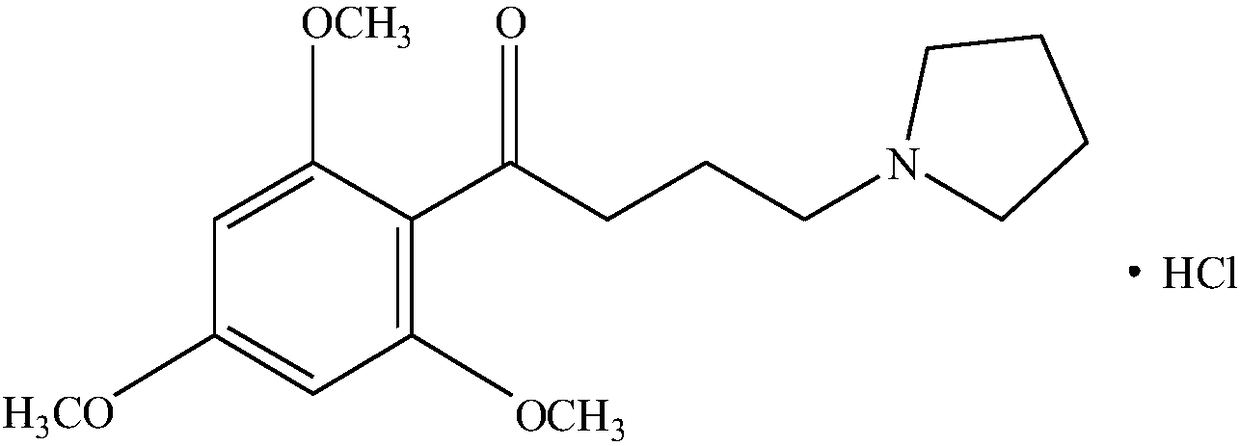
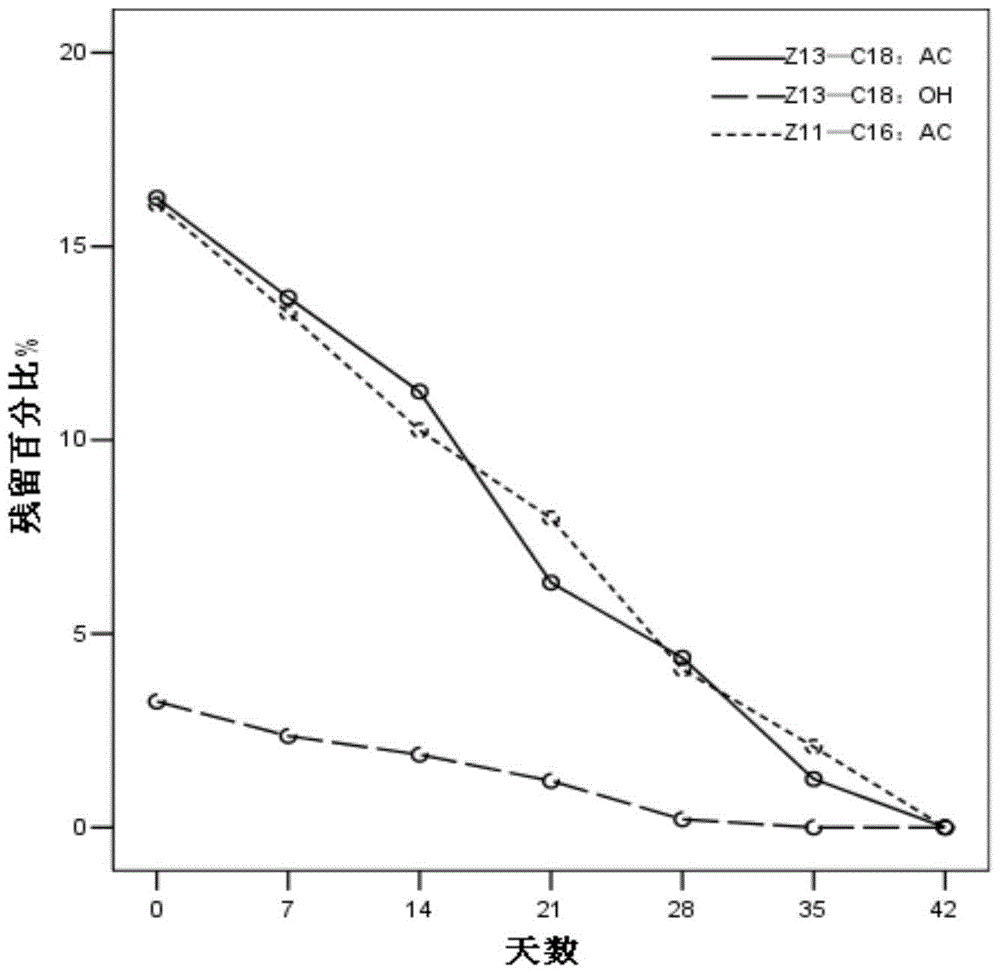
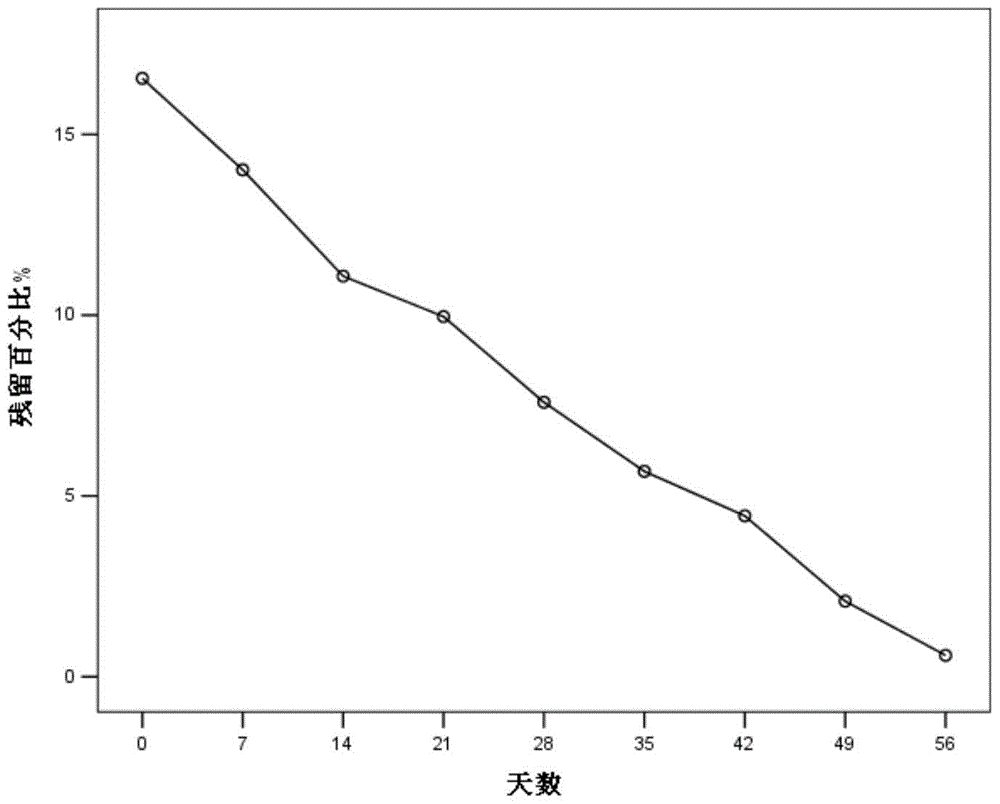
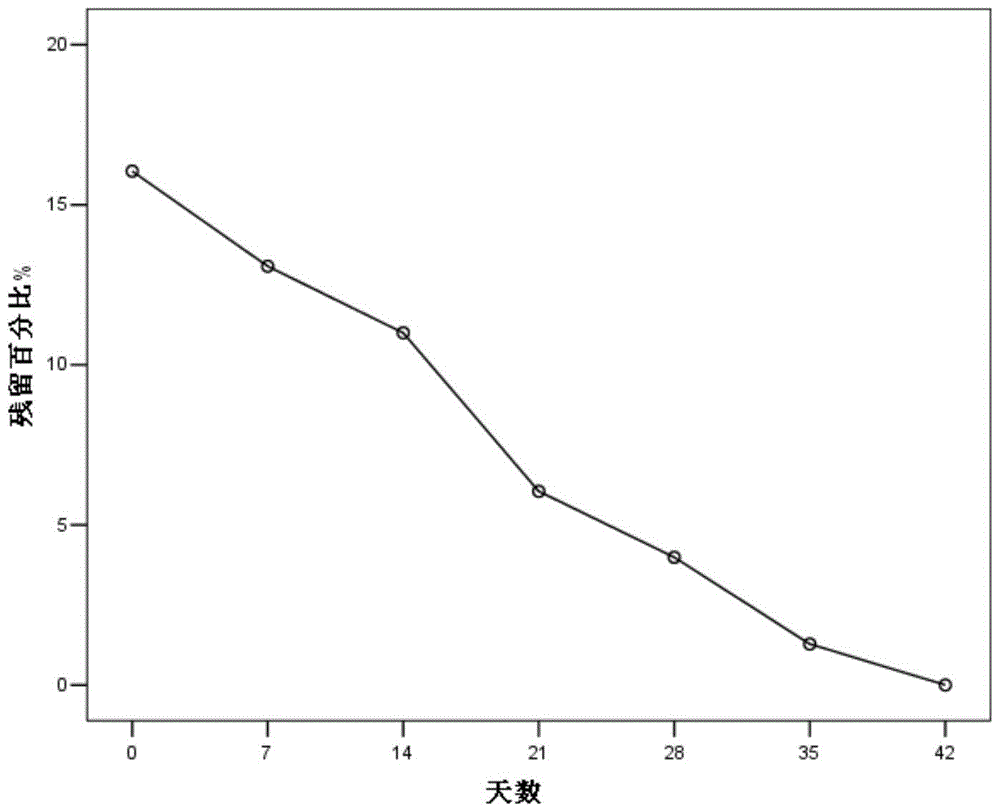
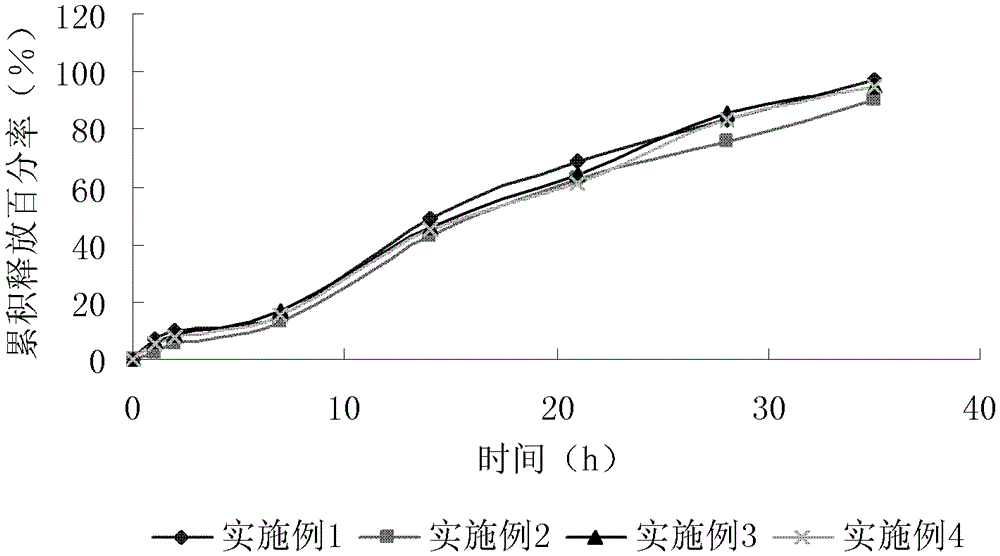
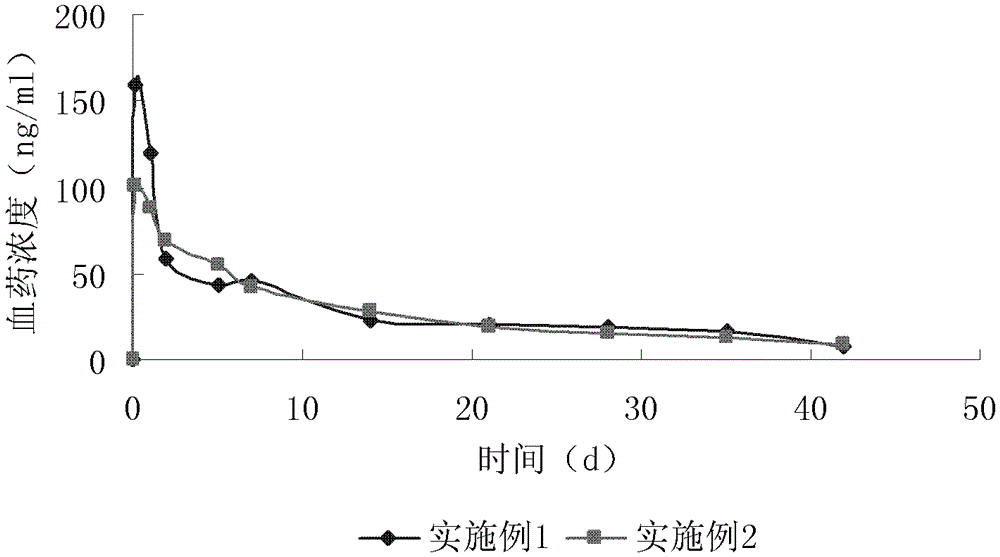
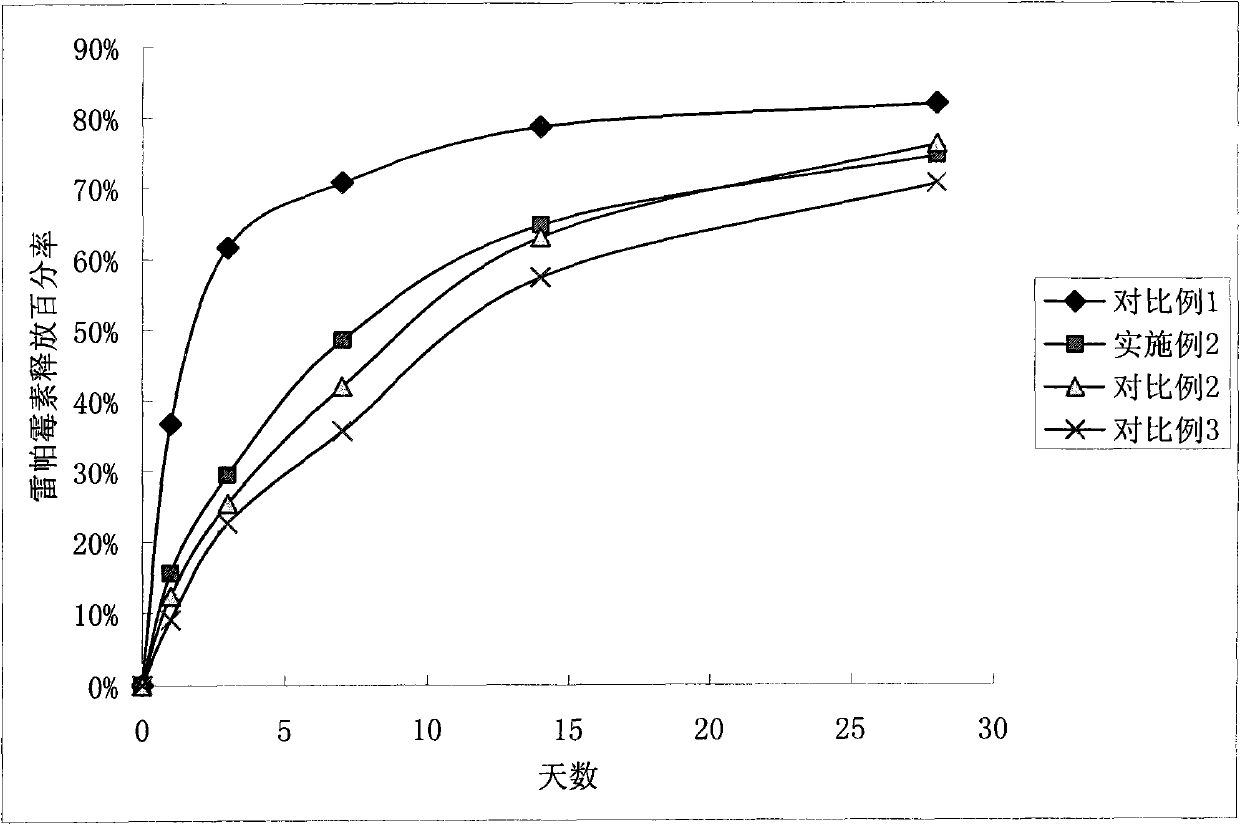
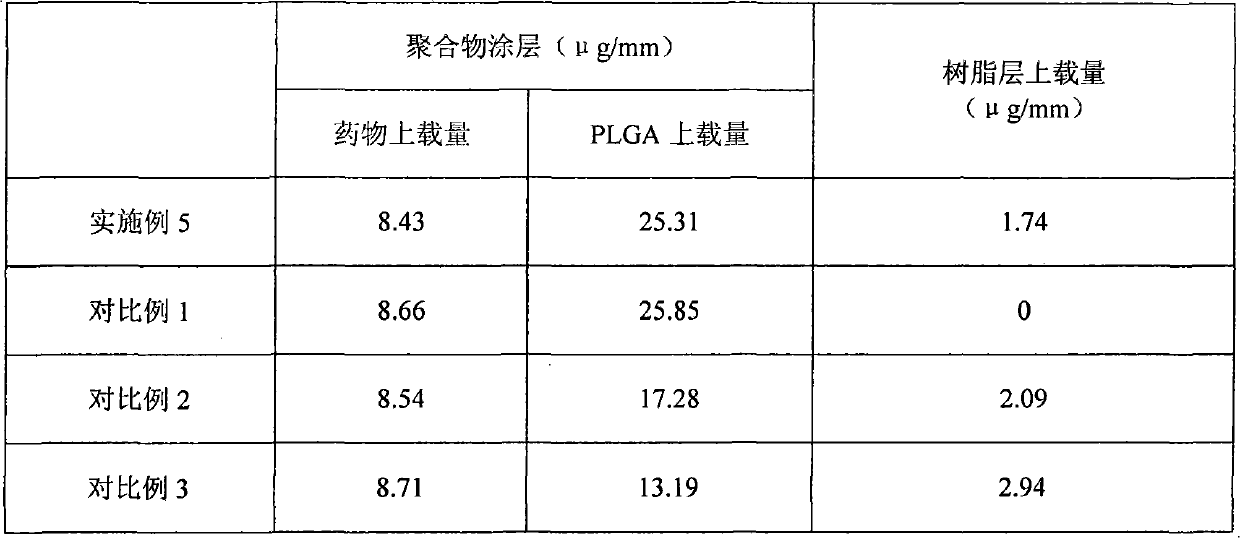
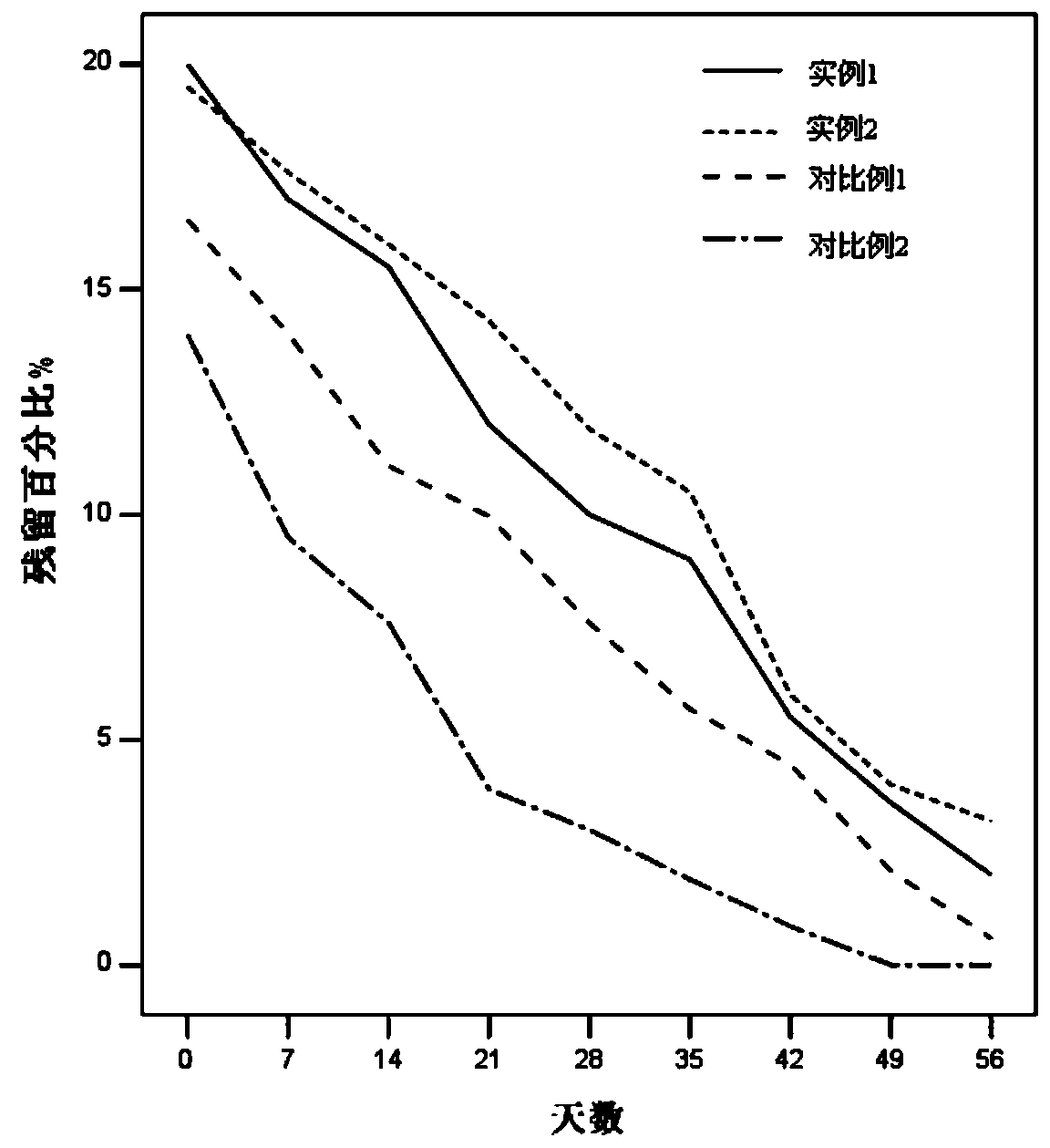
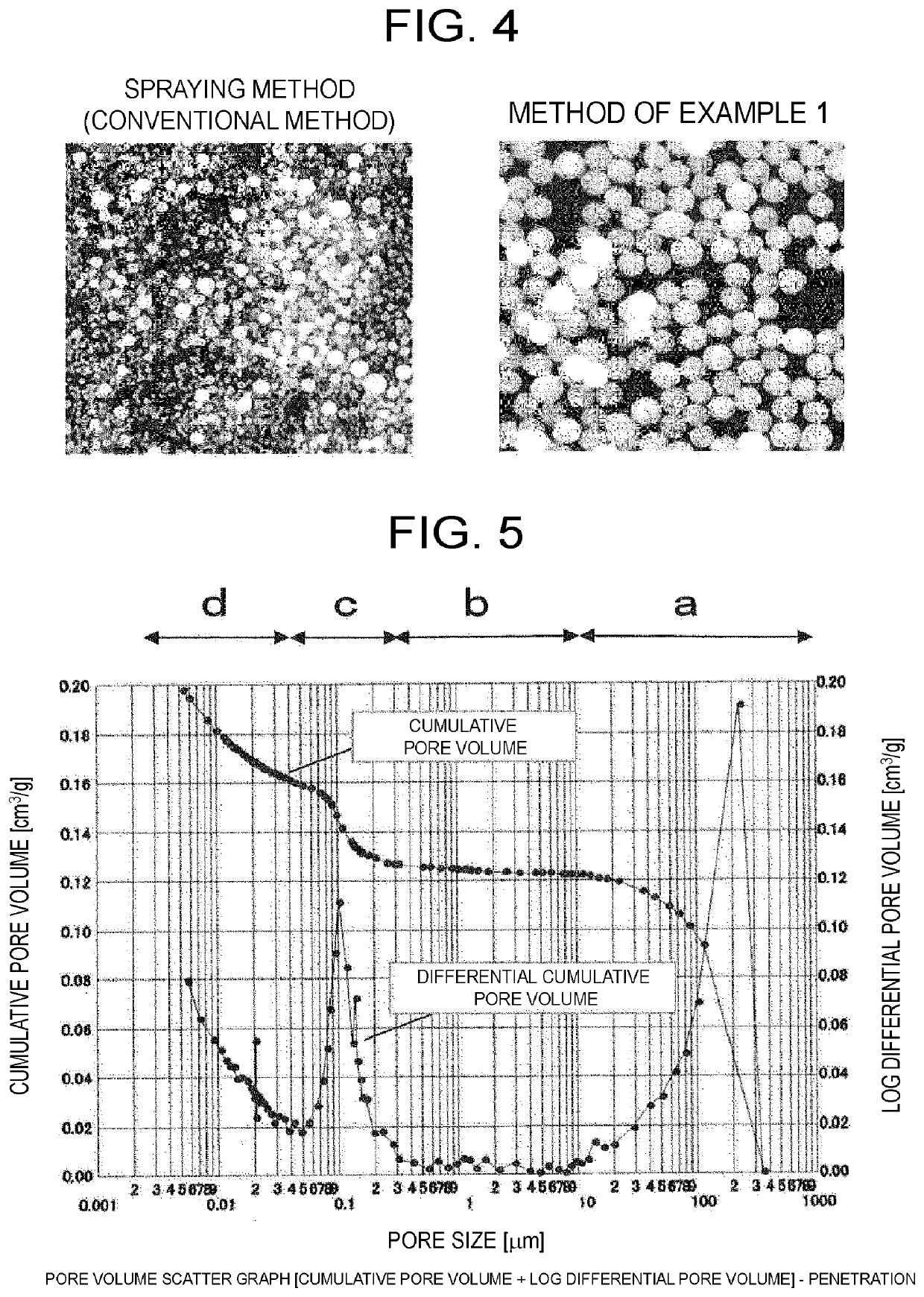
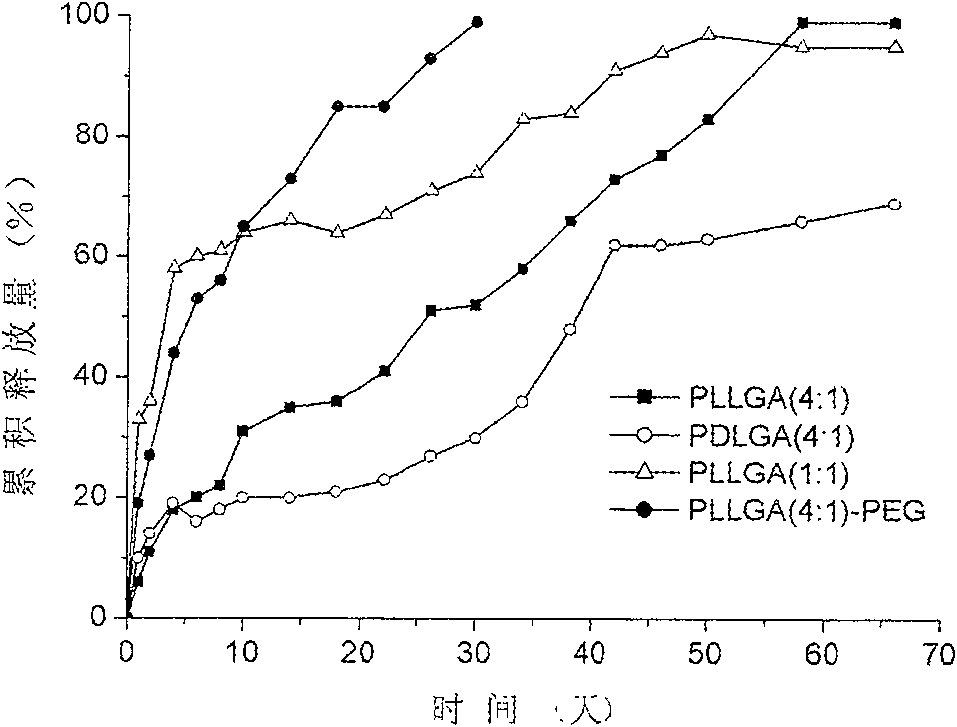
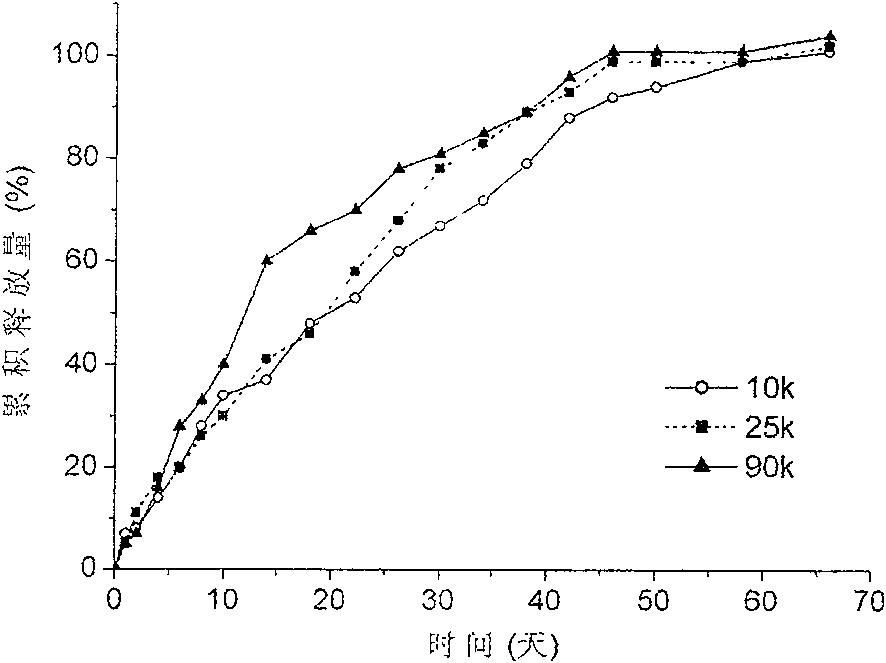
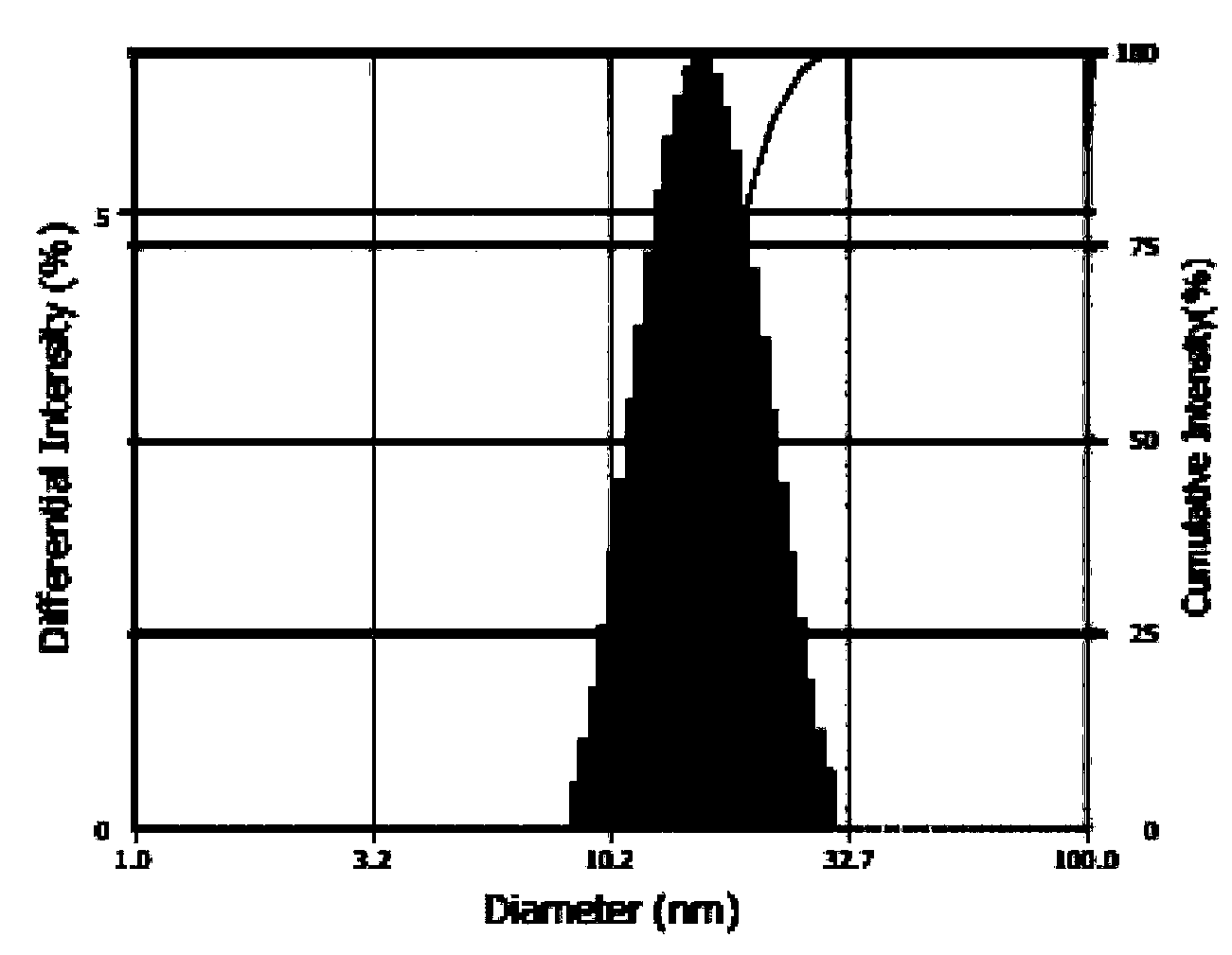
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "Improve release behavior" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
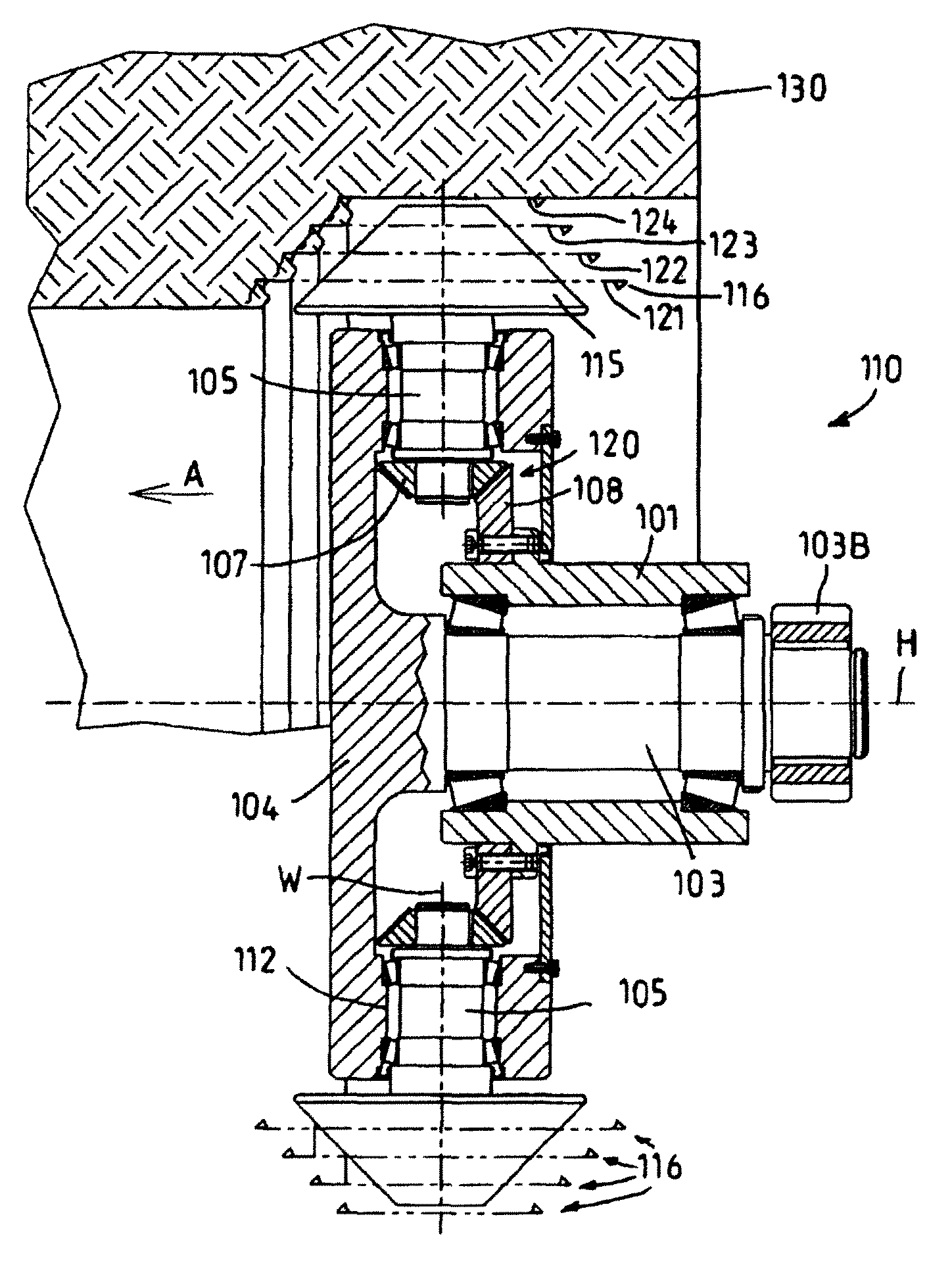
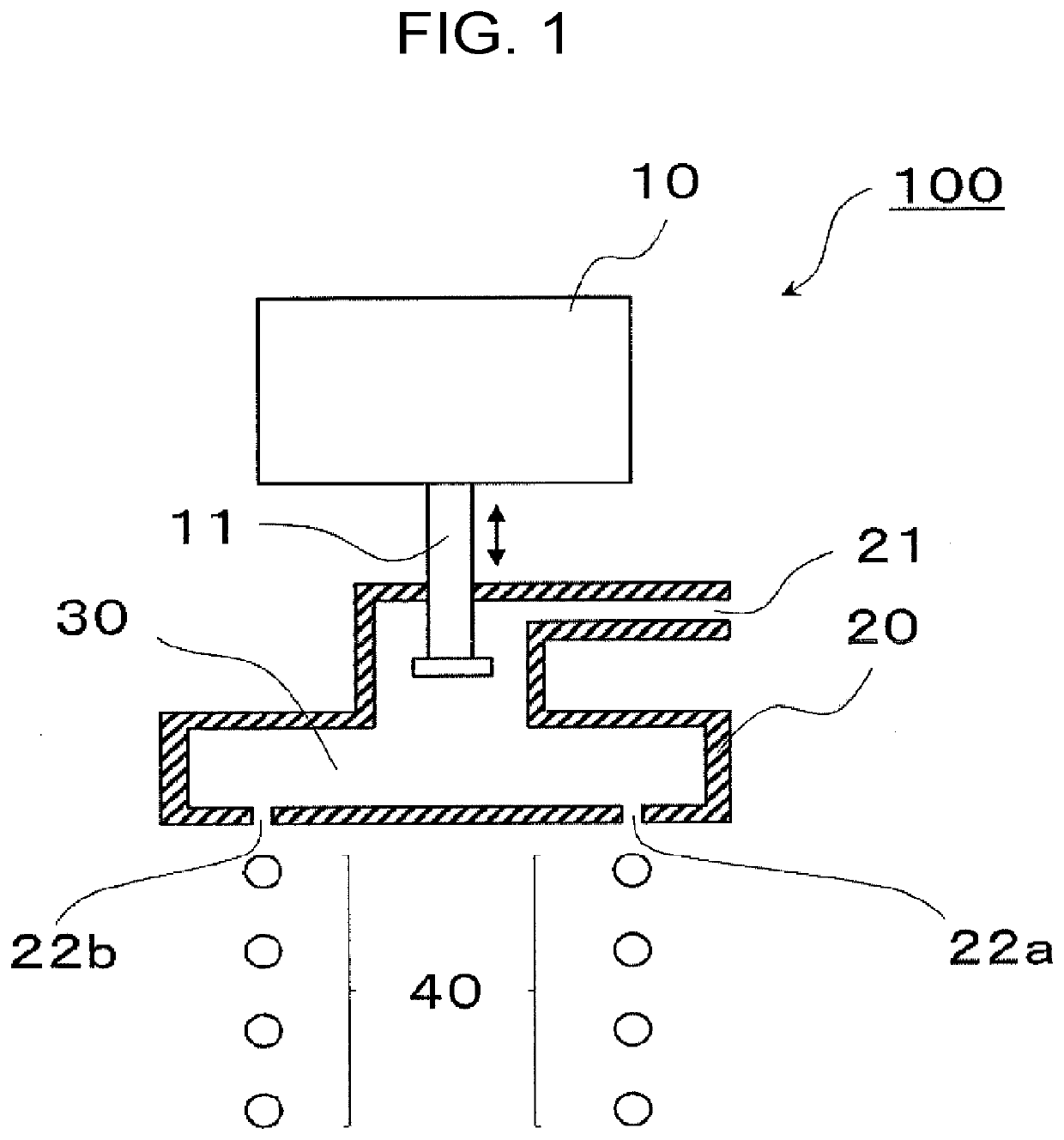
Method and apparatus for the milling cutting of materials
ActiveUS7896445B2High strengthLarge removal surfaceConstructionsDisloding machinesGear driveEngineering
An apparatus for the milling and / or drilling cutting of materials, in particular for the removal of rock, minerals or coal, with a tool drum which is mounted on a drum carrier rotatably about a drum axis, in which a plurality of tool shafts, which carry cutting tools at their ends projecting from the tool drum, are rotatable drivable mounted, at least two of the tool shafts being drivable by a common gear drive which has power take-off gearwheels arranged fixedly in terms of rotation on the tool shafts, and a common drive element which cooperates with the power take-off gearwheels. The drive element and the tool drum being rotatable in relation to one another and the shaft axes of the tool shafts standing transversely to the drum axis.
