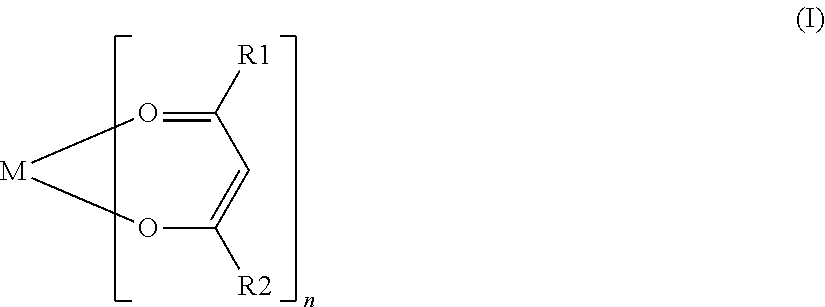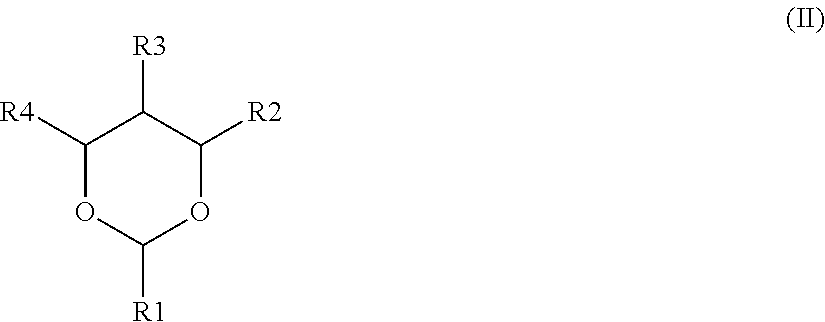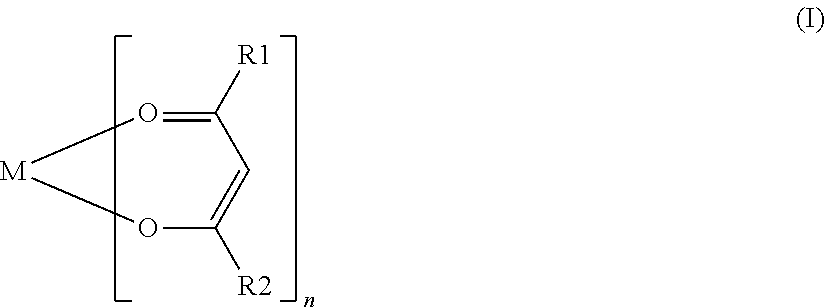Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "Potential oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
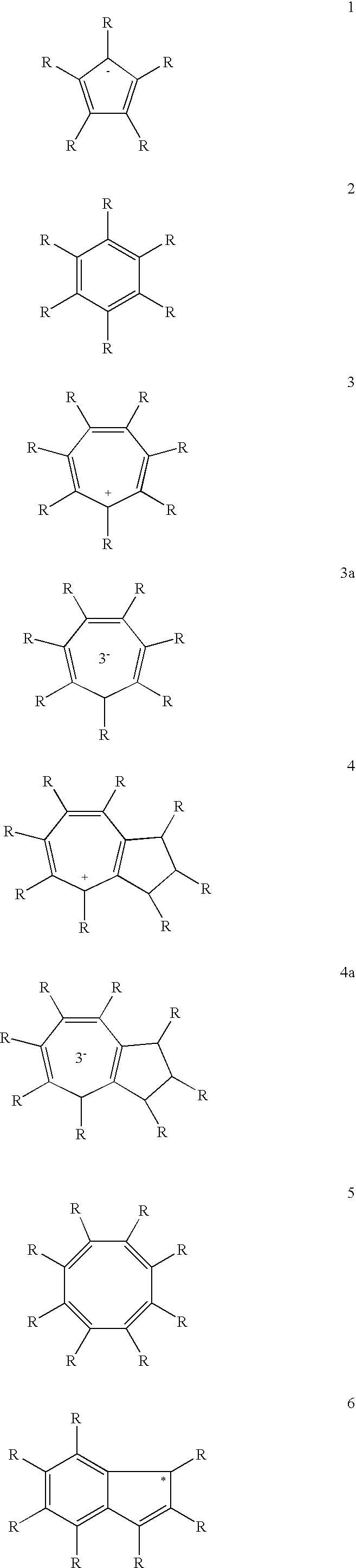
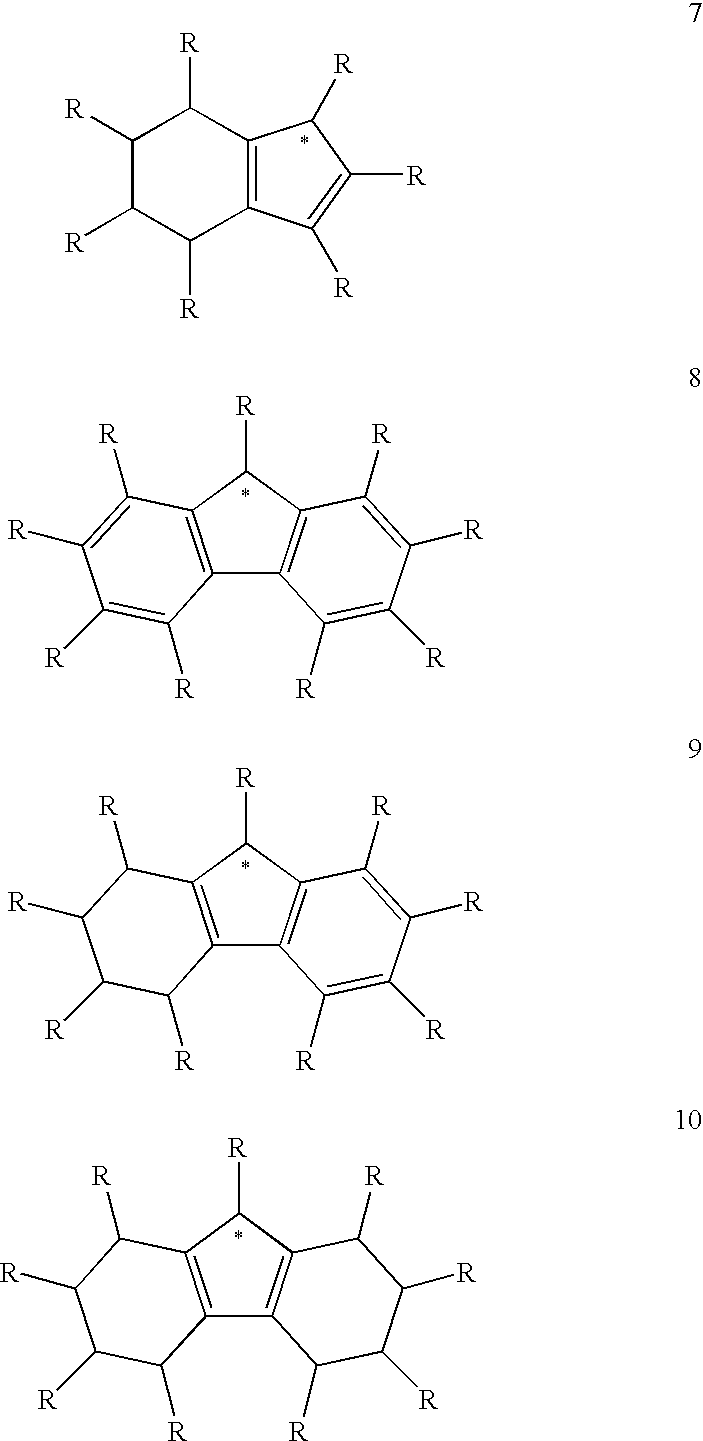
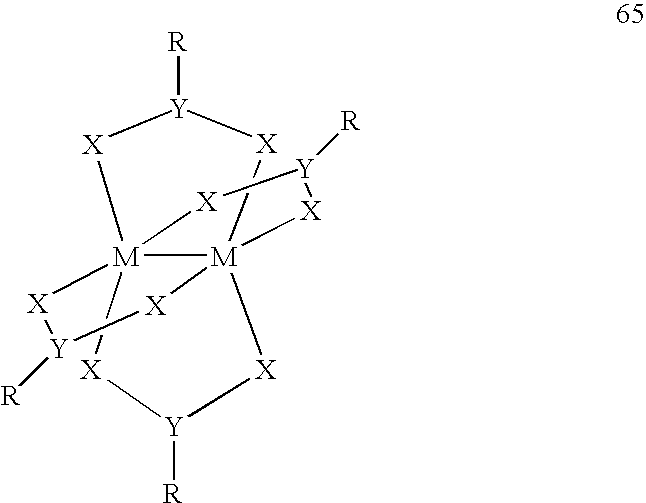
Use of a Metal Complex as an N-Dopant for an Organic Semiconducting Matrix Material, Organic of Semiconducting Material and Electronic Component, and also a Dopant and Ligand and Process for Producing same
ActiveUS20090212280A1Low oxidation potentialEasy to chargeGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsCarbanionValence electron
A method of using a metal complex as an n-dopant for doping an organic semiconducting matrix material in order to alter the latter's electrical characteristics is provided. In order to provide n-doped organic semiconductors with matrix materials having a low reduction potential, while achieving high conductivities, the n-dopant is a neutral electron-rich metal complex with a neutral or charged transition metal atom as a central atom and having at least 16 valence electrons. The complex can be polynuclear and can possess at least one metal-metal bond. At least one ligand can form a π complex with the central atom, which can be a bridge ligand, or it can contain at least one carbanion-carbon atom or a divalent atom. Methods for providing the novel n-dopants are provided.
Owner:NOVALED GMBH
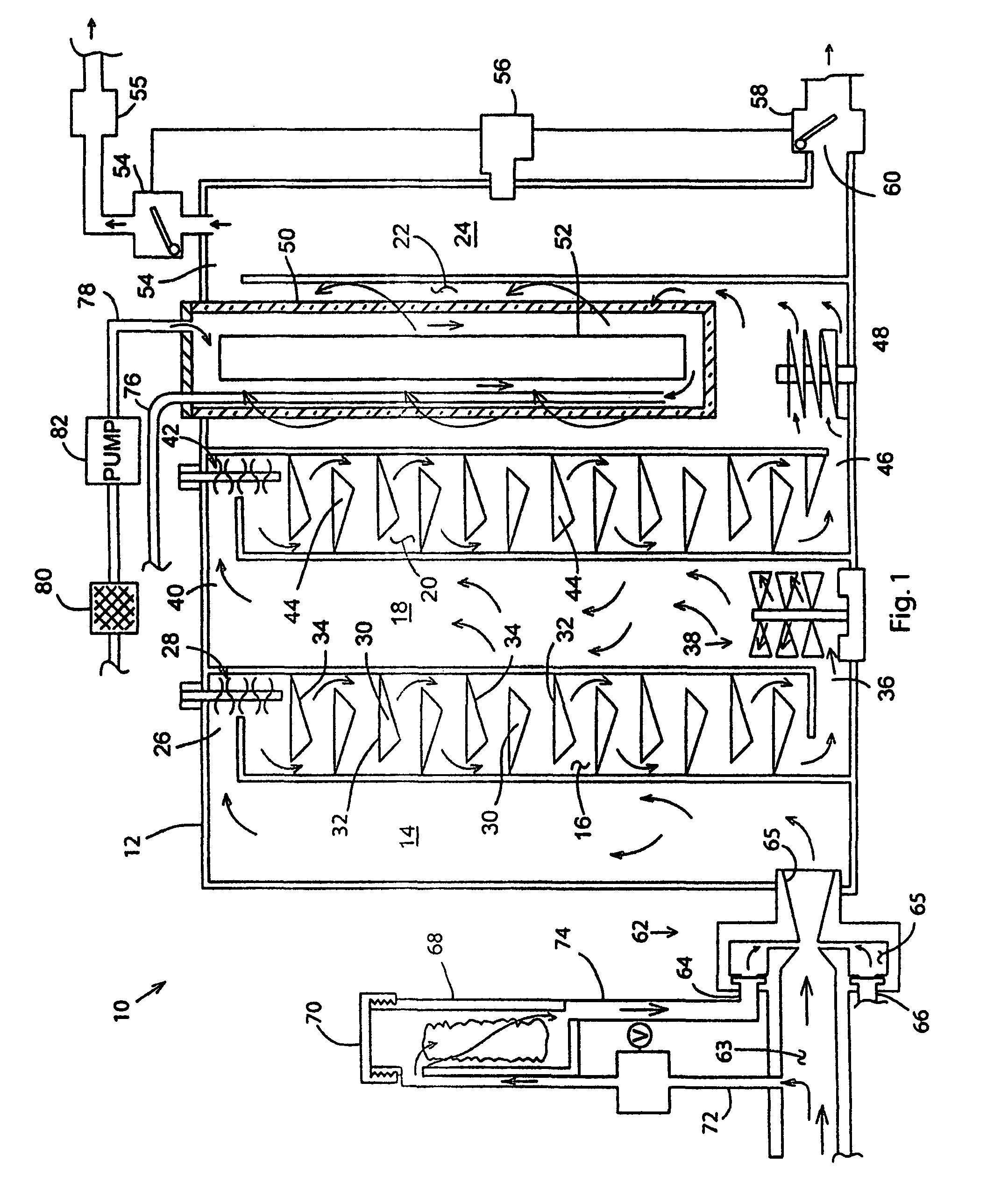
Joining of bipolar plates in proton exchange membrane fuel cell stacks
InactiveUS6887610B2Reduce material costsAvoid contactFuel cells groupingElectrode carriers/collectorsEngineeringProton exchange membrane fuel cell
A bipolar plate assembly for a proton exchange membrane fuel cell stack advantageously connects electrically conductive plate surfaces, without the requirement to weld or braze the plate pairs. Each plate has alternating coolant channels and lands formed on an inside facing surface. An electrically conductive layer is deposited over at least the coolant channels and lands. Pairs of plates are aligned having facing electrically conductive layers. A fluid seal is disposed between the inside facing surfaces about a perimeter of the coolant channels. Each plate pair is compressed to form a plurality of electrical bond lines between adjacent lands within the perimeter seal. The perimeter seal prevents stack reactant gas oxygen from contacting and oxidizing the electrically conductive layer. A dielectric coolant is also used to reduce oxidation of the electrically conductive layer.
Owner:GM GLOBAL TECH OPERATIONS LLC
Lithographic printing method and presensitized plate
ActiveUS20040197701A1Practical amount of energyIncrease impressionRadiation applicationsPhotomechanical apparatusDigital dataNitrogen
Disclosed is a presensitized plate composed of a support having thereon an image recording layer which includes: an infrared absorber (A) that is a cyanine dye having at least one fused ring composed of a nitrogen-containing heterocycle in combination with an aromatic ring or a second heterocycle, and having on the aromatic ring or second heterocycle an electron-withdrawing group or a heavy atom-containing group, a radical generator (B), and a radical-polymerizable compound (C), and which is removable with printing ink and / or dampening water. The presensitized of the present invention can be imaged with an infrared light-emitting laser to directly record an image from digital data on a computer or the like and is then subjected to on-machine development without carrying out a development step, which is capable of providing a large number of good impressions with a practical amount of energy.
Owner:FUJIFILM CORP
Assembly for purifying water
InactiveUS6881331B1Improve solubilityDissolve more quicklyFlow mixersMixing methodsContact timeWater flow
An ozone-based water purification system is disclosed. In this system, a mixing venturi injects ozone and a liquid sanitizer into a stream of water to be treated. Following ozone and sanitizer injection, the stream of water is passed to a contact region where the ozone and sanitizer are allowed to react with contaminants and biota. The stream is then mixed by several mixing devices to allow residual ozone and sanitizer to further react with contaminants and biota. Particularly a counterflow system is employed wherein the stream is directed downward at several points so that bubbles containing ozone are forced to flow upward against the flow, lengthening ozone contact time. Also, the ultraviolet lamp used to generate ozone is mounted so that ultraviolet light therefrom is exposed to the flow water, providing additional sterilizing effects. In addition, a novel mixing venturi is disclosed that mixes a gas containing ozone and a liquid sanitizer into a motive flow of water.
