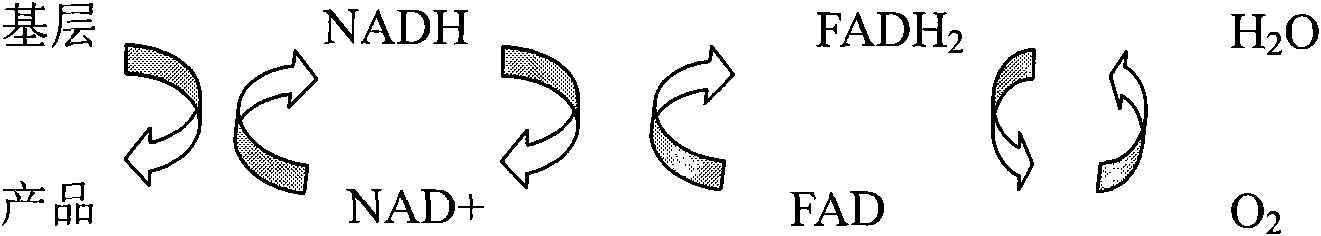
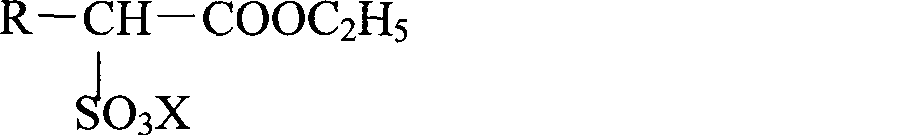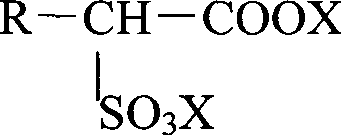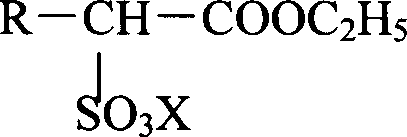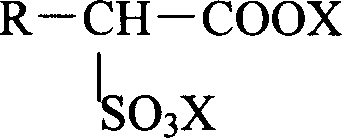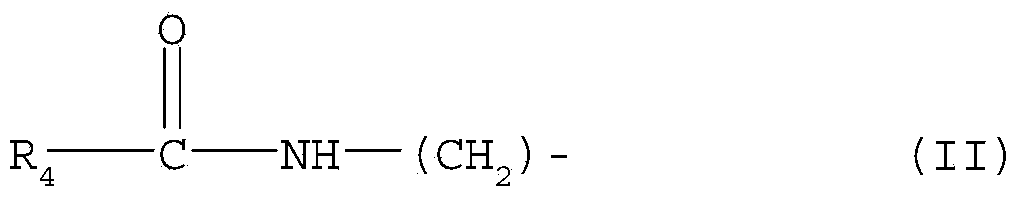Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Catalpic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalpic acid is a conjugated polyunsaturated fatty acid. The melting point of this fatty acid is 32 °C. Catalpic acid occurs naturally in the seeds of Catalpa ovata and Southern Catalpa (Catalpa bignonioides). Seeds of Catalpa species contain about 40% catalpic acid.
Production method and application of humic acid and fulvic acid extracted from humus and organic salts of mineral compounds thereof
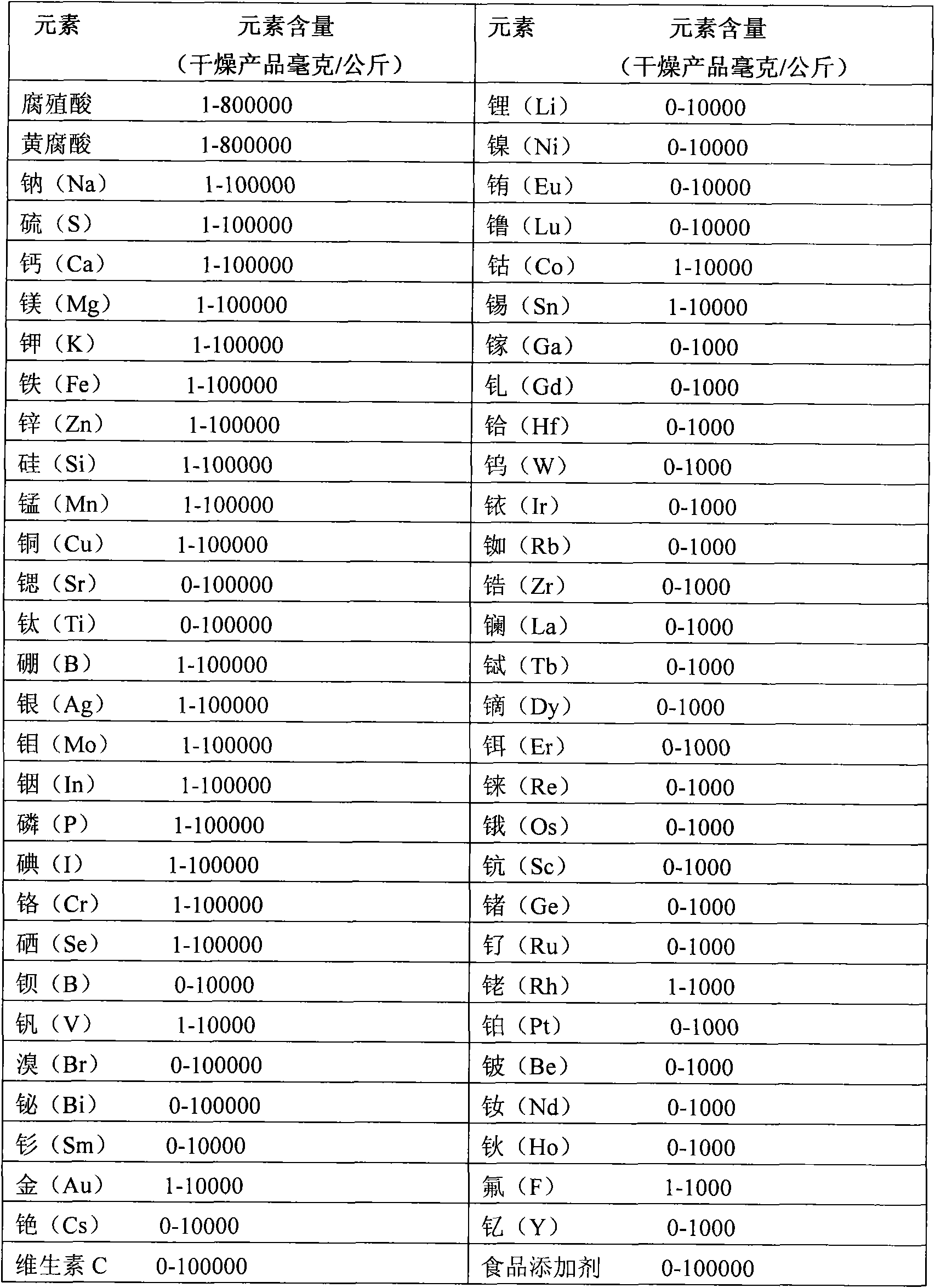
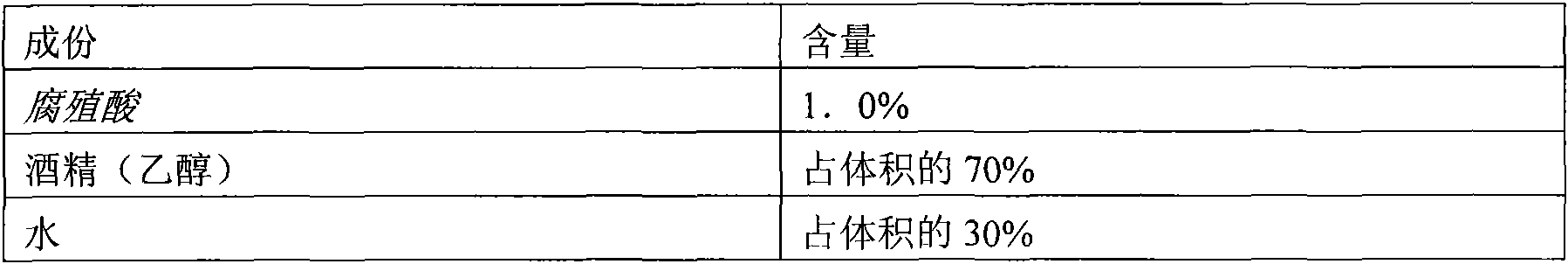
The invention discloses a method for extracting humic acid and fulvic acid from humus, which comprises the following steps: adopting the humus containing mineral substances as a natural source; preparing an alkaline extractant, the pH of which is between 8 and 13; and then, according to the total weight of extracting liquid, computing and adding the humus containing the mineral substances, whereinthe weight percentage of the humus is 10w / w%-30w / w%; stirring the liquid at the temperature of 30 DEG C-80 DEG C; after a determined time and when the liquid is stirred to reach a determined temperature, adding 50w / w% of hydrogen peroxide (H2O2), the weight percentage of which is 2%w / w%-8%w / w%, and stirring continuously; and after adding the hydrogen peroxide, carrying out solid-liquid separationand purification on the extracting liquid to obtain clear extracting liquid. The humic acid and the fulvic acid and the organic salts of the mineral compounds thereof obtained by the method of the invention can be widely applied to the application of the humus in agriculture, veterinary science, environmental protection, industrial additive, medicine and food additive, thereby having positive practical significance to nutrition supplement, nutrition absorption and health of human beings.
Owner:唐毅
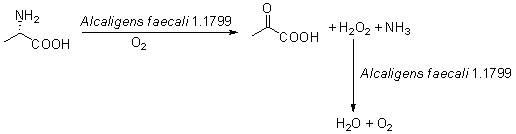
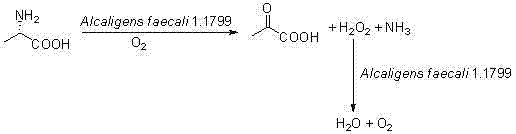
Biocatalysis method for preparing pyruvic acid from L-alanine
ActiveCN102586343ASimple separation processHigh chemical purityMicroorganism based processesFermentationL-amino-acid oxidaseAmmonia
The invention provides a biocatalysis method for preparing pyruvic acid from L-alanine. The method comprises the steps of: carrying out permeability treatment on Alcaligens faecalisl.1799 cells; catalyzing oxidative deamination of L-alanine by utilizing L-amino acid oxidase in the cells in the presence of air to form pyruvic acid, ammonia and hydrogen peroxide; catalyzing the hydrogen peroxide formed in the reaction by utilizing catalase in the cells to realize pyruvic acid accumulation without producing by-products. The method provided by the invention has the advantages of low cost, high product yield and purity and environment friendliness, and is suitable for industrial production of pyruvic acid.
