Jatropha curcas ribosome inactivating protein and its separation and purification method and application
A ribosome inactivation, separation and purification technology, applied in the field of plant protein separation and purification, can solve problems such as lack of targeted therapeutic drugs, and achieve obvious effects and obvious effects of in vitro proliferation inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In this example, it is confirmed by experiments that the Jatropha curcas ribosome inactivation protein Curcin C exists in the cotyledon and endosperm of Jatropha curcas seedlings after germination.
[0047] Soak Jatropha curcas seeds in tap water for 2 hours, then plant them on a culture bed made of vermiculite, germinate at a temperature of 25-30°C, 18 hours of light and 6 hours of darkness, and water properly to keep the culture bed moist , when the cotyledons are fully expanded, the seedlings are transplanted into the mixed soil with a volume ratio of vermiculite and nutrient soil of 1:1, and continue to grow under the same environmental conditions as for germination. From soaking the seeds, the cotyledon and endosperm of Jatropha curcas were collected every day until the cotyledon and endosperm fell off from the Jatropha curcas seedlings, wherein, the cotyledons fell off from the Jatropha curcas seedlings about 49 days after germination, and the endosperm fell off on...
Embodiment 2
[0051] In the present embodiment, from the cotyledons and endosperm of germinated Jatropha curcas seedlings, Curcin C is isolated and purified, and the steps are as follows:
[0052] (1) Preparation of crude extract
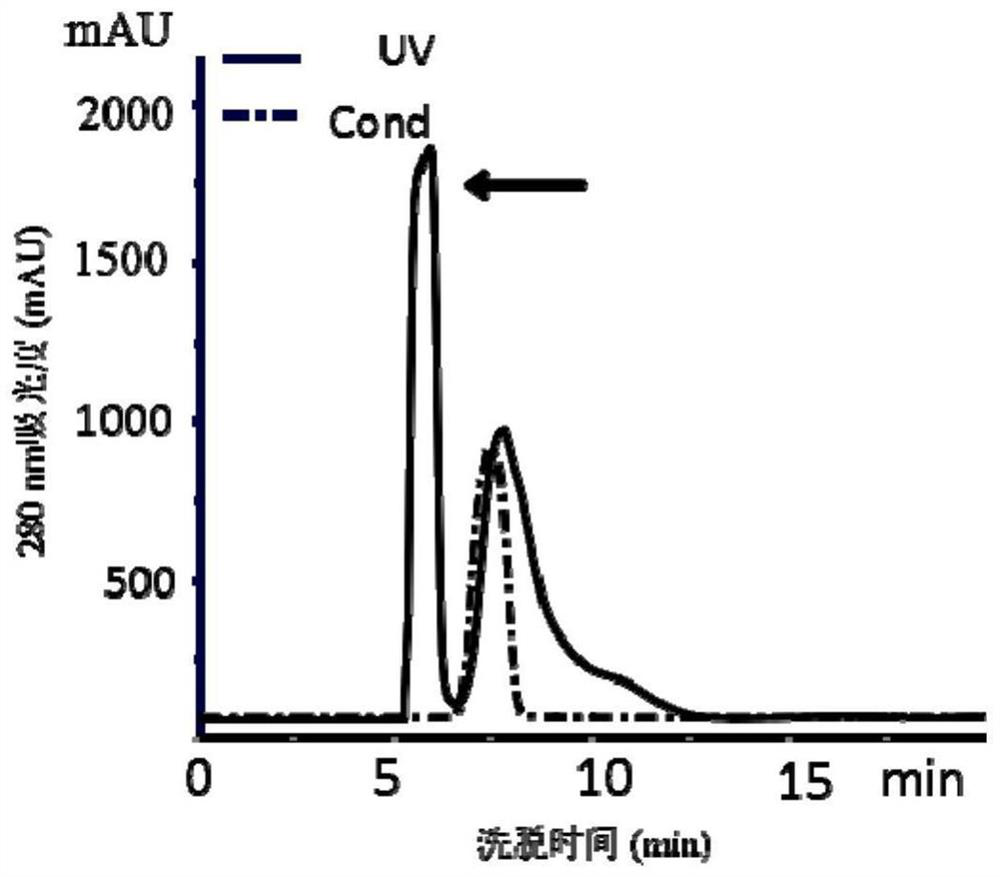
[0053] ① Germinate Jatropha curcas seeds, collect 40 g of cotyledons and endosperms of Jatropha curcas seedlings that have germinated for 4 to 49 days, grind the collected cotyledons and endosperms with liquid nitrogen respectively to powder, and then add 120 mL of Dissolve the powdered material in buffer A at 4°C, homogenize at 4°C, homogenize for 30 seconds every time, stop for 30 seconds, and homogenize for 5 minutes, rotate the homogenate at 4°C overnight, and rotate at 4°C at 10,000 rpm Centrifuge at a rotational speed for 30 minutes and collect the supernatant (that is, the homogenate crude extract);
[0054]②Add powdered ammonium sulfate to the supernatant under stirring at 4°C until the saturation of ammonium sulfate in the supernatant is 60%, continue s...
Embodiment 3
[0073] In this example, the N-terminal amino acid residue sequence of Curcin C was determined, and compared with the N-terminal amino acid residue sequence of Jatropha curcas ribosome inactivation protein reported in the existing literature.
[0074] The sequence of the N-terminal amino acid residues of Curcin C was obtained by the following method: the Curcin C obtained in Example 2 was electrophoresed on a denatured polyacrylamide gel, then transferred to a PVDF membrane, and Shimadzu PPSQ-33A type full-body The sequence of the N-terminal amino acid residues of Curcin C was determined by an automatic protein peptide sequencer, and the result was: -Ala Gly Ser ThrPro Thr Leu Ala Ile Thr Tyr Asp Ala Thr Thr.
[0075] Compared the sequence of N-terminal amino acid residues of Curcin C protein with the sequence of N-terminal amino acid residues of RIPs on NCBI, it was found that there were three groups of proteins with sequences close to the N-terminal amino acid residues of Curc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com