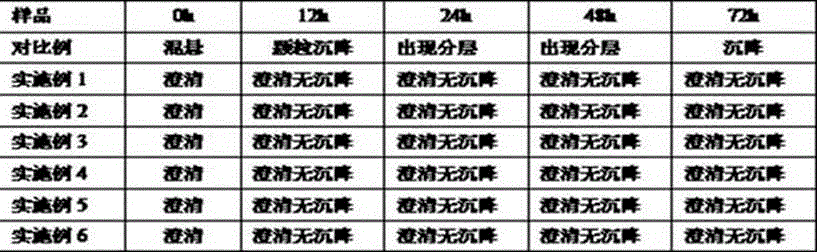
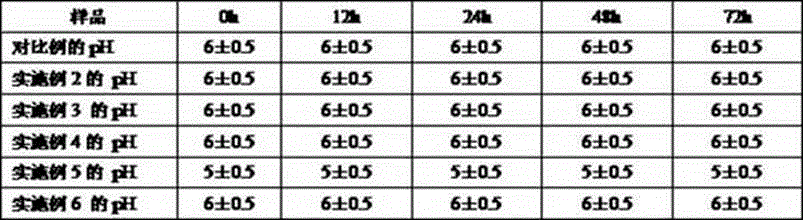
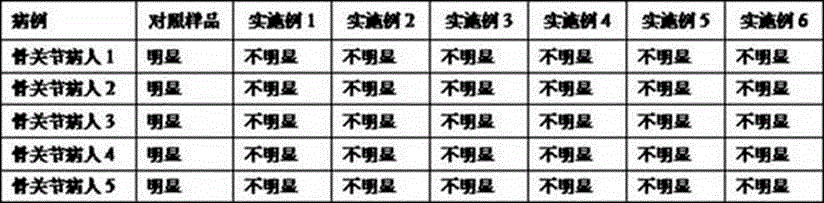
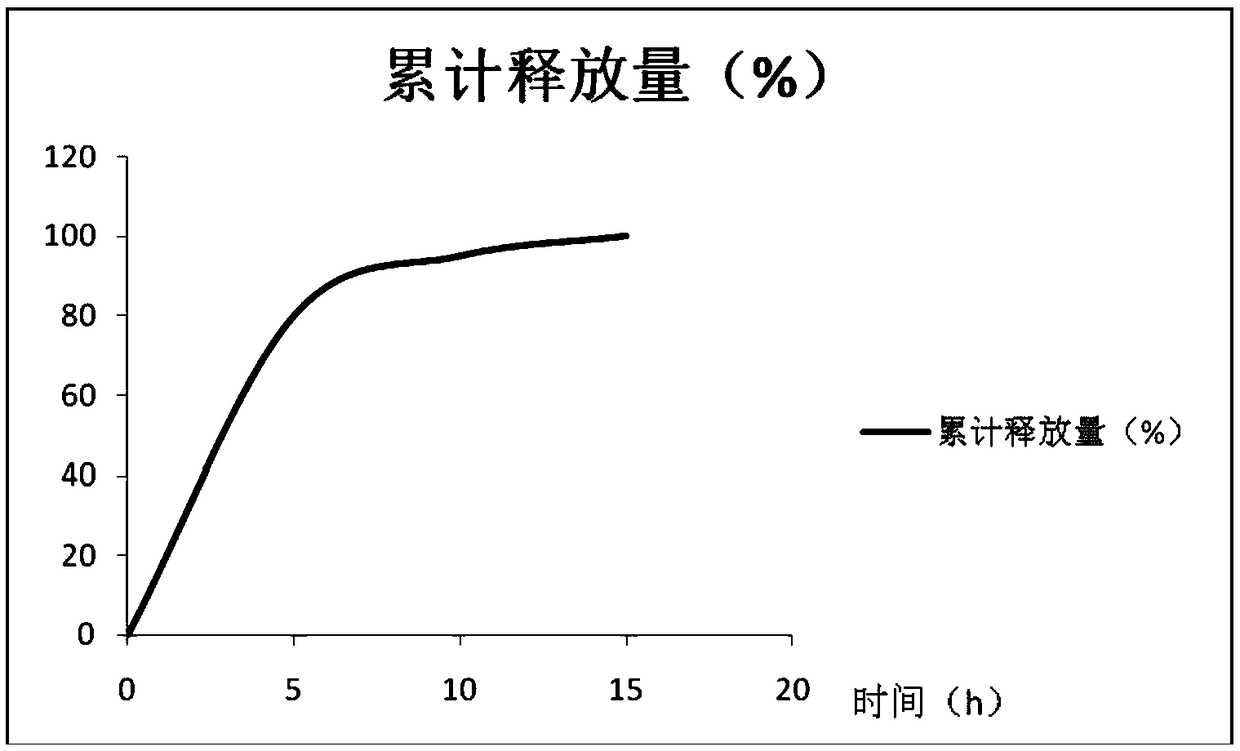
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Triamcinolone diacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triamcinolone diacetate is a synthetic glucocorticoid corticosteroid and a corticosteroid ester.