Owner:CATERPILLAR INC
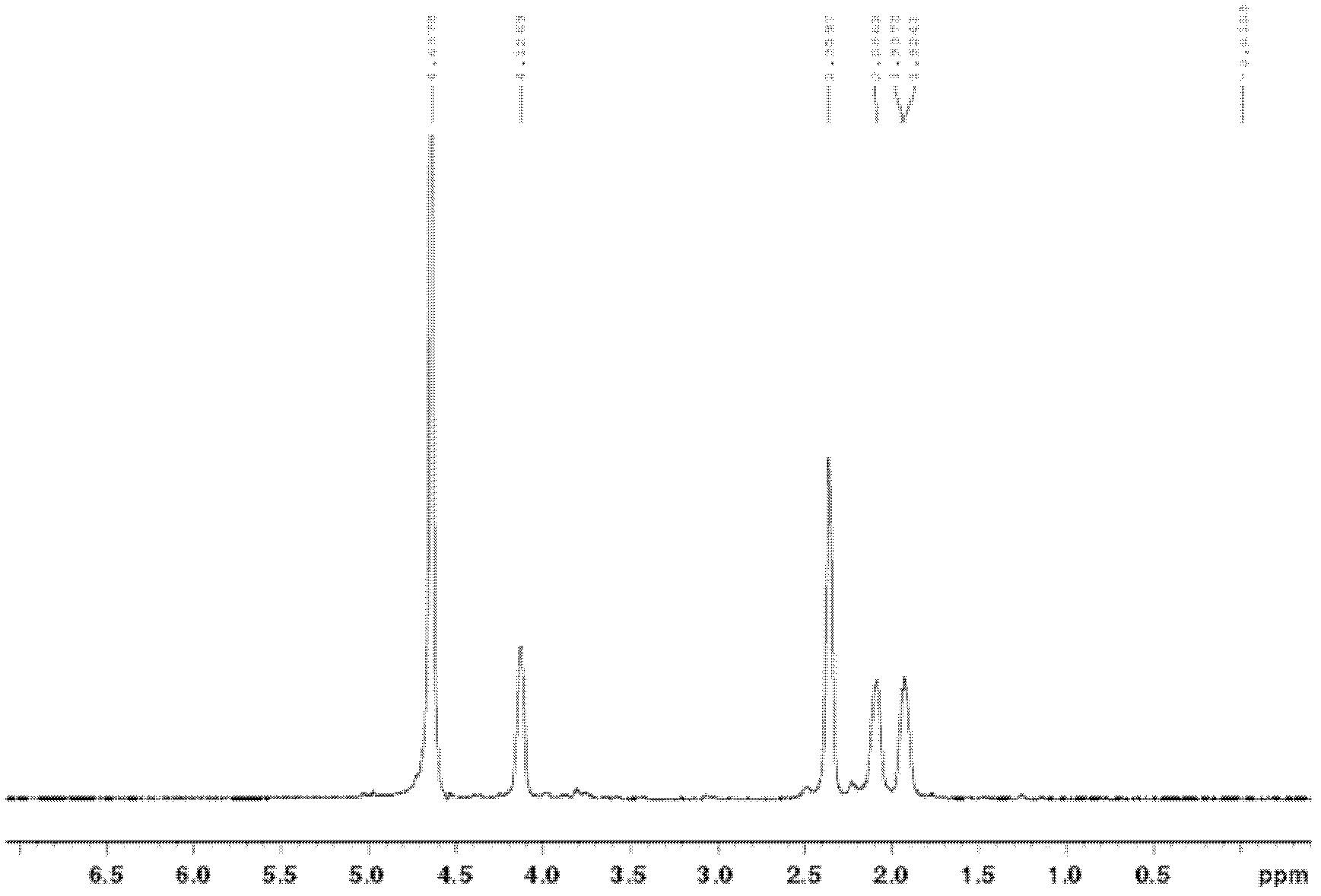
Anti-tumor platinum pro-drug and nanometer hydrogel drug and preparation method thereof
InactiveCN105713046AQuick releaseLittle side effectsOrganic active ingredientsHeavy metal active ingredientsHydrophilic monomerMicrosphere
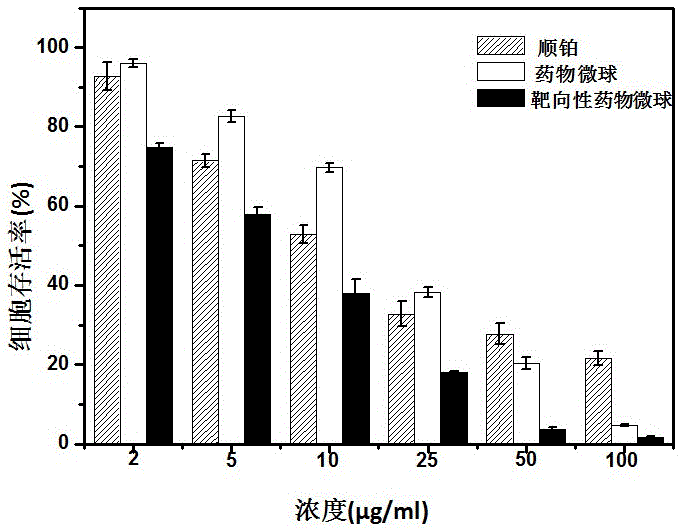
The invention belongs to the technical field of biological medicines and particularly provides an anti-tumor platinum pro-drug and a nanometer hydrogel drug and a preparation method thereof.The pro-drug is obtained by conducting hydrogen peroxide oxidation on an anti-tumor platinum drug to obtain a Pt(IV) sexadentate complex and then making the sexadentate complex react with anhydride or carboxylic acid containing double bonds.The pro-drug can be in self-polymerization or can be copolymerized with other hydrophilic monomers for the preparation of drug micro-spheres having high drug loading capacity.The micro-spheres are uniform in particle size and controllable in size and can keep long-term stability in an aqueous solution by using a stabilizer.The preparation method is simple, an obtained nanometer hydrogel drug carrier has glutathione stimulus responsibility, so that nanometer hydrogel is kept stable in a low-glutathione-concentration environment outside tumor cells, the drug is rarely leaked after long-time cycle, the nanometer hydrogel is rapidly degraded in the reducing environment within the tumor cells and converted into a linear chain of small molecular weight, the drug is quickly released, and the targeted killing effect on the target tumor cells is achieved.
Owner:FUDAN UNIV
Medicament eluting stent and preparation method thereof
ActiveCN101711710AReduce dosageReduce adverse reactions such as restenosisStentsMedical devicesGlycolic acidMedicine
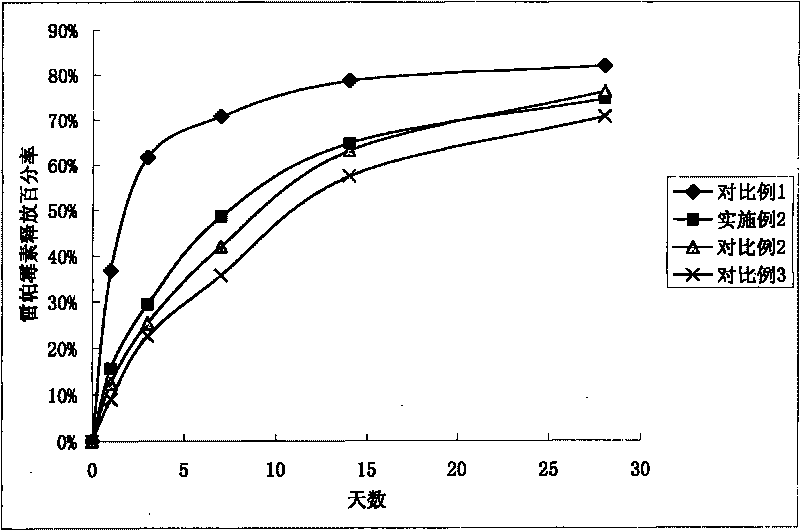
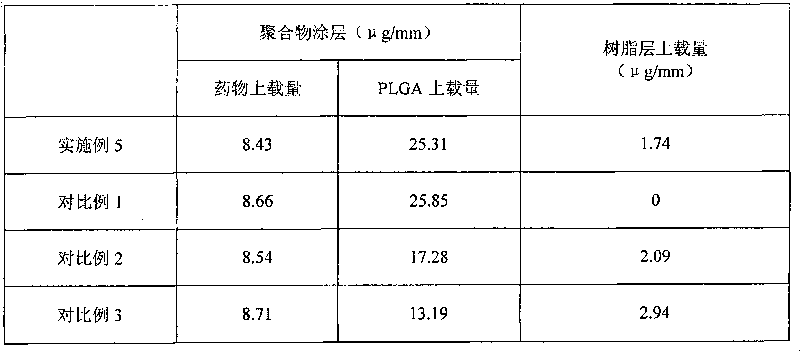
The invention relates to a medicament eluting stent, which consists of a bare stent and a coating coated on the bare stent. The medicament eluting stent is characterized in that the coating at least comprises a polymer layer and a resin layer, wherein the polymer layer comprises polylactic-co-glycolic acid (PLGA) and medicaments; and the resin layer is coated on the surface of the polymer layer. A resin of the invention is coated on the surface of the medicament-loaded polymer coating. Due to the sustained release effect of the resin, on the premise of guaranteeing medicament dosage, the dosage of the polymer is reduced so as to obvious reduce an inflammatory reaction, reduce the generation of adverse reactions such as late intravascular restenosis and the like, and avoid forming late thrombosis.
Owner:SHENZHEN SALUBRIS BIOMEDICAL ENG CO LTD
Sustained release microsphere containing risperidone and risperidone analogues and preparation method thereof
InactiveCN103126997AReduce porosityReduce burstOrganic active ingredientsNervous disorderEmulsionChronic mental disease