Owner:BARNES RONALD L
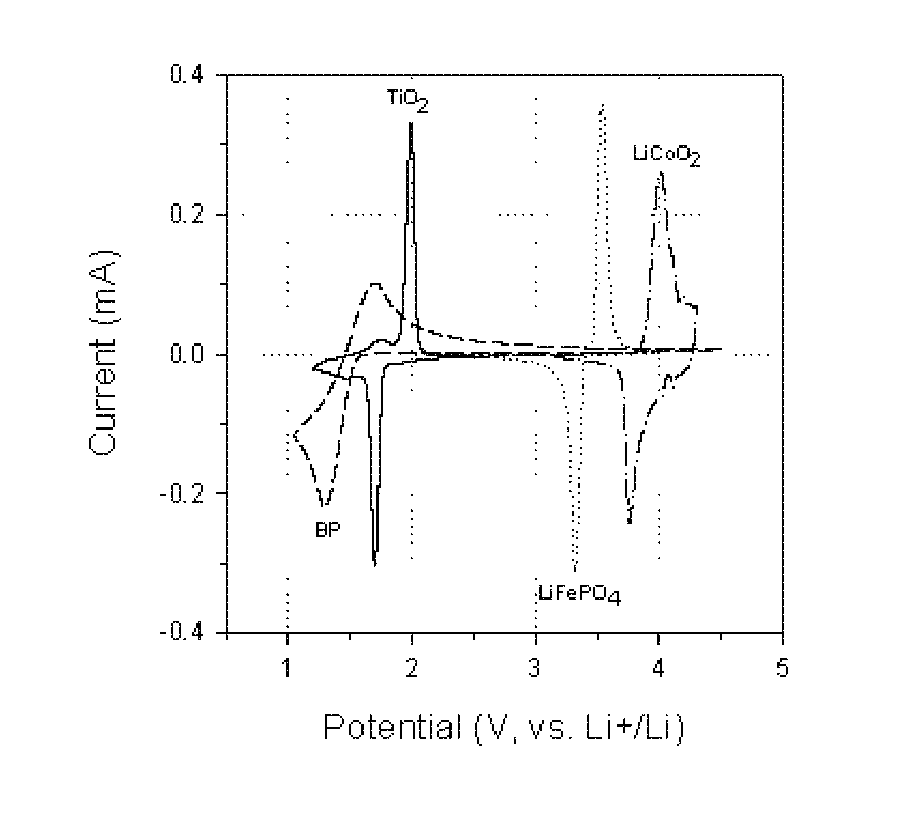
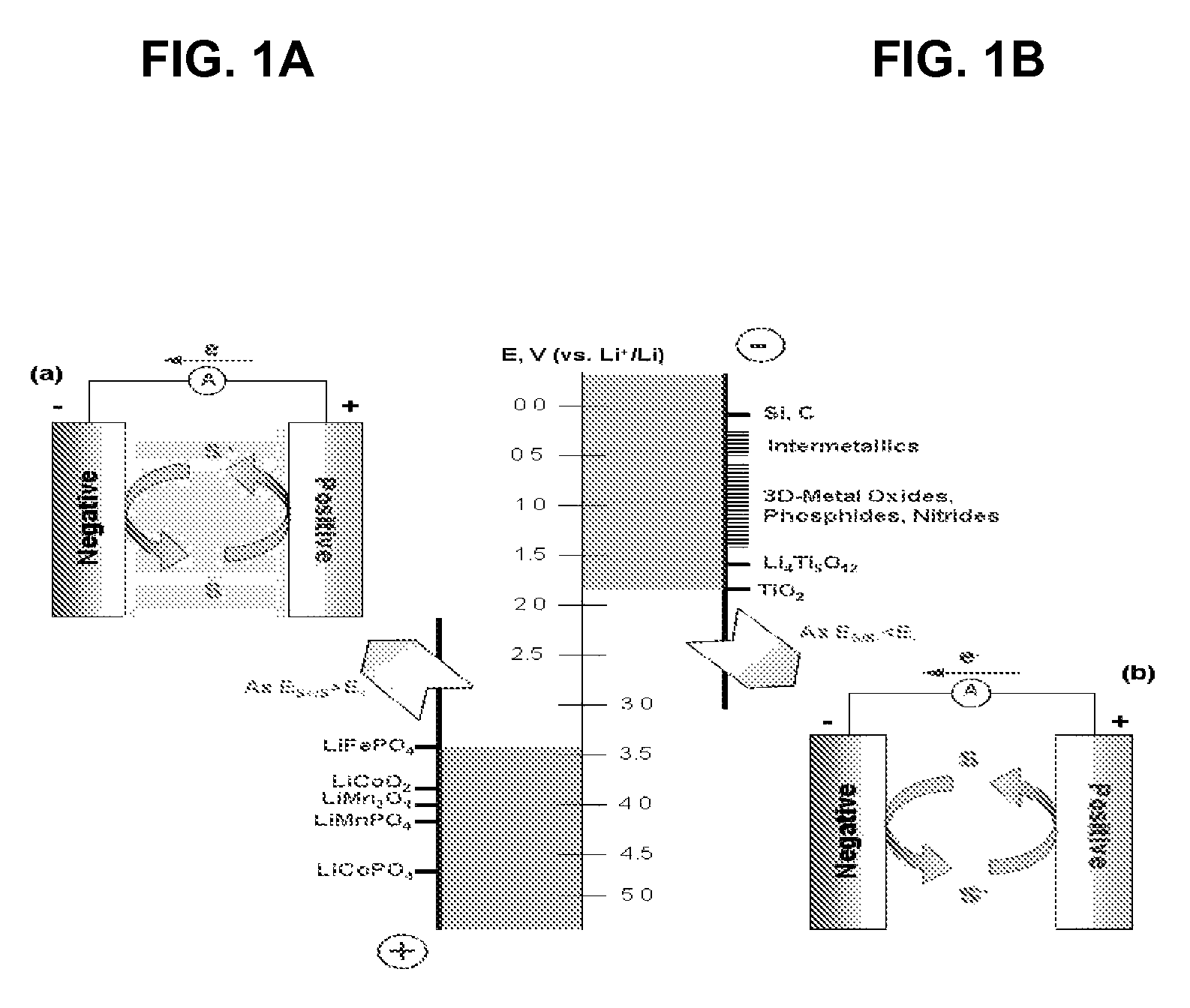
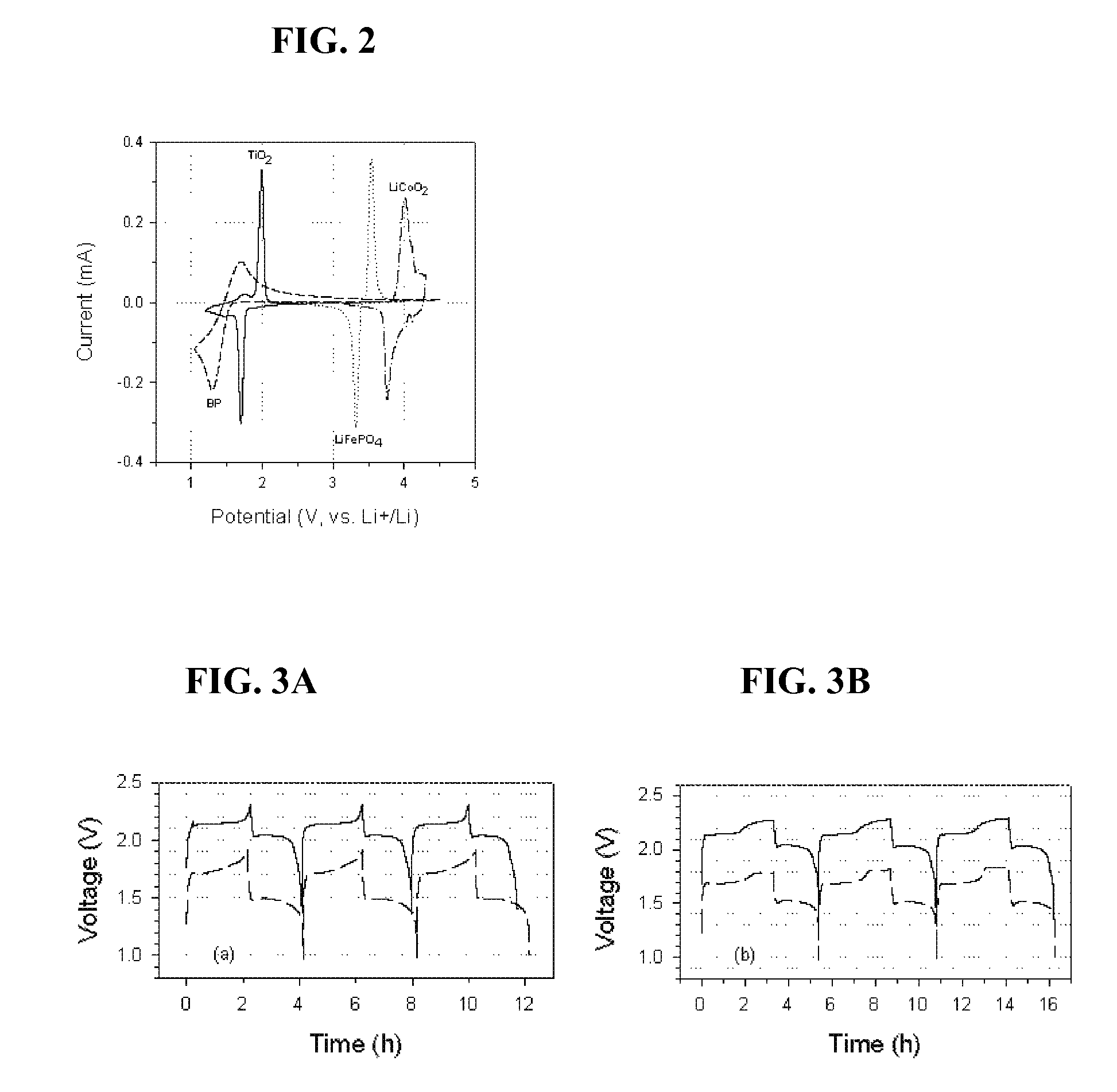
Overcharge and overdischarge protection in lithium-ion batteries
InactiveUS20100081059A1Efficient level of safetyEffective levelingMaterial nanotechnologySecondary cellsRedoxEngineering
A lithium-ion battery comprising a first electrode made of cathodic material, a second electrode made of anodic material and an electrolyte, said lithium-ion battery containing an overcharge protection material consisting of redox molecules, characterized by the fact that said redox molecules have a reduction potential which is lower than said anodic material.
Owner:DOW GLOBAL TECH LLC
Residual chlorine measuring method and residual chlorine measuring device
InactiveUS20070114137A1Easy to measureTotal current dropWeather/light/corrosion resistanceVolume/mass flow measurementPhysicsResidual chlorine
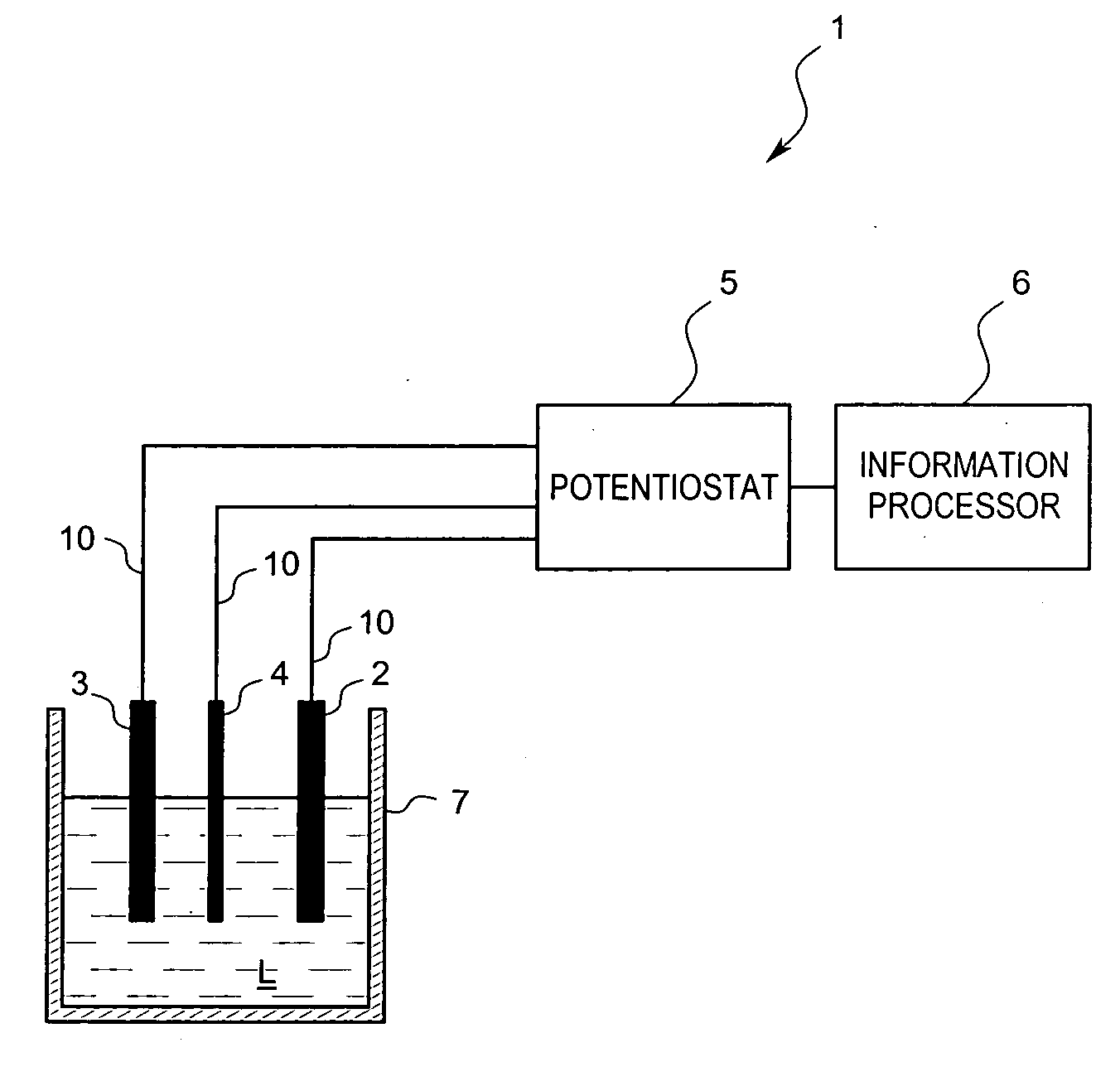
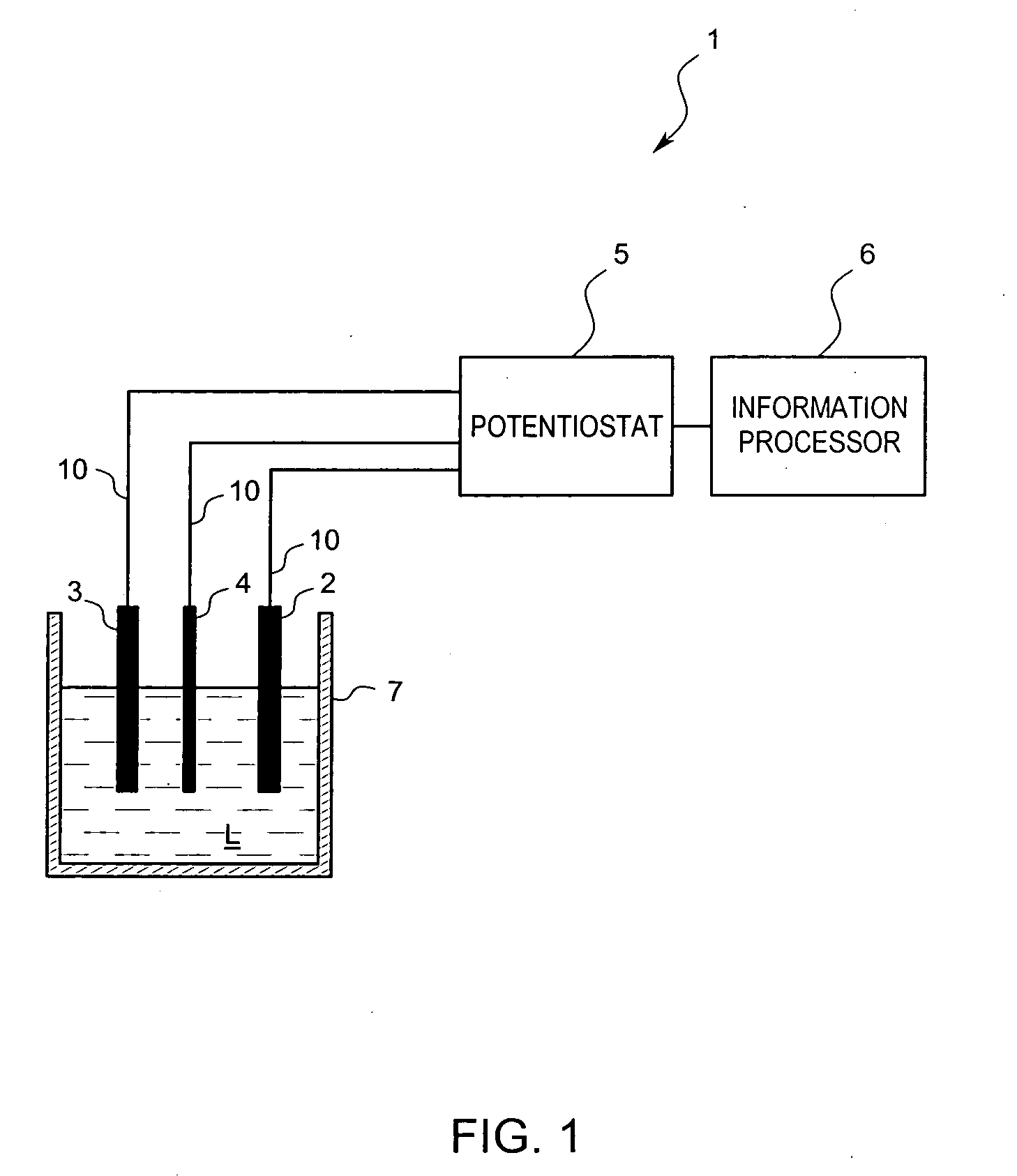
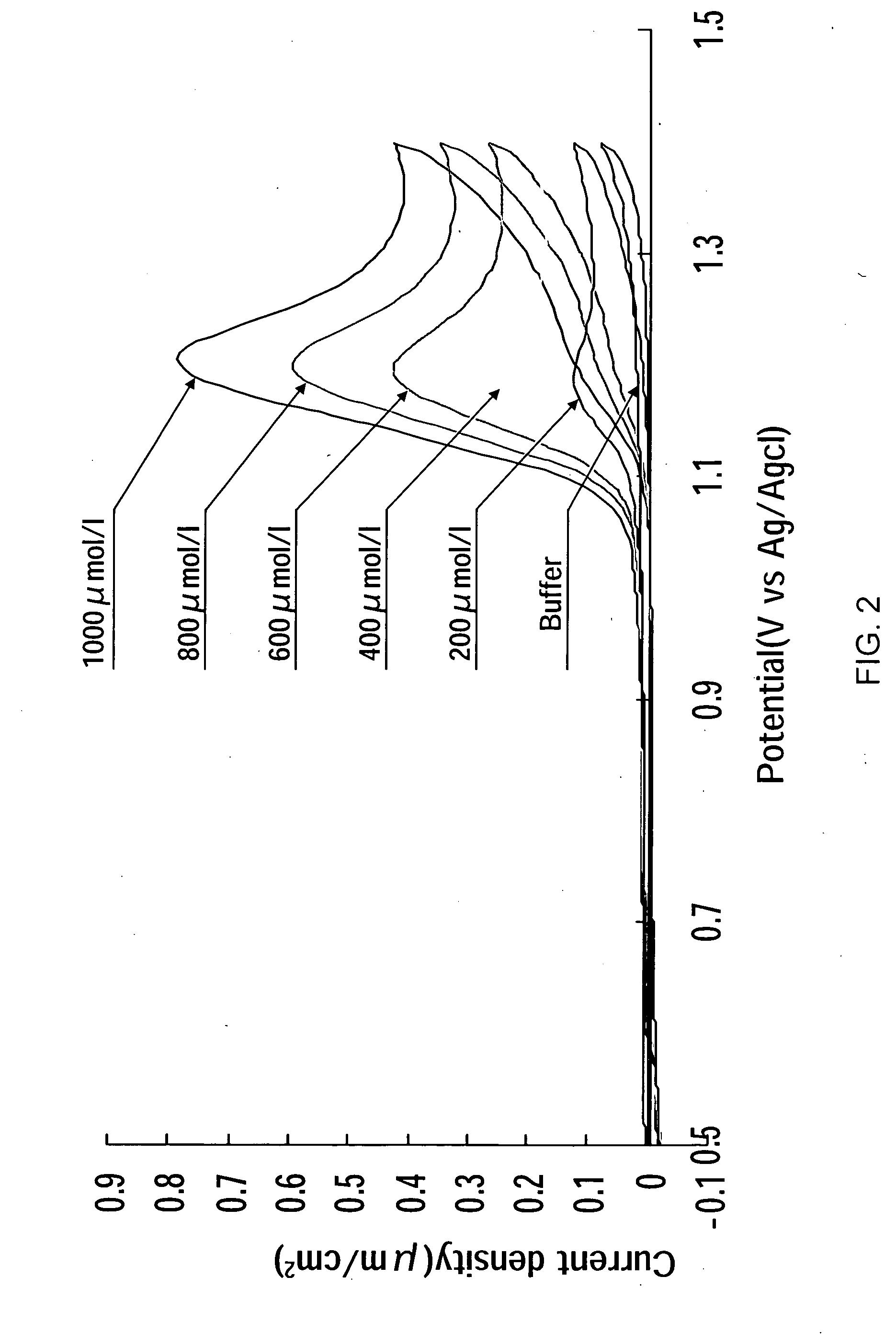
A residual chlorine measuring method includes the steps of bringing a counter electrode, a working electrode and a reference electrode into contact with a sample solution containing a residual chlorine. Applying a voltage between the counter electrode and the working electrode and measuring a current value to calculate a concentration of the residual chlorine. The working electrode is an electrically conductive diamond electrode to which an element selected from a group of boron, nitrogen and phosphorus is doped into a diamond coating. The reference electrode is a silver / silver chloride electrode. A current value, when setting the potential of the electrically conductive diamond electrode to the silver / silver chloride electrode, is in the range of +0.5 V to +1.5 V for measurements.
