Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "ELAV Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ELAV-like protein 1 or HuR (human antigen R) is a protein that in humans is encoded by the ELAVL1 gene. The protein encoded by this gene is a member of the ELAVL protein family. This encoded protein contains 3 RNA-binding domains and binds cis-acting AU-rich elements.
Arylsulfonamide compounds
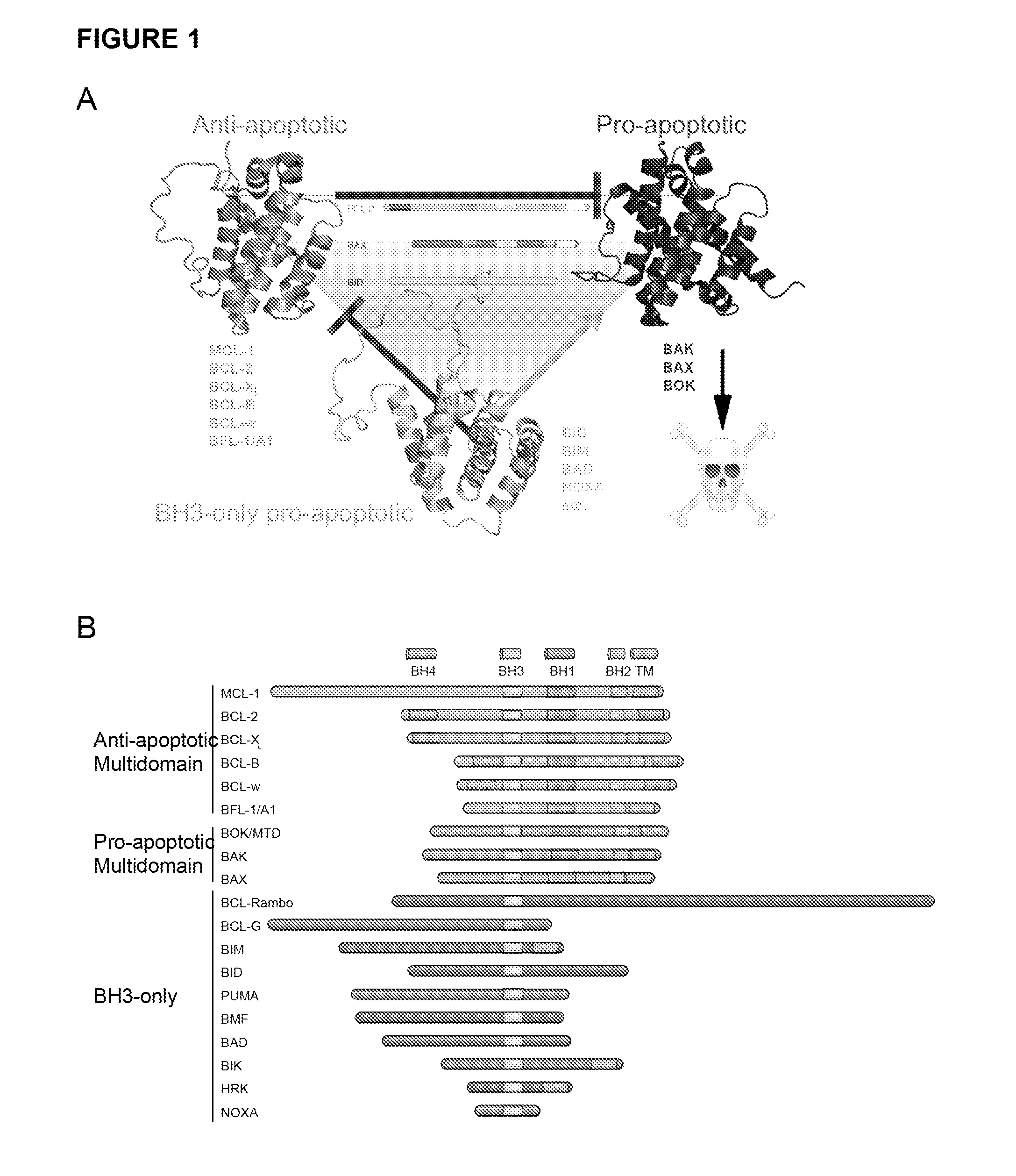
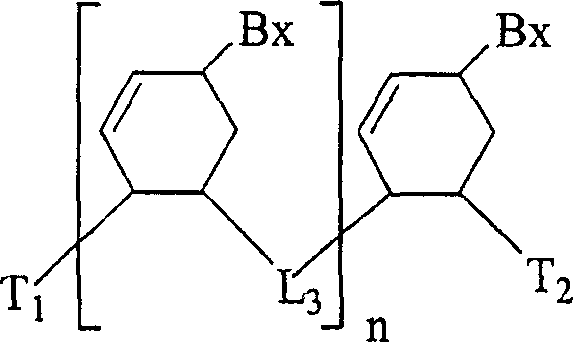
The invention relates generally to small molecules that mimic the biological activity of certain peptides and proteins, to compositions containing them and to their use. In particular, the invention relates to compounds of the general formula (I) that mimic the biological activity of BH3-only proteins and are capable of binding to and neutralizing pro-survival Bcl-2 proteins:wherein A1, A2, B1, B2, B3, X, Z, R1, R2, R3 and t are as described herein. The invention also relates to processes of preparing the benzenesulfonamide compounds that mimic portions of peptides and proteins, and to the use of such compounds in the regulation of cell death and the treatment and / or prophylaxis of diseases or conditions associated with the deregulation of cell death.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES +1
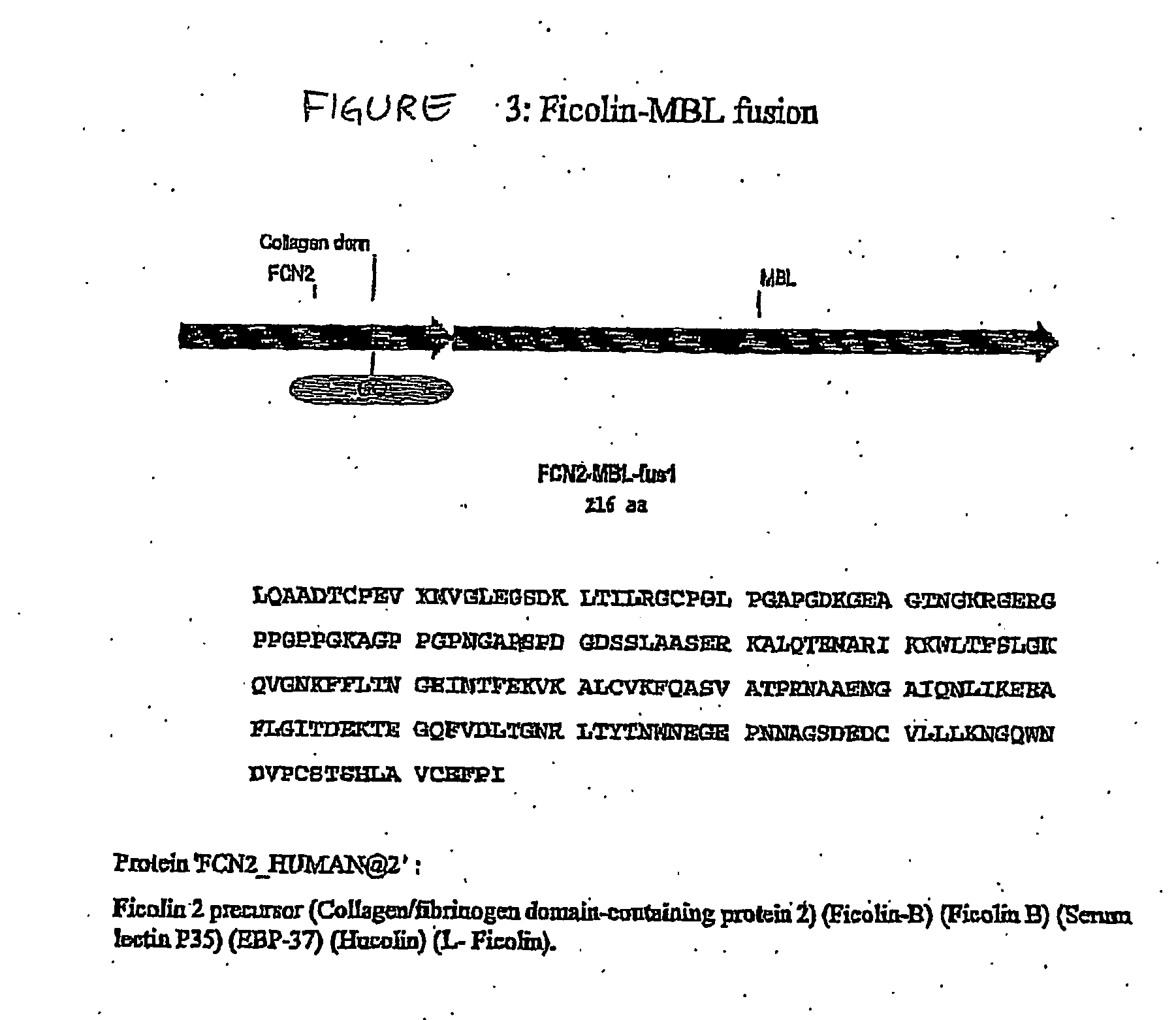
Collectin-complement activating protein chimeras
InactiveUS20060188963A1Improve abilitiesUseful in treatmentAntibacterial agentsPeptide/protein ingredientsCollectinComplement system
The present invention relates to a fusion protein capable of activating the complement system, the fusion protein comprising a first polypeptide sequence derived from a lectin-complement pathway activating protein or a functional homologue thereof; and a second polypeptide sequence derived from a collectin or a functional homologue thereof; wherein said complement activating protein is not a collectin. A preferred fusion protein comprises amino acids of the L-ficolin sequence of FIG. 1 and amino acids of the MBL sequence shown in FIG. 2. The fusion protein is suitable for use in treatment consisting of creation, reconstitution, enhancing and / or stimulating the opsonic and / or bactericidal activity of the complement system, i.e. enhancing the ability of the immune defence to recognise and kill microbial pathogens, and accordingly, the invention relates to a medicament comprising the fusion protein, methods for producing said fusion protein and methods for treating diseases, in particular infections.