Owner:CHONGQING UNIV OF POSTS & TELECOMM +1
Method for compounding epoxy fatty acid ester with unsaturated fatty acid ester
InactiveCN102875492AHigh selectivityHigh yieldOrganic chemistryChemical recyclingEpoxyUnsaturated fatty acid ester
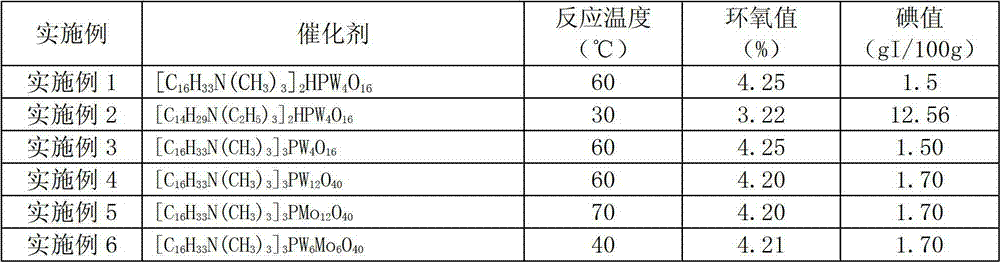
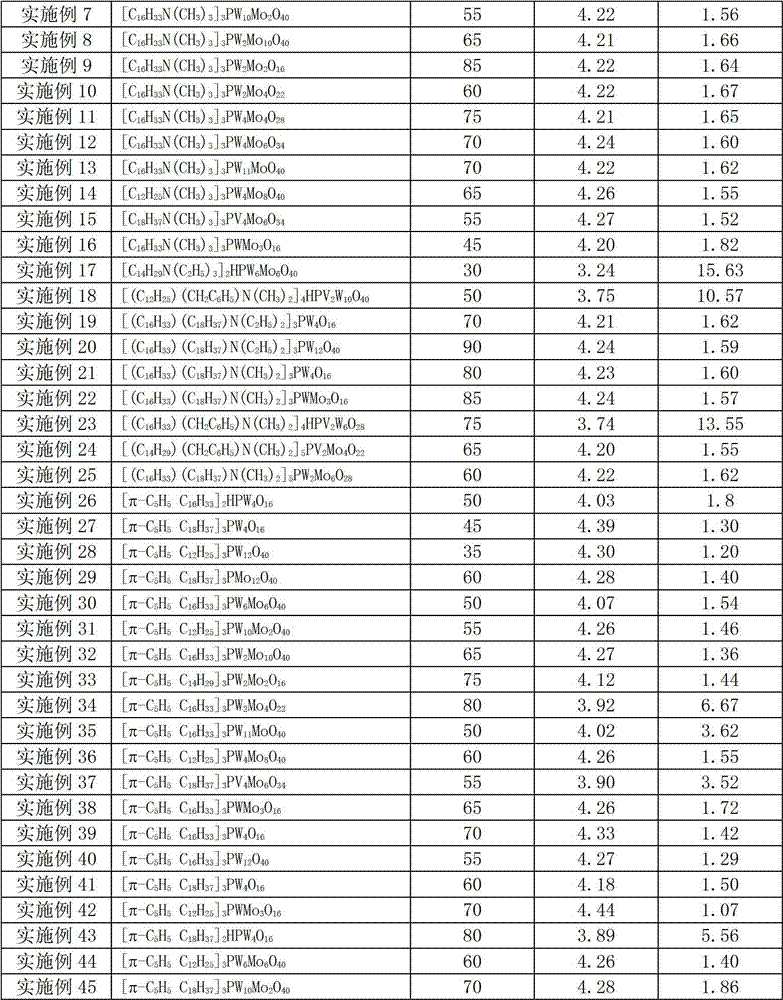
The invention provides a method for compounding epoxy fatty acid ester with unsaturated fatty acid ester. The method is characterized in that heteropoly acid metal compounds are used as catalyst, and hydrogen oxide aqueous solution is used as an oxygen source. When no other organic solvent or organic peroxide acid exists, the unsaturated fatty acid ester is oxidized selectively to compound epoxy fatty acid ester, wherein the unsaturated fatty acid ester is unsaturated fatty acid methyl ester, unsaturated fatty acid ethyl ester, unsaturated fatty acid acrylic ester or unsaturated fatty acid butyl ester; and the structural formula of the catalyst is as follows: