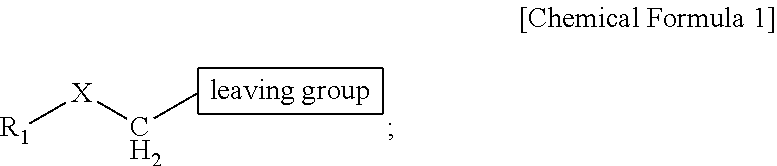Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about "Isotope introduction to steroids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ursodeoxycholic acid compound for preventing or treating FXR (farnesol X receptor)-mediated disease
ActiveCN106008639AGood pharmacokinetic parametersImprove securityOrganic active ingredientsMetabolism disorderFatty liverEnantiomer
Owner:SHENZHEN TARGETRX INC
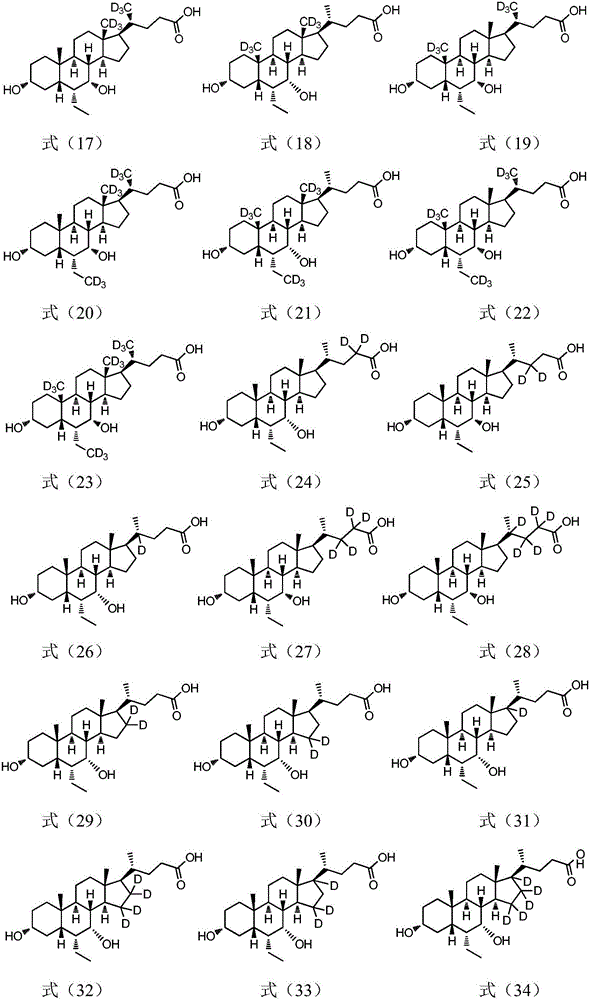
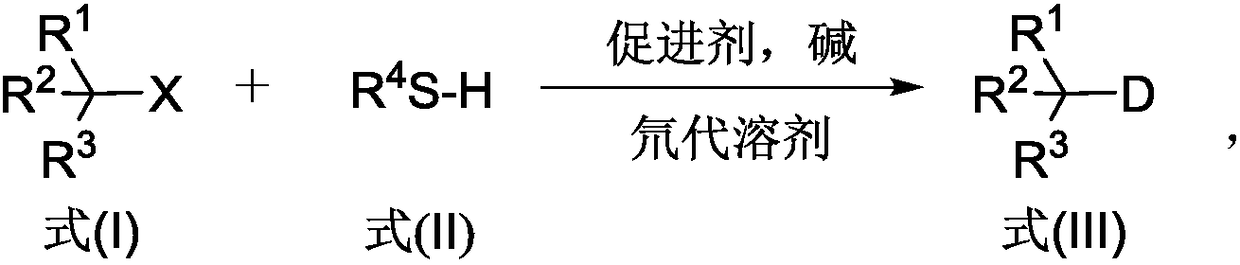
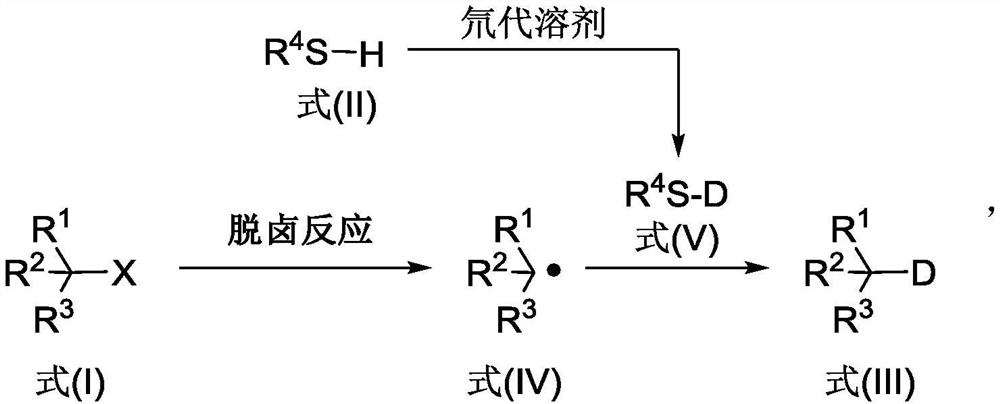
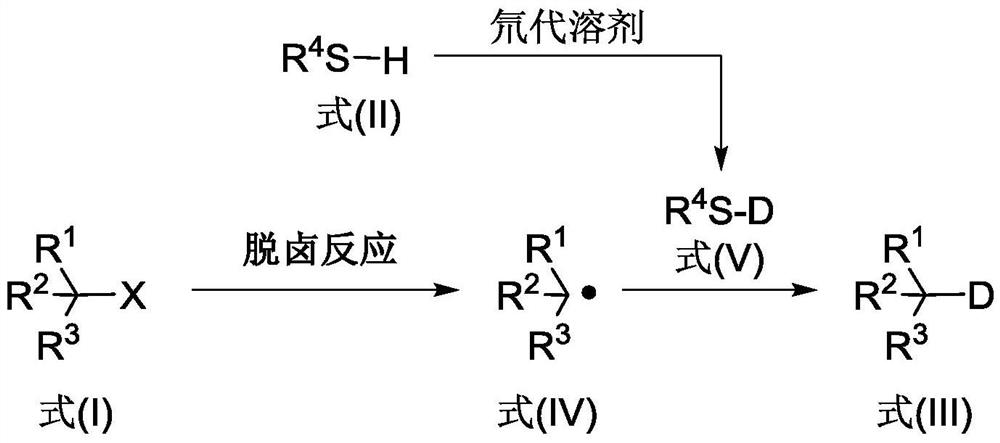
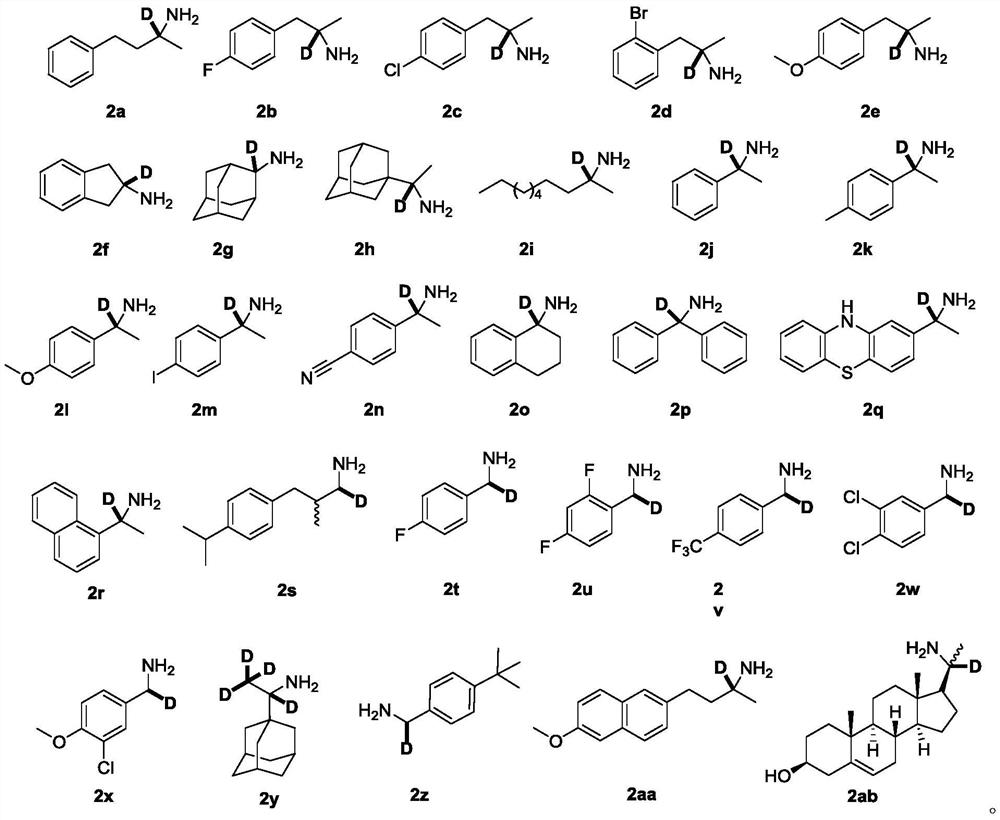
Deuterated compound synthesis method
ActiveCN109265304AImprove applicabilityMild conditionsIsotope introduction to sugar derivativesIsotope introduction to heterocyclic compoundsArylHydrogen atom
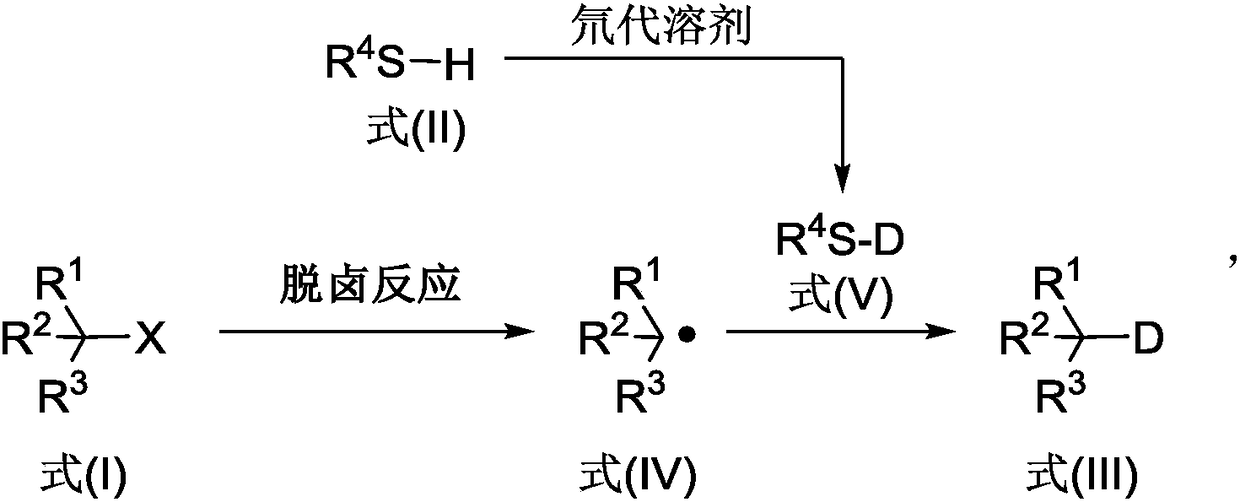
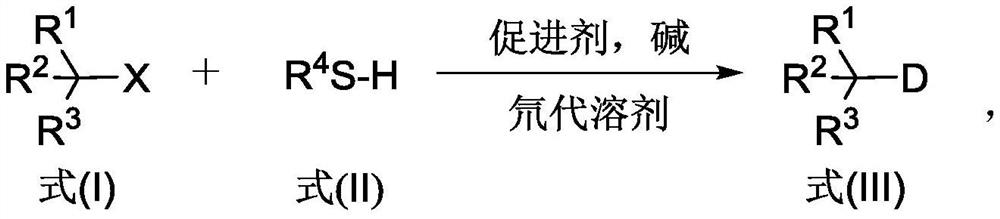
The invention discloses a deuterated compound synthesis method, which comprises: carrying out a mixing reaction on a halogenated compound represented by a formula (I), a sulfide represented by a formula (II), a promoter, an alkali and a deuteration solvent to obtain a reduced deuterated product represented by a formula (III), wherein the reaction equation of the reaction is defined in the specification, R<1>, R<2>, R<3> and R<4> respectively are at least one selected from alkyl, aryl, acyl, hydrogen atom and deuterium atom, and X is at least one selected from iodine, bromine and chlorine. According to the present invention, the deuteration marking of the compound is efficiently achieved by using the common heavy water and the deuterated alcohol as the deuterium source through the simple reduction reaction, such that the use of expensive, toxic, flammable and explosive deuterium source reagents is not required compared with the prior art. The formulas I, II and III are defined in the specification.