Owner:ENZON PHARM INC
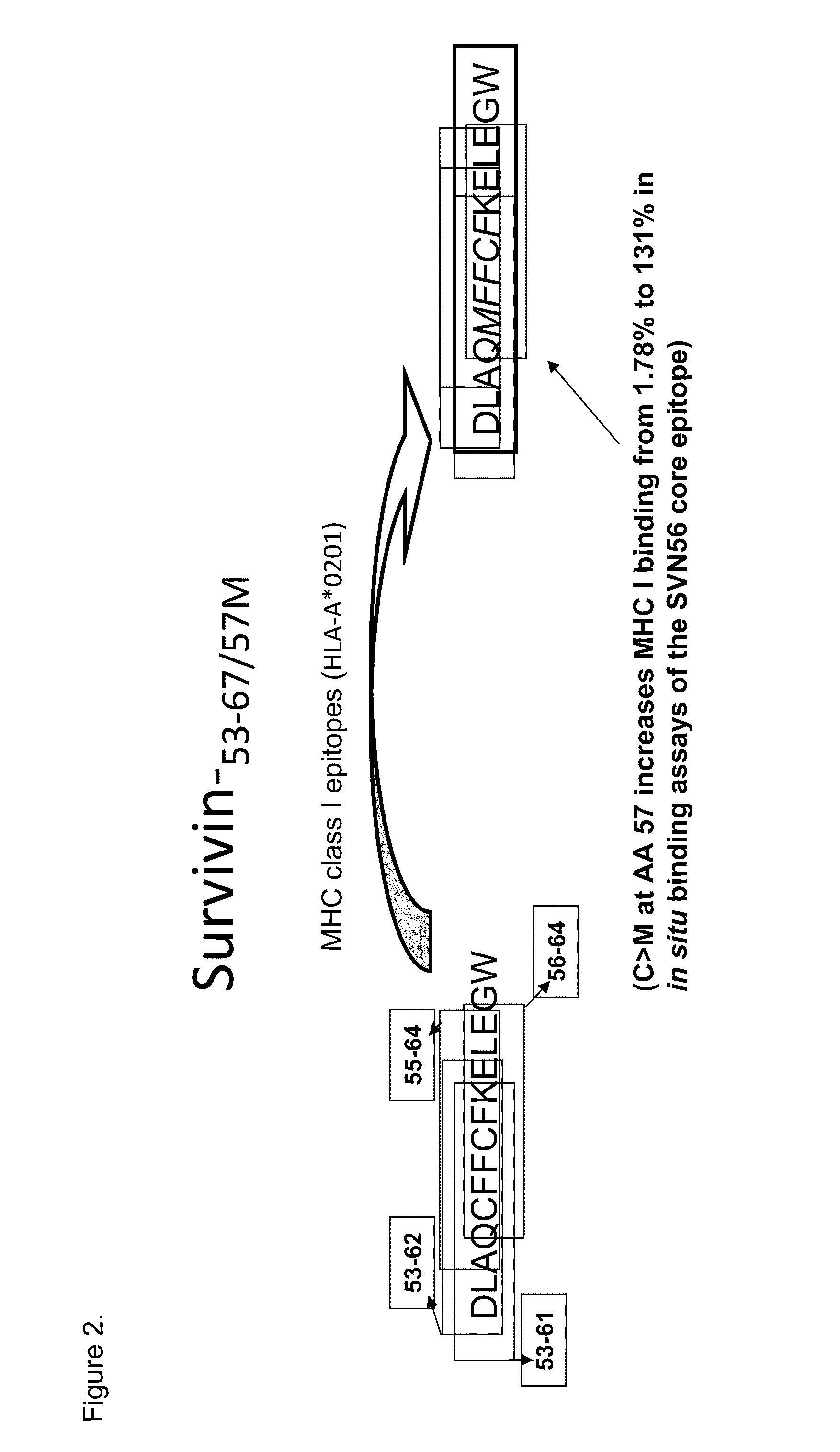
Survivin Peptides As Cancer Vaccines
ActiveUS20090041732A1Improved human cell mediated immune responseImprove survivalBiocidePeptide/protein ingredientsCancer cellCancer research
Provided are compositions and methods for treating survivin expressing cancers. The compositions contain peptide survivin peptide mimics with improved MHC-I binding characteristics. The method involves administering a survivin peptide mimic with improved MHC-I binding characteristics to an individual to effect inhibition of the growth of survivin expressing cancer cells in the individual.
Owner:HEALTH RES INC
Methods and compositions for specific modulation of MCL-1
ActiveUS9079970B2Regulating MCL-1 activityApoptosis is enhancedAntibacterial agentsOrganic active ingredientsDiseasePharmacometrics
A series of stapled BCL-2 family peptide helices were identified as able to target the survival protein MCL-I with high affinity and a subset with unprecedented selectivity. Agents and methods for selective pharmacologic neutralization of MCL-I are provided for drug discovery and therapeutic uses, including use in overcoming the apoptotic resistance of cancer and other diseases associated with impaired cell death.
Owner:DANA FARBER CANCER INST INC
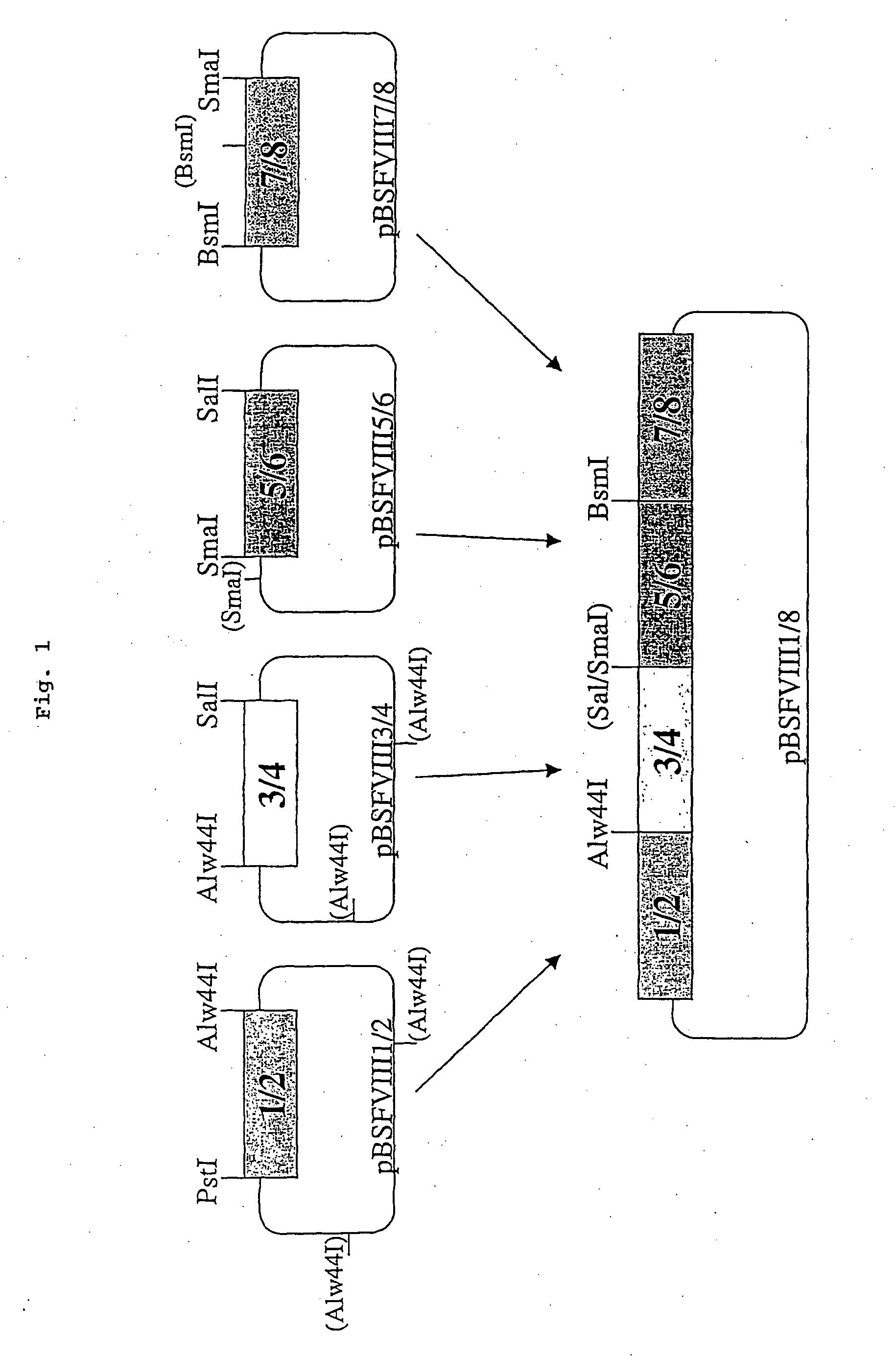
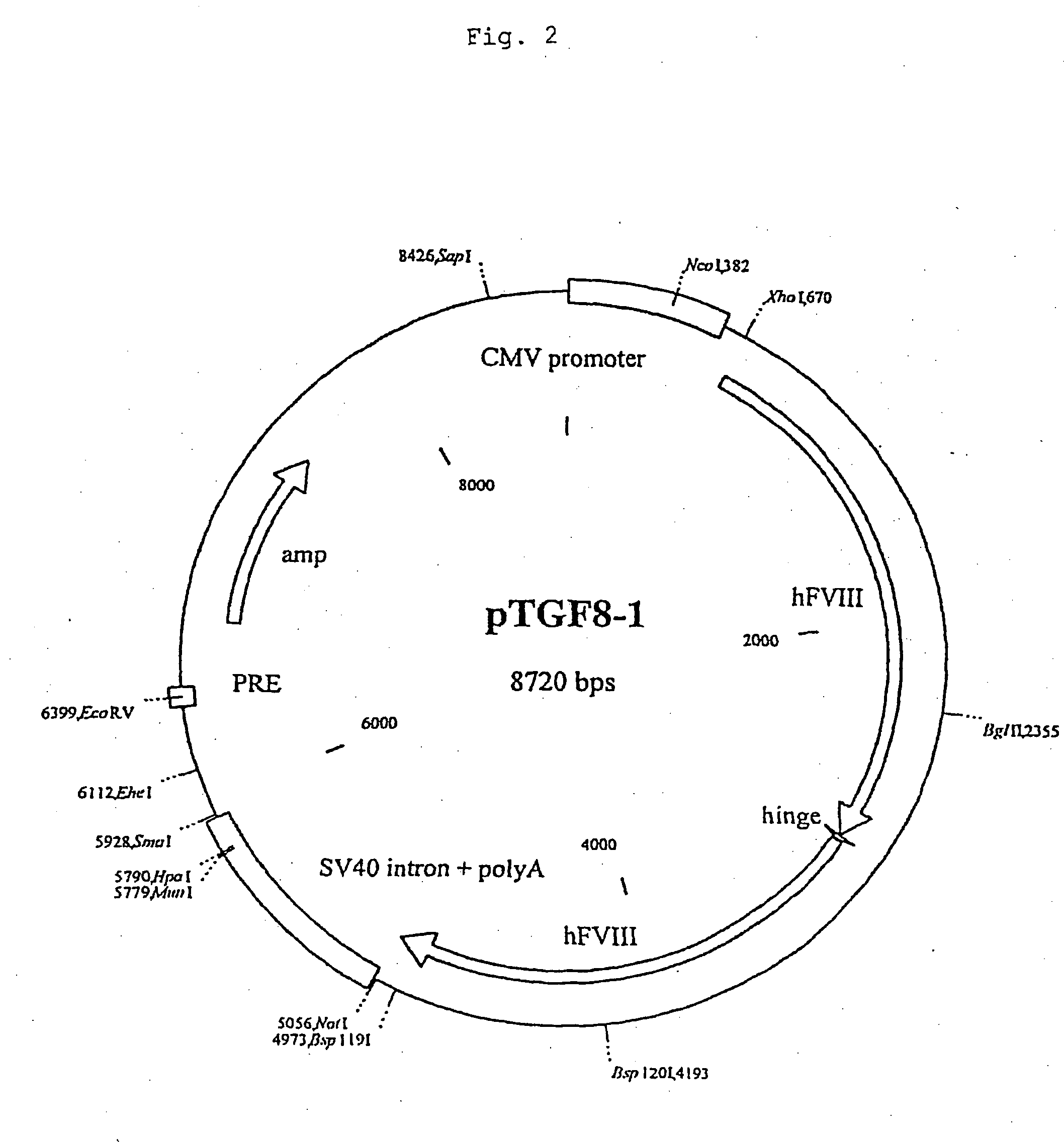
Production of recombinant blood clotting factors in human cell lines
ActiveUS20040023333A1Increased activationStable against proteolytic inactivationFactor VIIFungiHuman cellA-DNA
The present invention relates to an improved method for the production of recombinant human blood clotting factors, in particular of factor VIII and factor IX, utilizing an immortalized human cell line stably expressing viral transcription activator proteins and carrying a vector having a promoter functionally linked to a DNA sequence coding for a blood coagulating factor, provided that said promoter is not a viral promoter which is stimulated by said viral transcription activator proteins; an immortalized human cell line carrying said vector, factor VIII muteins particularly suitable for the above production method; pharmaceutical compositions comprising such factor VIII muteins and the use of such factor VIII muteins for preparing a medicament for treating hemophilia.
Owner:OCTAPHARMA +1
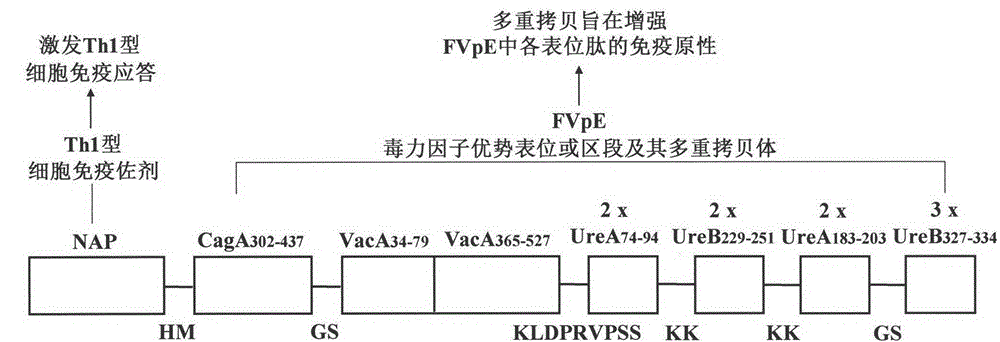
Helicobacter pylori tetravalent virulence factor multi-epitope vaccine and preparation method thereof
ActiveCN105106945ASmall molecular weightLow immunogenicityDigestive systemAntibody medical ingredientsVirulence factorELAV Proteins
