Docetaxel-loading mixed micelle preparation and preparation method thereof
A technology for loading docetaxel and docetaxel, which is applied in the field of docetaxel-loaded mixed micelles and its preparation, can solve the problems of low bioavailability, increase drug loading, solve poor water solubility, and promote penetration The effect of retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 4mg docetaxel, 40mg MPEG 3000 -PLA 2000 Copolymer, 20mg TPGS, add 4mL acetonitrile, ultrasonically dissolve the carrier material and drug completely, and remove the organic solvent acetonitrile by rotary evaporation, so that the material (MPEG-PLA copolymer and TPGS) and drug form a uniform film on the vessel wall. Weigh 10mg of CSO with amino substitution degree 50% 5000 -SA was dissolved in 2mL of water to form an aqueous solution, and the aqueous solution was transferred to a film-forming container. The aqueous solution was magnetically stirred to disperse the film at a temperature of 30°C. After stirring for 30 minutes, the resulting solution was centrifuged to obtain the supernatant docetaxel mixed micelle preparation , 4 ℃ airtight storage.
[0036] Results: The prepared docetaxel mixed micelles had a drug loading capacity of 4.2% and an encapsulation efficiency of 77.1%.
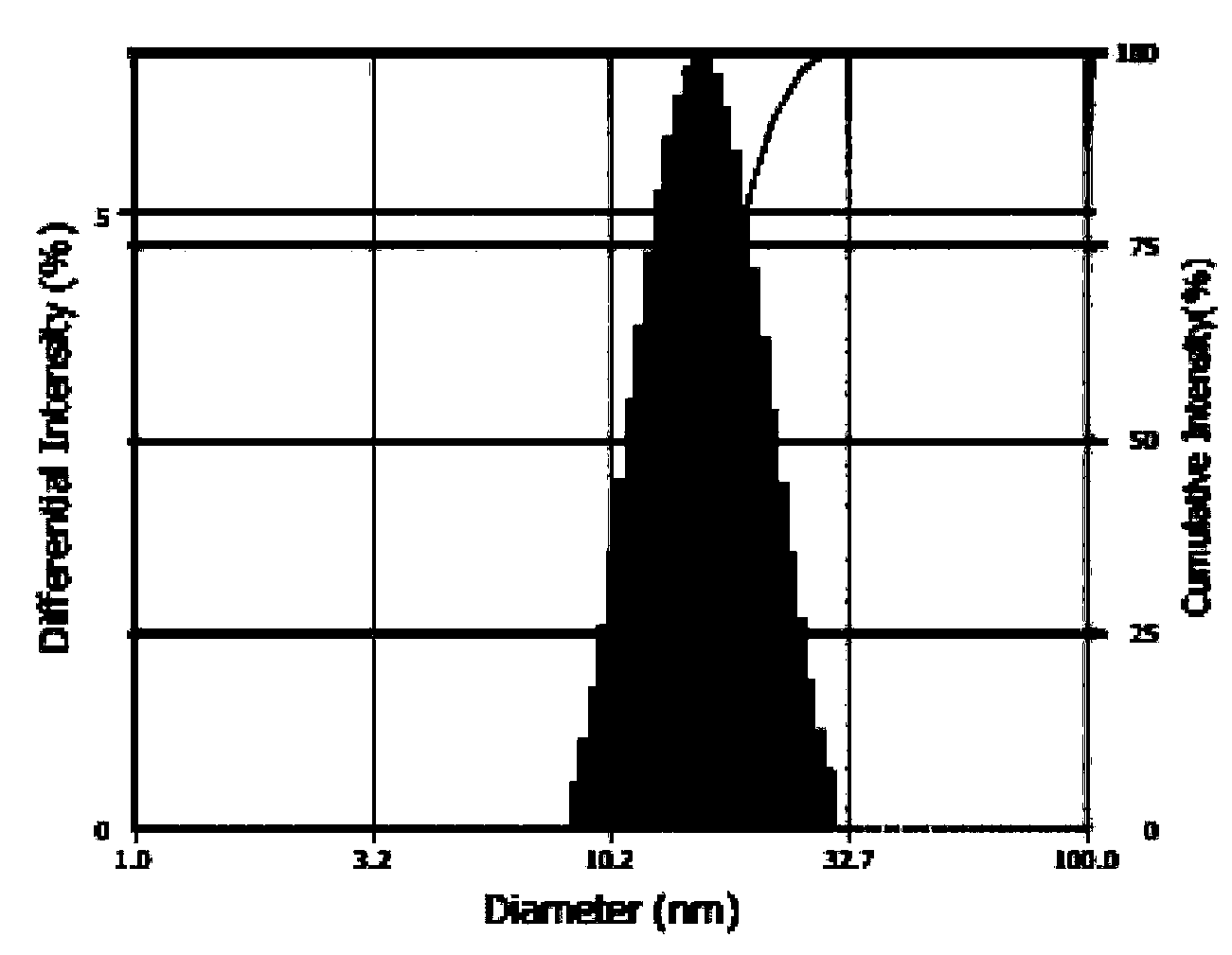
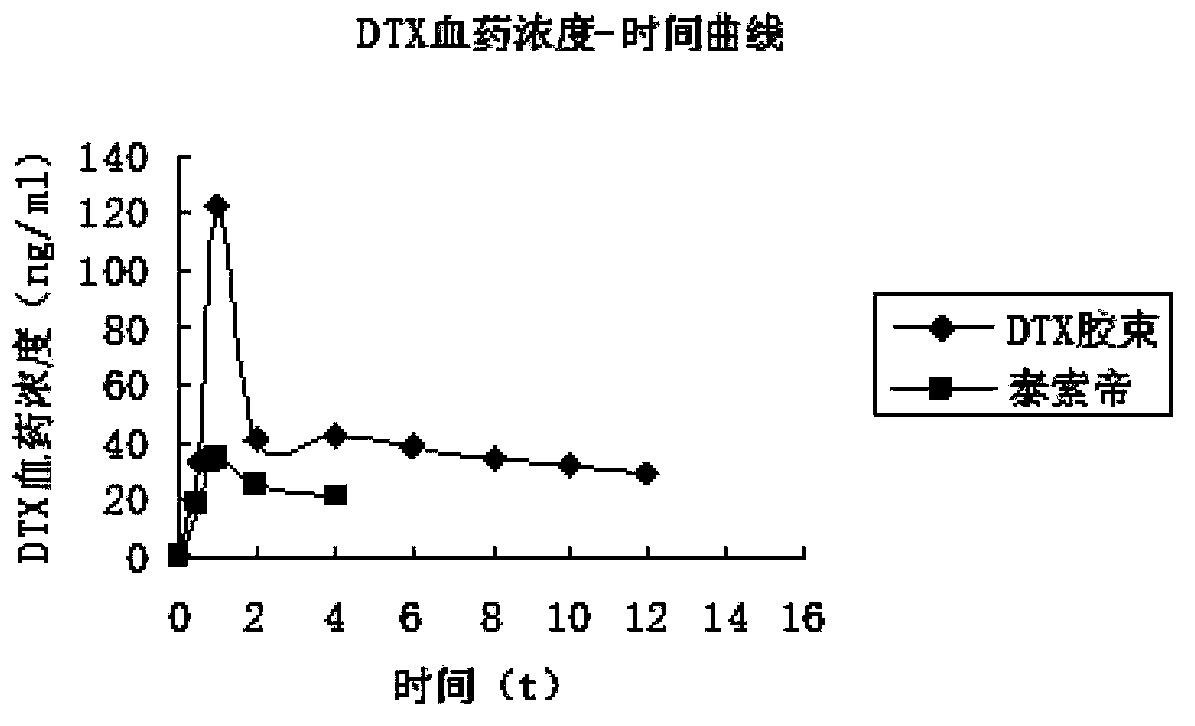
[0037] The particle size distribution figure of the prepared loaded docetaxel mixe...
Embodiment 2
