Process for producing recombinant human vascular endothelial inhibitor composition sustained-release microsphere
A slow-release microsphere preparation and vascular endothelial technology, which is applied in drug combinations, microcapsules, and pharmaceutical formulations, can solve the problems of easy drug leakage, limited drug loading, and reduced yield of preparation encapsulation, so as to reduce drug administration The effect of increasing the number of times, improving compliance, and facilitating adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
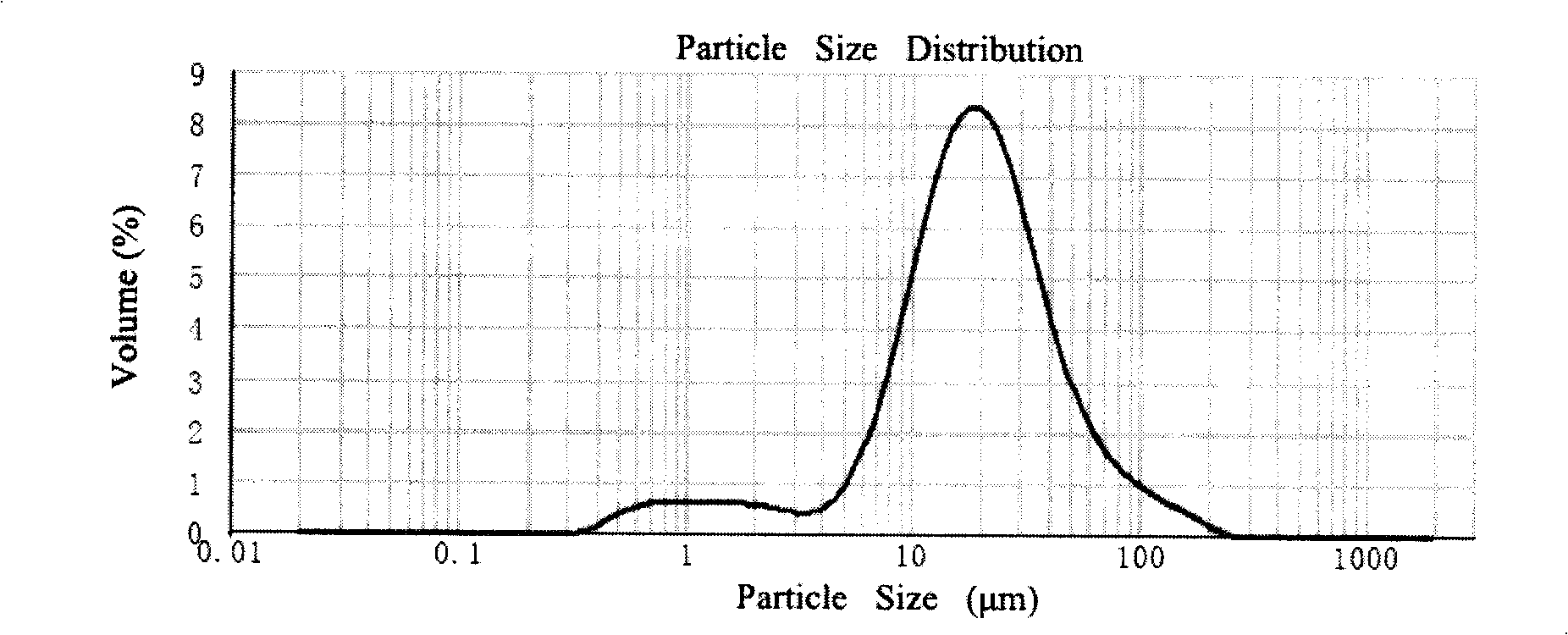
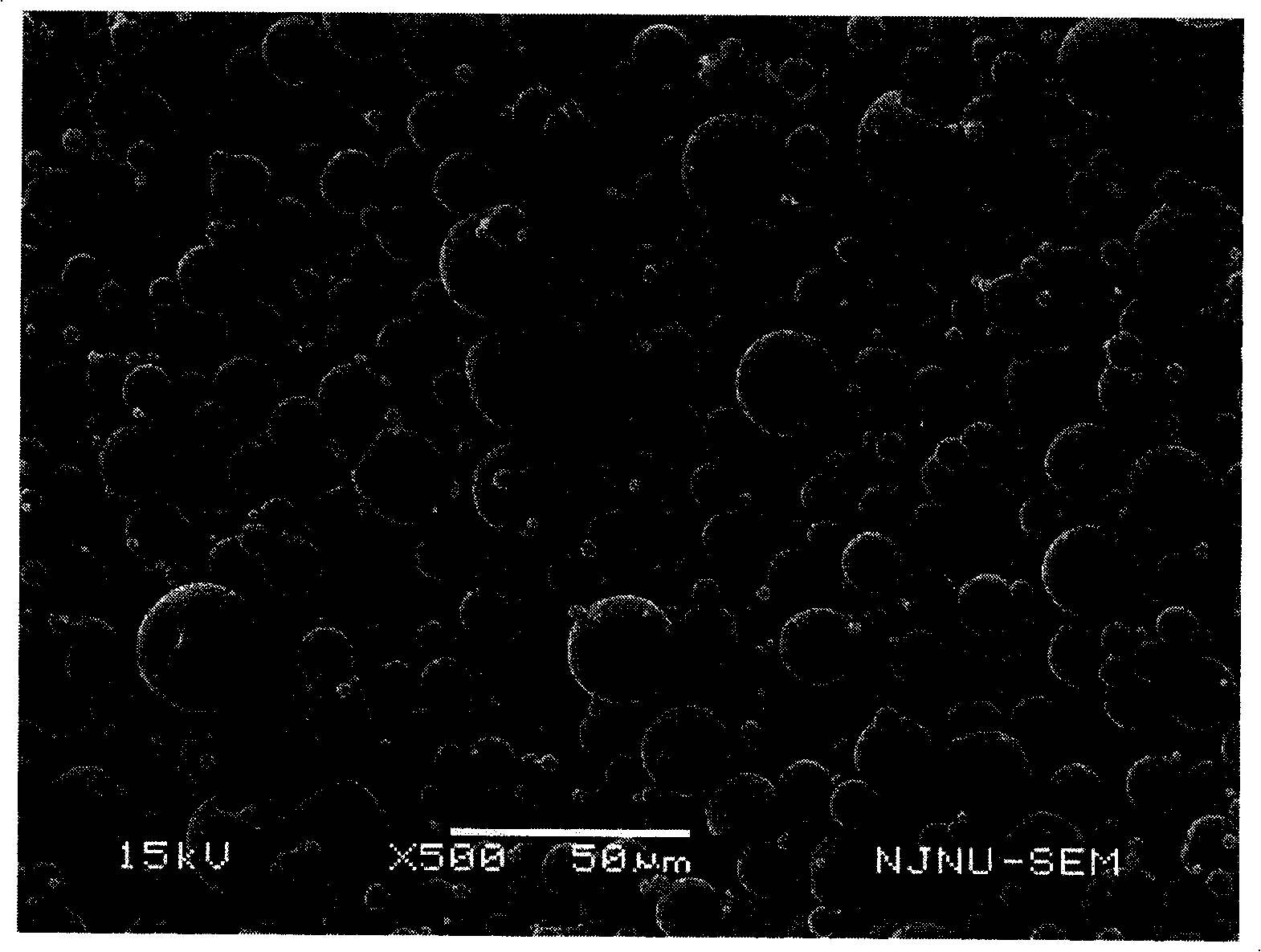
[0040] Accurately weigh 10 mg Endostar and 300 mg glucose and dissolve in 1 ml PBS buffer solution of pH 7.0, freeze-dry the solution to obtain composition powder. 4 g of PLGA (Mw=50000, lactic acid:glycolic acid=50:50) was dissolved in 20 ml of ethyl acetate to form an organic phase. Suspend the powder of the above composition in the organic phase, disperse at a high speed of 10000rpm for 20s, to form an S / O two-phase dispersion system, then pour this colostrum into 1L containing 3% polyvinyl alcohol 1788 (the degree of polymerization is about 1700, alcohol In the outer aqueous phase with a solution degree of 88%), at 20 degrees Celsius, the solvent was evaporated under normal pressure by mechanical stirring for 6 hours, and the microspheres were obtained by filtering with a 0.8 μm microporous membrane, washed three times with pure water, and dried to obtain the finished microspheres.
[0041] Accurately weigh 20 mg of the finished microsphere, dissolve it in 1 ml of dichloro...