QmHnPM(I)xM(II)yO4+3(X+Y). According to the invention, the epoxy fatty acid ester can be obtained with low cost, high productivity and high selectivity; all the epoxide number, the iodine number and the acid number meet the requirement; the stability of the product is high; and the catalyst can be recycled.
Owner:DALIAN POLYTECHNIC UNIVERSITY
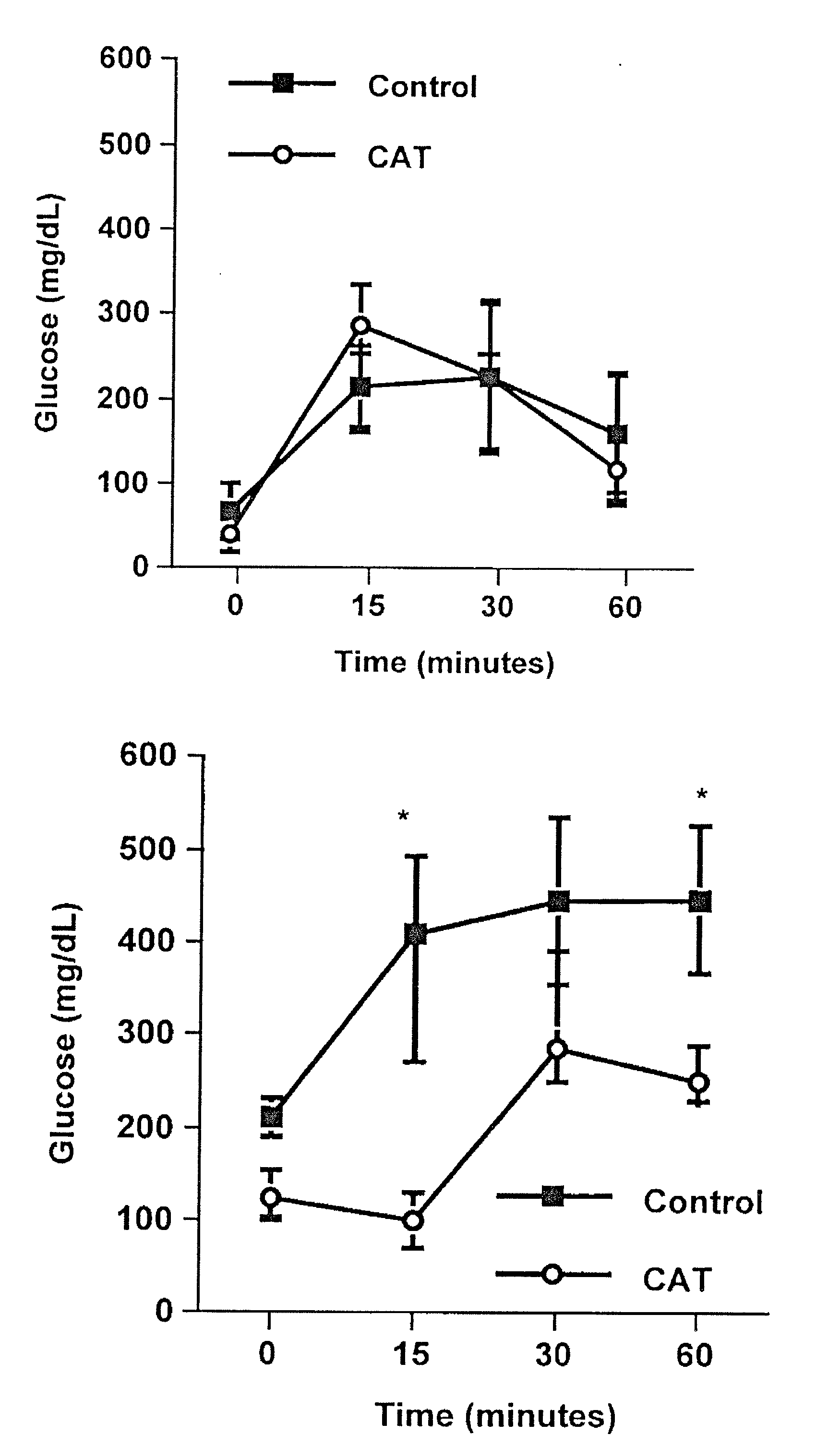
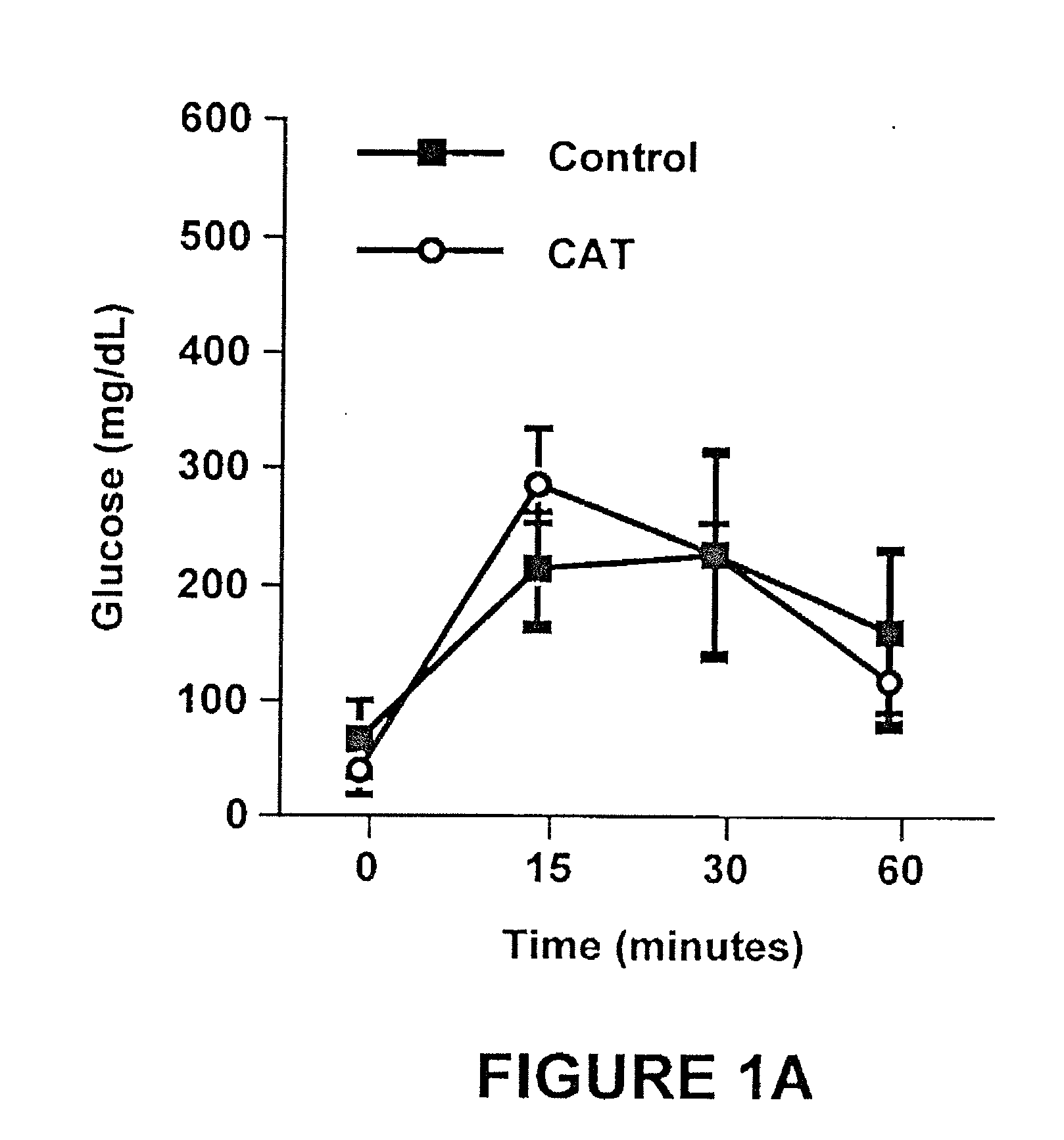
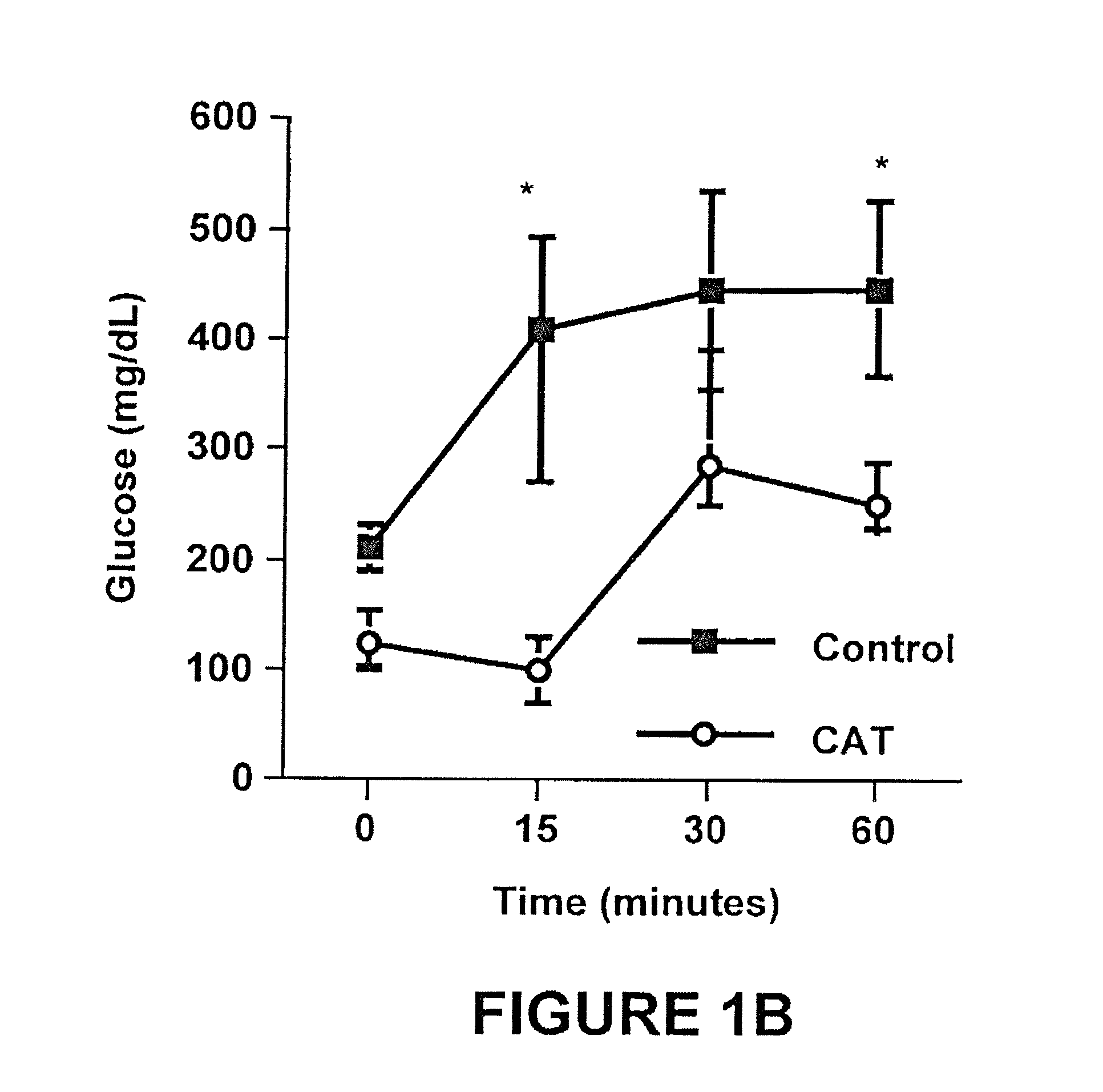
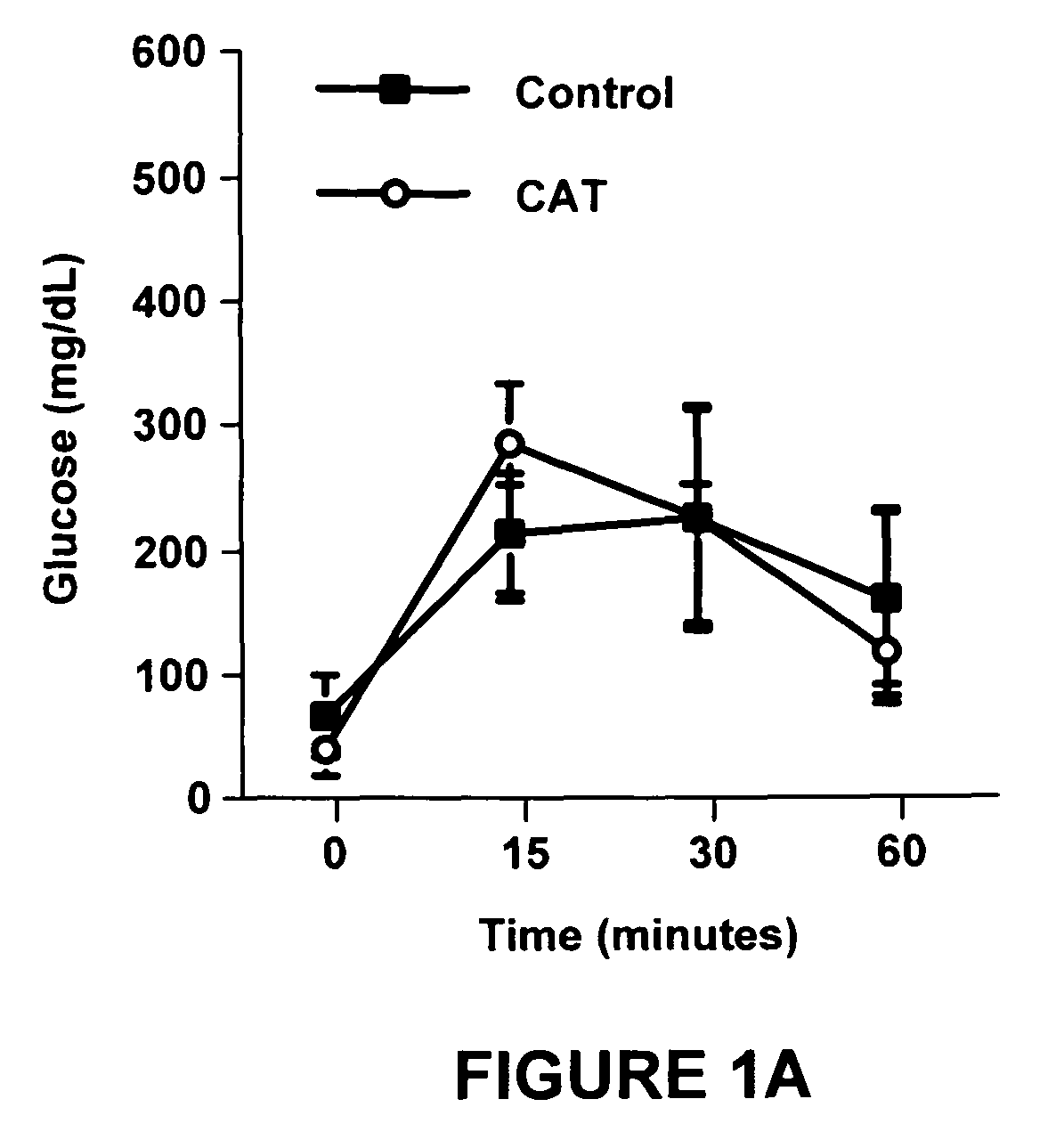
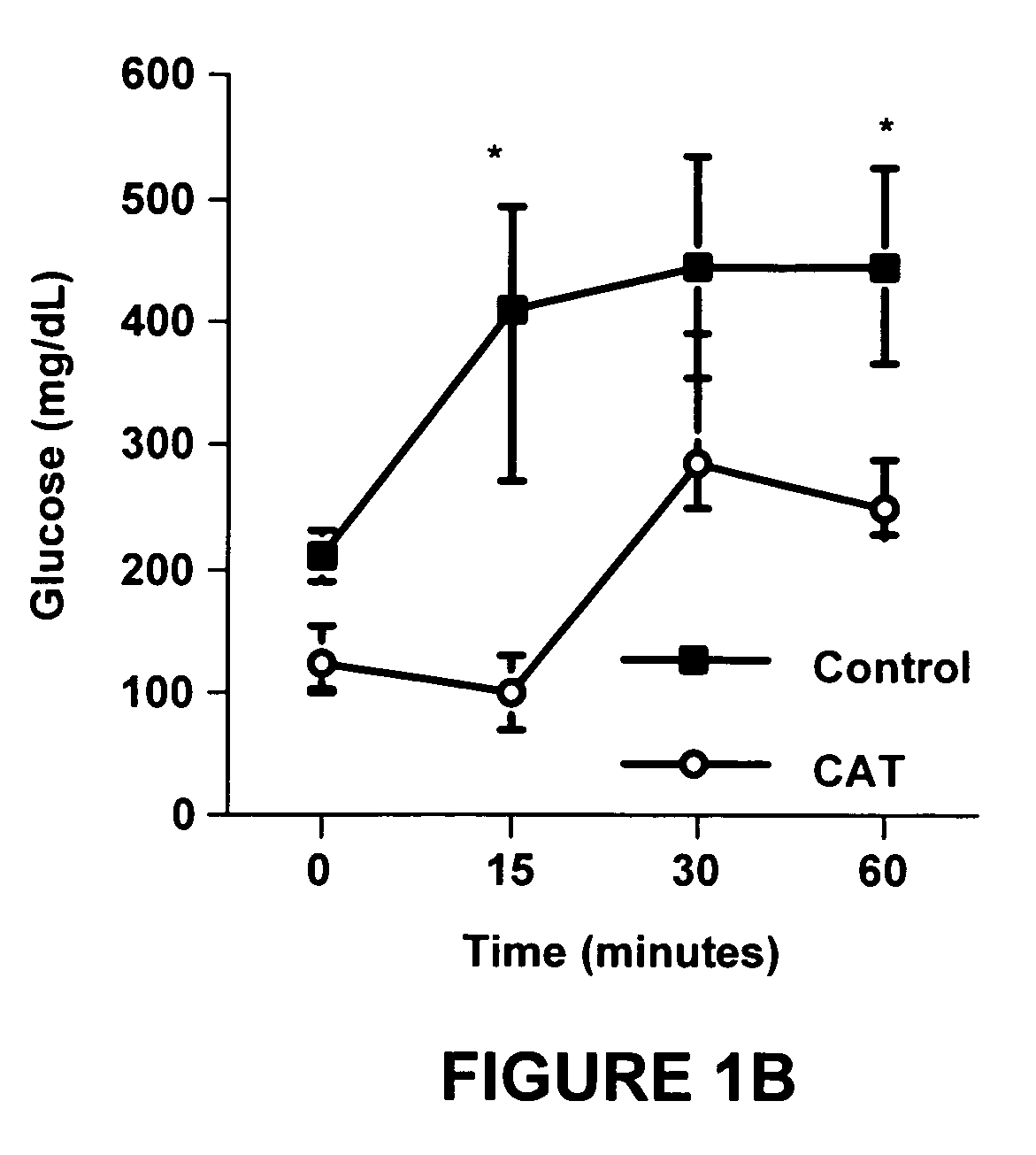
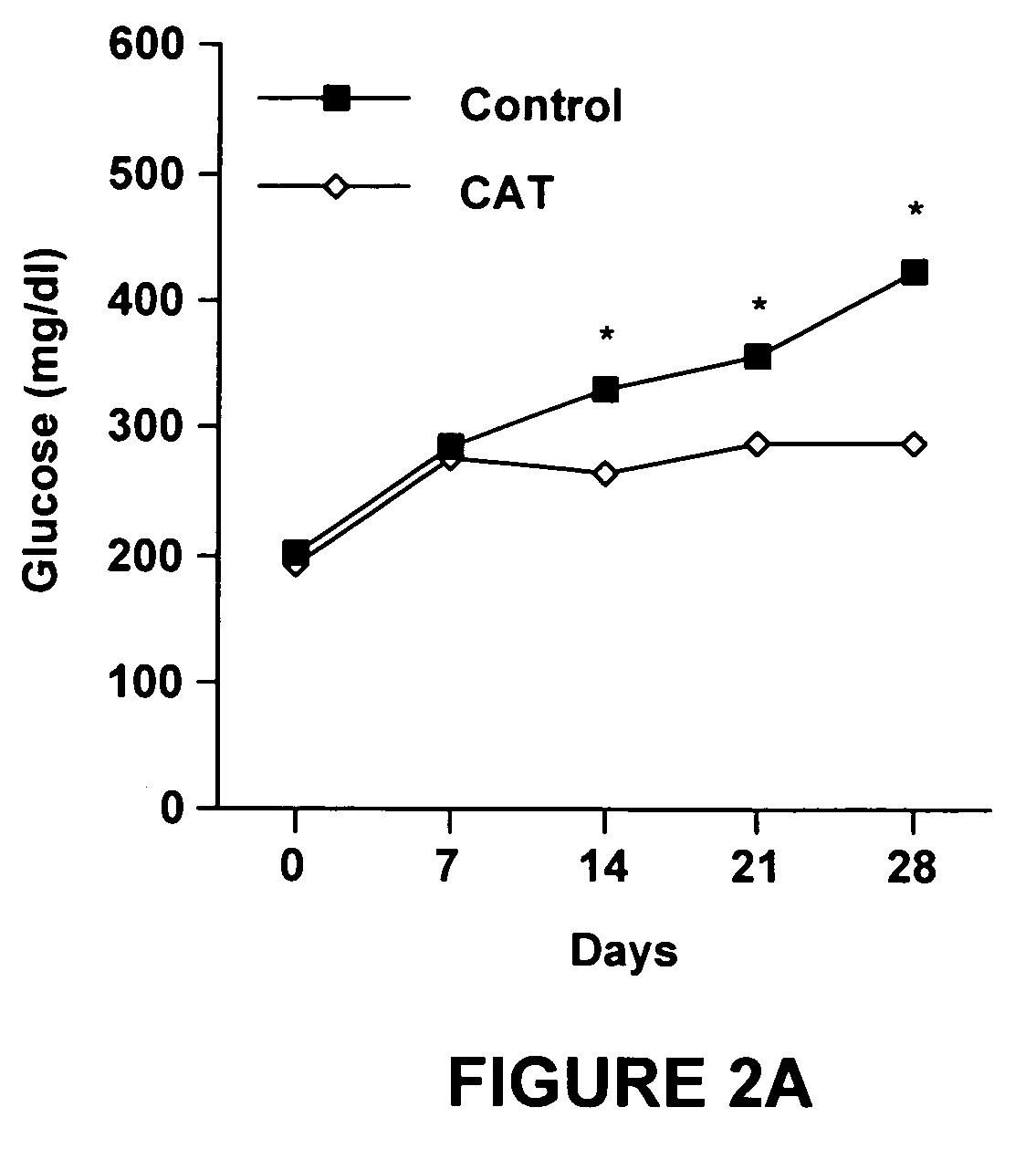
Method of using catalpic acid to treat and prevent type 2 diabetes and associated disorders
ActiveUS20060160898A1Impaired glucose tolerancePrevent hyperglycemiaBiocideMetabolism disorderDiseaseObesity
A method of treating and preventing type 2 diabetes and obesity an animal, including mammals and humans, in which a therapeutically effective amount of catalpic acid to the animal is administered orally or parentally.
