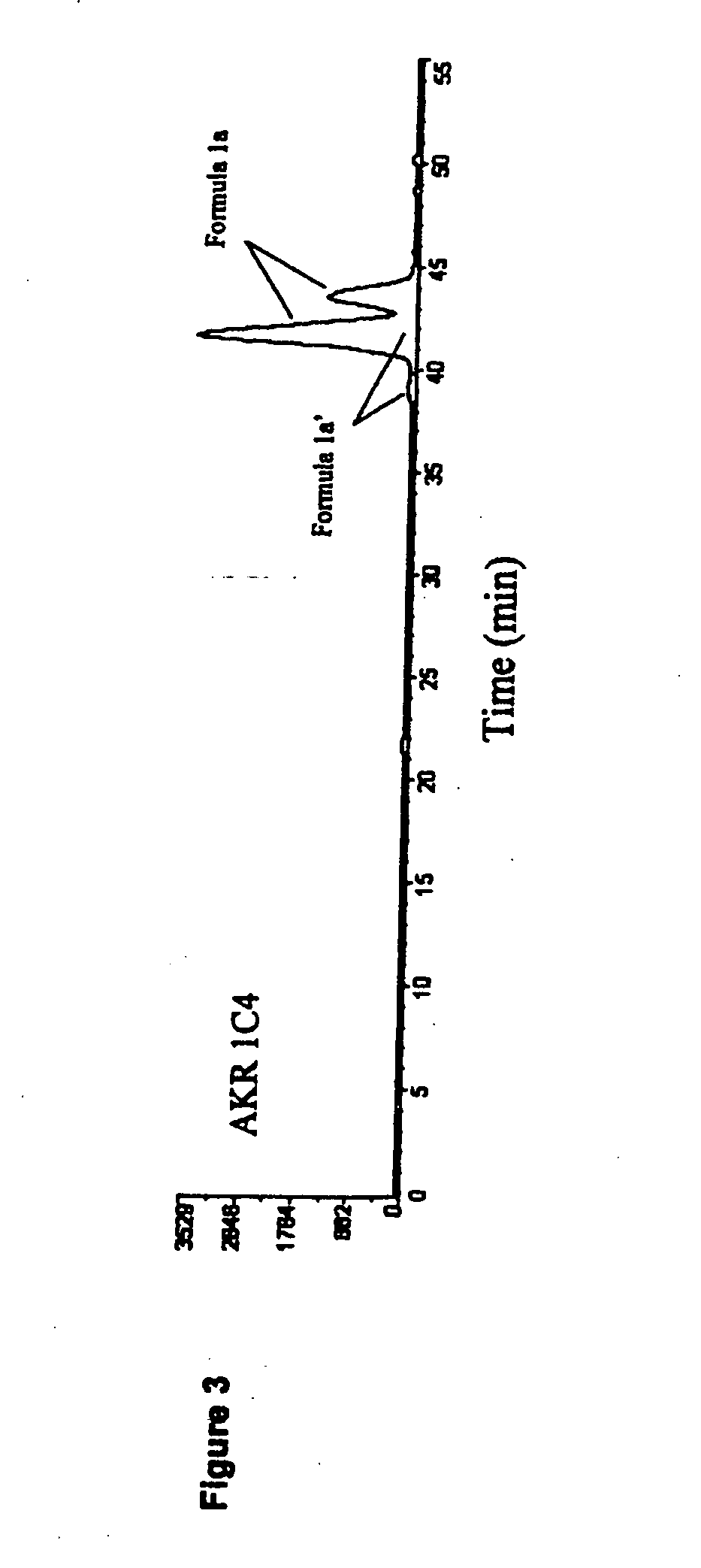
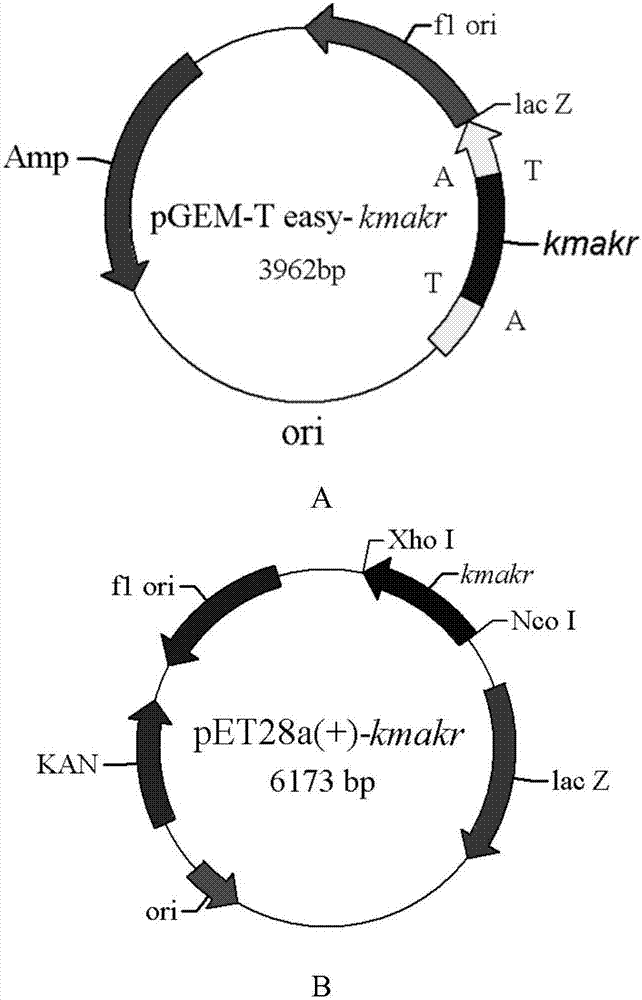
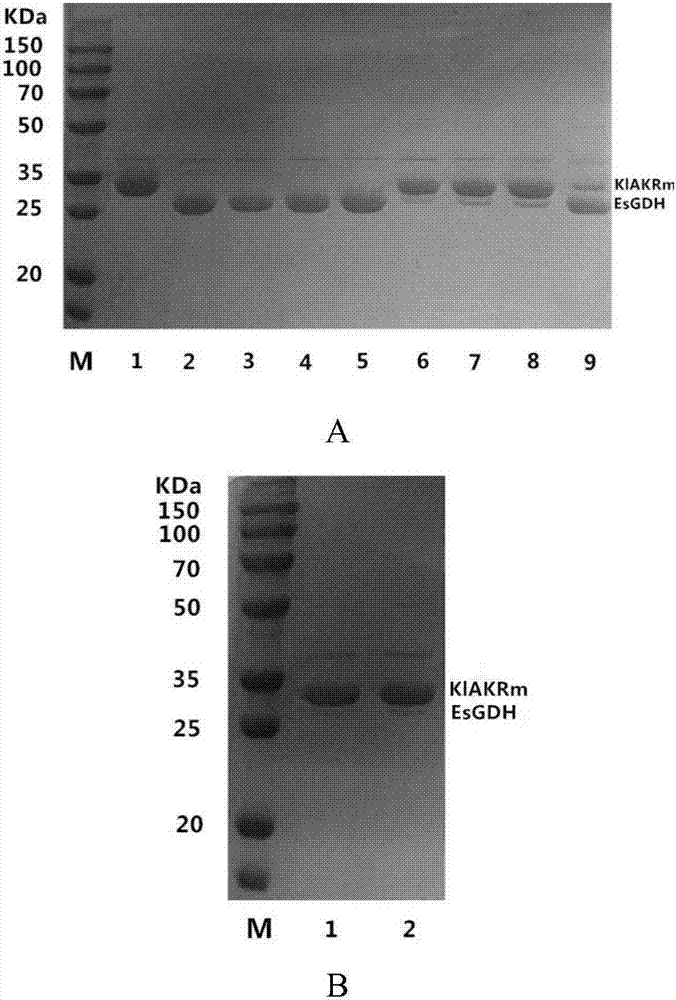
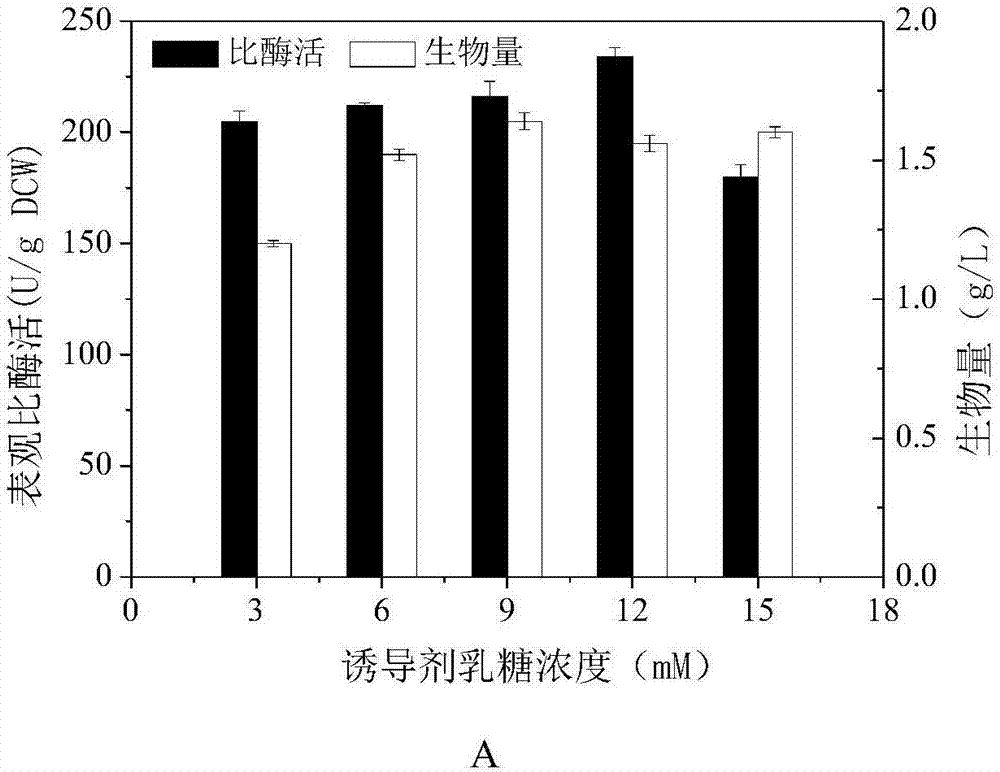
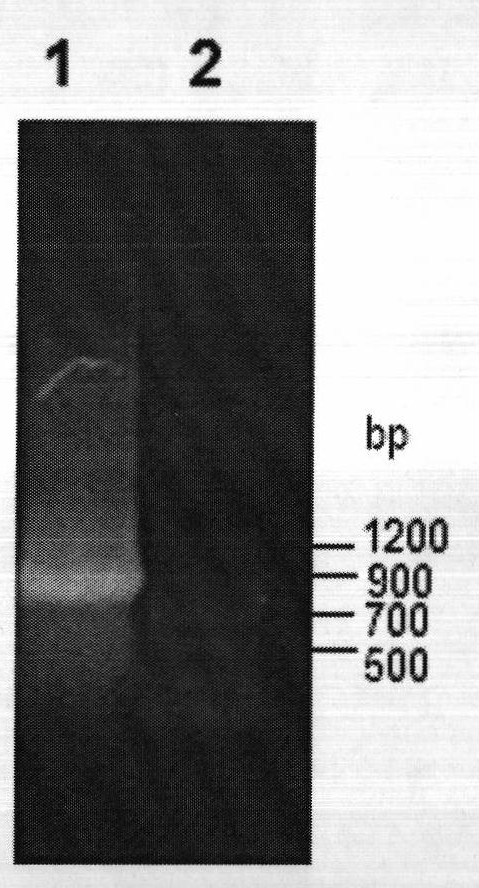
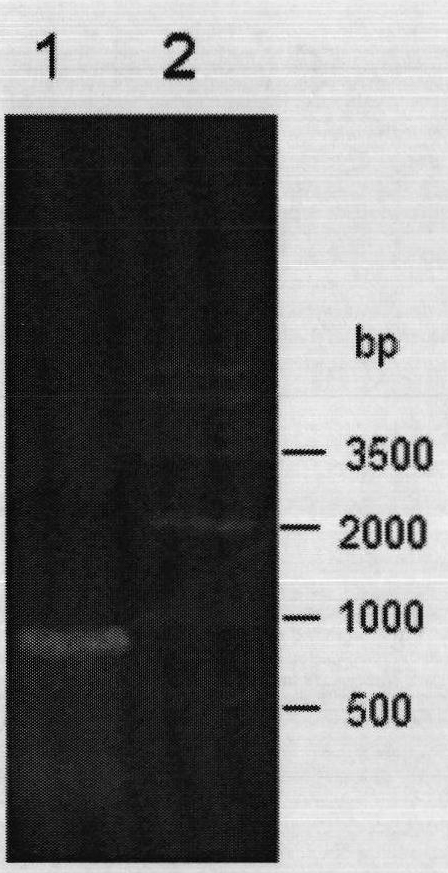
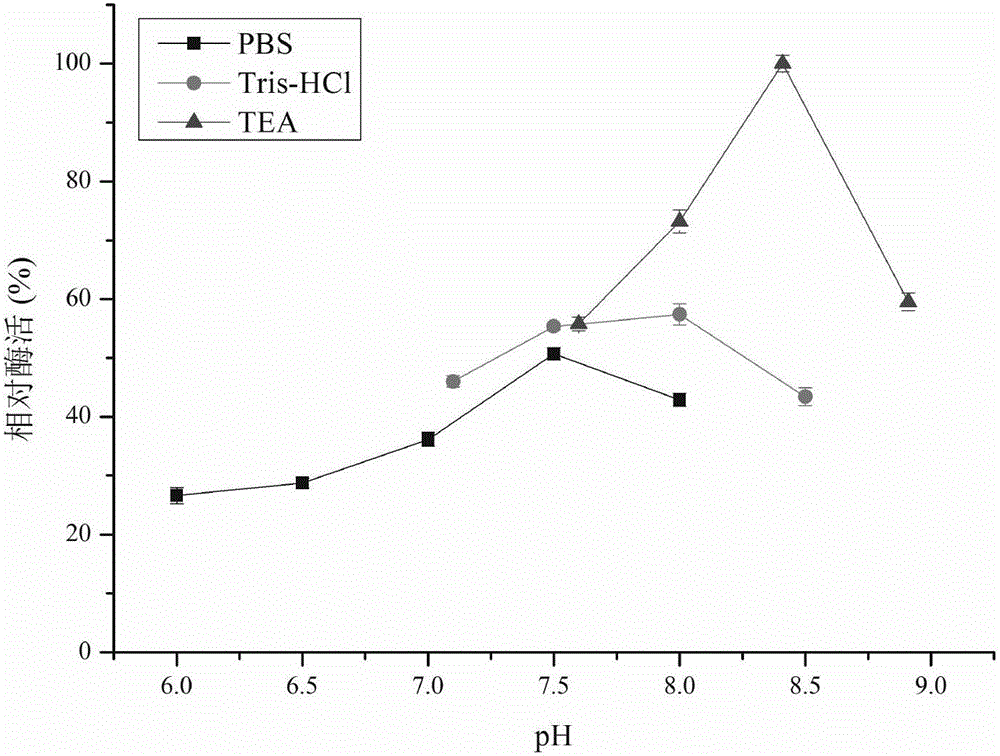
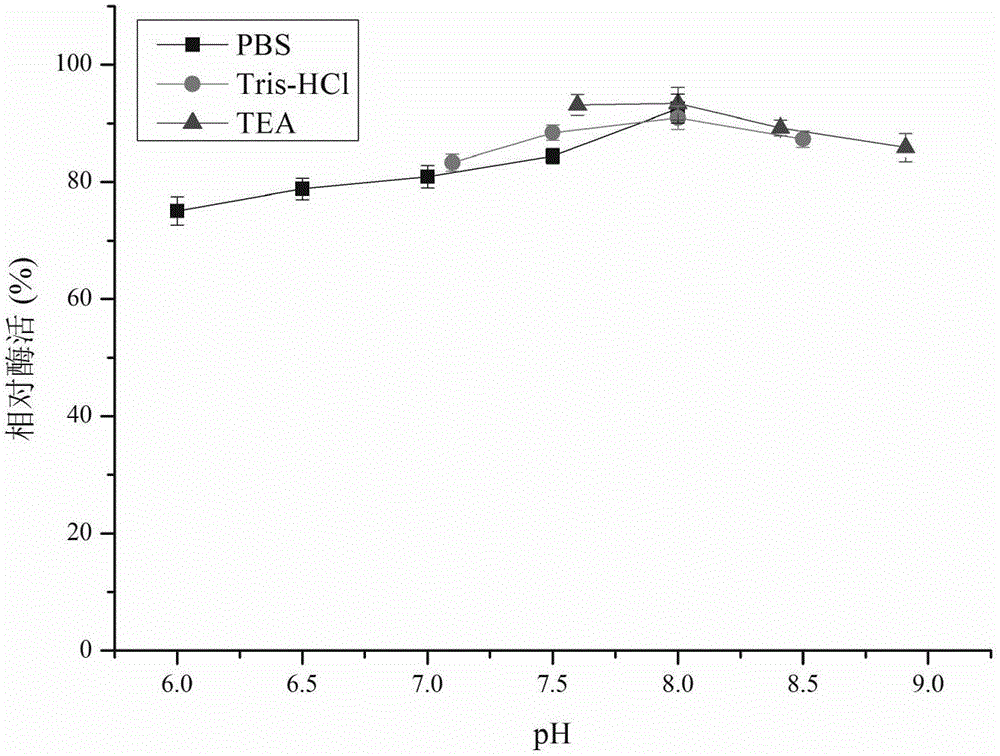
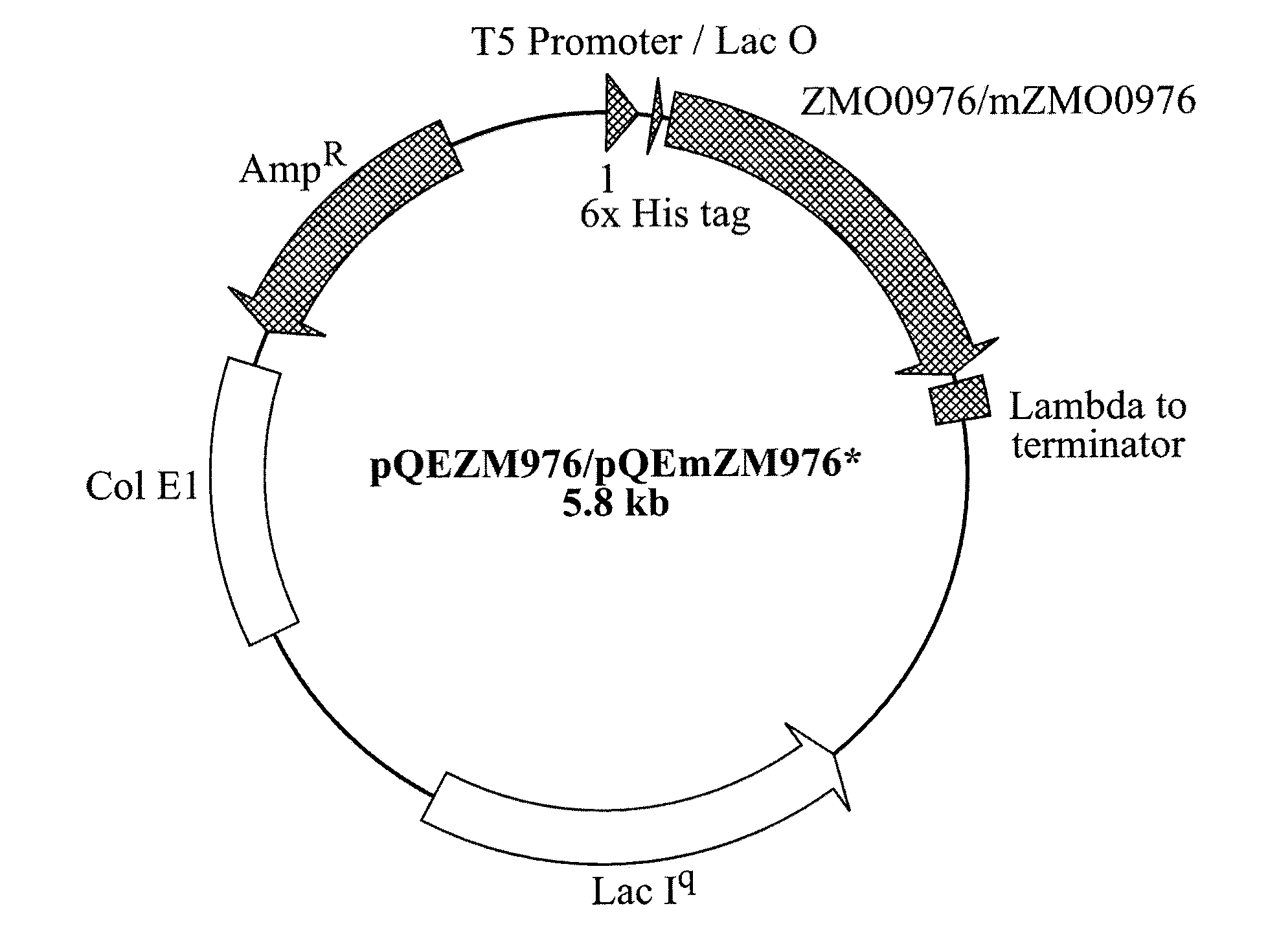
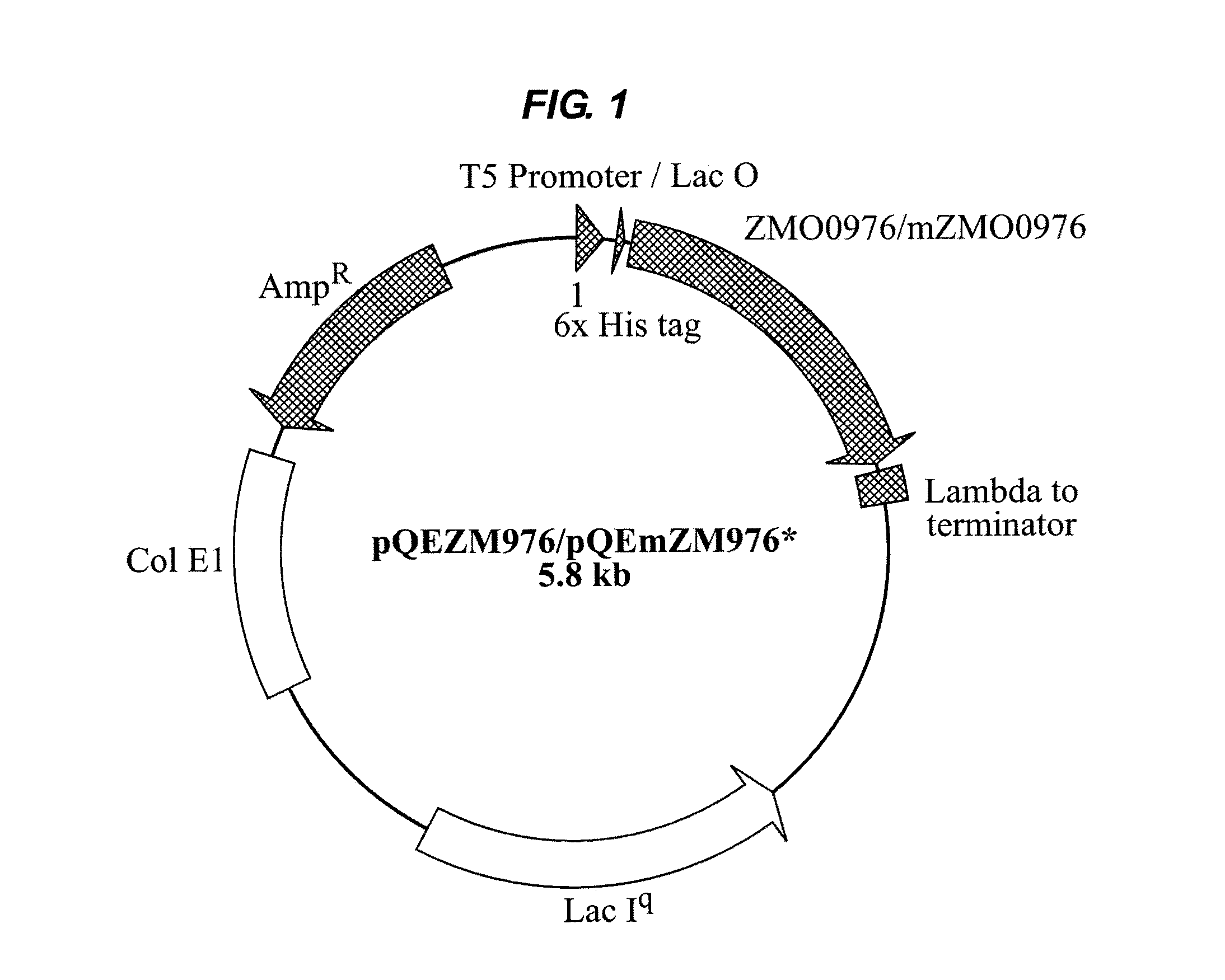
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Aldo-keto reductase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
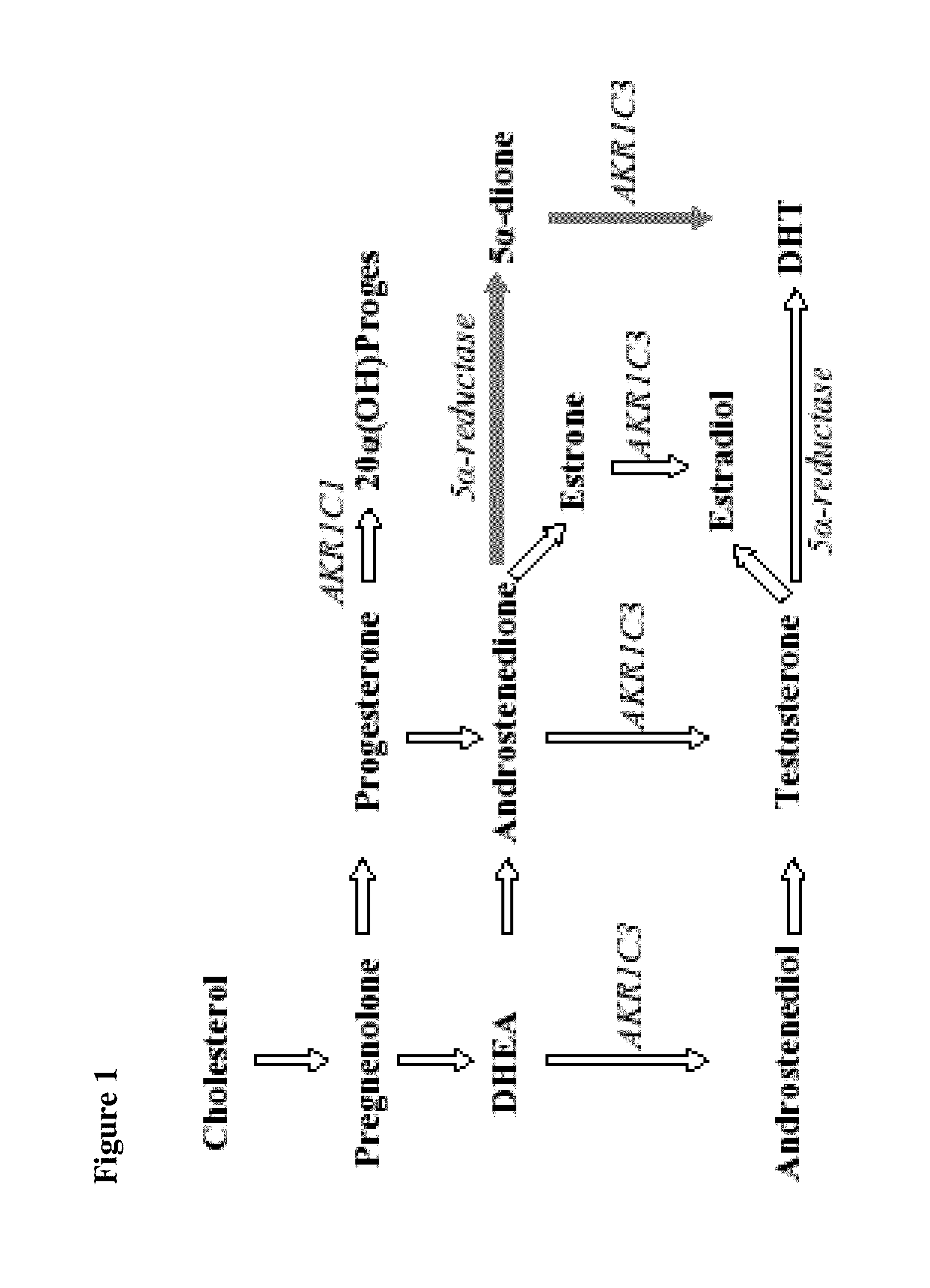
The aldo-keto reductase family is a family of proteins that are subdivided into 16 categories; these include a number of related monomeric NADPH-dependent oxidoreductases, such as aldehyde reductase, aldose reductase, prostaglandin F synthase, xylose reductase, rho crystallin, and many others.
Medicaments and methods combining a HCV protease inhibitor and an AKR competitor
InactiveUS20070207949A1Improve bioavailabilityImprove drug bioavailabilityOrganic active ingredientsBiocideDiseaseProteinase activity
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations of a hepatitis C virus (HCV) protease inhibitor and an aldo-keto reductase (AKR) competitor, for concurrent or consecutive administration in treating, preventing, or ameliorating one or more symptoms of HCV, treating disorders associated with HCV, or inhibiting cathepsin activity in a subject.
Owner:SCHERING CORP
Medicaments and methods combining a HCV protease inhibitor and an AKR competitor
InactiveUS20070232527A1Improve efficacyExtended durationBiocideDipeptide ingredientsDiseaseCathepsin
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations of a hepatitis C virus (HCV) protease inhibitor and an aldo-keto reductase (AKR) competitor, for concurrent or consecutive administration in treating, preventing, or ameliorating one or more symptoms of HCV, treating disorders associated with HCV, or inhibiting cathepsin activity in a subject.
