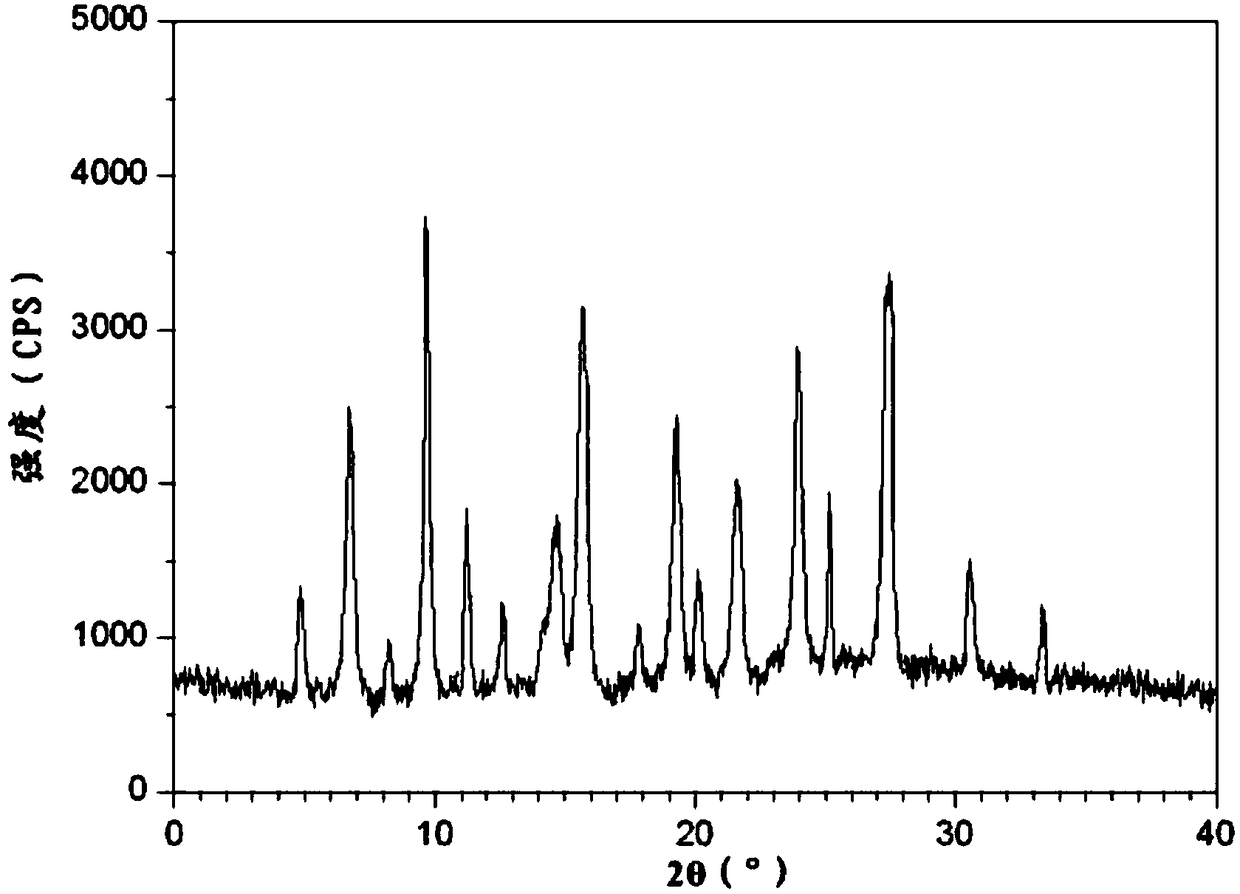
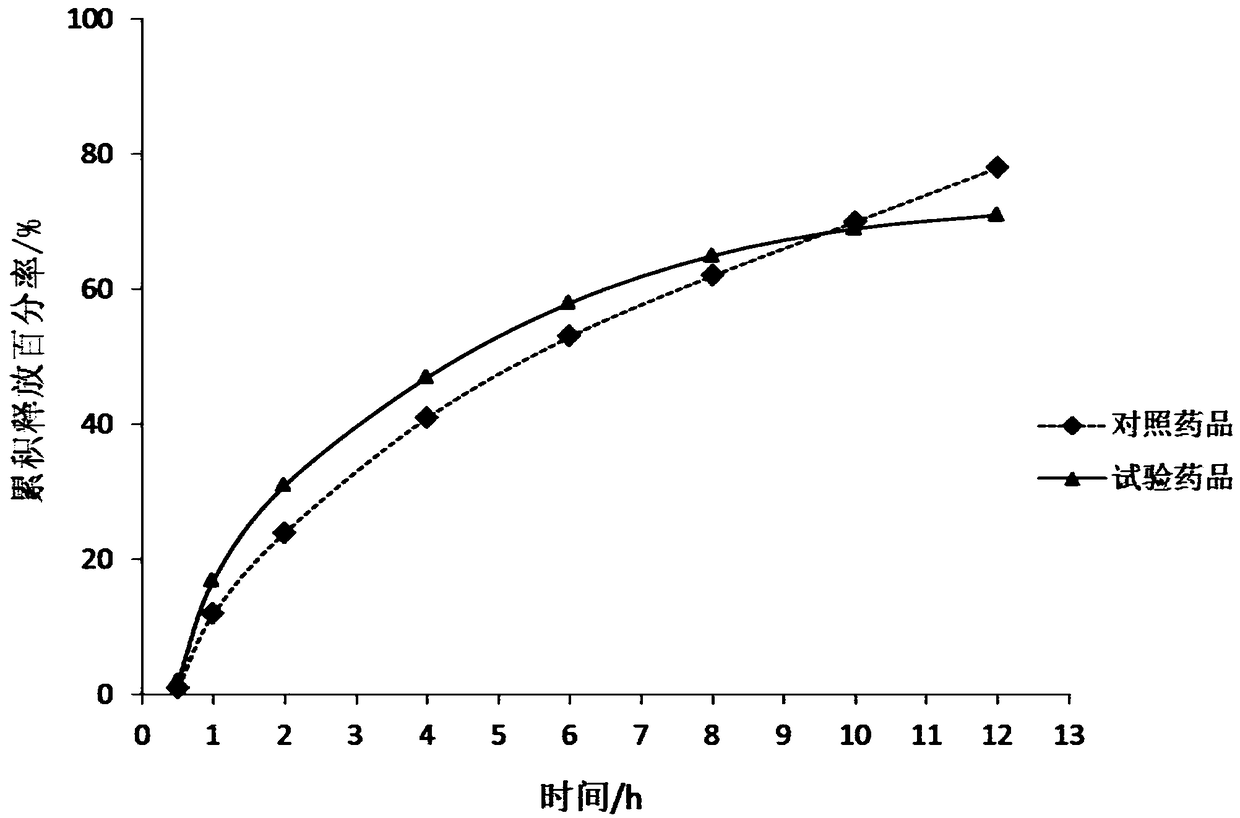
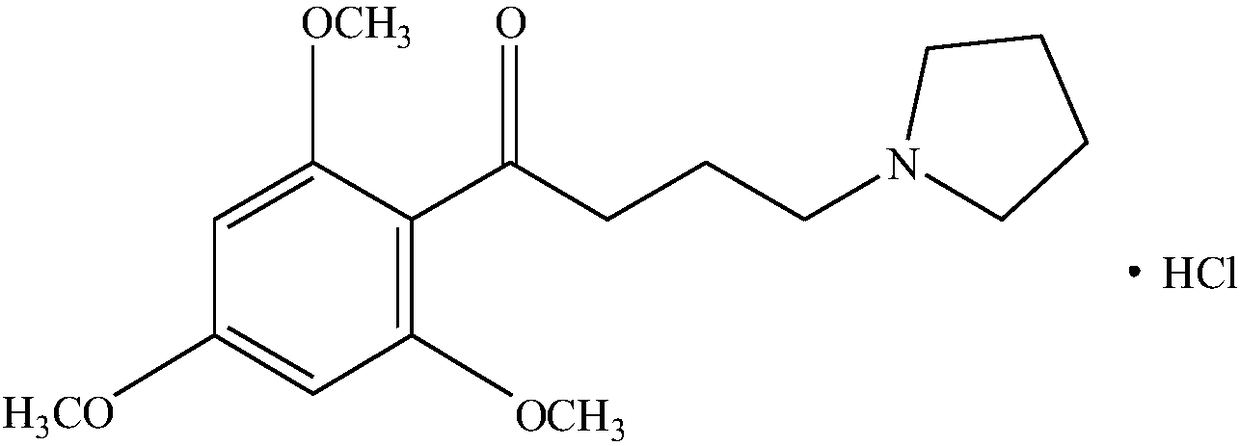
A kind of buflodil hydrochloride compound and pharmaceutical composition thereof
A technology of buflomedil hydrochloride and its compound, which is applied in the field of medicine, can solve problems affecting product stability, effectiveness and safety, internal quality of drug effect, decreased fluidity, and unsatisfactory effect, etc., to achieve excellent drug release behavior, Effect of reducing solubility and improving hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] [embodiment 1] the preparation of buflomedil hydrochloride compound
[0047] 1) Preparation of solution A: Sterile filter 600L of mixed solvent A of tetrahydrofuran and n-butanol (the volume ratio of tetrahydrofuran and n-butanol is 3:1) into a crystallization tank, cool down to 5°C and wait for use, which is solution A;
[0048] 2) Preparation of solution B: at room temperature, add 110L of mixed solvent B of water and methanol (the volume ratio of water and methanol is 5:1) into the dissolving tank, then add buflomedil hydrochloride crude product 30kg, dissolve and add activated carbon 0.45kg, stirring and decolorizing for 25 minutes, sterile filtering, washing with water, collecting the filtrate and washing liquid to obtain solution B;