The invention provides a helicobacter pylori tetravalent virulence factor multi-epitope vaccine and a preparation method thereof. The active constituent of the vaccine is polypeptide. The vaccine mainly comprises the dominant Th and B cell epitopes or sections of a urease A subunit, a urease B subunit, cytotoxin associated protein A and vacuolating cytotoxin associated protein A, and neutrophil activating protein. The gene synthesis and molecular cloning technology is used for building a fused gene containing the dominant Th and B cell epitopes or sections of ureases, the cytotoxin associated protein A and the vacuolating cytotoxin associated protein A, and the neutrophil activating protein. Escherichia coli is used for expressing the fused gene, and the tetravalent virulence factor multi-epitope vaccine is obtained after protein purification. The vaccine can stimulate a body to generate T cell immunity response and specific antibody humoral immunity response aiming at the ureases, the cytotoxin associated protein A, the vacuolating cytotoxin associated protein A and the neutrophil activating protein and can be used for preventing and treating diseases related to helicobacter pylori infection.
Owner:NINGXIA MEDICAL UNIV
Survivin-directed RNA interference-compositions and methods
InactiveUS20060276423A1Poor prognosis of survivalHigh sensitivityBiocideOrganic active ingredientsDiseaseDNA
The present invention is directed to compositions and methods for inhibiting the expression of survivin in cells expressing survivin. The invention is also directed to methods of treating conditions associated with elevated survivin, such as hyperproliferative disorders. More particularly, the invention is directed to inhibition of survivin expression using short interfering RNAs (si-RNAs) or through administration of DNA sequences yielding the expression of short hairpin RNAs (sh-RNAs).
Owner:ALTURA RACHEL +2
Compositions and methods for inducing gene expression
InactiveUS20060127361A1Increased formationHigh expressionOrganic active ingredientsBiocideA-DNABiological activation
The present invention provides recombinant nucleic acid molecules encoding a chimeric transactivator protein including a DNA binding domain of a DNA binding protein and a protein domain capable of transcriptional activation. The present invention also provides recombinant viral and non-viral vectors that are able to infect and / or transfect and sustain expression of a biologically active chimeric transactivator proteins in mammalian cells. Also provided are host cell lines and non-human transgenic animals capable of expressing biologically active chimeric transactivator proteins. In another aspect, compositions and methods for treating or preventing ischemic damage associated with hypoxia-related disorders are provided.