Owner:NUTRITION THERAPEUTICS
Hydrogen peroxide stabilizing agent in Chinese red pine paper-pulp bleaching process
InactiveCN101429744AReduce Catalytic DecompositionReduce decomposition rateBleaching agents additionFiberEthylenediamine
The invention provides a hydrogen peroxide stabilizer used in the masson pine paper pulp bleaching process, which comprises the following raw materials in weight percentage: 5 to 10 percent of ethylenediamine tetraacetic acid, 5 to 7 percent of polyacrylic acid, 5 to 10 percent of diethylene triamine pentacetic acid, 5 to 10 percent of hydroxyl ethidene bis phosphoric acid, proper amount of sodium hydroxide, and the balance being deionized water. The preparation method comprises the following steps: the ethylenediamine tetraacetic acid, the polyacrylic acid, the diethylene triamine pentacetic acid and the hydroxyl ethidene bis phosphoric acid are mixed according to the ratio, added with the sodium hydroxide to adjust the pH value to between 6.0 and 6.5, and added with the deionized water until the blending ratio is 100 percent. The hydrogen peroxide stabilizer used in the masson pine paper pulp bleaching process can be used under the conditions of low alkalinity, medium alkalinity and high alkalinity, chelate metallic ions of manganese, iron and the like make the rate of dissolution of the stabilizer less than 15 percent; when the stabilizer is used for bleaching at a temperature of between 60 and 75 DEG C, the optical brightness value ISO of the masson pine paper pulp is more than 65; moreover, the stabilizer can avoid the fouling of system fibers and gluing matters.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Mixture containing fatty acid ethyl sulfonate and preparation method thereof
The invention relates to a mixture with aliphatic acid carbethoxy sulfonate salt, and relative production, wherein said mixture comprises 90-98 deals of aliphatic acid sulfonate salt, 1-4 deals of aliphatic acid sulfonate salt, 0.5-4 deals of sulfate, 0.1-2 deals of fatty acid ester, 0.3-3 deals of ethylsulfuric acid salt, and 1-50 deals of water. The production comprises that: 1, sulfonating the fatty acid ester and sulfur trioxide at 1:1-1:1. 1 mol ratios; 2, adding alcohol into mixture to be aged; 3, adding auricome into mixture to poach; 4, adding extractive into the mixture to extract; 5, adding alkali metal mixture into mixture to be neutralized to obtain the mixture. The inventive production is safe while the product purity is higher than 90%.
Owner:谢仁华
Mixture containing fatty acid ethyl ester sulfonate and preparation method thereof
The invention relates to a mixture with aliphatic acid carbethoxy sulfonate salt, and relative production, wherein said mixture comprises 90-98 deals of aliphatic acid sulfonate salt, 1-4 deals of aliphatic acid sulfonate salt, 0.5-4 deals of sulfate, 0.1-2 deals of fatty acid ester, 0.3-3 deals of ethylsulfuric acid salt, and 1-50 deals of water. The production comprises that: 1, sulfonating the fatty acid ester and sulfur trioxide at 1:1-1:1. 1 mol ratios; 2, adding alcohol into mixture to be aged; 3, adding auricome into mixture to poach; 4, adding extractive into the mixture to extract; 5, adding alkali metal mixture into mixture to be neutralized to obtain the mixture. The inventive production is safe while the product purity is higher than 90%.
Owner:谢仁华
Process for double-enzyme coupling-chemical synthesis of epsilon-caprolactone
InactiveCN106701859AHigh activityHigh selectivityImmobilised enzymesHydrolasesHigh concentrationChemical synthesis
The invention relates to a process for double-enzyme coupling-chemical synthesis of epsilon-caprolactone; hydrogen peroxide generated in situ from GOD oxidation of glucose by a GOD / CALB or GOD / ROL double-enzyme cross-linked enzyme aggregate is used as an oxidant, at the same time, peroxy acid is generated in situ through catalyzing oxidation of an acyl donor by CALB or ROL in the cross-linked enzyme aggregate, and then epsilon-caprolactone (epsilon-CL) is generated through oxidation of cyclohexanone by peroxy acid. The yield of the epsilon-caprolactone reaches 97% or more, and the selectivity of the epsilon-caprolactone is 100%. The process reduces enzyme inactivation caused by too high concentration of hydrogen peroxide, avoids an easily explosiveness risk possibly caused by direct addition of peroxy acid, reduces the influence of decrease of enzyme micro-environment pH on the enzyme activity, and can reduce the inhibition of a substrate on an enzyme.
Owner:QINGDAO UNIV OF SCI & TECH
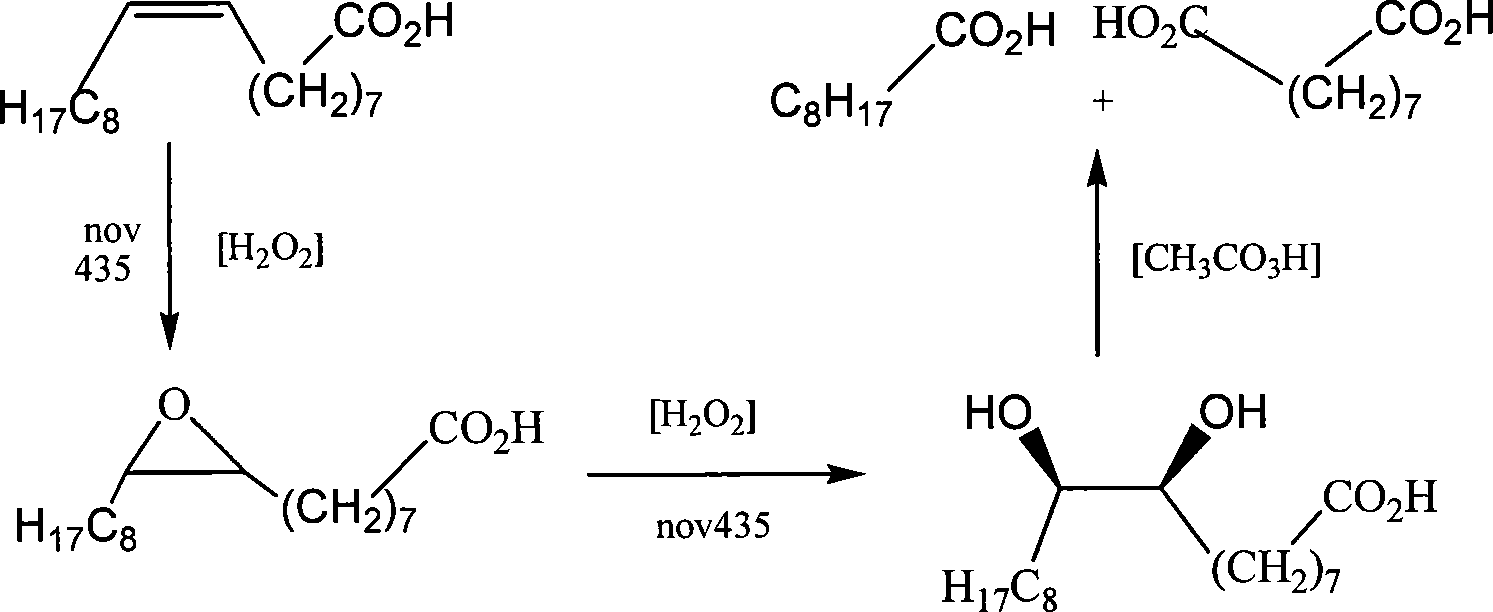
Method for preparing azelaic acid by enzyme catalysis of hydrogen dioxide oxygenated oleic acid
The present invention relates to a method of preparing for nonane diacid by an enzymatic catalysis oxidation system. The method is that oleic acid, lipase and solvent are stirred and mixed uniformly; hydrogen peroxide solution is dripped to react at the room temperature and to be filtered to recover catalyst; the solvent is recovered by rotary vaporization; ethyl acetate is used for recrystallizing to obtain 9, 10-dihydroxyl stearic acid; acetic acid peroxide solution is added into an oil phase of the 9, 10-dihydroxyl stearic acid to be extracted, filtered and dried to obtain the product of the nonane diacid. The highest yield of the nonane diacid can be 60 percent to 70 percent (counted according to the oleic acid). The method of the present invention has clean technology and friendly environment and does not pollute the environment; the first step of the epoxidation does not need heating, adding high-concentration hydrogen peroxide and noble metal catalyst and does not need being provided with oxygen and ozone for long time; no high pressure is required during the reaction; the lipase can be recycled for repeating use after the reaction; the implementing condition is simple, which is convenient for the large scale production.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
A harmless extraction process for preparing agricultural biochemical fulvic acid from livestock and poultry manure
ActiveCN102816332BTo achieve the extraction effect of separationEliminate hidden dangersOrganic chemistryFertilizer mixturesAntibiotic YFermentation
The invention relates to a harmless extraction process for preparing agricultural biochemical fulvic acid from livestock and poultry manure. The process includes an acid-soluble extraction process of fulvic acid and a Fenton oxidation degradation process. The process of acid-soluble extraction of fulvic acid is to combine the acid-soluble extractant of group A containing 0.1%~2.5% ferrous sulfate, pH value ≤ 2, and 1%~2.5% sulfuric acid with fermented livestock and poultry manure at a ratio of 1:5~15 The proportion of the solution is mixed into a suspension solution, stirred at room temperature for 30min~60min, the dissolved fulvic acid contains a chelate of organic acid and ferrous ion; the Fenton oxidation degradation process is to extract the material through acid dissolution, and Value ≤ 2, containing 15% hydrogen peroxide and B group acid soluble extractant containing 1% ~ 2.5% sulfuric acid mixed, the control material contains 0.5% ~ 3.0% hydrogen peroxide, after the two groups of materials are mixed, chlorine peroxide is Under the catalysis of ferrous ions, the Fenton oxidation degradation reaction is generated, and the residual antibiotics, hormones, pesticides, and pathogenic bacteria, decay-causing bacteria, and parasite eggs are rapidly degraded to achieve the effect of harmless extraction.
