New photocatalytic fixed-point deuteration method for carbon-carbon unsaturated bonds
A new method, photocatalytic technology, applied in the direction of organic chemical methods, chemical instruments and methods, carbon-based compound preparation, etc., can solve the problem of low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
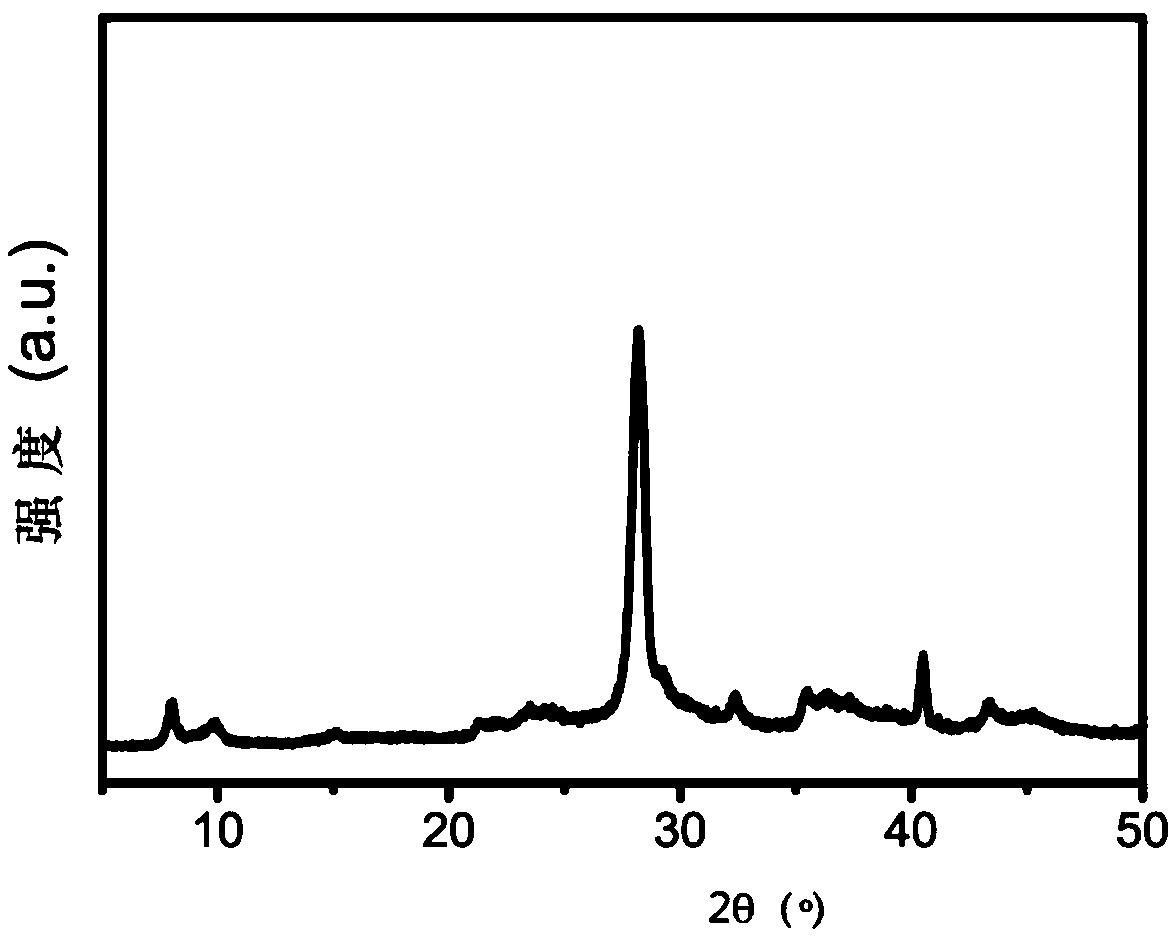
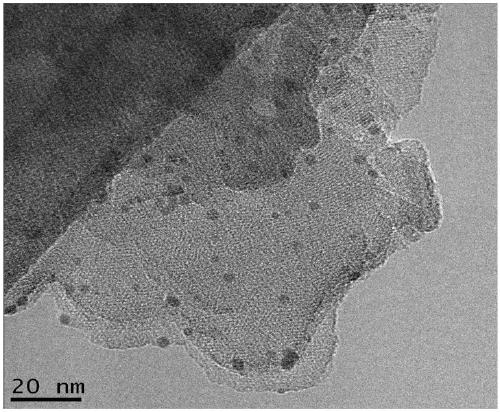
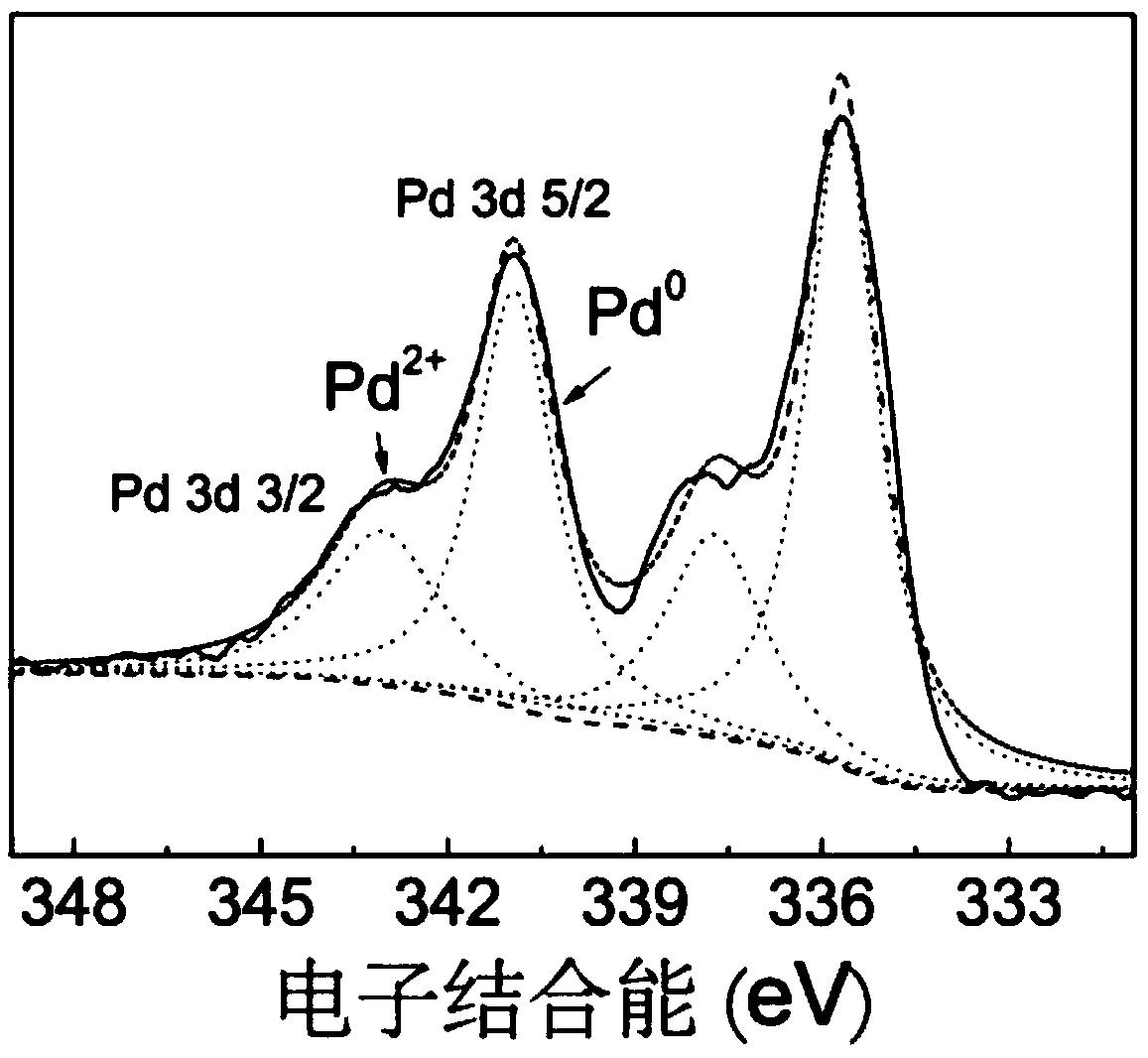
[0049] (1) Preparation of photocatalytic materials
[0050] The photocatalytic material is taken as an example of palladium (Pd)-loaded carbon nitride (PCN), and its preparation method is as follows:
[0051] Mix 2 g of melamine and 20 g of potassium bromide evenly and grind for 5 minutes, transfer to an alumina ceramic crucible, place it in a tube furnace, set the roasting program, and heat up to 550°C for 2 hours. Naturally cooled to room temperature, the obtained yellow solid was dispersed in 200 mL of 80°C hot water, stirred for 30 minutes, filtered while hot, and washed three times with deionized water. The obtained carbon nitride solid was dried in an oven at 80 degrees for 24 hours for later use.
[0052] Take 0.3 g of the above-mentioned carbon nitride solid, disperse it in 80 mL of deionized water, add 20 mL of ethylene glycol solution, stir and sonicate for 2 hours. Add 28 μL, 1 mol / L chloropalladium acid solution to the obtained mixed liquid with a pipette, and re...
Embodiment 1
[0055] Embodiment 1: the photocatalytic hydrogenation reaction of styrene
[0056]