Owner:GENZYME CORP
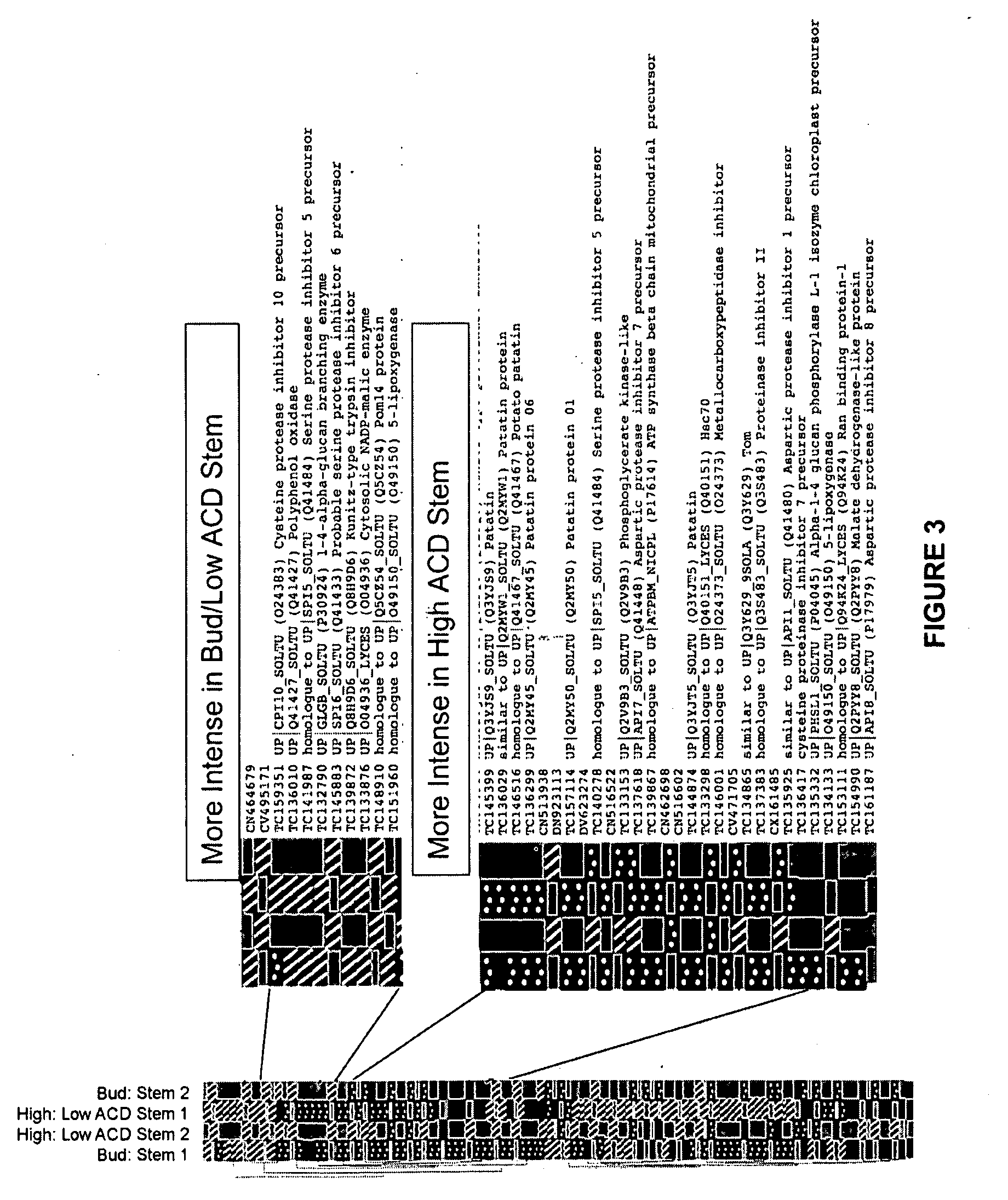
Proteins Involved in After-Cooking Darkening in Potatoes
InactiveUS20090241216A1Reducing ACDHigh activityMutant preparationFermentationSolanum tuberosumMolecular biology
Owner:DALHOUSIE UNIV +1
Benzothiazole compounds
The present invention relates to benzothiazole compounds that mimic the activity of BH3 only proteins and are capable of binding to and neutralizing pro survival Bcl 2 proteins. The invention also relates to the use of such compounds in the regulation of cell death or cell survival and the treatment and / or prophylaxis of diseases or conditions associated with the deregulation of cell death or cell survival.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Pumpkin protein 2 and preparation method and application thereof
InactiveCN101386644ASave manual and tedious operationsReduce cumbersome stepsPeptide/protein ingredientsAntiviralsPolyethylene glycolSingle crystal
The invention discloses a Cucurbita moschata protein 2 and a preparation method and application thereof, which relates to the field of biochemistry. The invention discloses a new ribosome inactivated protein extracted from flesh of Cucurbita moschata, namely the Cucurbita moschata protein 2 (type 2 Cucurbita moschata ribosome inactivated protein cucurmosin 2). The molecular weight of the Cucurbita moschata protein 2 is determined as 2, 7183Da by ESI-MS. The Cucurbita moschata protein 2 is put into phosphate buffer, citric acid buffer and Tris-HCl buffer in advance, and a single crystal for the diffraction of X-ray is produced taking carbowax as a precipitator. Through preliminary analysis, the crystal structure shows that the crystal is a type 1 ribosome inactivated protein with the functions of tumor and virus resistance, in particular human immunodeficiency virus resistance, thereby laying a material foundation for obtaining a protein for medicine use or preparing immunotoxin.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Antithrombotic thrombin variants
InactiveUS7223583B2Decreased cleavage activityStrong specificityPeptide/protein ingredientsMicrobiological testing/measurementProtein activationThrombin activity
The present invention relates to novel antithrombotic variants of thrombin or fragments thereof that are capable of proteolytically activating protein C, but which are substantially free of fibrinogen cleavage activity. The present invention further relates to variant polypeptides that may be cleaved to yield active thrombin variants. The present invention also relates to methods of inhibiting thrombus formation in an animal or human subject by delivering an antithrombotic variant thrombin of the present invention to the blood of the subject. The present invention relates also to methods that use the novel variant thrombins for determining the level of protein C activation in a blood sample, or the thrombogenic potential of a patient.
Owner:EMORY UNIVERSITY +1
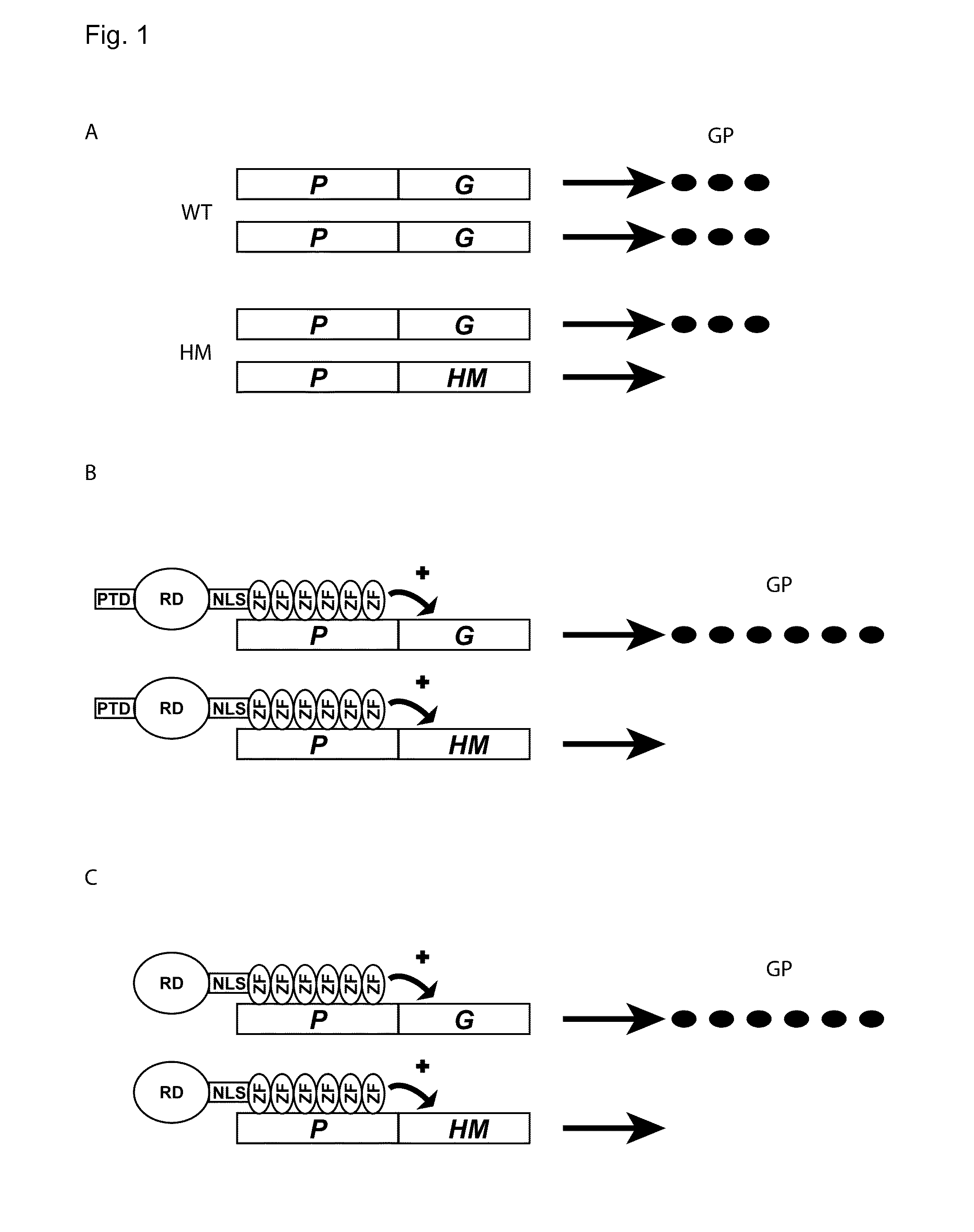
Artificial transcription factors for the treatment of diseases caused by opa1 haploinsufficiency
InactiveUS20160039893A1High expressionIncrease generationSenses disorderNervous disorderDiseaseHaploinsufficiency
The invention relates to an artificial transcription factor comprising a polydactyl zinc finger protein targeting specifically the OPA1 promoter fused to an activatory protein domain, and a nuclear localization sequence. Artificial transcription factors directed against the OPA1 promoter are useful for the treatment of diseases associated with OPA1 haploinsufficiency, such as autosomal dominant optic atrophy, syndromic autosomal dominant optic atrophy plus and normal tension glaucoma.
Owner:ALIOPHTHA
Bispecific antigen binding molecules comprising anti-FAP clone 212
ActiveUS20200190207A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingBinding domain
The invention relates to novel bispecific antigen binding molecules, comprising (a) at least one antigen binding domain capable of specific binding to Fibroblast Activation Protein (FAP) comprising FAP clone 212 or variants thereof, and (b) at least one antigen binding domain capable of specific binding to CD40, and to methods of producing these molecules and to methods of using the same.