Method for determining contents of miconazole nitrate and triamcinolone acetonide in Qumicin cream
InactiveCN1614410AImprove quality controllabilityEasy to separateComponent separationPreparing sample for investigationMicronazoleNitrate
A method for determining content of triamcinolone acetonide acetate and micronazole nitrate in qumixing emulsifiable paste utilizes high efficiency liquid plase external standard method to determine simultaneously out content of triamcinolone acetonide acetate and miconazole nitrate in qumixing emulsifiable paste.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Process for synthesizing triamcinolone acetonide acetate
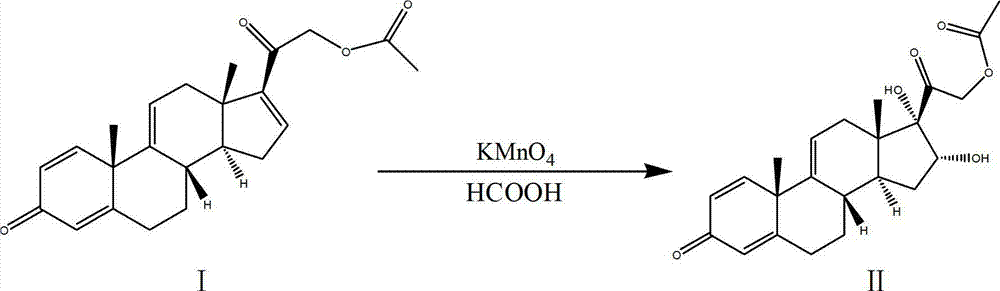
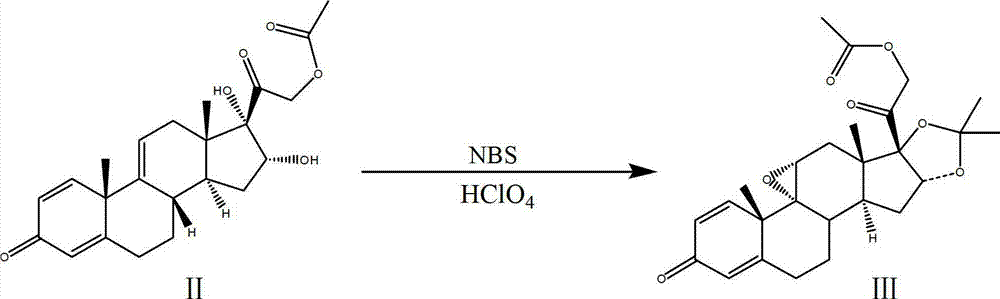
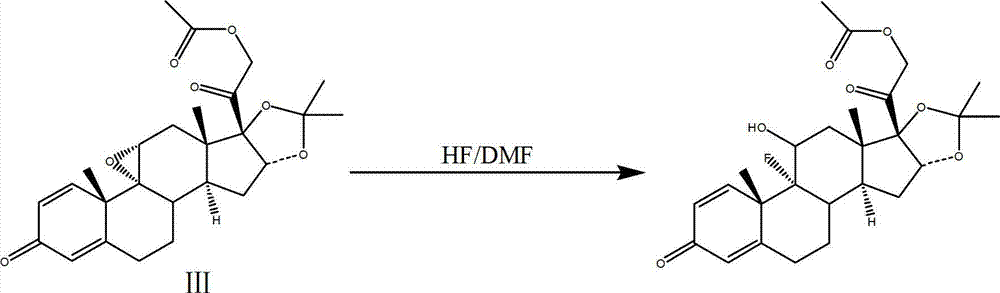
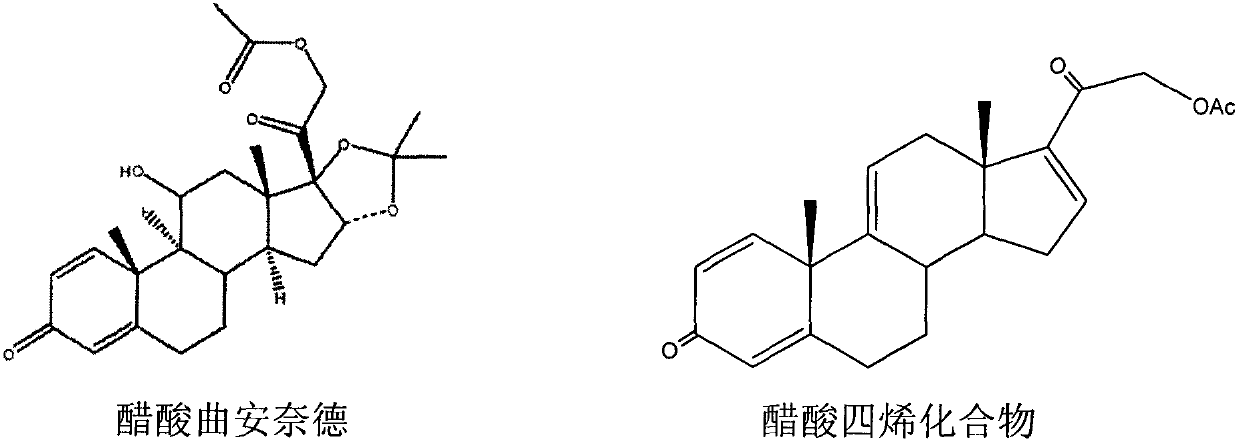
The invention discloses a process for synthesizing triamcinolone acetonide acetate. The process comprises the steps of firstly, taking an acetic acid tetraene material as a raw material, and obtaining an oxide through an oxidation reaction under the effect of methanoic acid and potassium permanganate; secondly, taking the oxide as a raw material, and obtaining a ring-reducing material through a ring-reducing reaction under the effect of perchloric acid and N-bromosuccinimide; thirdly, taking the ring-reducing material as a raw material, and conducting a fluorination reaction under the effect of fluorine hydride and dimethylformamide to obtain a triamcinolone acetonide acetate crude product; and fourthly, purifying the triamcinolone acetonide acetate crude product to obtain the triamcinolone acetonide acetate. The process is mild in reaction