The invention provides a sustained release microsphere containing risperidone and risperidone analogues and a preparation method thereof, wherein the sustained release microsphere containing the risperidone and the risperidone analogues can be used for injections. The sustained release microsphere comprises an active substance risperidone, the risperidone analogues, biodegradable medicinal high polymer materials, plasticizers, antioxygen and the like. The preparation method of the sustained release microsphere containing the risperidone and the risperidone analogues can be an emulsion process, a spray drying process, a low-temperature spray extraction method, a supercritical fluid method and the like. A blank medicinal high polymer material layer can also be wrapped on the surface of the microsphere on the basis. The microsphere preparation solves the problems, of dumping, lag phase and the like, existing in a reported or a listed product. The composition is mainly used in treating acute and chronic mental diseases.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Metformin hydrochloride sustained-release tablet and preparation method thereof
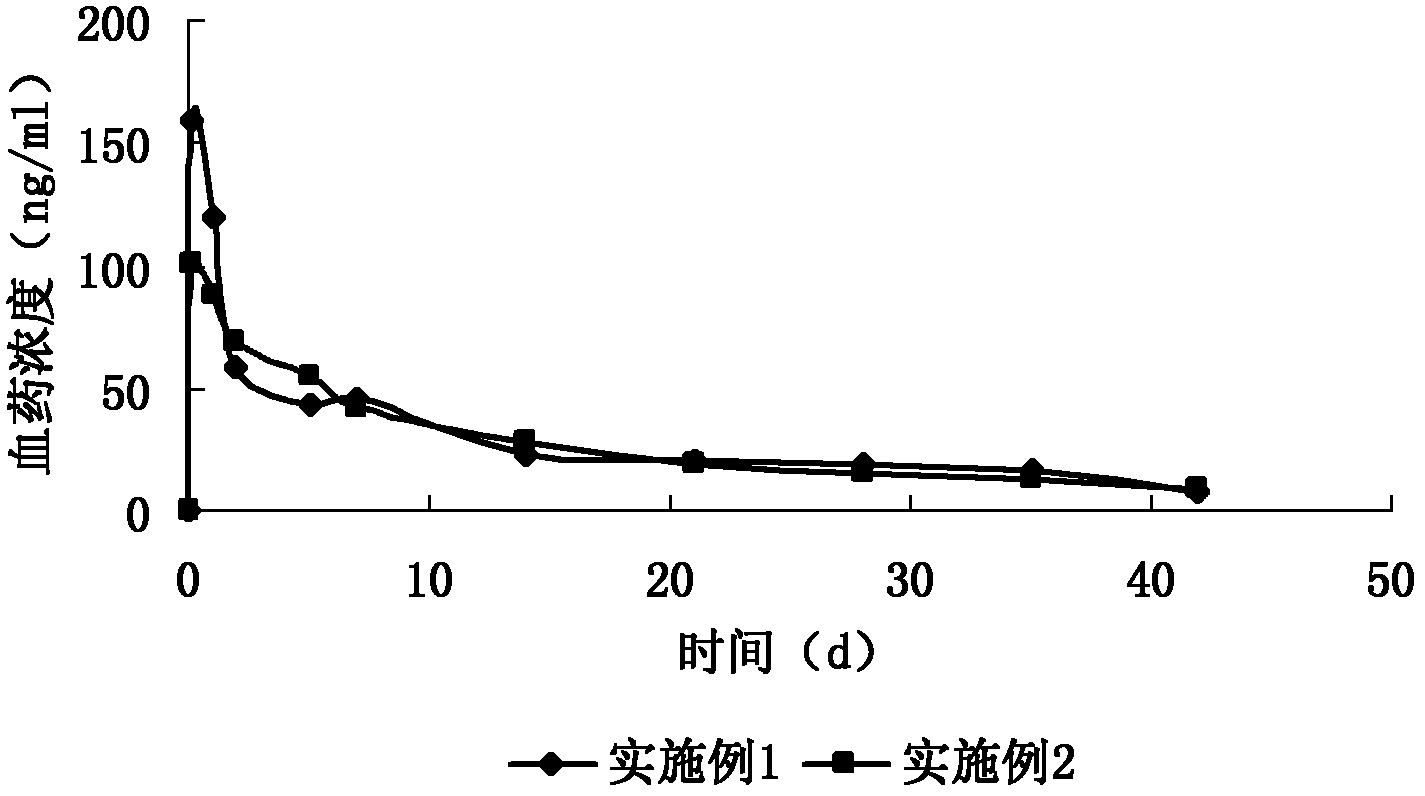
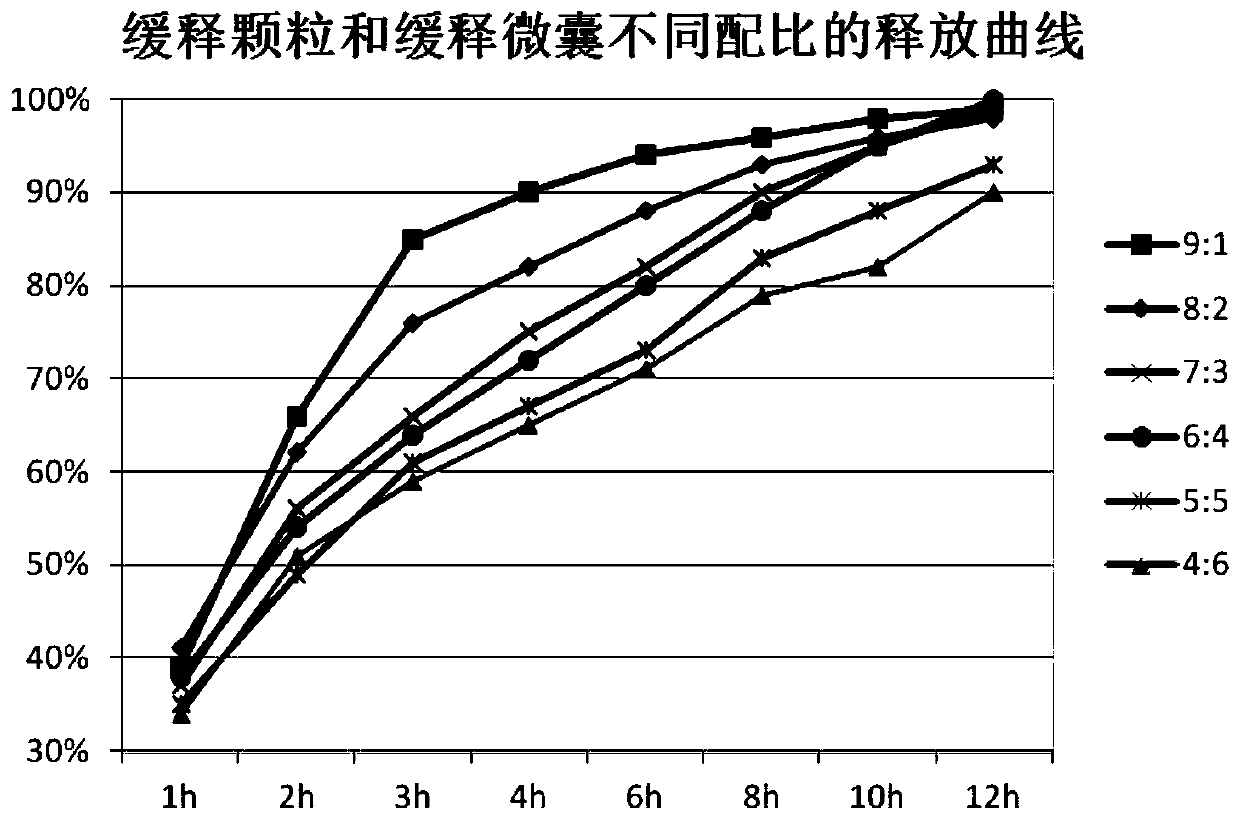
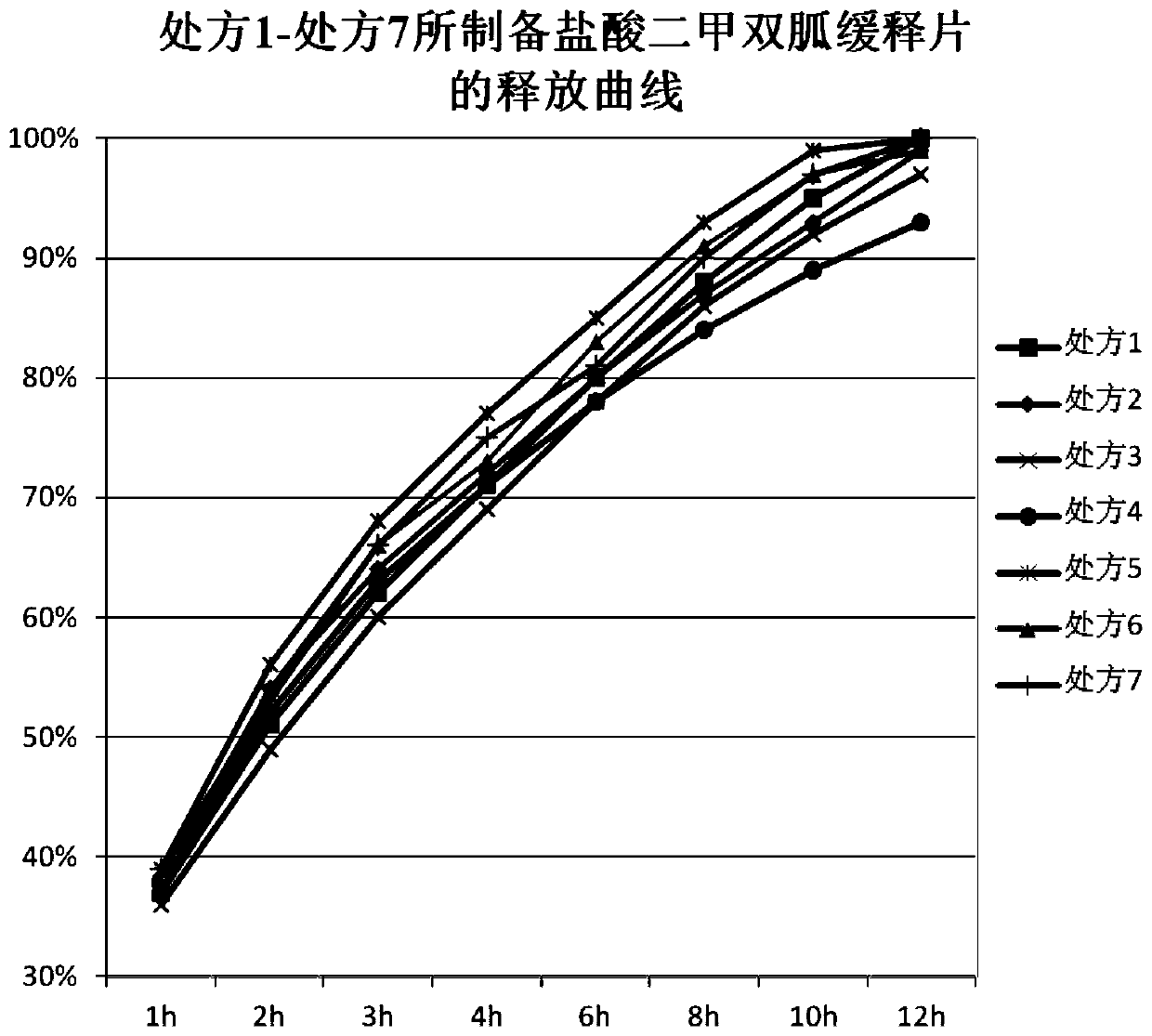
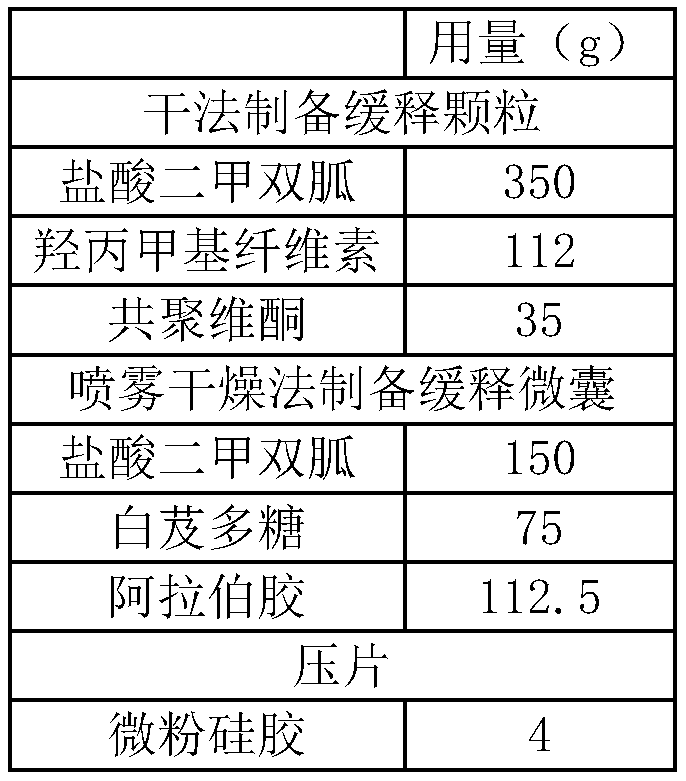
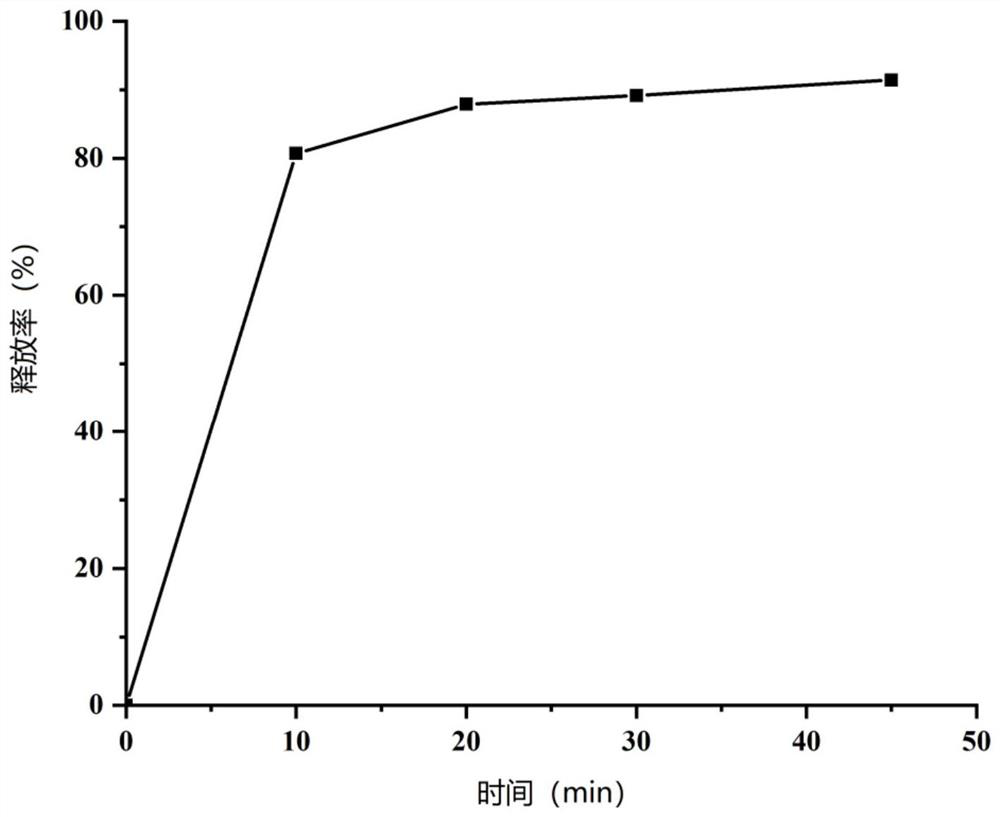
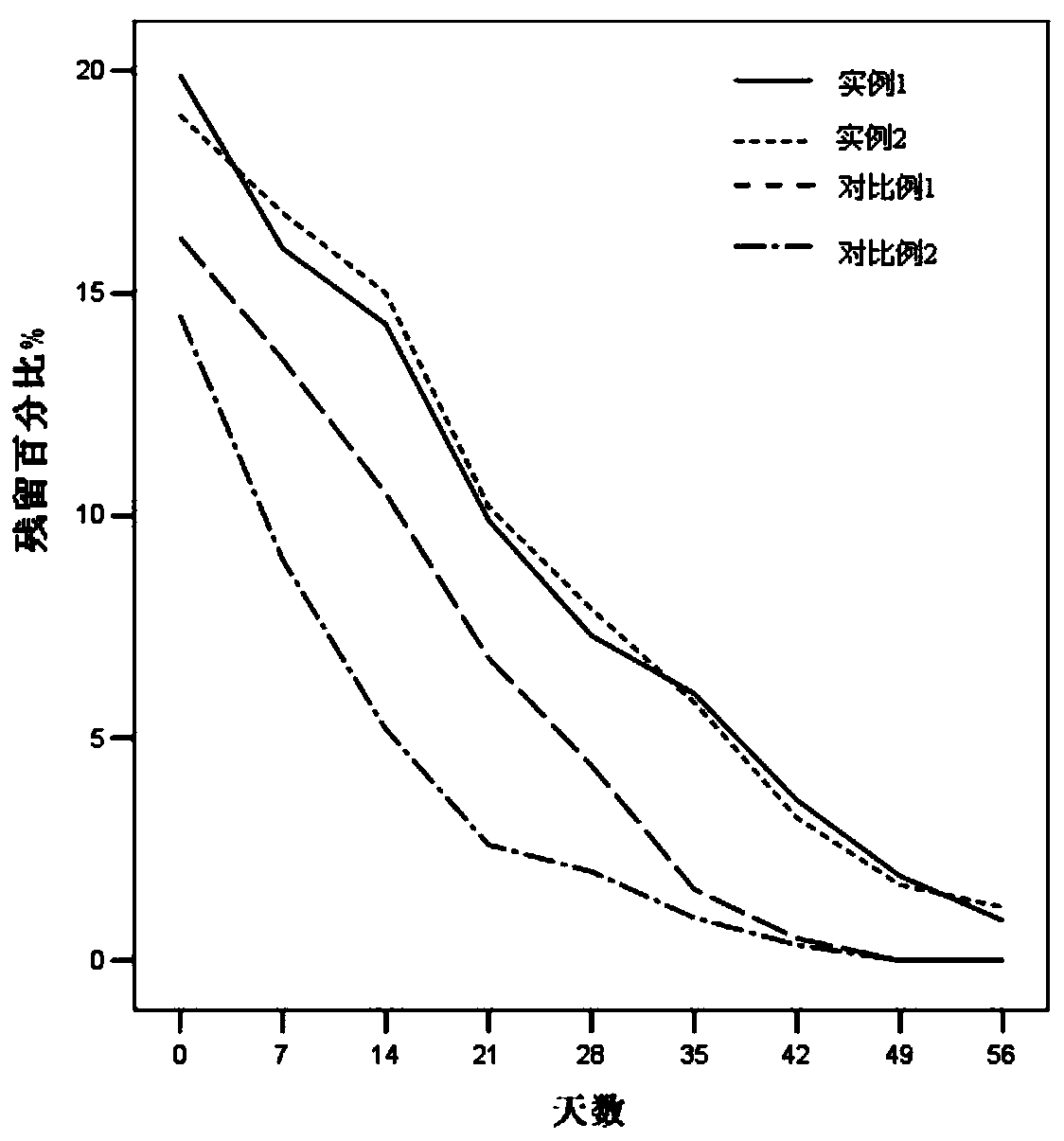
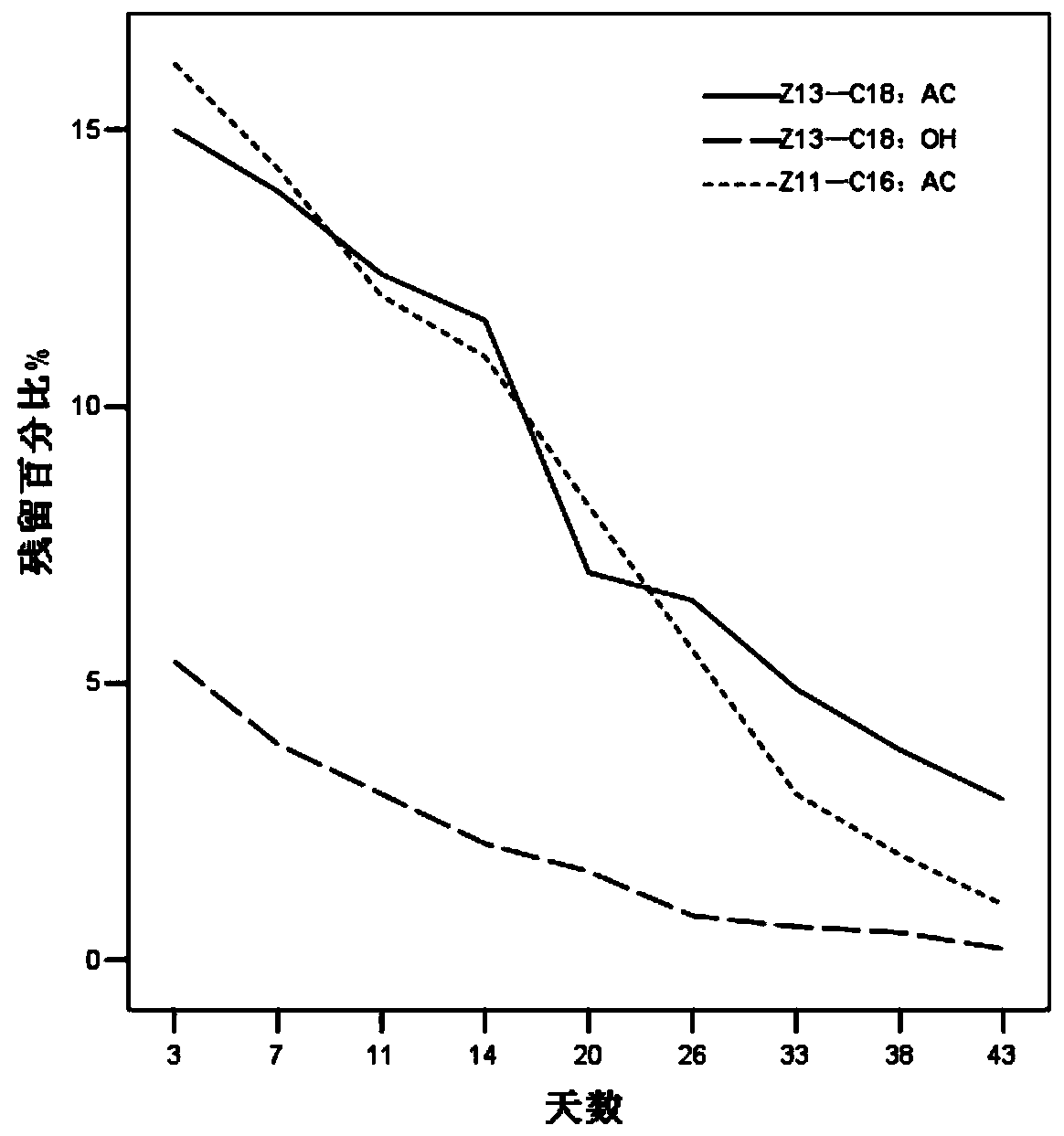
ActiveCN110354090AImprove release behaviorOvercome sudden releaseOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
The invention relates to a metformin hydrochloride sustained-release tablet and a preparation method thereof, and belongs to the technical field of medicine. The metformin hydrochloride sustained-release tablet is composed of sustained-release granules, sustained-release microcapsules and a lubricant, and is prepared through tabletting, wherein the weight ratio of the metformin hydrochloride in the sustained-release granules and the metformin hydrochloride in the sustained-release microcapsules is (3:7)-(6:4), and the lubricant is selected from superfine silica powder or magnesium stearate. According to the preparation method, a traditional spray drying method is improved, by combining a dry granulation process, the sustained-release microcapsules and sustained-release granules of different processes are prepared, tabletting is further carried out to prepare the metformin hydrochloride sustained-release tablet, the specific ratio of the sustained-release microcapsules prepared by the spray drying process to the sustained-release granules prepared by the dry granulation process is explored, and the metformin hydrochloride sustained-release tablet of which the inner portion has the structure of the sustained-release microcapsules and the sustained-release granules is finally obtained. The metformin hydrochloride sustained-release tablet and the preparation method thereof achievea good release behavior of the sustained-release tablet, overcome the shortcomings of sudden release and incomplete release, obtain a better release curve, and also greatly reduce the impurity content.