Owner:F HOFFMANN LA ROCHE & CO AG
ANTAGONISTS OF Bcl-2 AND USES THEREOF IN INDUCTION OF APOPTOSIS
InactiveUS20150018285A1Peptide/protein ingredientsMicrobiological testing/measurementMedicineApoptosis
The present invention provides an antagonist of a Bcl-2 pro survival protein containing a BH3-like domain. The antagonist of the invention comprises ARTS and any fragment or peptide that comprises a BH3-like domain. The invention further provides compositions, combined compositions and kits as well as methods for treating Bcl-2 over-expressing disorders.
Owner:CARMEL HAIFA UNIV ECONOMIC
Screening and use of agents which block or activate intein splicing utilizing natural or homologous exteins
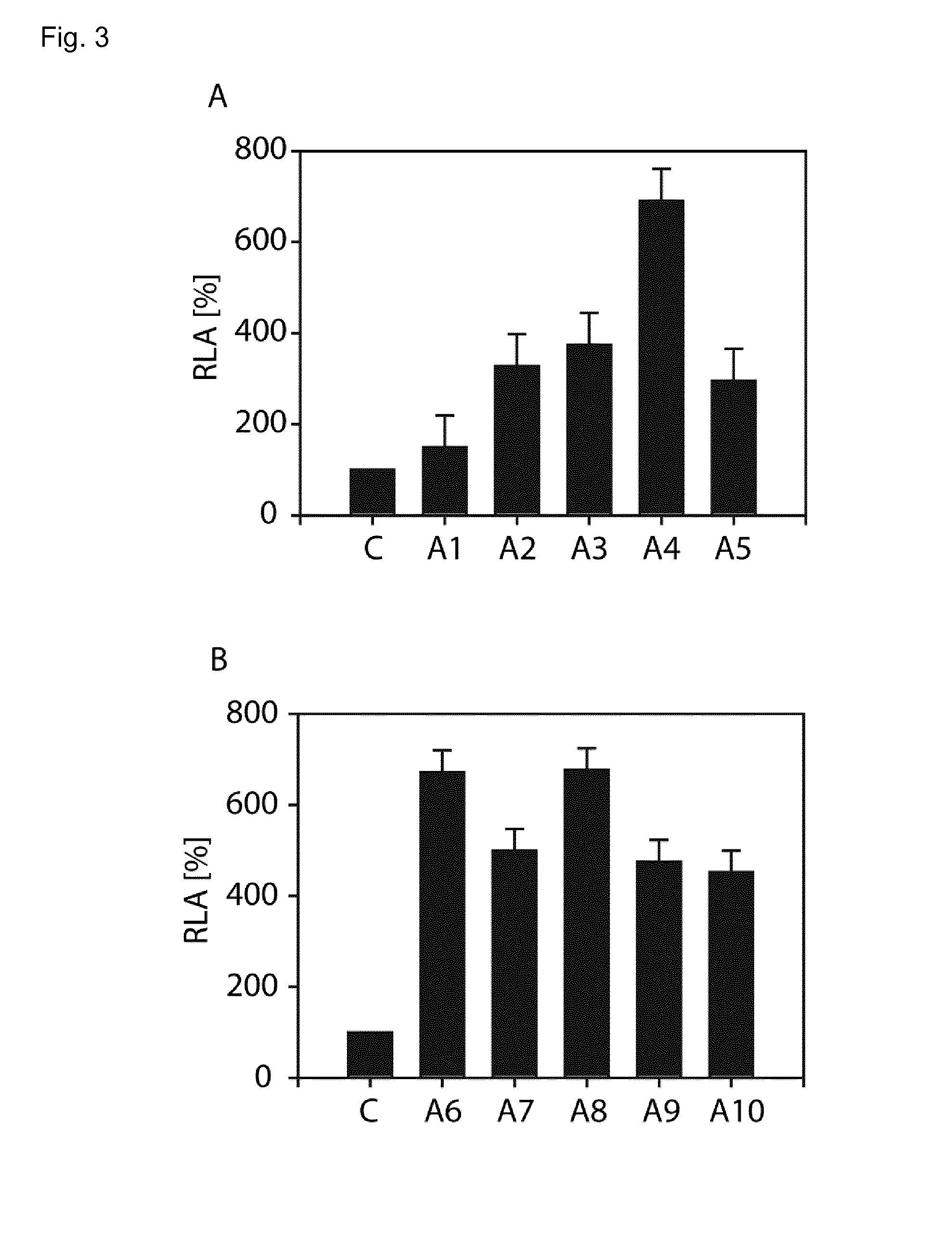
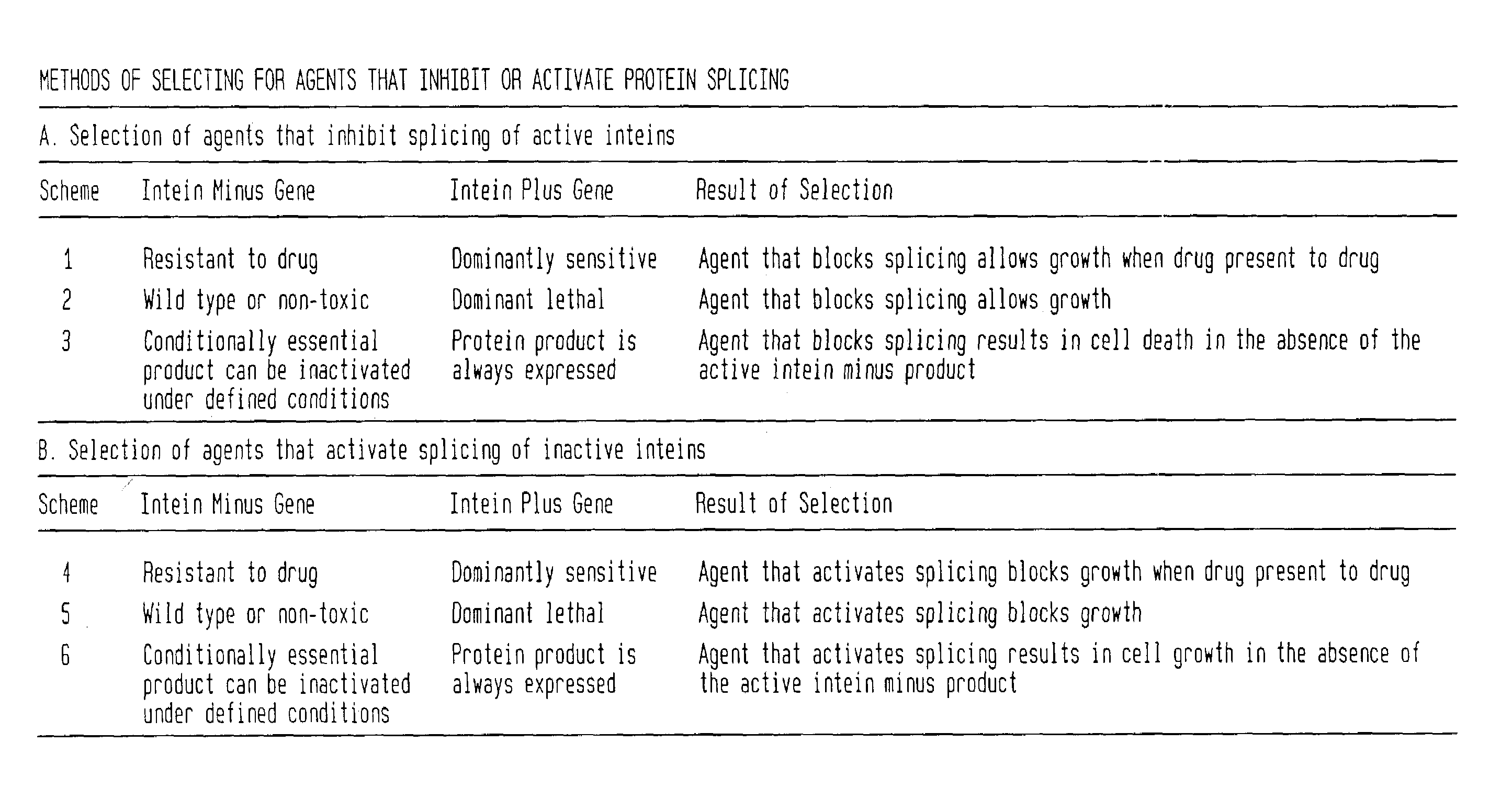
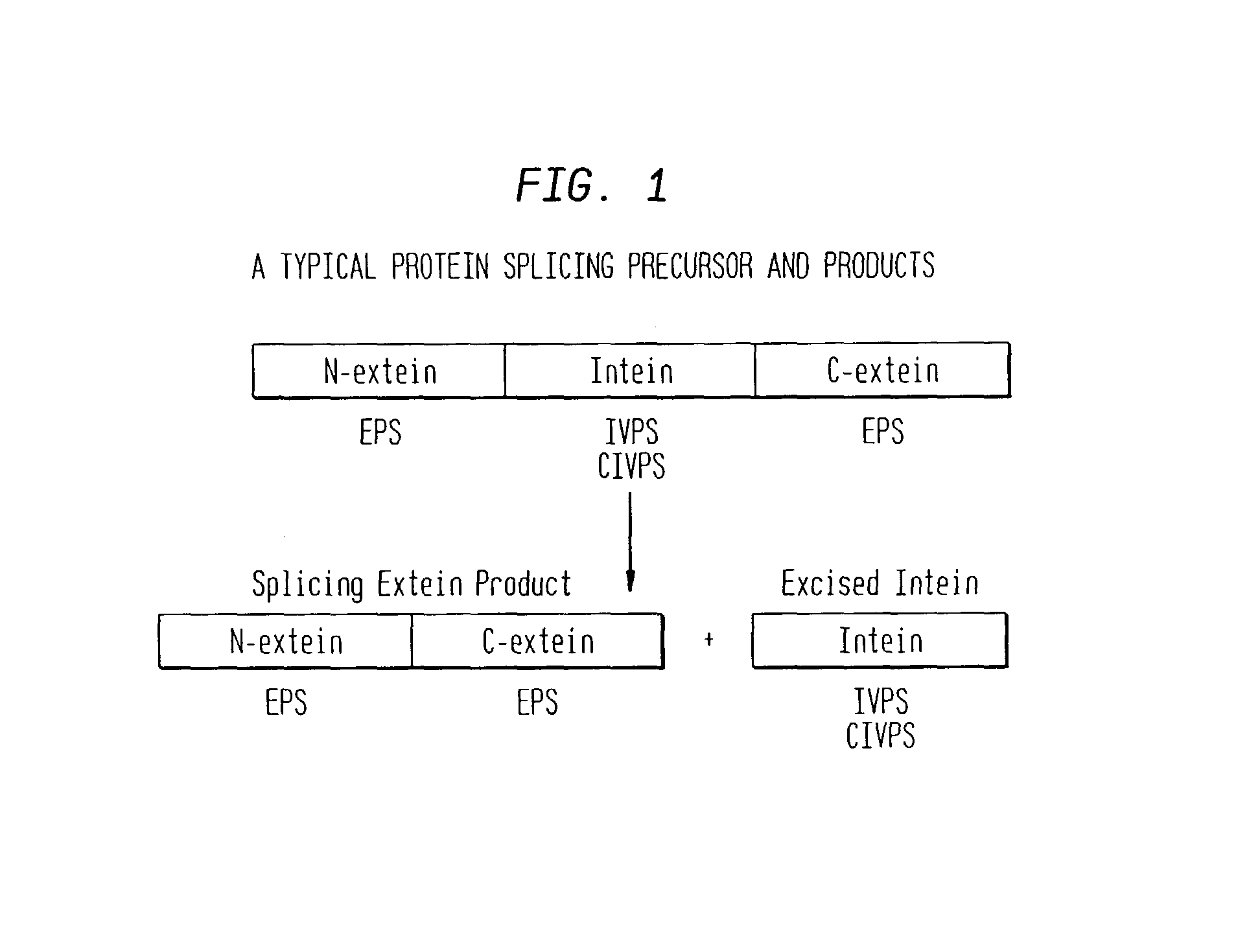
In accordance with the present invention, there are provided selection systems and methods for screening for agents that control splicing of inteins in their native host protein (extein) or in homologous exteins. Specifically, there are provided positive genetic selection systems for the screening of agents which inhibit or activate protein splicing which comprise: a host cell containing a chromosomal gene encoding either a drug-resistant form of a target enzyme or a wild-type target enzyme, and a plasmid-borne gene encoding either a drug-sensitive form of the target enzyme, which is dominantly cytotoxic upon interaction with the drug, or a dominantly cytotoxic form of the target enzyme. In these systems the plasmid-borne gene contains an intein, and the inhibition or activation of splicing of the dominant cytotoxic form of the target enzyme by a given reagent results in the survival or death of the host cell. More specifically, positive genetic selection systems which utilize the M. xenopi GyrA intein or M. tuberculosis DnaB helicase intein are provided. Similar reporter systems utilizing native or homologous exteins and systems utilizing controllable inteins are provided, as are methods of controlling in vivo expression of proteins by modulating protein splicing with inhibiting or activating agents, and methods of controlling the delivery of proteinaceous drugs in vivo by modulating protein splicing.
Owner:NEW ENGLAND BIOLABS
Protein marker detection kit combining gold nanoprobe and CRISPR-Cas and detection method
PendingCN112725343AEasy to operateHigh sensitivityMaterial analysisDNA/RNA fragmentationImmune profilingAnalyte
The invention relates to the technical field of chemistry and biology, in particular to a protein marker detection method combining a gold nanoprobe and CRISPR-Cas. Immunoassay, a nanotechnology and a CRISPR detection technology are combined, and an aptamer and a CRISPR activation chain are covalently connected to gold nanoparticles to obtain the gold nanoprobe. Through the specific recognition effect of the antibody and the aptamer on the protein marker, a sandwich structure of the antibody-analyte-gold nanoprobe is formed in the 96-hole elisa plate. The activation chain on the gold nanoprobe can activate