Recombined aldehyde ketoreductase mutant, gene, carrier, engineering bacteria and application of recombined aldehyde ketoreductase mutant
A technology of genetically engineered bacteria and mutants, applied in genetic engineering, oxidoreductase, applications, etc., can solve the problems of borane compounds such as flammability, low optical purity of products, high energy consumption, etc., and achieve stereoselectivity and activity improvement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
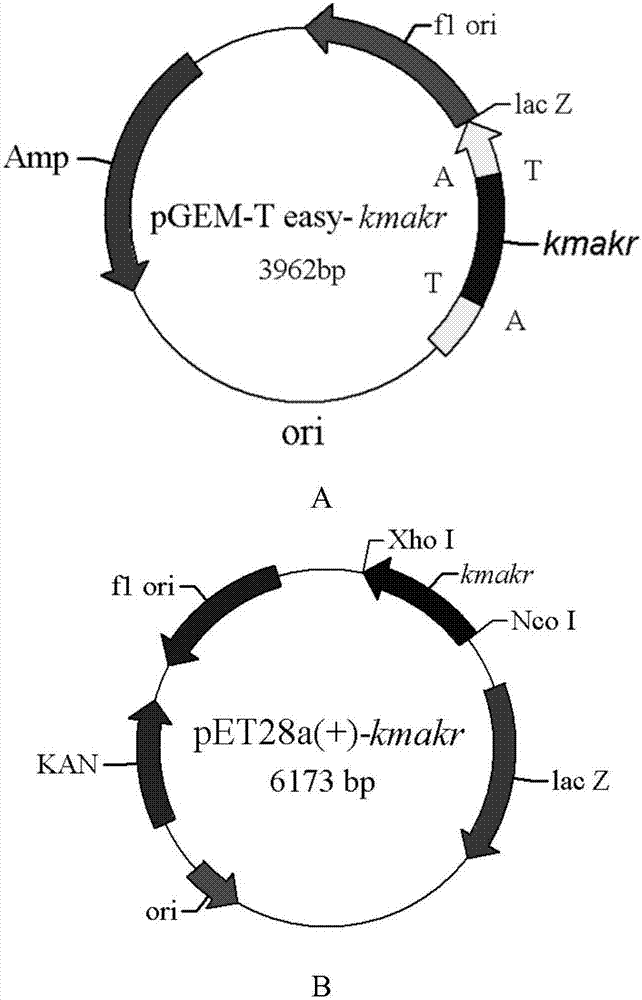
[0032] Embodiment 1: the construction of wild-type aldehyde and ketone reductase KmAKR gene clone and its recombinant expression vector
[0033] (1) Cloning of the target gene
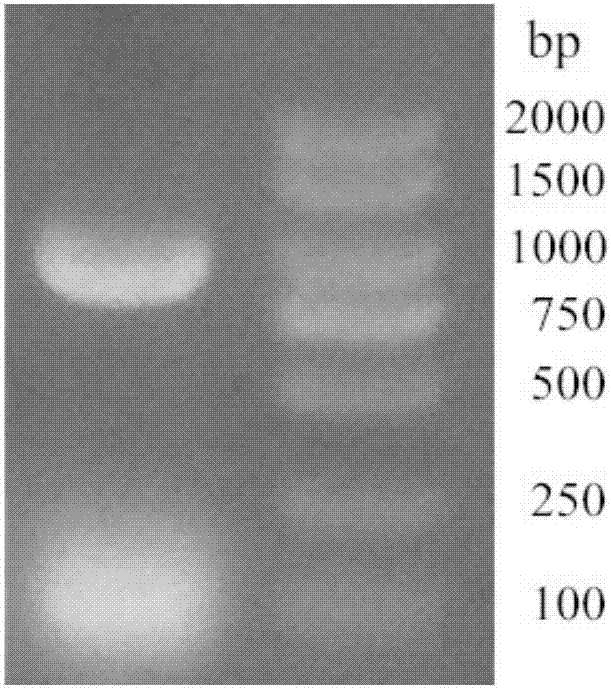
[0034] Using Kluyveromyces marxianus (CICC32920) genomic DNA as a template, through the upstream primer: 5'- CCATGG CCATGACAAAACCAAAAGTTCTTTACTT-3', downstream primer: 5'- GCGGCCGC TCACTTCTGGGATTCAGAAT-3' was amplified by PCR to obtain the amplified product. Take 10 μL of the PCR amplification product and detect it by 0.9% agarose gel electrophoresis. figure 1 As shown, a band appeared around 1000bp;
[0035] Composition of PCR reaction system (total volume 100 μL): 10 times Pfu DNA polymerase buffer (containing Mg 2+ ) 10 μL, 0.5 μL of 10 mM dNTP mixture (dATP, dCTP, dGTP, dTTP each 2.5 mM), 0.5 μL each of the clone upstream primer and downstream primer at a concentration of 50 μM, 1 μL of genomic DNA, 1 μL of Pfu DNA polymerase, and 86.5 μL of nucleic acid-free water. Using a Biorad PCR instru...
Embodiment 2
[0038] Example 2 Construction of aldehyde and ketone reductase mutant KmAKR-W297H recombinant expression vector
[0039] Based on the pET28a(+)-kmakr constructed in Example 1, in order to improve the stereoselectivity of the original type aldehyde and ketone reductase and the substrate 6-cyano-(5R)-hydroxyl-3-oxoylhexanoic acid tert-butyl Two rounds of point-saturation mutagenesis were performed on the ester activity. In the first round of mutation, the gene sequence of pET28a(+)-kmakr was used as a template, and W297-F and W297-R were used as upstream and downstream primers (Table 1) for PCR amplification (PCR reaction parameters: 95°C for 4min; 95°C for 15s , 55°C for 15s, 72°C for 7min, repeat 30 cycles; 72°C for 10min). The PCR product recovered after amplification was digested with DpnI for 3 h, purified by using a purification kit, and then transformed into E.coliJM109 recipient bacteria, spread on LB solid plates with a final concentration of 50 μg / mLKan, 37 After cul...
Embodiment 3
[0043] Example 3 Purification of wild type aldehyde and ketone reductase KmAKR and recombinant aldehyde and ketone reductase mutants KmAKR-W297H, KmAKR-Y296W / W297H
[0044] According to the method in embodiment 1 and 2, obtain E.coli BL21 (DE3) / pET28a (+ )-kmakr-W297H, and the E.coli BL21(DE3) / pET28a(+)-kmakr-Y296W / W297H of the double-site mutation gene expression vector were cultured.
[0045] (1) Induction culture: E.coli BL21(DE3) / pET28a(+)-kmakr, E.coli BL21(DE3) / pET28a(+)-kmakr-W297H and E.coli BL21(DE3) / pET28a(+) -kmakr-Y296W / W297H, respectively inoculated into Lysogeny-Broth (LB) liquid medium containing Kan at a final concentration of 50 μg / mL, cultured at 180 rpm for 10-12 hours at 37°C, and then inoculated with an inoculum volume concentration of 2% to contain The LB liquid medium with a final concentration of 50 μg / mL Kan was used for expansion culture, and cultured at 37°C and 180 rpm to the OD of the culture medium 600 Between 0.6 and 0.8, add a final concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com