Owner:HORIBA LTD +1
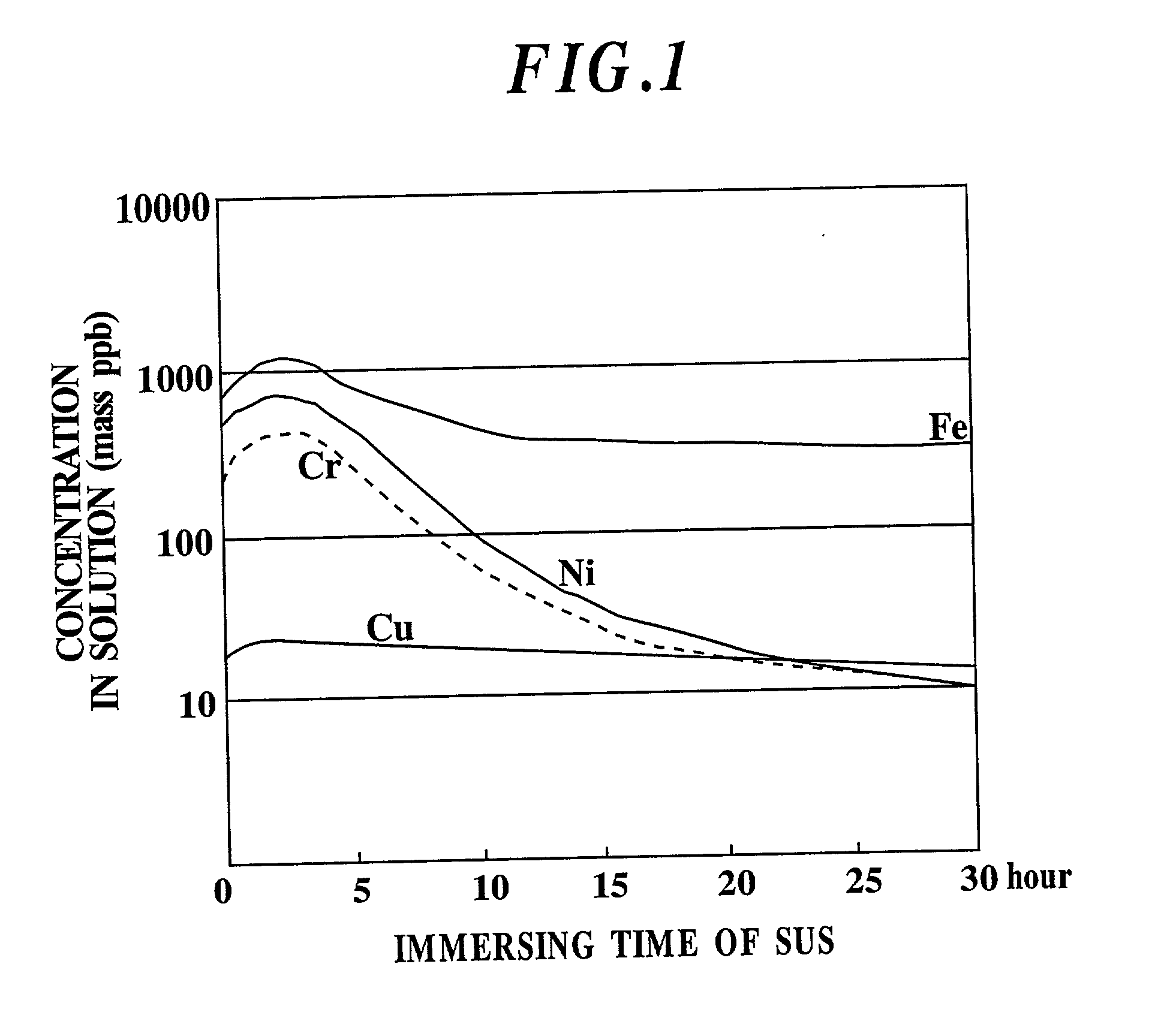
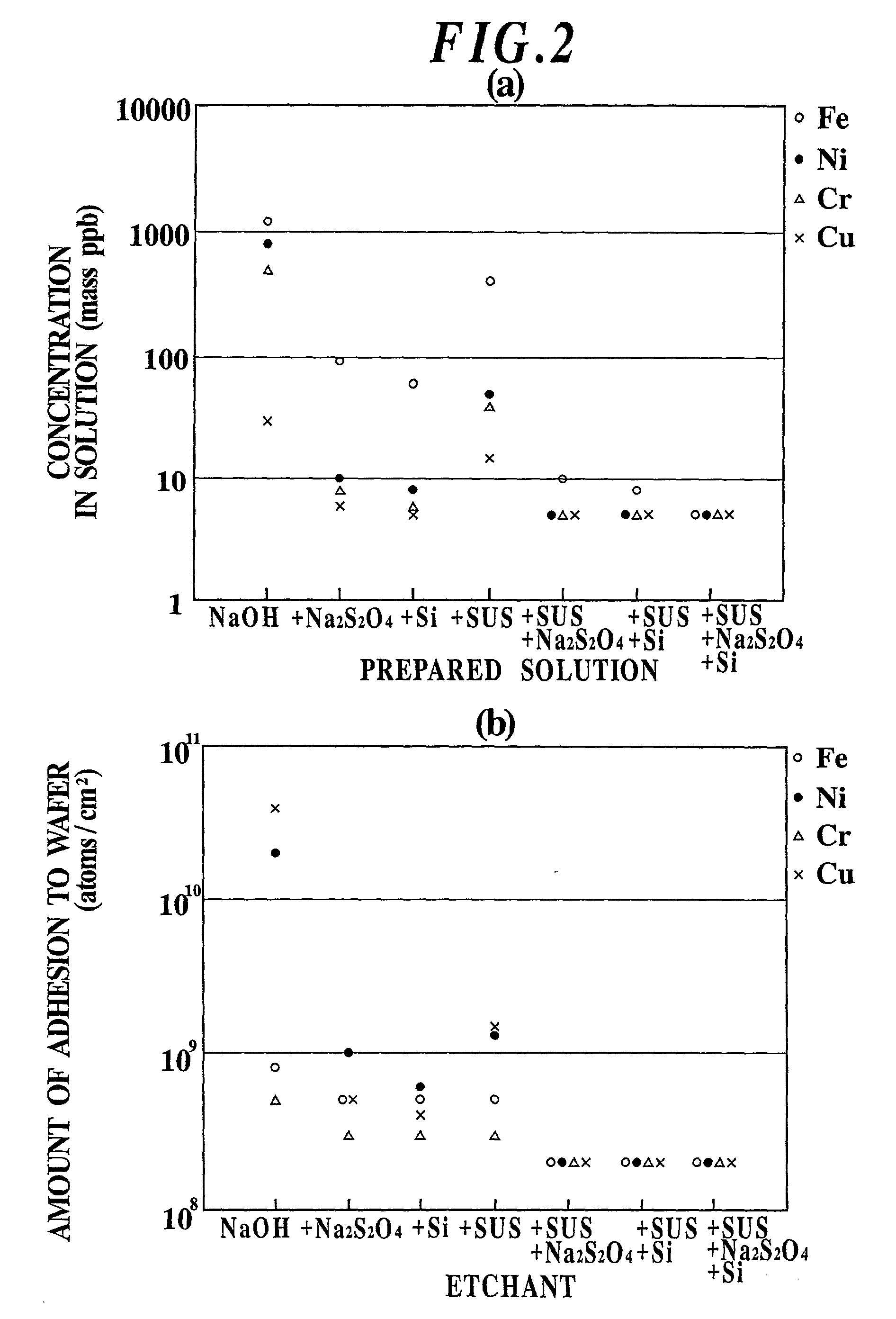
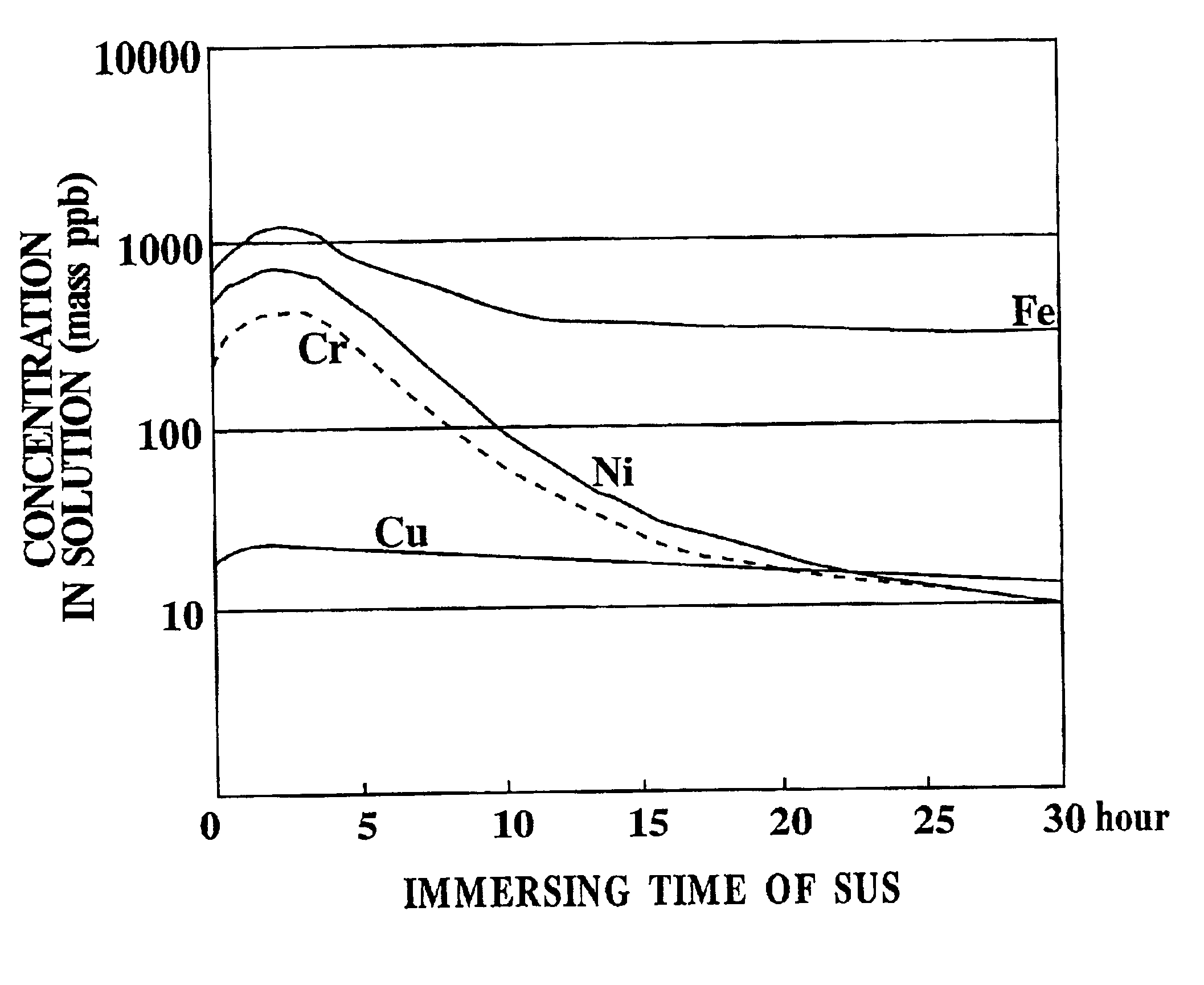
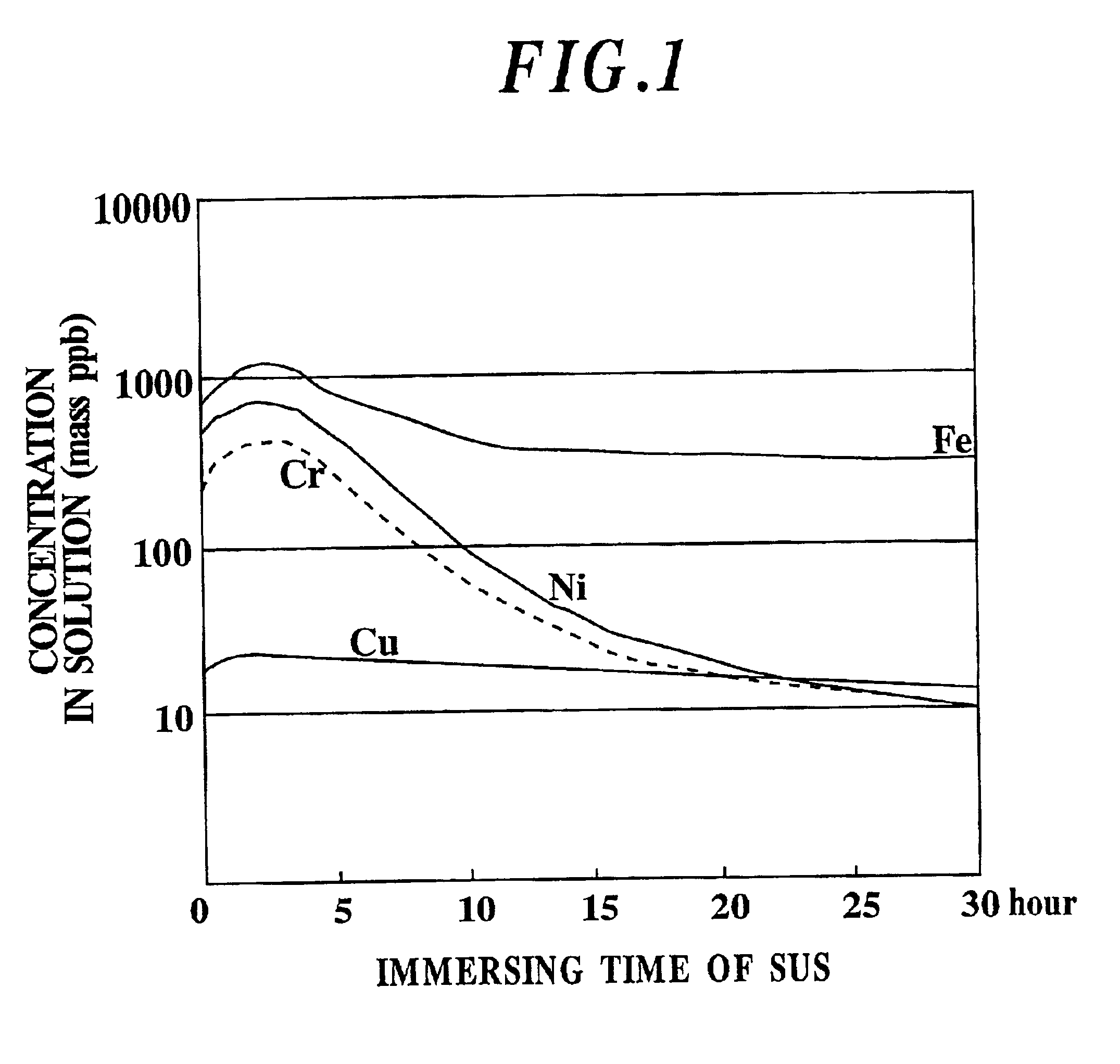
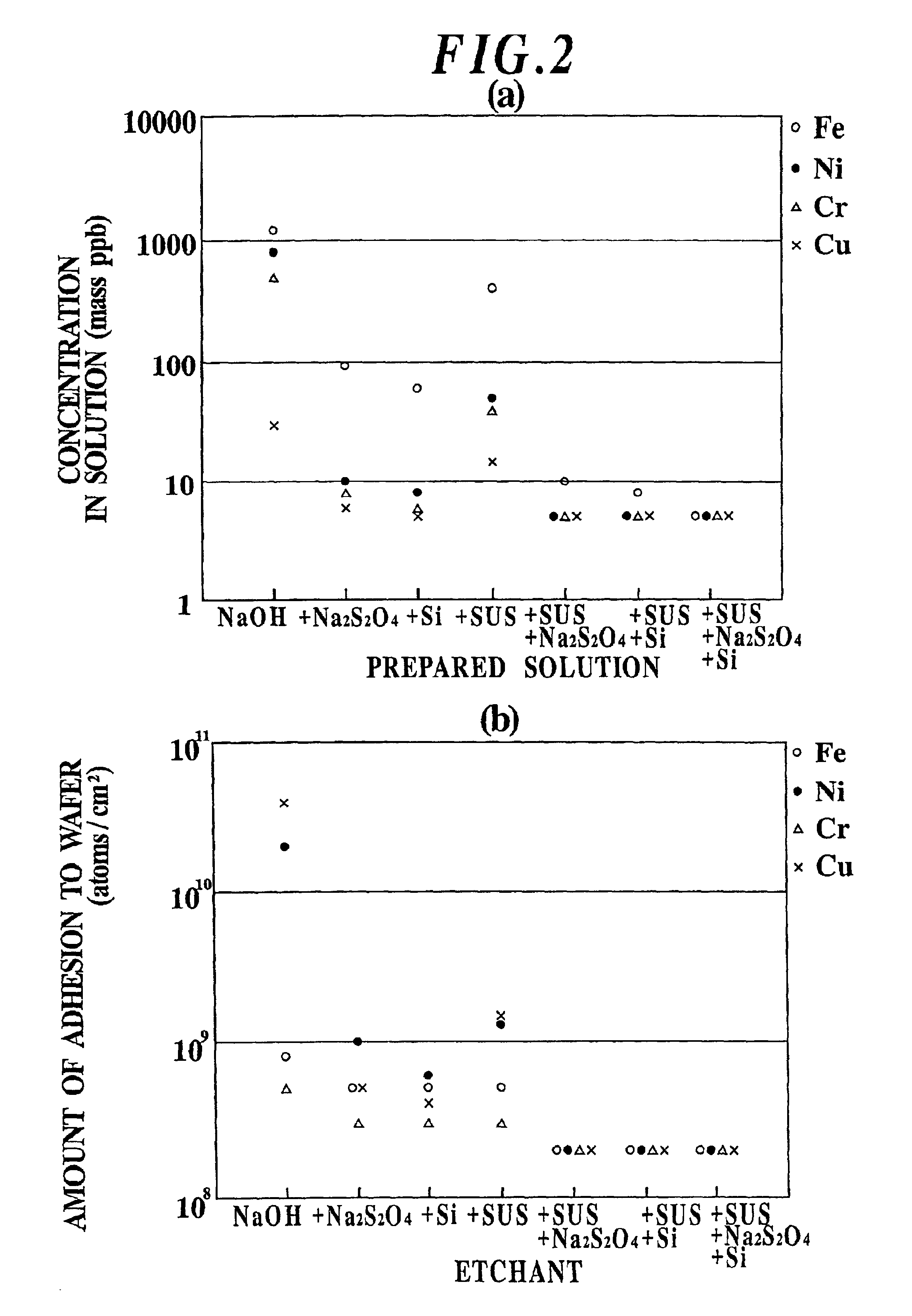
Etchant, etching method and semiconductor silicon waffer
InactiveUS20030008504A1Reduce metal pollutionReduce concentrationPolycrystalline material growthAfter-treatment detailsMetal contaminationAqueous solution
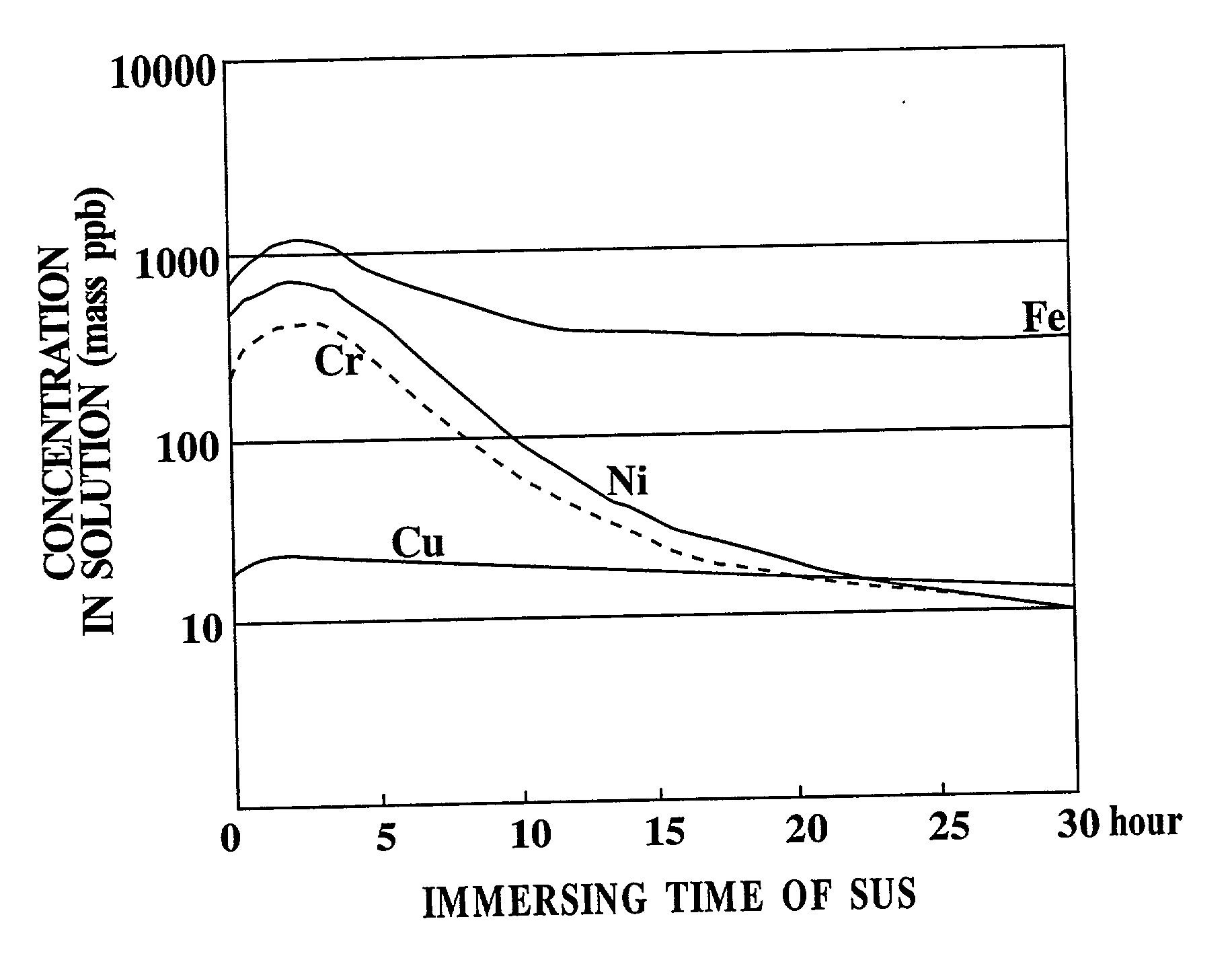
An etchant and an etching method that contribute to prevention of metal contamination of a semiconductor silicon wafer, and a semiconductor silicon wafer in which metal contamination is extremely reduced, are provided. The etchant according to the present invention is prepared by immersing stainless steel in an alkali aqueous solution for not less than 10 hours. In the etching method according to the present invention, a semiconductor silicon wafer is etched by using the etchant. Thereby, the semiconductor silicon wafer according to the present invention, in which metal contamination is extremely reduced, is obtained.
Owner:SHIN-ETSU HANDOTAI CO LTD
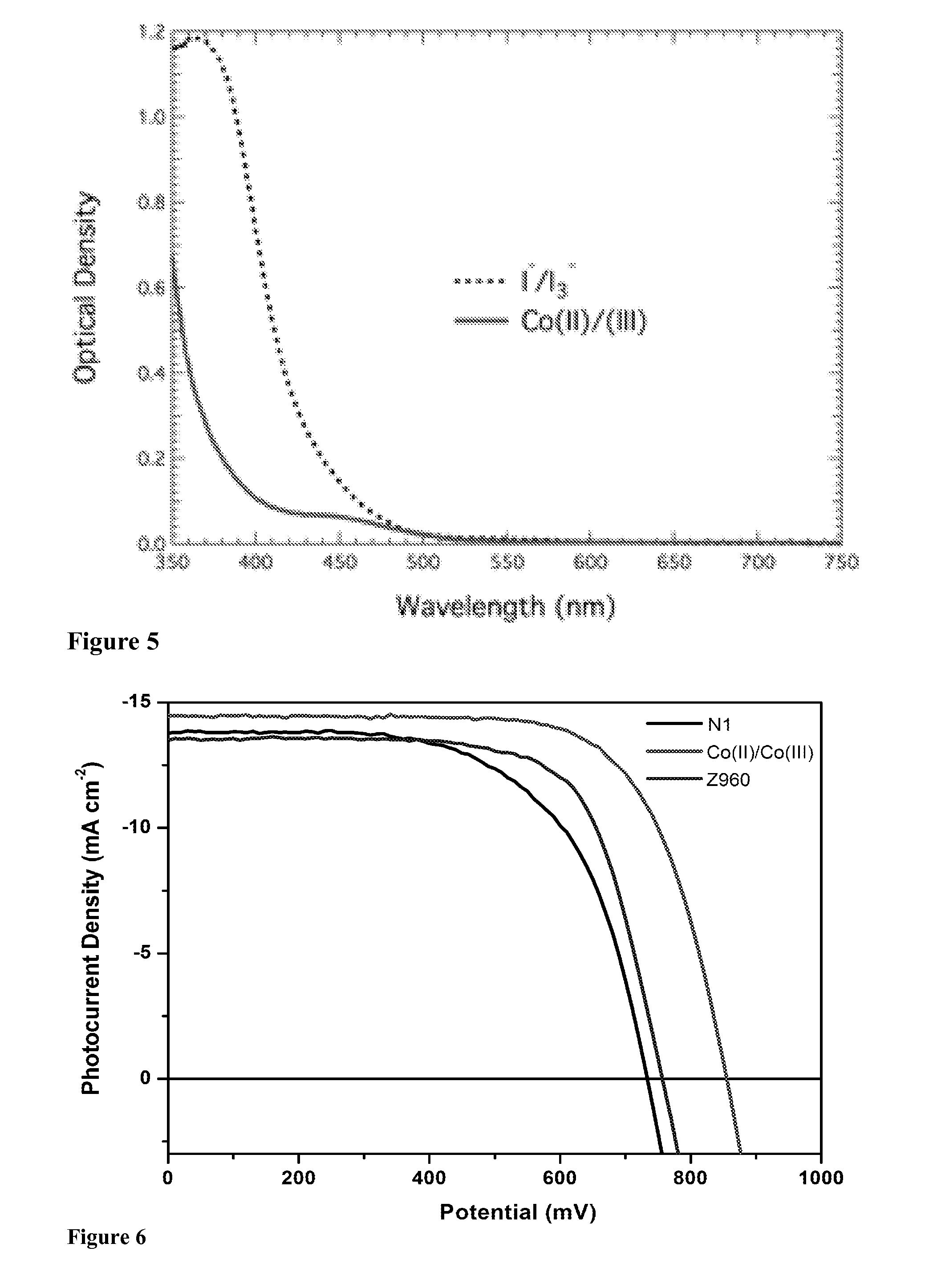
Redox couple for electrochemical and optoelectronic devices
InactiveUS20140060641A1Process stabilityImproved and high conversion efficiencyLight-sensitive devicesCell electrodesHeteroatomDouble bond
The present invention provides an improved redox couple for electrochemical and optoelectronic devices. The redox couple is based on a complex of a first row transition metal, said complex containing at least one mono-, bi-, or tridentate ligand comprising a substituted or unsubstituted ring or ring system comprising a five-membered N-containing heteroring and / or a six-membered ring comprising at least two heteroatoms, at least one of which being a nitrogen atom, said five- or six-membered heteroring, respectively, comprising at least one double bond. The invention also relates to electrolytes and to the devices containing the complex, and to the use of the complex as a redox couple. The invention further provides electrochemical and / or optoelectronic devices comprising a first and a second electrode and, between said first and second electrode, a charge transport layer, said a charge transport layer comprising tetracyanoborate ([B(CN)4]−) and a cationic metal complex functioning as redox-couple.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Overcharge and overdischarge protection in lithium-ion batteries
InactiveUS8003260B2Effective levelingImprove stabilityMaterial nanotechnologyActive material electrodesRedoxEngineering
A lithium-ion battery comprising a first electrode made of cathodic material, a second electrode made of anodic material and an electrolyte, said lithium-ion battery containing an overcharge protection material consisting of redox molecules, characterized by the fact that said redox molecules have a reduction potential which is lower than said anodic material.