the para-cleavage activity of the CRISPR protein and cleave the fluorescence reporter molecule to generate a fluorescence signal, thereby quantitatively detecting the analyte. According to the present invention, with the gold nanometer probe, the conversion from the analyte identification signal to the CRISPR side cleavage activity signal is achieved, and the amplification of the activation chain signal is achieved; the detection method is simple to operate, high in sensitivity, strong in specificity and wide in detection linear range.
Owner:NANJING UNIV OF TECH
Modulation of survivin expression
Compounds and compositions are provided for modulating the expression of survivin. The compounds, exemplified by those acting through an RNAi antisense mechanism of action, include double-stranded and single-stranded constructs, as well as siRNAs, canonical siRNAs, blunt-ended siRNAs and single-stranded antisense RNA compounds. Methods of using these compounds for modulation of survivin expression and for treatment of diseases associated with expression of survivin are provided.
Owner:IONIS PHARMA INC +1
Construction and application of marine nannochloropsis oculata transcriptional activation CRISPRa system
ActiveCN114480474ATranscriptional activationEasy to captureHydrolasesUnicellular algaeBiotechnologyNannochloropsis salina
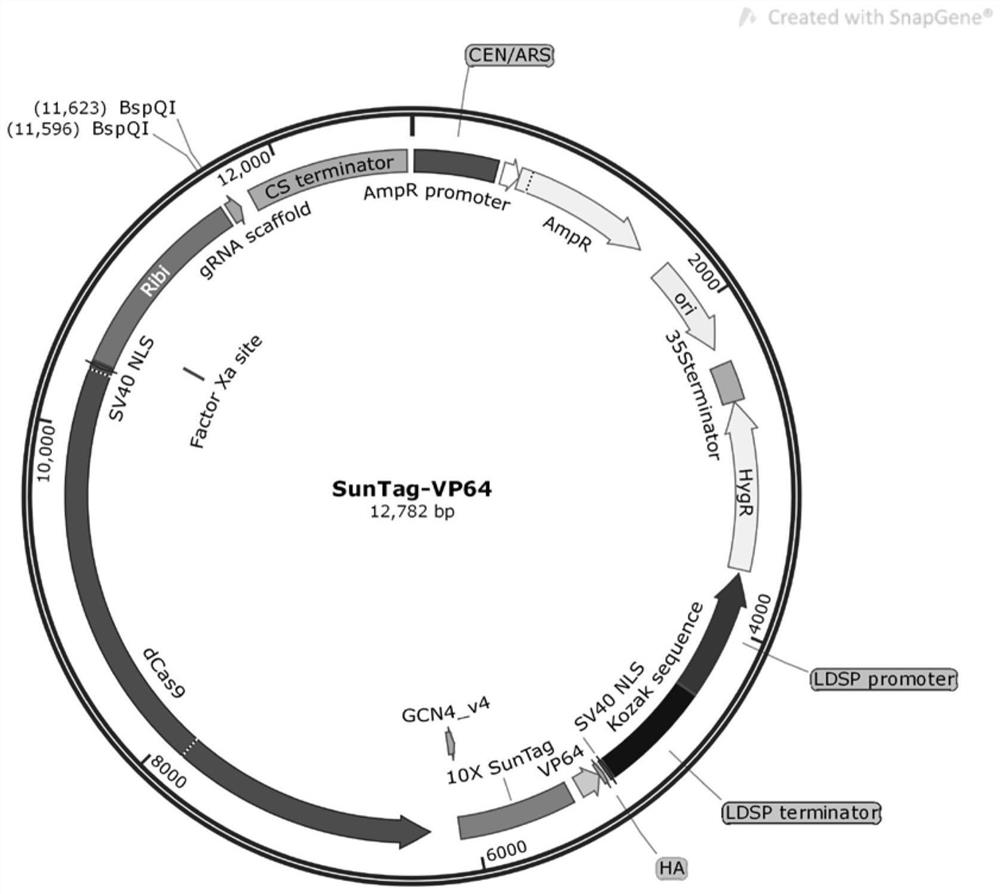
The invention belongs to the technical field of biology, and particularly relates to a marine nannochloropsis oculata transcriptional activation CRISPRa system and a construction method and application thereof. The system vector contains a Cas9 inactivated protein, a transcriptional activation effect protein VP64, a resistance selection marker gene, at least one gRNA scaffold sequence and a specific sequence for enhancing transcriptional activation (SunTag sequence). The invention also provides the application of the CRISPRa vector for the transcription activation of the nannochloropsis oculata gene in gene transformation. The invention provides a new model and a feasible method for gene function research of nannochloropsis oculata.
Owner:HAINAN NORMAL UNIV
Cyclic AMP response element activator proteins and uses related thereto
The present invention discloses newly identified cyclic AMP response element activator proteins (CREAP proteins). It is mentioned herein that the protein is a suitable drug target for developing new therapeutic agents to prevent, treat or alleviate pathological conditions related to abnormal activation of CRE-dependent gene expression or abnormal activation of chemokines. The present invention relates to a method for preventing, treating or alleviating said pathological conditions and a pharmaceutical composition comprising a regulator having an inhibitory effect on CREAP protein activity and / or CREAP gene expression. The present invention also relates to methods of identifying compounds useful in the treatment of said pathological conditions, comprising identifying compounds capable of inhibiting CREAP protein activity and / or CREAP gene expression.