Owner:HUAZHONG UNIV OF SCI & TECH
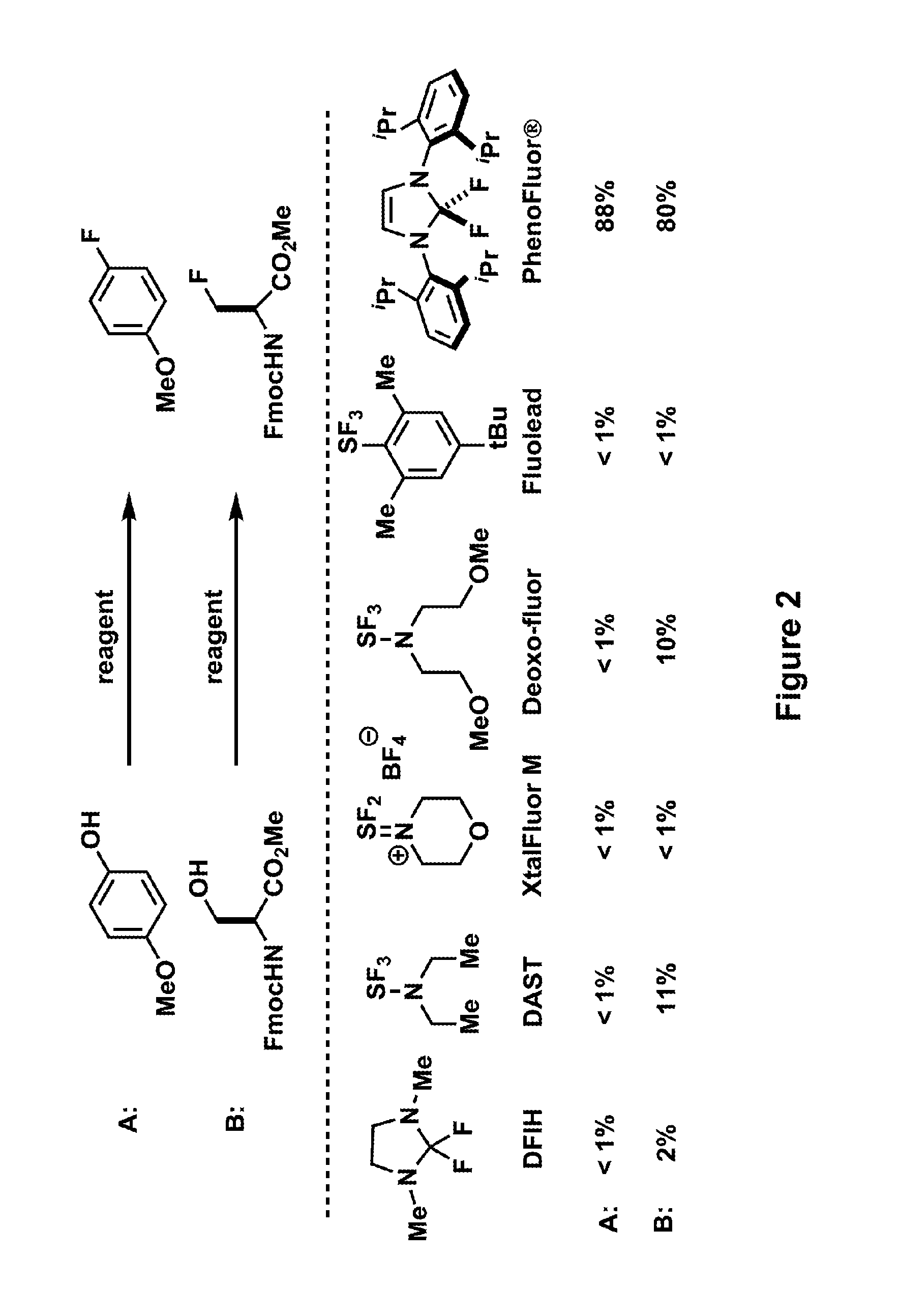
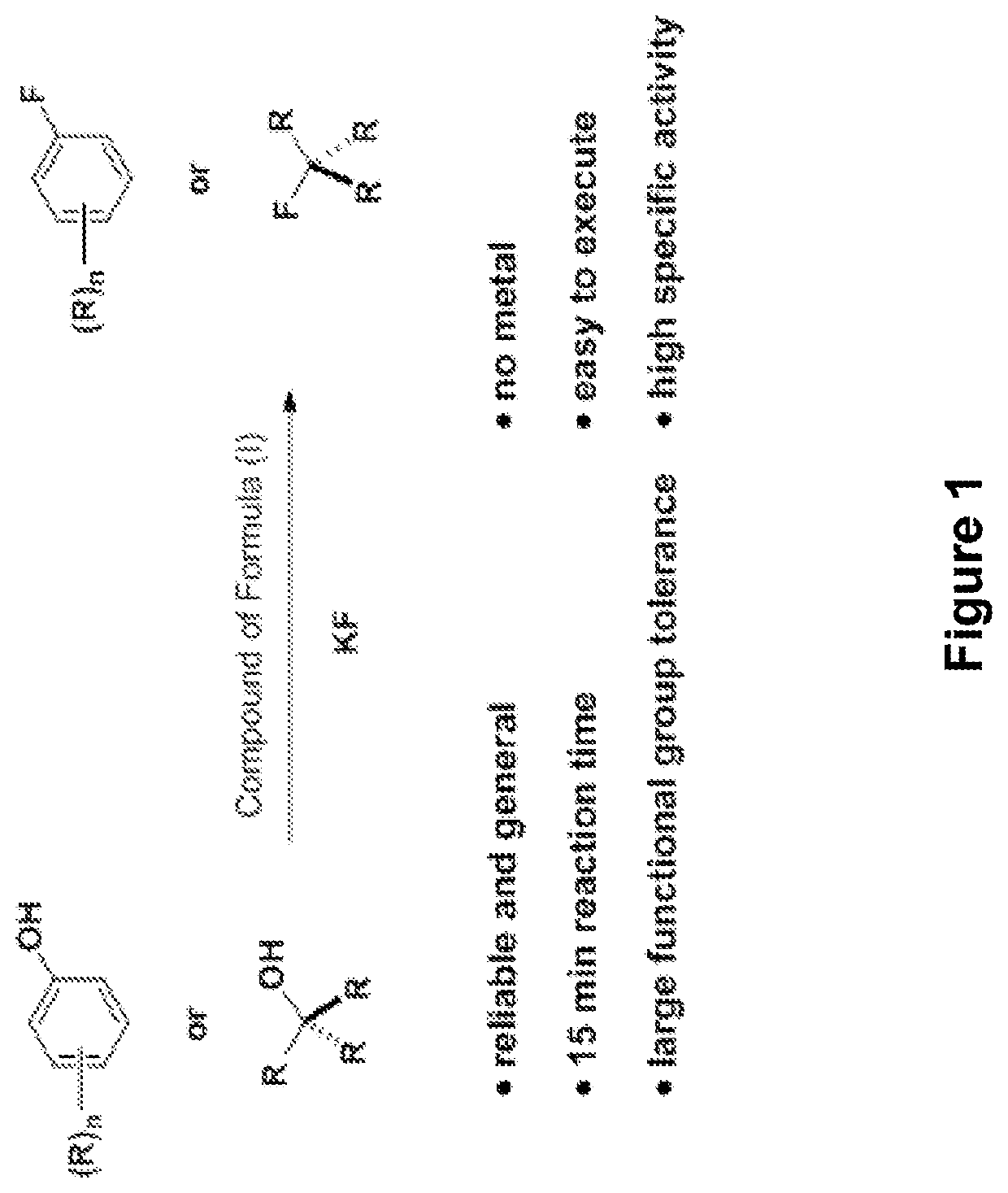
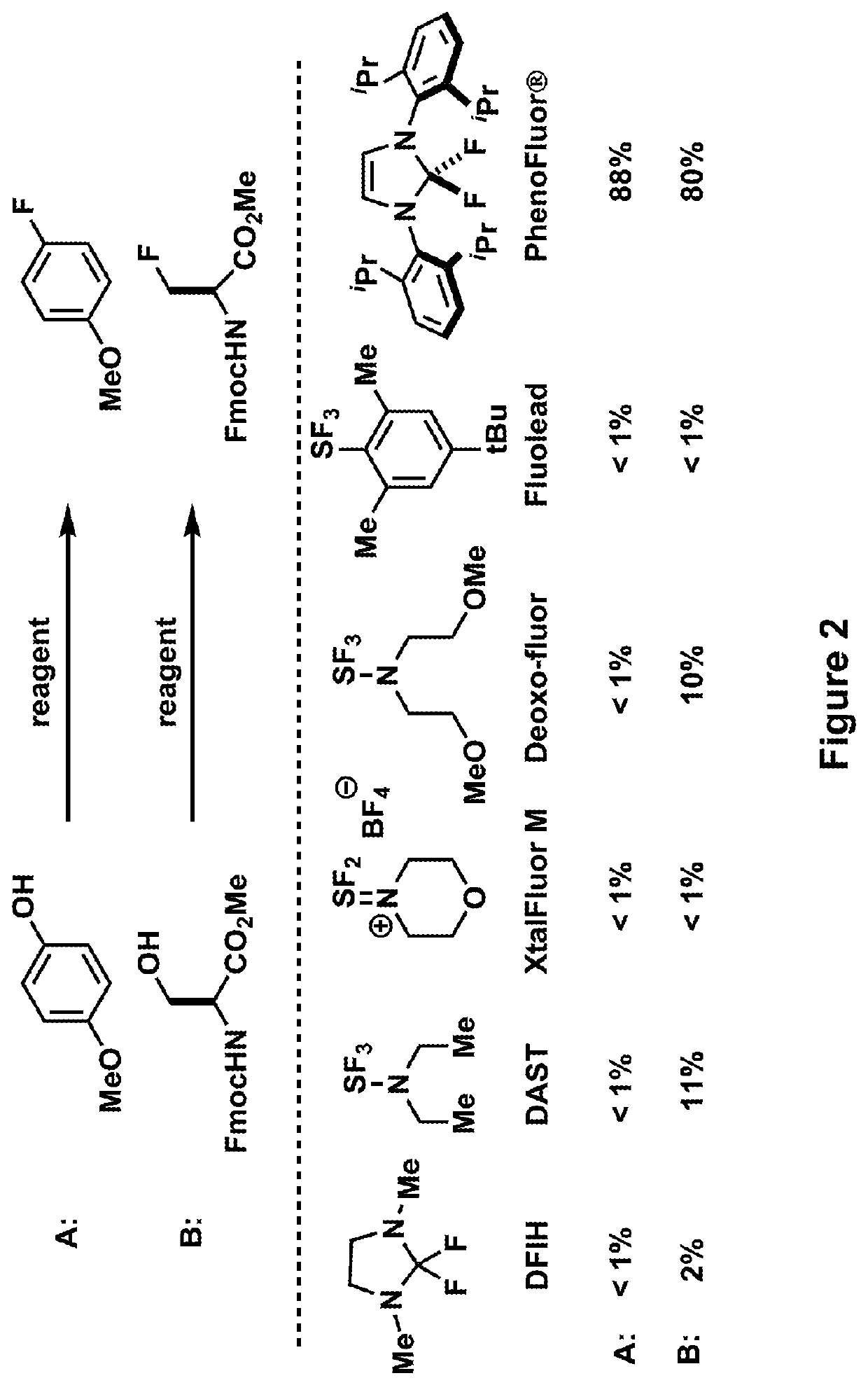
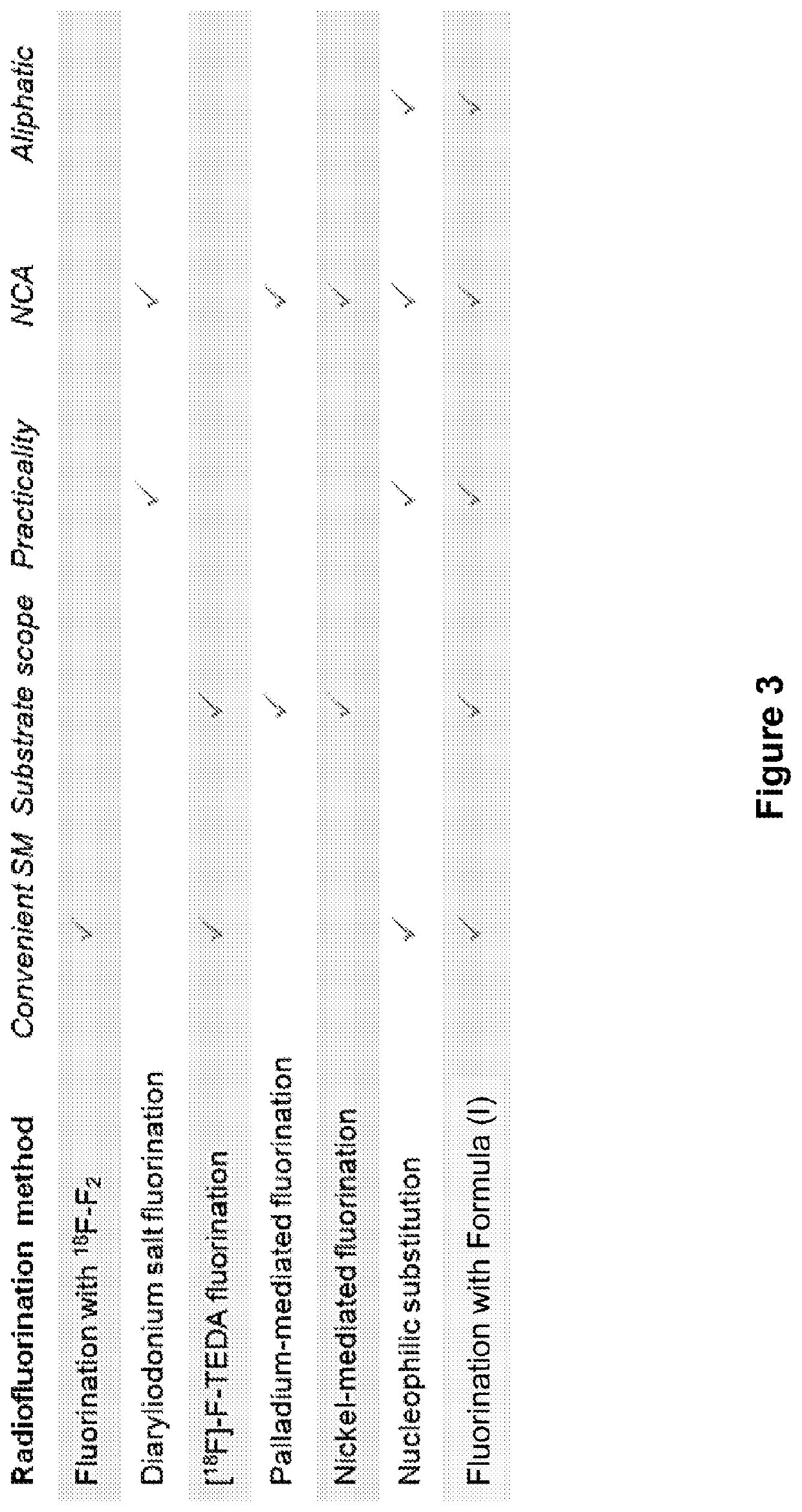
Fluorination of organic compounds
ActiveUS20160272593A1Easy accessSimplify the development processIsotope introduction to steroidsRadioactive preparation carriersSimple Organic CompoundsPet imaging
Methods for fluorinating organic compounds utilizing a novel organic reagent are described herein. The invention further discloses the utility of this reagent for incorporation of the 18 F isotope into hydroxyl group-containing organic molecules for PET imaging studies. Preparation of the reagents is described along with isolable intermediate structures from reaction of the reagent with a hydroxyl group-containing organic molecule.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
New photocatalytic fixed-point deuteration method for carbon-carbon unsaturated bonds
ActiveCN109180411AOrganic compound preparationIsotope introduction to steroidsReaction temperatureAlkyne Compound
The invention relates to a new photocatalytic fixed-point deuteration method for carbon-carbon unsaturated bonds. The method is characterized in that an olefin or alkyne compound and a deuterium source undergoes a deuteration reaction under the catalysis of a light source and a photocatalyst to obtain a deuterated product, wherein the deuterium source is deuterated water, deuterated alcohol or deuterated acid, and the reaction temperature is between room temperature and 80 DEG C. The fixed-point deuteration reaction of the olefin or alkyne compound is realized under the photocatalysis action of the photocatalyst with environmentally-friendly and cheap deuterated water or a deuteration reagent as a deuterium source to substitute deuterium gas. Compared with traditional deuteration reactions, the method has a higher selectivity, milder reaction conditions and higher economical suitability, and is suitable for large-scale deuterated chemical substance production.