Owner:FUJIAN CHAODA GROUP
Epoxy linseed oil and citric acid compound plasticizer
The invention discloses an epoxy linseed oil and citric acid compound plasticizer. The epoxy linseed oil and citric acid compound plasticizer is characterized by comprising citric acid, n-butyl alcohol, sulfuric acid, linseed oil, petroleum ether, formic acid, hydrogen peroxide, catalyst, sodium hydroxide solution, acetic acid and acetic anhydride, wherein the weight part ratio of the citric acid, the n-butyl alcohol and the sulfuric acid is 1 to (1 to 2) to 0.01; the molar ratio of the citric acid and the acetic anhydride is 1 to 2.5; the mass part ratio of the linseed oil, the petroleum ether, the formic acid, the hydrogen peroxide and the catalyst is 10 to 3 to 5 to 13 to (0.2 to 0.6); the molar ratio of the linseed oil, the sodium hydroxide solution and the acetic acid is 1 to (0.5 to 1.5) to (1 to 3). The epoxy linseed oil and citric acid compound plasticizer has the advantages that by adopting ether bonds, compared with traditional citric easters plasticizer, the compatibility is better, and the easiness in separation is avoided; by combining epoxy linseed oil and citric easters compound plasticizer, the property is excellent.
Owner:成都米特瑞新材料科技有限公司
Treatment method of N-p-aminobenzoyl-L-glutamic acid medical intermediate wastewater
InactiveCN110330153AGuaranteed separation effectEasy to operateMultistage water/sewage treatmentWater/sewage treatment by neutralisationWater solubleAqueous solution
The invention discloses a treatment method of N-p-aminobenzoyl-L-glutamic acid medical intermediate wastewater, and belongs to the technical field of pharmaceutical wastewater treatment. The treatmentmethod comprises the following steps: 1, adjusting pH: adjusting the pH of raw wastewater to 2-6 to obtain wastewater A; 2, performing Fenton oxidation: adding a mixture of water-soluble ferrous saltand an aqueous hydrogen peroxide solution or the aqueous hydrogen peroxide solution into the wastewater A to obtain wastewater B, performing solid-liquid separation to obtain a supernatant of the wastewater B; 3, performing coagulating sedimentation: adjusting the pH of the wastewater B to 7.5-9, adding a flocculant to obtain a flocculent precipitate and wastewater C; and 4, performing electrocatalytic oxidation: sending the wastewater C to an electrocatalytic oxidation system and discharging water to obtain purified water. By the treatment method, treatment of the wastewater is achieved andthe wastewater is discharged after standards are met.
Owner:杭州胜于蓝环保科技有限公司
Oxidizing system containing hydrogen peroxide, acid/peracid, stabilizer, amine oxide and essential oil, composition comprising such a system and use in the field of disinfection
The present invention relates to, as a novel industrial product, an aqueous system usable in the field of disinfection, said system, which comprises hydrogen peroxide, acids, an amine N-oxide substance and an essential oil component, being characterized in that: (1) it is homogeneous and consists of a single phase, and (2) said essential oil component is chosen from the collection consisting of (a) water-soluble essential oils, (b) terpinen-4-ol, and (c) mixtures thereof. The invention also relates to a process for preparing such a system.
Owner:SILVER INTERVEST
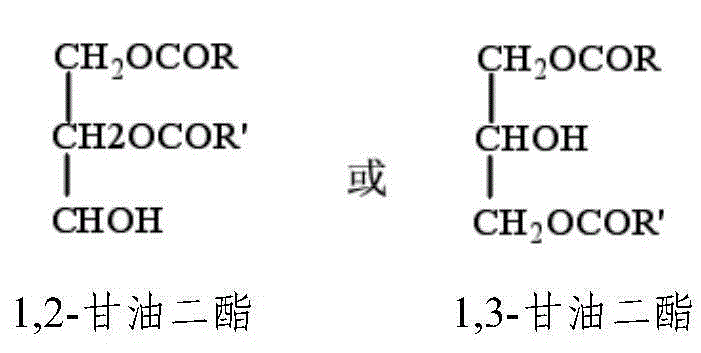
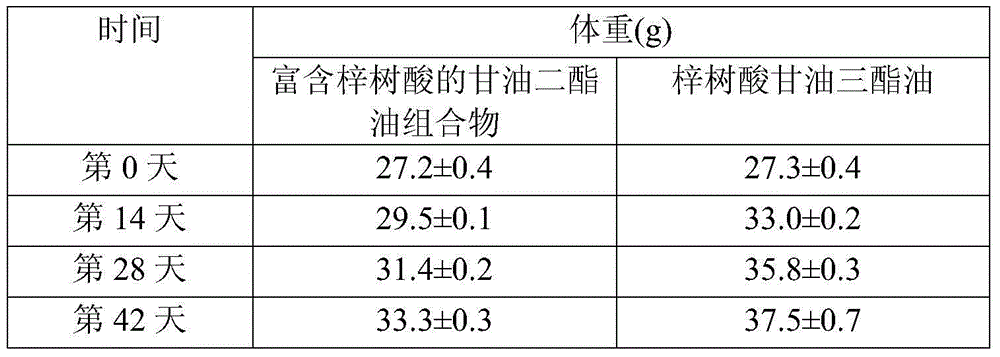
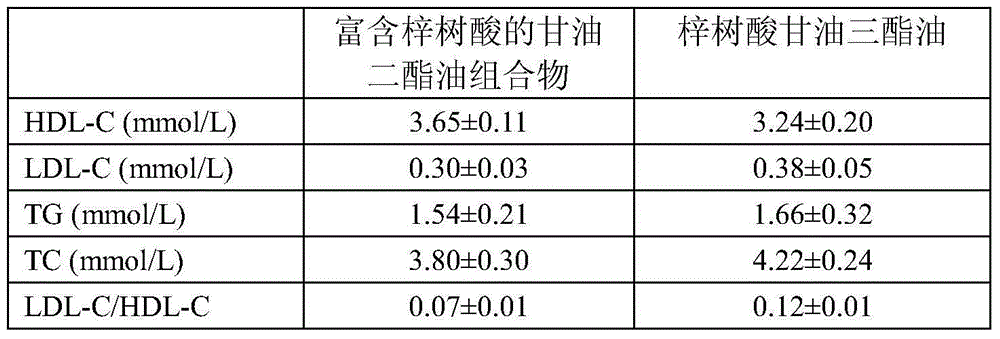
Diglyceride oil composition rich in catalpic acid and preparation method and application thereof
InactiveCN106135902ARich sourcesImprove food safetyOrganic active ingredientsOxygen-containing compound preparationMonoglycerideTriglyceride
The invention relates to a diglyceride oil composition rich in catalpic acid; the diglyceride oil composition is composed of the following components according to the mass percentage: 50-90% of diglyceride, 9-45% of triglyceride, 0.1-10% of monoglyceride and 0.02-10% of residues; in the total glycerides composed of monoglyceride, diglyceride and triglyceride, the mass ratio of catalpic acid to fatty acid is (0.2-0.8):1, and the residues comprise free fatty acid and glycerol. The invention also relates to a preparation method of the composition and an application of the composition in functional food and drugs for controlling weight, improving blood lipid and increasing the immunity. The diglyceride oil composition rich in catalpic acid not only has an anti-obesity efficacy of diglyceride but also has anti-inflammatory and anti-obesity efficacies of catalpic acid, so a large number of the composition can be applied in the functional food and drugs for controlling weight, improving blood lipid and increasing the immunity; and in short, the preparation process of the composition is simple, is low in cost, and has broad application prospects.