condition, easy to control, low in toxicity and dangerousness of used auxiliary materials, low in pollution and applicable to industrial production, the triamcinolone acetonide acetate which is synthesized by the process is subjected to high performance liquid chromatography (HPLC) detection, the purity can exceed 99%, and the yield is high.
Owner:BAOJI KANGLE BIOTECH
Triamcinolone acetonide acetate sustained release microsphere and preparation method thereof
ActiveCN110237052ALarge particle sizeRound shapeOrganic active ingredientsAntipyreticPoor complianceEmulsion
The invention relates to a triamcinolone acetonide acetate sustained release microsphere and a preparation method thereof. The method comprises the following steps that triamcinolone acetonide acetate and a degradable polymer with a hydrophobic chain segment are dissolved in an organic solvent, and a polymer solution with drugs is obtained; under the stirring condition, the polymer solution with drugs is added to a water phase to form an O / W emulsion, and the organic solvent in the emulsion is removed; the water phase contains a stabilizer, and the proportion of the stabilizer to the water phase is 0.1-5% (w / v); under the stirring condition, the obtained dispersion system is added into water for solidification, and the triamcinolone acetonide acetate sustained release microsphere is obtained. The triamcinolone acetonide acetate sustained release microsphere is proper in particle diameter, round in shape, high in encapsulation efficiency and good in drug loading capacity, and the problems of short release time of a preparation and poor compliance of a patient can be effectively solved.