Owner:SHENZHEN UNIV
Receptor molecularly-targeted imaging agent and preparation method and application thereof
ActiveCN106632571AHas an estradiol skeletonGood chemical stabilityIsotope introduction to steroidsRadioactive preparation carriersBiological propertyPolyethylene glycol
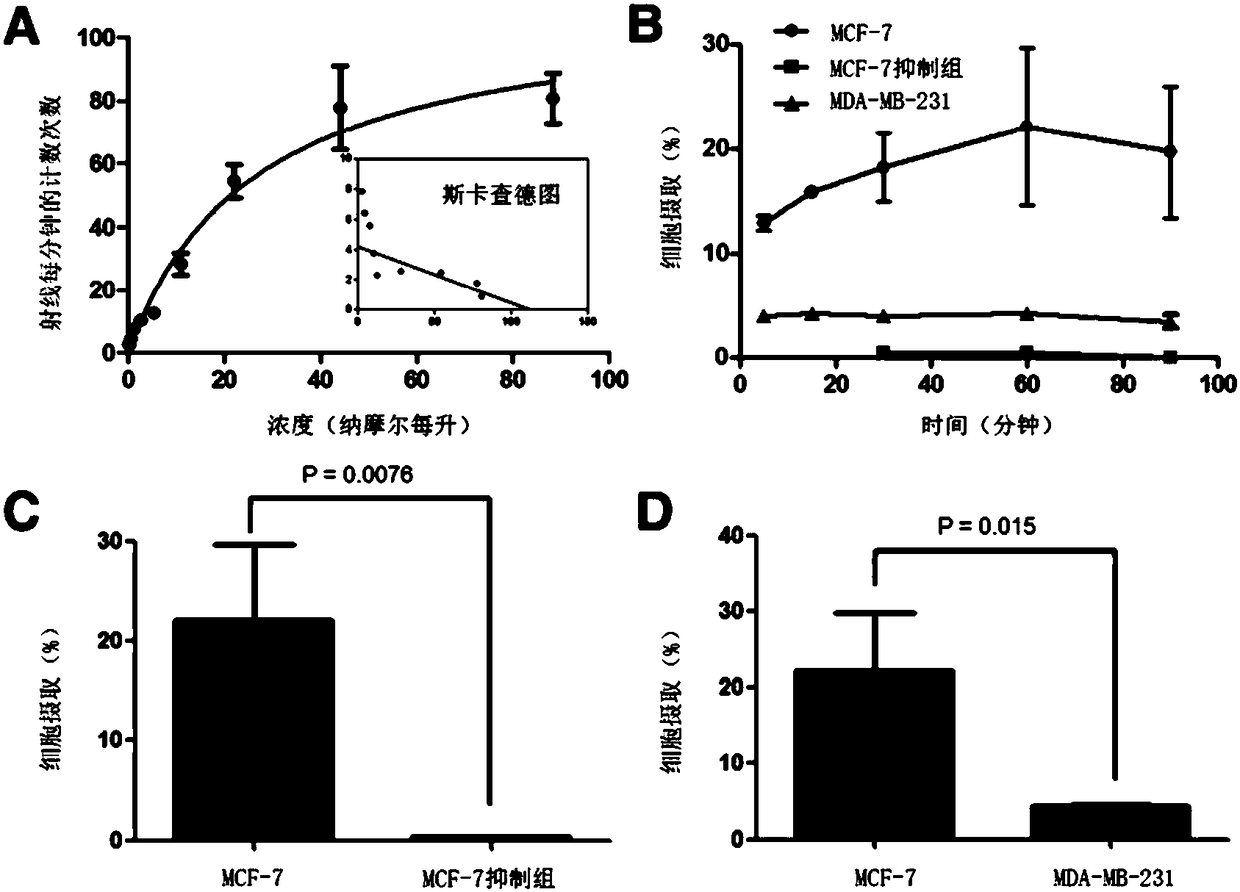
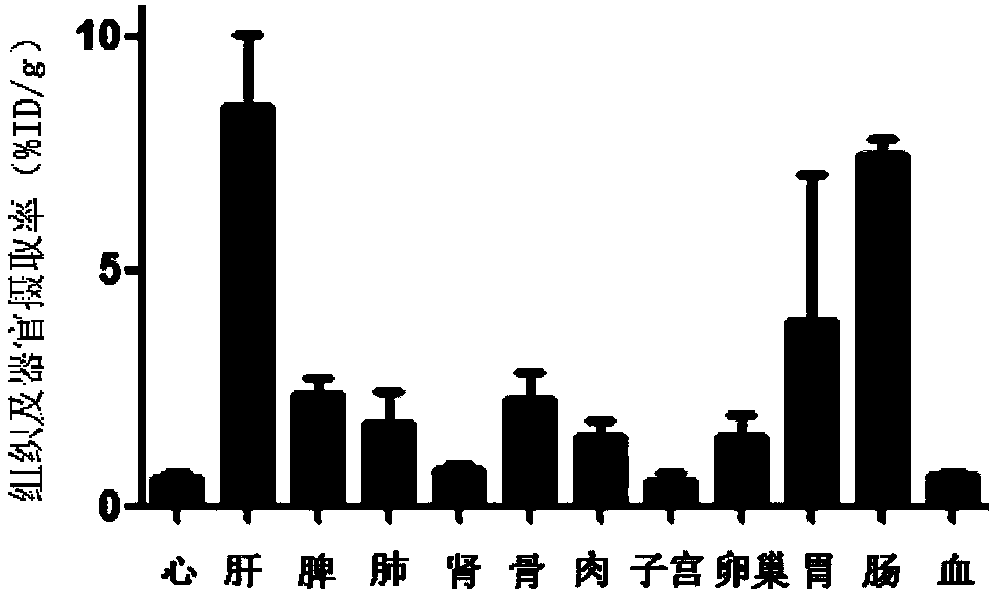
The invention discloses a receptor molecularly-targeted imaging agent and a preparation method and application thereof. The receptor molecularly-targeted imaging agent comprises a polyethylene glycol short chain which is linked with 17alpha-ethinyloestradiol of a target estrogen receptor through 'click' reaction. The receptor molecularly-targeted imaging agent is shown as a concrete general molecular formula in the description, wherein the 17alpha-ethinyloestradiol serves as a targeting group, polyethylene glycol serves as a hydrophilic group, and <18>F serves as a radioactive group. The receptor molecularly-targeted imaging agent has excellent biological properties and high specificity, is high in uptake when used in positive breast cancer tumors of the estrogen receptor and capable of distinguishing between estrogen positive receptors and estrogen negative receptors, and meets the conditions as a breast cancer receptor imaging agent.
Owner:XIAMEN UNIV
Method for synthesizing radiopharmaceuticals using a cartridge
ActiveUS9550704B2Guaranteed performancePresent inventionIsotope introduction to sugar derivativesIsotope introduction to heterocyclic compoundsRadioactive drugBiomedical engineering
Owner:FUTURECHEM
Method for synthesizing radiopharmaceuticals using a cartridge
ActiveUS20150232392A1Guaranteed performancePresent inventionGlycosidesAmino-hyroxy compound preparationCombinatorial chemistryPolymer
The present invention relates to a method for synthesizing a radiopharmaceutical using a cartridge, which makes it possible to carry out several steps of reaction required for synthesizing a radiopharmaceutical in the cartridge filled with a polymer. A method for synthesizing a radiopharmaceutical according to the present invention enables each step's reaction to be carried out with the solution confined in the cartridge so as not to flow out, thus being simplified compared to the conventional methods for synthesizing radiopharmaceuticals, and expediting the synthesis thereof.
Owner:FUTURECHEM
Deuterated compounds
InactiveUS20160222052A1Nervous disorderIsotope introduction to steroidsMotor neurone diseaseOrganic chemistry
Compounds of general formula (I) wherein (I) R1-R4 are each independently selected from H and deuterium; and at least one of R1-R4 is deuterium. The compounds have been found to be particularly useful for treating neurodegenerative conditions and in particular but not exclusively conditions such as motor neurone disease.
Owner:SWANSEA UNIV
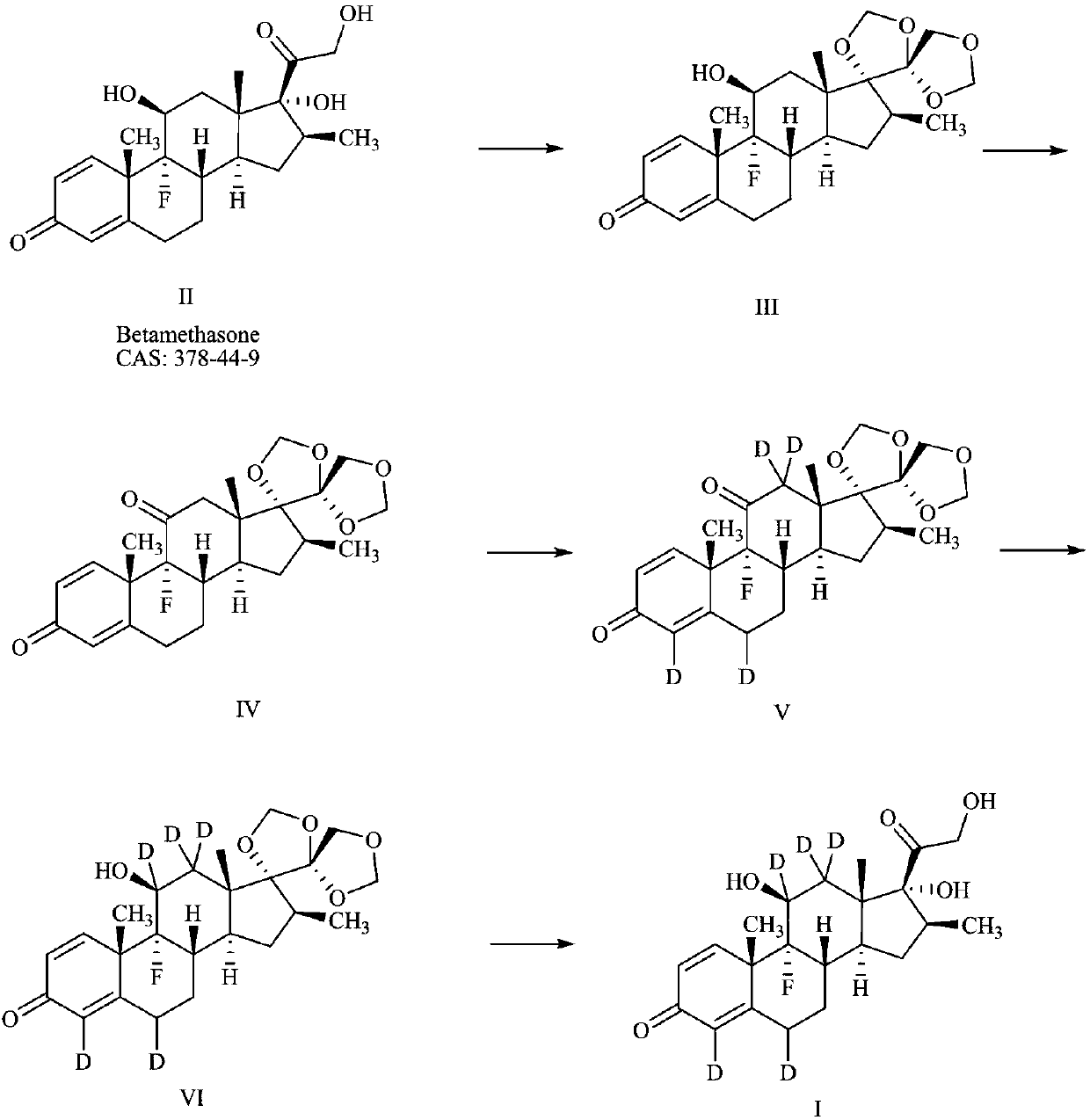
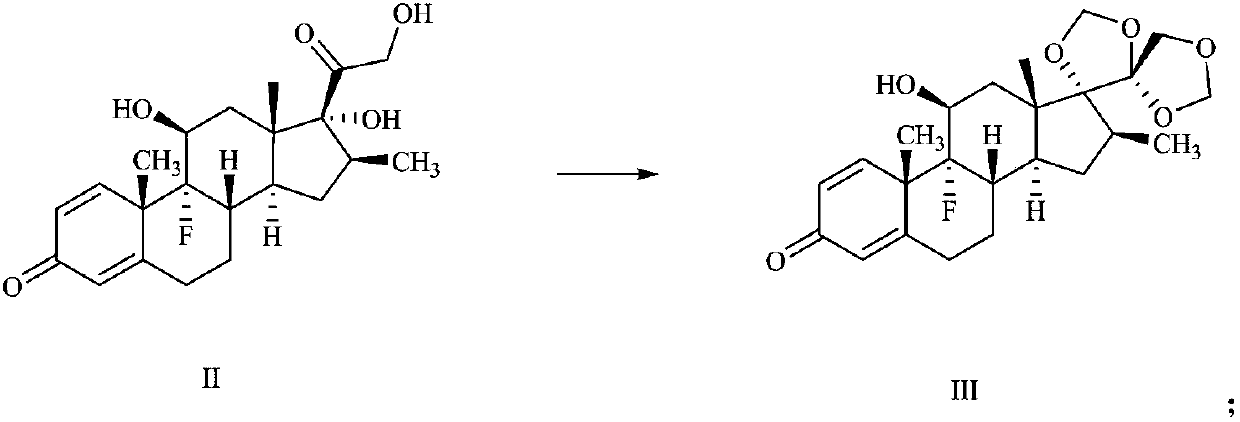
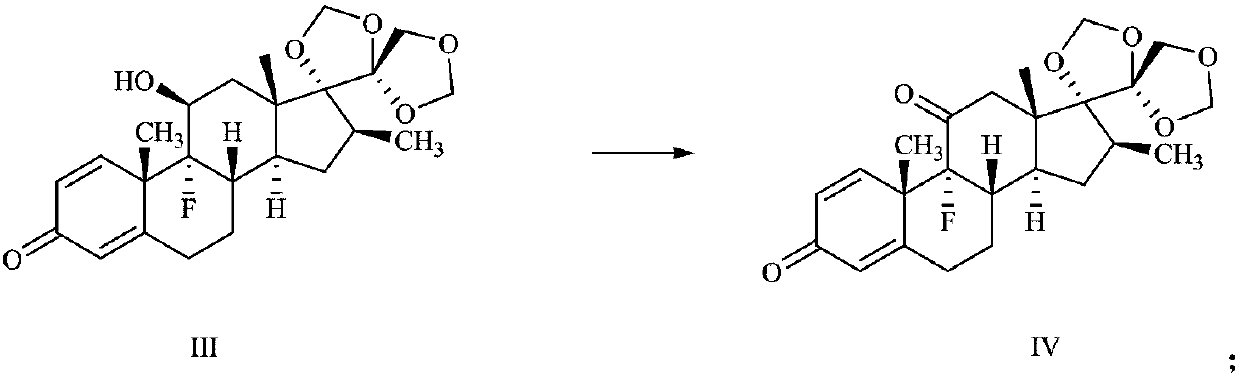
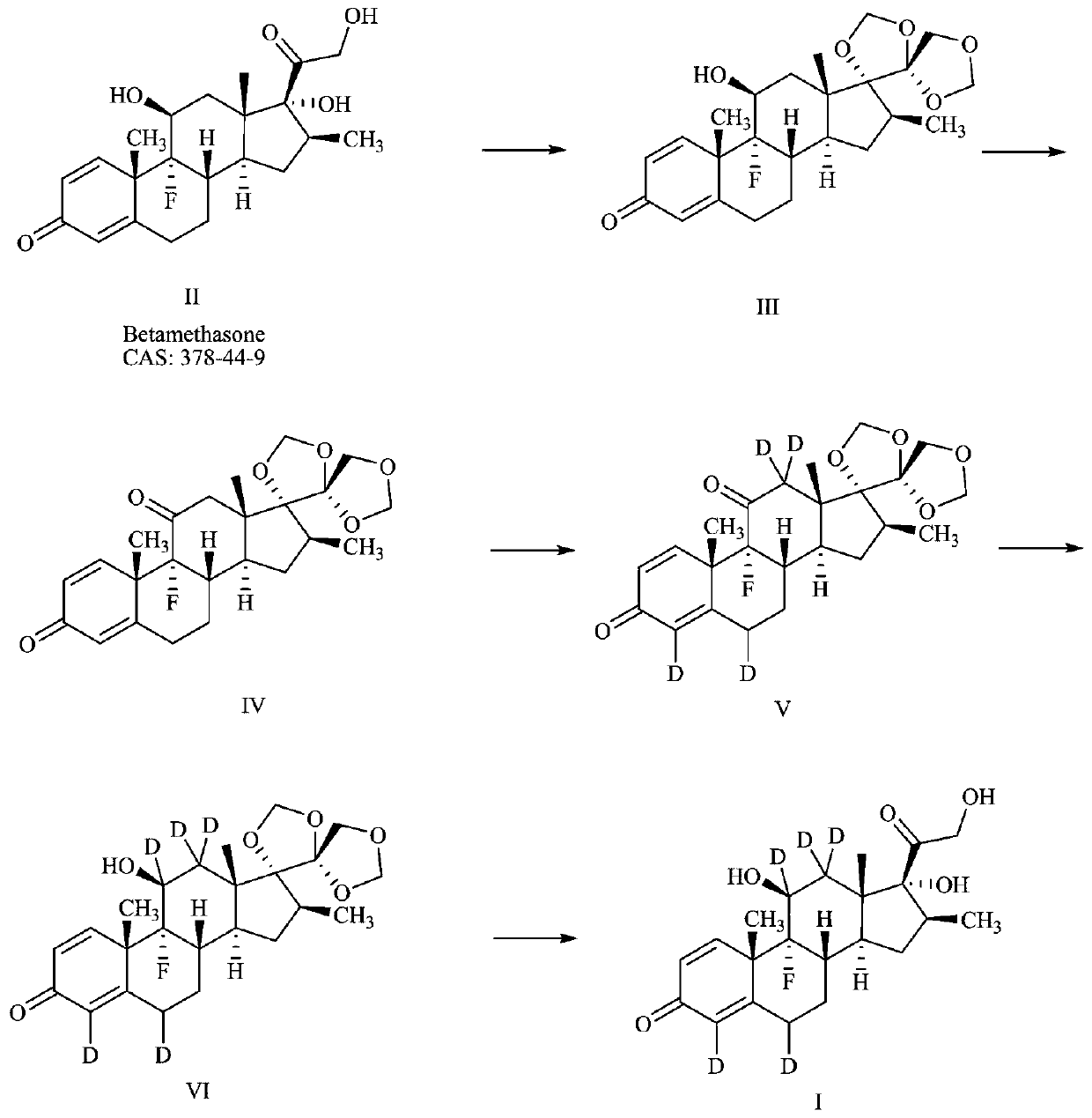
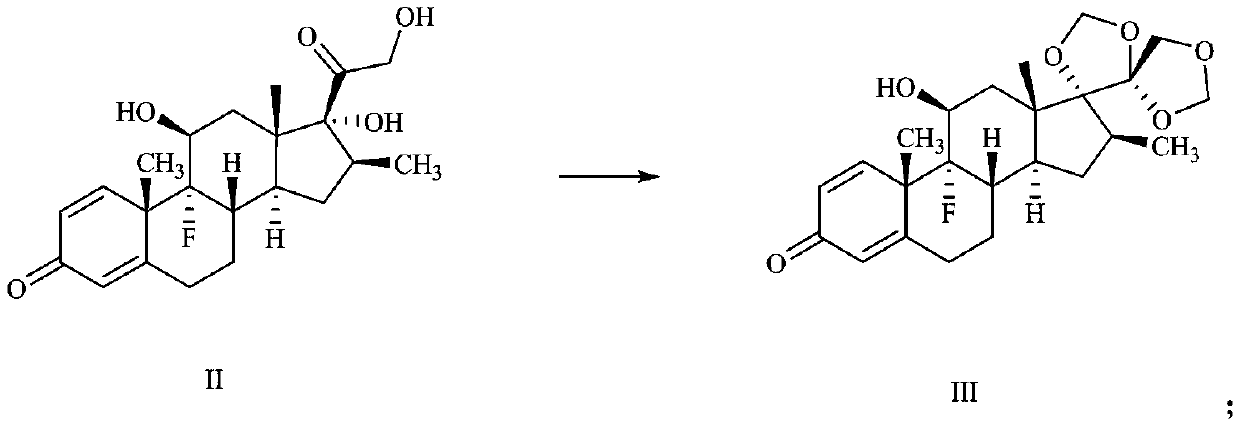
Preparation method of deuterium-labeled betamethasone