Owner:CSPC OUYI PHARM CO LTD
Anti-malarial medicinal composition and preparation method and application thereof
ActiveCN102283829BPromote absorptionGood curative effectOrganic active ingredientsPill deliveryLumefantrineCurative effect
Owner:恩威医药股份有限公司
Apremilast gel for injection to articular cavity and preparation method of apremilast gel
InactiveCN106924174AImprove disadvantagesReduce adverse reactionsOrganic active ingredientsAerosol deliveryArticular cavityAqueous solution
The invention mainly provides apremilast gel for injection to the articular cavity and a preparation method of the apremilast gel. According to the apremilast gel and the preparation method, polymer with the temperature-sensitive reverse gel property is adopted as a carrier material, and medicines are dispersed or dissolved in an aqueous solution of the carrier material. When the temperature is lower than the body temperature, a sample exists in the form of sol, and is injected into the body through a common syringe; when the temperature of the injected part rises to the body temperature, the sol is transformed into semi-solid gel, along with the slow dissolving of the carrier material, the medicines are released from the gel at the low rate, and the release of the medicines can last for several weeks to several months. The release ratio of the medicines can be realized by changing the variety, concentration, proportion and molecular weight of the gel material. The dosing interval of the gel is long, the preparation process is simple, the preparation process is mature, and the method is suitable for industrial mass production.
Owner:CHONGQING UNIV
Tofacitinib or tofacitinib salt sustained release preparation and preparation method thereof
PendingCN111728953AControlled Physical StabilityImprove stabilityOrganic active ingredientsSkeletal disorderControl releaseTofacitinib
The present invention relates to a tofacitinib or tofacitinib salt sustained release preparation, which sequentially comprises a permeation tablet core composition containing a release accelerant, a controlled release coating, and an unnecessary aesthetic coat from inside to outside, wherein based on the total weight of the tablet core composition, the tablet core composition comprises 6.4wt%-16wt% of a pharmaceutical active component, 20wt%-77wt% of the release accelerant, 0wt%-56wt% of an osmotic pressure accelerant, 16wt%-50wt% of an adhesive and 0.5wt%-7wt% of other pharmaceutical auxiliary materials; and the release accelerant is a cyclic oligosaccharide cyclodextrin derivative. The tofacitinib sustained release preparation prepared from the osmotic tablet core composition containingthe release accelerant can increase the in-vivo exposure amount of tofacitinib and has improved bioavailability.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Mesalazine slow-release granules and preparation method thereof
ActiveCN103989638AImprove coating efficiencyImprove release behaviorOrganic active ingredientsDigestive systemPlasticizerMedicine
The invention relates to mesalazine slow-release granules and a preparation method thereof. The slow-release granules are composed of the following active compositions and auxiliary agent in percent by weight: 40-60% of mesalazine, 18-36% of pill-preparing mother nucleuses, 3-7% of a bonding agent, 0.5-1% of a plasticizer, 15-35% of a coating material, 0.5-1% of a lubricant and 0.5-2% of a flow aid. The slow-release granules are characterized in that the mesalazine slow-release granules are prepared by performing medicine-charging pill preparation for two times and performing coating for two times, and the preparation technology successively comprises the following medicine-charging pill preparation and coating steps: (1) performing first medicine-charging pill preparation on the pill-preparing mother nucleuses, so as to obtain first medicine-charged pills; (2) performing first coating on the first medicine-charged pills, so as to prepare first-coated medicine pills; (3) performing secondary medicine-charging pill preparation on the first medicine-charged pills, so as to prepare secondary medicine-charged pills; and (4) performing secondary coating on the secondary medicine-charged pills. According to the slow-release granules prepared through the specific technology, the coating efficiency and the release behavior of mesalazine are improved.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4
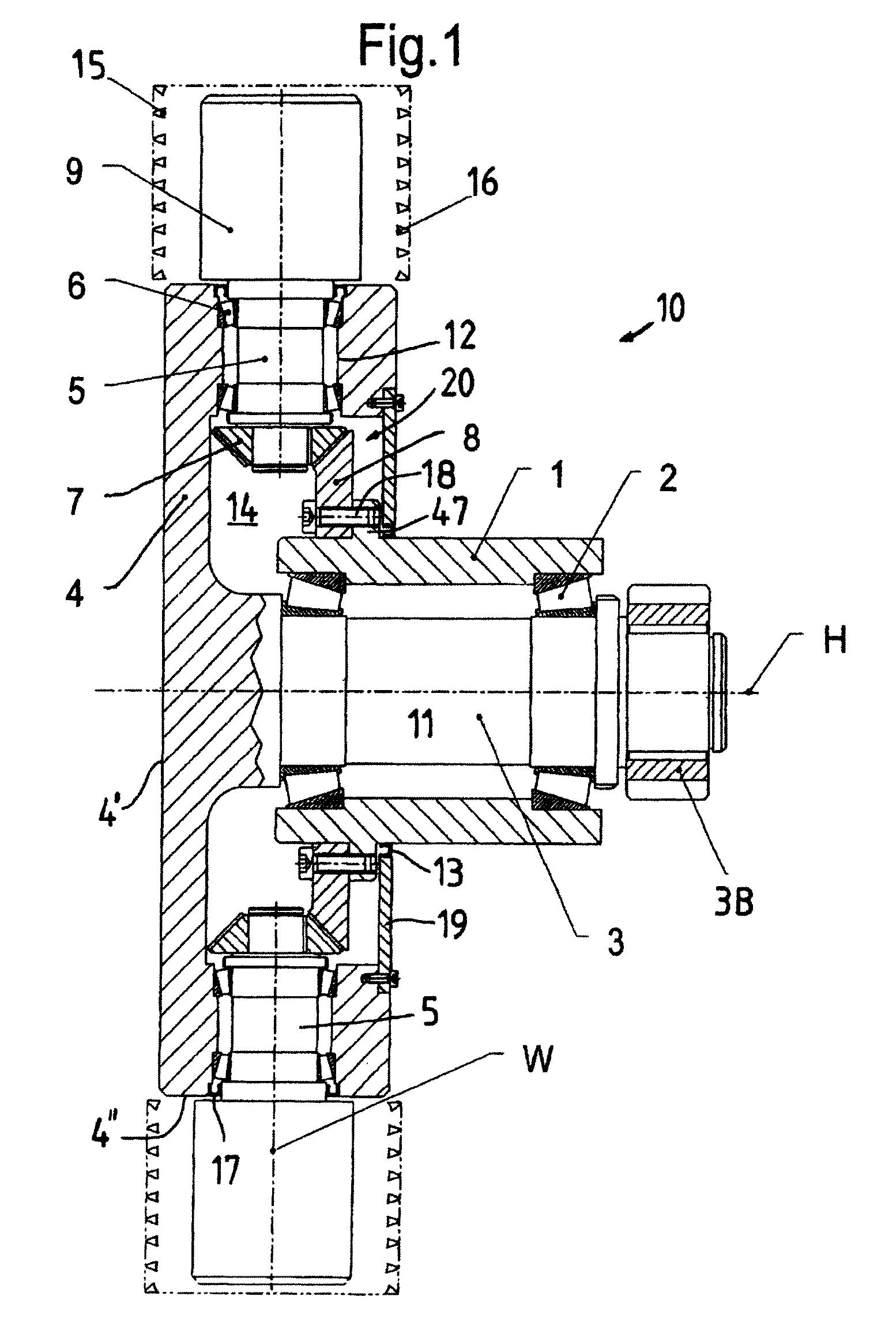
Electromechanical brake booster
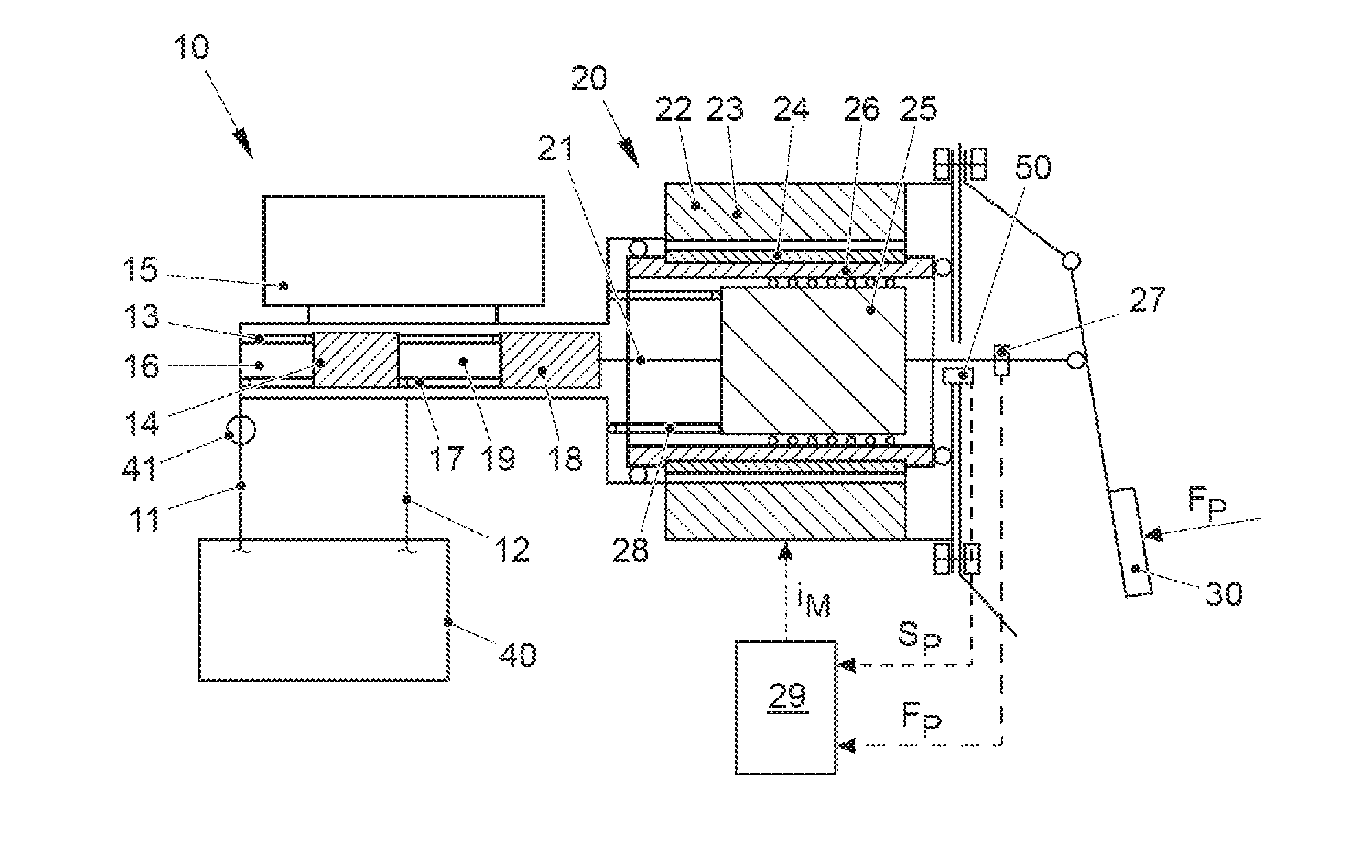
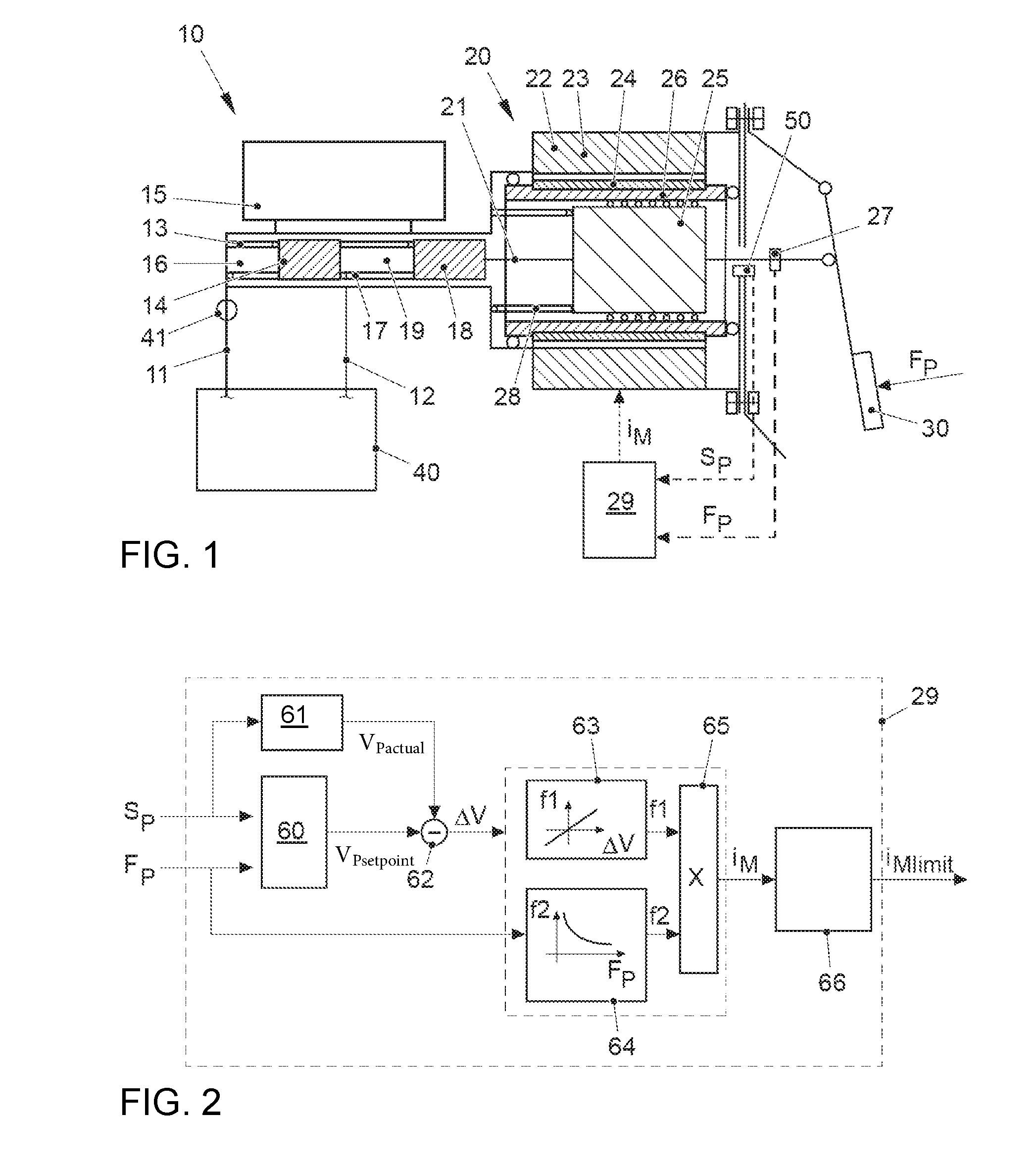
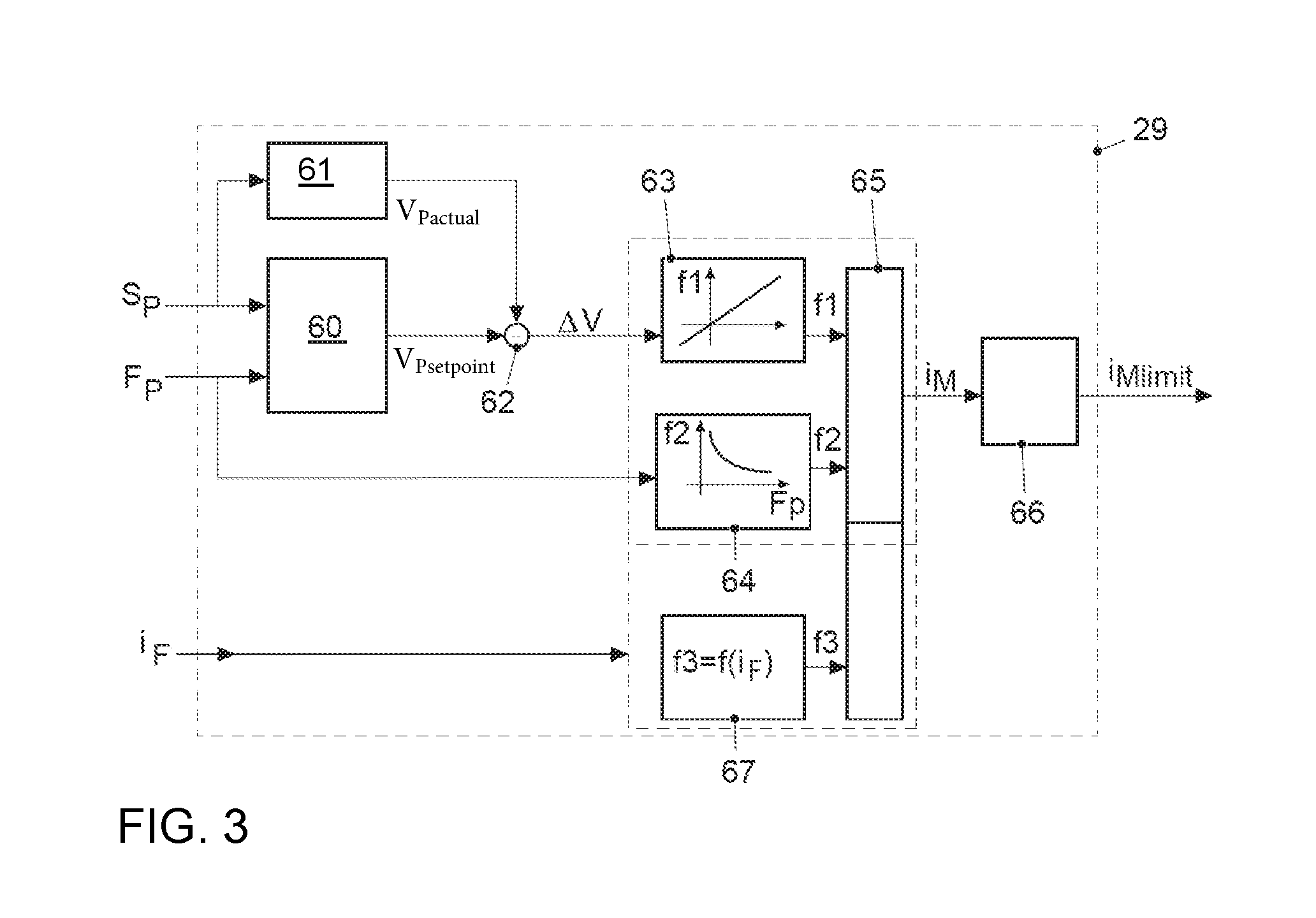
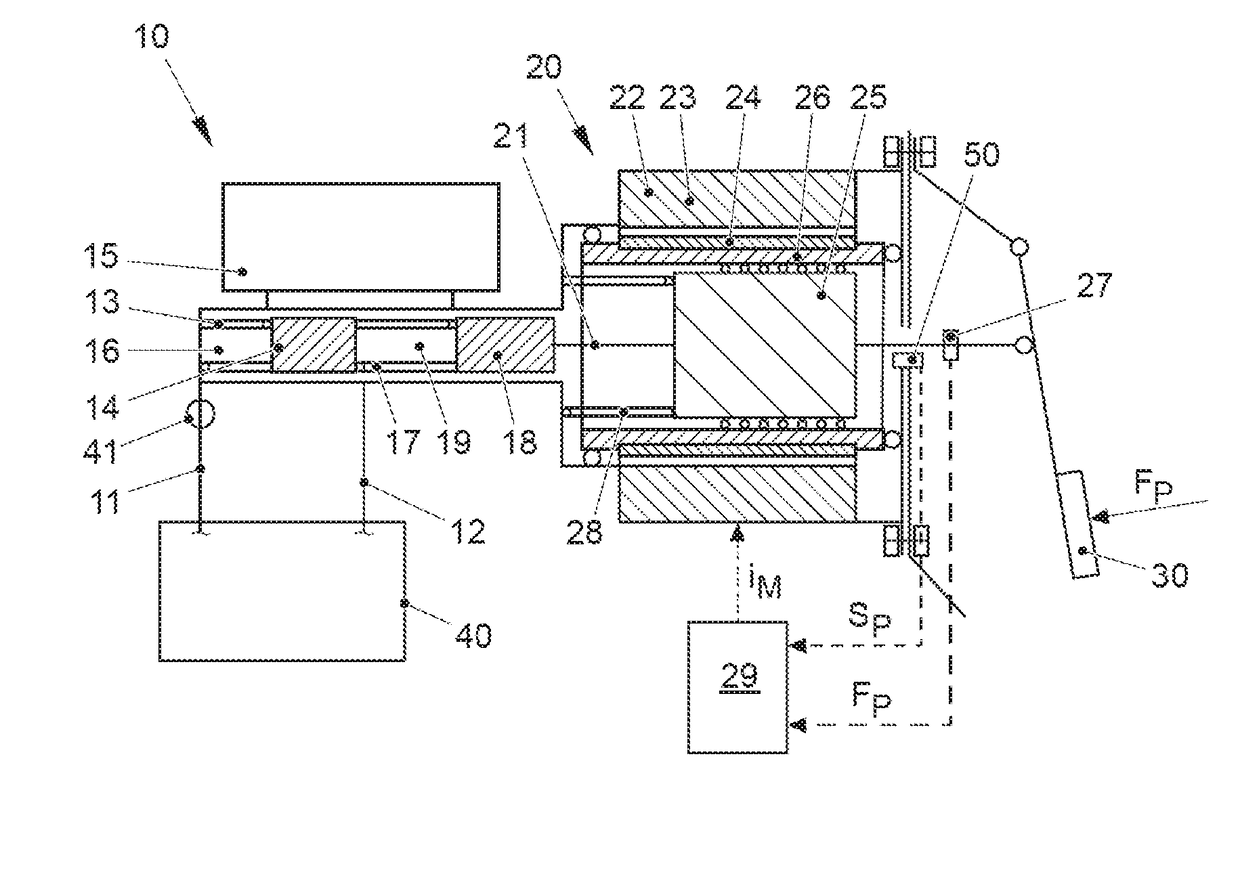
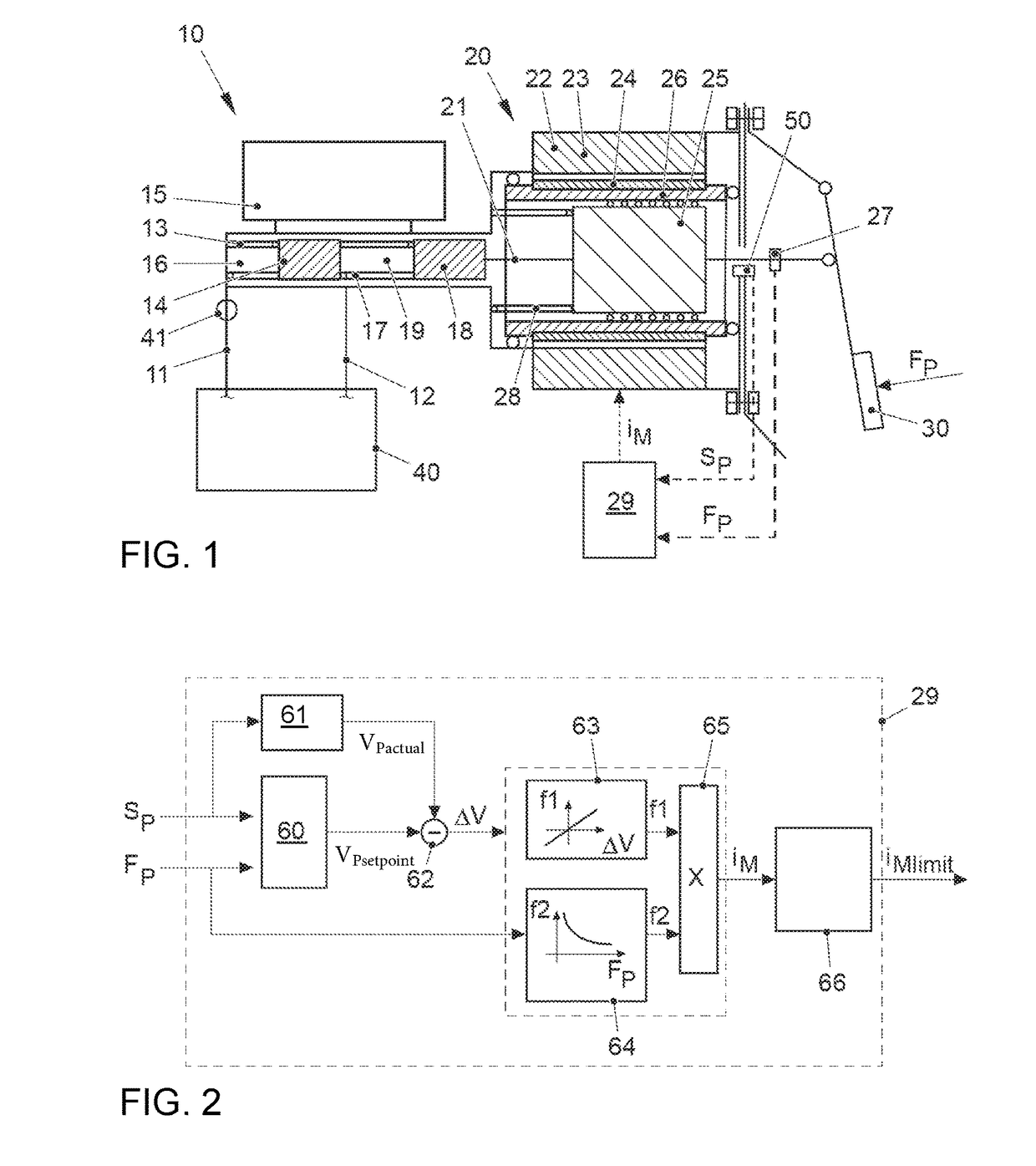
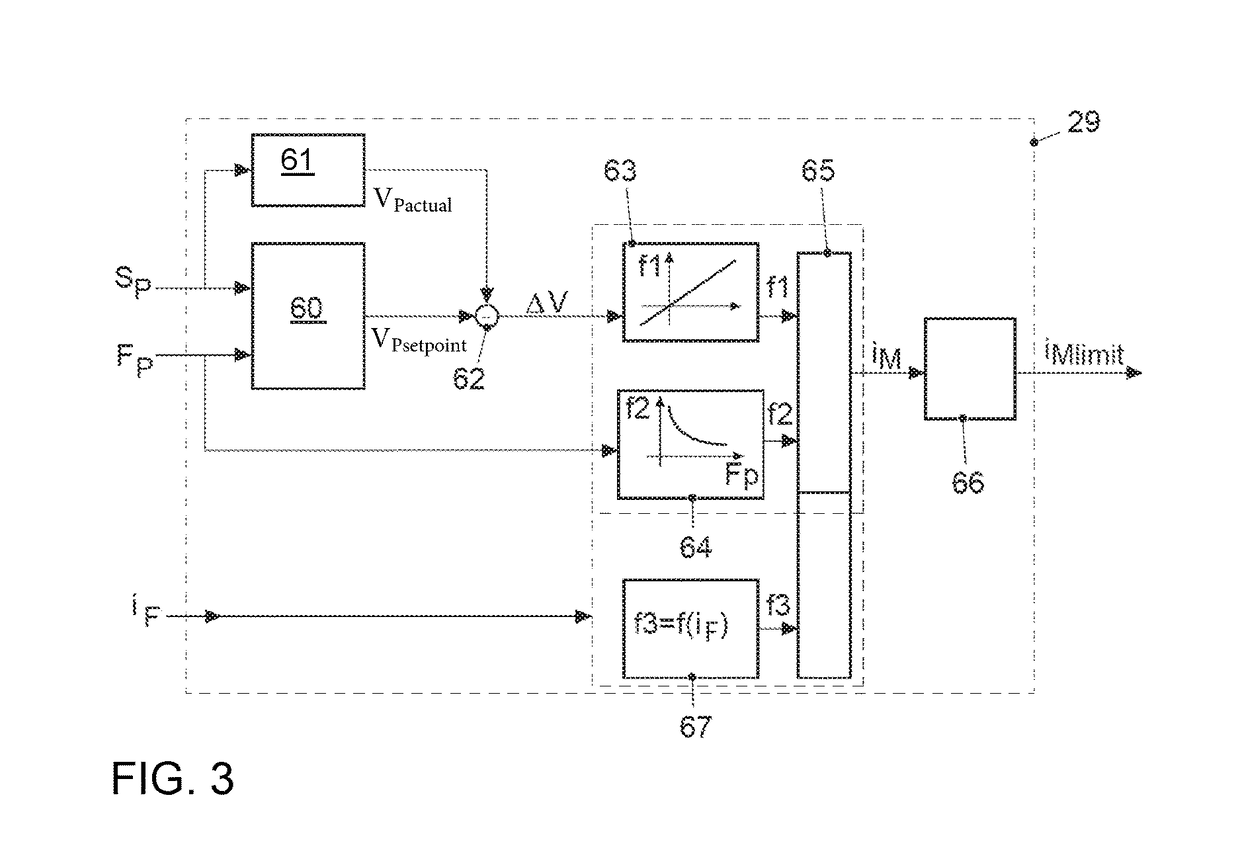
ActiveUS20170021816A1Improve returnImprove release behaviorBraking action transmissionMaster cylinderBiological activation