Owner:JIANG SU PHARMAMAXCORP
Medical product containing solution-type triamcinolone acetonide acetate
ActiveCN104971039ASimple production processSmall local irritationOrganic active ingredientsAerosol deliveryIrritationMedicine
The invention provides a medical product containing solution-type triamcinolone acetonide acetate. The medical product contains triamcinolone acetonide acetate, sulfobutyl ether-beta-cyclodextrin and sodium hyaluronate. The medical product containing solution-type triamcinolone acetonide acetate is small in local irritation in clinic use, especially when the medical product is applied to intra-articular injection, the product is easy to absorb, the particle deposition on the surface of the periosteum existing in the suspension type triamcinolone acetonide acetate medical product is avoided, and the periosteum injury caused by particle deposition is reduced.
Owner:上海正大通用药业股份有限公司
Method for measuring content of triamcinolone acetonide chloromycetin solution
InactiveCN101196495AQuantitative analysis methods are sensitiveQuantitative analysis method is fastComponent separationTesting medicinal preparationsAcetic acidBULK ACTIVE INGREDIENT
The invention provides a method for measuring the high efficiency liquid chromatography by the content of triamcinolone acetonide chloramphenicol solution, which can simultaneously measuring the content of three active ingredients comprising acetic acid triamcinolone acetonide, chloramphenicol and naphazoline hydrochloride. The method is characterized in high accuracy, good specificity, high sensitivity, easy operation and low cost. Compared with the prior art, the content measuring method provided in the invention not only increases the method for controlling the content of the naphazoline hydrochloridem but also brings a better effect of each component separation and more sensitive and accurate measuring which can avoid the complicated and time-consuming operation, less specificity and no content measuring of the naphazoline hydrochloride prescribed in the active standard, and guarantees the advancement and accuracy of the quality standard, thus further effectively ensure the safe and effective use of the medicine for the people.
Owner:安军永
Compound terbinafine emulsifiable paste and preparation method thereof
InactiveCN101181275AStrong medicineQuick effectOrganic active ingredientsAntimycoticsRegimenSkin fungal infection
The invention discloses a terbinafine compound preparation for treating eczema, dermatitis, body ringworm, tinea cruris, tinea manus and pedis and the preparation method; the invention mainly uses terbinafine hydrochloride and clobetasol propionate or triamcinolone acetonide acetate, which is further matched with O / W emulsion matrix for the preparation of cream. The invention is characterized by fast onset of action, short treatment course and low recurrence rate for treating skin fungal infection with local inflammatory or chronic eczema.
Owner:林华清
Triamcinolone acetonaide acetate nano controlled-release formulation, preparation method thereof and artificial lens containing same
InactiveCN102327212ATurbidity preventionImprove bioavailabilityOrganic active ingredientsAntipyreticPolyvinyl alcoholLate complication
The invention relates to a triamcinolone acetonaide acetate nano controlled-release formulation, a preparation method thereof and an artificial lens containing the same for preventing inflammation and late complication after cataract operation, wherein the triamcinolone acetonaide acetate nano controlled-release formulation is prepared from the triamcinolone acetonaide acetate-chloroform solution with the triamcinolone acetonaide acetate concentration of 1%-3%, the lactic acid-glycolic acid copolymer (PLGA)-dichloromethane solution with the lactic acid-glycolic acid copolymer (PLGA) concentration of 2%-5%, and the polyvinyl alcohol (PVA) with the concentration of 0.5%-1.5%. The invention provides the preparation method of the triamcinolone acetonaide acetate nano controlled-release formulation which can effectively prevent posterior capsular opacity, has simple preparation process, and is convenient to use and the artificial lens containing the triamcinolone acetonaide acetate nano controlled-release formulation.
Owner:严宏 +1
Compound triamcinolone acetonide film coating agent and preparation method thereof
InactiveCN105030733AHas antibacterial propertiesEnhance immune functionOrganic active ingredientsAntisepticsGram-Positive CocciNeurodermatitis
The invention discloses a compound triamcinolone acetonide film coating agent and a preparation method thereof. The compound triamcinolone acetonide film coating agent is suitable for various skin diseases such as neurodermatitis, eczema and psoriasis. Triamcinolone acetonide acetate has effects of diminishing inflammation and resisting allergy. Zinc glutamine has effects of convergence and antibiosis and can participate in synthesis of a plurality of enzymes in the body, improve immunity and promote growth and development. The film coating agent prepared from triamcinolone acetonide acetate and zinc glutamine as main raw materials has effects of resisting gram positive cocci and gram negative bacilli. The compound triamcinolone acetonide film coating agent prepared from chitosan has no oily feel, can be spread easily, has strong adhesion and a certain tearing strength, is convenient for use, and has no irritation on skin. The preparation method has simple processes, realizes stable quality, utilizes high performance liquid chromatography to determine triamcinolone acetonide content, prevents accessory material-caused interference on determination, has simple processes, can produce a reliable result and is suitable for preparation quality control.