The invention discloses a preparation method of deuterium-labeled betamethasone. The method is characterized in that betamethasone is used as an initiator and the deuterium-labeled betamethasone is synthesized through five-step reaction. According to the preparation method disclosed by the invention, optimal preparation steps and reaction conditions are screened out through a large number of experiments; the whole technology is reasonable in design and the operability is high. The deuterium-labeled betamethasone prepared by the preparation method disclosed by the invention has the characteristics that the purity can reach 98 percent or above, the yield is high, and isotope abundance is greater than 97 percent. The deuterium-labeled betamethasone prepared by the preparation method disclosedby the invention has the advantages that test and control samples can be provided for the study on a metabolic mechanism and an anti-inflammatory mechanism of the betamethasone; in addition, the deuterium-labeled betamethasone has important potential anti-inflammatory value and other application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
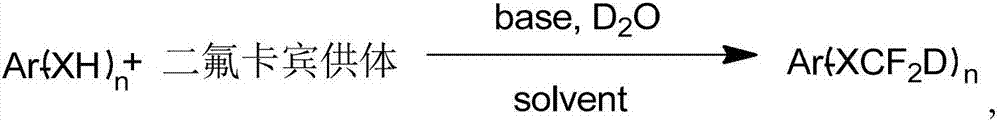
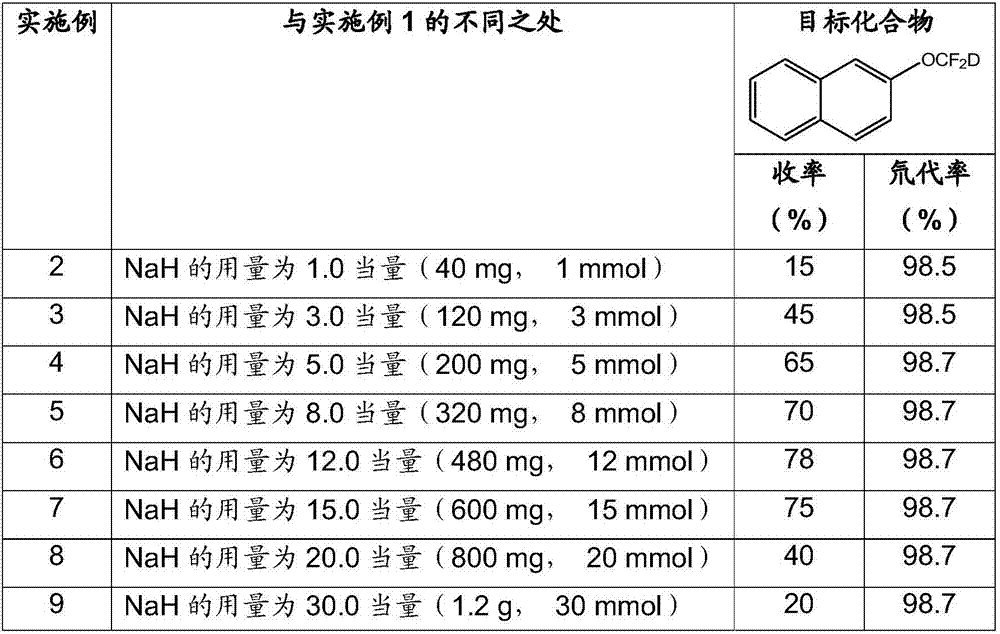
Synthesis method of difluorodeuteromethoxy(thio) function group-containing aromatic compound
InactiveCN107226769AHigh rate of deuteriumHigh yieldIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationThio-Synthesis methods
The invention belongs to the technical field of deuterium-containing compound synthesis and discloses a synthesis method of an aromatic compound containing a difluorodeuteromethoxy functional group or a difluorodeuterothio functional group. In the presence of an alkali, a difluorocarbene donor, a group shown in the description and heavy water undergo a reaction to produce a deuterated product shown in the description, wherein the difluorocarbene donor is BrCF2PO(OR)2 or (chlorodifluoromethyl)trimethylsilane or (bromodifluoromethyl)trimethylsilane and the alkali is selected from Na, K, Li, Mg, Zn, Al, NaH, KH, LiH, LiAlH4, KOD, NaOD, NaOMe, NaOEt, NaOBu, sodium carbonate, potassium carbonate and cesium carbonate. The method has the advantages of use of cheap and easily available raw materials, simple and mild reaction, high yield, high deuteration rate, and production amplification easiness.
Owner:TETRANOV PHARMA CO LTD
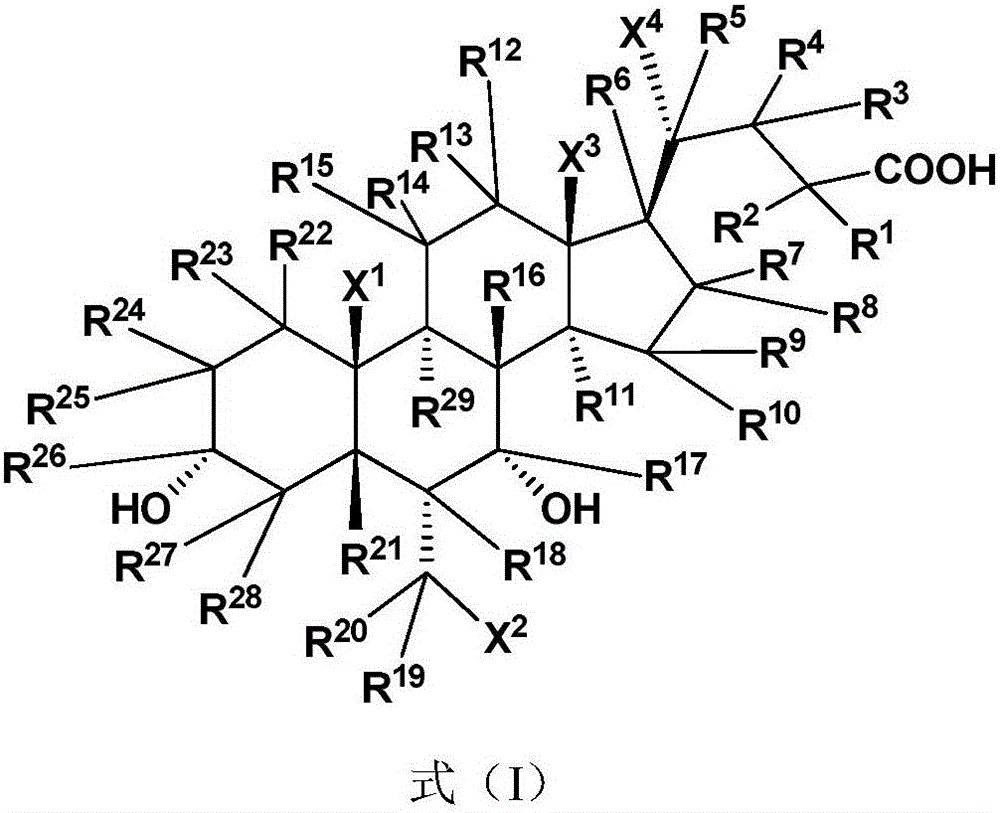
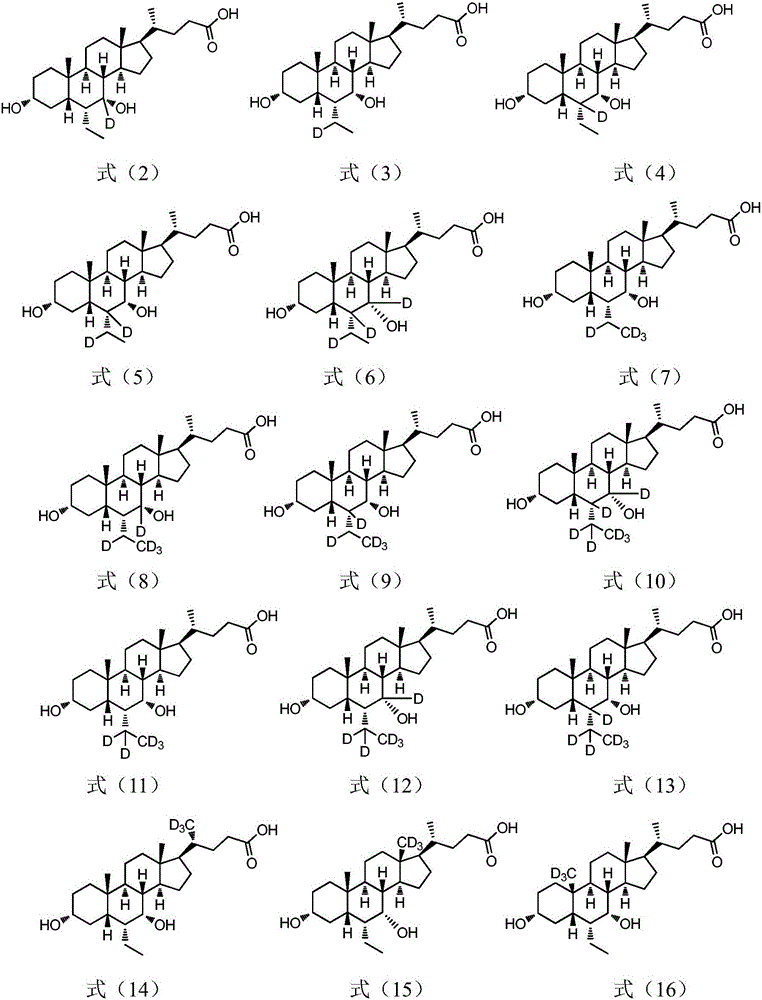
Deuterated chenodeoxycholic acid derivative and pharmaceutical composition comprising compound thereof
ActiveUS20180030083A1Improve the activation effectPharmacodynamics/pharmacokinetic propertyOrganic active ingredientsAntipyreticChenodeoxycholic acidCholesterol cholelithiasis
Disclosed are deuterated chenodeoxycholic acid derivatives and pharmaceutical compositions containing the deuterated chenodeoxycholic acid derivatives. In particular, disclosed is a deuterated chenodeoxycholic acid derivative of formula (I), or a crystal form, pharmaceutically acceptable salt, hydrate or solvate thereof, and a pharmaceutical composition containing the same. The deuterated chenodeoxycholic acid derivatives of formula (I) can be used to treat and / or prevent diseases related to the farnesoid X receptor (FXR) and / or G-protein coupled bile acid receptor, such as nonalcoholic steatohepatitis, nonalcoholic fatty liver diseases, gallstones, primary biliary cirrhosis, and cirrhosis.