Owner:SCHERING CORP
Medicaments and methods combining a HCV protease inhibitor and an AKR competitor
InactiveUS20060276404A1Improve efficacyExtended durationBiocideDipeptide ingredientsDiseaseEnzyme Inhibitor Agent
Disclosed are medicaments, pharmaceutical compositions, pharmaceutical kits, and methods based on combinations of a hepatitis C virus (HCV) protease inhibitor and an aldo-keto reductase (AKR) competitor, for concurrent or consecutive administration in treating, preventing, or ameliorating one or more symptoms of HCV, treating disorders associated with HCV, or inhibiting cathepsin activity in a subject.
Owner:SCHERING CORP
Recombined aldehyde ketoreductase mutant, gene, carrier, engineering bacteria and application of recombined aldehyde ketoreductase mutant
ActiveCN107058248AModerate stereoselectivityGeneral stereoselectivityBacteriaMicroorganism based processesTryptophanMutant
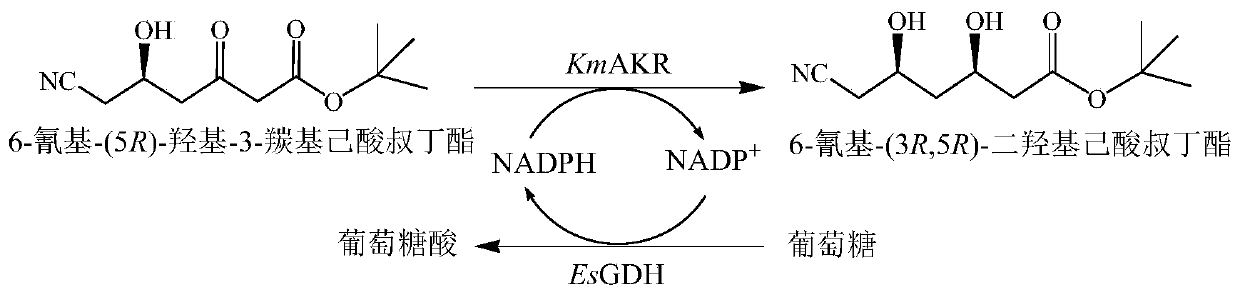
The invention discloses a recombined aldehyde ketoreductase mutant, a gene, a carrier, an engineering bacteria and application of the recombined aldehyde ketoreductase mutant in preparing 6-cyan-(3R, 5R)-tert-butyl dihydroxycaproate; the mutant is obtained by mutating the 297th bit tryptophan of amino acid sequence shown in SEQ ID No.1 to histidine, mutating the 296th bit tyrosine to tryptophan and the 297th bit tryptophan to histidine. The aldehyde ketoreductase mutants W297H and Y296W / W297H have significantly improved three-dimensional selectivity and activity to 6-cyan-(3R, 5R)-tert-butyl dihydroxycaproate; the conversion rate is up to 99%; the product reaches the optical purity with the d.e. value greater than 99.5%.
