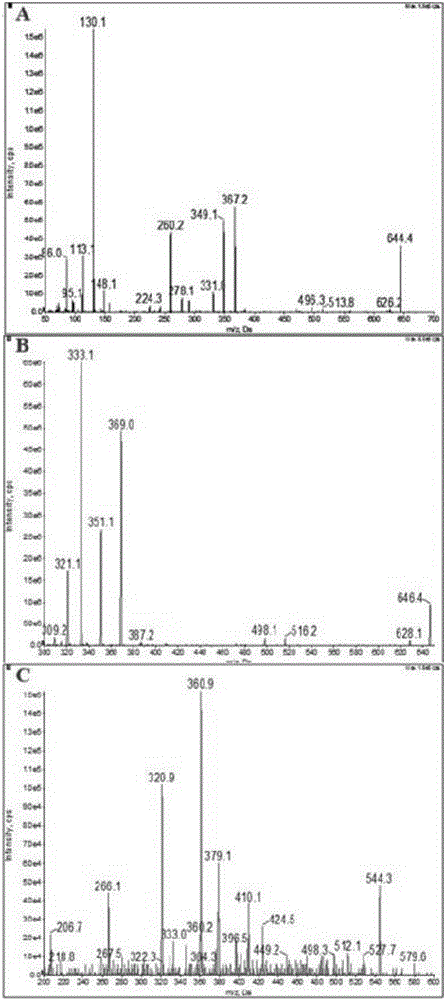
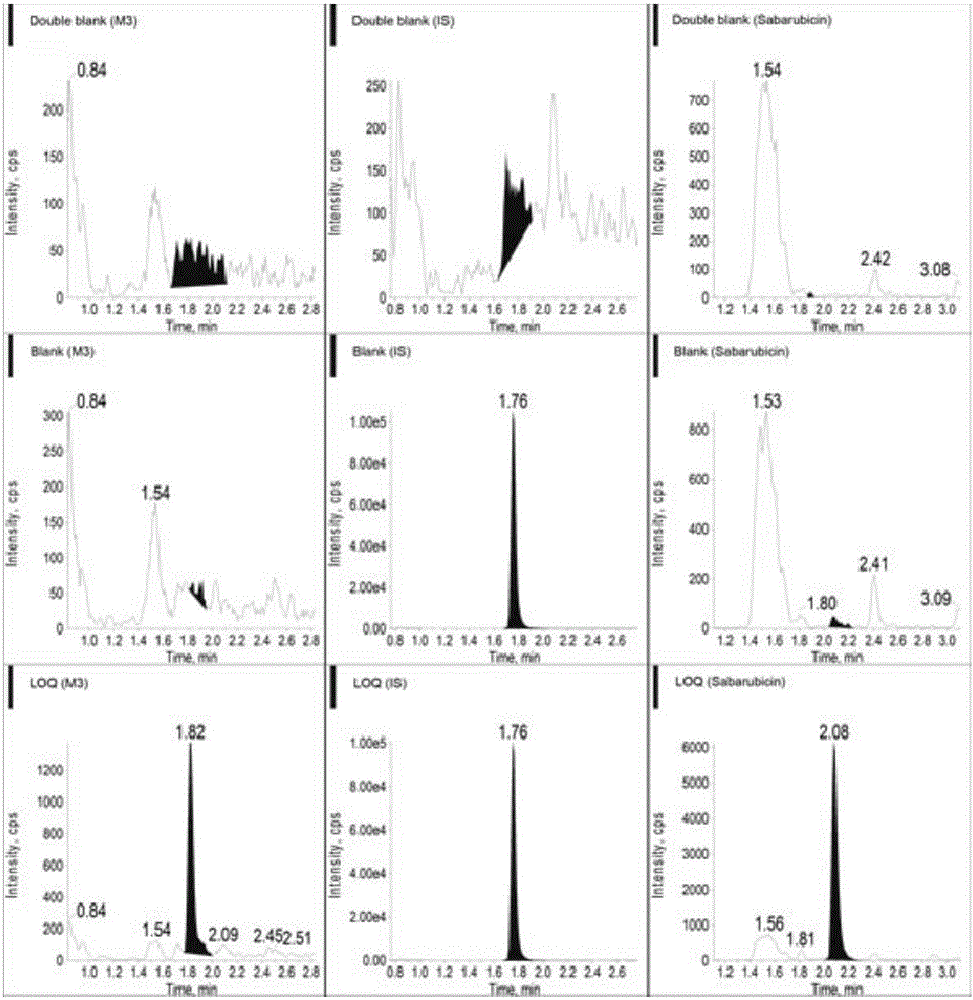
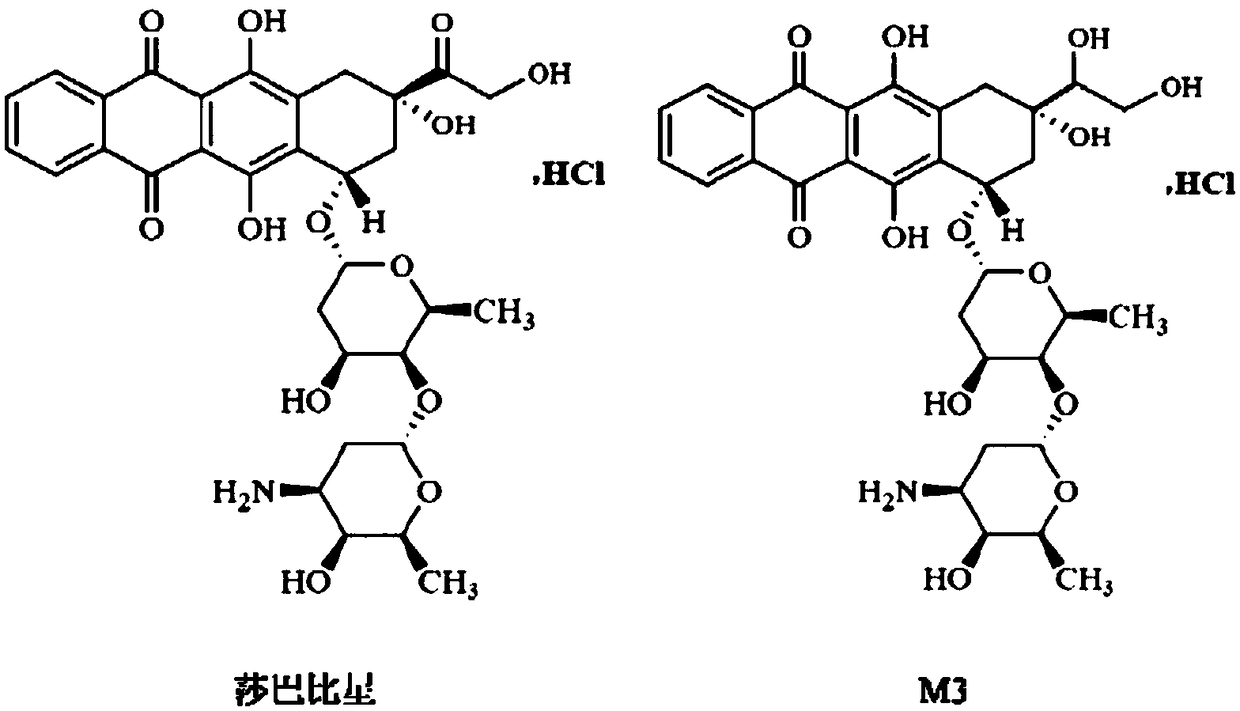
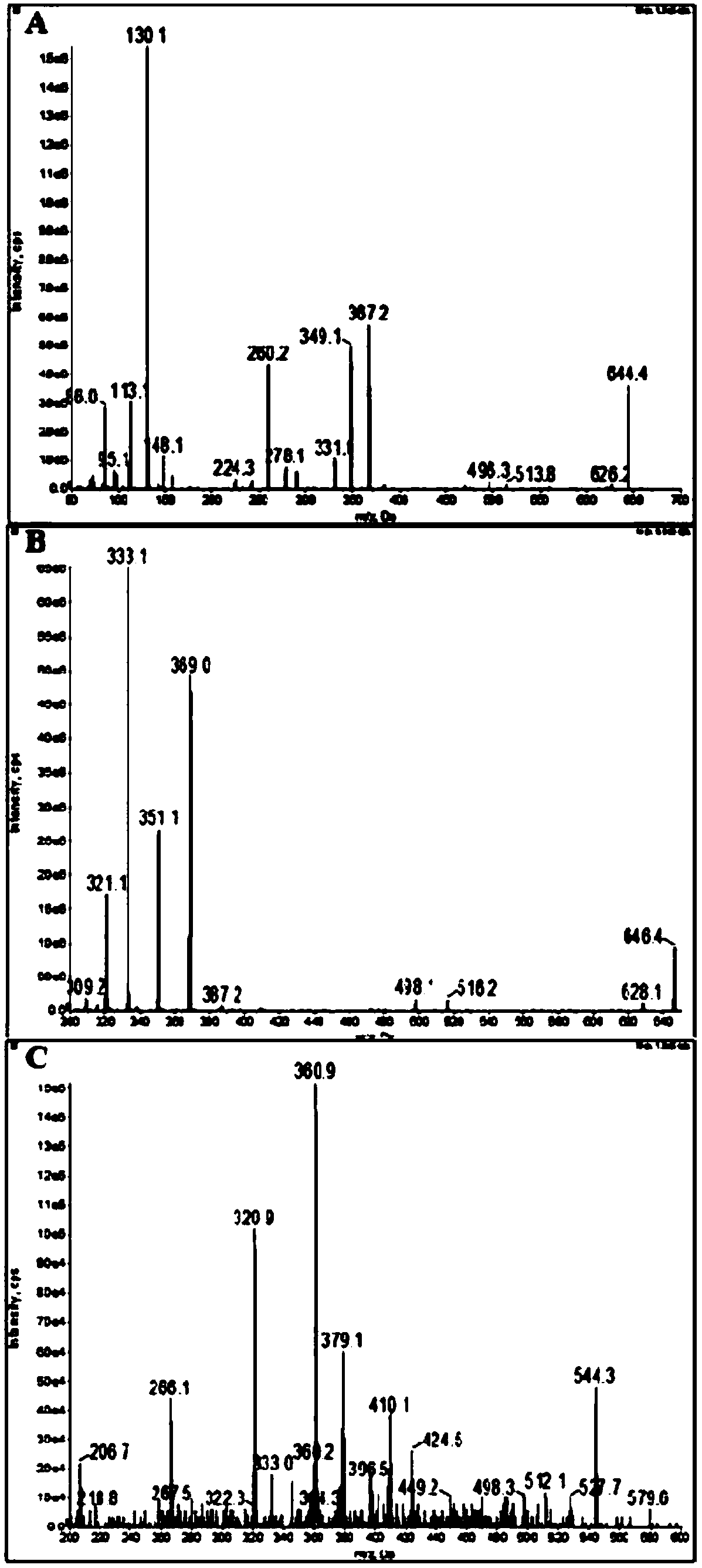
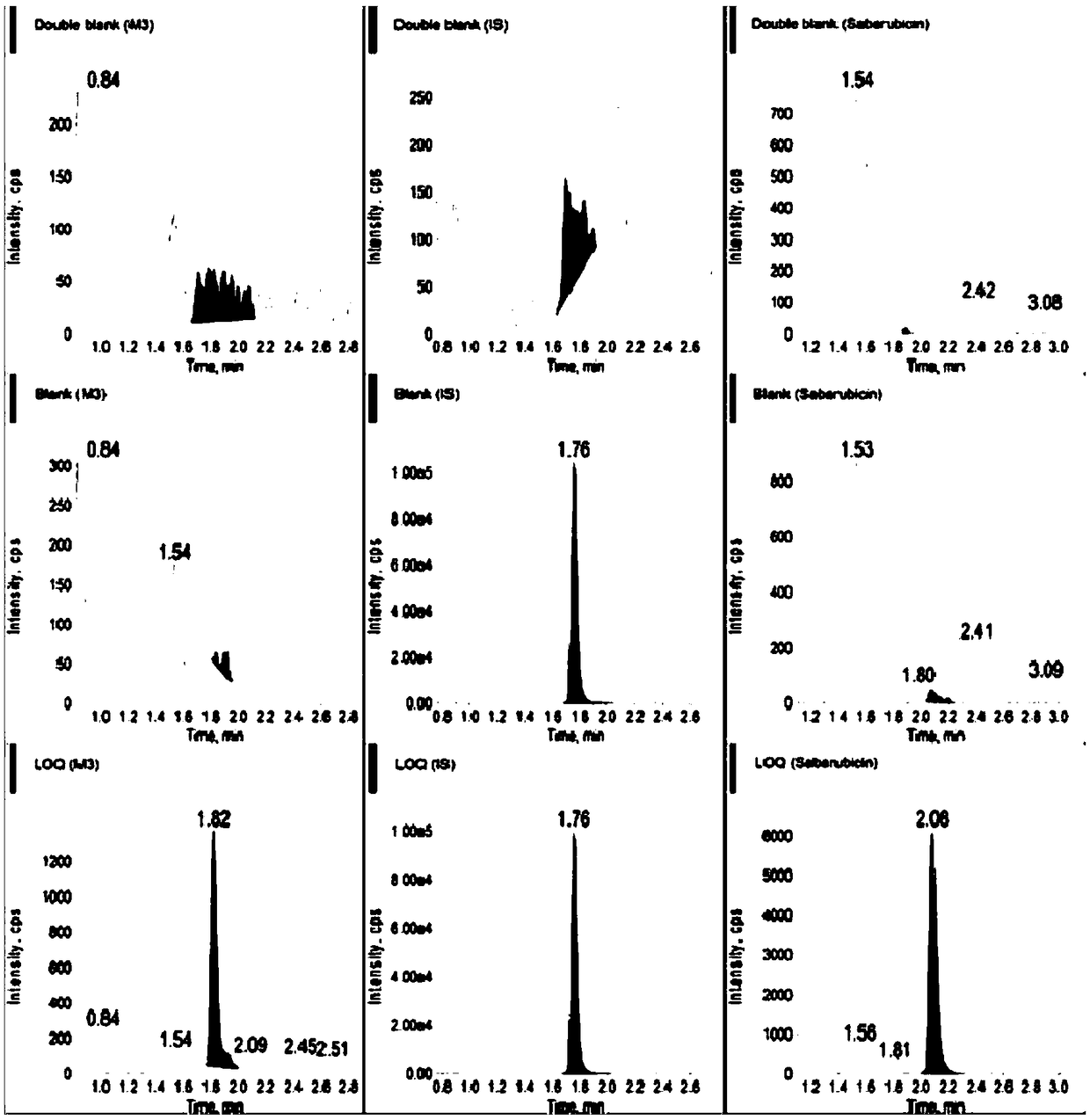
Ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma
The invention relates to an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma and provides a method for determining the concentration of doxorubicin analogue and C13 alcohol metabolite M3 in human plasma through UPLC-MS / MS. The method is carried out with formic acid water-acetonitrile as a mobile phase. The method for detecting the doxorubicin analogue and the metabolite M3 provided by the invention is high in sensitivity and good in repeatability, and can meet the requirements of quantitative analysis of clinical pharmacokinetic biological samples for treating malignant parenchymatous tumors by the doxorubicin analogue.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Use of hepatocyte nuclear factor 1a in preparation of drug for treating malignant solid tumor disease
InactiveUS20140010786A1Good biological propertiesRetard growth of tumor cellBiocidePeptide/protein ingredientsTherapeutic effectTumor cells
A use of a hepatocyte nuclear factor 1α gene and / or protein and a recombinant expression vector containing a hepatocyte nuclear factor 1α in preparation of drugs for treating malignant solid tumor diseases and in preparation of differentiation inducing reagents or composition for inducing differentiation of malignant solid tumor cells. The hepatocyte nuclear factor 1α gene can improve the biological properties of tumor cells, and retard the growth of tumor cells, and up-regulation of expression thereof has therapeutic effects on animal models with malignant solid tumors.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