Embodiment 2
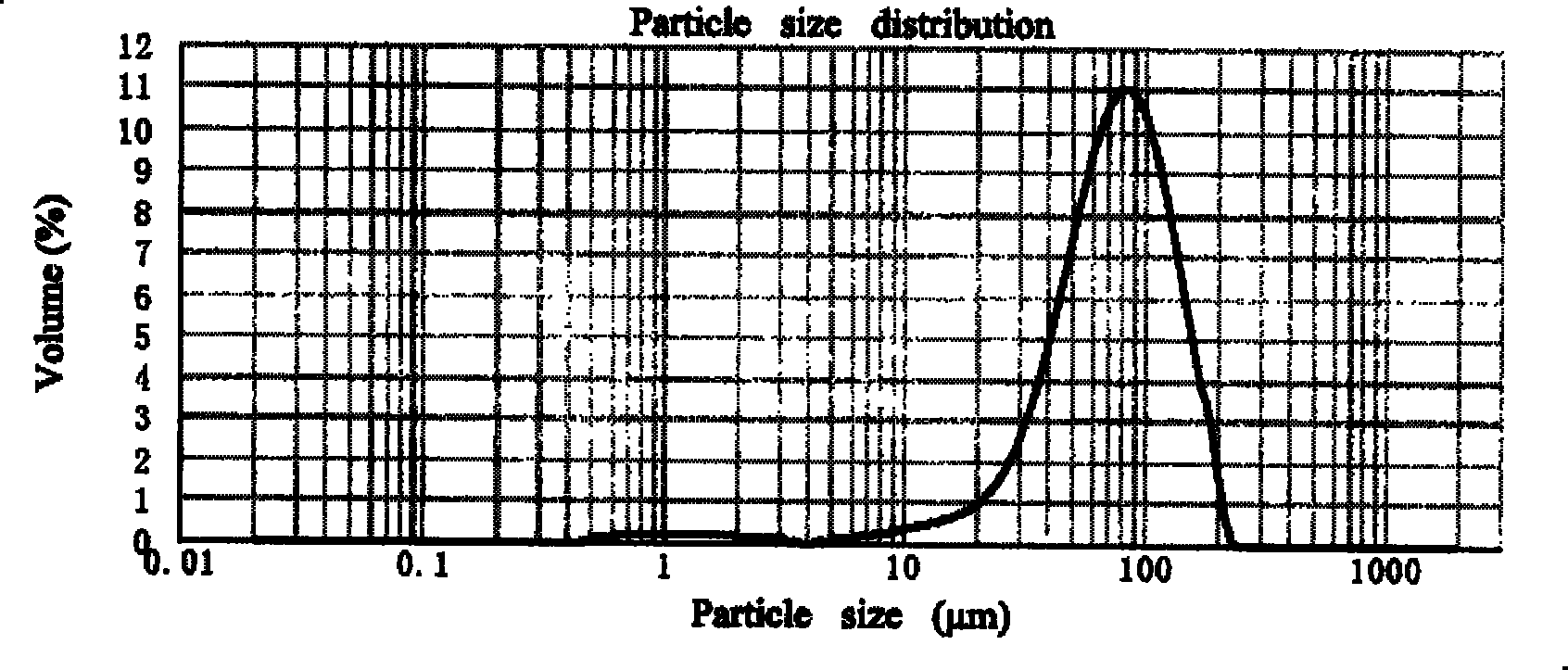
[0044] Accurately weigh 300 mg Endostar and 10 mg mannitol and dissolve in 1 ml pH 3.0 glycine-hydrochloric acid buffer, freeze-dry the solution to obtain composition powder. 3 g of PLGA (Mw=20000, lactic acid:glycolic acid=75:25) was dissolved in 20 ml of dichloromethane to form an organic phase. Suspend the powder of the above composition in the organic phase, ultrasonicate with a 200W probe for 40 seconds to form an S / O two-phase dispersion system, and then pour the colostrum into 150ml of the external aqueous phase containing 0.02% Tween 80, at 0°C , mechanically stirred at normal pressure to evaporate the solvent for 24 hours, filtered through a 0.8 μm microporous membrane to obtain microspheres, washed three times with pure water, and dried to obtain finished microspheres.
[0045] Precisely weigh 20 mg of the finished microsphere, dissolve it in 1 ml of dichloromethane, then add 10 ml of phosphate buffered saline (PBS) with pH=7.4 to extract the drug, and use the BCA me...
Embodiment 3
[0048] Accurately weigh 200mg Endostar and 200mg PEG 4000 and dissolve in 1ml Tris buffer solution with pH 8.0, freeze-dry the solution to obtain composition powder. 600 mg of PLGA (Mw=7000, lactic acid:glycolic acid=50:50) was dissolved in 20 ml of dichloromethane to form an organic phase. Suspend the powder of the above composition in the organic phase, disperse at a high speed of 4000rpm for 60 seconds to form an S / O two-phase dispersion system, then pour this colostrum into 1L containing 1% PVA 0486 (the degree of polymerization is about 400, the degree of alcoholysis 86%) in the external water phase, start from 0°C and heat up at a rate of 1°C / min, and finally keep it at 30°C, mechanically stir and volatilize the solvent for 2 hours, and control the pressure at 20kpa throughout the process. The microspheres were obtained by filtering with a 0.8 μm microporous membrane, washed three times with pure water, and dried to obtain the finished microspheres.
[0049] Precisely w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume average particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com