[0039]Weigh 4mg docetaxel, 20mg MPEG 3000 -PLA 10000 Copolymer, 40mg TPGS, add 4mL chloroform, ultrasonically dissolve the carrier material and drug completely, and remove the organic solvent by rotary evaporation, so that the material and drug form a uniform film on the vessel wall. Weigh 10mg of CSO with amino substitution degree 50% 10000 -SA was dissolved in 2mL of water to form an aqueous solution, and the aqueous solution was transferred to a film-forming container, and the aqueous solution was used to disperse the organic film under magnetic stirring at a temperature of 40°C. After stirring for 50 minutes, the resulting solution was centrifuged to obtain the supernatant docetaxel Mixed micellar preparations, sealed and stored at 4°C.
[0040] Results: The prepared docetaxel mixed micelles had a drug loading capacity of 3.1% and an encapsulation efficiency of 54.4%.
Embodiment 3
[0042] Weigh 4mg docetaxel, 20mg MPEG 1000 -PLA 8000 Copolymer, 40mg TPGS, add 3mL ethyl acetate, ultrasonically dissolve the carrier material and drug completely, and remove the organic solvent by rotary evaporation, so that the material and drug form a uniform film on the vessel wall. Weigh 10 mg of CSO with a substitution degree of 50% 8000 -SA was dissolved in 5mL water to form an aqueous solution, and the aqueous solution was transferred to a film-forming container. The aqueous solution was magnetically stirred to disperse the film at a temperature of 25°C. After stirring for 40 minutes, the resulting solution was centrifuged to obtain the supernatant docetaxel mixed micelles Preparations, sealed and stored at 4°C.
[0043] Results: The prepared docetaxel mixed micelles had a drug loading capacity of 5.2% and an encapsulation efficiency of 83.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com