Owner:ZHEJIANG UNIV OF TECH
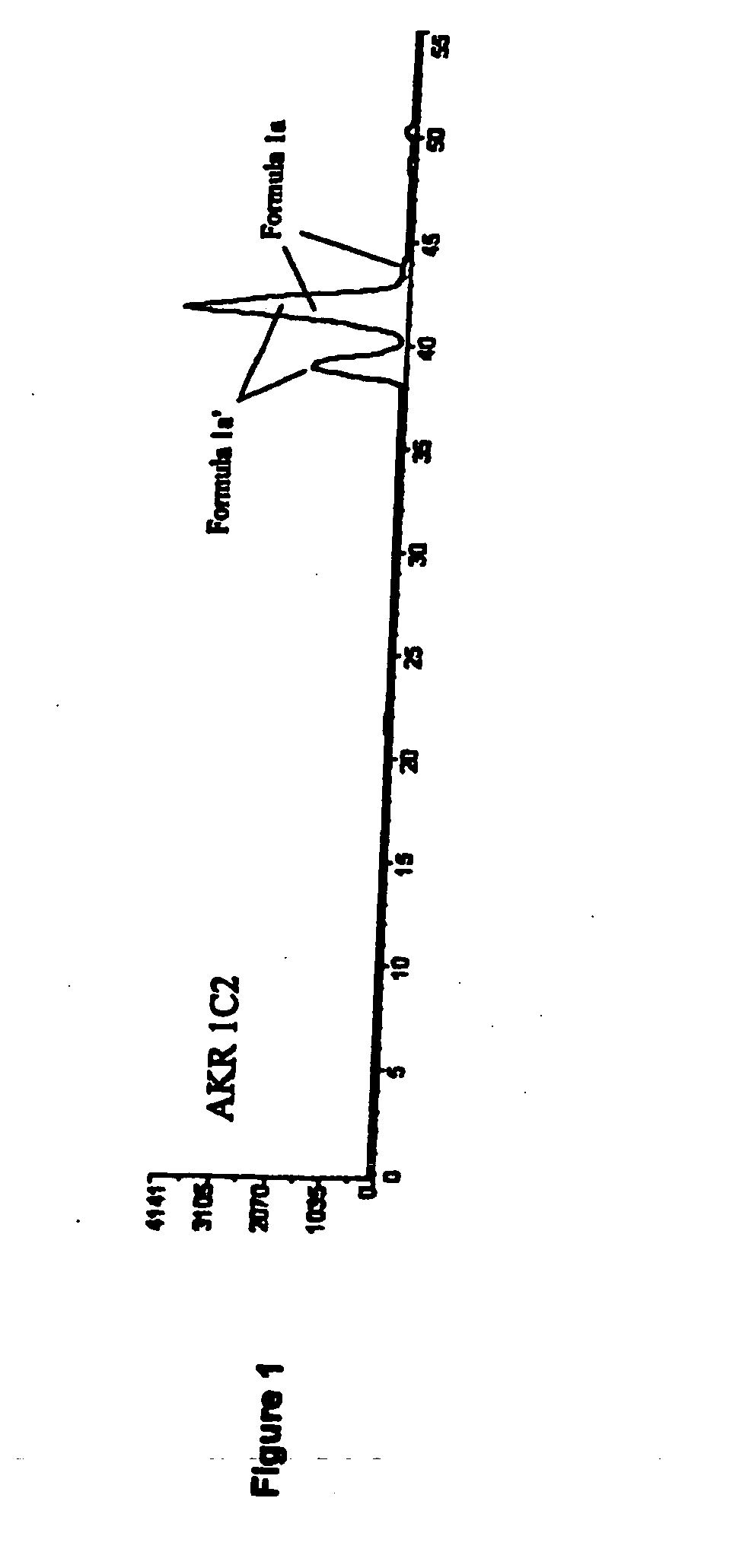
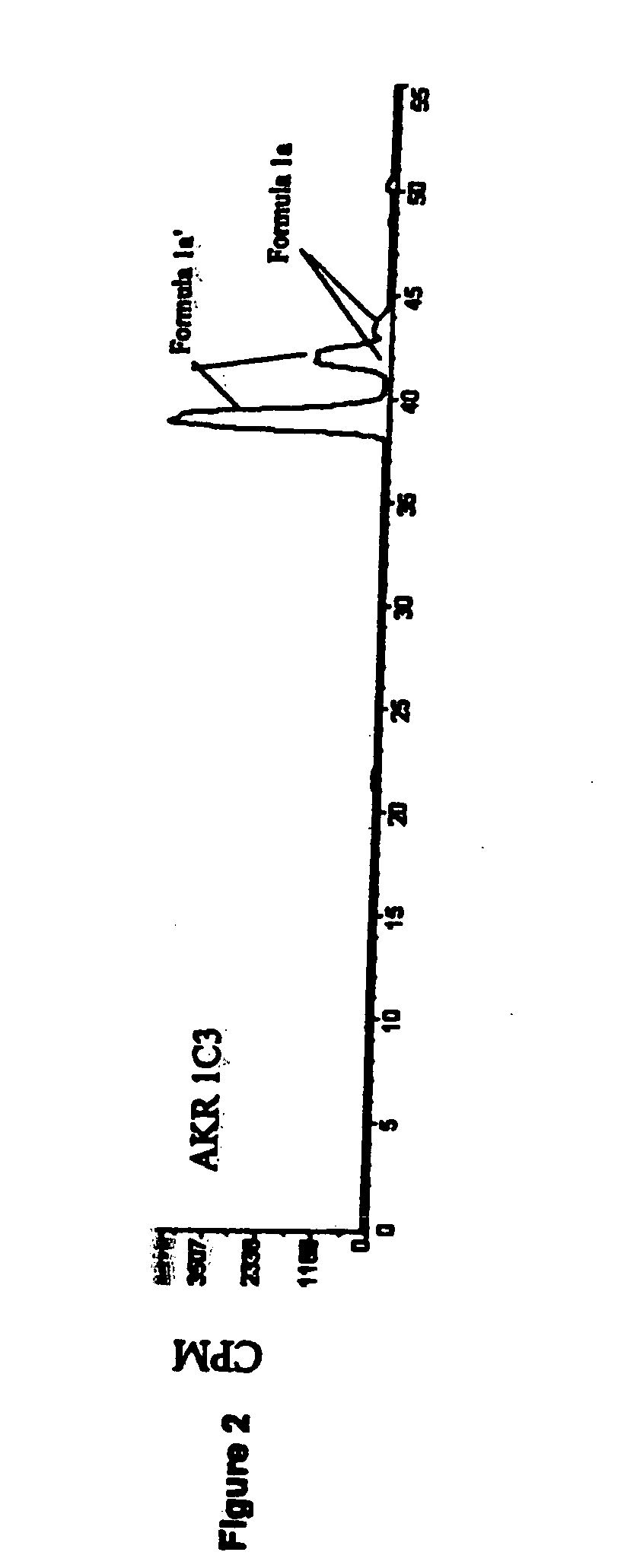
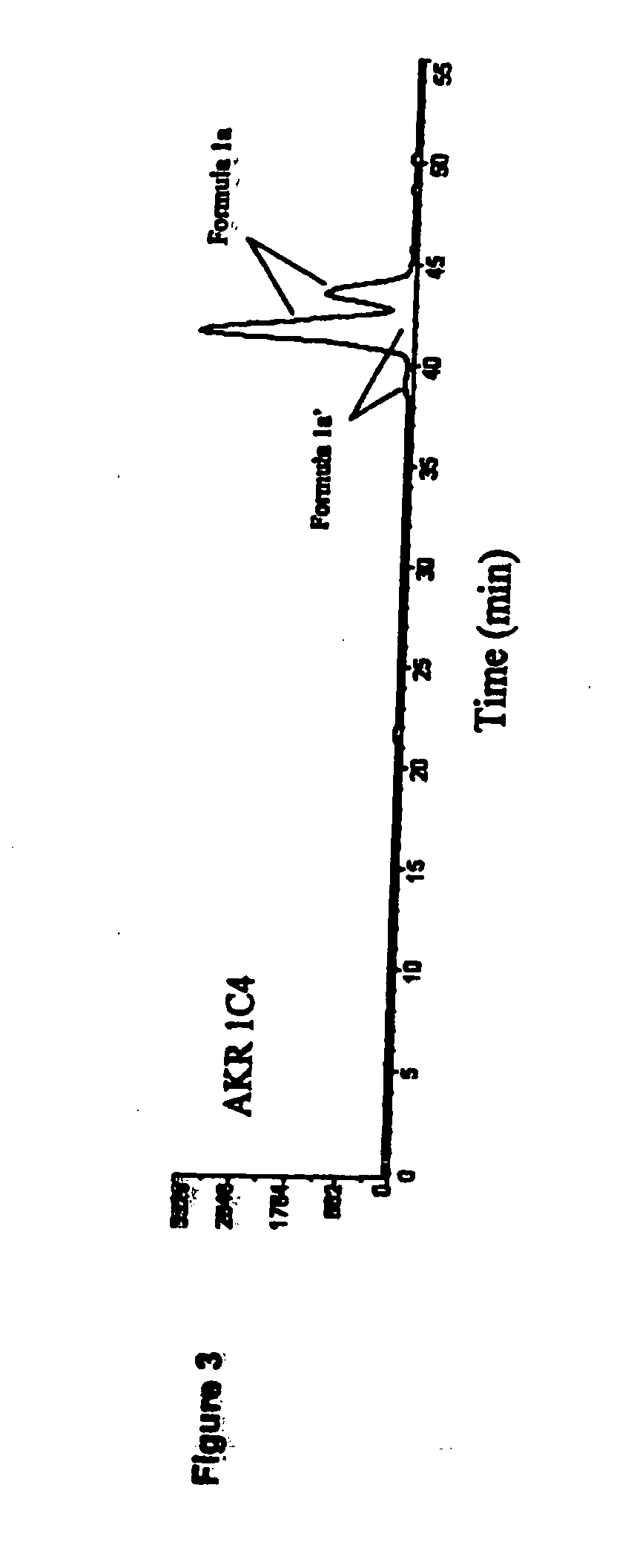
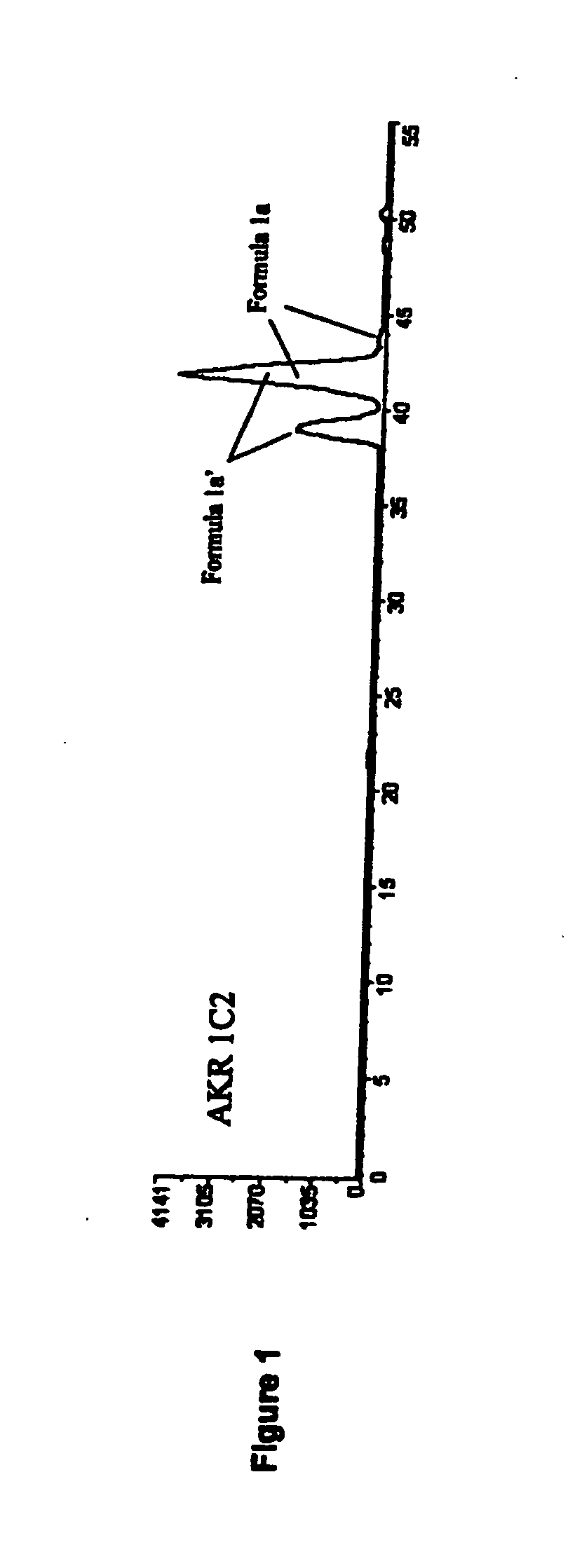
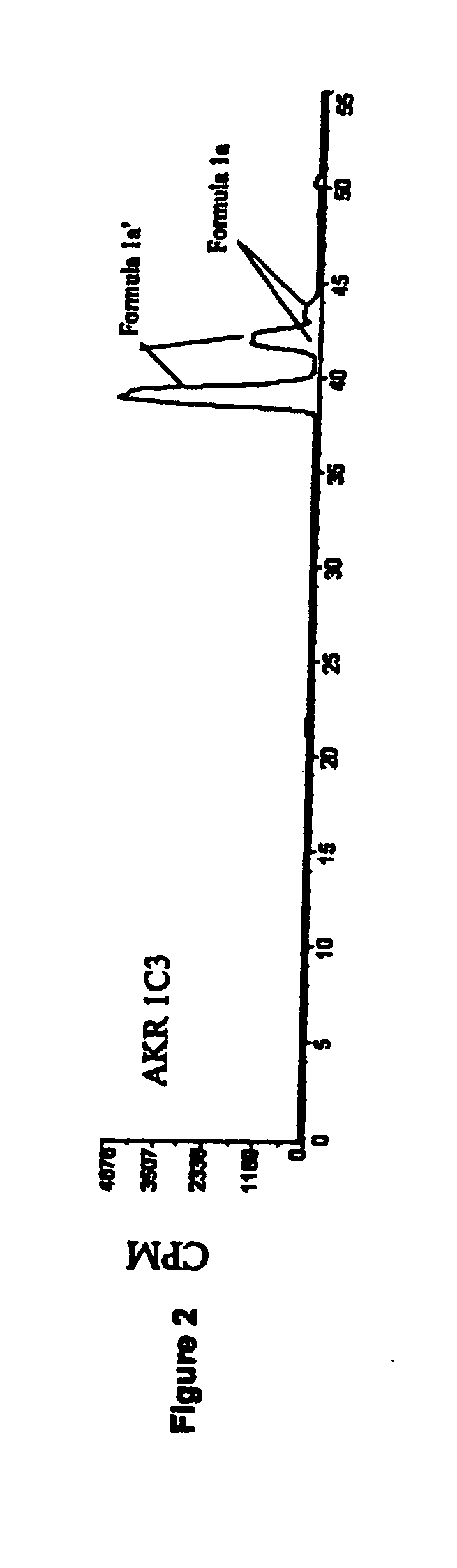
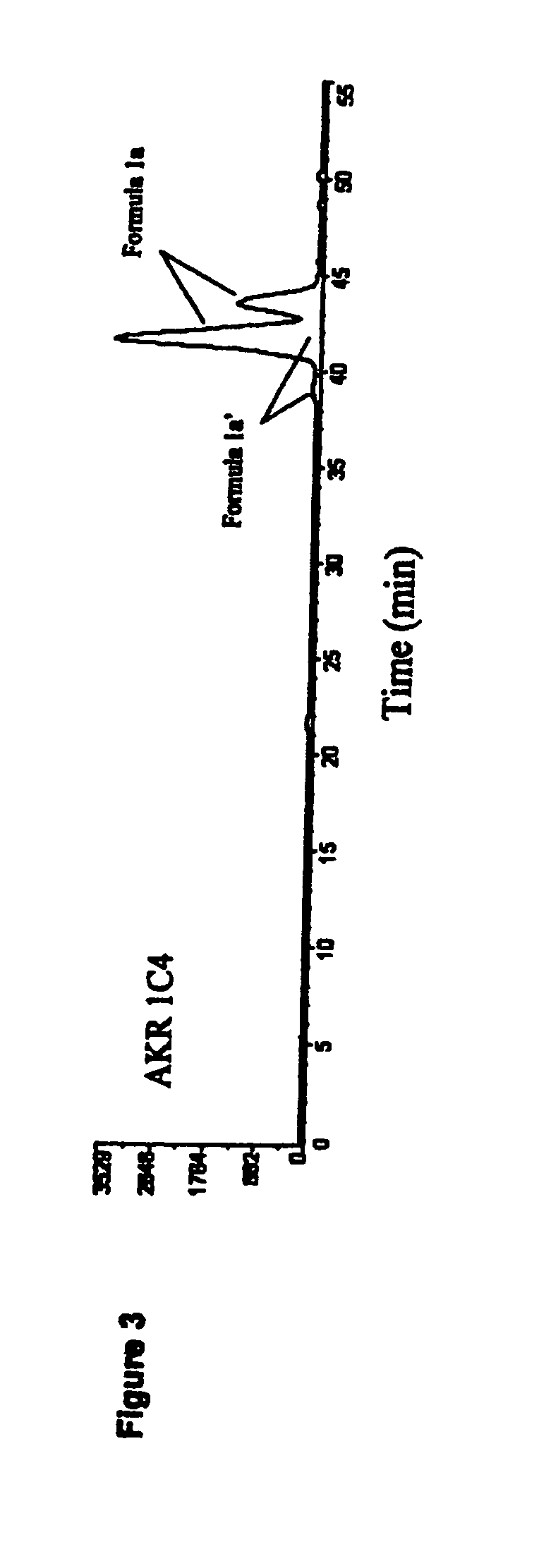
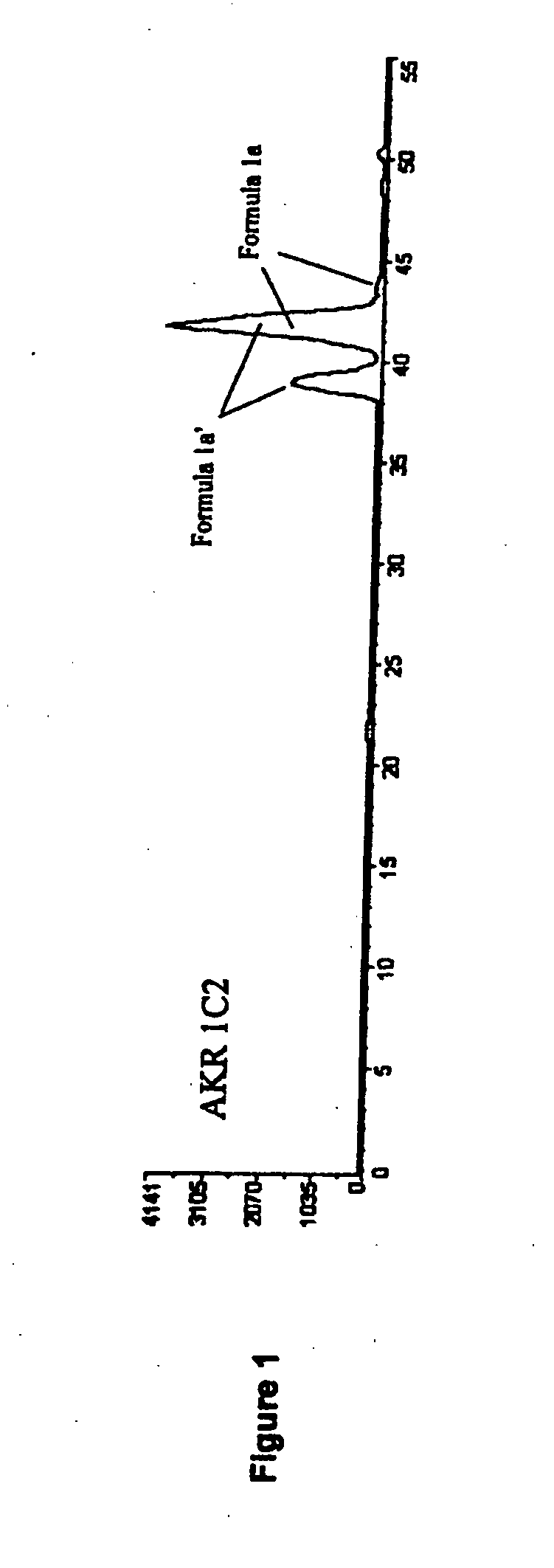
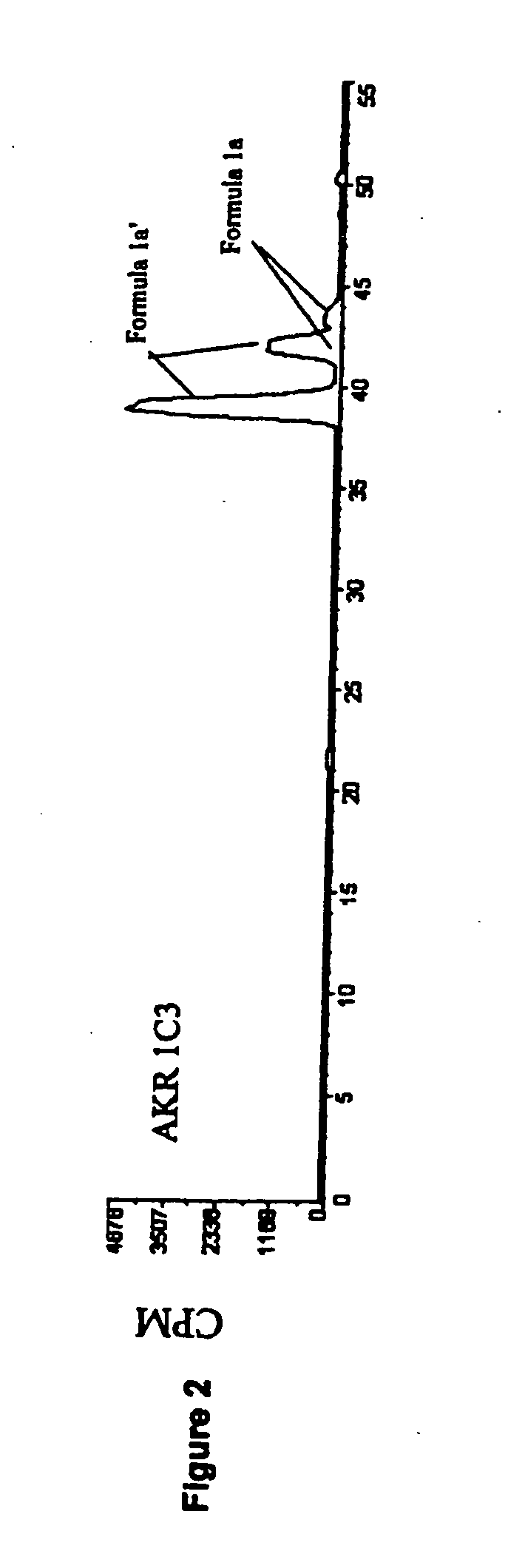
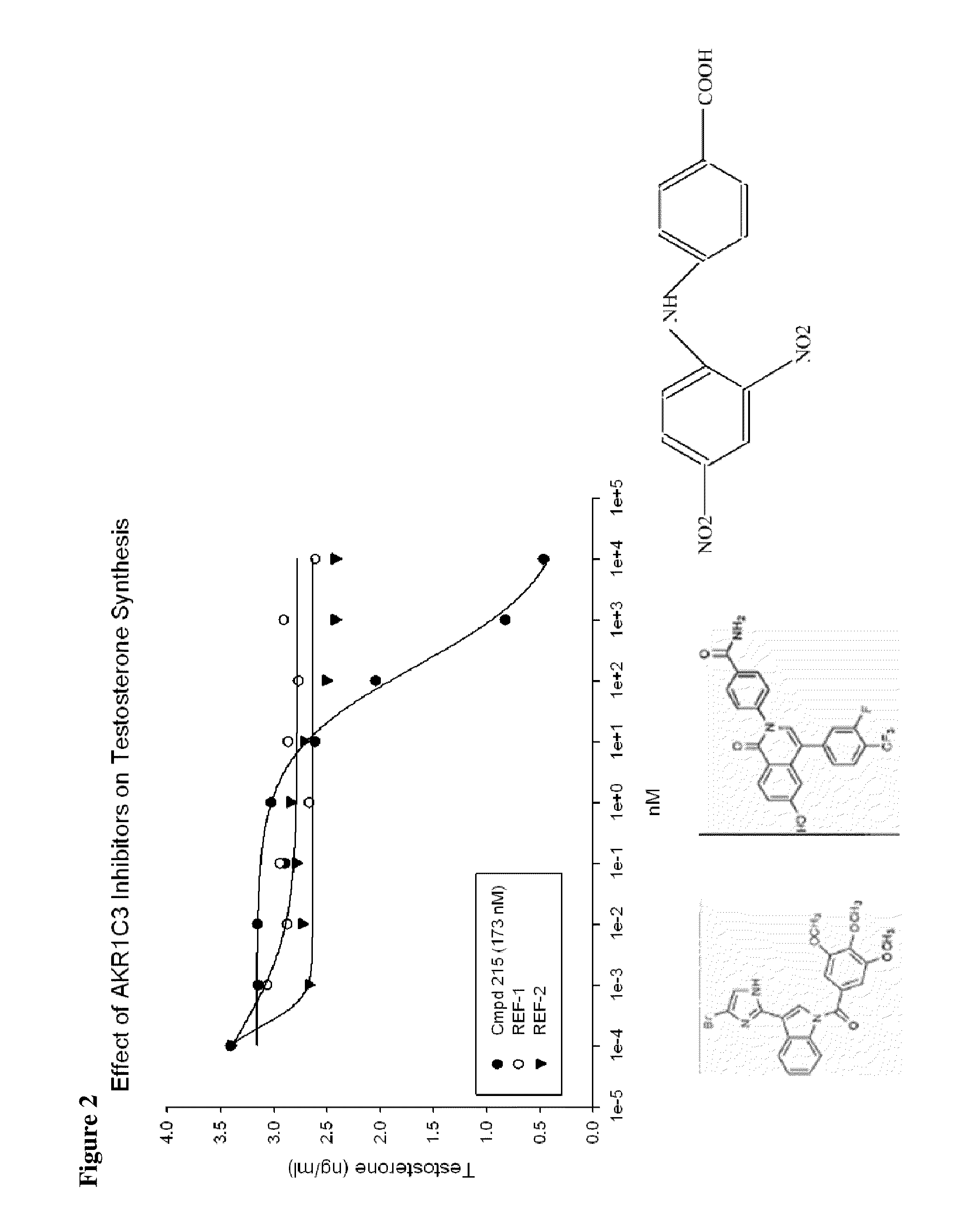
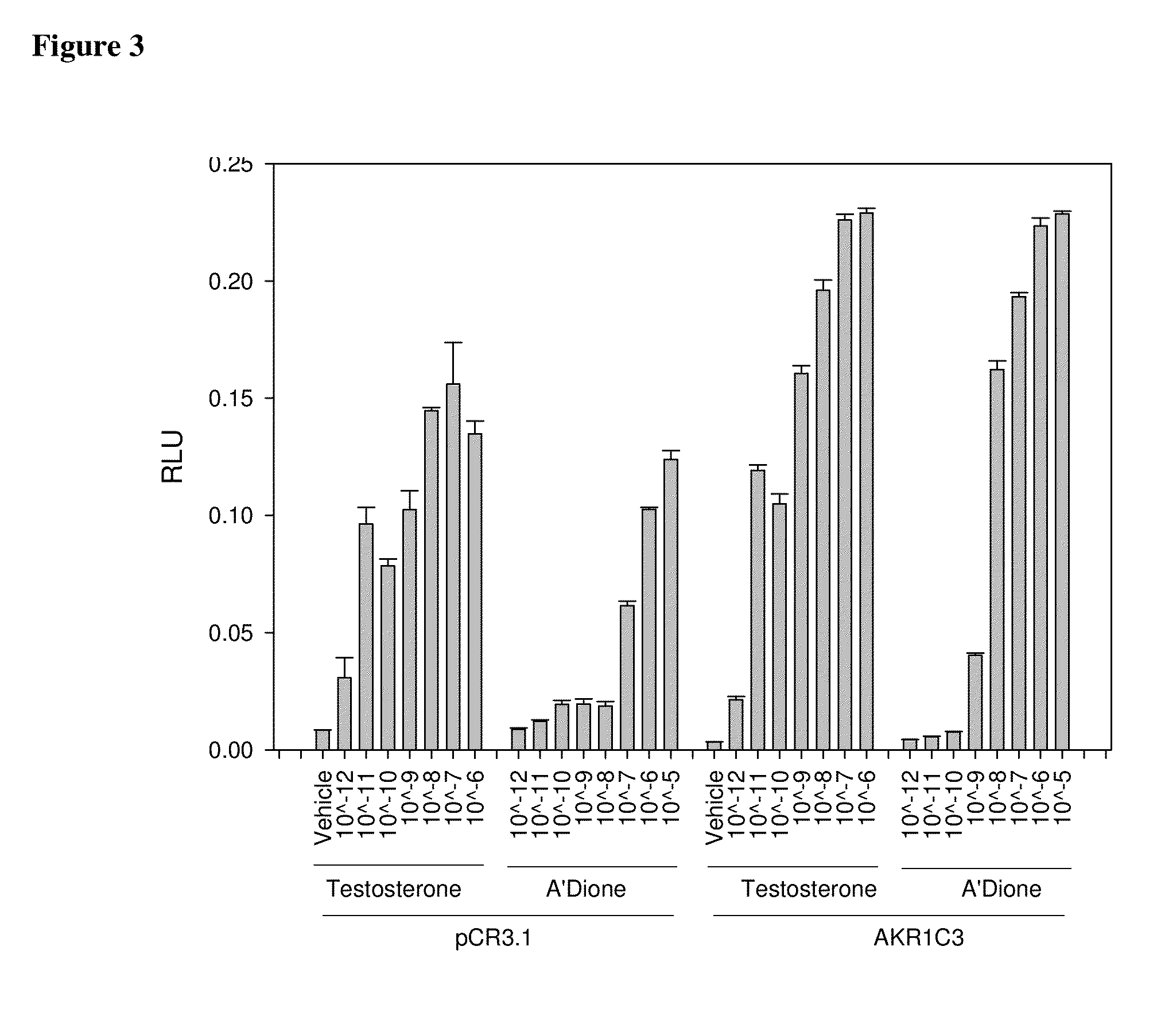
Aldo-keto reductase subfamily 1c3 (AKR1c3) inhibitors
The present invention relates to a novel class of AKR1C3 inhibitors, to compositions containing them, to methods for their preparation, and to methods of use thereof. The AKR1C3 inhibitors may be useful in the treatment of, for example, prostate cancer, benign prostate hyperplasia (BPH), lung cancer, acne, seborrhea, hirsuitism, baldness, alopecia, precocious puberty, adrenal hypertrophy, polycystic ovary syndrome, breast cancer, uterine cancer, uterine fibroids, endometriosis, myeloma and leiomyoma.
Owner:DALTON JAMES T +6
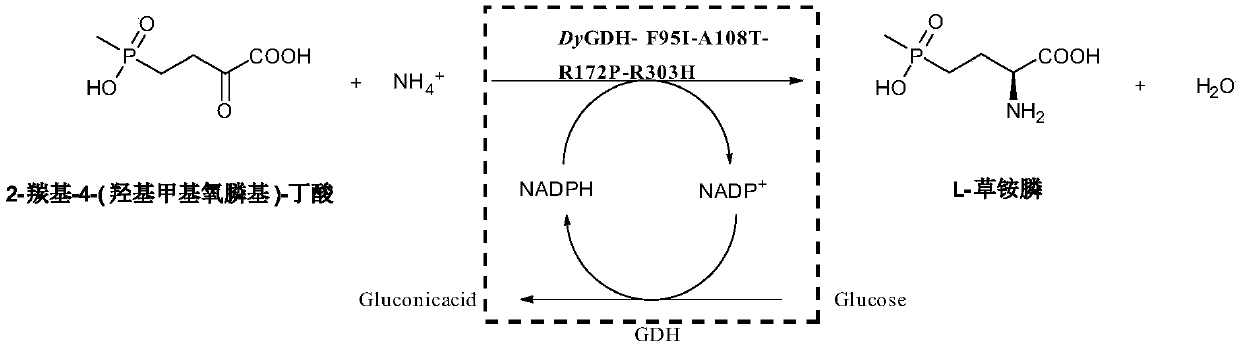
Amino acid dehydrogenase mutant and application of mutant in synthesizing L-glufosinate
The invention discloses an amino acid dehydrogenase mutant and application of the mutant in synthesizing L-glufosinate. The amino acid dehydrogenase mutant is obtained by carrying out single mutationor multiple mutation on the 95th, 108th, 172nd and 303rd amino acid as shown in SEQ ID No.2. The specific enzyme activity of the amino acid dehydrogenase mutant DyGDH-F95I-A108T-R172P-R303H prepared by the method is improved by 33 times than that of female parent aldo-keto reductase, and the maximum feeding amount of a substrate 2-carbonyl-4-(hydroxyl methyl phosphinyl)-butyric acid reaches 500 mM. The amino acid dehydrogenase mutant has better industrial application prospect. The amino acid dehydrogenase mutant is used for producing L-glufosinate, and the reaction time is remarkably shortened. The general process requires 20 hours, while the reaction time of the present invention takes only 120 minutes, which shows that the amino acid dehydrogenase mutant has a good Industrial applicationprospect.