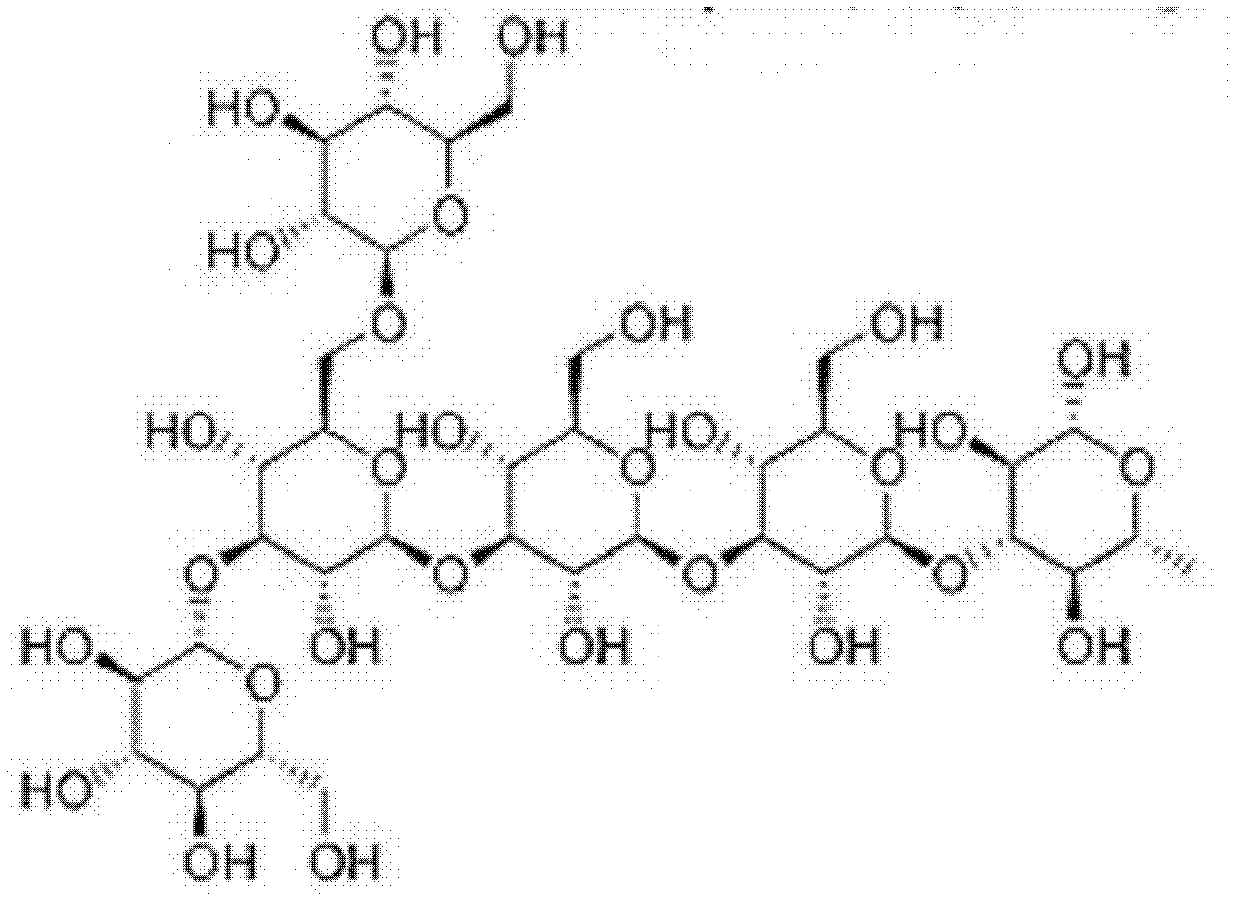
Application of beta-dextran in preparation of human dendritic cell tumor vaccine
ActiveCN102600467AImprove immunityImprove proliferative abilityBlood/immune system cellsCarrier-bound antigen/hapten ingredientsAbnormal tissue growthDendritic cell
The invention discloses an application of beta-dextran in preparation of human dendritic cell tumor vaccine. The application comprises culturing human peripheral blood mononuclear cells with culture medium containing GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-4 (interleukin-4) to obtain immature human dendritic cell; and inducing loading of tumor antigen onto the immature human dendritic cell through beta-dextran with concentration of 80-120 mug / mL, to obtain human dendritic cell tumor vaccine. According to the invention, the human dendritic cell tumor vaccine is obtained by inducing loading of tumor antigen onto the immature human dendritic cell through beta-dextran. The vaccine can improve immunizing potency of human dendritic cell, promote multiplication capacity of T-cells, especially can externally stimulate the T-cells to achieve specific tumor killing ability, thereby treating various malignant solid tumors.
Owner:戚春建
Determination of plasma concentrations of sababicin and its metabolite m3 in human plasma by uplc-ms/ms
ActiveCN105806973BMeet the requirements of quantitative analysisHigh sensitivityComponent separationMetaboliteBlood plasma
The invention relates to an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma and provides a method for determining the concentration of doxorubicin analogue and C13 alcohol metabolite M3 in human plasma through UPLC-MS / MS. The method is carried out with formic acid water-acetonitrile as a mobile phase. The method for detecting