Owner:ZHEJIANG OCEAN UNIV
Fresh-keeping method of living Chinese toon buds through salicylic acid solution treatment
InactiveCN110140760ADecreased vitamin C contentAvoid damageFruit and vegetables preservationSolution treatmentVitamin C
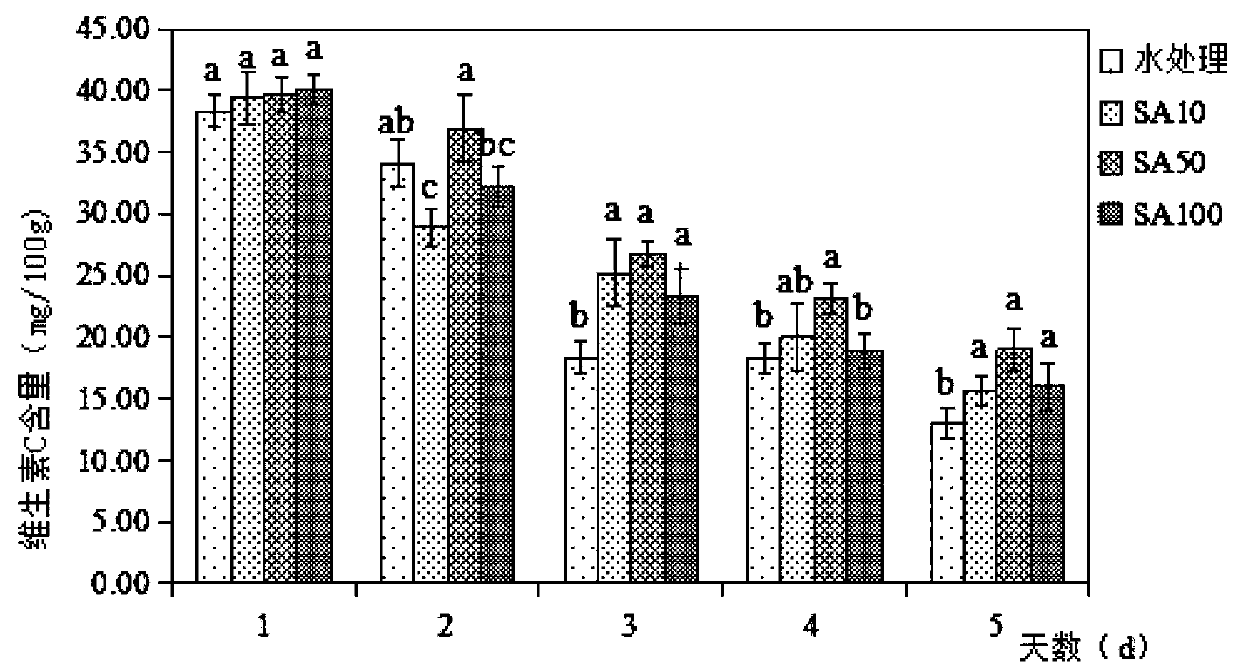
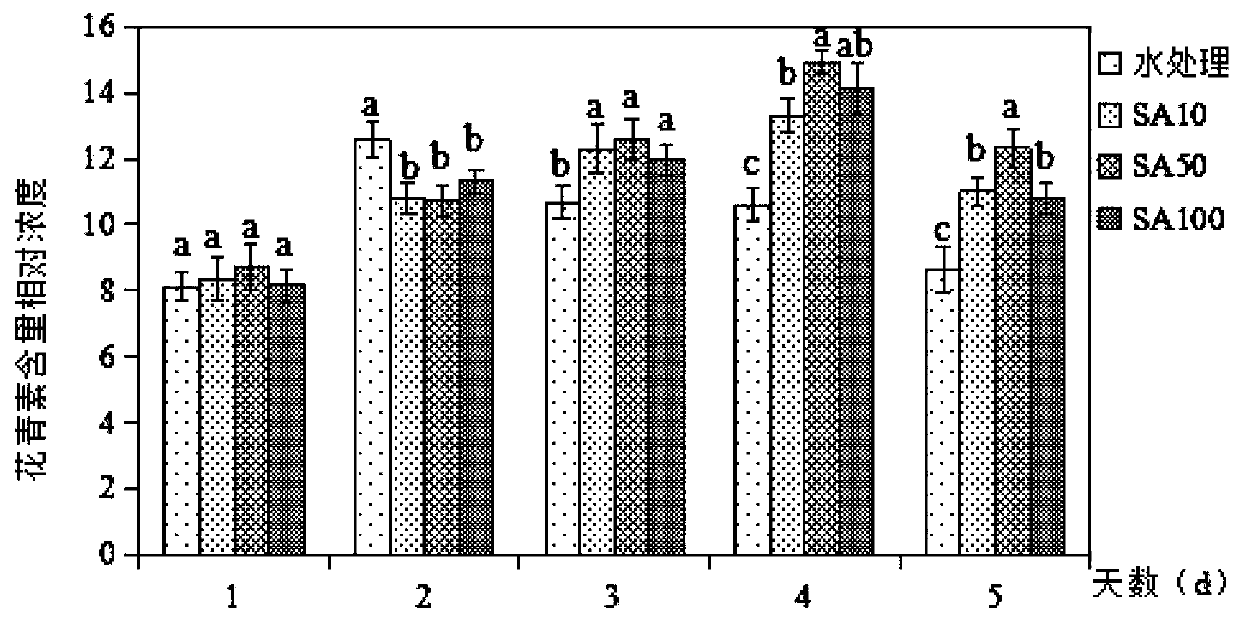
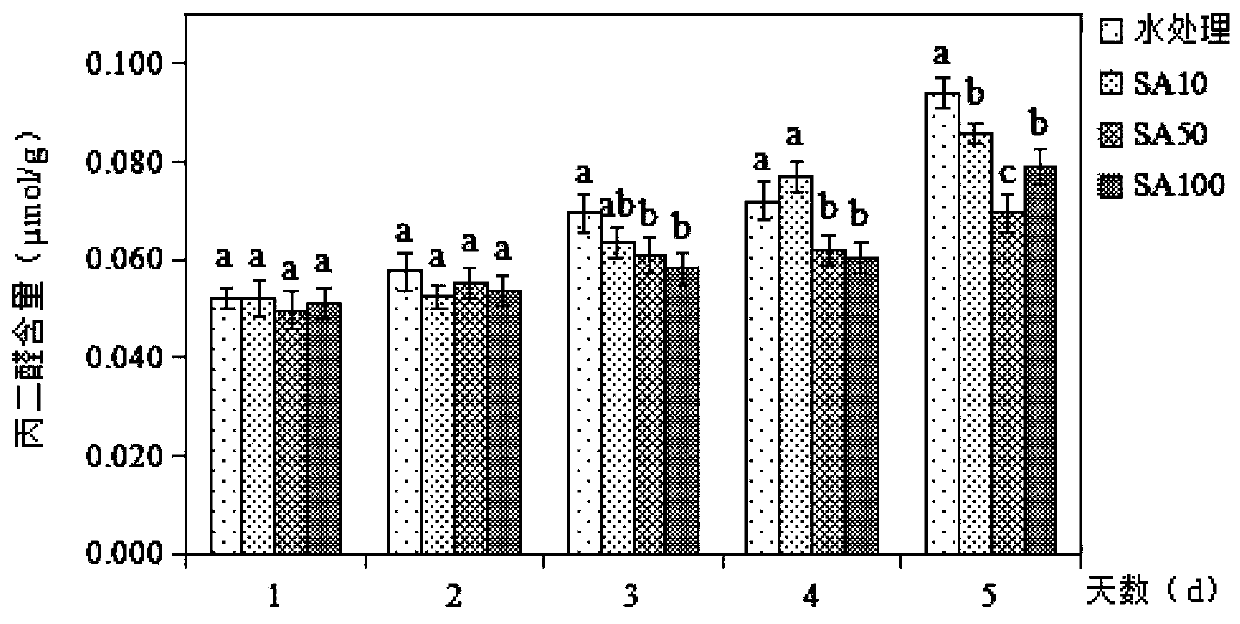
The invention discloses a fresh-keeping method of living Chinese toon buds through salicylic acid solution treatment. The method comprises the following specific steps: washing Chinese toon buds withrunning water, airing the buds in a shade place, soaking the buds in 84 disinfectant for half an hour, taking out the buds, and airing the buds in a shade place; and selecting 2000ml of 10-100 [mu]mol / L salicylic acid solution, soaking sterilized Chinese toon buds in the solution for 20 minutes, taking out and airing the Chinese toon buds, and storing the treated Chinese toon buds in a refrigerator at 4-8 DEG C. After being picked, the tender Chinese toon buds can age and brown along with the storage time. After being treated by salicylic acid, the content of vitamin C in the Chinese toon decreases, the content of anthocyanin firstly rises and then decreases, and the salicylic acid reduces the damage of the Chinese toon in the storage process, well maintains the nutritional quality of theChinese toon, and controls the increase of the content of malondialdehyde and hydrogen peroxide.
Owner:FUYANG NORMAL UNIVERSITY
Pseudoalteromonas sp.B3 and application thereof in biological oxidation of L-amino acid
ActiveCN102367430BSubstrate Broad SpectrumMild responseBacteriaMicrobiological testing/measurementMicroorganismL-amino-acid oxidase
Owner:菏泽建数智能科技有限公司
Reagent (kit) for diagnosing/determining amino acid and method for determining concentration of amino acid
InactiveCN101750324AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryAlanine dehydrogenase
The invention relates to a reagent (kit) for diagnosing / determining amino acid by using an enzyme multiplication method, an enzyme colorimetric method and an enzyme link method, and also discloses a method for determining the concentration of the amino acid, a composition and components of the reagent, belonging to the technical field of medicine / food / environmental test determination. The reagent (kit) comprises the main components: a buffer solution, a coenzyme, pyruvic acid, hydrogen peroxide, an amino acid oxidase, a glycine oxidase, an alanine dehydrogenase and a stabilizing agent. A sample and the reagent are mixed according to a certain volume ratio to generate a series of enzymatic reactions, and reactants are placed under an ultraviolet / visible light analyzer for detecting the speed of absorbance rise at the dominant wavelength of 340 nm, thereby measuring and calculating the concentration of the amino acid.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
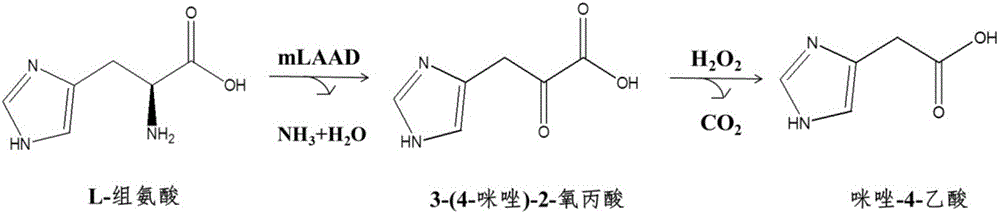
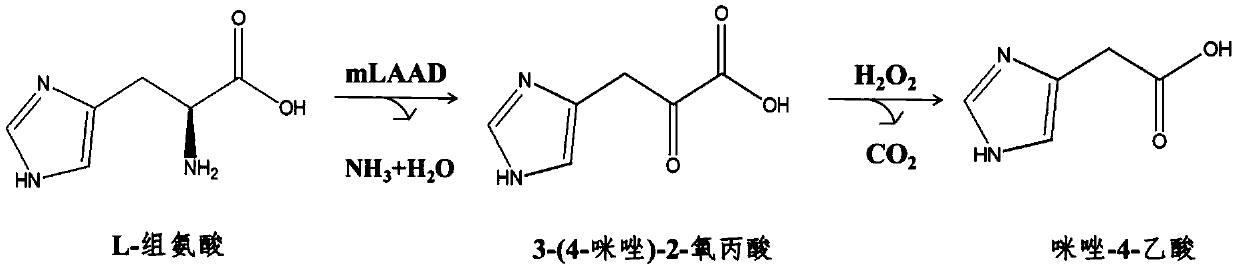
Biosynthesizing method of imidazole-4-acetic acid
ActiveCN107177642AWide variety of sourcesEasy to manufactureMicroorganism based processesFermentationPropanoic acidHistidine
The invention discloses a biosynthesizing method of imidazole-4-acetic acid. The biosynthesizing method comprises the following steps of using L-histidine as a substrate, and performing deaminizing reaction under the catalyzing action of membrane-linked L-amino acid deaminases, so as to obtain reaction liquid containing an intermediate product, namely 3-(4-imidazole)-2-oxo propionic acid; adding a hydrogen peroxide solution into the reaction liquid, and reacting, so as to obtain the imidazole-4-acetic acid. The biosynthesizing method has the characteristics that the steps are simple, the reaction conditions are mild, the sources of raw materials are wide, the preparation of a biological catalyst is easy, the requirement on equipment is low, and the conversion rate of the substrate is high; the organic solvent and the toxic reagent are not needed; compared with the existing chemical method, the efficiency is high, the cost is low, and the environment-friendly effect is realized.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