Owner:CHENGDU YICHUANGSI BIOLOGICAL SCI & TECH
Composition of bifonazole and its use
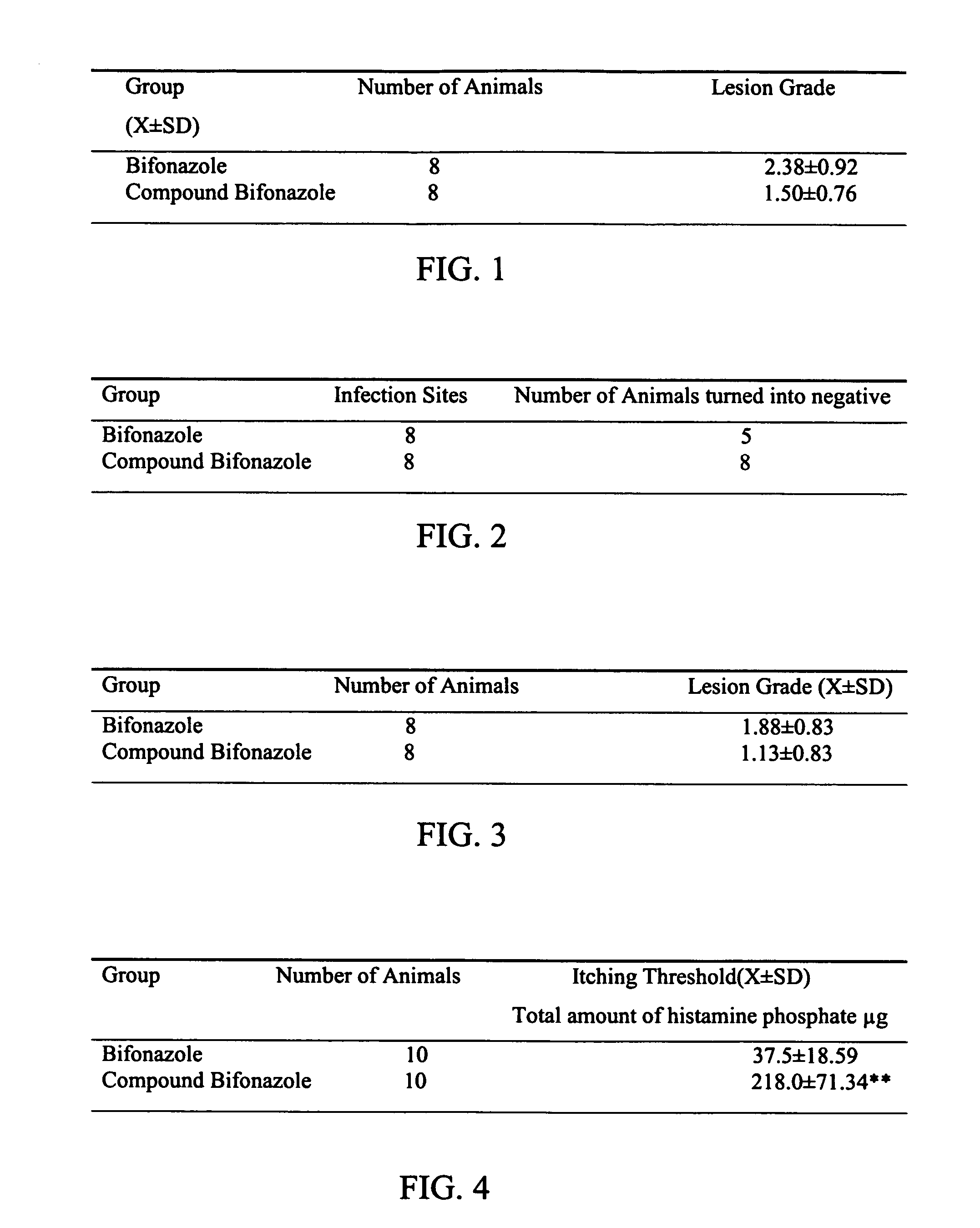
A medical composition includes Bifonazole and Triamcinolone acetonide acetate, and is mixed with pharmaceutical acceptable accessory. The medical composition of the present invention is used in treatment of skin superficial fungal infections, and also has the dual role of anti-inflammatory, anti-allergy and anti-itching functions.