[0057] Weigh 0.1mmol styrene, 0.1mmol AlCl respectively 3 , 10.0mg of Pd / PCN photocatalyst was added to a 5mL reaction flask, and a mixed solution of ethyl acetate / water / methanol (2mL / 1.5mL / 1.5mL) was added to replace the reaction system into an argon protection state, and then the reaction The bottle was placed under a 420nm light source for light reaction for 4 hours. After the reaction was completed, the light source was removed, and the reaction mixture was washed with 5.0mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. GC-MS analysis was performed and the yield of the reaction was determined in conjunction with a standard curve of the target product.
[0058] According to the same synthesis method, other compounds can be catalytically hydrogenated to obtain corresponding hydrogenated products, see Table...
Embodiment 14
[0063] Example 14: Preparation of deuterated chemicals by photocatalytic deuterium addition of (E)-1,2-stilbene with deuterium water as deuterium source
[0064]
[0065] Weigh 0.1mmol (E)-1,2-stilbene, 0.1mmol AlCl 3 , 10.0mg of Pd / PCN nanosheets were added to a 5mL reaction bottle, and a mixed solution of ethyl acetate / deuterium water / deuterated methanol (2mL / 1.5mL / 1.5mL) was added with a syringe to replace the reaction system into an argon protection state , and then the reaction bottle was placed under a 420nm light source for 4.0 hours of light reaction. After the reaction, the light source was removed, and the reaction mixture was washed with CH 2 Cl 2 (5.0mL×3) extraction, combined organic phases, dried with anhydrous sodium sulfate, vacuum desolvated, column chromatography separation to obtain the target product. The resulting product was analyzed by GC-MS, HRMS, 1 HNMR, C-NMR, FT-IR and other tests to confirm the structure.
[0066] According to the same synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com