Owner:DOW GLOBAL TECH LLC
Adjustable venturi
InactiveUS8061387B1Improve solubilityDissolve more quicklyFlow mixersDissolving using flow mixingMixing chamberWaste management
An adjustable Venturi is disclosed, and having a plurality of inlet ports providing substances to be mixed with a flow of fluid through the Venturi. In one embodiment, the Venturi is constructed having an inlet portion, an outlet portion, with an annular mixing chamber coupled to inlet ports so that substances provided through inlet ports are mixed in the mixing chamber, and such mixture introduced into the flow. A gasket sealably disposed between inlet portion and outlet portion is selectable in thickness, for adjusting width of a Venturi interface between the inlet portion and outlet portion. In another embodiment, the inlet portion and outlet portion are fitted in an elongated opening, and are replaceable to change operational characteristics of the Venturi. A replaceable disk having a central opening and substance-providing passageways is disposed between the inlet portion and outlet portion, and may be changed to change characteristics of the Venturi.
Owner:BARNES RONALD L
Ink jet ink, ink jet recording method, ink cartridge, recording unit and ink jet recording apparatus
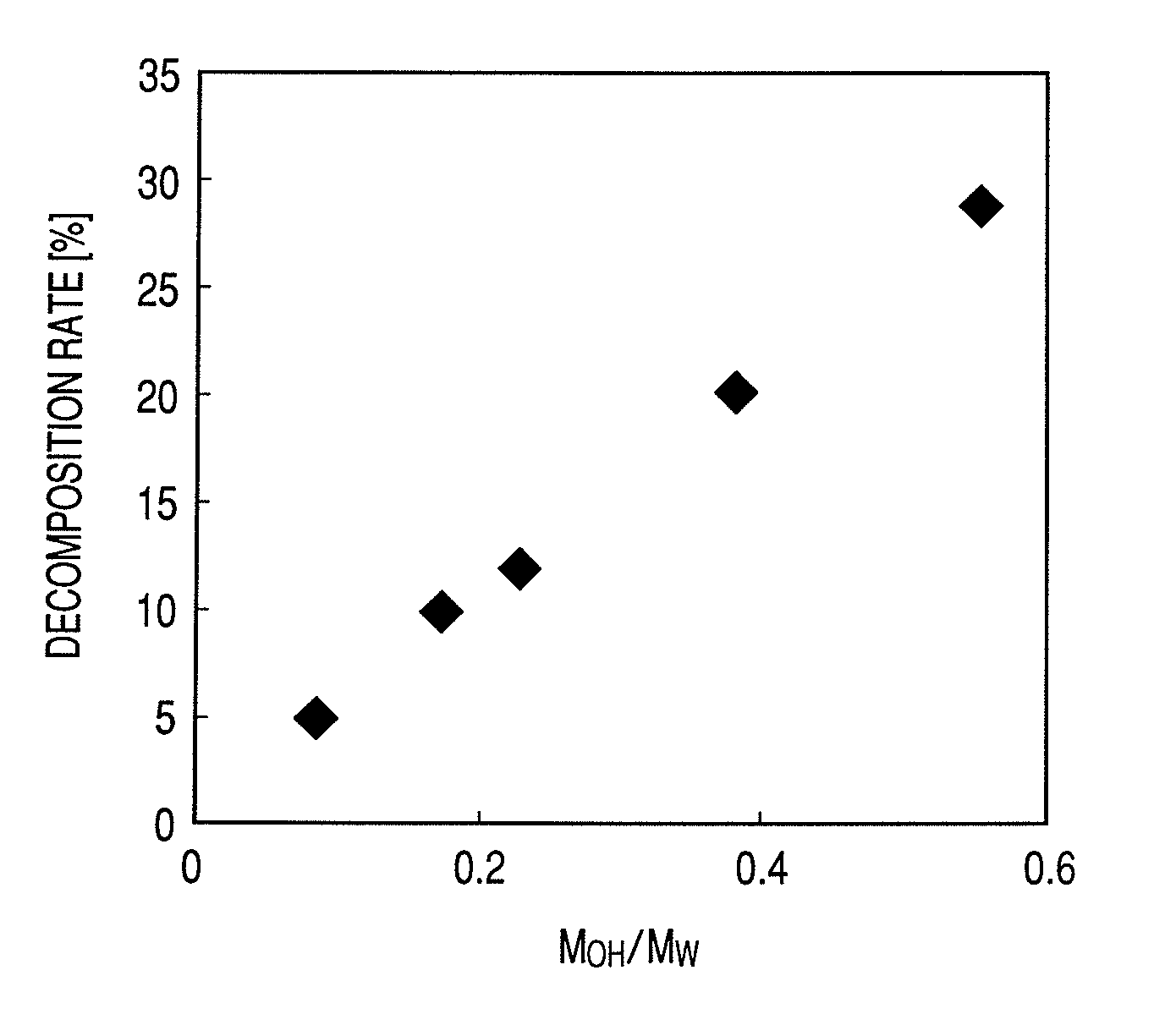
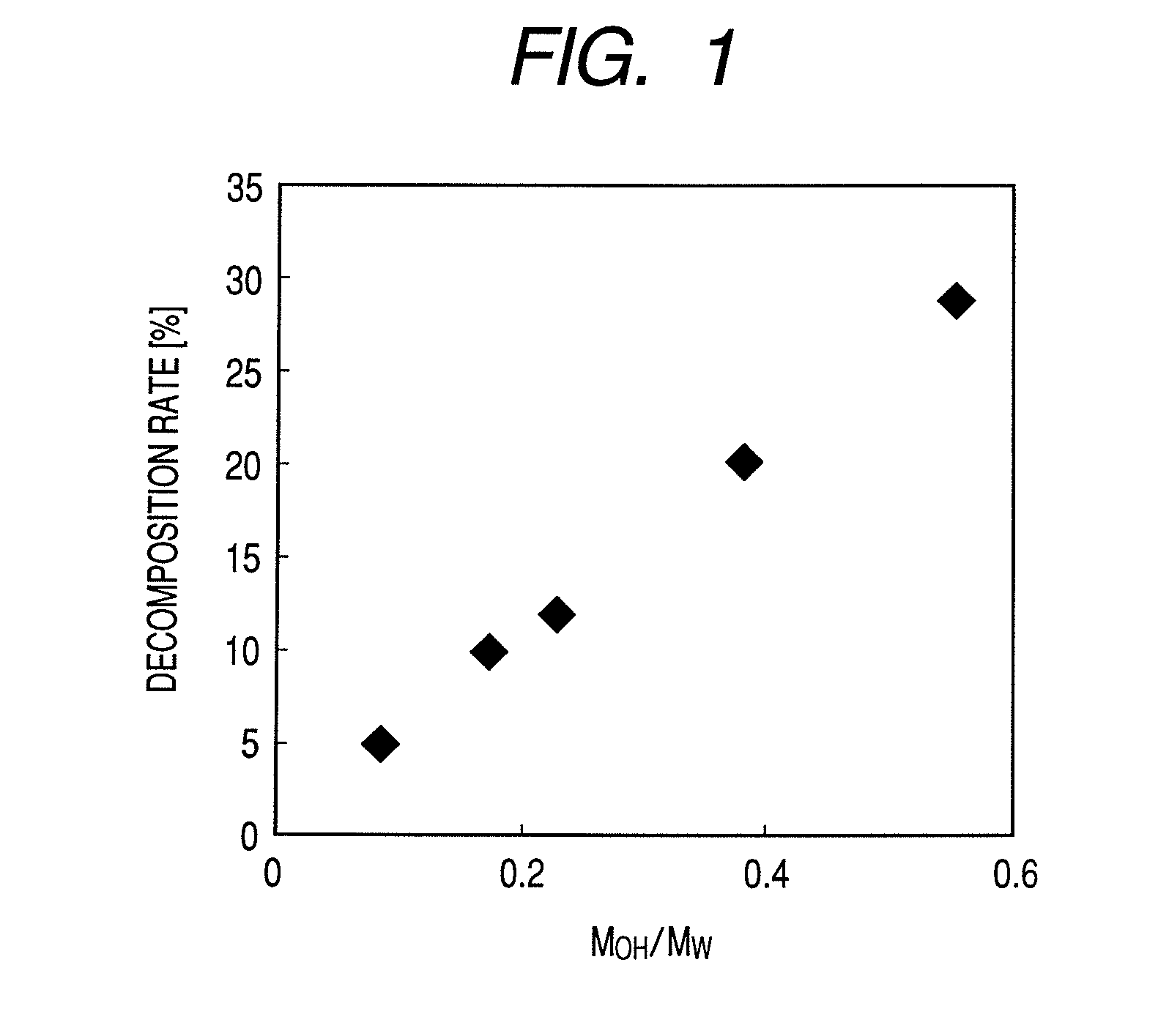
ActiveUS8114208B2Coloring materialPotential oxidationDisazo dyesMeasurement apparatus componentsOrganic solventNitrogen
An ink jet ink including at least a compound represented by the following general formula (I) and organic solvents. When the organic solvents are classified by MOH / MW indicating a proportion of the molecular weight of hydroxyl group to the molecular weight of the organic solvents into an organic solvent A whose MOH / MW is 0 or more and less than 0.2, an organic solvent B whose MOH / MW is 0.2 or more and less than 0.4, and an organic solvent C whose MOH / MW is 0.4 or more and less than 1.0, the ink contains at least one organic solvent A, at least one organic solvent B and at least one organic solvent C. The at least one organic solvent A contains a nitrogen-containing organic solvent in an amount of 80.0% by mass or more of the at least one organic solvent A.
Owner:CANON KK
Method for Controlling Chlorinated Nitrogen-Containing Disinfection By-Product In Water
ActiveUS20190055148A1Efficient removalRapid aerationBiocideWater/sewage treatment by irradiationDisinfection by-productHydrogen
Disclosed is a method for controlling a chlorinated nitrogen-containing disinfection by-product in water, including the following steps: firstly, the pH value of a water sample is controlled, ultraviolet irradiation treatment is used and a persulfate or hydrogen persulfate is added at the same time, then chlorination disinfection is carried out, and finally, aeration treatment is carried out, so that a water sample from which the chlorinated nitrogen-containing disinfection by-product in water is removed and obtained. The method can effectively control the formation of chlorinated N-DBPs, and can effectively reduce the amount of trichloromethane in the subsequent chlorination disinfection process.
Owner:TONGJI UNIV
Nonaqueous electrolytic solution and lithium secondary battery
InactiveUS20040096748A1Suppress productionSafety be ensureOrganic electrolyte cellsElectrolytesCycloalkaneKetone
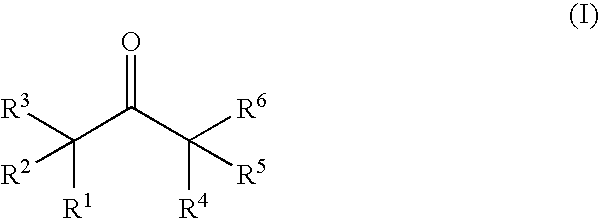
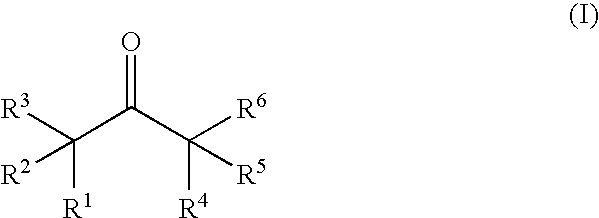
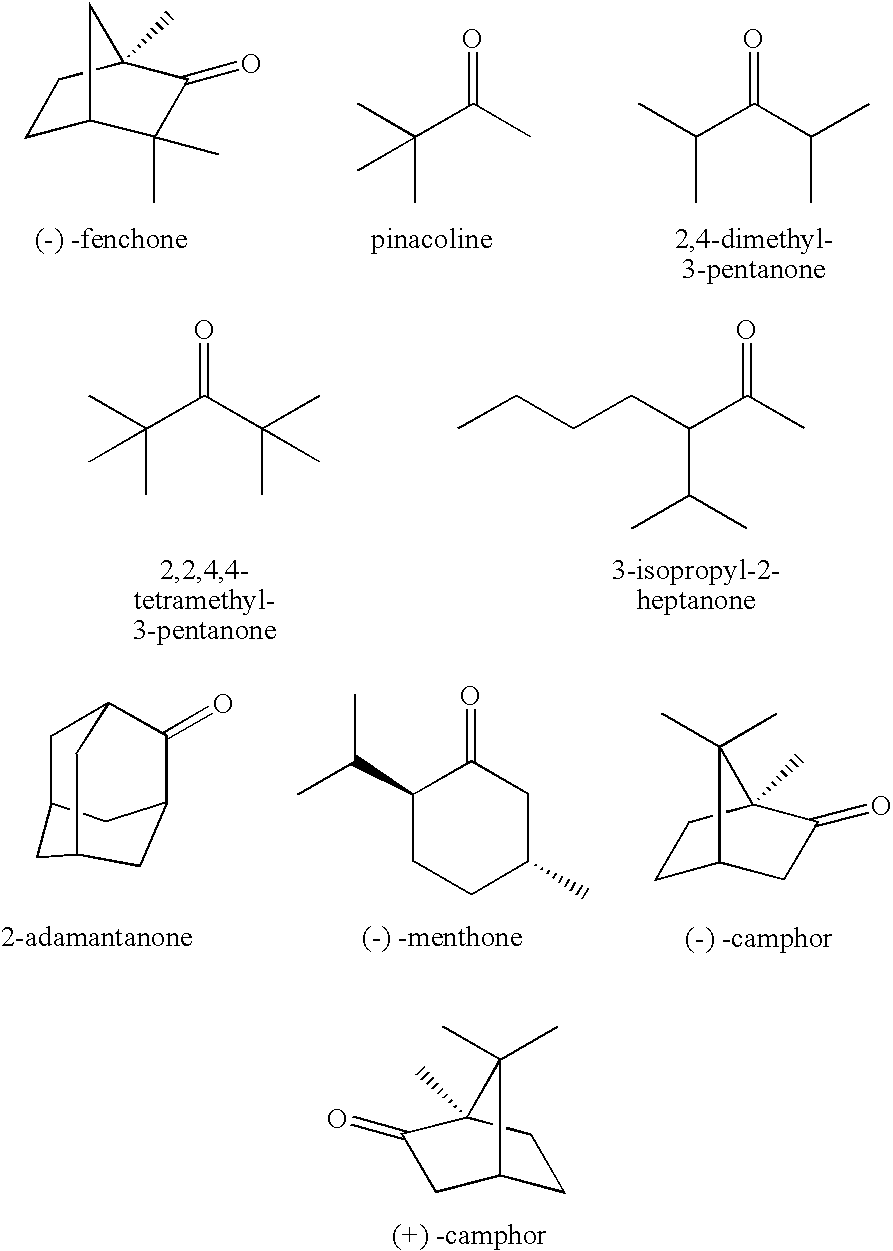
A non-aqueous electrolytic solution containing a ketone compound having the formula (I): [each of R<1 >and R<2 >independently represents a linear or branched alkyl group; and each of R<3>, R<4>, R<5 >and R<6 >independently represents a hydrogen atom or a linear or branched alkyl group; provided that R<1 >and R<4 >can be combined to form a cycloalkanone ring in conjunction with a propanone skeleton to which R<1 >and R<4 >are connected, that two or more of an alkyl group of R<2>, an alkyl group of R<5>, a branched chain of an alkyl group of R<1>, and a branched chain of an alkyl group of R<1 >can be combined to form a cycloalkane ring, or that an alkyl group of R<1 >and an alkyl group of R<2 >and / or an alkyl group of R<1 >and an alkyl group of R<5 >can be combined to each other to form a cycloalkane ring] is favorably employed as a constituent material for preparing a lithium secondary battery that is excellent in the battery performances and cycle performance.