An electromechanical brake booster having a plunger rod for connecting a brake pedal lever to a brake master cylinder, a gear motor, which is coupled with the plunger rod, and a control device for driving the gear motor. A pedal force variable representing the pedal force and a plunger rod motion variable representing the motion of the plunger rod are supplied to the control device as input variables. The control device is configured to determine a setpoint return velocity for the plunger rod with the aid of the plunger rod motion variable and an actual return velocity for the plunger rod and the pedal force variable from the setpoint return velocity and to generate an activation signal for the gear motor. This makes it possible to improve the brake release behavior and to reduce high Bernoulli forces at the end stop for the starting position of the brake pedal lever.
Owner:VOLKSWAGEN AG
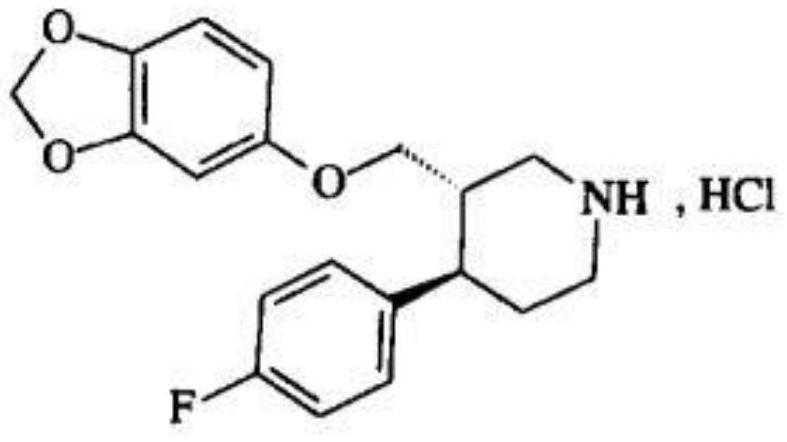
Paroxetine hydrochloride suspension and preparation method thereof
ActiveCN113209017AHigh drug loadingImprove stabilityOrganic active ingredientsNervous disorderParoxetine hclMedicine
The invention relates to a paroxetine hydrochloride suspension and a preparation method thereof. The paroxetine hydrochloride suspension is prepared from the following raw materials: paroxetine hydrochloride, cation exchange resin, an impregnant and pharmaceutic adjuvants; and the cation exchange resin is composed of strong acid cation exchange resin and weak acid cation exchange resin. According to the paroxetine hydrochloride suspension disclosed by the invention, the cation exchange resin composed of the strong acid cation exchange resin and the weak acid cation exchange resin is creatively adopted as a carrier of the medicine paroxetine hydrochloride; the medicine is loaded on a resin material; the bitter taste of the medicine can be covered; the paroxetine hydrochloride suspension is easier to swallow by a patient; meanwhile, the drug loading capacity of paroxetine hydrochloride is improved; and the stability, the redispersibility and the drug release behaviour are further improved.
Owner:SHANGHAI MEIYOU PHARMA
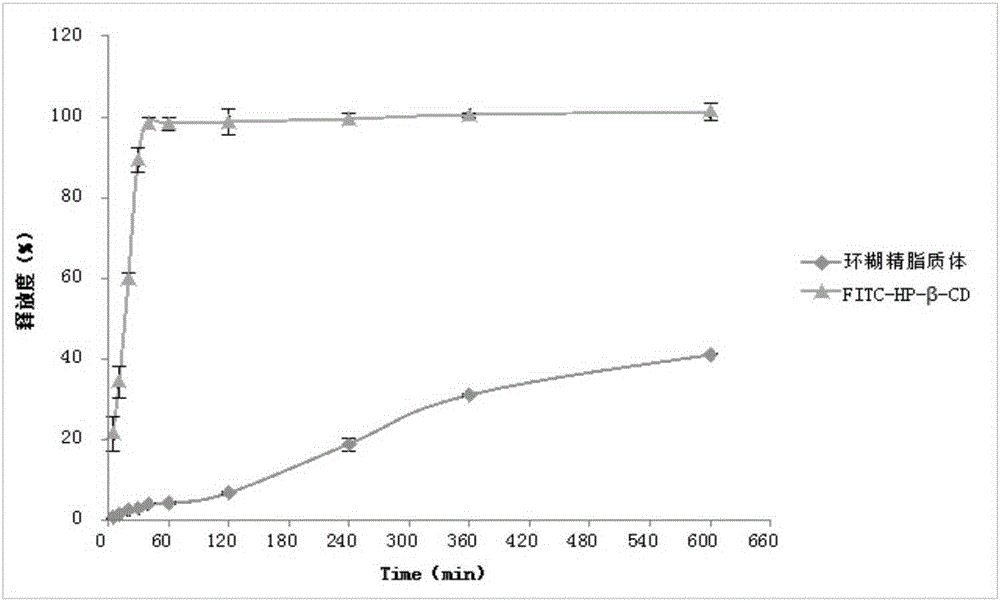
Fluorescent probe modified cyclodextrin, and preparation method and applications thereof
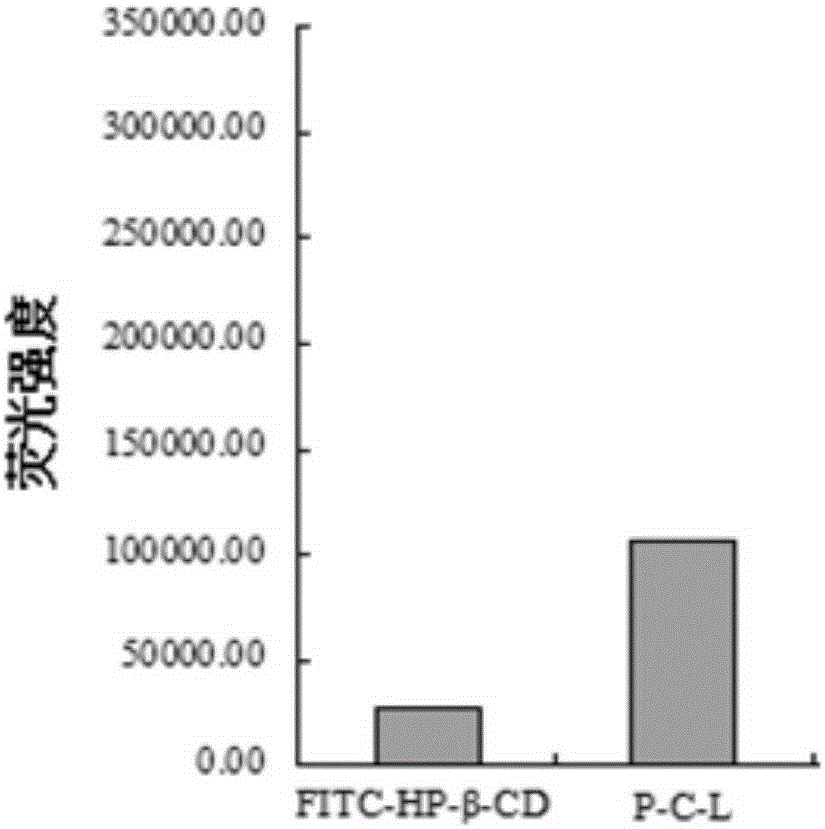
ActiveCN105237658AConvenience ingestion behaviorHigh sensitivityMacromolecular non-active ingredientsLuminescent compositionsLower limitFluorescein isothiocyanate
The invention discloses a method used for modifying cyclodextrin with fluorescent probe fluorescein isothiocyanate (FITC) so as to solve technical problems of the prior art that cyclodextrin content determination method is low in sensitivity, and evaluation of endocytosis behavior is impossible to realize. According to the method, molecule mole ratio of FITC / cyclodextrin is 0.1-20:1, preferably 0.5-10:1, and most preferably 1:1, and hydroxypropyl-beta-cyclodextrin is used preferably. Compared with the prior art, drug inclusion effects are not influenced by the FITC-modified cyclodextrin, cyclodextrin content can be determined via FITC fluorospectrophotometry, concentration lower limit of cyclodextrin ranges from 27.5 to 110ng / ml, and sensitivity is increased substantially compared with that cyclodextrin determination using a high performance liquid chromatography-differential refraction detector (concentration lower limit is 0.1mg / ml). The method is capable of solving a technical problem that cyclodextrin quantification is difficult to realize; and application in cyclodextrin new dosage form content determination, pharmaceutical property evaluation such as releasing rate evaluation, and cyclodextrin endocytosis action and target drug delivery effect is wide.
Owner:JINGCHU UNIV OF TECH
Chilo venosatus sex pheromone liquid slow-release agent and preparation method thereof.
ActiveCN108124867AHigh initial contentLow costBiocidePest attractantsWater basedPoly(butylene succinate)
The invention relates to a chilo venosatus sex pheromone liquid slow-release agent and a preparation method thereof. The liquid slow-release agent is prepared from the following raw materials in partsby weight: 0.7-1.2 parts of chilo venosatus sex pheromone, 3-6 parts of a biodegradable polymer, 0.7-1.5 parts of an oily anti-UV agent, 0.7-1.5 parts of a water-based anti-UV agent, 0.7-1.5 parts ofbutyl hydroxyl anisole, and 130-160 parts of a solvent, wherein the biodegradable polymer is selected from polylactic acid and / or poly(butylene succinate); and the solvent is a mixed solution of dichloromethane and a hydroxypropyl methyl cellulose aqueous solution, the mass ratio of the dichloromethane to the hydroxypropyl methyl cellulose aqueous solution is 1:(1-2), and the mass concentration of the hydroxypropyl methyl cellulose in the hydroxypropyl methyl cellulose aqueous solution is 0.7-1.3%. The liquid slow-release agent provided by the invention has a long release lasting period, caneffectively prevent and control chilo venosatus pests, and can save labor and time through mechanical spraying.
