Strain and method for biosynthesis of isopentyldiol
A technology for biosynthesis and isoprene glycol, applied in the direction of microorganism-based methods, biochemical equipment and methods, botany equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 bacterial strain, plasmid and substratum
[0027] The Escherichia coli strains and plasmids used in the present invention are listed in Table 1. Escherichia coli strains DH5a and BL21 were used for gene cloning and expression. Methylobacillus flagellatus KT and lactobacillus lactis were purchased from Leibniz Institute DSMZ (https: / / www.dsmz.de / ) and used to amplify the pathway enzymes for the synthesis of IPDO.
[0028] The pET28a(+) plasmid was used for protein expression in E. coli BL21 strain, and the primers for constructing the plasmid are shown in Table 2.
[0029] Shake flask fermentation: a single colony was cultured overnight in 5mL LB medium, and then inoculated in 10mL Fe II medium (30g / L glucose, 0.5g / L MgSO 4 ·7H 2 O,3g / L KH 2 PO 4 ,12g / L K 2 HPO 4 ,4g / L(NH 4 ) 2 SO 4 , 1g / L yeast extract, 2g / L monosodium citrate and 0.1g / L FeSO 4 ·7H 2 Cultivate at 37° C. in (0), the rotating speed is 200 rpm, and the fermentation time is 48 hour...
Embodiment 2
[0034] Embodiment 2 is used for the plasmid construction of IPDO production
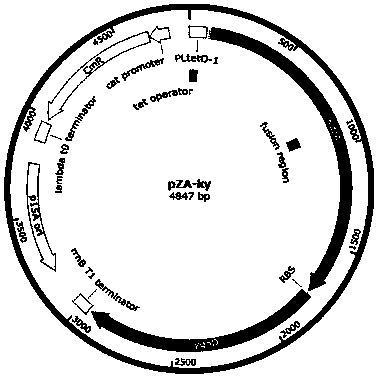
[0035] Taking the construction of pZA-ky as an example, the steps of plasmid pZA-ky construction are as follows: use primers pky-kdc-LF / LR and pky-yqhd-LF / LR to amplify (2-keto acid decarboxylase) kdc and (aldehyde ketone reductase) yqhD genes. Using pZA as a template, a linearized vector with terminal extension of 15-20bp was obtained by PCR using primers pky-vec-LF / LR. After the three fragments were processed for gel recovery, the fusion HD cloning kit (Clontech, TaKara Bio USA Inc., USA) was used to ligate the PCR fragment and the linearized vector in a fusion cloning reaction.
Embodiment 3
[0036] Embodiment 3 gas chromatography analyzes IPDO (GC)
[0037] The concentration of isopentyl glycol-phenylboronic acid was determined by GC (Agilent 7890B, USA). The non-polar capillary column used here is HP5ms UI, 30m x 250μm x 0.25μm, and the carrier gas helium is constant at 80kpa. The FID temperature was set at 350 °C, the injector temperature was 270 °C, the injection volume was 0.2 μL, and the separation was 1:5. The temperature was maintained at 100 °C for 2 min and increased to 270 °C with a gradient of 20 °C / min. Add 1,3-propanediol (1,3-PDO) as an internal reference while testing the IPDO standard.
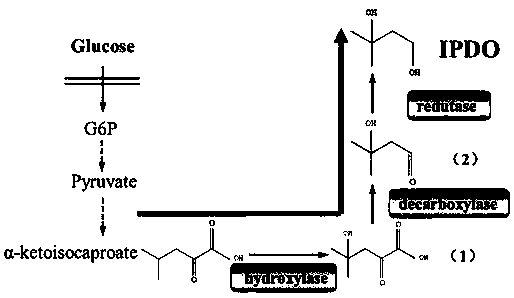
[0038] The effect example is constructed by the synthetic pathway from α-KIC to IPDO
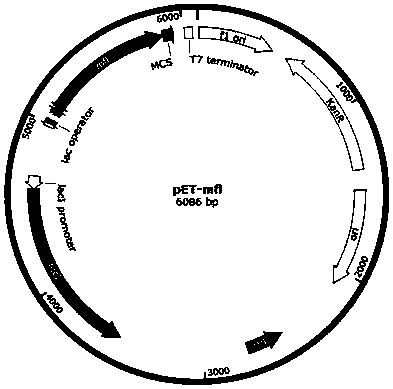
[0039] The mfl gene was amplified from Methylflagellate KT and connected to pET28a vector to obtain pET-mfl. The plasmid map of pET-mfl is as follows figure 2 shown. The kdc and yqhD genes were amplified from the genomes of Lactobacillus and Escherichia coli, respectively, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com