Owner:UBE IND LTD
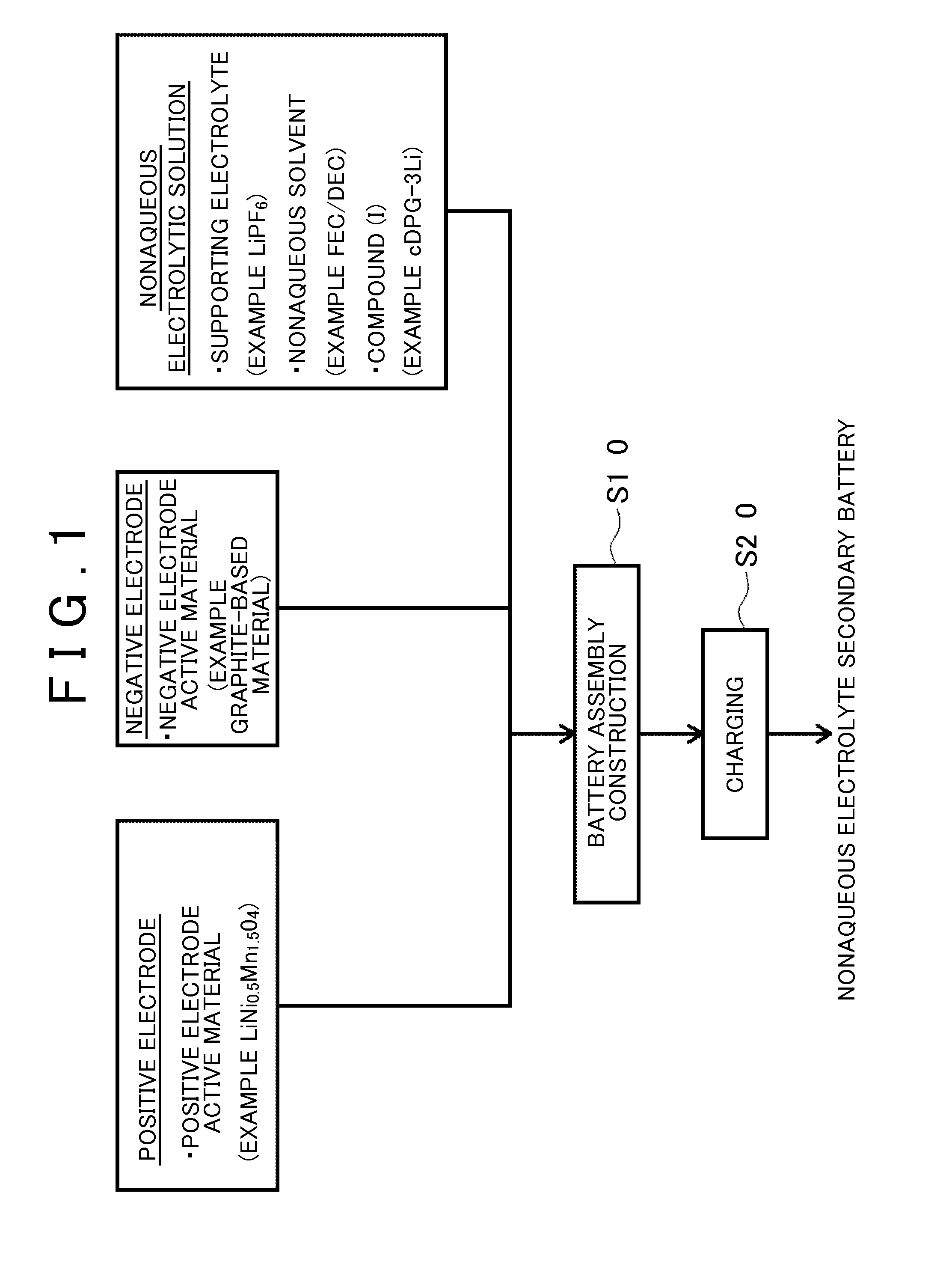
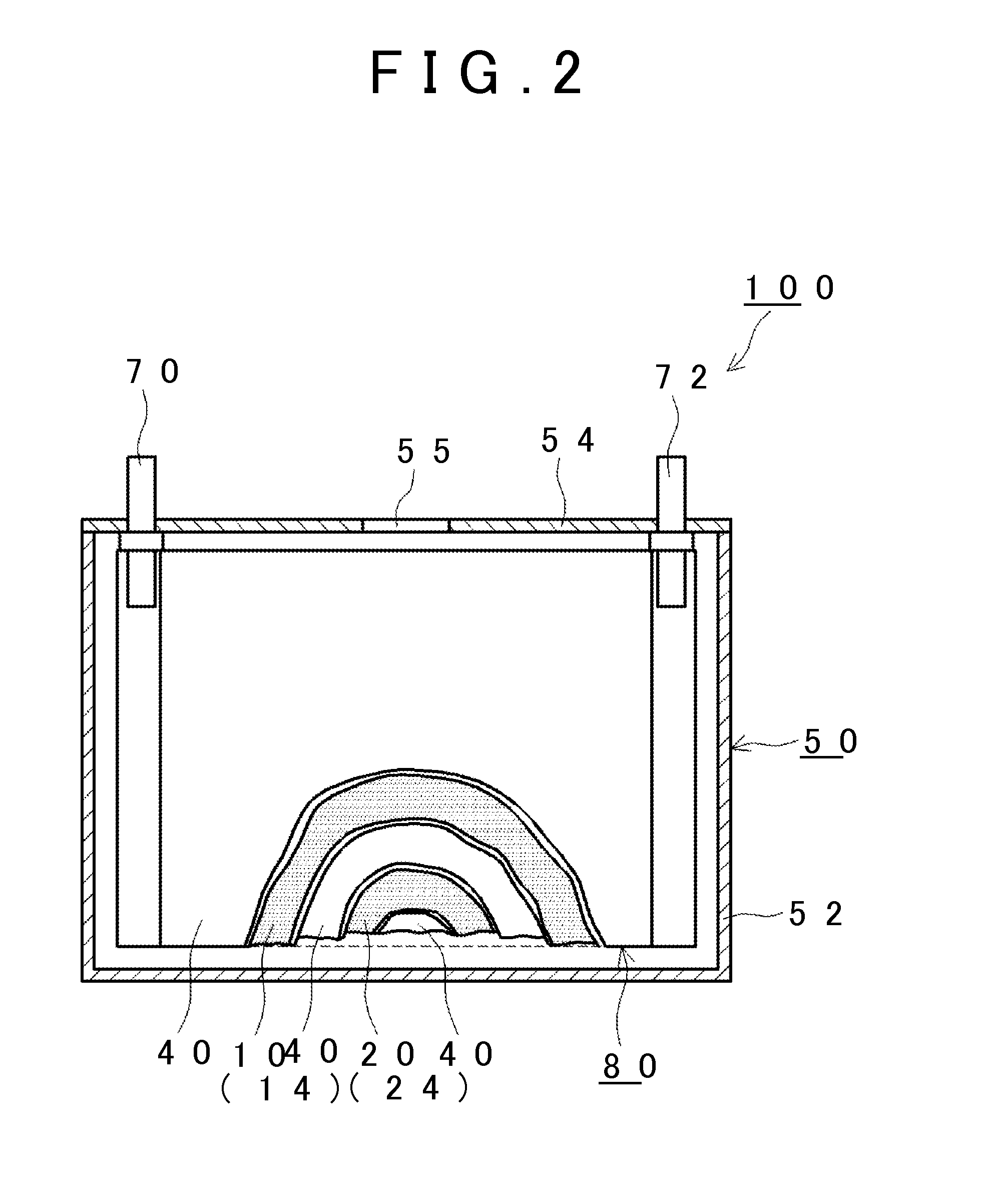
Nonaqueous electrolyte secondary battery, method of manufacturing the same, and nonaqueous electrolytic solution
ActiveUS20160020490A1High energy densityReduce decompositionFinal product manufactureElectrode carriers/collectorsEngineeringElectrolyte
A method of manufacturing a nonaqueous electrolyte secondary battery includes the following steps of: constructing a battery assembly using a positive electrode, a negative electrode, and a nonaqueous electrolytic solution containing a compound represented by a specific formula; and forming a film on a surface of the positive electrode by charging the battery assembly such that the compound is decomposed. In an embodiment, during the construction of the battery assembly, a content of the compound is adjusted to be 0.1 mass % or more with respect to 100 mass % of a total amount of the nonaqueous electrolytic solution.
Owner:TOYOTA JIDOSHA KK
Nonaqueous secondary battery
InactiveUS20110183214A1Low costPromote resultsOrganic electrolyte cellsSecondary cellsCouplingAlkoxy group
A positive electrode active material has an average particle diameter of 4.5 to 15.5 μm and a specific surface area of 0.13 to 0.80 m2 / g. A positive electrode mixture layer contains at least one of a silane coupling agent and / or at least one of aluminum, titanium, or zirconium based coupling agent having an alkyl or an alkoxy groups having 1 to 18 carbon atoms at a content of 0.003% by mass or more and 5% by mass or less with respect to the mass of the positive electrode active material. The nonaqueous electrolyte contains a 1,3-dioxane derivative at a content of 0.05% by mass or more with respect to the total mass of the nonaqueous electrolyte. Thus a nonaqueous secondary battery that has good high-temperature cycle characteristics and suppresses an increase in self-discharge after repetition of charge and discharge cycles at high temperature is provided.