Owner:ZHEJIANG UNIV OF TECH
Application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate
InactiveCN104726507ADisinhibition effectImprove conversion abilityBacteriaOxidoreductasesHydroxybutyric acidPtru catalyst
The invention discloses an application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate. With aldo-keto reductase of which an amino acid sequence is as shown in SEQ ID NO:2 as a catalyst, with 4-ethyl acetoacetate as a substrate and NADPH as a cofactor, the (R)-4-chlorine-3-hydroxy ethyl butyrate is prepared by asymmetric reduction. The aldo-keto reductase of which the amino acid sequence is as shown in SEQ ID NO:2 is applied to the 4-ethyl acetoacetate to prepare the (R)-4-chlorine-3-hydroxy ethyl butyrate in an asymmetric reduction manner for the first time, so that a good effect is obtained; the enzyme activity can be up to 8.1U / mg; the conversion ratio of the aldo-keto reductase on the substrate can be up to 99.8%; the enantiomer excess value of the product is greater than 995; the yield is high; and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Aldo-keto reductase and application thereof in synthesis of (2S,3R)-2-benzoylaminomethyl-3-hydroxybutyrate
ActiveCN105624125AHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationMethyl palmoxirate
The invention provides a new aldo-keto reductase mutant and a method using the recombinant aldo-keto reductase mutant or a lyophilized powder thereof to carry out asymmetric reduction. When the new aldo-keto reductase is used to catalyze a substrate with the concentration reaching up to 1000g / L, the optical purity of products still reaches up to 98% or above, and extra addition of an expensive coenzyme is avoided. Compared with other asymmetric reduction preparation methods, a method using the aldo-keto reductase has the advantages of high concentration of the products, high optical purity of the products, mild reaction conditions, environmental protection, simple operation and easy industrial amplification, so the aldo-keto reductase has very good industrial application prospect.