Owner:SUZHOU ZELGEN BIOPHARML
Aldehyde deuteration and application of aldehyde deuteration in preparation of deuterated aldehyde
PendingCN114075108AIsotope introduction to heterocyclic compoundsCarbamic acid derivatives preparationPtru catalystOrganosolv
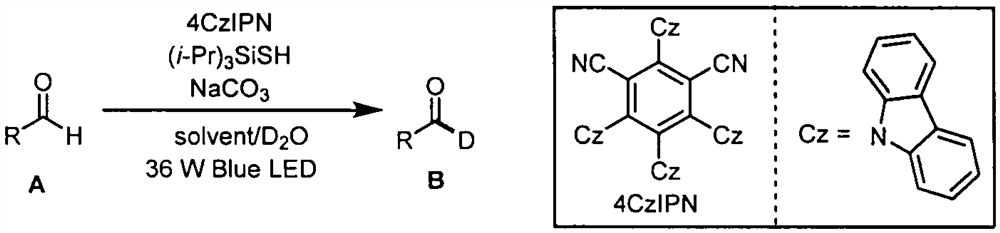
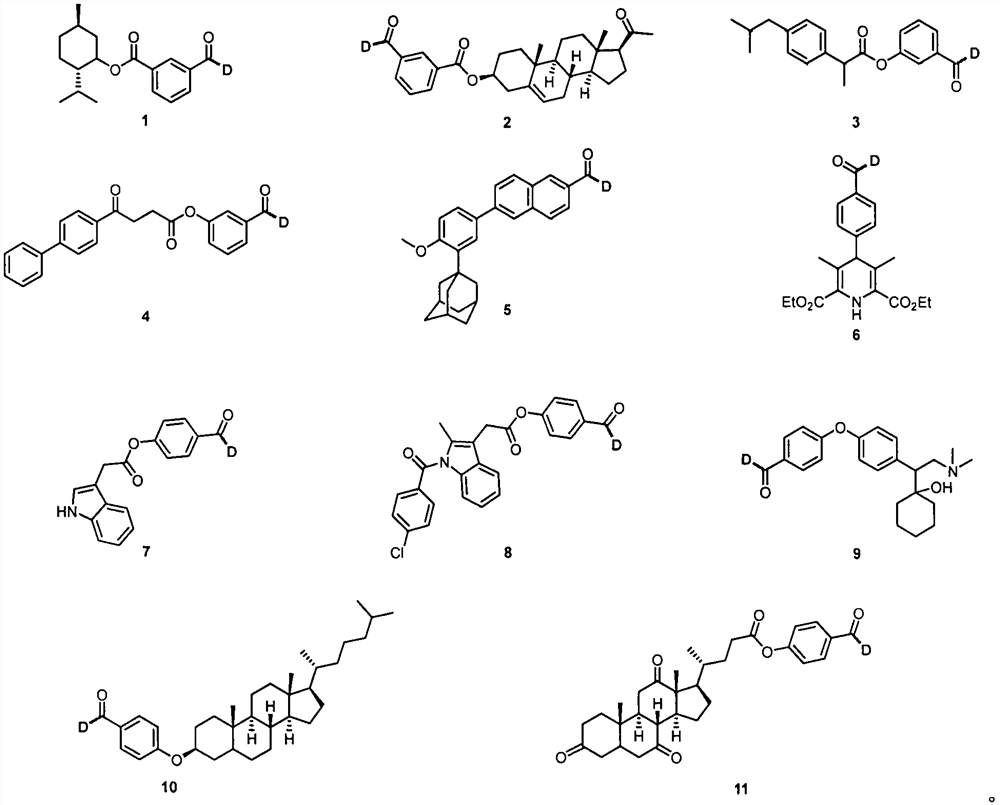
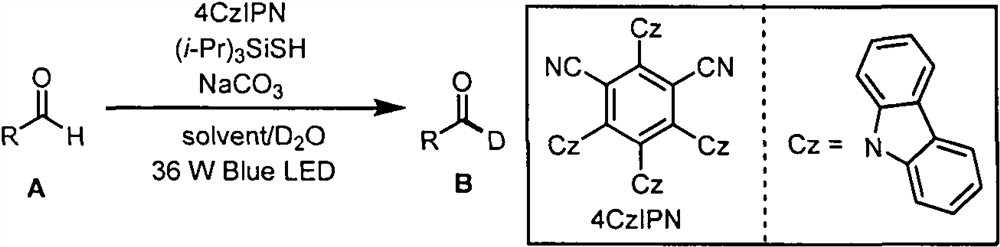
The invention belongs to the technical field of fine chemicals, and particularly relates to a method for preparing deuterated aldehyde through a deuteration reaction of aldehyde under synergistic catalysis of visible light and small organic molecules. The method comprises the following steps: mixing aldehyde with a photocatalyst 4CzIPN, an organic micromolecular catalyst triisopropylsilyl mercaptan ((i-Pr)3SiSH), sodium carbonate (NaCO3), deuterated water and an organic solvent, blowing argon into a reaction bottle, conducting reacting under the irradiation of blue light with a power of 36 W and a wavelength of 470 nm, removing the solvent through spinning, and carrying out column chromatography to obtain a pure product.
Owner:NANKAI UNIV
A kind of synthetic method of deuterated compound
ActiveCN109265304BImprove applicabilityMild conditionsIsotope introduction to sugar derivativesIsotope introduction to heterocyclic compoundsArylAlcohol
The invention discloses a deuterated compound synthesis method, which comprises: carrying out a mixing reaction on a halogenated compound represented by a formula (I), a sulfide represented by a formula (II), a promoter, an alkali and a deuteration solvent to obtain a reduced deuterated product represented by a formula (III), wherein the reaction equation of the reaction is defined in the specification, R<1>, R<2>, R<3> and R<4> respectively are at least one selected from alkyl, aryl, acyl, hydrogen atom and deuterium atom, and X is at least one selected from iodine, bromine and chlorine. According to the present invention, the deuteration marking of the compound is efficiently achieved by using the common heavy water and the deuterated alcohol as the deuterium source through the simple reduction reaction, such that the use of expensive, toxic, flammable and explosive deuterium source reagents is not required compared with the prior art. The formulas I, II and III are defined in the specification.
Owner:HUAZHONG UNIV OF SCI & TECH
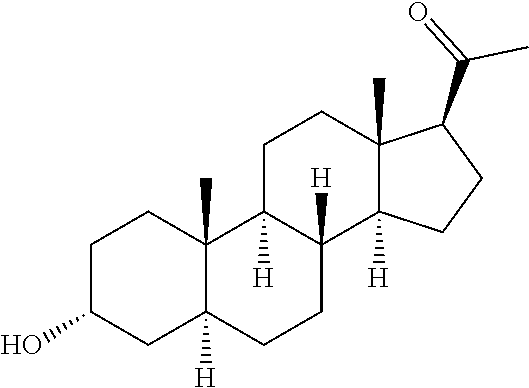
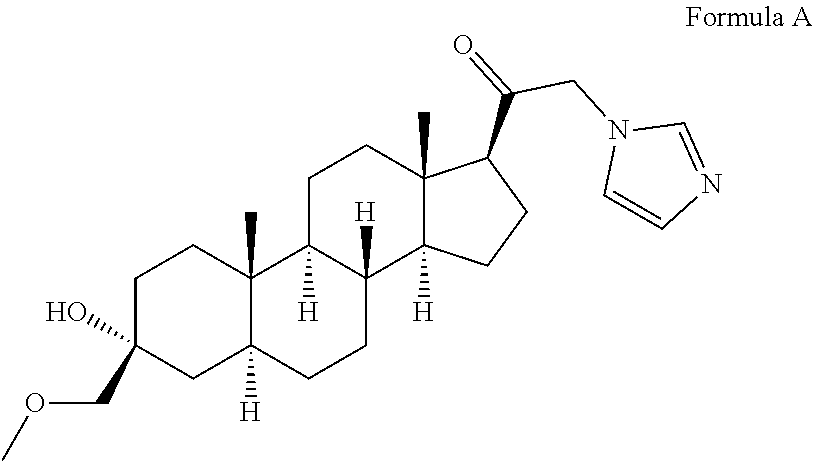
Organic compounds
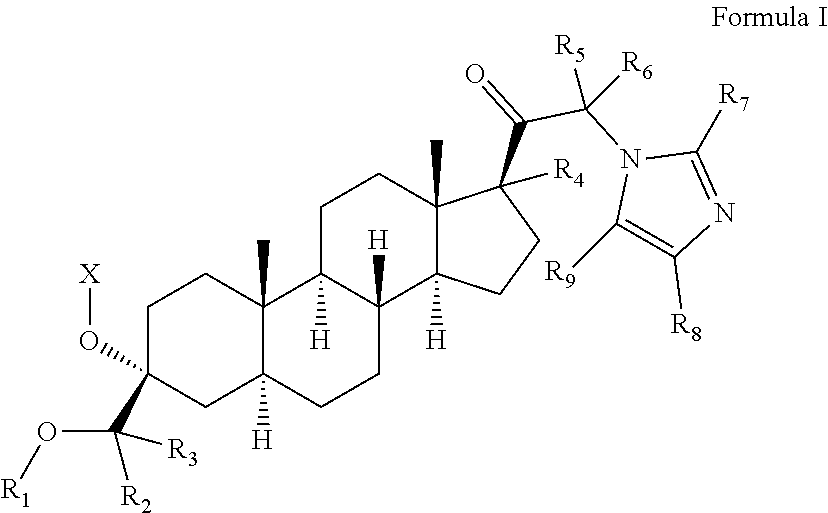
PendingUS20220073558A1Convenient treatmentOrganic active ingredientsNervous disorderAnti-Anxiety AgentsPharmaceutical medicine
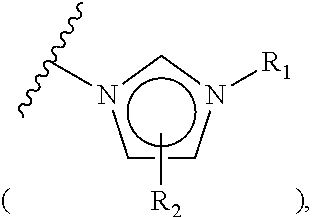
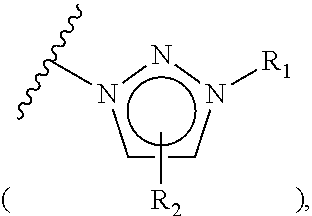
The invention relates to particular prodrugs and analogs of (3α,5α)-3-hydroxy-21-(1H-imidazol-1-yl)-3-methoxymethyl)-pregnan-20-one, in free or pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use as sedatives, hypnotics, anxiolytics, and / or anesthetics, and methods for treatment of depression, anxiety, insomnia, epilepsy, and other central nervous system disorders, as well as to combinations with other agents.
Owner:INTRA CELLULAR THERAPIES INC
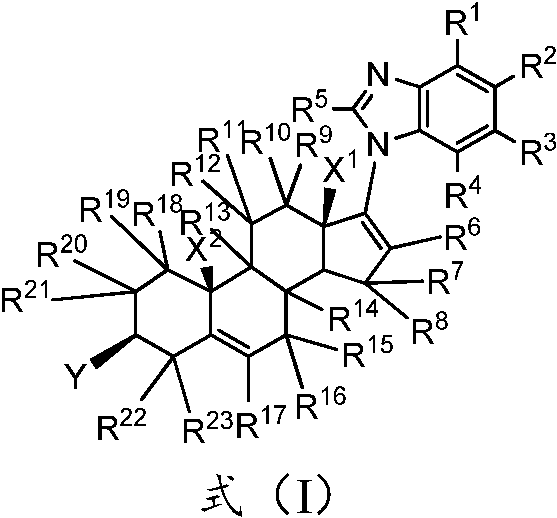
A kind of substituted steroid compound and its application
ActiveCN108350024BEnhanced inhibitory effectGood curative effectOrganic active ingredientsAntipyreticAndrogen Receptor GeneEfficacy
The invention provides a substituted steroid compound and its application. Specifically, the substituted steroid compound of the present invention is a steroid compound represented by formula (I), or its crystal form, pharmaceutically acceptable salt, prodrug, stereoisomer, hydrate or solvent compound. The substituted steroidal compound disclosed in the present invention and the composition containing the compound have excellent inhibitory properties on CYP17 enzyme and androgen receptor (AR), and have better pharmacokinetic parameter characteristics, and can improve the Drug concentration in animals to improve drug efficacy and safety.