the doxorubicin analogue and the metabolite M3 provided by the invention is high in sensitivity and good in repeatability, and can meet the requirements of quantitative analysis of clinical pharmacokinetic biological samples for treating malignant parenchymatous tumors by the doxorubicin analogue.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
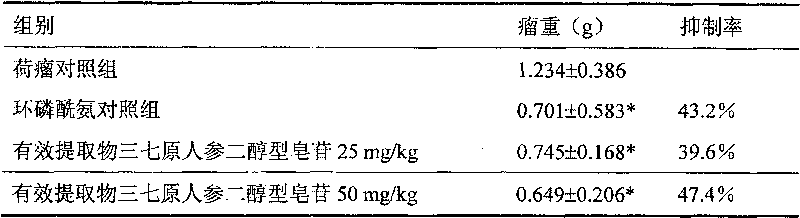
Preparation method of pseudo-ginseng protopanoxadiol saponin
InactiveCN101156882BEfficient removalIncrease contentAntineoplastic agentsPlant ingredientsProtopanaxadiolGradient elution
The invention provides a preparation method for extractive originating from an araliaceae plant, notoginseng. The extractive of the category mainly contains extractive of dammarane type, tetracyclic triterpene category, protopanaxadiol type and saponin category, wherein the content of ginsenoside Rb1 and Rd is not lower than 60 percent, and the content of ginsenoside Re, Rg1, and notoginseng saponin R1 is not higher than 5 percent. The notoginseng is extracted by using alcohol containing water as the solvent, chromatography to the extracting solution through a chromatographic column is performed to obtain elutriant, reversed phase and liquid phase gradient elution is performed, the elutriant is collected, and then the target extractive is obtained through concentration. The extraction process of the invention can effectively remove impurities of sugar, protein, amino acid, and the saponin of the protopanaxadiol type of the notoginseng, etc., and improve the content of effective components. The effective components are definite, the process is simple, the quality is controllable, and the invention is suitable for industrialized production, thereby being applied for preparing medicines treating malignant solid tumors.