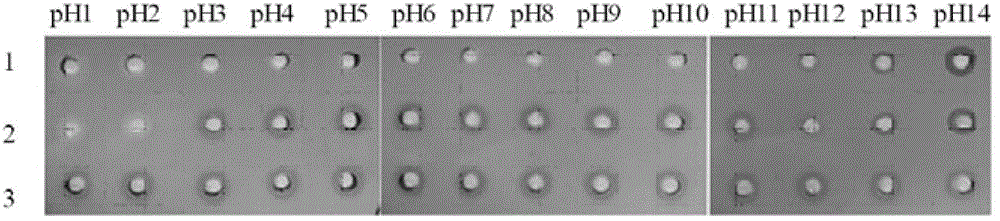
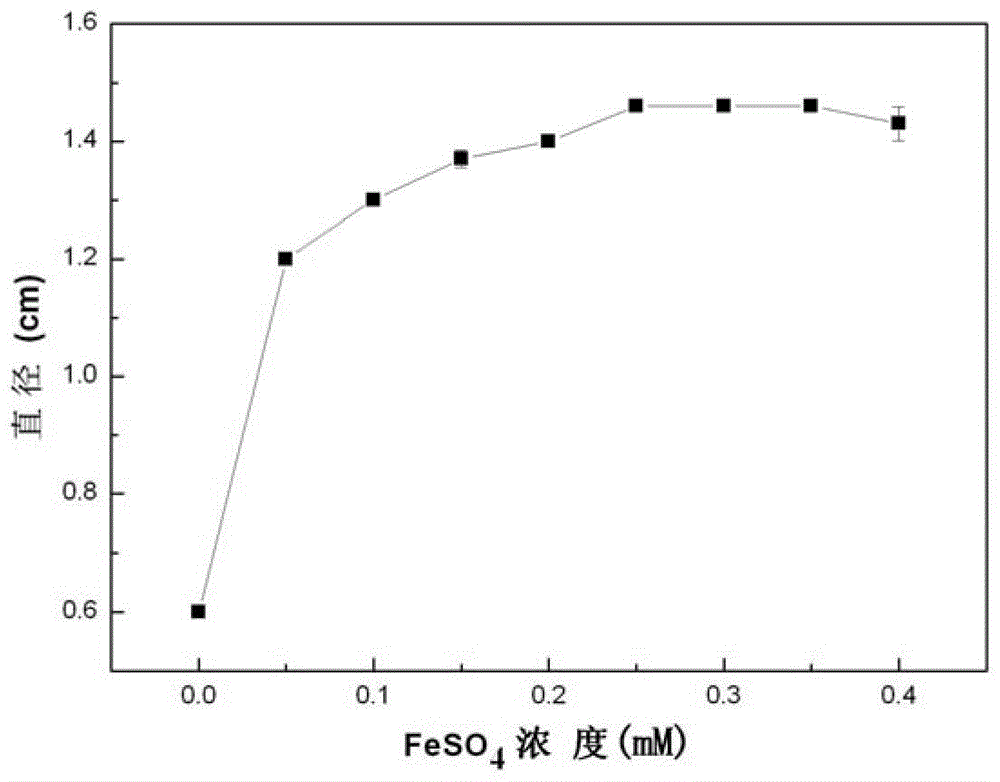
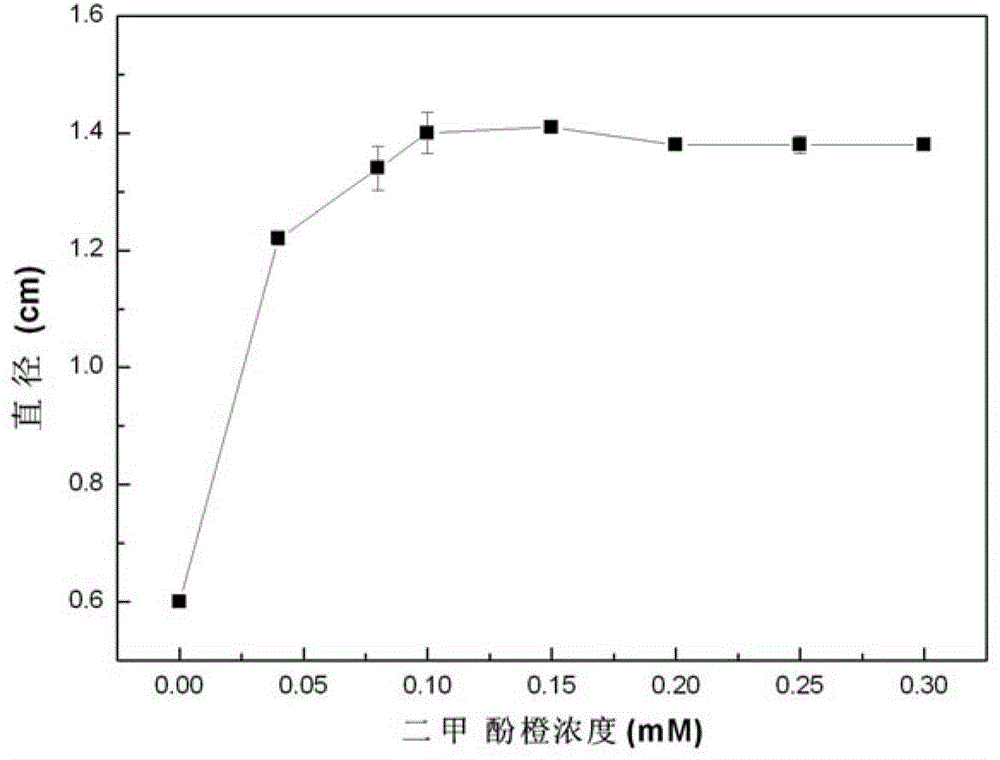
A kind of quantitative detection method of L-amino acid oxidase activity
ActiveCN103361397BHigh sensitivityEasy to operateMicrobiological testing/measurementPurplish redRoom temperature
The invention provides a method for quantitatively detecting the activity of L-amino acid oxidase by utilizing a xylenol orange agar flat plate. The method comprises the following steps of: punching small holes with the diameter of 4-10mm in the xylenol orange agar flat plate by using a hole puncher, then adding a solution to be detected into the small holes, standing at room temperature for 20-100min, determining the diameter of a purple-red circle, determining the concentration of hydrogen peroxide in the solution to be determined by a standard straight line made according to a log value of the concentration of the hydrogen peroxide and the diameter of the purple-red circle, and further determining the activity of the L-amino acid oxidase, wherein the solution to be detected takes an enzyme solution to be detected of the L-amino acid oxidase as an enzyme source, 0.2-20mmol / L of L-leucine is taken as a substrate, the reaction is performed for 0.5-2h under the conditions that the temperature is 25-50 DEG C and the pH value is 5-9, then the hydrogen peroxide is produced, and an obtained reaction solution is diluted to obtain the solution to be detected. The method provided by the invention has the characteristics of very high sensitivity, low cost, simplicity and convenience in operation, good stability, wide range of application, environmental friendliness and the like.
Owner:SHANGHAI BAILANG BIOTECHOLOGY
Efficient retarding and water reducing agent for highway concrete and preparation method thereof
The invention relates to the technical field of water reducing agents, in particular to an efficient retarding water reducing agent for highway concrete and a preparation method thereof. The efficient retarding and water reducing agent for the highway concrete is prepared from the following components: isobutene alcohol polyoxyethylene ether, maleic anhydride, alpha methacrylic acid, allyl trimethyl ammonium chloride, ammonium persulfate, ascorbic acid, calcium lignosulphonate, hydrogen peroxide, an acid regulator, an alkali regulator and distilled water. Through molecular design, much -COO- is introduced into molecules of the polycarboxylate superplasticizer, so that the retarding effect of the superplasticizer is enhanced, and alpha-methacrylic acid is used for replacing acrylic acid, so that the self-polymerization degree of a monomer is reduced. By adding the reducing agent ascorbic acid, the temperature of the reaction system is reduced from about 80 DEG C to room temperature, and the energy consumption is reduced under the condition that the performance is basically unchanged. By adding the modified calcium lignosulphonate, the synthesis cost of the polycarboxylate superplasticizer is greatly reduced under the condition of small performance loss.
Owner:丁一飞 +4
Salicylic acid amide derivative and preparation method thereof
Owner:SHENZHEN WANHE PHARMA
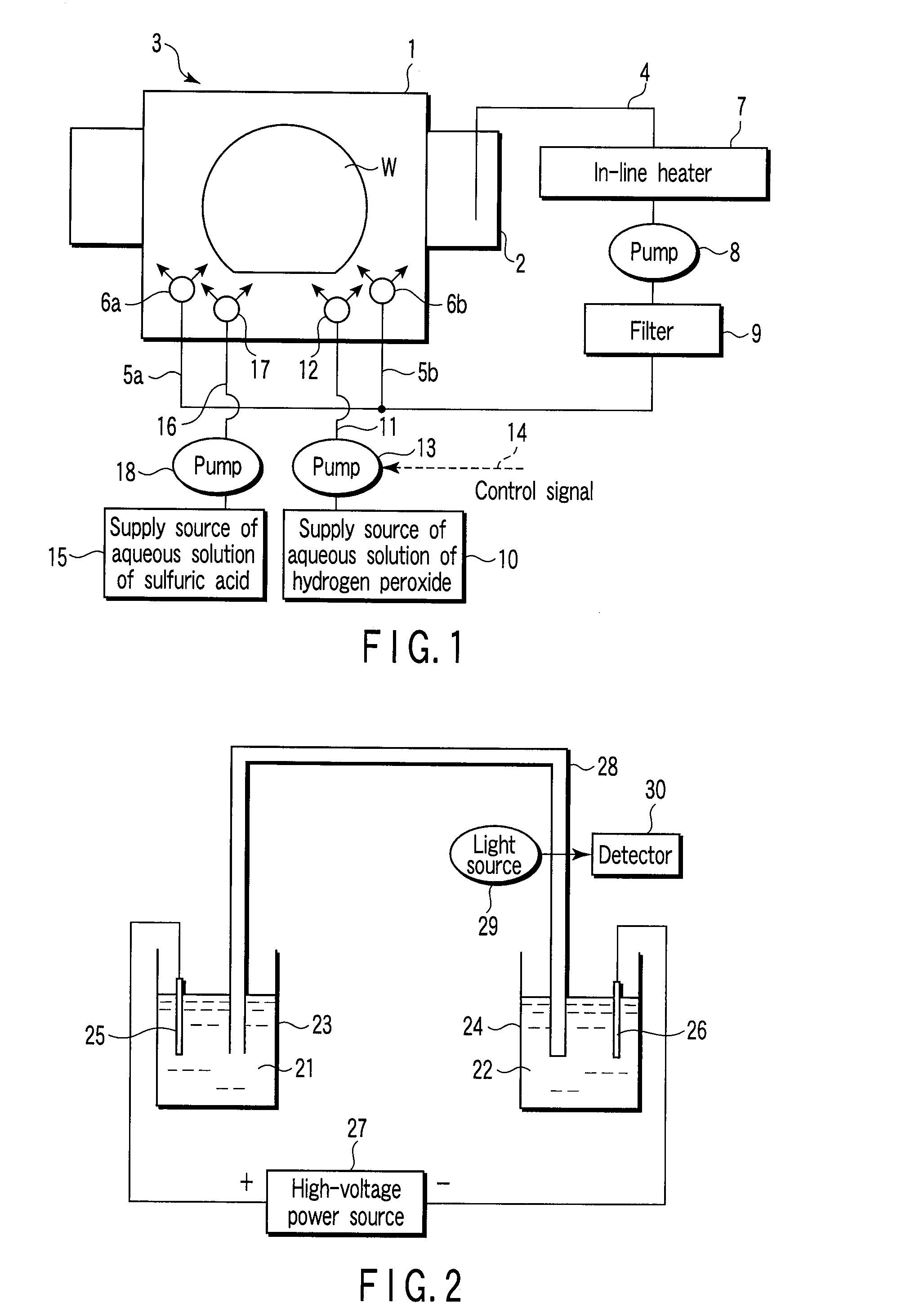
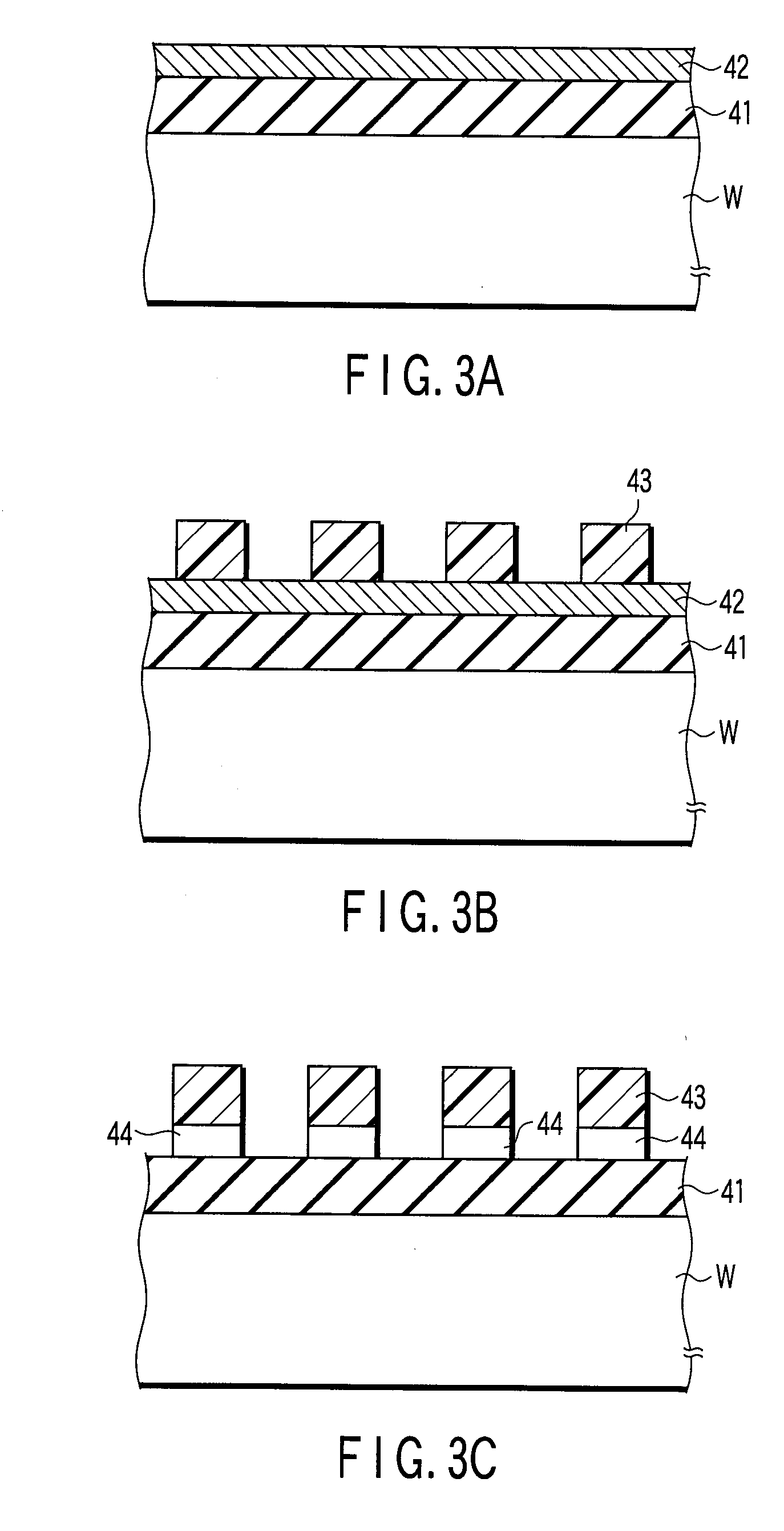
Substrate-processing method and method of manufacturing electronic device
InactiveUS20080053478A1Photomechanical apparatusSemiconductor/solid-state device manufacturingAqueous solutionChemistry
A method of manufacturing an electronic device includes dipping a substrate in a solution containing sulfuric acid, which is accommodated in a processing vessel. An aqueous solution of hydrogen peroxide supplies the sulfuric acid accommodated in the processing vessel for generating peroxomonosulfuric acid (Caro's acid). Therefore, an organic material present on the surface of the substrate is removed by the action of Caro's acid within the processing vessel. The time for supplying the aqueous solution of hydrogen peroxide into the processing vessel is set on the basis of the change with time in the concentration of Caro's acid measured in advance so as to permit the peak in the concentration of Caro's acid to appear while the substrate is kept dipped in the processing solution.