Owner:弈柯莱生物科技(集团)股份有限公司
Ligands for Aldoketoreductases
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Microwave-assisted co-immobilization method of aldehyde ketone reductase and glucose dehydrogenase
ActiveCN104988132AIncrease forceReduce manufacturing costOn/in inorganic carrierMulti-enzyme systemsThermal stabilityGlucose dehydrogenase
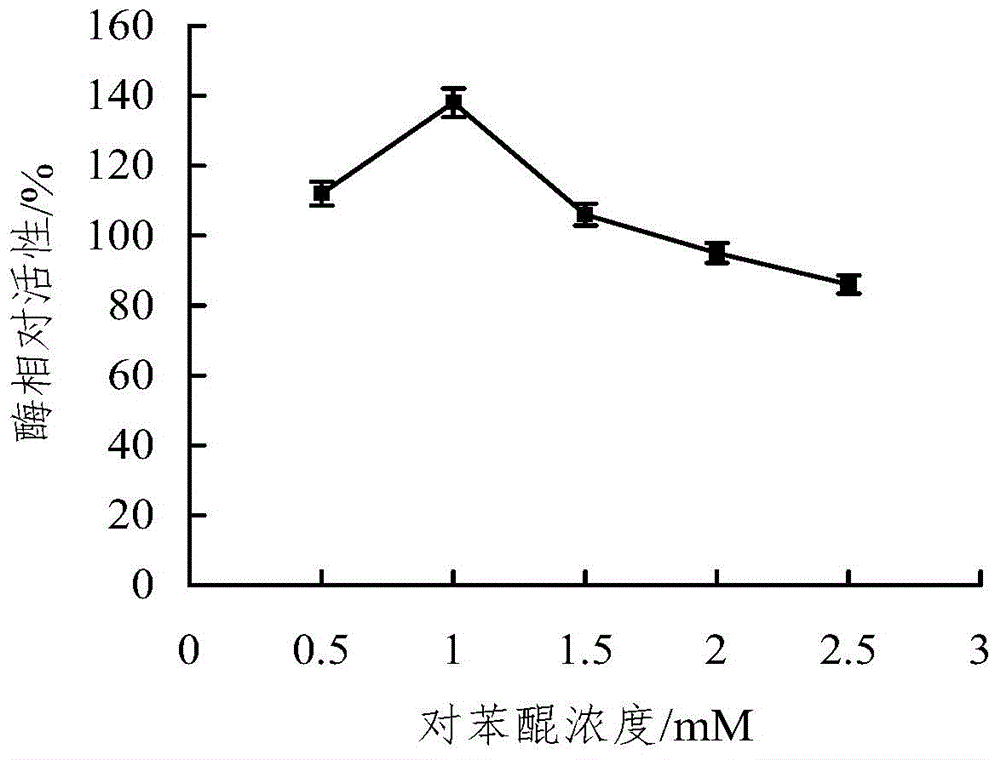
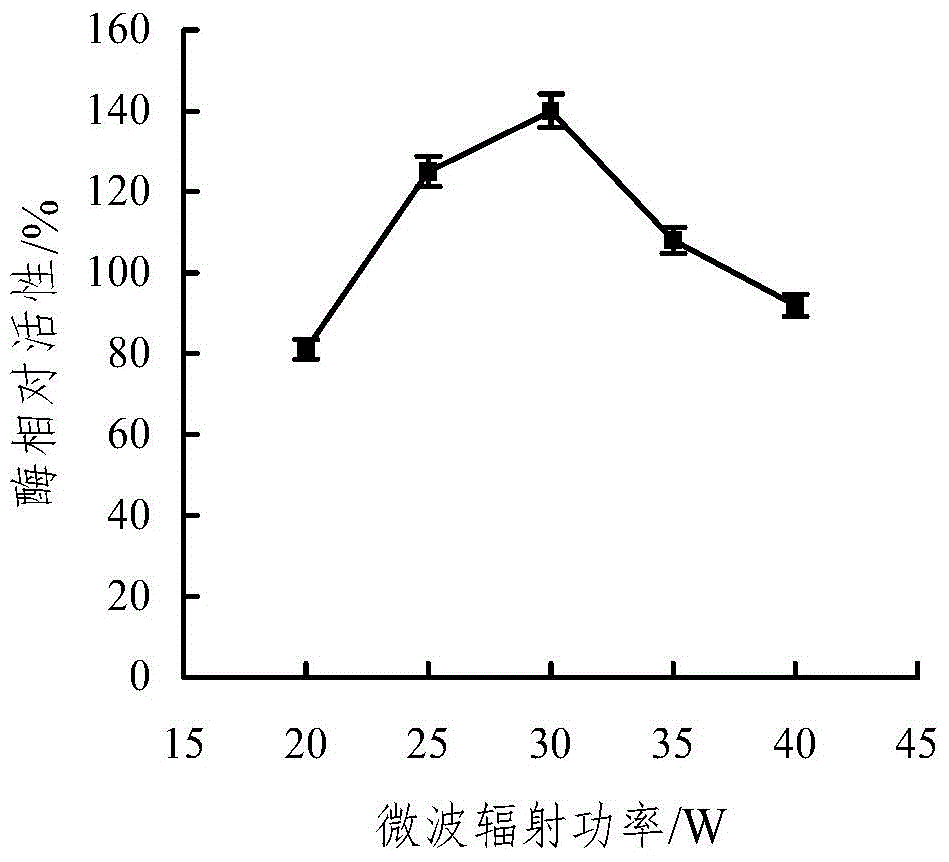
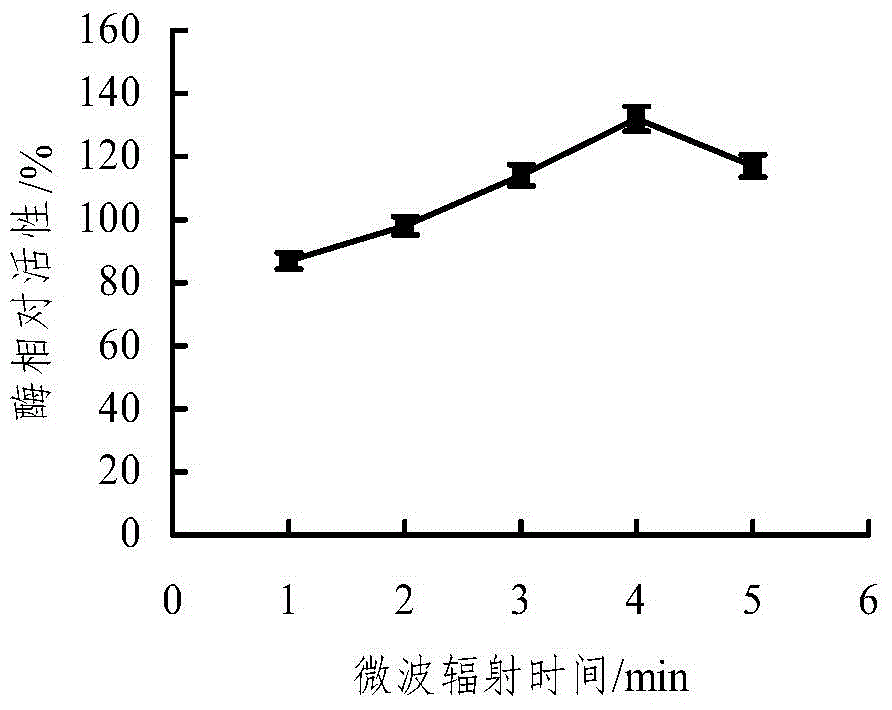
The invention discloses a microwave-assisted co-immobilization method of aldehyde ketone reductase and glucose dehydrogenase. The method includes the steps that a carrier and a crosslinking agent are mixed and activated, centrifuging is conduced, sediments are taken and washed through a PBS solution, centrifuging is conducted, and the sediments are re-dispersed in the PBS solution; the aldehyde ketone reductase and the glucose dehydrogenase are added to a dispersed solution, the mixed liquid is irradiated at the temperature of 0 DEG C to 10 DEG C under the 20-45 W microwave condition for 1 min to 5 min, centrifuging is conducted, sediments are taken and washed through the PBS solution, and co-immobilized enzyme is obtained; compared with free enzyme, the catalytic activity, heat stability and PH stability of the co-immobilized enzyme prepared through the method are improved; the glucose dehydrogenase serves as a coenzyme regeneration system of the glucose dehydrogenase to provide NAD(P)H required by a reaction, the cost is lowered, and industrial production is facilitated.
Owner:HANGZHOU NORMAL UNIVERSITY
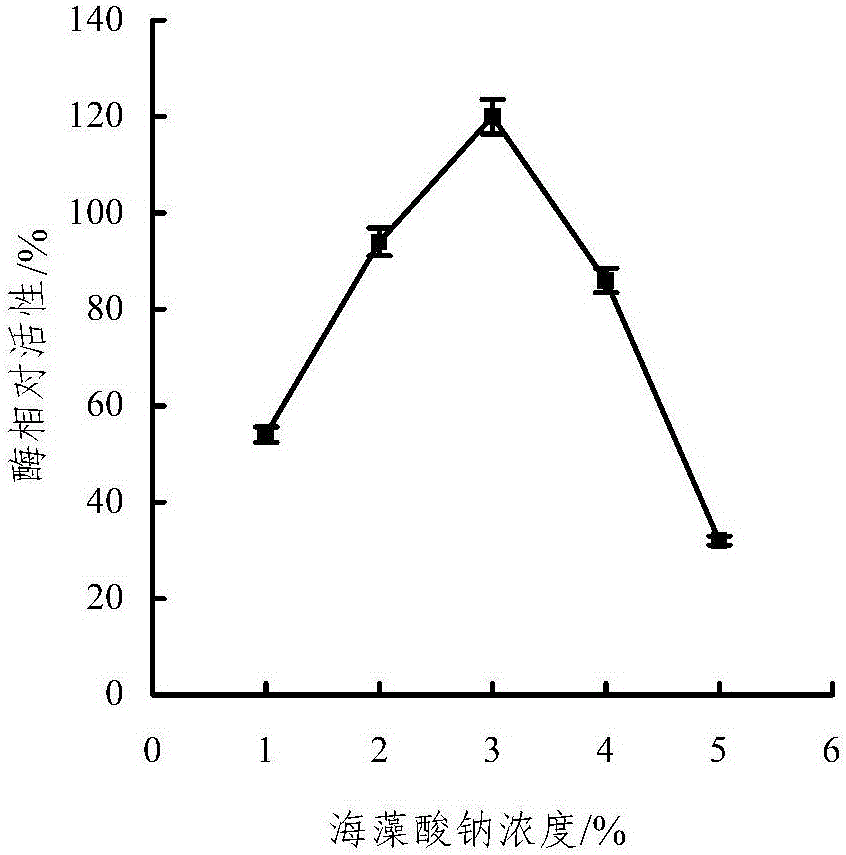
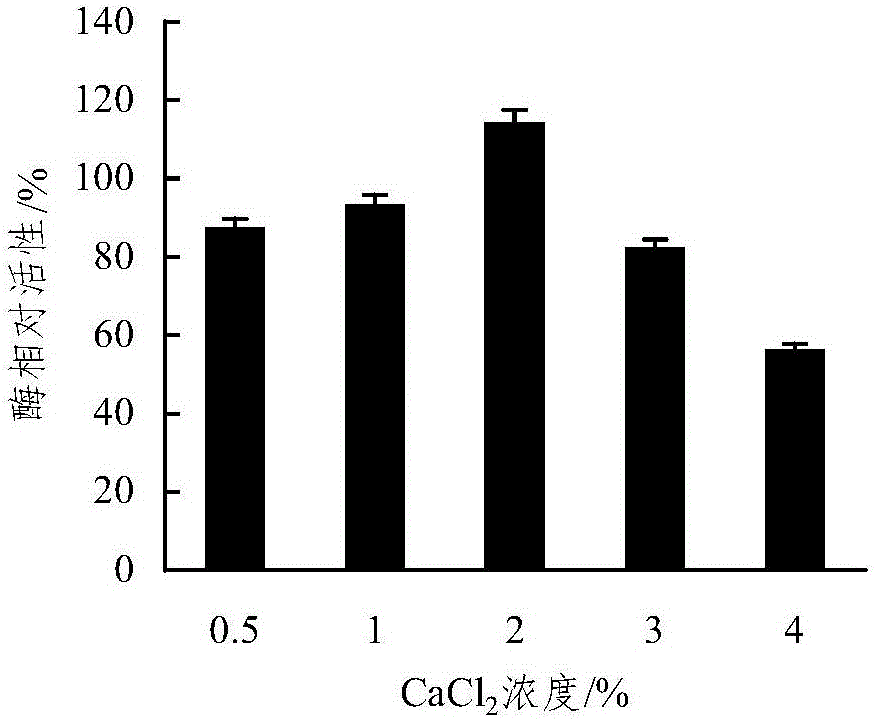
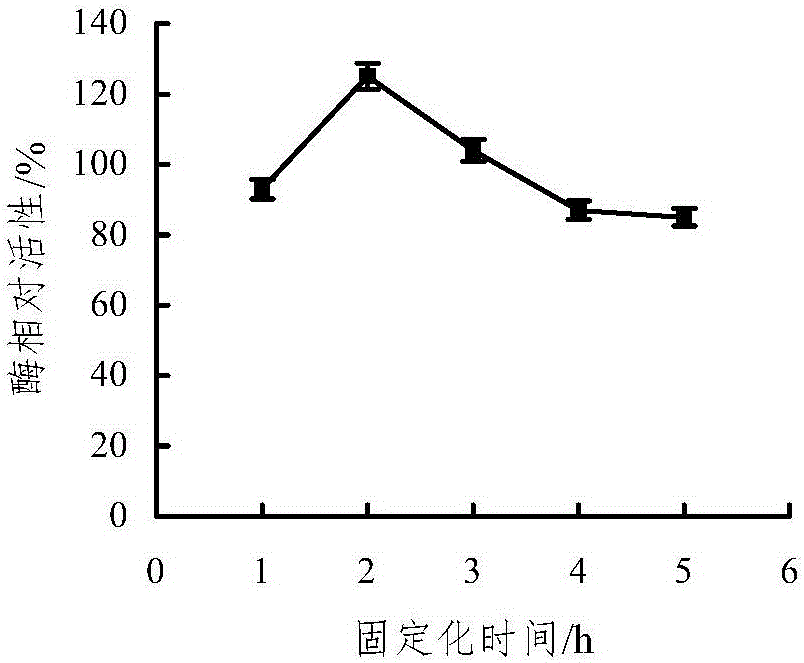
Embedding and co-immobilization method of aldehyde ketone reductase and glucose dehydrogenase
ActiveCN104988133AHigh catalytic activityImprove thermal stabilityOn/in organic carrierMulti-enzyme systemsFiltrationRefrigerated temperature
The invention discloses an embedding and co-immobilization method of aldehyde ketone reductase and glucose dehydrogenase. The method includes the steps that the aldehyde ketone reductase and the glucose dehydrogenase are mixed and then added to a sodium alginate aqueous solution to be stirred and mixed evenly, then the mixture is dropwise added to a Cacl2 aqueous solution, the mixture is made to stand in a refrigerator at the temperature of 4 DEG C, washing is conducted through distilled water, vacuum filtration is conducted, and immobilized particles are obtained. Compared with free aldehyde ketone reductase, the catalytic activity of the co-immobilized enzyme prepared through the method is increased by 1.32 times, the heat stability, the PH stability and other performance of the co-immobilized enzyme prepared through the method are improved as well, and meanwhile, the cost is lowered due to reuse of the co-immobilized enzyme.