Owner:ZHEJIANG UNIV
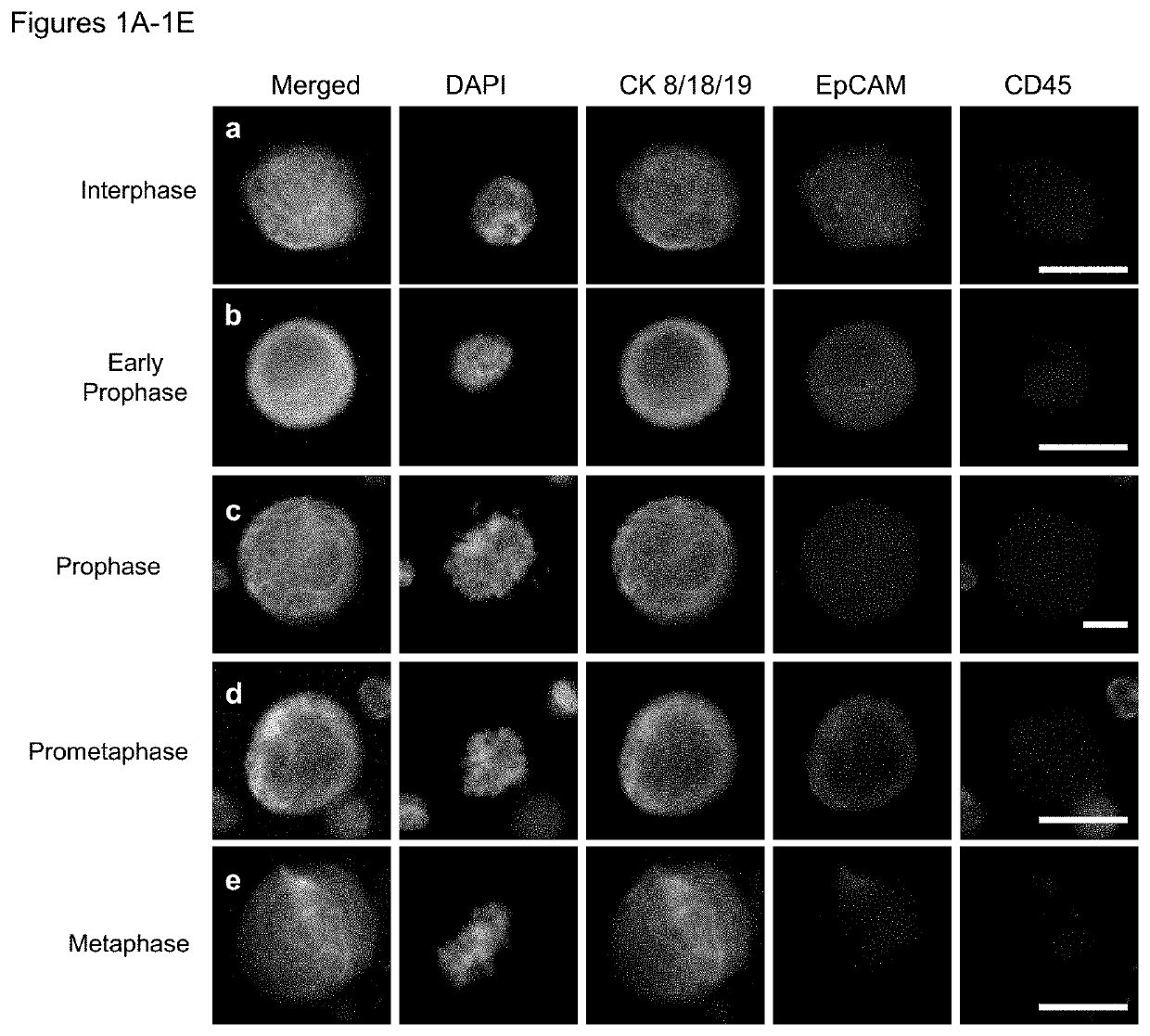
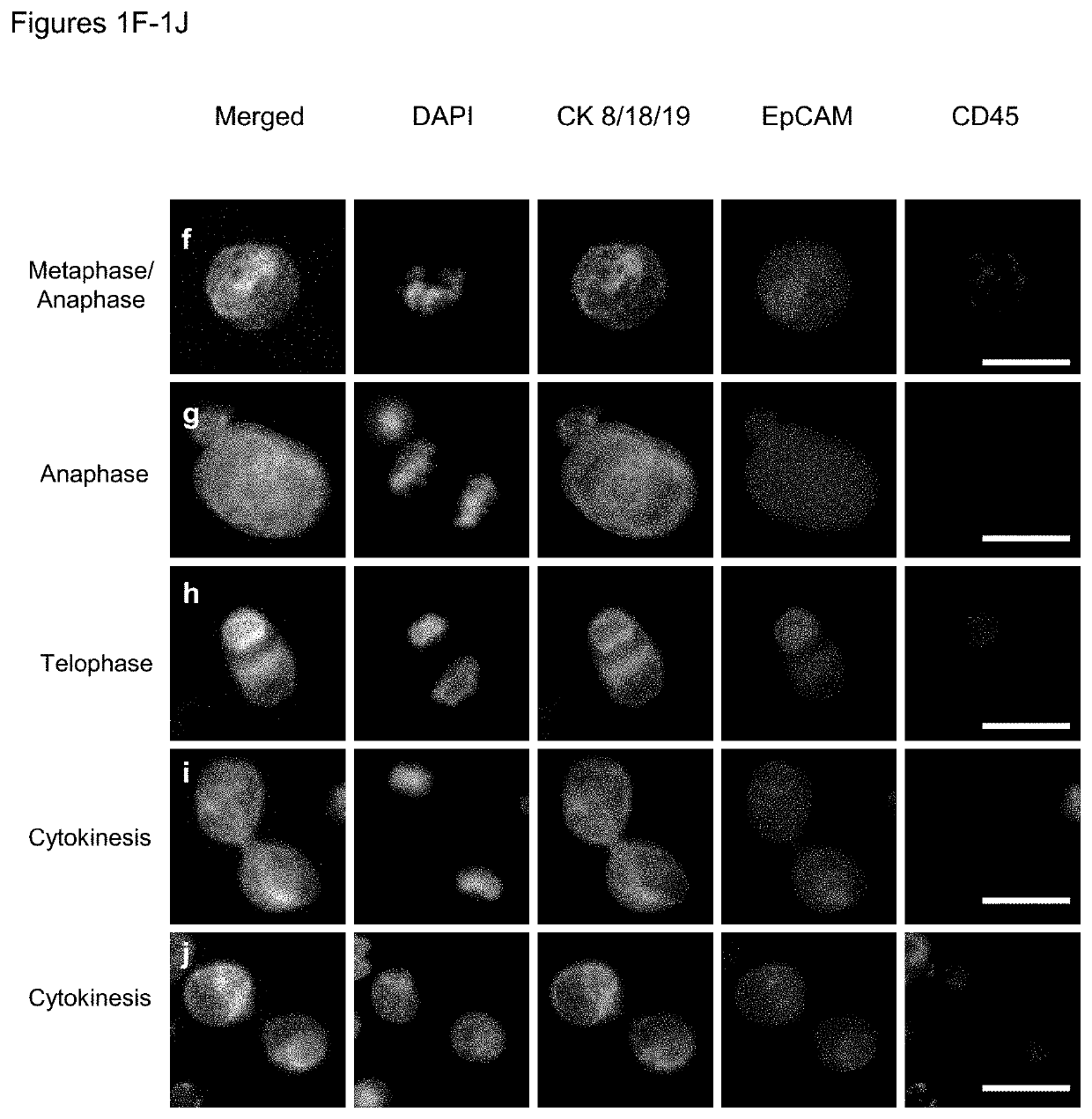
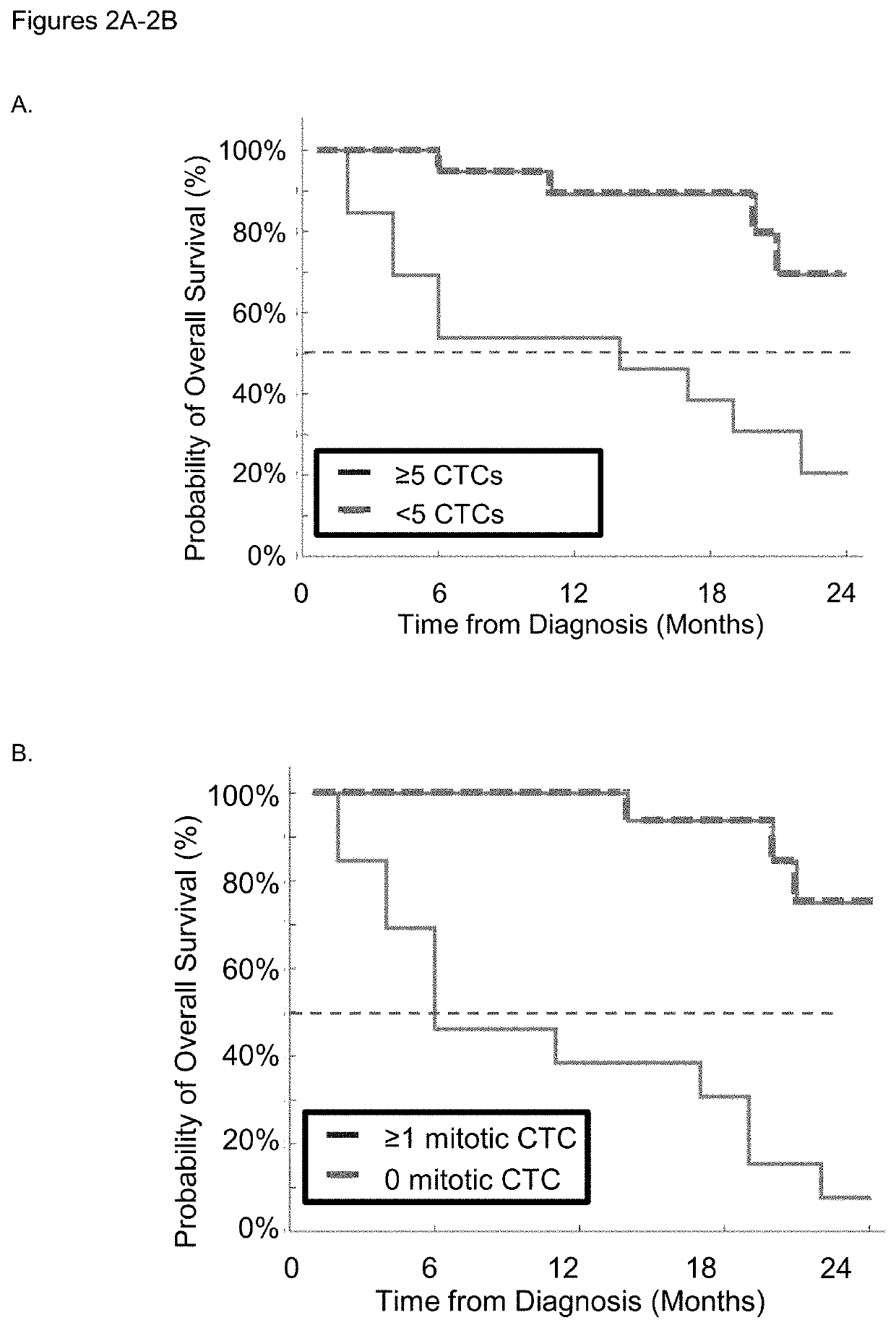
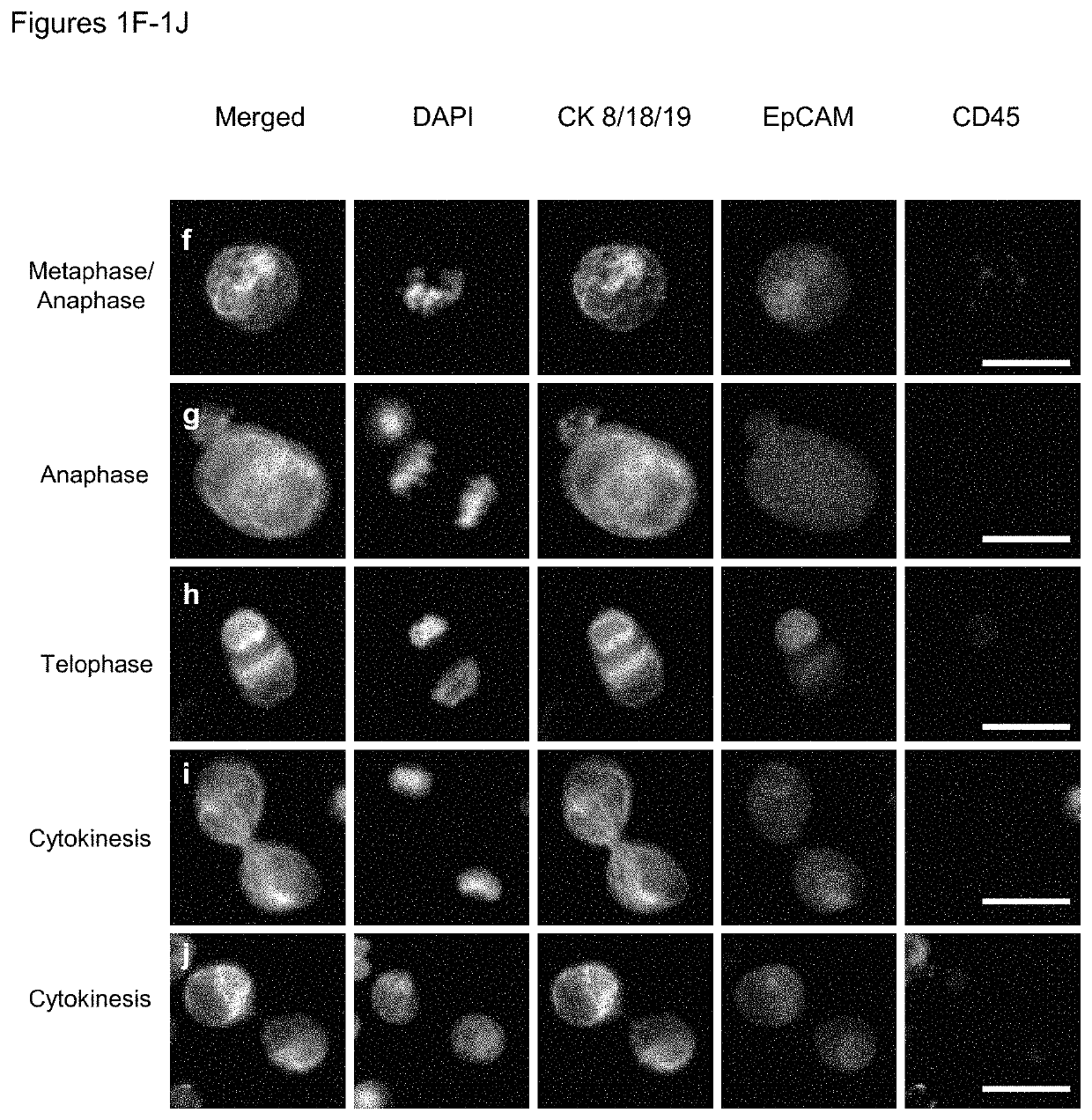
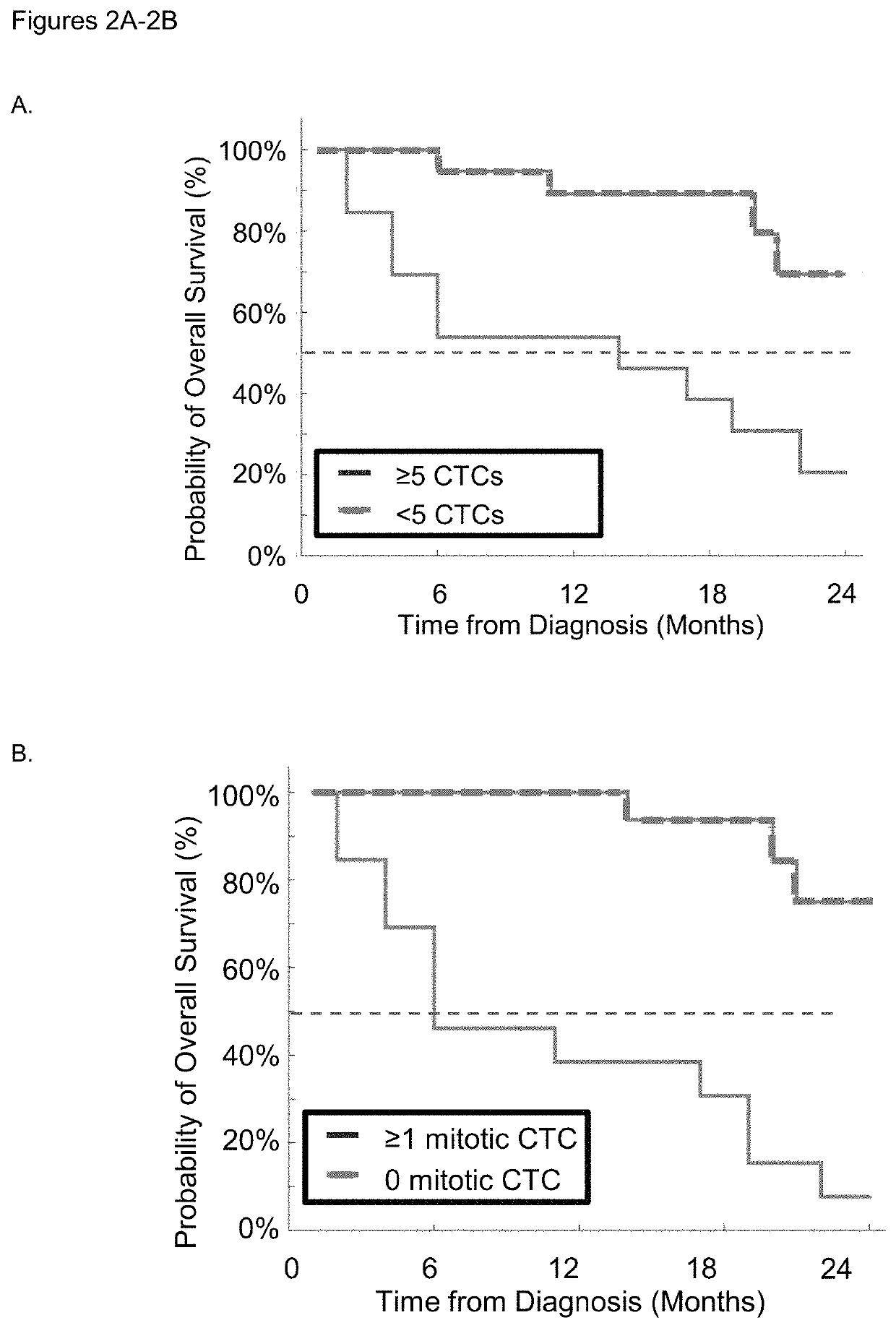
Use of circulating tumor cell mitotic index in cancer stratification and diagnostics
PendingUS20220034888A1Long term survival of the subject is less likelyMonitor the effectiveness of a given treatmentDisease diagnosisBiological testingCirculating cancer cellOncology
Circulating tumor cells (CTCs) are associated with metastasis of malignant solid tumors in a patient. Presented here is evidence that CTCs exhibit cell cycle phase variability and that there is a strong correlation between the number of CTCs in a mitotic cell cycle phase and the prospects for long term survival of the subject from which the cells were obtained. Also presented herein