Aldo-keto reductase and application thereof
A reductase, aldehyde and ketone technology, applied in the direction of oxidoreductase, the use of carriers to introduce foreign genetic material, viruses/bacteriophages, etc., can solve the problems of low product concentration, low enzyme catalytic activity, poor substrate tolerance, etc., and achieve high The effect of catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The establishment of embodiment 1 genetically engineered bacteria
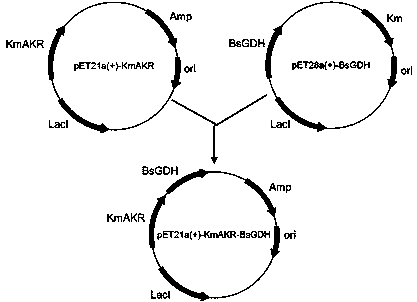
[0035] Using the protein PDB database and NCBI database to screen the aldehyde and ketone reductase gene sequence, obtained the Kluyveromyces marxianus Aldoketone reductase sequence of DMKU3-1042 (Genbank: XP 022677552.1). According to the amino acid sequence of the aldehyde and ketone reductase, combined with the codon preference of Escherichia coli for codon optimization, the gene fragment SEQ ID NO.2 was artificially synthesized and the gene was integrated into the pET21a(+) vector (enzyme Cutting sites are NdeI and XhoI). The glucose dehydrogenase BsGDH (preserved in our laboratory) and the pET21a(+)-KmAKR backbone in the pET28a(+) recombinant plasmid were amplified by PCR technology, and the above two The fragments were integrated to construct the pET21a(+)-KmAKR-BsGDH recombinant plasmid, and the integrated recombinant plasmid was transferred into Escherichia coli BL21(DE3) to establish the al...
Embodiment 2
[0039] Embodiment 2 The cultivation of recombinant escherichia coli
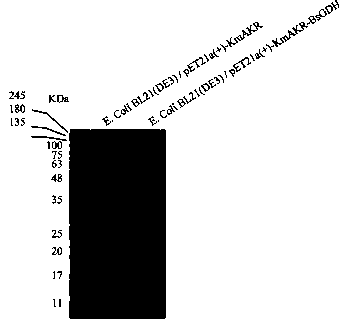
[0040] Inoculate the recombinant Escherichia coli obtained in Example 1 into 20 mL of LB medium containing ampicillin (100 μg / mL) (peptone 10 g / L, yeast extract 5 g / L, sodium chloride 10 g / L, pH 7.0), incubate at 37°C for 10 h in a shaker at 200 rpm. Transfer 1% bacterial culture solution to a shake flask filled with 100 mL LB medium, and shake culture under the same conditions. When the cell OD 600 When it reaches 0.5-0.8, add the inducer isopropyl-β-D-thiogalactoside (IPTG) at a final concentration of 0.2 mM, and induce the expression in the culture medium at 25°C for 10 h. After culturing, the cells were collected by centrifugation (8000 rpm, 10 min, 4°C), washed twice with phosphate buffer (pH 6.8), and centrifuged again to collect the cells (5000 rpm, 10 min, 4°C) for use. Take a small amount of cells for SDS-PAGE to observe the expression of aldehyde ketone reductase and glucose dehydrogenase (see a...
Embodiment 3
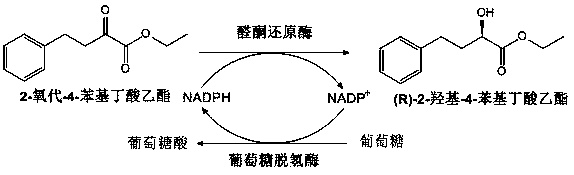
[0041] Example 3 Effects of Different Bacterial Concentrations on the Synthesis of (R)-2-Hydroxy-4-Phenylbutyrate Ethyl Ester
[0042] In a 100 mL reaction vial, add 40 mL of phosphate buffer (pH6.8) with a final concentration of 0.1 M, followed by NADPNa 2 (final concentration of 0.2 mM), glucose (final concentration of 1.2 M) and ethyl 2-oxo-4-phenylbutyrate (final concentration of 0.8 M), recombinant Escherichia coli (final concentration of 80 g / L , 120 g / L, 160 g / L). The reaction temperature was maintained at 30-37°C with a water bath, and the pH was maintained at 6.5-6.8 with a 2 M sodium carbonate solution. The reaction was terminated after 8 h. An equal volume of ethyl acetate was added for extraction, and the organic phase was collected after centrifugation (8000 rpm, 10 min, 4°C). The organic phase was rotary evaporated to a constant volume, and a certain amount of anhydrous sodium sulfate was added to dry overnight to obtain the final product (R)-ethyl 2-hydroxy-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com