Owner:SHENZHEN TARGETRX INC
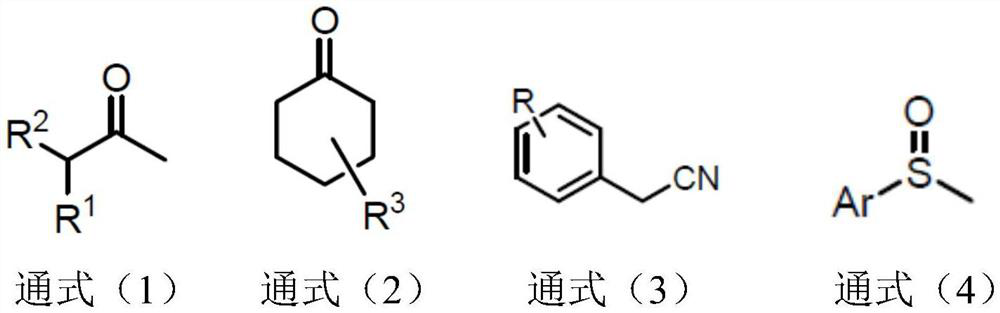
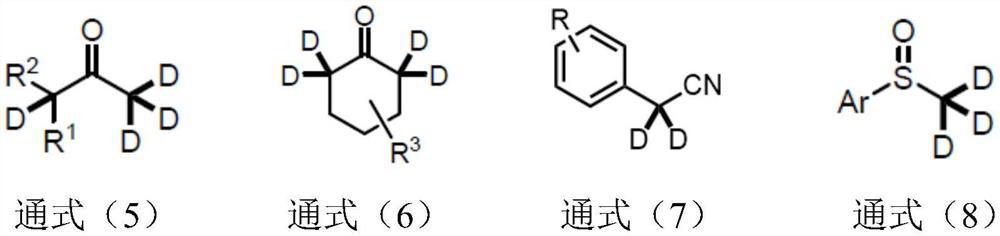
Synthetic method of alpha-deuterated carbonyl compound
PendingCN114773174AEasy to operateOperational securityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystRegioselectivity
The invention belongs to the technical field of preparation of deuterated compounds, and particularly relates to a synthesis method of alpha-deuterated carbonyl compounds. Inorganic base barium oxide BaO or barium hydroxide Ba (OH) 2 is used as a catalyst, a deuterium supply reagent is used as a deuterium source, and deuteration of the alpha position of the carbonyl compound is efficiently completed. According to the method disclosed by the invention, the H / D conversion reaction can be carried out at the alpha position of carbonyl at high selectivity without influencing other positions, the applicable substrates are wide, and the target product can be obtained at high yield and high deuteration rate in both cyclic ketone and chain ketone reactions. For the reaction of nitrile compounds and sulfoxide compounds, the target product can be obtained with high yield and medium deuteration rate, and the method has the characteristics of simplicity, high efficiency and good regioselectivity.
Owner:CHANGZHOU UNIV
Receptor molecular targeting imaging agent and its preparation method and application
ActiveCN106632571BHas an estradiol skeletonGood chemical stabilityIsotope introduction to steroidsRadioactive preparation carriersBiological propertyPolyethylene glycol
Owner:XIAMEN UNIV
Deuterated compounds
InactiveUS20180305396A1Nervous disorderIsotope introduction to steroidsDiseaseCombinatorial chemistry
Owner:SWANSEA UNIV
A kind of preparation method of deuterium-labeled betamethasone
ActiveCN108440628BHigh purityHigh yieldIsotope introduction to steroidsSteroidsBetamethasoneMedicinal chemistry
The invention discloses a preparation method of deuterium-labeled betamethasone. The method is characterized in that betamethasone is used as an initiator and the deuterium-labeled betamethasone is synthesized through five-step reaction. According to the preparation method disclosed by the invention, optimal preparation steps and reaction conditions are screened out through a large number of experiments; the whole technology is reasonable in design and the operability is high. The deuterium-labeled betamethasone prepared by the preparation method disclosed by the invention has the characteristics that the purity can reach 98 percent or above, the yield is high, and isotope abundance is greater than 97 percent. The deuterium-labeled betamethasone prepared by the preparation method disclosedby the invention has the advantages that test and control samples can be provided for the study on a metabolic mechanism and an anti-inflammatory mechanism of the betamethasone; in addition, the deuterium-labeled betamethasone has important potential anti-inflammatory value and other application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
Synthetic method of alpha-monodeuterated alcohol compound and deuterated drug
InactiveCN112939732AHigh rate of deuteriumGood location selectivityIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationDeuterated drugAlcohol
The invention provides an alpha-monodeuterated alcohol compound and a reduction deuteration method of an aldehyde ketone compound for preparing the alpha-monodeuterated alcohol compound. The aldoketone compound shown in a general formula (1) reacts with a divalent lanthanide transition metal compound and a deuterium donor reagent in an organic solvent I to generate the alpha-monodeuterated alcohol compound shown in a general formula (2). The invention establishes a reduction deuteration method of an aldehyde ketone compound based on a single electron transfer reduction deuteration reaction, and the method is used for preparing the alpha-monodeuterated alcohol compound shown in a general formula (2) as well as deuterated derivatives of aldehyde ketone drugs, hormones and natural products of the alpha-deuterated alcohol compounds. The method has the advantages of high product deuteration rate, good regioselectivity, good chemical selectivity, low reagent price, simple operation, mild conditions and wide substrate application range. Compared with an existing H / D exchange method, the deuterium donor reagent is small in dosage, the cost can be remarkably reduced, and the utilization rate of deuterium atoms is increased.
Owner:CHINA AGRI UNIV
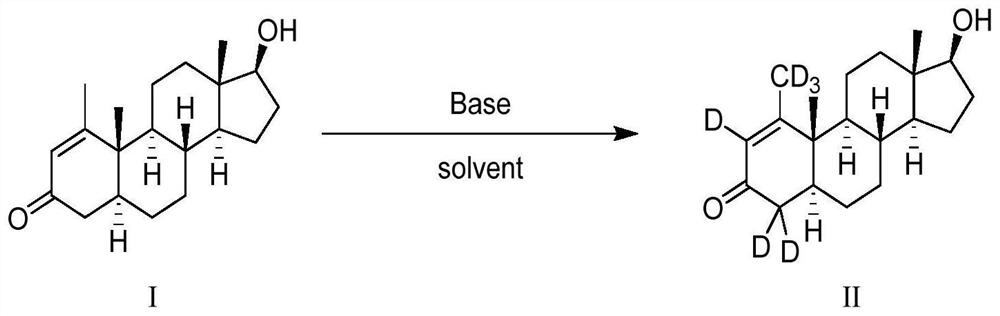
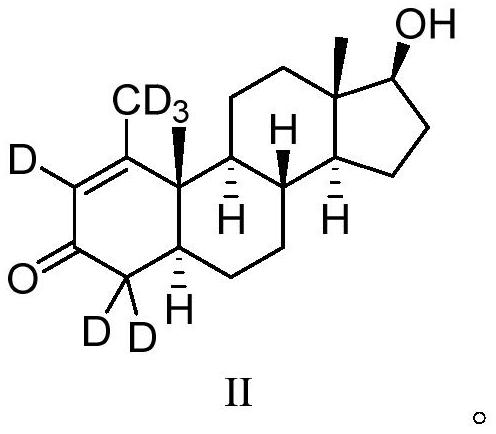
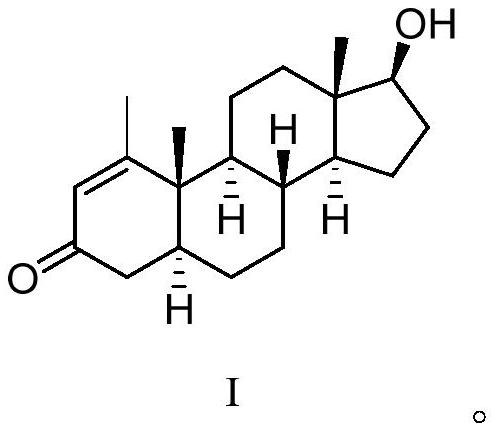
16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method
InactiveCN106946962AThe synthetic route is simpleReaction raw materials are cheap and easy to obtainIsotope introduction to steroidsSteroidsRefluxSynthesis methods
The present invention relates to a 16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method, which comprises: (A1) mixing 1,4-androstenedione and deuterated water in a first solvent, and carrying out heating reflux under the catalytic effect of a weak alkaline salt; (A2) extracting the reflux reaction liquid, and concentrating the extracted organic phase to obtain an intermediate product 16[alpha],16[beta]-di-deuterium-1,4-androstenedione; (A3) mixing the intermediate product and sodium borodeuteride in a second solvent, and carrying out selective reduction under an ice bath condition; (A4) acidifying the reduction reaction liquid to achieve the pH value of less than or equal to 2; and (A5) extracting the acidified reduction reaction liquid, and separating the organic phase by using a column chromatography method to obtain the target product 16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione. The synthesis method of the present invention has advantages of simple reaction route, high product purity, low cost, and the like.
Owner:SHANGHAI ANPEL SCI INSTR
18F-labeled precursor of PET radioactive medical supplies, and preparation method thereof
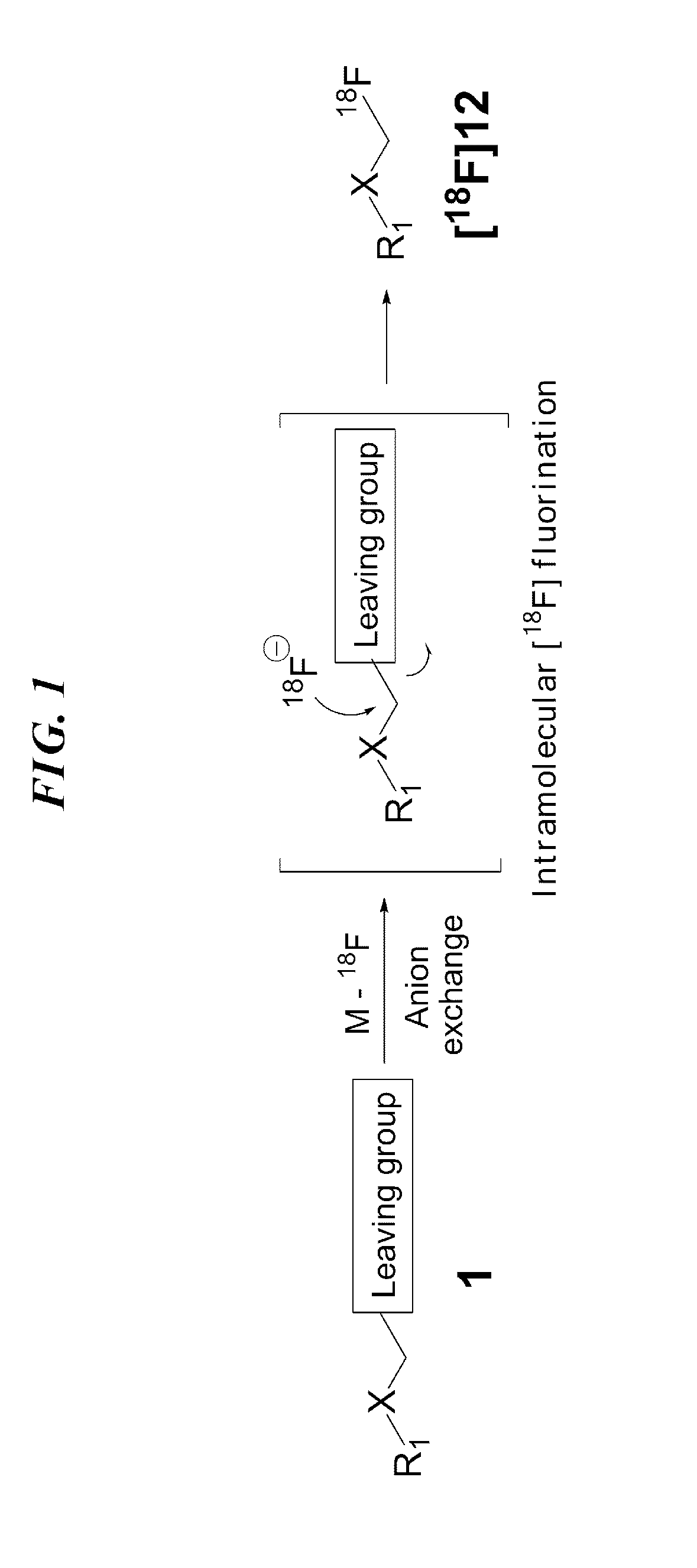
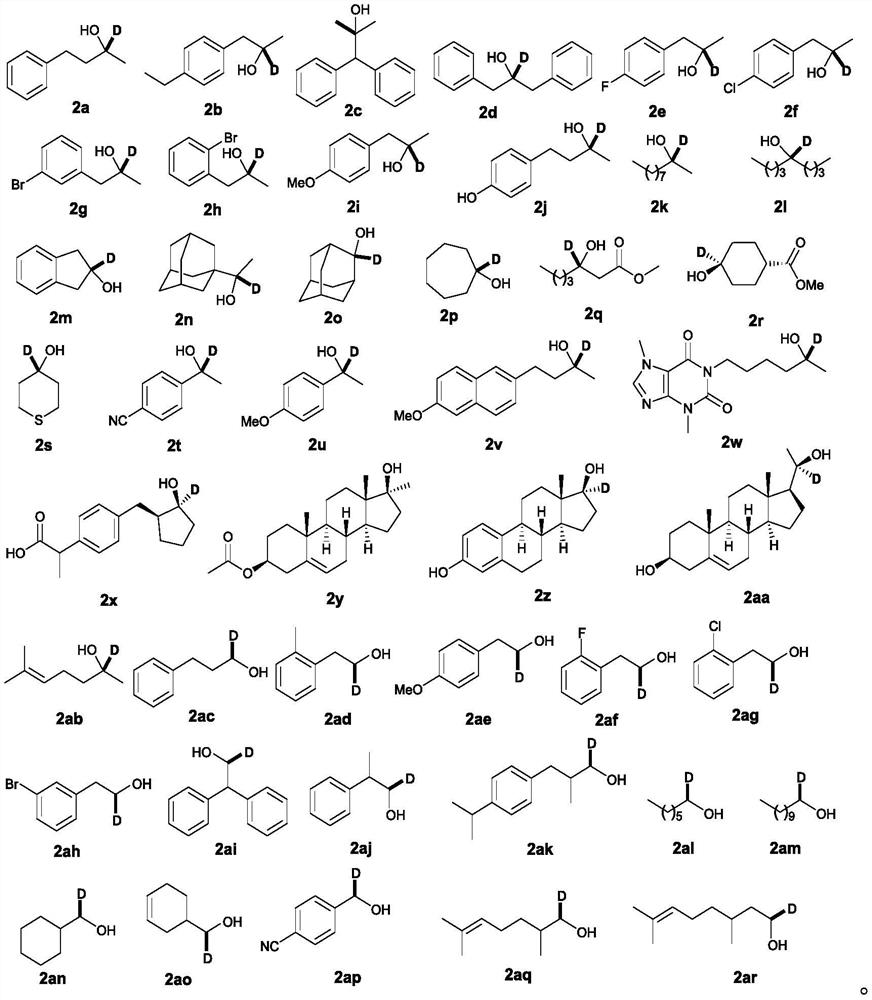
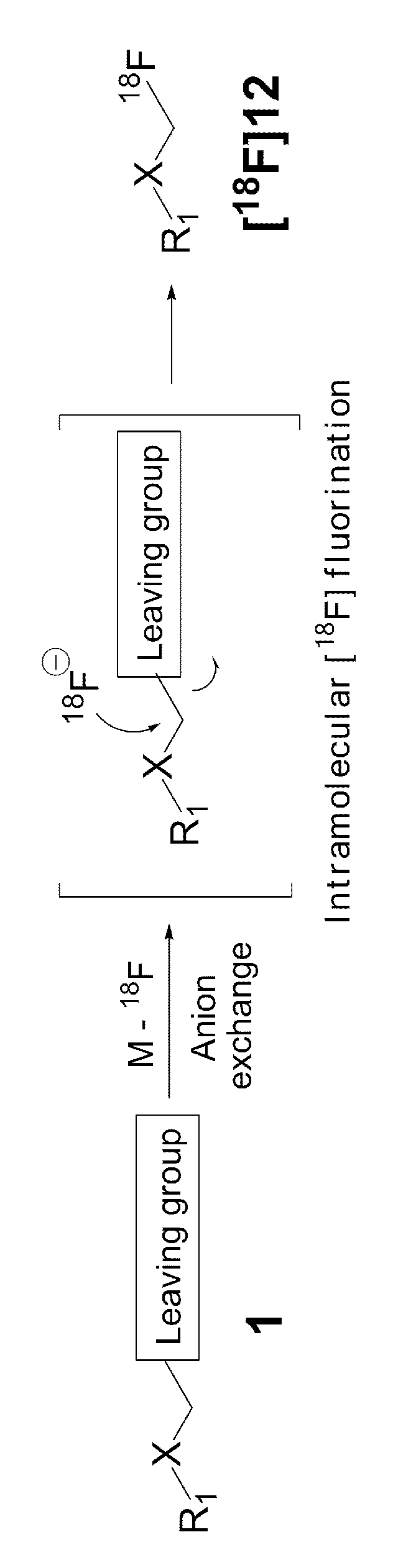
ActiveUS9505799B2Synthesis is complicatedLow production costOrganic compound preparationIsotope introduction to steroidsPtru catalystLeaving group
The present invention relates to a precursor of positron emission tomography (PET) radioactive medical supplies, a preparation method thereof, and an application thereof, and more specifically, to a precursor having a tetravalent organic salt leaving group, a preparation method, and a method for preparing desired PET radioactive medical supplies in a high radiochemical yield within a short preparation time by introducing 18F using the same through a single step. The precursor having a tetravalent organic salt leaving group of the present invention can simplify the known complex multistep preparation of radioactive medical supplies into a single step, can save production costs because an excessive amount of a phase transfer catalyst is not required, facilitates separation of a compound after reaction, and enables rapid reaction velocity. The features are appropriate for the mass production of PET radioactive medical supplies by an automated synthesis system.