are methods of determining the mitotic cell cycle phase of CTCs from a patient having cancer and using the information in grading malignant solid tumors and predicting the likelihood of survival of the patient.
Owner:CREATV MICROTECH
A fourth-generation car-t cell and its construction method and application
ActiveCN109504660BReduce cancer recurrencePromote proliferationAntibody mimetics/scaffoldsMammal material medical ingredientsProliferative capacityOncology
Owner:温州启星生物技术有限公司
A method for isolation and induction of tumor-infiltrating T-lymphocytes suitable for malignant solid tumors
ActiveCN109536444BDoes not affect biological activityEasy to operateCell dissociation methodsMammal material medical ingredientsMatrigelLymphocyte
The present invention provides a method for separating and inducing tumor-infiltrating T lymphocytes suitable for malignant solid tumors, specifically, including 1) processing the tumor tissue into a volume of 0.5-3mm 3 2) Matrigel is spread in the cell culture plate, and the tissue piece of the tumor tissue processed in step 1) is transferred to Matrigel for coating; 3) The coated tissue in step 2) Transfer the tissue block to another cell culture plate and incubate for 10-60 minutes; 4) add pre-amplification medium to the cell culture plate in step 3), and carry out pre-amplification culture for 10-30 days, every 2- For 3 days, half of the pre-amplification medium was used to change the medium. The invention provides an effective method for separating and inducing tumor infiltrating T lymphocytes for the specific biological treatment of malignant solid tumors. The tumor infiltrating T lymphocytes separated by the method of the invention have high purity and stronger tumor cell killing activity.