Owner:广东省科学院南繁种业研究所
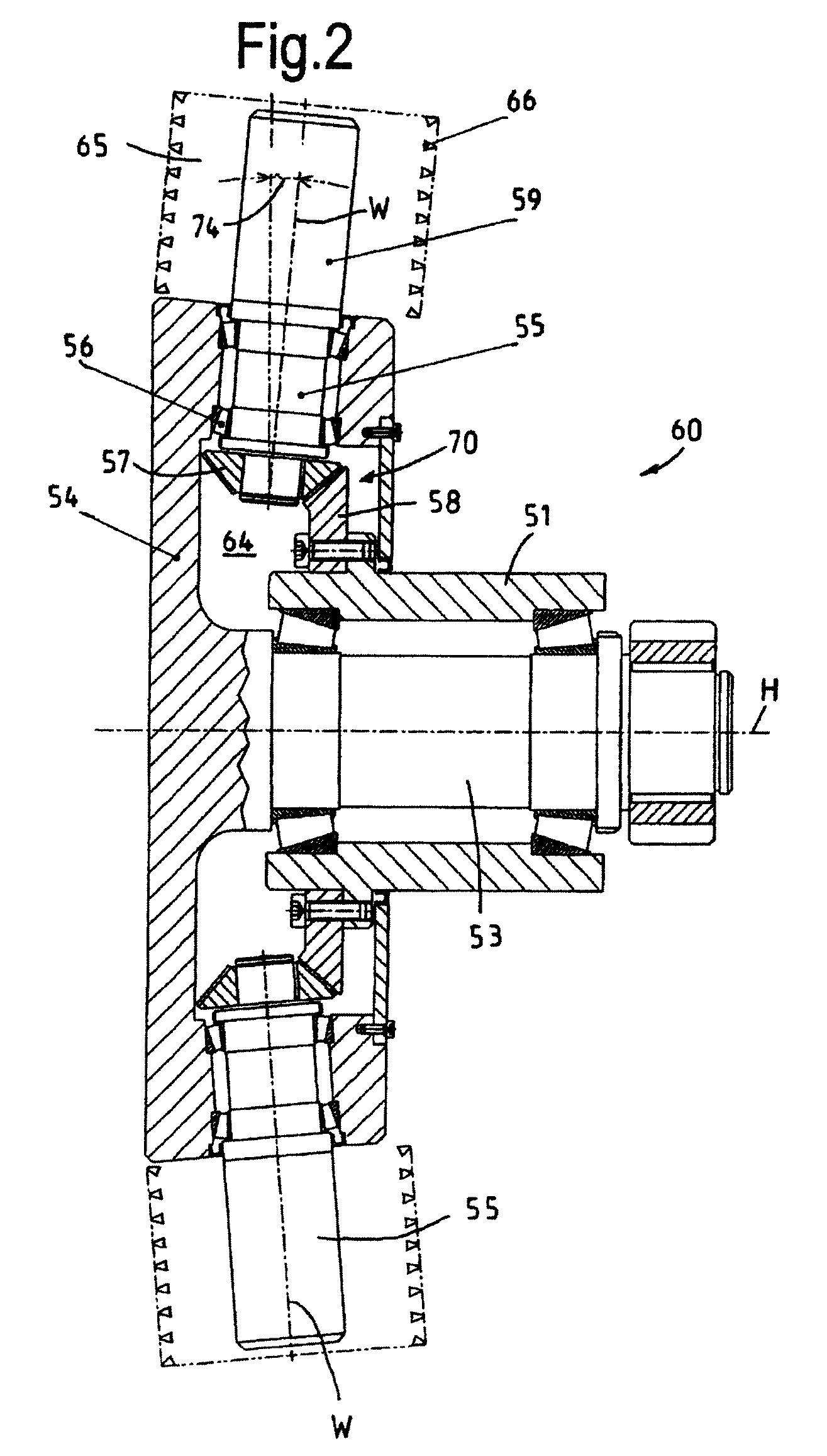
Electromechanical brake booster
ActiveUS9840244B2Improve returnImprove release behaviorBraking action transmissionMaster cylinderEngineering
An electromechanical brake booster having a plunger rod for connecting a brake pedal lever to a brake master cylinder, a gear motor, which is coupled with the plunger rod, and a control device for driving the gear motor. A pedal force variable representing the pedal force and a plunger rod motion variable representing the motion of the plunger rod are supplied to the control device as input variables. The control device is configured to determine a setpoint return velocity for the plunger rod with the aid of the plunger rod motion variable and an actual return velocity for the plunger rod and the pedal force variable from the setpoint return velocity and to generate an activation signal for the gear motor. This makes it possible to improve the brake release behavior and to reduce high Bernoulli forces at the end stop for the starting position of the brake pedal lever.
Owner:VOLKSWAGEN AG
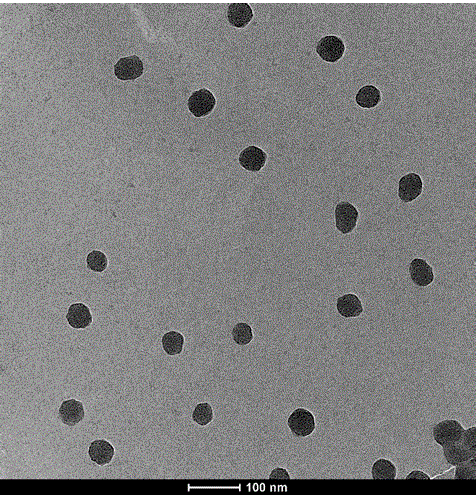
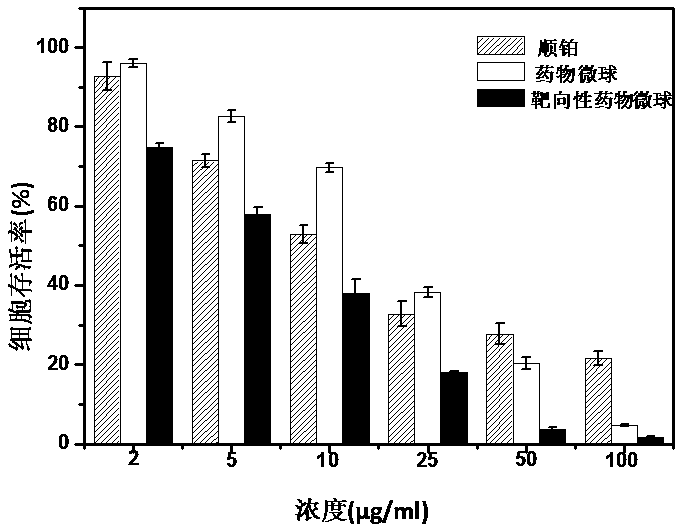
Macromolecule-cis-platinum compound, preparation method and application thereof
InactiveCN102499986BIncrease the effective binding rateHigh drug loadingHeavy metal active ingredientsPharmaceutical non-active ingredientsHemolysisSide chain
The invention relates to a macromolecule-cis-platinum compound, a preparation method and application thereof. The preparation method comprises the following steps of: degrading or purifying polyamino acid serving as a raw material to obtain a polyamino acid carrier with appropriate molecular weight; performing reaction of a small molecular acid serving as a modifier and side chain active groups of the small molecular acid, modifying a structure of the small molecular acid, and connecting cis-platinum to the polymer skillfully to prepare the medicine-carrying compound. The result of the study on the properties of the medicine-carrying compound indicates that the properties of the medicine-carrying compound are obviously superior to the conventional report, and the prepared compound is low in toxicity and does not have hemolysis.
Owner:FANTAI INST OF CHEM MEDICINES NANJING
A kind of platinum antitumor prodrug, its nano hydrogel drug and preparation method thereof
InactiveCN105713046BGood killing effectReduced survival rateHeavy metal active ingredientsOrganic active ingredientsHydrophilic monomerMicrosphere
The invention belongs to the technical field of biological medicines and particularly provides an anti-tumor platinum pro-drug and a nanometer hydrogel drug and a preparation method thereof.The pro-drug is obtained by conducting hydrogen peroxide oxidation on an anti-tumor platinum drug to obtain a Pt(IV) sexadentate complex and then making the sexadentate complex react with anhydride or carboxylic acid containing double bonds.The pro-drug can be in self-polymerization or can be copolymerized with other hydrophilic monomers for the preparation of drug micro-spheres having high drug loading capacity.The micro-spheres are uniform in particle size and controllable in size and can keep long-term stability in an aqueous solution by using a stabilizer.The preparation method is simple, an obtained nanometer hydrogel drug carrier has glutathione stimulus responsibility, so that nanometer hydrogel is kept stable in a low-glutathione-concentration environment outside tumor cells, the drug is rarely leaked after long-time cycle, the nanometer hydrogel is rapidly degraded in the reducing environment within the tumor cells and converted into a linear chain of small molecular weight, the drug is quickly released, and the targeted killing effect on the target tumor cells is achieved.
Owner:FUDAN UNIV
Method for preparing medicine encapsulating liposome
ActiveCN102188377BImprove release behaviorImprove bioavailabilityPharmaceutical non-active ingredientsLiposomal deliveryOrganic solventMedicine
The invention relates to a method for preparing a medicine encapsulating liposome, which comprises: preparing medicine solid dispersion; and uniformly dispersing the medicine solid dispersion and a liposome film-forming material in an organic solvent to prepare the medicine encapsulating liposome. When the method is used, a medicine can be uniformly encapsulated in the liposome, the medicine encapsulating rate is improved, and sudden release of the medicine encapsulating liposome is reduced. The liposome is suitable for encapsulating various medicines and can be used in a wide formulation range.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A kind of microencapsulated butylphthalide pharmaceutical composition and its preparation method and application
ActiveCN103417514BAvoid degradationImprove solubilityOrganic active ingredientsNervous disorderSolubilityButylphthalide
The invention relates to a microencapsulated butylphthalide pharmaceutical composition, a preparation method and application thereof. In order to improve the solubility of butylphthalide, prevent volatilization, develop clinically required solid preparations and liquid preparations, so as to better exert the therapeutic effect of butylphthalide, the present invention microencapsulates butylphthalide. The microencapsulated butylphthalide pharmaceutical composition of the present invention contains 1-55% butylphthalide active ingredient by mass percentage, and utilizes natural or synthetic polymer materials as capsule material or carrier material to wrap butylphthalide into Microcapsules can be used to prepare powders, capsules, chewable tablets, embedded tablets, buccal tablets, other oral compressed tablets, injections, liquid suspensions, lotions, powder injections, paints, films and other dosage forms.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
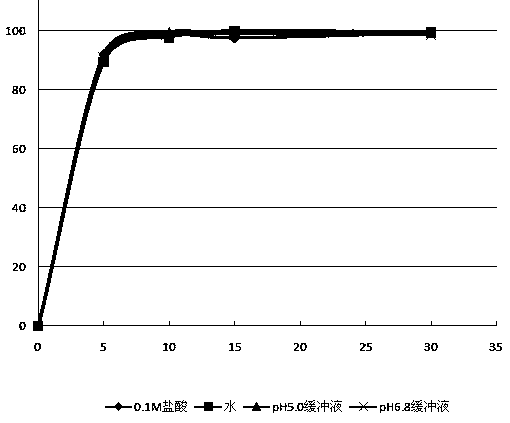
Water resistance type film coating composition and application thereof
InactiveCN102949371BImprove water resistanceGood sensitivity to pH changesPharmaceutical delivery mechanismEffervescent tabletAlcohol
The invention relates to a coating material, and particularly relates to a water resistance type film coating composition and an application thereof. The water resistance type film coating composition comprises the following components by weight parts: 3-15 parts of polyacrylic resin IV and 1-5 parts of plasticizer which is stearic acid. Before use, the composition is dissolved in an absolute ethyl alcohol, or is mixed together with the absolute ethyl alcohol to be stored or used. The water resistance type film coating composition provided by the invention has an excellent water resistance type performance and good sensitiveness to pH change; and after the medicine is coated according to a conventional film coating technology, the release behavior of the medicine can be obviously improved, the stability of dosage form effervescent tablets sensitive to water and products sensitive to water in the production and storage period is facilitated.
Owner:湖北华来生物科技有限公司
A kind of buflodil hydrochloride compound and pharmaceutical composition thereof
ActiveCN105924411BReduce solubilityGood hygroscopicityNervous disorderOrganic chemistry methodsSustained Release TabletSolubility
Owner:王珍 +1
Polyethylene glycol modified anti-tumor prodrug as well as preparation method and application thereof
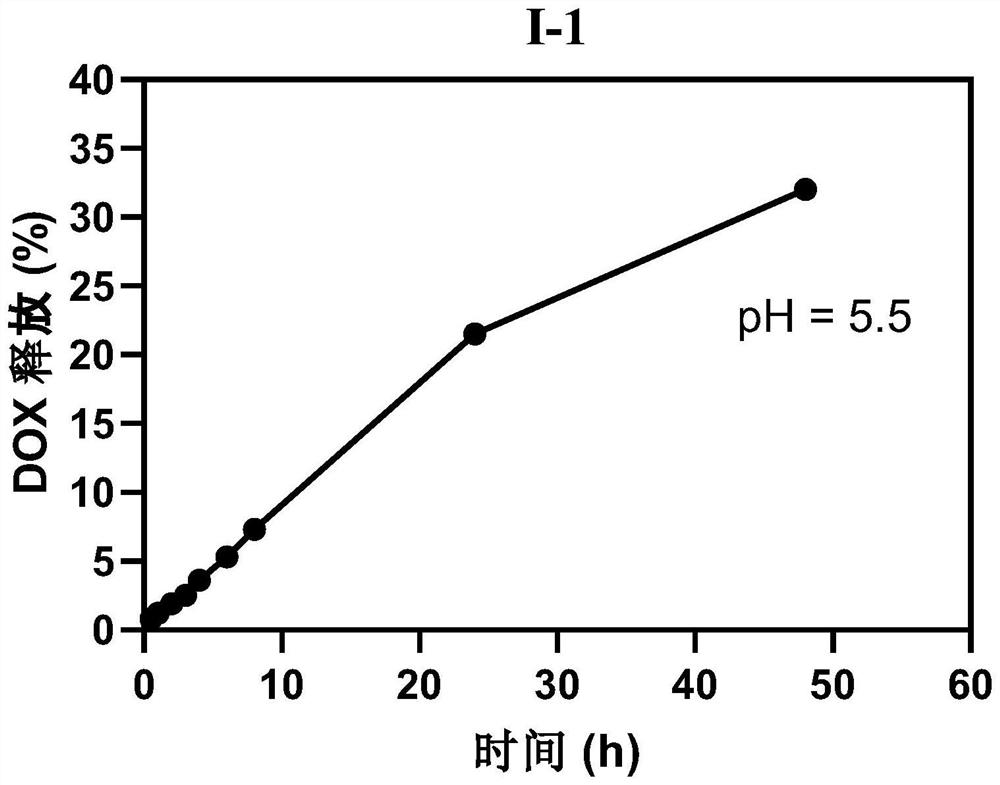
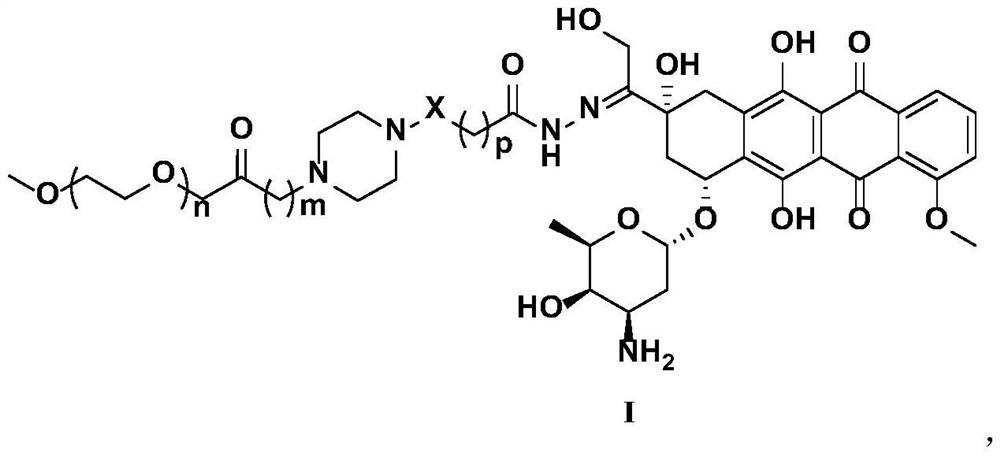
PendingCN114259570AExcellent release behaviorGood application prospectOrganic active ingredientsPharmaceutical non-active ingredientsDoxorubicinoneCombinatorial chemistry
The invention relates to a polyethylene glycol modified doxorubicin prodrug as well as a preparation method and application thereof, and belongs to the technical field of biological pharmacy. The polyethylene glycol modified doxorubicin prodrug contains a novel connecting arm of a hydrazide fragment connected by a piperazine heterocyclic ring, the prodrug has an excellent selective slow release behavior, and the preparation method of the prodrug has the advantages of mild reaction conditions, high yield, simplicity and convenience in operation and environmental friendliness. The polyethylene glycol modified doxorubicin prodrug disclosed by the invention has a good application prospect.
Owner:长沙创新药物工业技术研究院有限公司
Barrel sex pheromone microspheres and preparation method thereof
InactiveCN104472483BImprove release behaviorImprove stabilityBiocidePest attractantsPolyesterMicrosphere
The invention discloses a proceras venosatus sex pheromone microsphere and a preparing method thereof. The microsphere comprises, by weight, 9-11 parts of aliphatic polyester, 98-102 parts of solvent, 0.7-1.2 parts of proceras venosatus sex pheromone, 0.7-1.2 parts of antioxidant, 0.008-0.012 part of surfactant and 0.04-0.06 part of adhesive. The preparing method comprises the steps that the aliphatic polyester is pre-heated under the temperature of 95-105 DEG C, the proceras venosatus sex pheromone and the solvent are added to the aliphatic polyester, the proceras venosatus sex pheromone, the solvent and the aliphatic polyester are mixed evenly and cooled to the temperature of 55-65 DEG C, the antioxidant is added to the proceras venosatus sex pheromone, the solvent and the aliphatic polyester and heated to the temperature of 80-90 DEG C, the surfactant and the adhesive are added to the proceras venosatus sex pheromone, the solvent, the aliphatic polyester and the antioxidant and cooled to the indoor temperature, and a mixture is obtained; the mixture is stirred at the speed of 250-1500 rpm. The proceras venosatus sex pheromone microsphere is good in releasing behavior and stability and capable of effectively preventing the sugarcane proceras venosatus attack.