Owner:HANGZHOU NORMAL UNIVERSITY
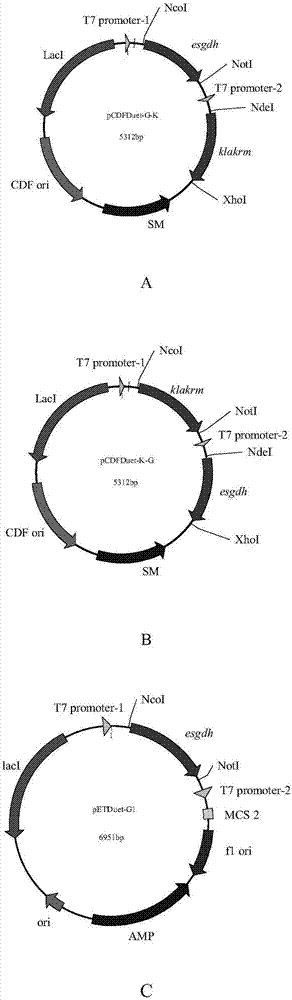
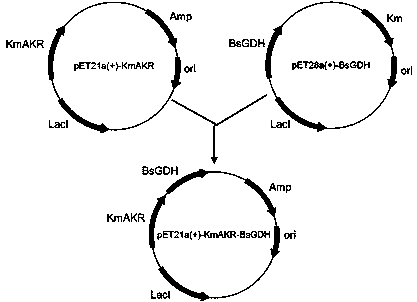
Aldo-keto reductase gene recombinant coexpression vector, engineering bacteria and application thereof
The invention discloses an aldo-keto reductase gene recombinant coexpression vector, engineering bacteria and application thereof. The aldo-keto reductase gene recombinant coexpression vector is based on plasmid pET-28b, plasmid pETDuet-1 or plasmid pCDFDuet-1 and comprises an aldo-keto reductase mutant gene of which the nucleotide sequence is shown in SEQ ID NO:1, and a glucose dehydrogenase gene of which the nucleotide sequence is shown in SEQ ID NO:3. On the basis that the maximum substrate feeding amount of a method that two enzymes are combined and catalyzed after being separated and independently expressed is 50g / L (which is disclosed in the patent application (201610124451.5)), the maximum substrate feeding amount is further increased to 60g / L or greater, meanwhile a two-enzyme coexpression system is beneficial to whole-cell immobilization, and relatively good industrial application prospects can be achieved.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of ricefield eel aldo-keto reductase polyclonal antibody
ActiveCN106478822AStrong specificityEstablishing an in vitro immunoassaySerum immunoglobulinsOxidoreductasesStainingMicroscopic observation
The invention discloses a preparation method of a ricefield eel aldo-keto reductase polyclonal antibody. The preparation method comprises the following steps of: (1) cloning of ricefield eel Eakr gene; (2) establishment of a recombinant expression vector of the ricefield eel Eakr gene; (3) inducible expression and purification of recombinant protein of ricefield eel Eakr; and (4) preparation and Western blot analysis of polyclonal antibody. In the invention, a recombinant expression vector of ricefield eel Eakr gene is established by molecular biology and genetic engineering to induce expression, and purified recombinant expression protein is obtained through affinity chromatography. The ricefield eel Eakr polyclonal antibody prepared by the method disclosed by the invention can meet the experimental requirements of immunohistochemistry and Western blot, realizes in-vitro immunoassay, studies the functions of ricefield eel Eakr as well as ricefield eel immunofluorescent staining laser confocal microscopic observation and also can be applied to the immunodetection of aldo-keto reductase of grass carp, pseudobagrus fulvidraco, megalobrama amblycephala, snakehead and crucian.
Owner:YANGTZE UNIVERSITY
Preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction
InactiveCN102206686AHigh catalytic efficiencyIncrease productivityBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The present invention discloses a preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction and used recombinant vectors and genetically engineered bacteria. The preparation method comprises the step of carrying out biotransformation reaction on methyl o-chlorobenzoylformate used as the substrate with genetically engineered bacteria wet cells or freeze-dried cells capable of coexpressing recombinant reductase and recombinant glucose dehydrogenase as the catalyst at pH 6-8 in the presence of glucose, wherein the recombinant reductase is a recombinant aldo-keto reductase. The genetically engineered bacteria whole cells can simultaneously express the aldo-keto reductase and glucose dehydrogenase and can achieve high-efficiency regeneration of intracellular coenzyme NADP<+>. By using the preparation method, high-concentrations methyl o-chlorobenzoylformate can be catalyzed and completely transformed into methyl (R)-o-chloromandelate with a single conformation, without adding a coenzyme. Because of expensive coenzyme, the preparation method provided by the invention greatly lowers the production cost, has mild reaction conditions, is environmentally-friendly and simple to operate, and has good industrial application prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
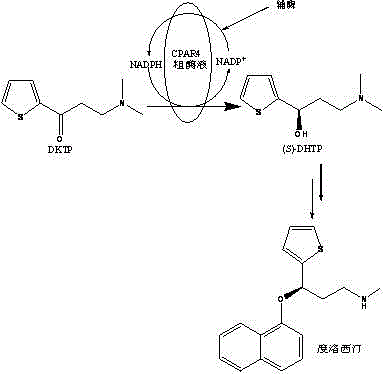
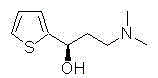
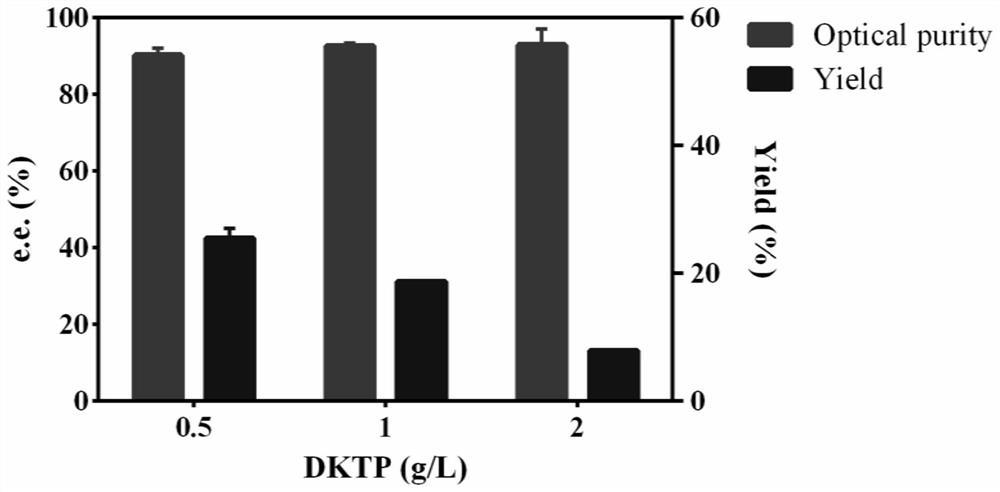
Method catalytically synthesizing (S)-N, N-dimethyl-3-hydroxy-(2-thiofuran)-1-propylamine((S)-DHTP) by aldehyde ketone reductase recombinant strain crude enzyme system
The invention discloses a method catalytically synthesizing (S)-N, N-dimethyl-3-hydroxy-(2-thiofuran)-1-propylamine((S)-DHTP) by aldehyde ketone reductase recombinant strain crude enzyme system, and belongs to the technical field of biological catalysis asymmetric conversion. An auxiliary substrate and original bulk coenzyme NADP<+> are added in the recombinant strain crude enzyme system to accelerate regeneration cycle of coenzyme NADPH in the system; the reaction substrate is N, N-dimethyl-3-ketone-(2-thiophene)-1-propylamine (DTKP); the recombinant strain is E. coliBL21(DE3)(pETCPAR4); aldehyde ketone reducing enzyme gene cpar4 comes from Candida parapsilosis; the gene coded aldehyde ketone reducing enzyme gene CPAR4 catalytically and asymmetrically reduces the DKTP into (S)-DHTP. The method utilizes the cell-free system to conduct catalytic reaction; coupling enzyme required by regeneration of the coenzyme is not extra added in the reaction system; direct acting efficiency of the enzyme and the substrate is improved; reaction time is shortened; the conversion effect is relatively good.
Owner:JIANGNAN UNIV
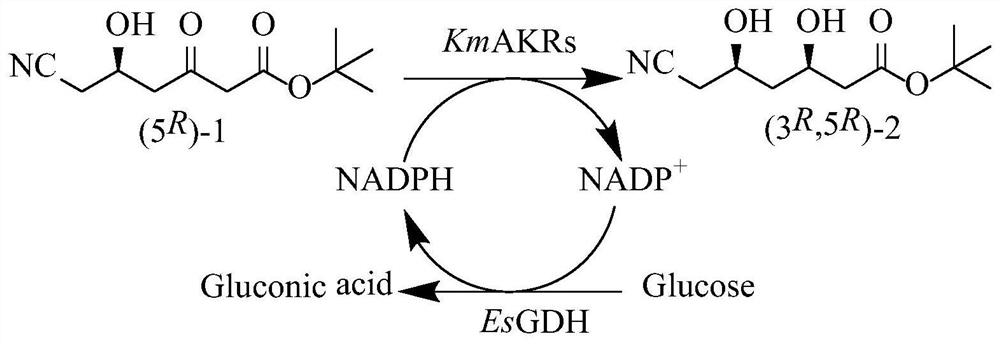
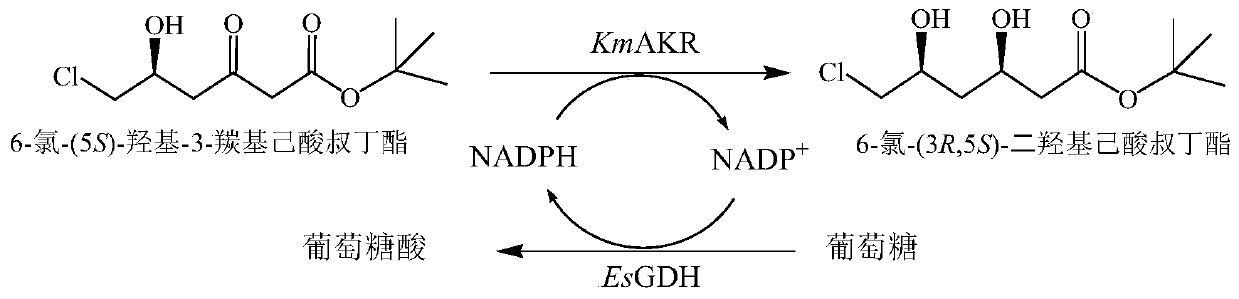
Aldehyde ketone reductase KmAKR mutant and application thereof in catalytic synthesis of chiral alcohol
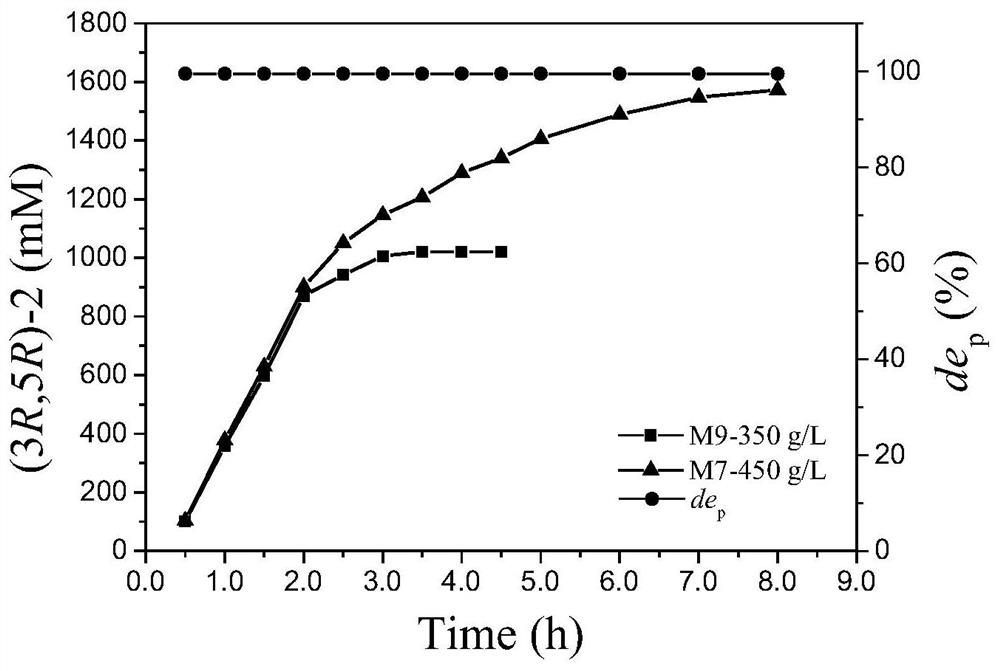
The invention discloses an aldehyde ketone reductase KmAKR mutant and application thereof in catalytic synthesis of chiral alcohol. The aldehyde ketone reductase KmAKR mutant is obtained by performing single-site or multi-site mutation on the 30 site, the 302 site, the 109 site, the 196 site, the 23<rd> site or the 213<rd> site of an amino acid sequence shown in SEQ ID NO. 1. The specific enzyme activities of the constructed aldehyde ketone reductase mutants M7 and M9 are increased by 1.1 times and 0.63 times respectively compared with those of an original strain, and the T5015 is increased by 6.3 DEG C and 4.9 DEG C respectively. For the mutant M9, when the feeding amount of a substrate 6-cyano-(5R)-hydroxy-3-carbonyl tert-butyl hexanoate can reach 350g / L, the reaction can be completed within 3.7h, the substrate conversion rate is greater than 99%, the dep value of the product is always kept at 99.5% or above, and the space-time yield reaches 1.82kg / L.d.
Owner:ZHEJIANG UNIV OF TECH
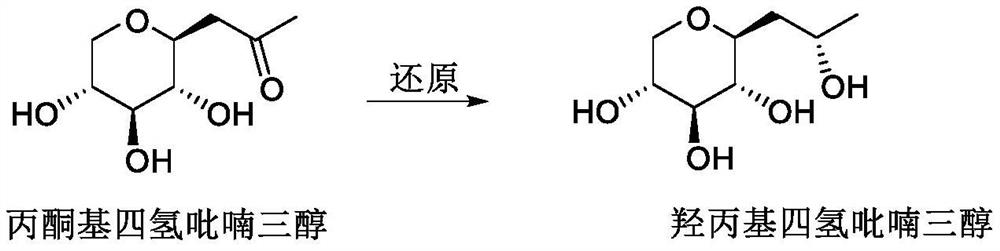
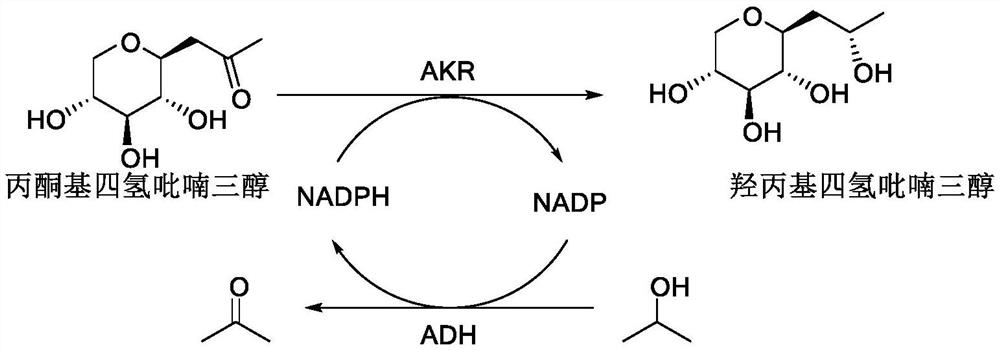
Synthetic method of hydroxypropyl tetrahydropyrantriol catalyzed by biological enzyme
PendingCN113416756AHigh puritySuitable for synthesisOxidoreductasesGenetic engineeringEthanol dehydrogenaseOrganic synthesis
The invention belongs to the field of organic synthesis, fine chemicals and daily chemicals, and particularly relates to a synthetic method of hydroxypropyl tetrahydropyrantriol catalyzed by a biological enzyme. According to the method, a double-enzyme circulating catalysis system containing aldoketo reductase (AKR) and ethanol dehydrogenase (ADH) is used, and in the presence of oxidized coenzyme II (NADP), isopropanol is used as a reducing agent, so that the synthesis of the hydroxypropyl tetrahydropyrantriol is realized. As biological enzyme catalysis is adopted, the method is mild in condition, simple and convenient to operate, small in pollution, green, safe, free of metal residues and high in product purity, and is particularly suitable for synthesis of active matters of medicines and cosmetics.
Owner:SHANGHAI COACHCHEM TECH
Method for asymmetric synthesis of duloxetine intermediate by carbonyl reductase
The invention belongs to the technical field of biocatalysis and discloses a method for asymmetric synthesis of duloxetine intermediate by carbonyl reductase.In a pure enzyme catalytic system, by control of reaction pH, reaction temperature and metal ions, biocatalysis performance is improved.N,N-dimethyl-3-keto-3-(2-thienyl)-1-propylamine (DKTP) serves as a reaction substrate, a recombinant strain refers to E.coli BL21 / Pet21c-cr2 expressing an aldo-keto reductase gene, a carbonyl reductase gene cr2 is derived from Candida macedoniensis AKU4588 and codes carbonyl reductase CR2, and catalysis of asymmetric reduction of DKTP is realized to obtain (S)-DHTP.By pure enzyme catalytic reaction and optimal control of the reaction pH, the reaction temperature and the metal ions, properties and functions of DKTP catalyzed by CR2 can be known, highly-stereoselective DKTP catalysis is realized, and optically-pure duloxetine key intermediate (S)-DHTP is prepared through asymmetric conversion reaction.
Owner:JIANGNAN UNIV
Industrial Applications of A Novel Aldo/Keto Reductase Of Zymomonas Mobilis
InactiveUS20120196342A1Reducing xylitol productionIncreased ethanol yieldBacteriaBiofuelsXylose fermentationFurfural
The present invention relates to methods of reducing the toxicity of lignocellulosic hydrolysates which comprise one or more inhibitors. One method reduces the amount of furfural inhibitor leading to a more effective process. Another method reduces the amount of xylitol produced during the fermentation of xylose present in lignocellulosic hydrolysates. Naturally occurring aldo / keto reductase enzymes, as well as, enzymes produced by recombinant cells or by selective adaptation may be employed.