Owner:NOVARTIS AG
Method for identifying modulators of g3bp activity
A method of identifying a lead or candidate compound that modulates the activity of GTPase-Activating Protein SH3 Domain-Binding Proteins (G3BP) is provided, which includes determining whether a compound modulates the interaction between the N-terminal Nuclear Transport Factor 2-like (NTF2L) domain of G3BP and FGDF peptide of ubiquitin specific protease 10 (USP10) or non-structural protein 3 (nsP3).
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Application of oridonin in preparing activator of tumor-suppressor protein FBW7
InactiveCN102526020AEasy to manufactureImprove applicabilityOrganic active ingredientsAntineoplastic agentsApoptosisInducer Cells
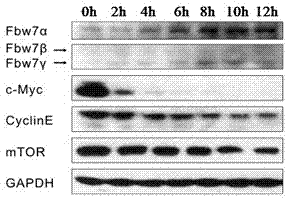
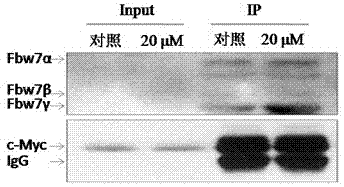
The invention discloses an application of oridonin in preparing an activator of tumor-suppressor protein FBW7 (F-box and WD repeat domain containing 7). According to a large number of studies, oridonin can activate the expression of FBW7 protein and enhance the affinity of FBW7 protein to a substrate protein, so that FBW7-medicated ubiquitination degradation pathway is rapidly and efficiently activated. Oridonin target-degrades cancer protein such as c-Myc, cyclinE and mTOR through activation of the FBW7-medicated ubiquitination degradation pathway, so as to inhibit the growth of a variety oftumor cells and induce cell apoptosis. Oridonin is mainly derived from traditional Chinese medical material Rabdosia rubescens, is easy to prepared, has low toxicity to human body, and has high applicability.
Owner:SUN YAT SEN UNIV
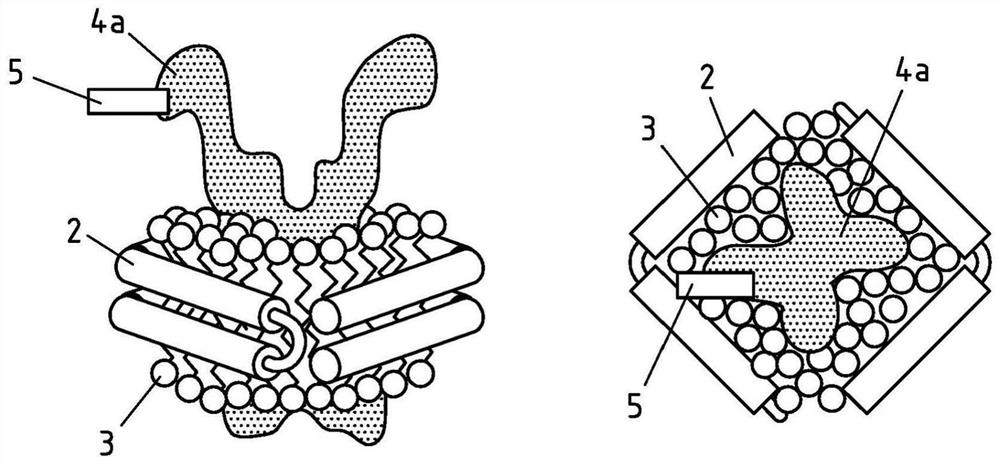
Production of salipro particles
PendingCN113873999AKeep dryBiological Functional EffectsMacromolecular non-active ingredientsLiposomal deliveryA lipoproteinLipoprotein particle
The invention relates to a process for preparing saposin lipoprotein particles, comprising a saposin-like protein, lipids and optionally a hydrophobic agent wherein the saposin-like protein or the hydrophobic agent is selectively bound to a support to allow the self-assembly of the saposin lipoprotein particles. The process of the invention comprises the step of a.) providing the hydrophobic agent and lipids, b. 1) / b.2) contacting the hydrophobic agent or the saposin-like protein with a support that is capable of selectively binding either of the two molecules to the support, c.1) / c.2) contacting the support-bound particle components with the remaining particle components, either the saposin-like protein or the hydrophobic agent, to allow fpr the self-assembly of the saposin lipoprotein particle on the support and d.) optionally eluting the support-bound saposin lipoprotein particles.
Owner:塞利普洛生物技术有限责任公司
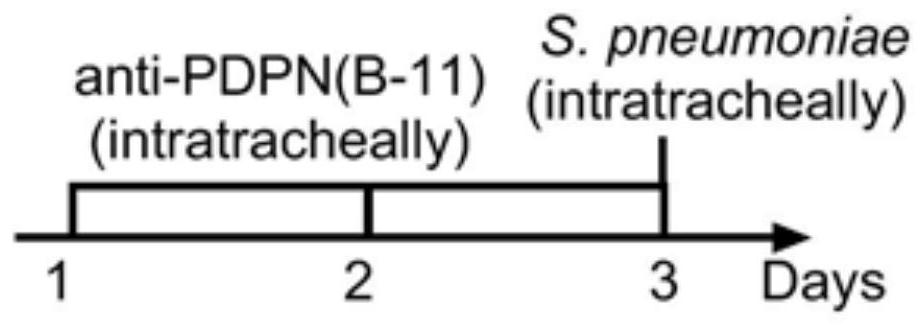
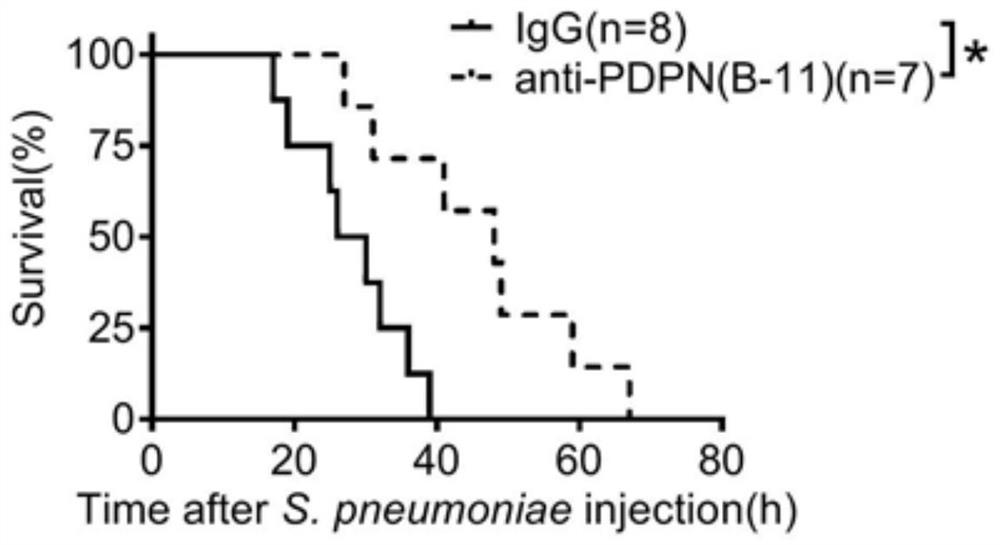
Application of SCGB3A2-PDPN-RhoA signal channel as drug target for inhibiting lung inflammatory factor storm
ActiveCN113058036AHigh strengthHigh expressionAntibacterial agentsAntiviralsInflammatory factorsGlucocorticoid
Research finds that exocrine protein SCGB3A2 in lung tracheal bronchus is combined with a receptor PDPN on the cell surface and activates RhoA protein, so that the strength of pneumonia and the expression quantity of inflammatory factors are enhanced, and lung inflammation can be relieved and expression of inflammatory factors and infiltration of lung inflammatory cells can be reduced by blocking combination of SCGB3A2 and PDPN through an antibody. Therefore, the invention provides a brand new target for controlling lung inflammatory factor storm caused by bacteria or viruses. The target spot has a relatively clear signal transmission path, so that the side effect of the medicine for inhibiting the inflammatory factor storm, which is prepared aiming at the target spot, is smaller than that of glucocorticoid.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
CRISPR-Cas13 nucleic acid detection kit based on lightened RNA aptamer
ActiveCN112609010ALow costEasy to operateMicrobiological testing/measurementMicroorganism based processesAptamerEnzyme digestion
The invention discloses a CRISPR-Cas13 nucleic acid detection kit based on a lightening type RNA aptamer. The kit comprises the lightening type RNA aptamer, Cas13 protein, crRNA and nucleic acid dye; the lightening type RNA aptamer can be combined with nucleic acid dye to emit fluorescence, and if the lightening type RNA aptamer is sheared by Cas13 protein, fluorescence is quenched; the crRNA is a section of RNA, the crRNA and Cas13 protein can form a crRNA-Cas13 compound, and after the Cas 13-crRNA is combined with a target RNA sequence, the enzyme digestion activity of the Cas13 protein is activated, and an RNA aptamer is excised. The kit can detect living pathogens and RNA markers by detecting RNA, and compared with an existing nucleic acid detection method based on CRISPR-Cas13, the method does not depend on reverse transcription and nucleic acid amplification, target RNA sequence detection is completed in one step, the detection cost is low, the operation steps are simple, high specificity and sensitivity can be guaranteed, and industrial application is facilitated.