Owner:FUTURECHEM
Method for hydrogenolysis of halides
PendingCN112142544AAchieve hydrogenolysisImprove compatibilityUrea derivatives preparationCarboxylic acid nitrile preparationHalogenCombinatorial chemistry
The invention discloses a method for hydrogenolysis of halides. The invention discloses a preparation method of a compound represented by a formula I. The preparation method comprises the following step: in a polar aprotic solvent, zinc, H2O and a compound represented by a formula II are subjected to a reaction as shown in the specification, wherein X is halogen; Y is -CHR<1>R<2> or R<3>; hydrogenin H2O exists in the form of natural abundance or non-natural abundance. According to the preparation method, halide hydrogenolysis can be simply, conveniently and efficiently achieved through a simple and mild reaction system, and good functional group compatibility and substrate universality are achieved.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
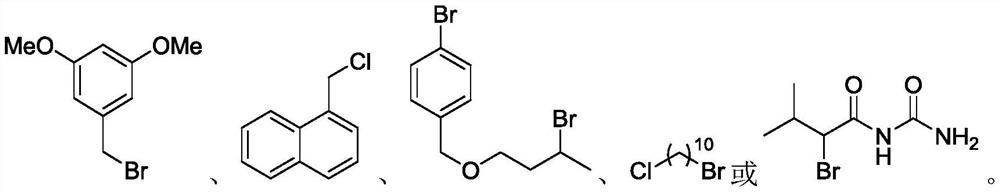
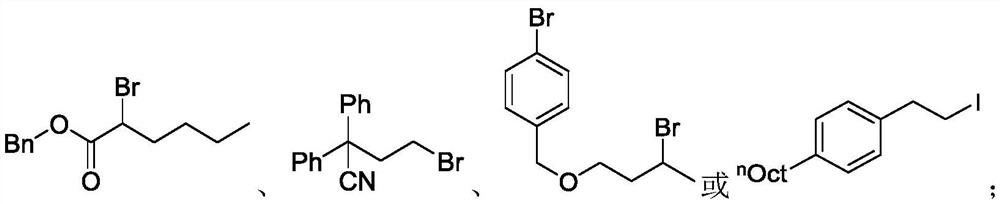
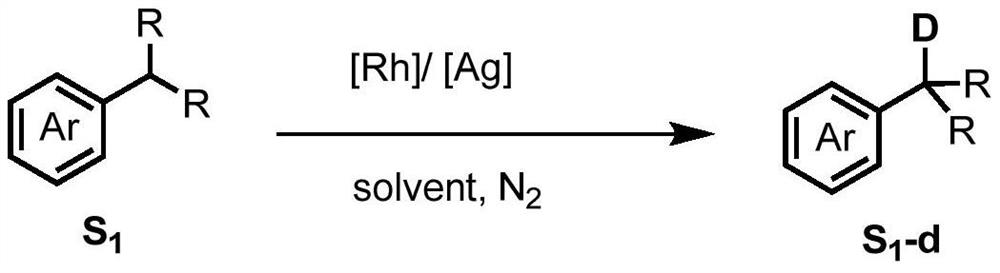
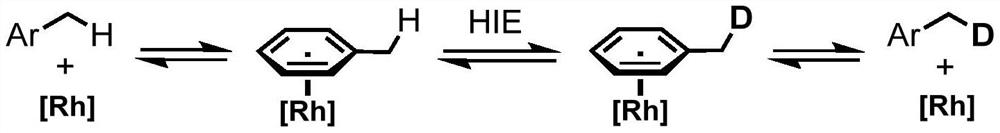
Method for selective deuteration of aromatic ring benzyl carbon-hydrogen bonds
ActiveCN113563147AEasy to handleSimple and fast operationGroup 5/15 element organic compoundsCarboxylic acid esters preparationRhodium MetallicumPtru catalyst
The invention discloses a method for selective deuteration of aromatic ring benzyl carbon-hydrogen bonds. According to the method, a metal rhodium catalyst is used for performing eta6 coordination activation on an aromatic ring, so that hydrogen-deuterium exchange can be selectively performed on the aromatic ring and a deuterated reagent at a benzyl position; strong acid or strong alkali does not need to be added, the cheap and easily available deuterated reagent is adopted as a deuterium source, so that the method has good universality for various aromatic hydrocarbons with different functional groups, can be applied to later selective deuteration of complex drug molecules, and has high application value.
Owner:WESTLAKE UNIV
Fluorination of organic compounds
ActiveUS10759764B2Impact patient careImproved diagnostic and prognostic criteriaIsotope introduction to steroidsRadioactive preparation carriersSimple Organic CompoundsChemical compound
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Alpha-monodeuterated amine compound, deuterated medicine and preparation method of same
ActiveCN112851520AHigh rate of deuteriumHigh regional selectivityIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationOrganic acidOrganosolv
The invention relates to an alpha-monodeuterated amine compound and a reduction deuteration method of an oxime compound for preparing the alpha-monodeuterated amine compound. The method is characterized in that an oxime compound as shown in a general formula (1) reacts with a divalent lanthanide transition metal compound and a deuterium donor reagent in an organic solvent I to generate the alpha-monodeuterated amine compound as shown in a general formula (2), optionally, the compound shown in the general formula (2) is added into an organic acid and / or inorganic acid-organic solvent solution to obtain the ammonium salt shown in the general formula (2). The invention overcomes the defects that the preparation method of the alpha-monodeuterated amine compound in the prior art needs to adopt an expensive and highly toxic transition metal catalyst or an expensive and flammable metal deuteride and generates toxic by-products.
Owner:CHINA AGRI UNIV
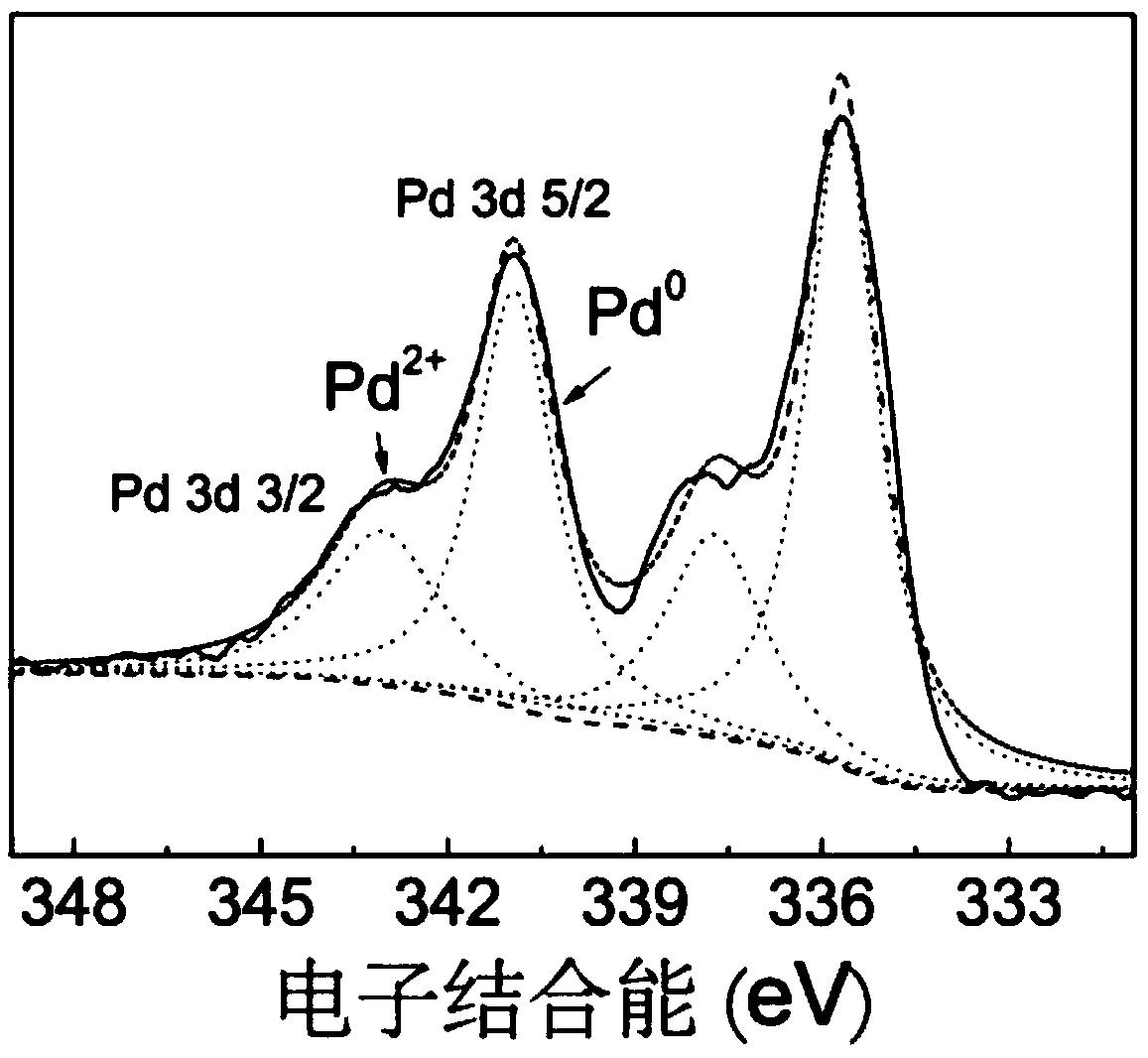
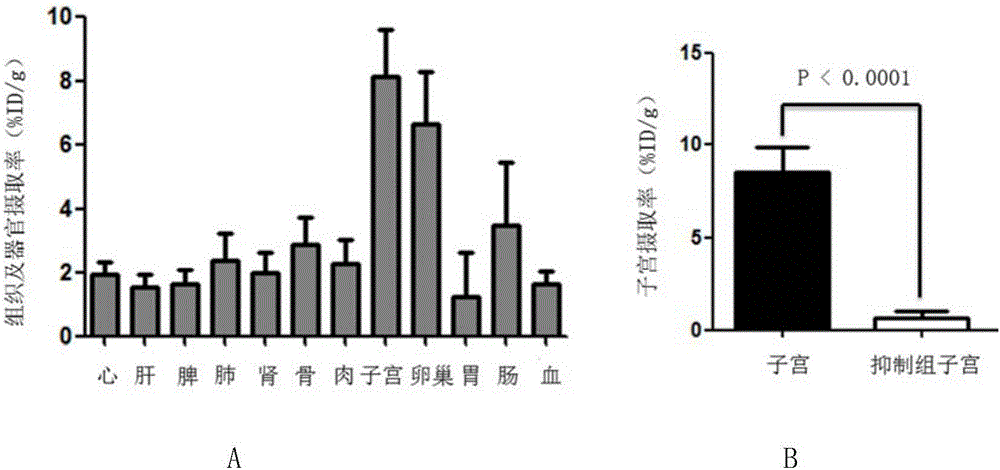
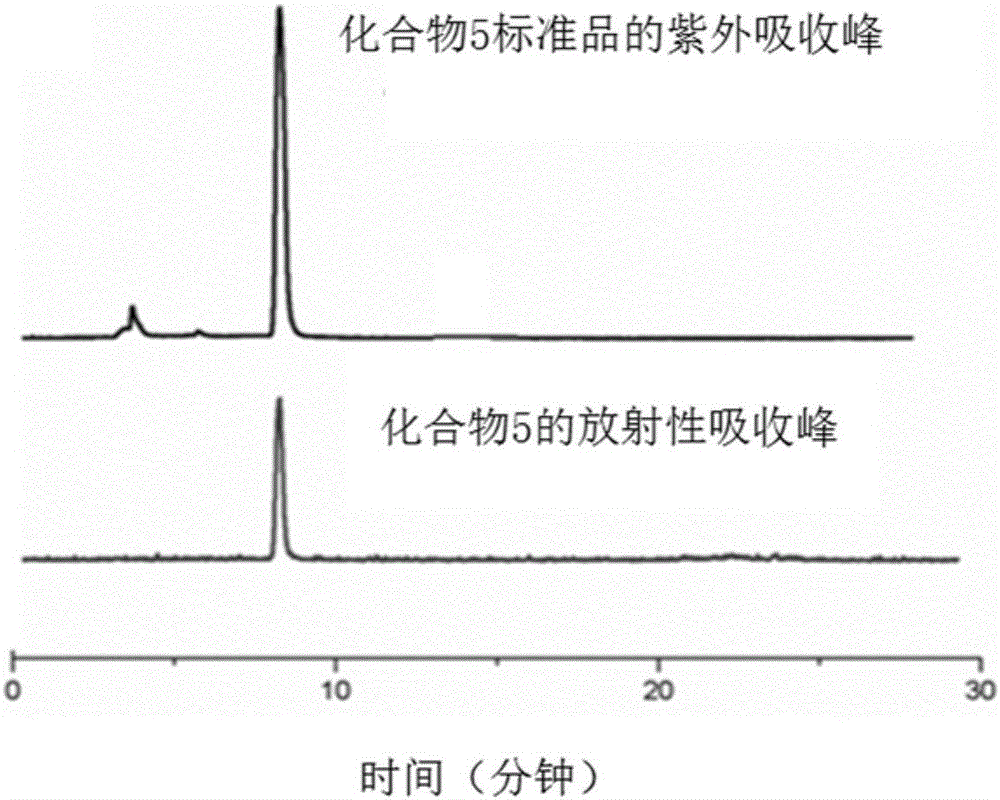
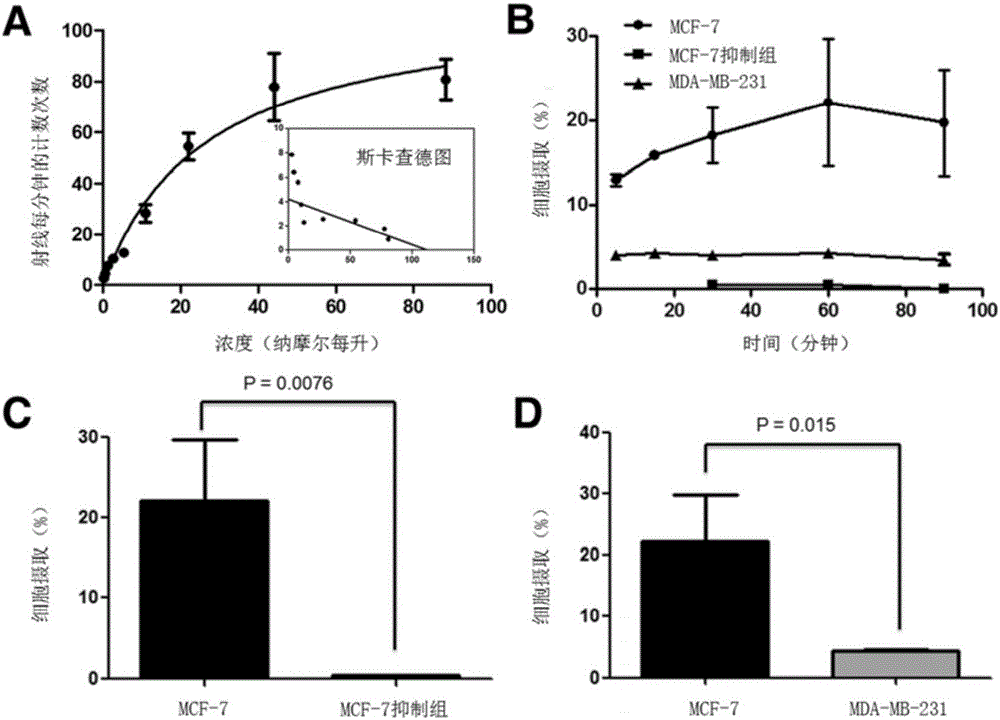
16α-[ 18 f] The automatic synthesis method of fluoro-17β-estradiol
ActiveCN108250260BReduce separation and purification stepsHigh degree of automationIsotope introduction to steroidsEstrane derivativesMeth-Fluid phase
The invention provides an automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol (18F-FES). The automatic synthesis method comprises the following steps: performing [18F] nucleophilic fluorination reaction of precursor 3-O-(methoxymethyl)-16,17-O-sulfonyl-16-estradiol (MMSE), hydrolysis reaction of a non-separated [18F] fluorinated intermediate and an alcohol-containing acidic solution as well as liquid-phase separation and purification through a substituted solid-phase column in the same reaction flask of an automatic synthesis module, thereby obtaining the product 18F-FES. Theautomatic synthesis method can realize automatic production of 16 alpha-[18F]fluoro-17 beta-estradiol by adopting a conventional FDG synthesis module and is short in total synthesis time and high in radiochemical yield.
Owner:广州市原子高科同位素医药有限公司
Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol
ActiveCN108250260AEfficient removalMeet the requirementsIsotope introduction to steroidsEstrane derivativesAlcoholSynthesis methods