Owner:GEORGIA TECH RES CORP
Multi-point immobilization method based on modification of enzyme with unnatural amino acid
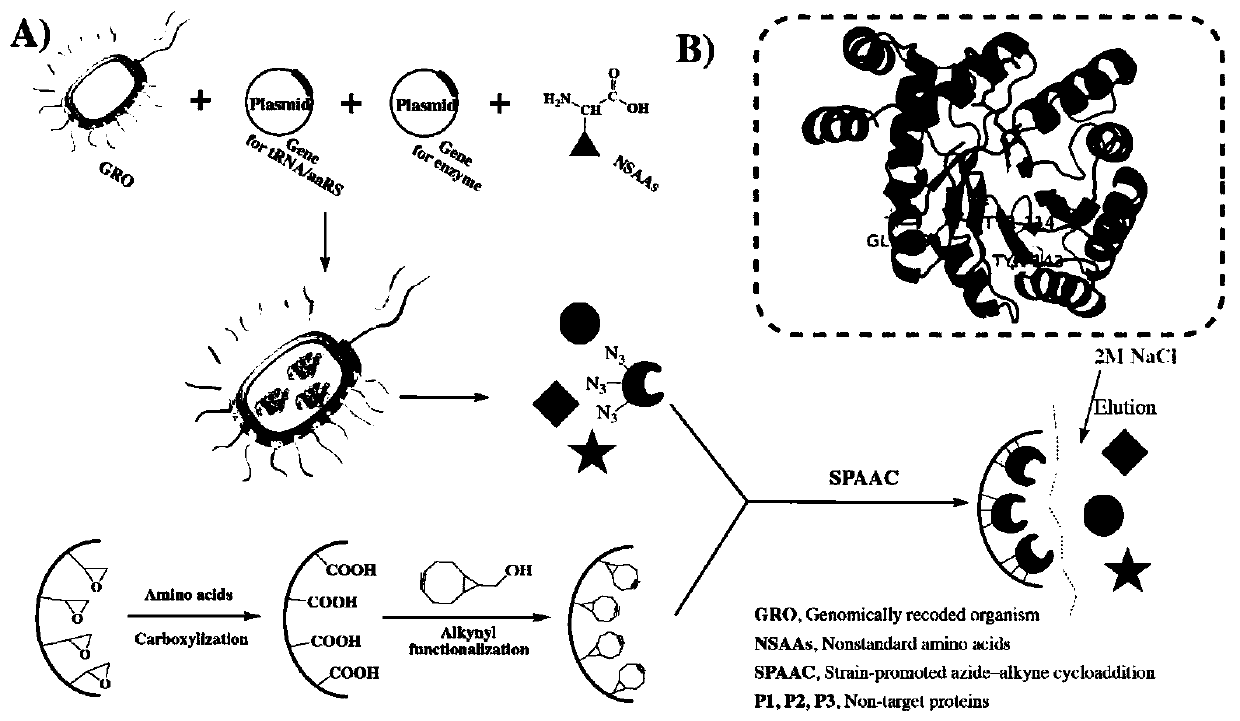
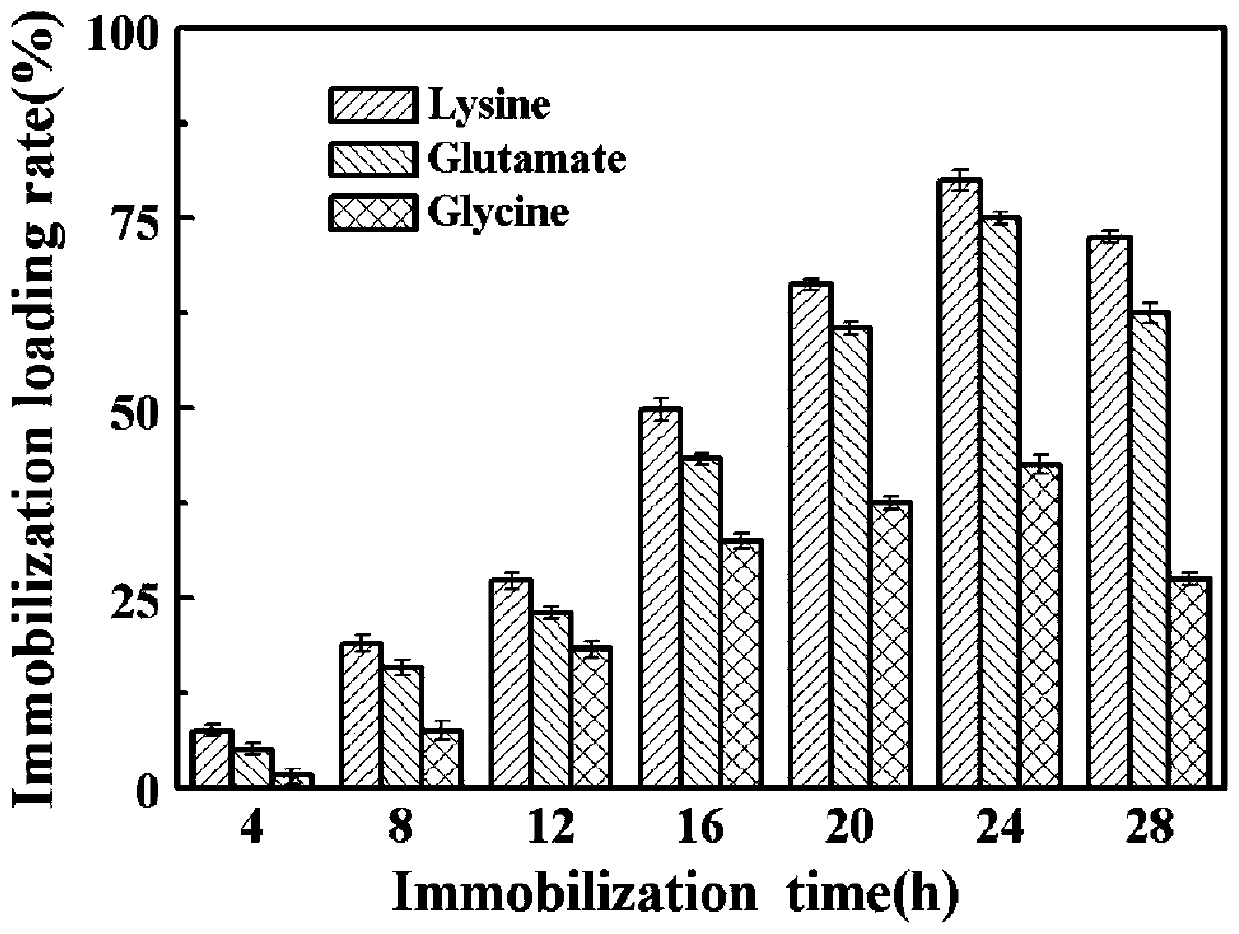
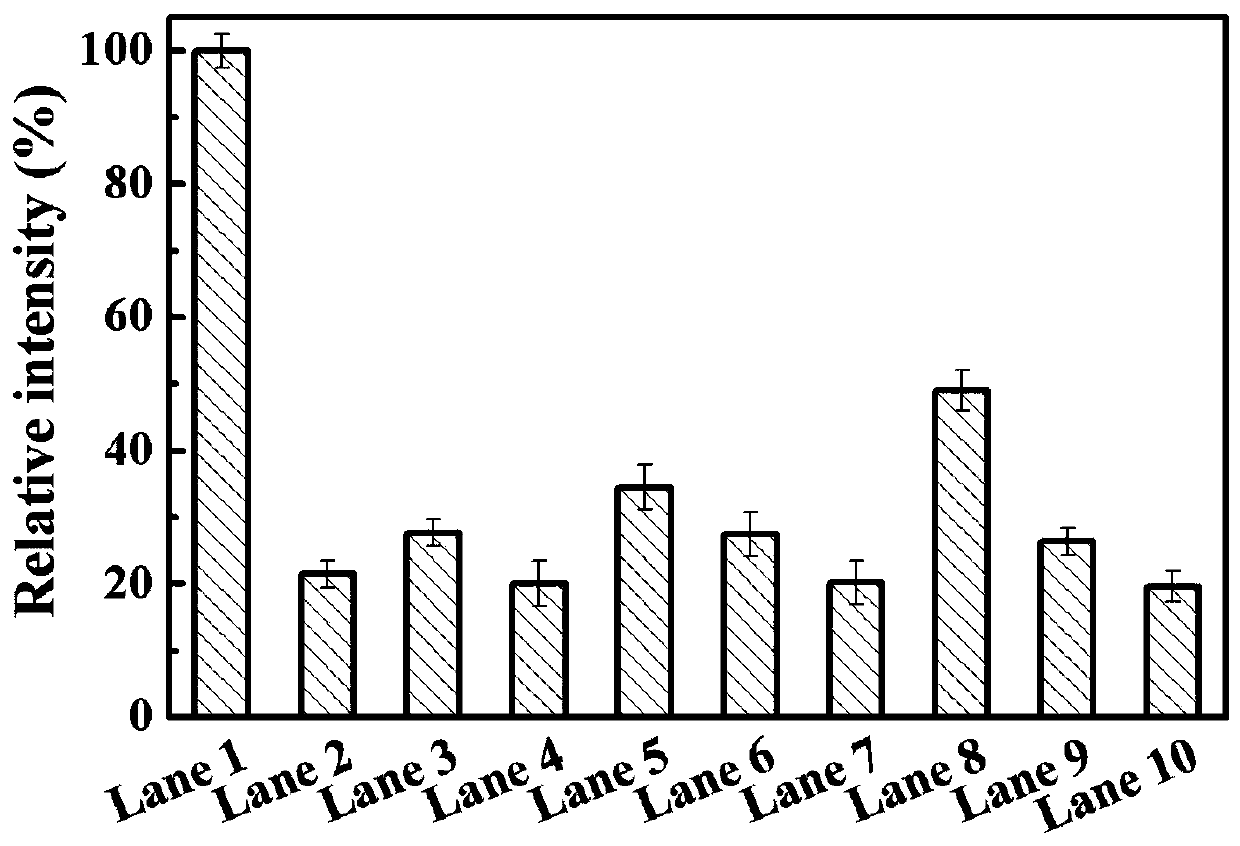
PendingCN110669753AReduce pollutionIncrease enzyme activityOxidoreductasesOn/in organic carrierEpoxyCycloaddition
The invention relates to a multi-point immobilization method based on modification of an enzyme with an unnatural amino acid. According to the method, an aldo-keto reductase is modified with an unnatural amino acid, p-azido-L-phenylalanine, the unnatural amino acid-modified aldo-keto reductase is immobilized with a cyclooctyne-modified amino-activated epoxy polymer as a solid carrier to achieve multi-point covalent linkage, and one-step purification and immobilization of the enzyme is completed by alkyne-azide cycloaddition reaction. The immobilized aldo-keto reductase modified by the unnatural amino acid prepared by the method has greatly improved enzyme activity compared with free enzyme. Through a copper-free click reaction, a traditional tedious purification method is eliminated for the aldo-keto reductase, and the five-point immobilization can achieve a purification efficiency of 60%. The method is safe and reliable, has less environmental pollution, greatly saves time and cost consumed by purification, and is suitable for industrial production.
Owner:HANGZHOU NORMAL UNIVERSITY
Kluyveromyces marxianus aldehyde ketone reductase KmAKR mutant and application thereof
The invention discloses a kluyveromyces marxianus aldehyde ketone reductase KmAKR mutant and an application thereof. The aldehyde ketone reductase mutant is obtained through performing fixed-point saturation mutation on a 63rd site of an amino acid sequence as shown in SEQID NO.2. The specific enzyme activity of the constructed aldehyde ketone reductase mutant M5-A is increased by 1.1 times than that of aldehyde ketone reductase in a control group, the specific enzyme activity of the constructed aldehyde ketone reductase mutant M5-L is increased by 3.2 times than that of the aldehyde ketone reductase in the control group, the specific enzyme activity of the constructed aldehyde ketone reductase mutant M5-M is increased by 4.1 times than that of the aldehyde ketone reductase in the controlgroup, wherein the mutant KmAKR-Y296W / W297H / K29H / Y28A / T63M has remarkably-improved catalytic activity on 6-cyano-(5R)-hydroxy-3-carbonyl hexanoate tert butyl, 6-chloro-(5S)-hydroxy-3-carbonyl hexanoate tert butyl and the like. The feeding quantity of the largest substrate namely the 6-cyano-(5R)-hydroxy-3-carbonyl hexanoate tert butyl can reach 450g / L, the substrate conversion rate is higher than99%, the de value of products is always maintained to be 99.5% or above, and the time and space yield in the biocatalysis process is as high as 1224.3g / L d.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing chiral duloxetine intermediate by aldehyde ketone reductase and asymmetric reduction
PendingCN111979207AEasy to operateHigh optical purityOxidoreductasesFermentationDuloxetineBacillus megaterium
The invention discloses a method for preparing chiral duloxetine intermediate by aldehyde ketone reductase and asymmetric reduction, and belongs to the technical field of asymmetric synthesis of chiral compounds by biological method. The aldehyde ketone reductase obtained by the invention comes from Bacillus megaterium BM1-1. Through strain culture, gene cloning, engineering bacterium construction, aldehyde ketone reductase expression and purification, with the addition of NADPH and auxiliary substrates, aldehyde ketone reductase catalyzes the substrate 3-(dimethylamino)-1-(2-thienyl)-1-acetone(DKTP) to produce (S)-3-(dimethylamino)-1-(2-thienyl)-1-propanol(S-DHTP). According to the present invention, the reaction system and reaction conditions based on the catalyzed asymmetric reduction of DKTP. The method of the invention has convenient operation and simple equipment, has good industrial application prospects in the field of biocatalytic preparation of chiral duloxetine intermediates, and has important significance for the development of special enzyme sources for biocatalysis and the research of chiral compound synthesis methods in the future.
Owner:HUAQIAO UNIVERSITY
Monopterus albus AKR (aldo-keto reductase) gene and in-vitro expression method thereof
The invention relates to the field of genetic engineering, in particular to a monopterus albus AKR (aldo-keto reductase) gene with nucleotide and amino acid sequences shown in SEQ ID NO:1 and SEQ ID NO:2. A recombinant expression vector of a monopterus albus Eakr gene is constructed with methods in molecular biology and genetic engineering, induction expression and affinity chromatography are performed, and purified recombinant expression protein is obtained. Monopterus albus Eakr recombinant protein can catalyze different substrates such as methylglyoxal, acetone, progesterone, methyltestosterone and the like and has theoretical guidance significance and application reference value for industrial application of AKR. Besides, the invention further provides an in-vitro expression method of the monopterus albus AKR gene.
Owner:YANGTZE UNIVERSITY
Ezetimibe synthesis intermediate and preparation method and application thereof
ActiveCN104860980AIncrease concentrationHigh optical purityGroup 4/14 element organic compoundsFermentationEnzymeAldo-keto reductase
The invention discloses an ezetimibe synthesis intermediate and a preparation method and application thereof, the ezetimibe synthesis intermediate has the structure as shown in formula III, and is synthesized by enzyme-chemical method, a chiral hydroxyl compound is produced by asymmetric reduction reaction of a raw material compound under the catalysis effect of aldehyde ketone reductase, and a product can be obtained by cyclization reaction. The ezetimibe synthesis intermediate is used in the preparation of ezetimibe, the process is simple, the concentration of the obtained product is high, and the product has the advantages of high optical purity, mild reaction conditions, environmental friendliness, simple operation, easy industrial amplification, and very good industrial application prospect.
Owner:ABIOCHEM BIOTECH CO LTD
Aldo-keto reductase and application thereof
InactiveCN110387361AImprove catalytic performancePotential for industrial applicationsBacteriaMicroorganism based processesNucleotideNucleotide sequencing
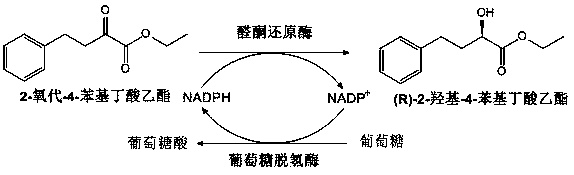
The invention relates to analdo-keto reductase, and application of recombinant expression transformant of the aldo-keto reductaseas a biocatalyst in asymmetric reduction of 2-carbonyl-4-phenylbutanoate to synthesis (R)-2-hydroxyl-4-phenylbutyrate, and belongs to the technical field of bioengineering. The amino acid sequence of the aldo-keto reductase is shown in SEQ ID NO.1. The encodinggene the aldo-keto reductase corresponds to the nucleotide sequence of the amino acid sequence of the SEQ ID NO.1 and is shown in SEQ ID NO.2. The novel aldo-keto reductaseis high in catalytic performance.
