Method for preparing azelaic acid by enzyme catalysis of hydrogen dioxide oxygenated oleic acid
A technology of lipase catalyzing hydrogen peroxide and hydrogen peroxide, which is applied in the direction of fermentation, etc., can solve the problem of no strict separation, etc., and achieve the effect of high-efficiency utilization, simple implementation conditions and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
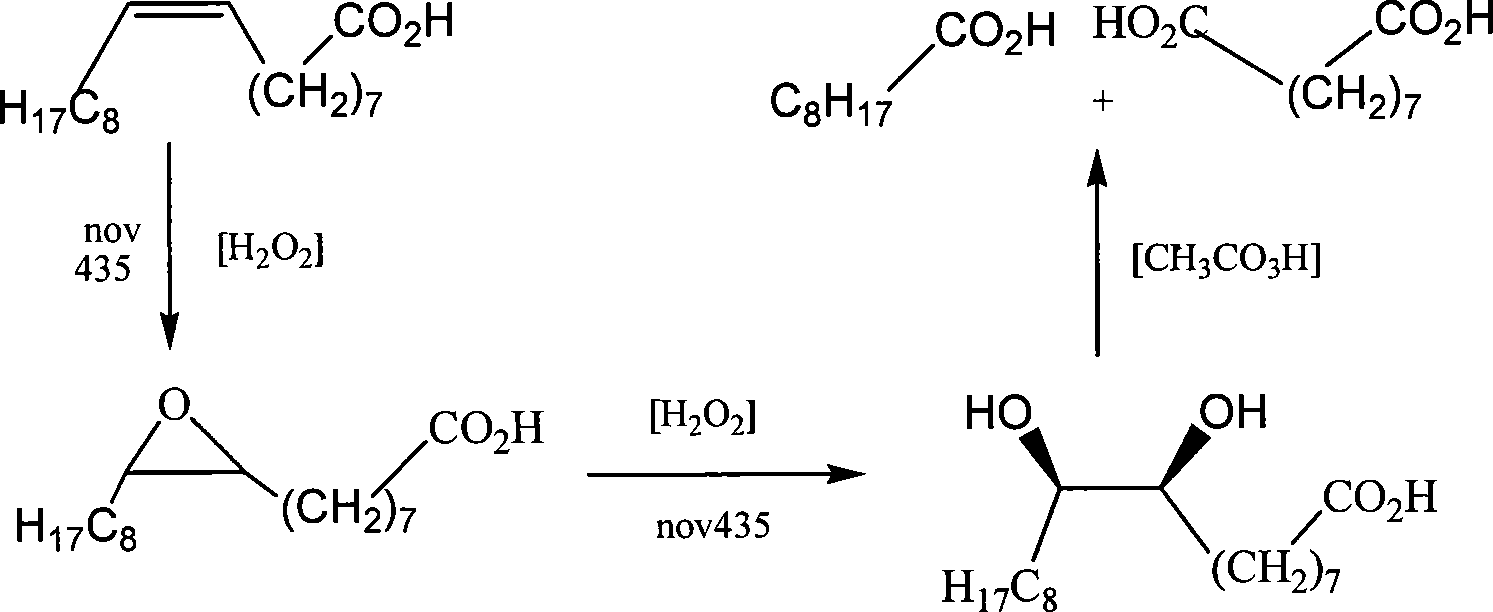
[0038] a, 9, the preparation of 10-dihydroxystearic acid:
[0039] Stir and mix oleic acid, lipase nov435 and toluene according to 1:0.01:1.2 (mass ratio), and add dropwise an 8% hydrogen peroxide solution at a concentration equal to that of oleic acid, and complete the dropwise addition within 2 hours. The reaction was carried out for 10 hours, the lipase nov435 was recovered by filtration, the solvent was recovered by rotary evaporation, and recrystallized with ethyl acetate to obtain 9,10-dihydroxystearic acid;
[0040] b, the synthesis of azelaic acid:
[0041] In the container that 9,10-dihydroxystearic acid is housed, add peracetic acid mixed solution, the add-on of peracetic acid mixed solution is (mass ratio) take step a oleic acid as base, oleic acid: peracetic acid: Hydrogen peroxide: acetic acid: water: sulfuric acid = 1: 3: 0.5: 2: 0.5: 0.01, react at a temperature of 90 ° C for 2 hours, remove the solvent by rotary evaporation after the reaction, and extract the ...
Embodiment 2
[0044] a, 9, the preparation of 10-dihydroxystearic acid:
[0045] Stir and mix oleic acid, lipase nov435 and toluene according to 1:0.1:2 (mass ratio), dropwise add a 20% hydrogen peroxide solution with a concentration 1.5 times the amount of oleic acid, and complete the dropwise addition within 3 hours. React at room temperature for 14 hours, filter and recover lipase, rotary evaporate and recover solvent, use ethyl acetate to recrystallize to obtain 9,10-dihydroxystearic acid;
[0046] b, the synthesis of azelaic acid:
[0047] In the container that 9,10-dihydroxystearic acid is housed, add peracetic acid mixed solution, the add-on of peracetic acid mixed solution is (mass ratio) take step a oleic acid as base, oleic acid: peracetic acid: Hydrogen peroxide: acetic acid: water: sulfuric acid = 1: 0.5: 2: 2: 1.5: 0.3, react at a temperature of 92 ° C for 3 hours, remove the solvent by rotary evaporation after the reaction, and extract the product azelaic acid and by-products...
Embodiment 3
[0050] a, 9, the preparation of 10-dihydroxystearic acid:
[0051] Stir and mix oleic acid, lipase nov435 and toluene according to 1:1.0:3 (mass ratio), and add dropwise a 40% hydrogen peroxide solution with a concentration twice that of oleic acid (mass ratio), within 4 hours. Complete, react at room temperature for 18 hours, filter and recover lipase, rotary evaporate and recover solvent, use ethyl acetate to recrystallize to obtain 9,10-dihydroxystearic acid;
[0052] b, the synthesis of azelaic acid:
[0053] In the container that 9,10-dihydroxystearic acid is housed, add peracetic acid mixed solution, the add-on of peracetic acid mixed solution is (mass ratio) take step a oleic acid as base, oleic acid: peracetic acid: Hydrogen peroxide: acetic acid: water: sulfuric acid = 1: 1.5: 3: 1: 2.5: 0.1, react at a temperature of 95°C for 3.5 hours, remove the solvent by rotary evaporation after the reaction, extract the product azelaic acid and by-products from the reaction sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com