Owner:SANYO ELECTRIC CO LTD
Electrode Active Material and Lithium Secondary Battery
ActiveUS20100183923A1Improve discharge characteristicsAvoid problemsElectrode manufacturing processesActive material electrodesLithiumElectronegativity
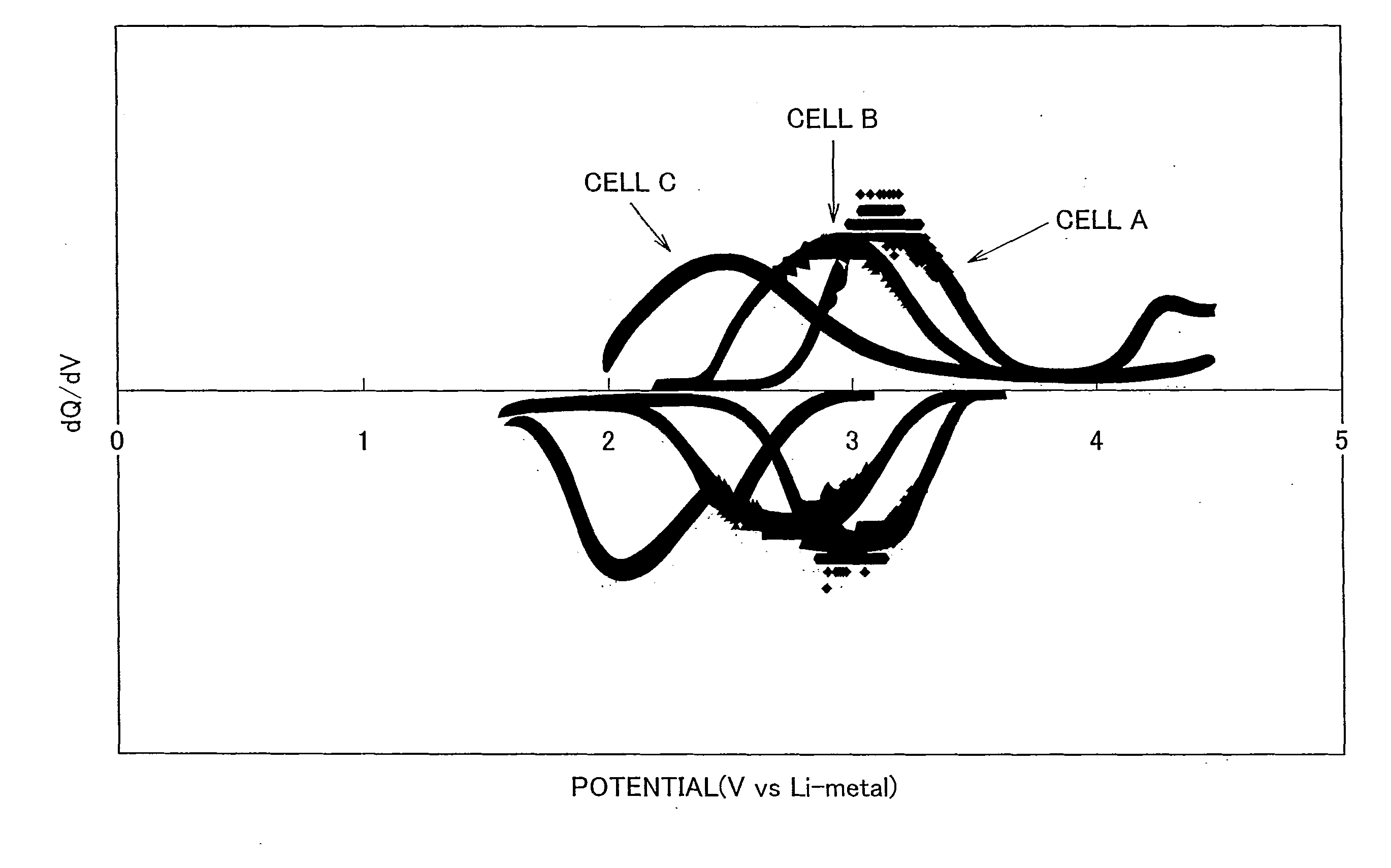
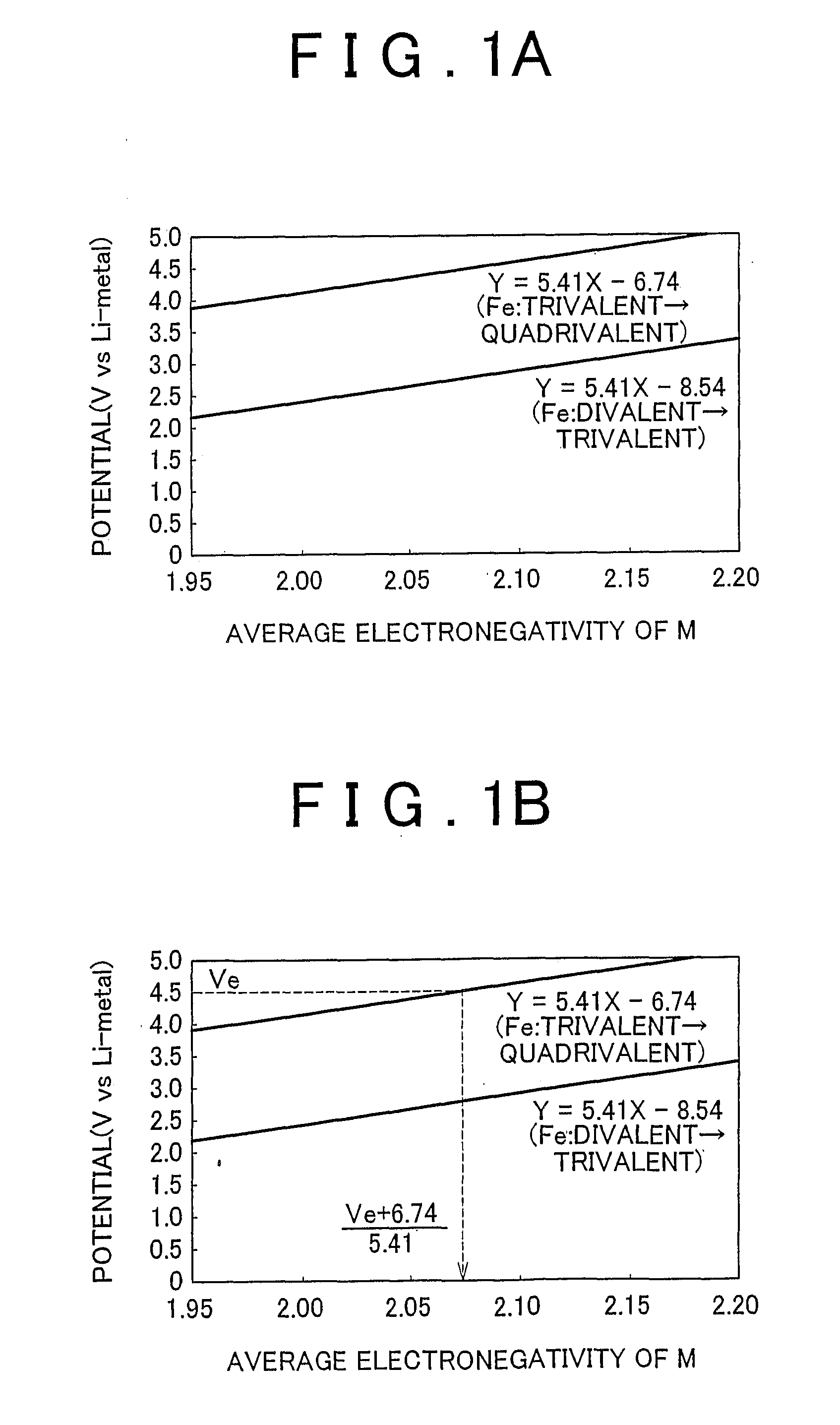
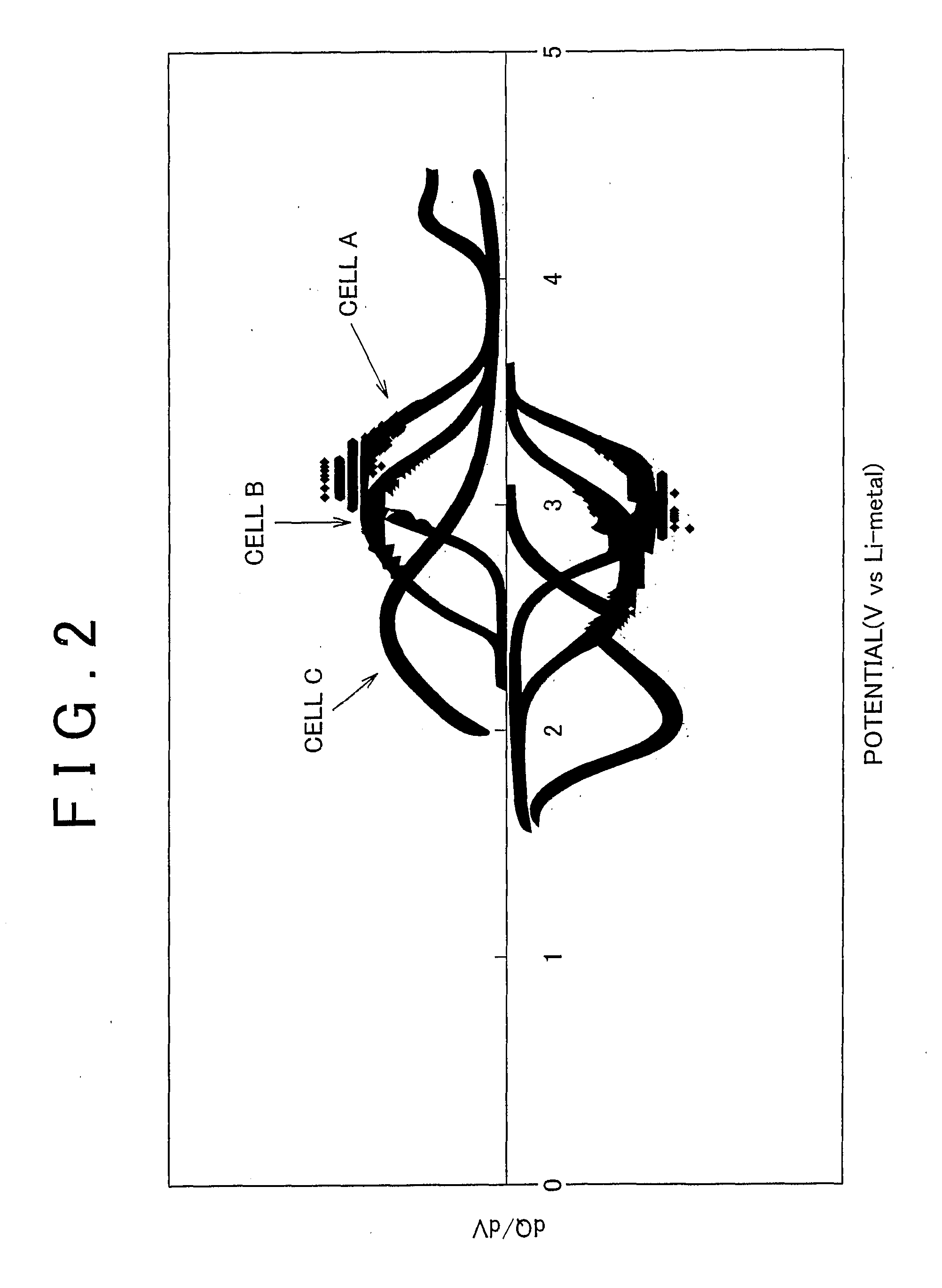
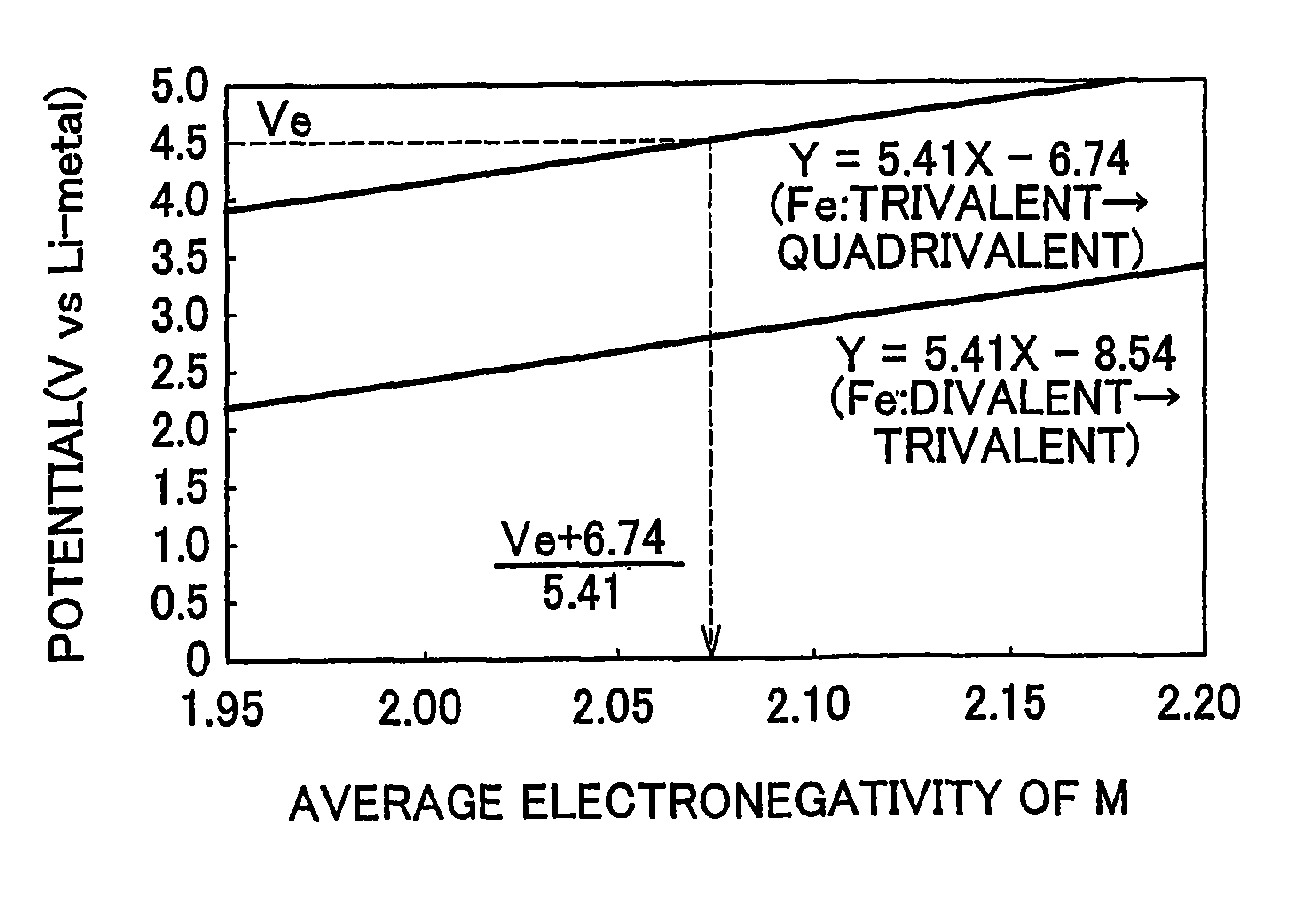
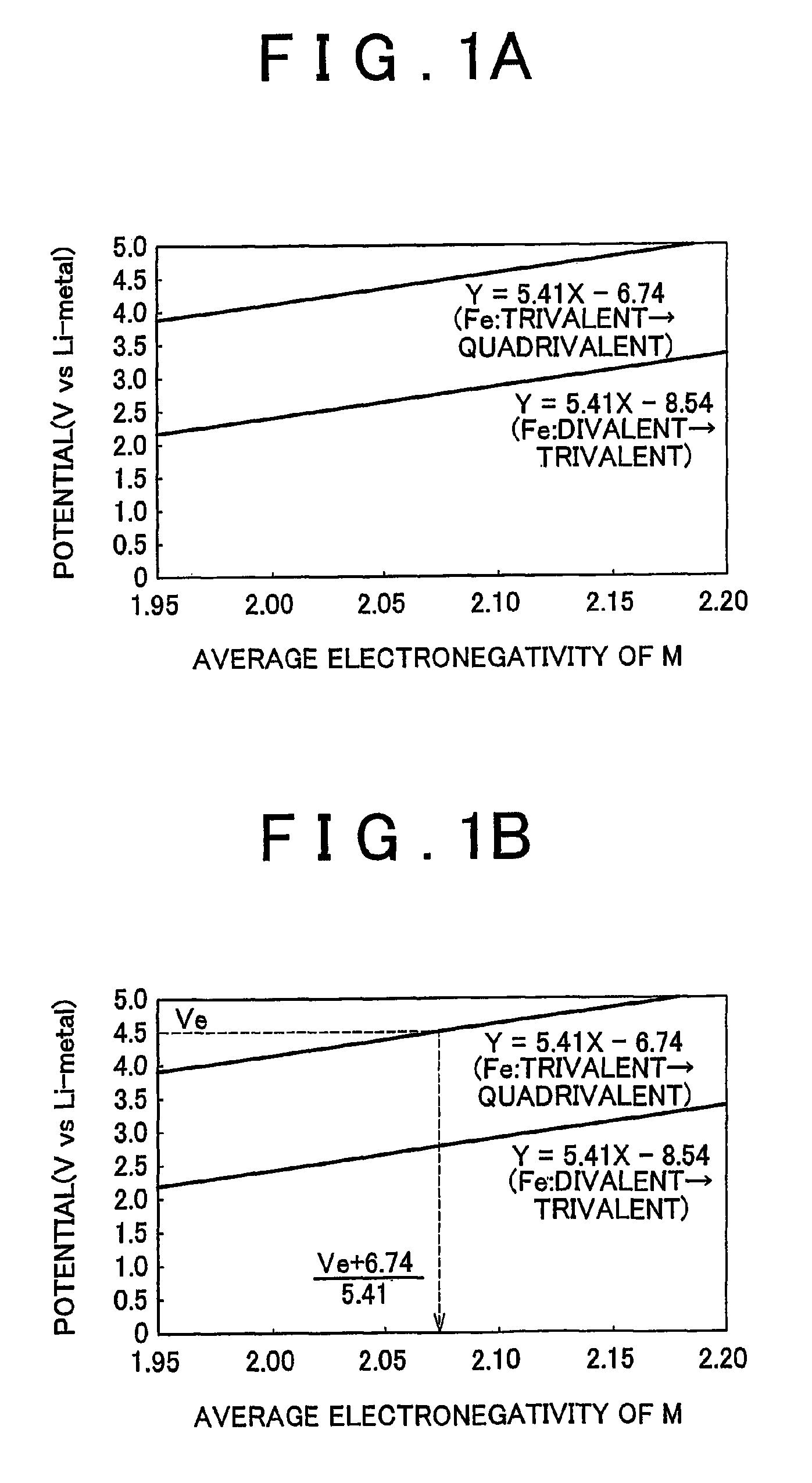
Electrode active material that is used together with an electrolyte solution having an electrolyte decomposition potential Ve is represented by the general expression LixFeMyO2 and is amorphous. In the expression, x and y are values which independently satisfy 1<x≦2.5 and O<y≦3, respectively, and z=(x+(valence of Fe)+(valence of M)×y) / 2 to satisfy stoichiometry, and M represents one or two or more types of glass former element. The average electronegativity of M is less than (Ve+6.74 / 5.41.
Owner:KYUSHU UNIV +1
Acidic ferrate composition and methods of making ferrate
ActiveUS20200270145A1Little to no degradation of oxidative activitySimpler and convenient ferrate synthesisSpecific water treatment objectivesWater contaminantsAqueous solutionOxidizing agent
Various embodiments relate to an acidic ferrate composition and methods of making ferrate. A method of forming ferrate includes treating an iron source with an oxidizer in an aqueous solution having a pH of less than 7 under conditions sufficient to form ferrate.
Owner:NUQUATIC LLC
Microfluidic device comprising electrolysis device for cell lysis and method for electrochemically lysing cells using the same
ActiveUS20060134777A1Potential oxidationBioreactor/fermenter combinationsBiological substance pretreatmentsElectrolysisLysis
Provided are a microfluidic device including an electrolysis device for cell lysis which includes an anode chamber, a cathode chamber and a separator, in which the separator is installed between the anode chamber and the cathode chamber, the anode chamber includes an inlet and an outlet for an anode chamber solution and an electrode, and the cathode chamber includes an inlet and an outlet for a cathode chamber solution and an electrode, and a method of electrochemically lysing cells using the same.