Owner:天津迪嘉医药技术开发有限公司 +1
Salt responsive genes useful for generating salt resistant transgenic plants
ActiveUS20090217411A1Confer salt-resistanceLimited successBryophytesSugar derivativesInitiation factorPlant cell
The present invention relates to a transgenic plant comprising one or more plant cells transformed with exogenous nucleic acid encoding a Dunaliella salt-inducible or salt-responsive protein selected from the group consisting of elongation initiation factor 3 (eIF3), NADPH dependent quinone reductase (QOR), aldo-keto reductase (AKR), bifunctional aspartate kinase-homoserine reductase (AK-HSD) and mitochondrial import membrane translocase subunit (TIM9), or a fragment, homolog or variant thereof. The transgenic plant has increased tolerance to salt as compared to a corresponding non-transgenic plant. The present invention further relates to nucleic acids, vectors and constructs encoding the Dunaliella salt-inducible or salt-responsive proteins, and to a method of producing a transgenic plant having an increased tolerance to salt, a method of modifying a plant capacity to survive salt shock, and a method of modifying plant recovery after exposure to salt stress, by introducing the nucleic acids, constructs and / or vectors into one or more cells of the plant.
Owner:HAZERA GENETICS LTD +2
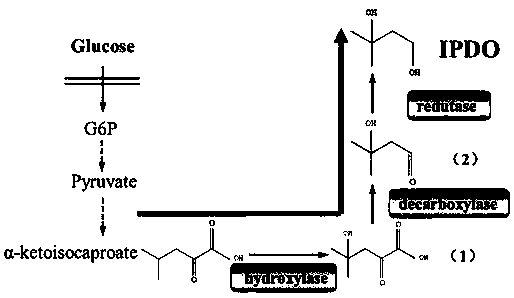
Strain and method for biosynthesis of isopentyldiol
The invention relates to the field of biosynthesis, in particular to a strain and method for biosynthesis of isopentyldiol. According to the strain, by taking alpha-ketoisocaproate (alpha-KIC) as a reaction substrate, the isopentyldiol (IPDO) can be produced by over-expression of isoleucine dioxygenase (IDO), 2-keto acid decarboxylase (Kdc) and aldo-keto reductase. After escherichia coli BL21 (DE3) is introduced into the path, the isopentyldiol (IPDO) of 21.57 mg / L is produced after a finally constructed recombinant escherichia coli engineering strain IP01 is added into a shake flask with the50mM substrate alpha-KIC and is cultured for 48h.
Owner:HUAANTANG BIOTECH GRP CO LTD
Method for synthesizing fludarabine phosphate through biological catalysis
PendingCN113584104AImprove conversion rateHigh yieldTransferasesOxidoreductasesReaction temperaturePhosphoric acid
The invention relates to a method for preparing fludarabine phosphate by an enzyme method. Fludarabine phosphate is prepared by taking fludarabine as a raw material through an enzyme catalytic reaction in the presence of inorganic salt and coenzyme; an enzyme used in the enzyme catalysis reaction is selected from at least one of phosphotransferase, aldoketoreductase, alcohol oxidase or alcohol dehydrogenase; the pH value of the enzyme catalytic reaction is 7.3-7.6, the reaction temperature is 37 DEG C, and the reaction time is 1-4 hours; the coenzyme is ATP. According to the invention, fludarabine phosphate is prepared by adopting a novel enzymatic process, so that the technical problems existing in some existing chemical processes are solved, and the process is improved compared with the existing enzymatic preparation process; the production cost is reduced; the process is environment-friendly and reaction conditions are mild.
Owner:JIANGSU OCEAN UNIV +1
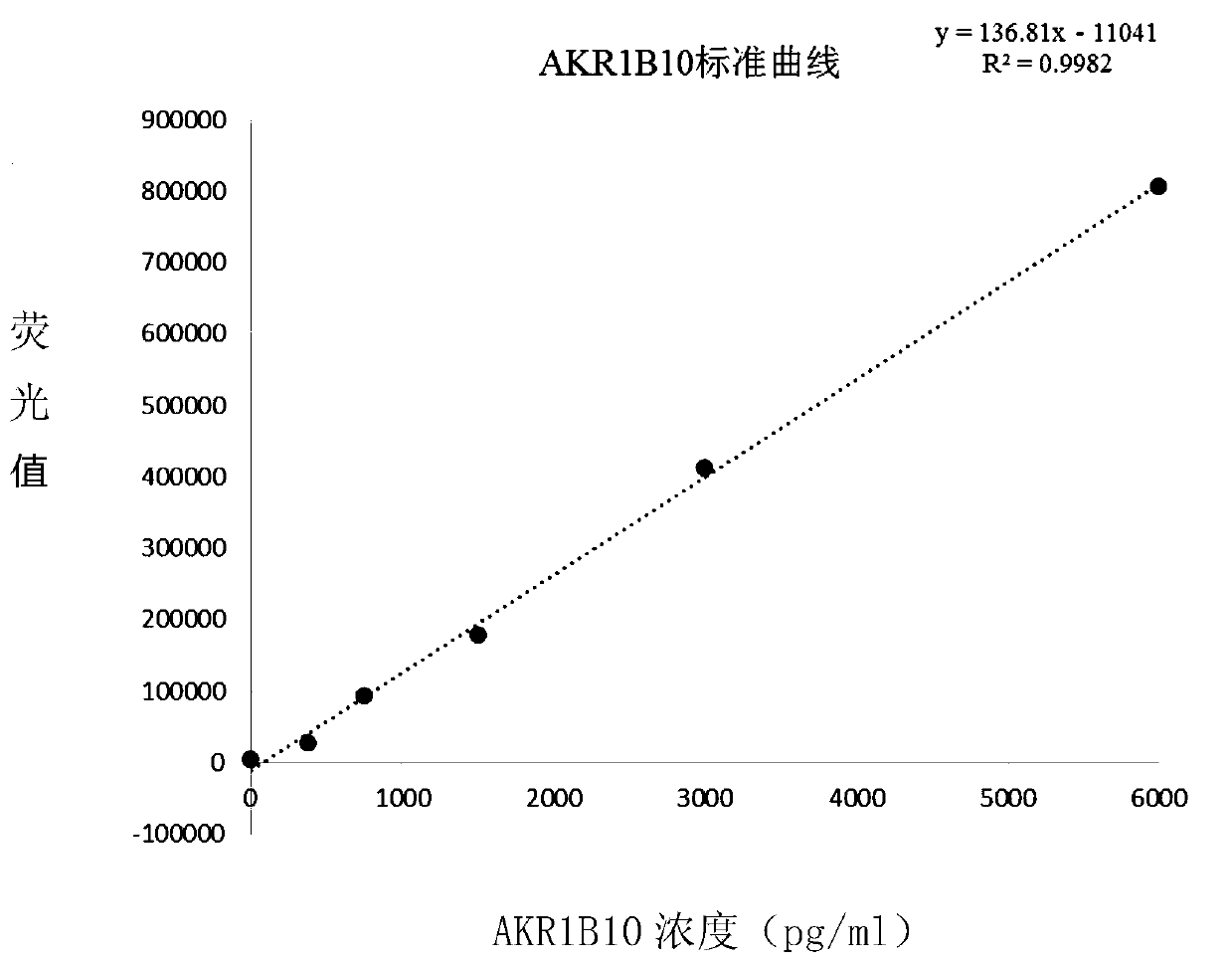
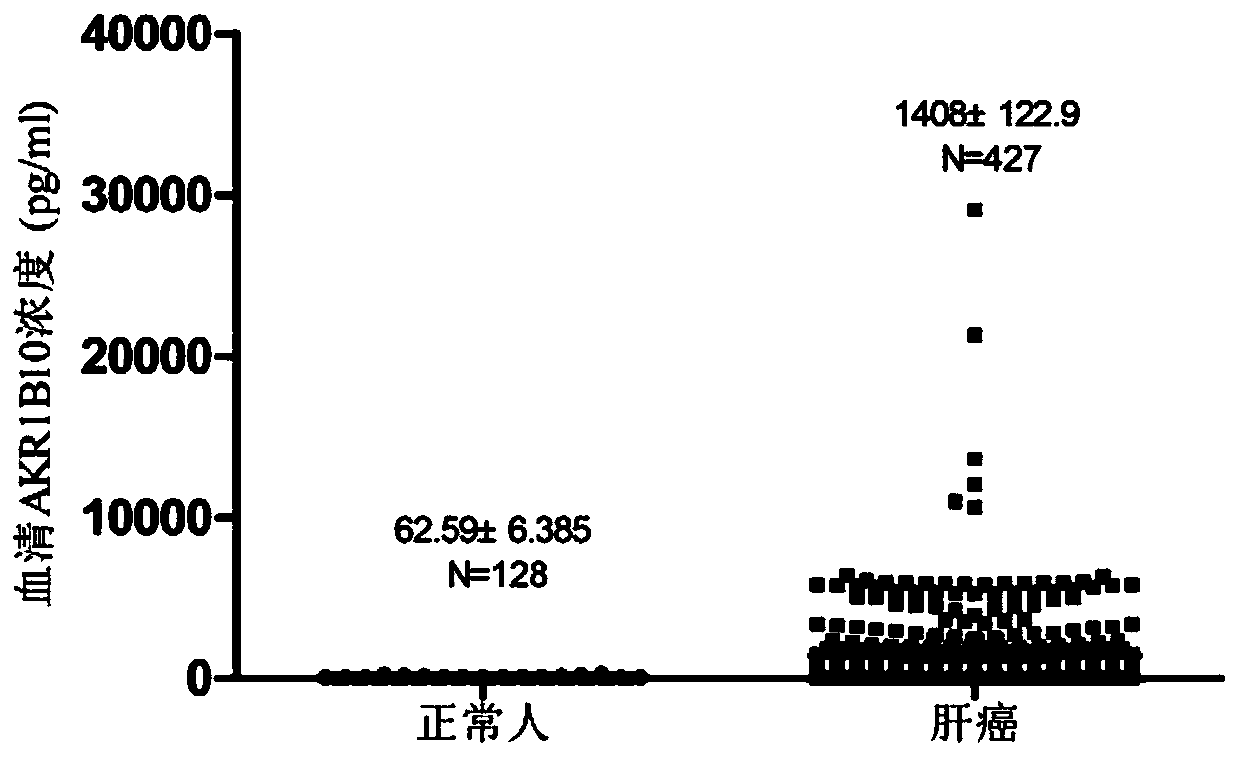
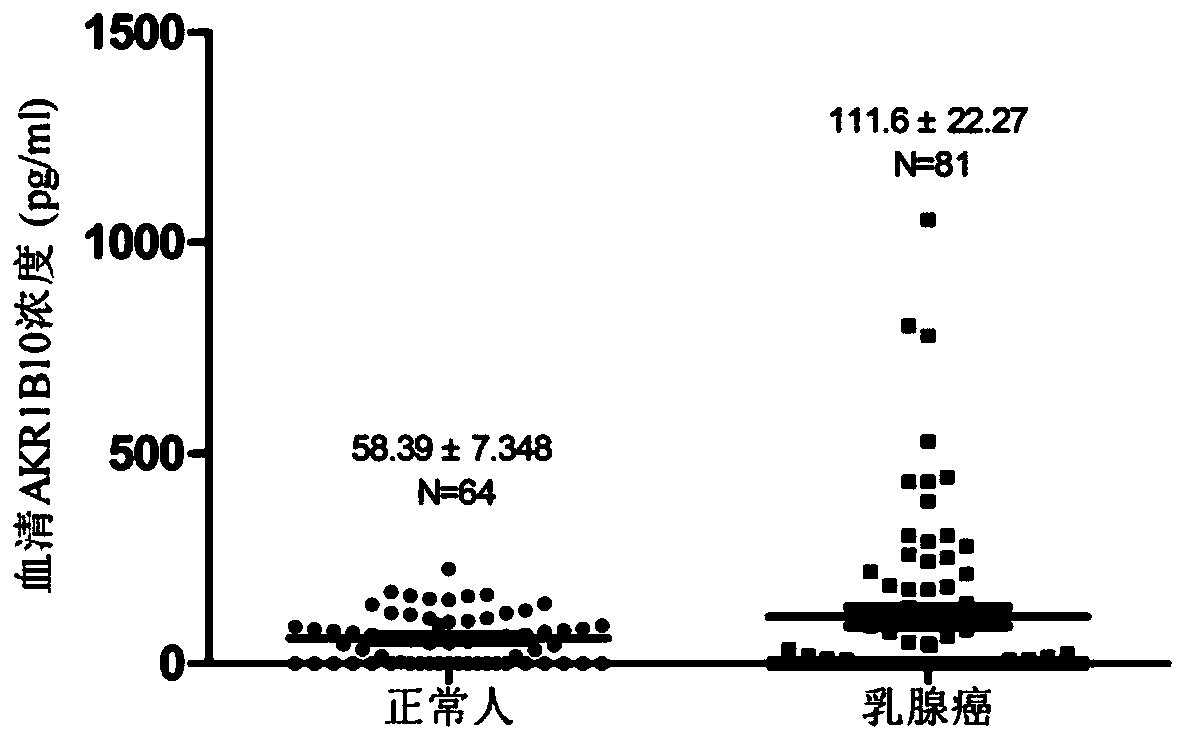
AKR1B10 Chemiluminescence quantitative detection kit and application thereof
PendingCN110579609AHigh sensitivityEasy to mixChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexMagnetic bead
The invention relates to the technical field of medical biology, in particular to a quantitative kit for detecting protein content of Aldo-Keto Reductase 1B10 (AKR1B10) by using a magnetic particle chemiluminescence method, which is used for screening, diagnosing, curative effect judging, prognosis evaluation or relapse monitoring of cancers. The kit comprises an AKR1B10 calibration product, an AKR1B10 quality control product, streptavidin magnetic beads, a biotin labeled antibody, an acridinium ester labeled antibody, an excitation liquid 1 and an excitation liquid 2. The AKR1B10 in serum tobe detected is combined with the biotin labeled antibody and the acridinium ester labeled antibody to form a double-antibody sandwich compound, streptavidin magnetic beads are added into the double-antibody sandwich compound, the excitation liquid 1 and the excitation liquid 2 are used for exciting and detecting luminescence values, and the concentration of AKR1B10 in a sample is calculated by means of a standard curve. The AKR1B10 Chemiluminescence quantitative detection kit adopted for detection has the advantages of good stability, high sensitivity, wide linear range and the like.
Owner:湖南莱拓福生物科技有限公司
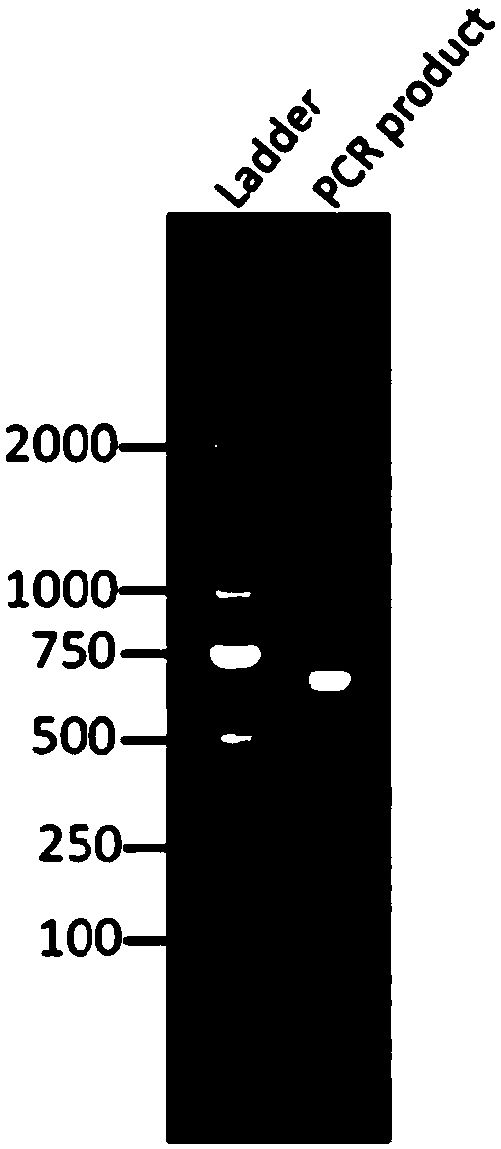
Method for cloning aldo-keto reductase gene of unknown bacterium
InactiveCN109385420ARich candidate gene resourcesMicrobiological testing/measurementDNA preparationBacteroidesGenomic DNA
The invention relates to the field of molecular biology, in particular to a method for cloning an aldo-keto reductase gene of an unknown bacterium. The method for cloning the aldo-keto reductase geneof the unknown bacterium comprises the following steps: 1), amplifying by a PCR technology, wherein a primer adopts a first degenerate primer and a template is a genomic DNA of the unknown bacterium;2), performing BLAST comparison on a part of the amplified aldo-keto reductase gene to obtain a culture with the closest genetic relationship and the sequence of the intact aldo-keto reductase gene ofthe culture with the closest genetic relationship; 3), according to the sequence of the intact aldo-keto reductase gene of the culture with the closest genetic relationship in the step 2), designinga second degenerate primer, and amplifying the intact aldo-keto reductase gene of the unknown bacterium by the PCR technology, wherein in the PCR amplification technology in the step, the primer is the second degenerate primer and a template is a genomic DNA of the unknown bacterium.
Owner:HUBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com