The invention provides an automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol (18F-FES). The automatic synthesis method comprises the following steps: performing [18F] nucleophilic fluorination reaction of precursor 3-O-(methoxymethyl)-16,17-O-sulfonyl-16-estradiol (MMSE), hydrolysis reaction of a non-separated [18F] fluorinated intermediate and an alcohol-containing acidic solution as well as liquid-phase separation and purification through a substituted solid-phase column in the same reaction flask of an automatic synthesis module, thereby obtaining the product 18F-FES. Theautomatic synthesis method can realize automatic production of 16 alpha-[18F]fluoro-17 beta-estradiol by adopting a conventional FDG synthesis module and is short in total synthesis time and high in radiochemical yield.
Owner:广州市原子高科同位素医药有限公司
18f-labeled precursor of pet radioactive medical supplies, and preparation method thereof
ActiveUS20140194620A1Simplifying conventional complicated multi-step synthesisFaster in reaction velocityOrganic compound preparationIsotope introduction to steroidsPtru catalystLeaving group
The present invention relates to a precursor of positron emission tomography (PET) radioactive medical supplies, a preparation method thereof, and an application thereof, and more specifically, to a precursor having a tetravalent organic salt leaving group, a preparation method, and a method for preparing desired PET radioactive medical supplies in a high radiochemical yield within a short preparation time by introducing 18F using the same through a single step. The precursor having a tetravalent organic salt leaving group of the present invention can simplify the known complex multistep preparation of radioactive medical supplies into a single step, can save production costs because an excessive amount of a phase transfer catalyst is not required, facilitates separation of a compound after reaction, and enables rapid reaction velocity. The features are appropriate for the mass production of PET radioactive medical supplies by an automated synthesis system.
Owner:FUTURECHEM
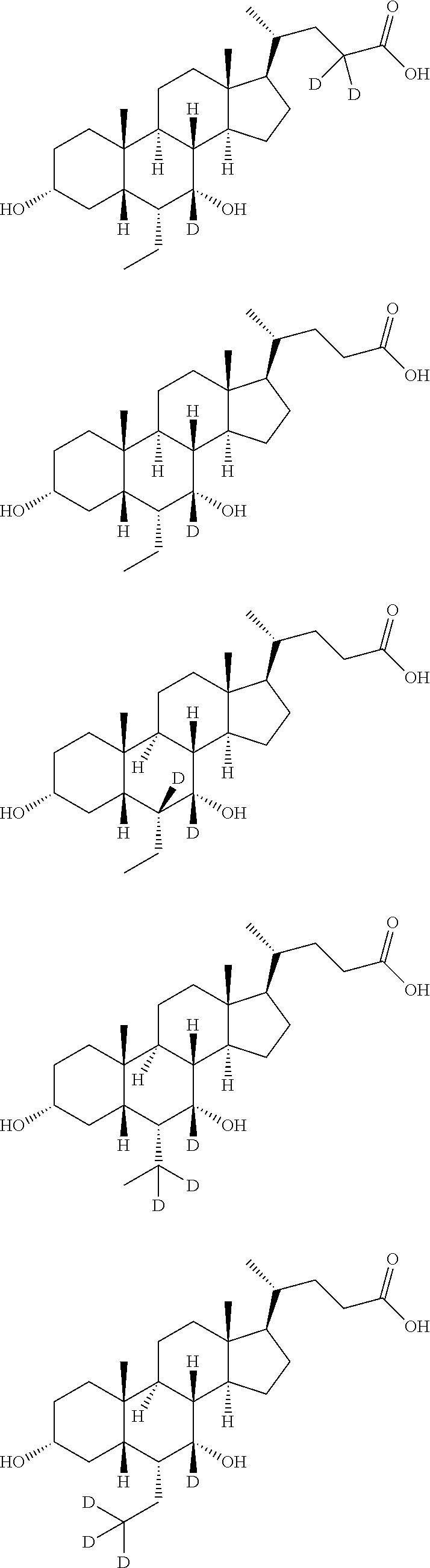
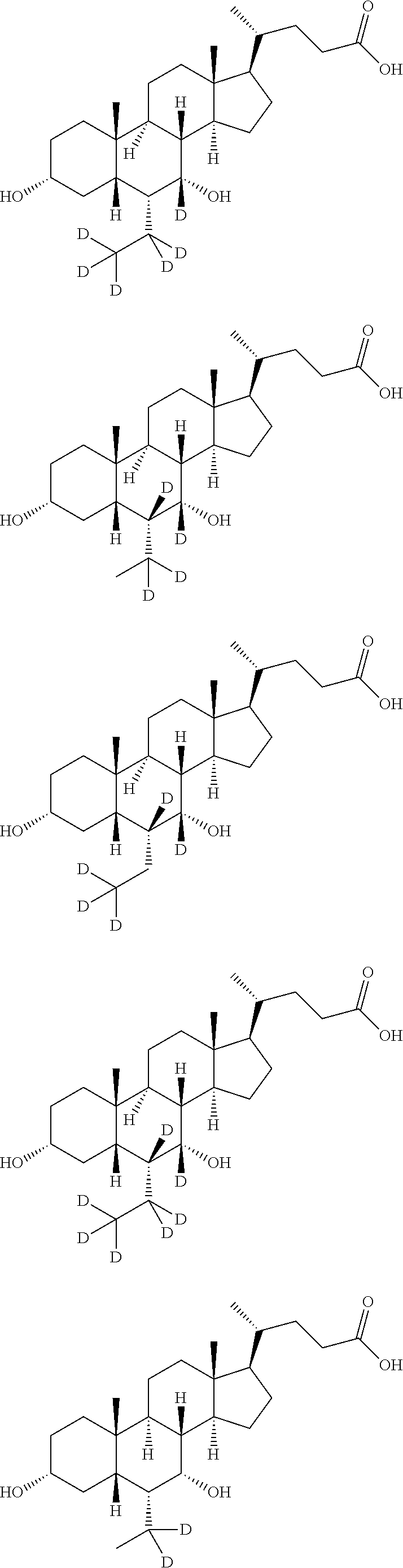
Deuterium-labeled metenolone stable isotope labeled compound
InactiveCN113061070AMeet physical requirementsHigh purityIsotope introduction to steroidsSteroidsMeth-Structural formula
The invention provides a deuterium-labeled metenolone stable isotope labeled compound. The structural formula of the deuterium-labeled metenolone stable isotope labeled compound is shown as a formula II, and the deuterium-labeled metenolone stable isotope labeled compound is obtained by taking a compound I as a raw material and carrying out H-D exchange reaction in a deuterium-labeled solvent, namely a deuterium source, in the presence of alkali at a proper temperature. According to the deuterium-labeled metenolone stable isotope labeled compound disclosed by the invention, deuterium replacement is carried out on 1-position methyl-H, 2-position olefinic bond H at carbonyl alpha position and 4-position methylene H in a natural abundance metenolone structure, the deuterium-labeled metenolone stable isotope labeled compound is 6 mass numbers different from that of metenolone, and the mass spectrum response data is obviously different, the physical property requirements of the internal standard substance required by isotope dilution mass spectrometry are completely met, the preparation method is reasonable in process design, controllable in experimental process and simple and convenient to operate, the prepared target product is high in purity, the yield reaches about 90.0%, the isotope abundance of the final product reaches about 93.5%-95.0%, the reproducibility is good, and a standard sample can be provided for the food safety detection industry.
Owner:阿尔塔(天津)标准物质研究院有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method 16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/56bfe5aa-b756-4afa-8e79-601080f4c61c/HDA0001247959370000011.png)
![16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method 16[alpha],16[beta],17[beta]-tris-deuterium-1,4-androstenedione synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/56bfe5aa-b756-4afa-8e79-601080f4c61c/HDA0001247959370000012.png)
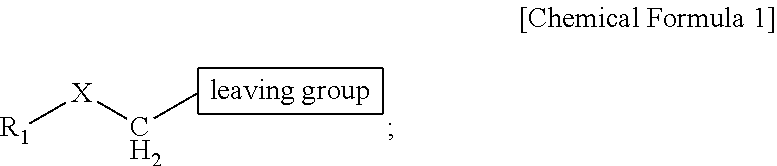




![16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol 16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ed9ab28-135f-4d3b-94b9-84f72c92379b/1802281701581.png)
![16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol 16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ed9ab28-135f-4d3b-94b9-84f72c92379b/1802281701582.png)
![16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol 16α-[ <sup>18</sup> f] The automatic synthesis method of fluoro-17β-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ed9ab28-135f-4d3b-94b9-84f72c92379b/1802281701583.png)
![Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acd6152b-3676-4cab-9e3a-e63f836fd41a/1802281701581.png)
![Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acd6152b-3676-4cab-9e3a-e63f836fd41a/1802281701582.png)
![Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol Automatic synthesis method of 16 alpha-[18F]fluoro-17 beta-estradiol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acd6152b-3676-4cab-9e3a-e63f836fd41a/1802281701583.png)