Owner:SICHUAN UNIV
Method for constructing ribosome inactivating protein gene virus vector and expressing active proteins in tumor cell through ribosome inactivated protein gene virus vector
PendingCN110747231AHas RNAN-glycosidase activityInhibit synthesisPeptide/protein ingredientsNucleic acid vectorPlant virusPlant cell
The invention belongs to the technical field of bioengineering and specifically discloses a method for constructing a ribosome inactivating protein gene virus vector and expressing active proteins intumor cells through the ribosome inactivated protein gene virus vector. The method comprises the following steps that first, suitable ribosome inactivated proteins are selected, and codon optimizationfor humanized expression is conducted on mature region genes of the ribosome inactivated proteins; second, an adenovirus carrier of wild type and optimized genes is constructed, and recombinant viruses are packaged; and third, recombinant adenoviruses infect the tumor cells, the recombinant adenoviruses express proteins in the tumor cells through detection, and the anti-tumor effect of the recombinant adenoviruses is detected. According to the method, an alpha-charantin gene is subjected to codon optimization, an expression vector system for the adenoviruses capable of infecting the tumor cells of mammals is constructed so as to be suitable for expressing the active proteins in the tumor cells, and the defects that in the prior art, a prokaryotic cell expression system can only be expressed in escherichia coli when being used, and a plant virus vector can only be expressed in plant cells but cannot be expressed in the tumor cells and kill tumors when being used are overcome.
Owner:成都富岱生物医药有限公司
New application of gamma-secretase activated protein gene and/or protein coded by gamma-secretase activated protein gene
ActiveCN112831560APeptide/protein ingredientsMicrobiological testing/measurementDiseaseAcute myeloid leukemias
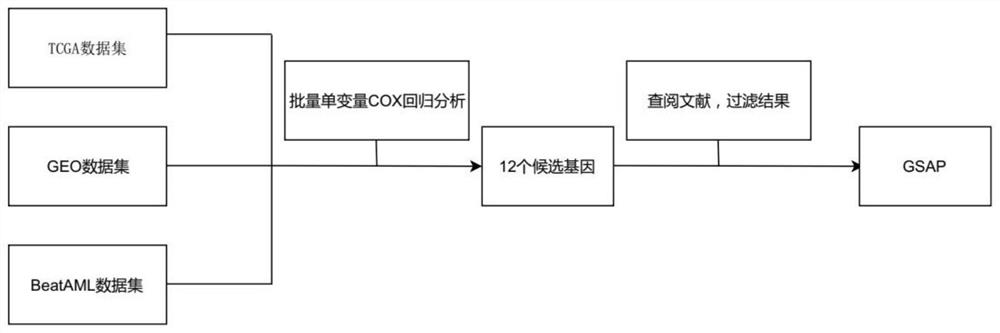
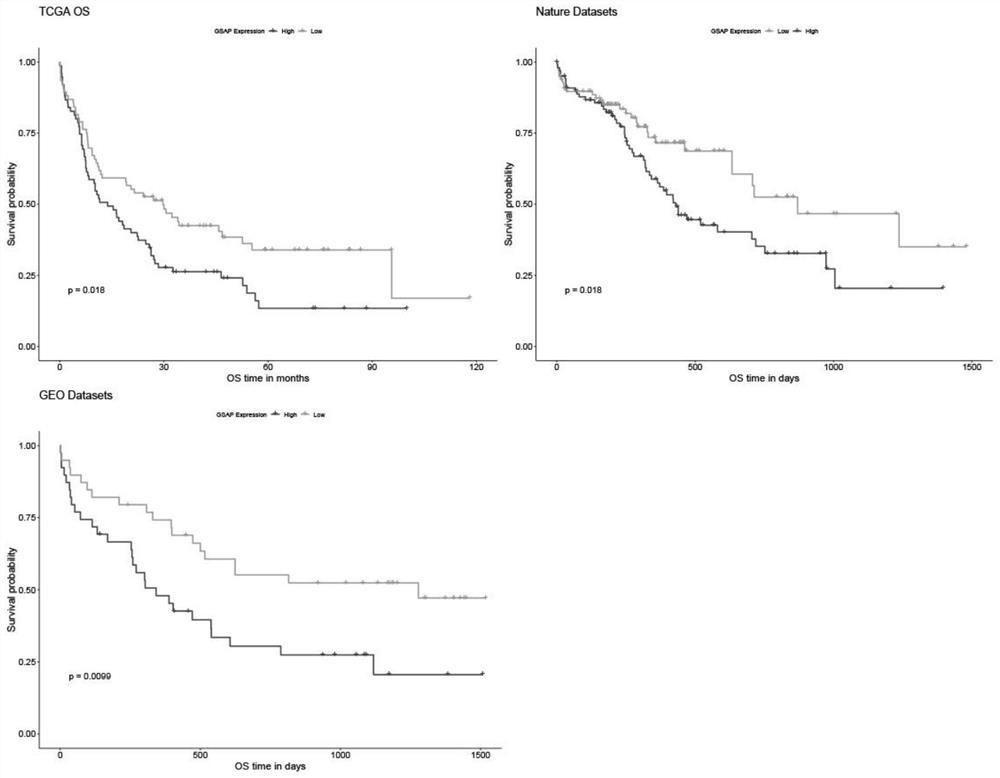
The invention relates to a novel application of gamma-secretase activated protein gene and / or protein coded by gamma-secretase activated protein gene. According to the application disclosed by the invention, by screening acute myelogenous leukemia (AML) prognosis related molecular markers, the screened gamma-secretase activated protein gene (GSAP) is closely related to the prognosis of acute myelogenous leukemia patients; the high expression of the GSAP prompts that the prognosis of the acute myelogenous leukemia patient is poor; the GSAP is related to FAB typing and cytogenetics risk stratification of an acute myelogenous leukemia patient; and the GSAP may participate in the disease progress of acute myelogenous leukemia through various ways such as PI3K-AKT, TLR and the like. The GSAP is used for preparing a treatment drug or a diagnostic reagent and a prognosis detection reagent for acute myelogenous leukemia.
Owner:SHANDONG UNIV QILU HOSPITAL
Jatropha curcas ribosome inactivating protein and its separation and purification method and application
ActiveCN107058261BStrong growth inhibitory effectObvious inhibitory effect on proliferation in vitroPeptide/protein ingredientsAntineoplastic agentsRibosome-inactivating proteinNon-small cell lung cancer (NSCLC)
The invention provides jatropha curcas L. ribosome-inactivating protein and a preparation method thereof. The jatropha curcas L. ribosome-inactivating protein is separated and purified from cotyledon and / or endosperm of jatropha curcas L. seedlings which are generated by jatropha curcas L. seeds germinating for 4 to 49 days, relative molecular weight is 31.398kDa, an isoelectric point is 7.12, and a sequence of amino acid residue of an N-terminal of the ribosome-inactivating protein is shown as SEQ ID No: 1 in a sequence table. The invention further provides application of the jatropha curcas L. ribosome-inactivating protein in preparing medicines for treating tumor. The jatropha curcas L. ribosome-inactivating protein has an obvious anti-proliferative effect on human osteosarcoma U20S, human non-small cell lung cancer A549, human non-small cell lung cancer H1975 and human colon cancer HCT116; an in-vitro proliferation inhibition effect of the jatropha curcas L. ribosome-inactivating protein on the human osteosarcoma U20S is superior to that of Curcin and taxol; furthermore, toxicity to human normal kidney cells is lower.
Owner:SICHUAN UNIV
LNA oligonucleotides and the treatment of cancer
InactiveUS8173428B2Organic active ingredientsSugar derivativesParanasal Sinus CarcinomaCarcinoma cell line
The present disclosure concerns LNA oligonucleotides having a (sub)sequence of the general formula 5′-(MeCx)(Tx)MeCxAsAstscscsastsgsgsMeCxAx(Gx)(c)-3′, and preferably of the general formula 5′-MeCxTxMeCxAsastscscsastsgsgsMeCxAxGxc-3′, wherein capital letters designate an LNA nucleotide analogue selected from β-D-oxy-LNA, β-D-thio-LNA, β-D-amino-LNA and α-L-oxy-LNA, small letters designate a deoxynucleotide, and underline designates either an LNA nucleotide analogue as defined above or a deoxynucleotide. Such LNA oligonucleotides exhibit surprisingly good properties with respect to inhibition of the expression of Survivin by means of an anti-sense mechanism, and thereby lead to reduction or inhibition of tumor development in vivo. The LNA oligonucleotides are superior to other LNA oligonucleotides targeting Surviving mRNA measured by functional read outs such as apoptosis induction and proliferation inhibition, and is potent in down-regulating Survivin mRNA and protein in transfected cancer cell lines, and induce apoptosis in combination with Taxol superior compared to other LNA oligonucleotides.
Owner:ENZON PHARM INC
Application of NRF2 protein in preparation of drug for regulating biorhythm
PendingCN111358936AQuick adjustabilityEasy to adjustNervous disorderDipeptide ingredientsAgonistPharmaceutical Substances
The invention discloses an application of NRF2 protein in preparation of a drug for regulating biorhythm. An NRF2 agonist is bortezomib. The application has the beneficial effects that rapid adjustment and no-interference adjustment of the biorhythm can be realized; specifically, the application is implemented in that 1) the NRF2 protein is directly activated, so that the NRF2 rapidly enters a cell nucleus to play a role, so that a slow-speed adjustment path by increasing the content of the NRF2 protein in a DNA-mRNA-protein process is avoided; and 2) the content of the biorhythm protein is directly increased by promoting the combination of the NRF2 protein and a biorhythm related gene, so that the biorhythm is rapidly adjusted, a slow-speed adjustment path by increasing the content of thebiorhythm content indirectly through a biorhythm feedback protein is avoided, and the interference of the biorhythm feedback protein on the biorhythm protein content is also avoided.
Owner:63919 TROOPS PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com