[0049] 3) Add solution B into solution A, cool down to 5°C, keep the temperature, and stir at a stirring speed of 18 rpm for 25 minutes;
[0050] 4) Then lower the temperature to -5°C, control the stirring speed to 10 rpm, and contr...
Embodiment 2
[0052] [embodiment 2] the preparation of buflomedil hydrochloride compound
[0053] 1) Preparation of solution A: sterile filter 600L of mixed solvent A of tetrahydrofuran and n-butanol (the volume ratio of tetrahydrofuran and n-butanol is 8:1) into a crystallization tank, cool down to 10°C and wait for use, which is solution A;
[0054] 2) Preparation of solution B: at room temperature, add 90L of mixed solvent B of water and methanol (the volume ratio of water and methanol is 7:1) into the dissolution tank, then add 30kg of crude buflomedil hydrochloride, and add activated carbon after dissolving 0.45kg, stirring and decolorizing for 35 minutes, sterile filtering, washing with water, collecting the filtrate and washing liquid to obtain solution B;
[0055] 3) Add solution B into solution A, cool down to 8°C, keep the temperature, and stir at a stirring speed of 22 rpm for 35 minutes;
[0056] 4) Then lower the temperature to 0° C., control the stirring speed to 15 rpm, cont...
Embodiment 3
[0058] [embodiment 3] the preparation of buflomedil hydrochloride compound
[0059] 1) Prepare A solution: Sterile filter 600L of mixed solvent A of tetrahydrofuran and n-butanol (the volume ratio of tetrahydrofuran and n-butanol is 6:1) into a crystallization tank, cool down to 8°C and set aside for use, which is A solution;
[0060] 2) Preparation of solution B: at room temperature, add 100L of mixed solvent B of water and methanol (the volume ratio of water and methanol is 6:1) into the dissolution tank, then add 30kg of crude buflomedil hydrochloride, and add activated carbon after dissolving 0.45kg, stirring and decolorizing for 30 minutes, sterile filtering, washing with water, collecting the filtrate and washing liquid to obtain solution B;
[0061] 3) Add solution B into solution A, cool down to 6°C, keep the temperature, and stir at a stirring speed of 20 rpm for 30 minutes;
[0062] 4) Then lower the temperature to -2°C, control the stirring speed to 12 rpm, and con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com