Owner:JILIN TUO HUA BIOTECH
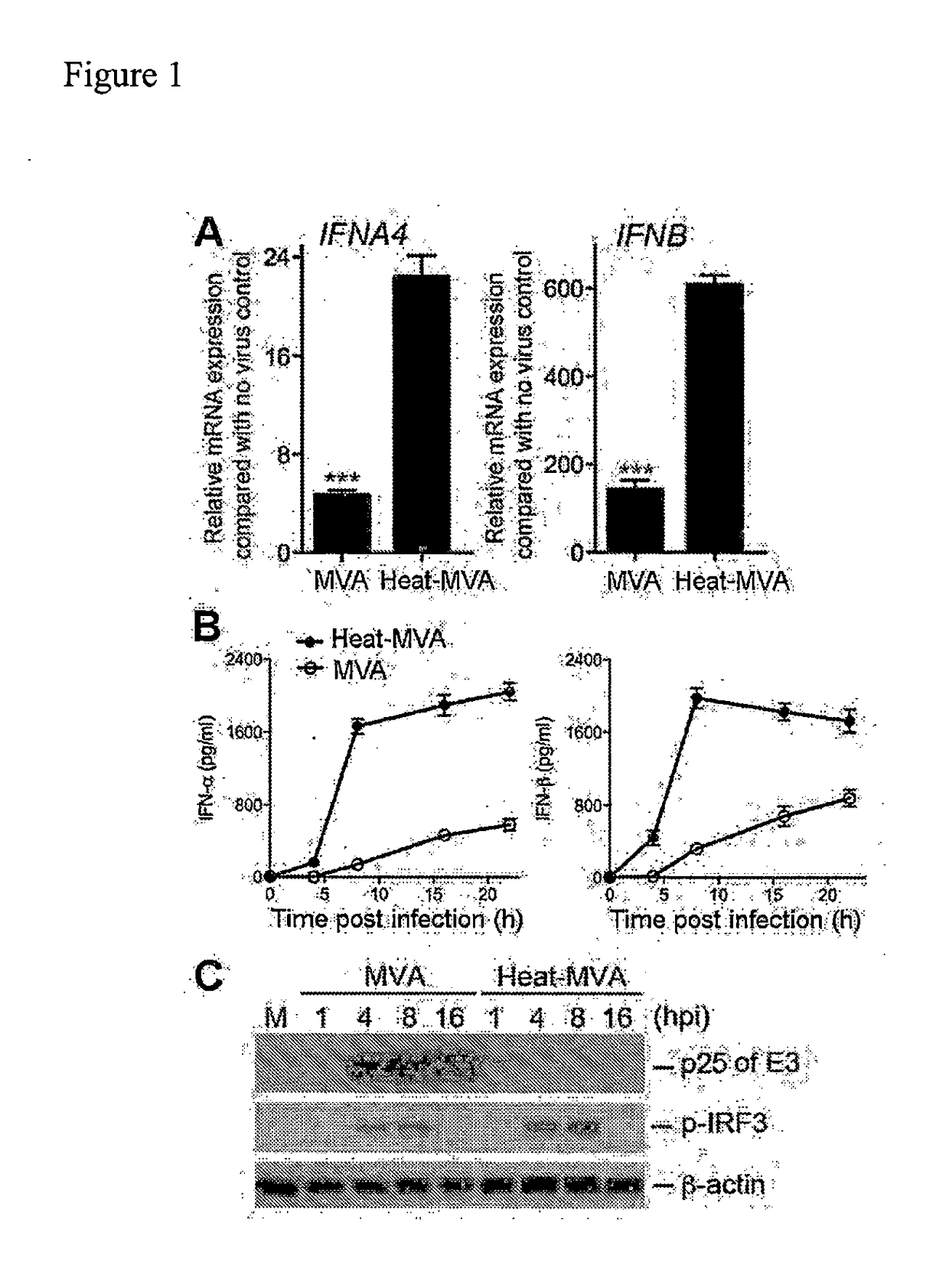
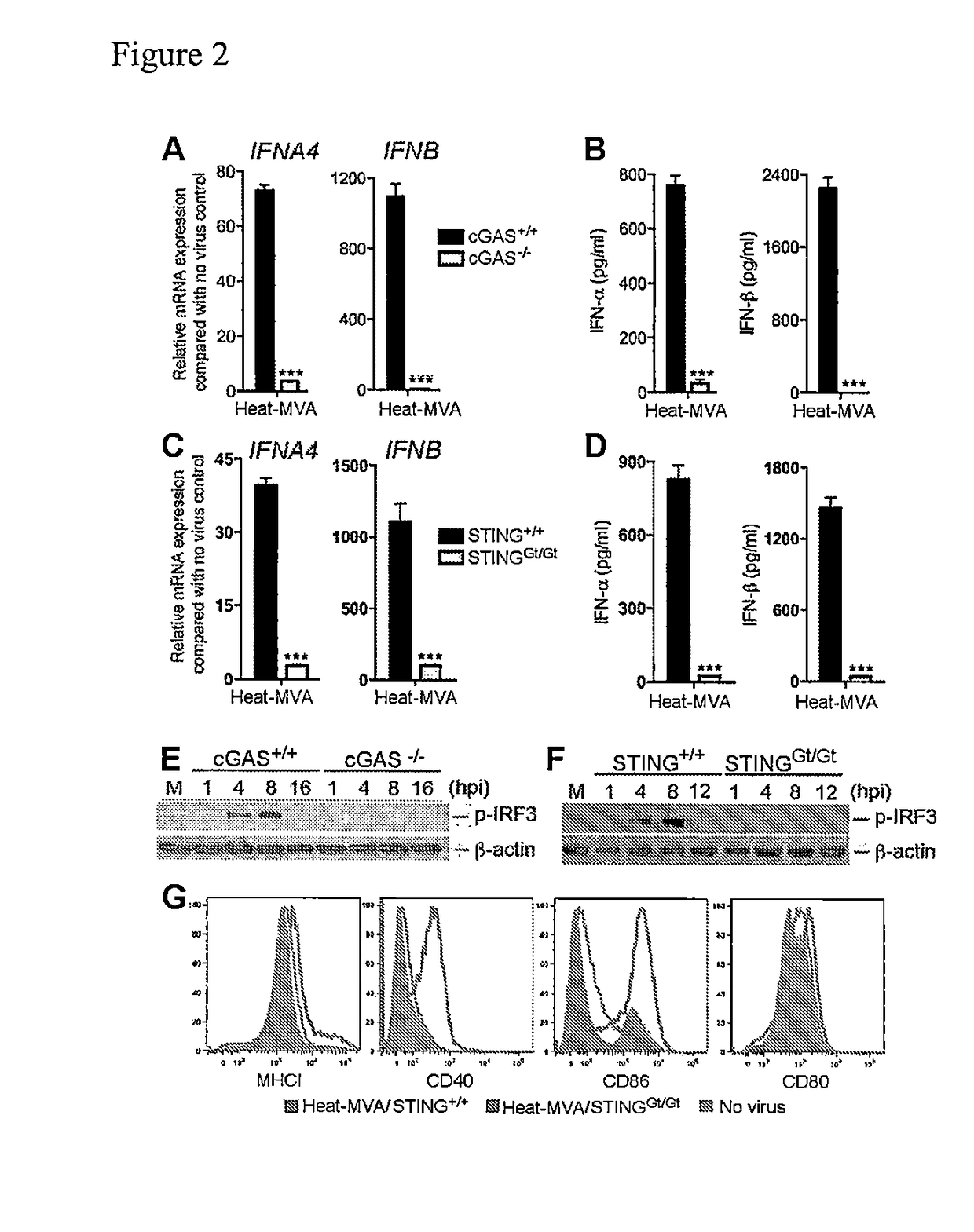
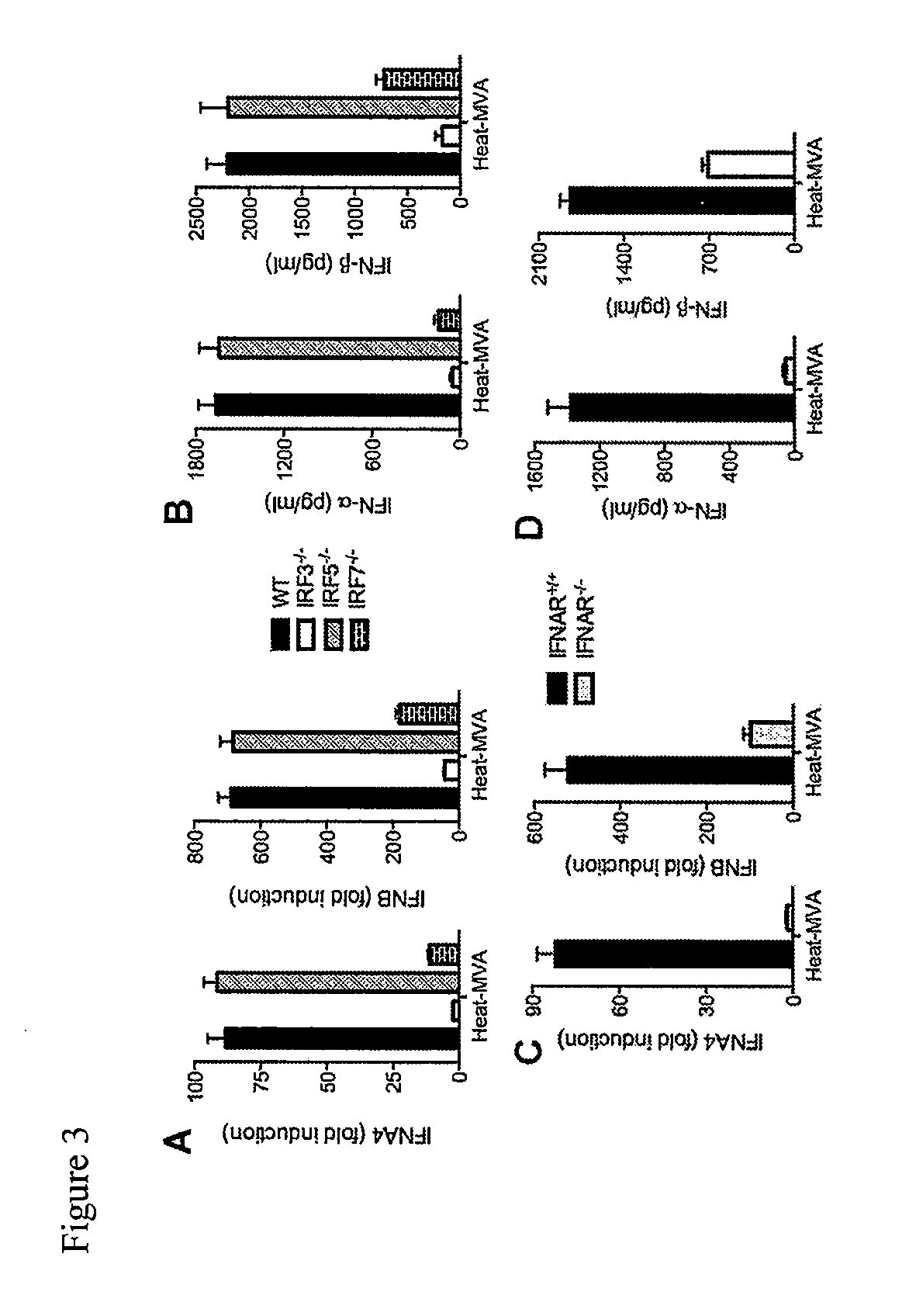
Use of inactivated nonreplicating modified vaccinia virus ankara (MVA) as monoimmunotherapy or in combination with immume checkpoint blocking agents for solid tumors
ActiveUS20180236062A1Reduce doseSynergistic effectViral antigen ingredientsDigestive systemModified vaccinia AnkaraSolid tumor
The present disclosure relates to infection-competent, but nonreplicative inactivated modified vaccinia Ankara (MVA) and its use as immunotherapy, alone, or in combination with immune checkpoint blocking agents for the treatment of malignant solid tumors. Particular embodiments relate to inducing an immune response in a subject diagnosed with a solid malignant tumor.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Topical natural lithraea caustica extract composition and use thereof in tumours
InactiveUS20190321429A1Ointment deliveryPharmaceutical non-active ingredientsDiabetes mellitusMelanoma
The invention relates to a natural composition for topical use, such as an ointment, comprising a Lithraea caustica extract and one or more pharmaceutically acceptable innocuous and non-toxic excipients, and to the use thereof in the treatment of benignant, premalignant and malignant solid tumors in the skin, including malignant melanoma. The extract is obtained via extraction with an organic solvent.
Owner:UNIV DE SANTIAGO DE CHILE
Use of circulating tumor cell mitotic index in cancer stratification and diagnostics
ActiveUS11150245B2Long term survival of the subject is less likelyMonitor the effectiveness of a given treatmentDisease diagnosisBiological testingCirculating cancer cellOncology