Owner:SAMSUNG ELECTRONICS CO LTD
Etching method for semiconductor silicon wafer
InactiveUS6844269B2Reduce metal pollutionReduce concentrationPolycrystalline material growthAfter-treatment detailsMetal contaminationAqueous solution
An etchant and an etching method that contribute to prevention of metal contamination of a semiconductor silicon wafer, and a semiconductor silicon wafer in which metal contamination is extremely reduced, are provided. The etchant according to the present invention is prepared by immersing stainless steel in an alkali aqueous solution for not less than 10 hours. In the etching method according to the present invention, a semiconductor silicon wafer is etched by using the etchant. Thereby, the semiconductor silicon wafer according to the present invention, in which metal contamination is extremely reduced, is obtained.
Owner:SHIN-ETSU HANDOTAI CO LTD
Lithographic printing method and presensitized plate
ActiveUS7282321B2Increase impressionIncreased printing lifeRadiation applicationsSemiconductor/solid-state device manufacturingDigital dataChemical compound
Disclosed is a presensitized plate composed of a support having thereon an image recording layer which includes:an infrared absorber (A) that is a cyanine dye having at least one fused ring composed of a nitrogen-containing heterocycle in combination with an aromatic ring or a second heterocycle, and having on the aromatic ring or second heterocycle an electron-withdrawing group or a heavy atom-containing group,a radical generator (B), anda radical-polymerizable compound (C),and which is removable with printing ink and / or dampening water.The presensitized of the present invention can be imaged with an infrared light-emitting laser to directly record an image from digital data on a computer or the like and is then subjected to on-machine development without carrying out a development step, which is capable of providing a large number of good impressions with a practical amount of energy.
Owner:FUJIFILM CORP
Novel light-activated compositions and methods using the same
ActiveUS20180043022A1Oxidation potentialReduce the overall heightAntibacterial agentsBacteria material medical ingredientsQuantum dotLight activated
Owner:UNIV OF COLORADO THE REGENTS OF
Non-aqueous electrolyte solution for lithium secondary battery and lithium secondary battery comprising the same
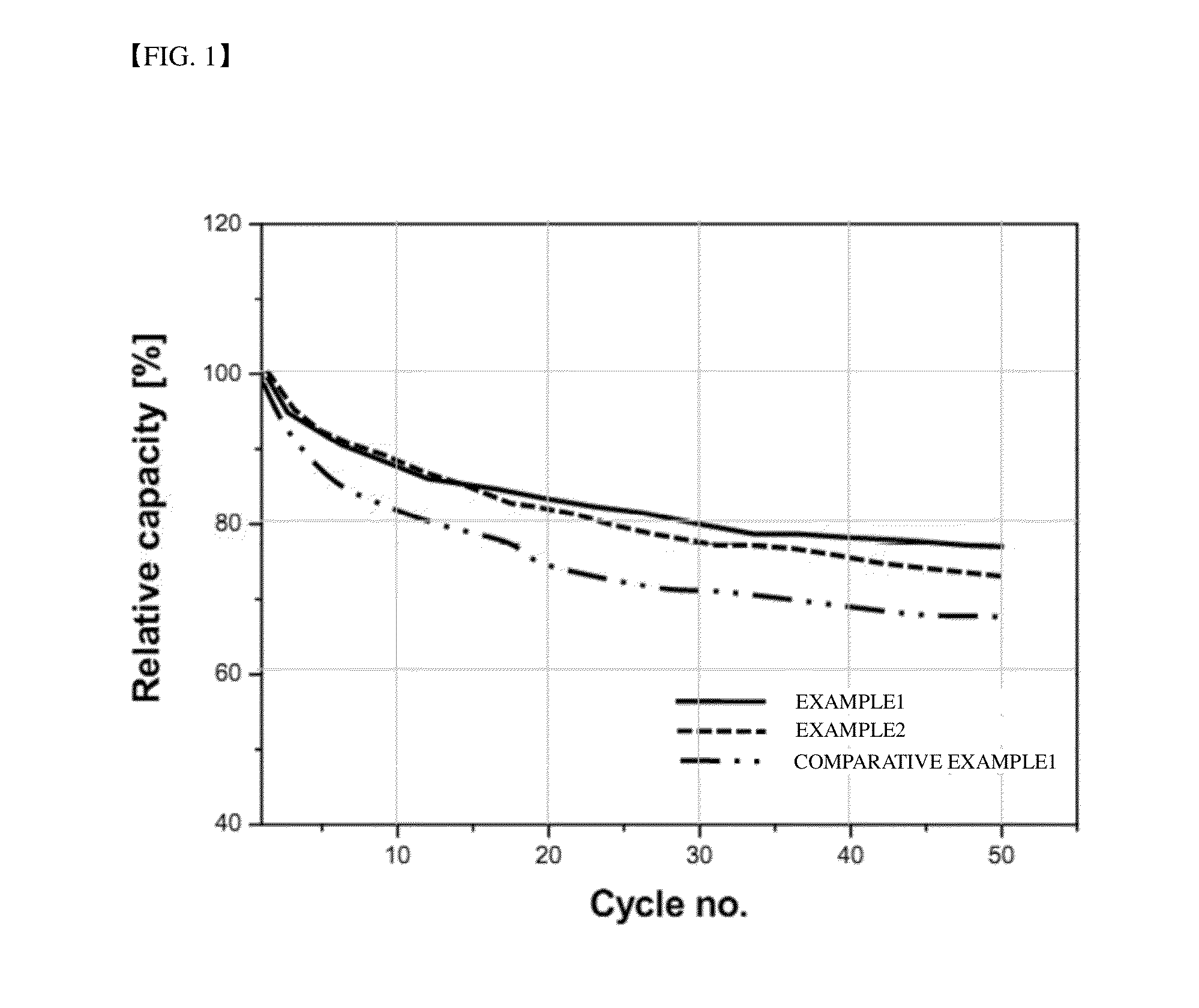
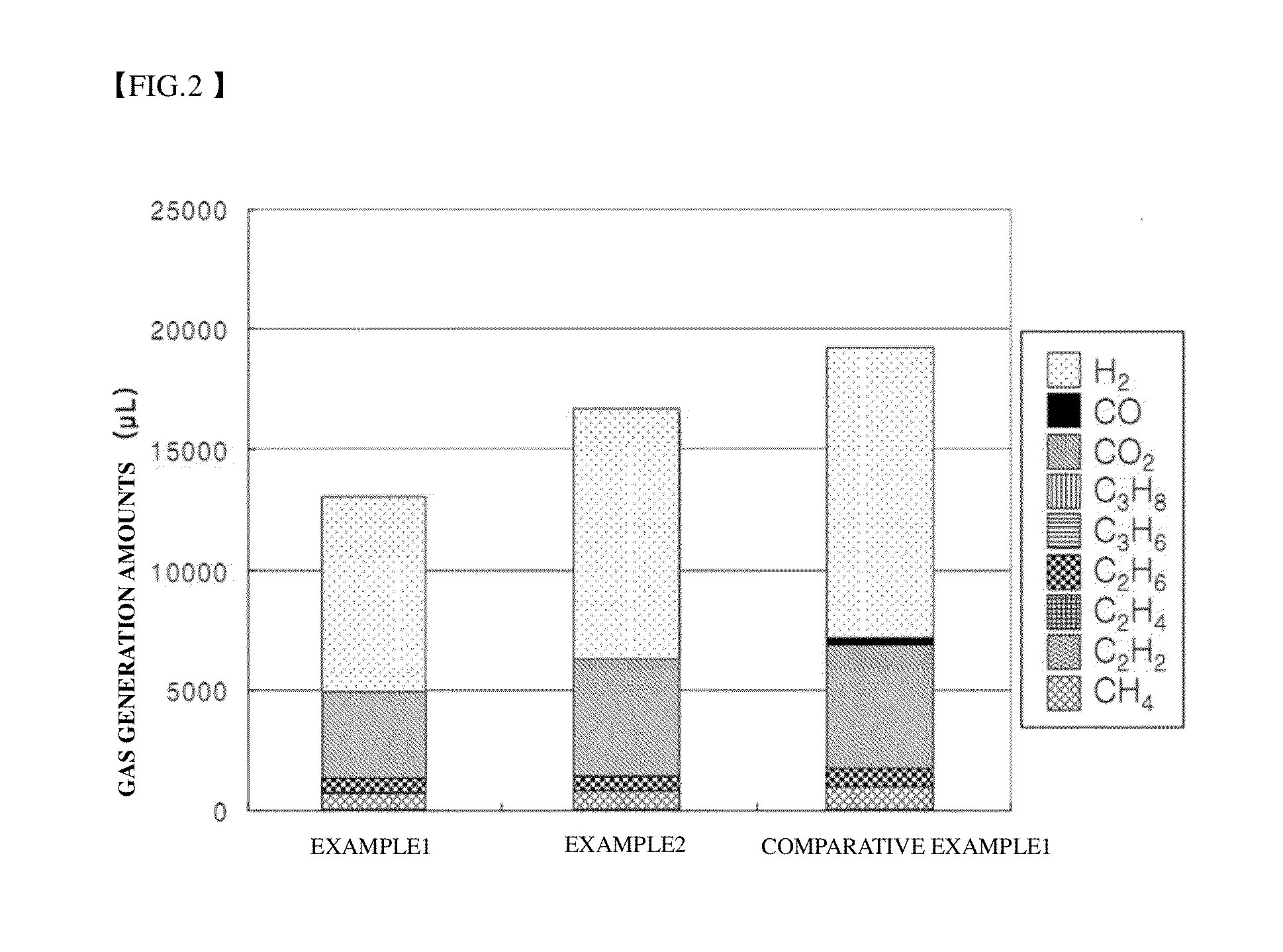
ActiveUS20110244339A1Reduce gas generationThickness of battery increaseOrganic electrolyte cellsElectrolytesDecompositionHigh rate
Disclosed are a non-aqueous electrolyte solution for a lithium secondary battery and a lithium secondary battery comprising the same. The non-aqueous electrolyte solution for a lithium secondary battery may include difluorotoluene having a lowest oxidation potential among components of the non-aqueous electrolyte solution. The lithium secondary battery may have improvement in basic performance including high rate charge / discharge characteristics, cycle life characteristics, and the like, and may remarkably reduce swelling caused by decomposition of an electrolyte solution under high voltage conditions such as overcharge.
Owner:LG ENERGY SOLUTION LTD
Nonaqueous Electrolytic Solution for Lithium Secondary Battery
ActiveUS20080248399A1Improve securityInhibit swellingElectrolytic capacitorsOrganic electrolyte cellsLithiumSolvent
Disclosed is a nonaqueous electrolytic solution useful for producing a lithium secondary battery having excellent cycle characteristics. Specifically disclosed is a nonaqueous electrolytic solution for lithium secondary batteries obtained by dissolving an electrolyte salt in a nonaqueous solvent which is characterized by containing 0.1 to 10 wt. % of a tert-alkylbenzene compound and also containing 0.001-0.5 wt. % of a benzene compound, wherein a hydrocarbon group having 1-4 carbon atoms is bonded to a benzene ring via the tertiary carbon atom, relative to the tert-alkylbenzene compound.
Owner:MU IONIC SOLUTIONS CORP
Lithium battery having higher performance
ActiveUS20140377656A1Lower performance requirementsImprove the immunityFinal product manufactureLi-accumulatorsEngineeringTitanium oxide
Disclosed is a lithium secondary battery including an electrode assembly including a cathode, an anode, and a separator disposed between the cathode and the anode and an electrolyte, wherein the anode includes a lithium titanium oxide (LTO) as an anode active material, and the lithium secondary battery has a charge cut-off voltage of 3.3 to 4 V and, when the charge cut-off voltage is reached, the anode has a potential of 0.75 to 1.545 V within a range within which a potential of the cathode does not exceed 4.95 V.
Owner:LG ENERGY SOLUTION LTD
Redox couple for electrochemical and optoelectronic devices
InactiveUS9779879B2Increase oxidation potentialEasy to adjustLight-sensitive devicesCell electrodesDouble bondElectrochemistry
The present invention provides an improved redox couple for electrochemical and optoelectronic devices. The redox couple is based on a complex of a first row transition metal, said complex containing at least one mono-, bi-, or tridentate ligand comprising a substituted or unsubstituted ring or ring system comprising a five-membered N-containing heteroring and / or a six-membered ring comprising at least two heteroatoms, at least one of which being a nitrogen atom, said five- or six-membered heteroring, respectively, comprising at least one double bond. The invention also relates to electrolytes and to the devices containing the complex, and to the use of the complex as a redox couple. The invention further provides electrochemical and / or optoelectronic devices comprising a first and a second electrode and, between said first and second electrode, a charge transport layer, said a charge transport layer comprising tetracyanoborate ([B(CN)4]−) and a cationic metal complex functioning as redox-couple.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Lithium ion secondary battery
ActiveUS10297814B2Accelerate decomposition reactionLower potentialFinal product manufactureCell electrodesLithium carbonatePhysical chemistry
Owner:TOYOTA JIDOSHA KK
Electrochromic element, and method for driving the same
PendingUS20220035216A1Potential oxidationStatic indicating devicesNon-linear opticsReduction potentialPhotochemistry
Provided is an electrochromic element including: a first electrode; a second electrode apart from and opposite to the first electrode; an electrolyte layer between the first electrode and the second electrode, containing an oxidizable substance or a reducible substance, or both; and at least one of an oxidizable electrochromic layer between the first electrode and the electrolyte layer, containing an oxidizable electrochromic compound, and a reducible electrochromic layer between the second electrode and the electrolyte layer, containing a reducible electrochromic compound, wherein an oxidation potential of the oxidizable substance is nobler than an oxidation potential of the oxidizable electrochromic compound, a reduction potential of the reducible substance is baser than a reduction potential of the reducible electrochromic compound, an oxidation reaction of the oxidizable substance is irreversible, and a reduction reaction of the reducible substance is irreversible.
Owner:RICOH KK
Lithium secondary battery with excellent performance
ActiveUS9564635B2Lower performance requirementsImprove the immunityLarge-sized flat cells/batteriesCell electrodesEngineeringComposite oxide
Disclosed is a lithium secondary battery including an electrode assembly including a cathode, an anode, and a separator disposed between the cathode and the anode and an electrolyte, wherein the cathode includes a spinel-structure lithium manganese composite oxide represented by Formula 1 below as a cathode active material, and the lithium secondary battery has a charge cut-off voltage of 3.3 to 4 V and, when the charge cut-off voltage is reached, the anode has a potential of 0.75 to 1.545 V within a range within which a potential of the cathode does not exceed 4.95 V:LixMyMn2−yO4−zAz (1)wherein 0.9≦x≦1.2, 0<y<2, and 0≦z<0.2;M is at least one element selected from the group consisting of Al, Mg, Ni, Co, Fe, Cr, V, Ti, Cu, B, Ca, Zn, Zr, Nb, Mo, Sr, Sb, W, Ti, and Bi; andA is at least one monovalent or divalent anion.
Owner:LG ENERGY SOLUTION LTD
Non-aqueous electrolyte solution for lithium secondary battery and lithium secondary battery comprising the same
ActiveUS8361660B2Reduce generationIncreasing the thicknessOrganic electrolyte cellsElectrolytesHigh rateDecomposition
Disclosed are a non-aqueous electrolyte solution for a lithium secondary battery and a lithium secondary battery comprising the same. The non-aqueous electrolyte solution for a lithium secondary battery may include difluorotoluene having a lowest oxidation potential among components of the non-aqueous electrolyte solution. The lithium secondary battery may have improvement in basic performance including high rate charge / discharge characteristics, cycle life characteristics, and the like, and may remarkably reduce swelling caused by decomposition of an electrolyte solution under high voltage conditions such as overcharge.
Owner:LG ENERGY SOLUTION LTD
Amorphous electrode active material and lithium secondary battery
ActiveUS8703334B2Avoid problemsReduce oxidationElectrode manufacturing processesSecondary cells charging/dischargingElectricityElectronegativity
Electrode active material that is used together with an electrolyte solution having an electrolyte decomposition potential Ve is represented by the general expression LixFeMyO2 and is amorphous. In the expression, x and y are values which independently satisfy 1<x≦2.5 and O<y≦3, respectively, and z=(x+(valence of Fe)+(valence of M)×y) / 2 to satisfy stoichiometry, and M represents one or two or more types of glass former element. The average electronegativity of M is less than (Ve+6.74 / 5.41.
Owner:KYUSHU UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com