Owner:KK TOSHIBA
Method for preparing urea peroxide containing organic titanium by recrystallization process
InactiveCN102557822BPromote absorptionEasy to operateUrea derivatives preparationOrganic compound preparationTitanium chlorideTitanium citrate
The invention relates to a method for preparing urea peroxide containing organic titanium by a recrystallization process. The method is characterized in that: new metatitanic acid precipitate of citric acid is prepared by neutralizing an iced solution of titanium salt by using alkali; the new metatitanic acid precipitate instead of titanium chloride and sulfate is used as raw materials for synthesizing citricacide-titatnium chelate; the citricacide-titatnium chelate is synthesized by a microwave hot melting chelation process by using the new metatitanic acid precipitate of citric acid as a titanium source and citric acid as an acid solvent and chelant; and the novel urea peroxide fertilizer containing the organic titanium is prepared by the steps of uniformly mixing hydrogen peroxide, acid citricacide-titatnium chelate and urea in a ratio, performing addition reaction on a mixture at the constant temperature of 60 DEG C, and recrystallizing at the temperature of less than or equal to 5 DEG C. The titanium element can strongly promote plant to absorb and convey nitrogen, phosphorus, potassium, other secondary elements and trace elements; the citricacide-titatnium chelate is reacted with hydrogen peroxide easily to form a titanium peroxide compound with high stability; and the citricacide-titatnium chelate can be used as stabilizer of urea peroxide, and synergist of urea peroxide nitrogenous fertilizer.
Owner:福建超大现代农业集团有限公司
Preparation method for high purity catalpic acid and series products like ester and salt thereof
InactiveCN105085239ARich sourcesImprove food safetyOrganic active ingredientsOxygen-containing compound preparationTriglycerideTwo step
The invention relates to a preparation method for high purity catalpic acid and series products like ester and salt thereof. According to the invention, catalpa seed is used as a raw material, and catalpic triglyceride is extracted; catalpic triglyceride undergoes saponification and acidification so as to obtain catalpic acid; catalpic acid is esterified so as to obtain catalpic acid ester, or catalpic acid ester is prepared by directly esterifying catalpic triglyceride; obtained catalpic acid and catalpic acid ester are subjected to separation through two-step freezing crystallization so as to obtain catalpic acid and catalpic acid ester with purity of 95 to 98%; and n catalpic acid and catalpic acid ester with purity of 95 to 98% undergo saponification so as to obtain catalpic acid salt with purity of 95 to 98%. Compared with the prior art, the method provided by the invention has the advantages of simple process, low cost, high purity of obtained catalpic acid and ester and salt thereof and wide application prospects.
Owner:ZHEJIANG OCEAN UNIV
Method of using catalpic acid to treat and prevent type 2 diabetes and associated disorders
ActiveUS20080306156A1Impaired glucose tolerancePrevent hyperglycemiaBiocideMetabolism disorderDiseasePrevention of diabetes mellitus type 2
A method of treating and preventing type 2 diabetes and obesity an animal, including mammals and humans, in which a therapeutically effective amount of catalpic acid to the animal is administered orally or parentally.
Owner:NUTRITION THERAPEUTICS
Method of using catalpic acid to treat dyslipidemia
ActiveUS8084500B2Impaired glucose tolerancePrevent hyperglycemiaBiocideAnimal repellantsDyslipidemiaLipid oxidation
Owner:NUTRITION THERAPEUTICS
A kind of preparation method of polyhydroxy modified soybean lecithin fatliquoring agent
The invention relates to a preparation method of a polyhydroxyl-modified soybean phosphatide fatliquoring agent. The soybean phosphatide fatliquoring agent is widely used for fatliquoring at present, while the traditional fatliquoring agent is relatively poor in water solubility and is also not good in filling property. The preparation method comprises the steps of placing 100 parts by mass of soybean phosphatide into a three-neck flask, then, adding 2-2.5 parts by mass of allyl glycidyl ether, heating to 40 DEG C, and reacting at the constant temperature for 2h; adding 5.4-7.7 parts by mass of formic acid into the system, dropwise adding 25-40 parts by mass of hydrogen peroxide within 1h by taking 0.5-1 part by mass of concentrated sulfuric acid as a catalyst, and reacting for 8-10h; heating the system to 75 DEG C, adding 40-65 parts by mass of sodium hydrogen sulfite, and reacting for 3h; and regulating the pH value of the system to 7.5-8 by using 40% NaOH, adding 60-DEG C hot water to regulate the solid content of the system to 60%, and stirring for 30min to obtain the improved fatliquoring agent. The soybean phosphatide fatliquoring agent prepared by using the method disclosed by the invention has better hydrophilicity.
Owner:SHAANXI UNIV OF SCI & TECH
A kind of biosynthesis method of imidazole-4-acetic acid
ActiveCN107177642BWide variety of sourcesEasy to manufactureMicroorganism based processesFermentationPropanoic acidHistidine
The invention discloses a biosynthesizing method of imidazole-4-acetic acid. The biosynthesizing method comprises the following steps of using L-histidine as a substrate, and performing deaminizing reaction under the catalyzing action of membrane-linked L-amino acid deaminases, so as to obtain reaction liquid containing an intermediate product, namely 3-(4-imidazole)-2-oxo propionic acid; adding a hydrogen peroxide solution into the reaction liquid, and reacting, so as to obtain the imidazole-4-acetic acid. The biosynthesizing method has the characteristics that the steps are simple, the reaction conditions are mild, the sources of raw materials are wide, the preparation of a biological catalyst is easy, the requirement on equipment is low, and the conversion rate of the substrate is high; the organic solvent and the toxic reagent are not needed; compared with the existing chemical method, the efficiency is high, the cost is low, and the environment-friendly effect is realized.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Biocatalysis method for preparing pyruvic acid from L-alanine
ActiveCN102586343BSimple separation processHigh chemical purityMicroorganism based processesFermentationL-amino-acid oxidasePyruvic acid
The invention provides a biocatalysis method for preparing pyruvic acid from L-alanine. The method comprises the steps of: carrying out permeability treatment on Alcaligens faecalisl.1799 cells; catalyzing oxidative deamination of L-alanine by utilizing L-amino acid oxidase in the cells in the presence of air to form pyruvic acid, ammonia and hydrogen peroxide; catalyzing the hydrogen peroxide formed in the reaction by utilizing catalase in the cells to realize pyruvic acid accumulation without producing by-products. The method provided by the invention has the advantages of low cost, high product yield and purity and environment friendliness, and is suitable for industrial production of pyruvic acid.
Owner:CHONGQING UNIV OF POSTS & TELECOMM +1
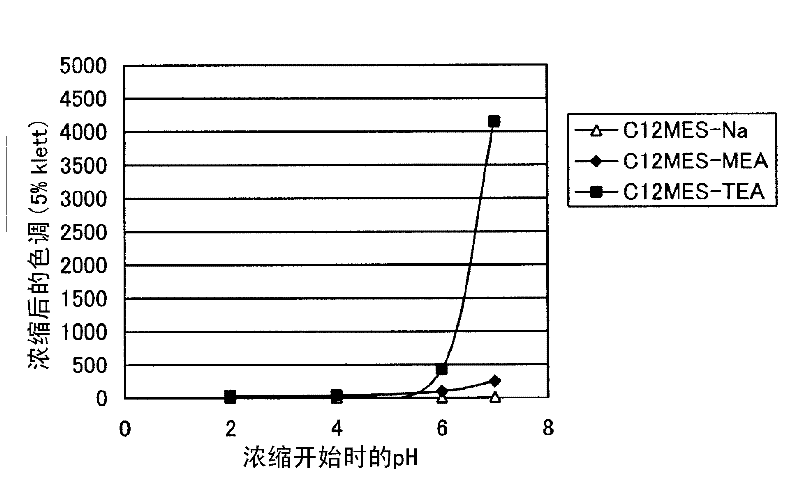
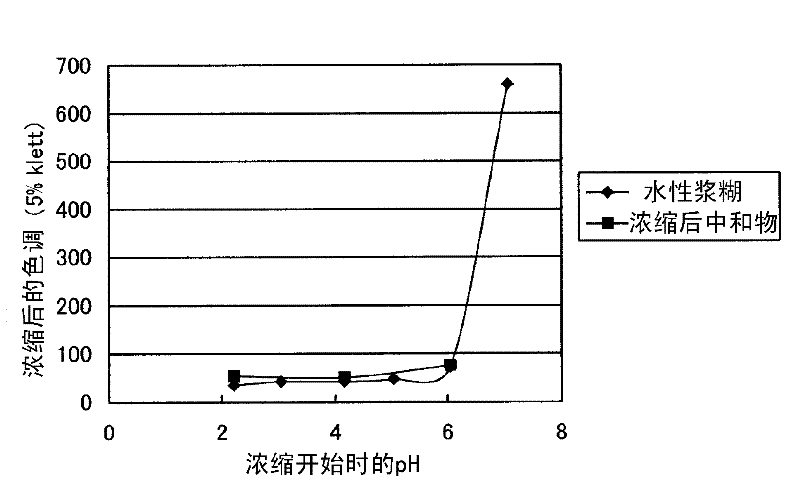
Method for producing alkanolamine salt of a-sulfo fatty acid alkyl ester
InactiveCN102414172ATo suppress color deteriorationOrganic compound preparationSulfonic acids salts preparationAlcoholFatty acid
Disclosed is a method for producing an alkanolamine salt of an a-sulfo fatty acid alkyl ester, comprising: a step of sulfonating a fatty acid alkyl ester to obtain a sulfonated compound; a step of reacting an alcohol and hydrogen peroxide with the sulfonated compound to obtain a reaction product; a step of adding an aqueous alkanolamine solution to the reaction product to obtain a neutralized product having a pH of 2 to 6.5; and a step of concentrating the neutralized product to obtain an aqueous paste containing an alkanolamine salt of an a-sulfo fatty acid alkyl ester. Accordingly, the deterioration of color tone at the time of concentration when preparing an aqueous paste of an alkanolamine salt of an a-sulfo fatty acid alkyl ester can be prevented.
Owner:LION CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com