Circulating tumor cells (CTCs) are associated with metastasis of malignant solid tumors in a patient. Presented here is evidence that CTCs exhibit cell cycle phase variability and that there is a strong correlation between the number of CTCs in a mitotic cell cycle phase and the prospects for long term survival of the subject from which the cells were obtained. Also presented herein are methods of determining the mitotic cell cycle phase of CTCs from a patient having cancer and using the information in grading malignant solid tumors and predicting the likelihood of survival of the patient.
Owner:CREATV MICROTECH
Targeted Radiotherapy Chelates for In Situ Immune Modulated Cancer Vaccination
ActiveUS20200330621A1Maintain good propertiesClean emission profilePeptide/protein ingredientsPharmaceutical delivery mechanismVaccinationImmunomodulating Agent
The disclosed method of treating a malignant solid tumor in a subject includes the steps of administering to the subject an immunomodulatory dose of a radioactive phospholipid metal chelate compound that is differentially retained within malignant solid tumor tissue, and performing in situ tumor vaccination in the subject by introducing into at least one of the malignant solid tumors one or more agents capable of stimulating specific immune cells within the tumor microenvironment, or by performing another method of in situ tumor vaccination. In a non-limiting example, the radioactive phospholipid metal chelate compound has the formula:wherein R1 comprises a chelating agent that is chelated to a metal atom, wherein the metal atom is an alpha, beta or Auger emitting metal isotope with a half life of greater than 6 hours and less than 30 days. In one such embodiment, a is 1, n is 18, m is 0, b is 1, and R2 is —N+(CH3)3.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com