Owner:GUANGDONG PROVINCIAL BIOENGINEERING INST (GUANGZHOU SUGARCANE IND RES INST)
Sustained-release microspheres containing risperidone and its analogs and preparation method thereof
InactiveCN103126997BReduce porosityReduce burstOrganic active ingredientsNervous disorderChronic mental diseaseEmulsion
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Medicament eluting stent and preparation method thereof
The invention relates to a medicament eluting stent, which consists of a bare stent and a coating coated on the bare stent. The medicament eluting stent is characterized in that the coating at least comprises a polymer layer and a resin layer, wherein the polymer layer comprises polylactic-co-glycolic acid (PLGA) and medicaments; and the resin layer is coated on the surface of the polymer layer. A resin of the invention is coated on the surface of the medicament-loaded polymer coating. Due to the sustained release effect of the resin, on the premise of guaranteeing medicament dosage, the dosage of the polymer is reduced so as to obvious reduce an inflammatory reaction, reduce the generation of adverse reactions such as late intravascular restenosis and the like, and avoid forming late thrombosis.
Owner:SHENZHEN SALUBRIS BIOMEDICAL ENG CO LTD
Liquid slow-release agent for sex pheromone of barber borer and its preparation method
Owner:广东省科学院南繁种业研究所
Production method for rumen-bypassing preparation, and granules obtained by means of production method for rumen-bypassing preparation
InactiveUS20200337338A1Low costResistance to dissolutionVitamin food ingredientsAcidic food ingredientsBiotechnologyInjection port
Provided is a method for producing a rumen-bypassing preparation which is produced at a low cost and simultaneously achieves the objects of protecting an active ingredient from release and decomposition in the first stomach and increasing release behavior in a target internal organ. A granular agent obtained thereby is also described. The method includes a step of applying vibration to a die head containing a melt of a coating agent for the rumen-bypassing preparation and a nutrient to bypass a rumen and having at least one injection port or to the melt, thereby injecting the melt from the injection port.
Owner:BIO SCI
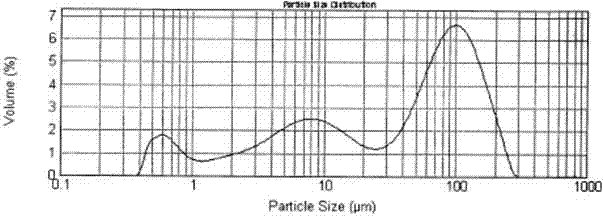
Preparation method of encapsulated drug lipid microparticles
ActiveCN102188711BReduce leakageHigh encapsulation efficiencyPowder deliveryPharmaceutical non-active ingredientsLipid formationOrganic solvent
The invention relates to a method for preparing medicine-encapsulating lipoid particles, which comprises the following steps of: preparing medicinal solid dispersoid, and dispersing the medicinal solid dispersoid and a film-forming material of the lipoid particles in organic solvent uniformly to prepare the medicine-encapsulating lipoid particles, so that medicines are encapsulated in the lipoid particles uniformly, the envelop rate of the medicines is improved, and the burst effect of the medicine-encapsulating lipoid particles is reduced. The medicine-encapsulating lipoid particles are suitable for encapsulating various medicines, and are wide in range of applicable formulations.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Process for preparing degradable polyester microsphere wrapping nano insulin
InactiveCN100544765CReduce churnHigh Package UtilizationPowder deliveryPeptide/protein ingredientsPolyesterN dimethylformamide
The invention provides a preparation method of bio-degradable polyester microsphere which encloses nano-insulin particles and can slowly release insulin. The invention is characterized in that: (1) prepare pure insulin nano-particles without additive through isoelectric-point precipitation and get insulin nano-particle solid powder through liquefied nitrogen freezing and drying; (2) disperse the nsulin nano-particles produced in step (1) evenly into non-aqueous solvent N,N-dimethylformamide (DMF) and add polyester polymers into the liquid to dissolve the DMF, then disperse the produced DMF solution in the another oily liquid which is not mutually soluble with the DMF to form double-organic-phase emulsion system without water; (3) add a third organic solvent in the emulsion system produced in step (2) to make the DMF in the liquid drops of the dispersed phase of the system be dispersed out to produce polyester microspheres with insulin. The microspheres which are low in roxic residual and have the capability of controlling and releasing the insulin for 0 to 50 days with the insulin content accounting for the total weight of the microspheres by the proportion of 20 to 100 mg / g can be produced by adopting the method.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Docetaxel-loading mixed micelle preparation and preparation method thereof
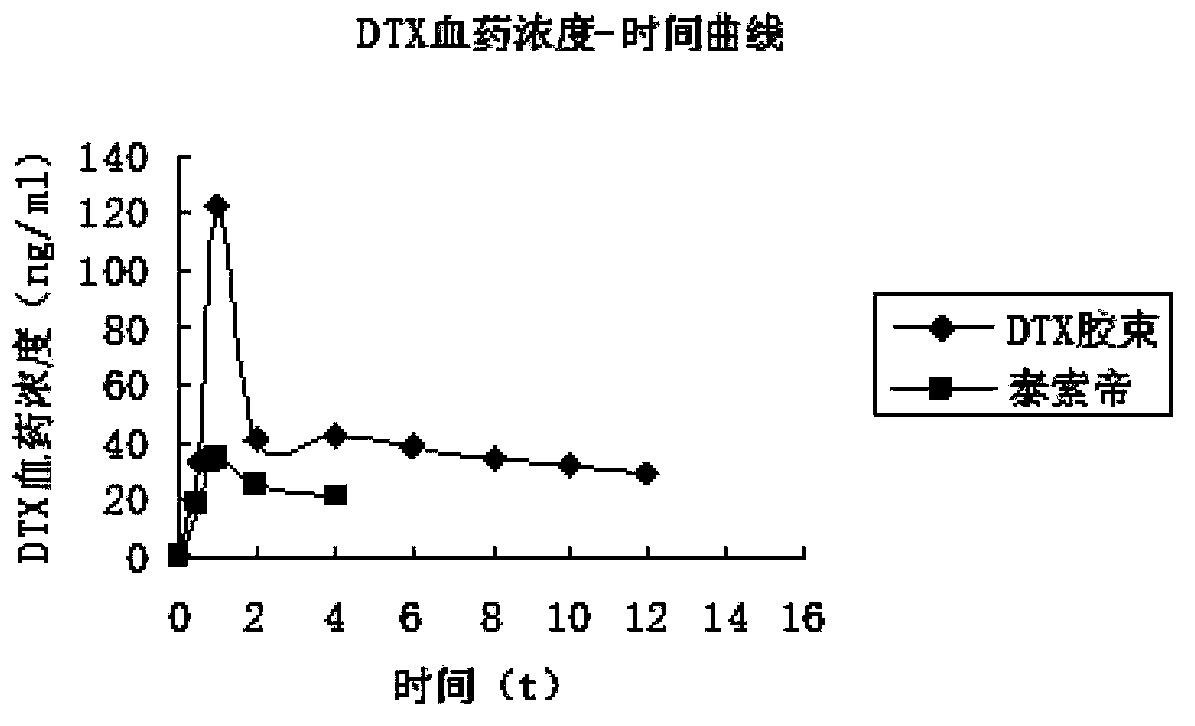
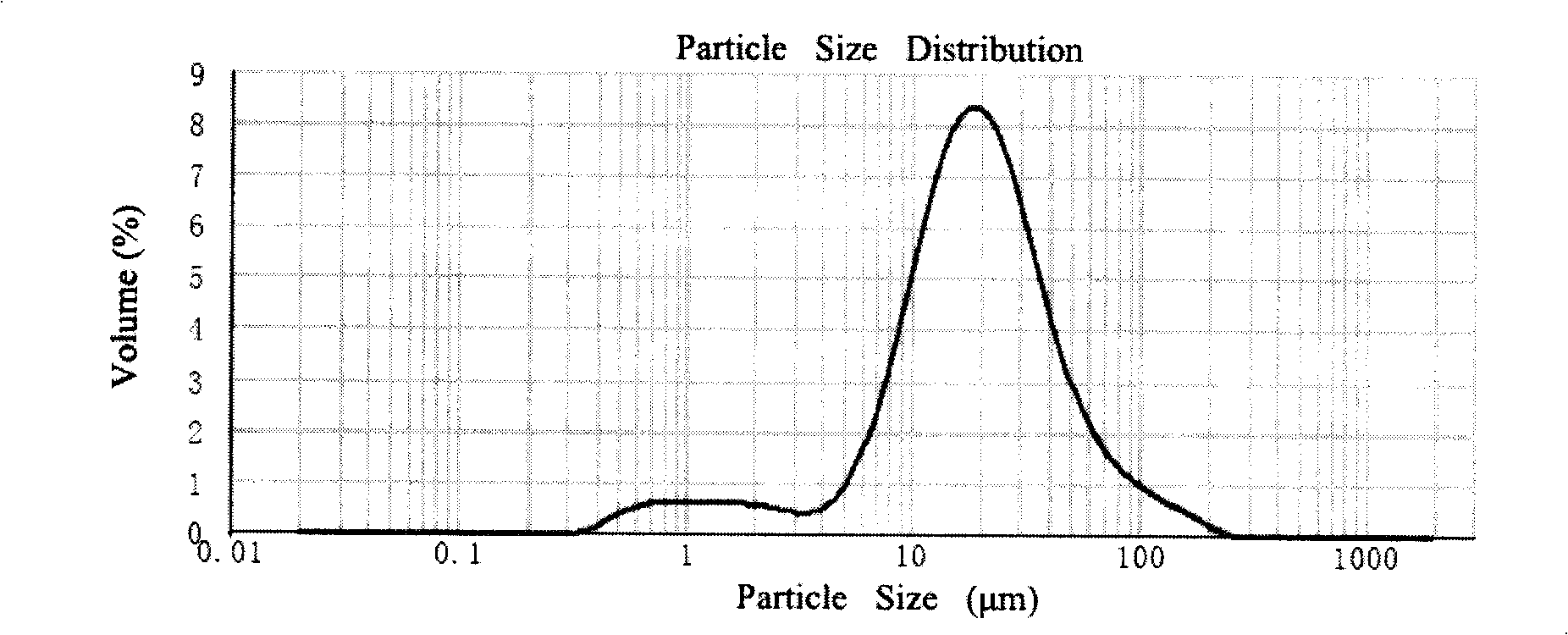
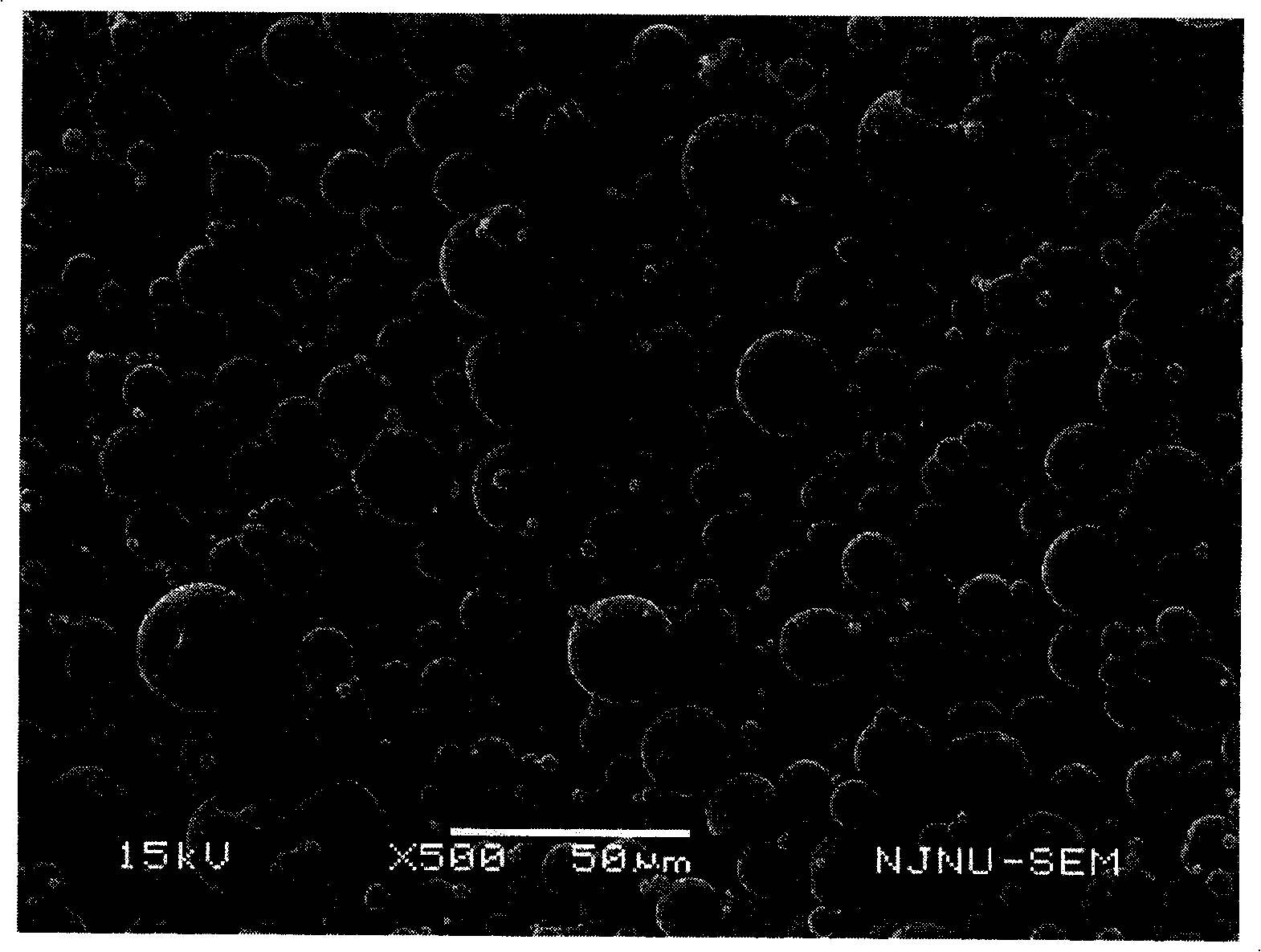
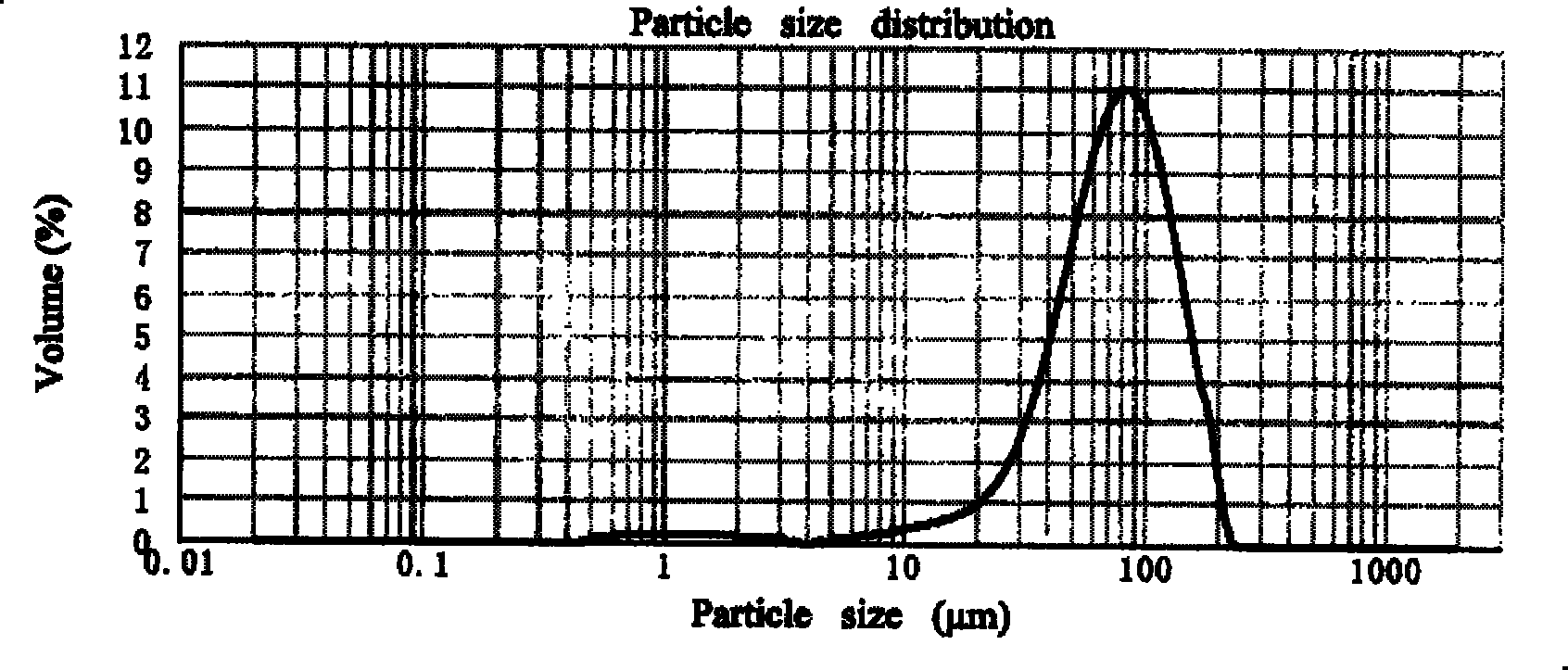
InactiveCN102885772BImprove solubilityHigh drug loadingOrganic active ingredientsSolution deliveryMixed micelleDocetaxel
The invention discloses a docetaxel-loading mixed micelle preparation and a preparation method thereof. The mixed micelle preparation is prepared by the following steps of: preparing docetaxel mixed micelle by using a thin-film dehydration method; and coating the docetaxel with hydrophobic nuclei by taking amphiphilic TPGS, MPEG-PLA and CSO-SA as carrier materials of the mixed micelle. The docetaxel-loading mixed micelle preparation is prepared by using the amphiohilic materials of TPGS, MPEG-PLA and CSO-SA, so that drug-loading rate is increased, solubility and oral bioavailability are improved, and the problems of poor water solubility, low medicine release speed, improper particle size and the like when hydrophilic medicines are prepared are solved.
Owner:SHANDONG UNIV
Process for producing recombinant human vascular endothelial inhibitor composition sustained-release microsphere
ActiveCN101428142BReduce the number of dosesImprove compliancePeptide/protein ingredientsPharmaceutical non-active ingredientsAcetic acidOrganic solvent
The invention relates a method for preparing a recombinant human vascular endothelial inhibin composition slow-release microsphere, which belongs to the technical field of pharmaceuticals. The constituents of the slow-release microsphere include recombinant human vascular endothelial inhibin which accounts for 1-30% in terms of weight percentage, hydrophilic substances 1-30% and lactic acid-glycolic acid polymers 69-98%. The preparation comprises the following steps: (a), adding the hydrophilic substance into a recombinant human vascular endothelial inhibin solution for free drying so as to obtain composition powder from the recombinant human vascular endothelial inhibin and the hydrophilic substance; (b), then suspending the power obtained in an organic solvent containing the lactic acid-glycolic acid polymers, which allows the power to disperse therein; (c), after dispersion, adding the solvent into a water phase containing emulsifier, stirring the solvent in a normal pressure or decompression environment to enable the solvent to be volatile and to change into microspheres, and keeping the temperature unchanged at a proper temperature as the solvent becomes volatile; and (d), subjecting the obtained microspheres to cleaning and drying so as to obtain slow-release microsphere preparations in the form of finished products. The preparation method provided by the invention can ensure that the recombinant human vascular endothelial inhibin can take a relatively stable form which effectively allows the recombinant human vascular endothelial inhibin to be encapsulated in the microspheres, thereby reducing the risk of possible degradation of the recombinant human vascular endothelial inhibin during the preparation process.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com