Owner:FIBONACCI BIOLOGICAL MEDICINE
Triamcinolone acetonide acetate particle, and preparation method and medicinal composition thereof
ActiveCN101618019AEffective control of crystal morphologyControl crystal morphologyPowder deliveryOrganic active ingredientsFree coolingMicroparticle
The invention discloses a triamcinolone acetonide acetate particle, and a preparation method and a medicinal composition thereof. The preparation method comprises the following steps: 1) heating and dissolving triamcinolone acetonide acetate with aqueous solution of ethanol, naturally cooling the obtained solution to obtain acicular crystals, washing away the ethanol from the crystals with water for injection, drying the washed crystals, and then adding the crystals into water for injection at a temperature of 0 to 10 DEG C; 2) linearly adding dropwise triamcinolone acetonide acetate solution dissolved with dimethyl formamide solution into the mixed solution of the water for injection at the temperature of 0 to 10 DEG C obtained in the step 1), simultaneously stirring the mixed solution, keeping the temperature of the mixed solution at 0 to 15 DEG C, and continuing stirring the mixed solution for 1 to 30 minutes to obtain a mixture of water and crystals; and 3) as usual, filtering the mixture of the water and the crystals obtained in the step 2) and vacuum-drying the obtained crystals. The invention adopts a crystal inoculation process, thus crystals of the prepared triamcinolone acetonide acetate particles have regular shapes and uniform particle sizes; crystallization is free from jet milling and the physicochemical property of the particle is stable; and no crystal expansion phenomenon generates in the prepared triamcinolone acetonide acetate suspension injection during storage.
Owner:上海正大通用药业股份有限公司
Triamcinolone acetonide acetate preparation method
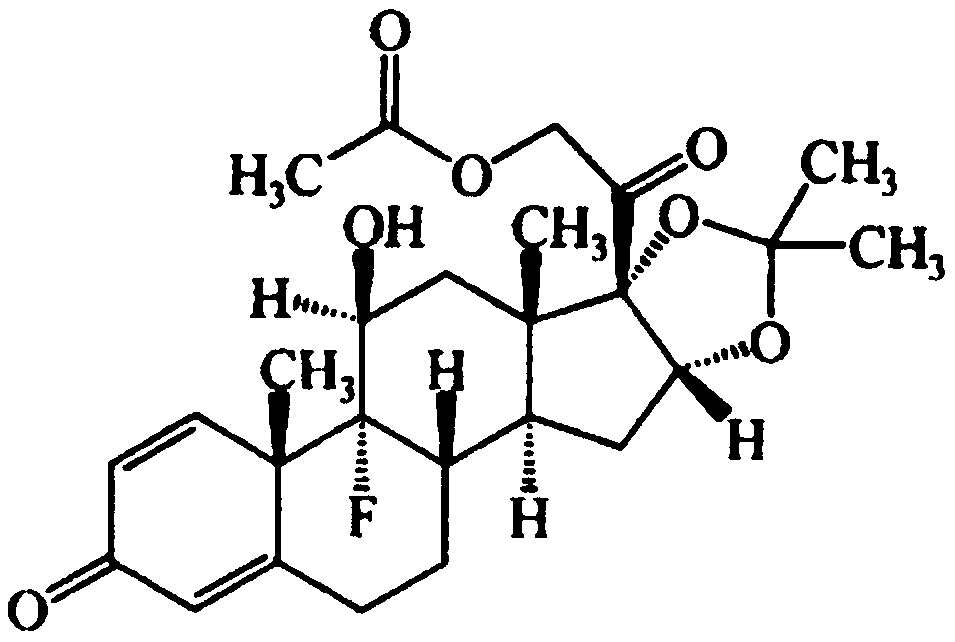
InactiveCN107778345ASimple reaction conditionsHigh yieldSteroidsBulk chemical productionDouble bondProtecting group
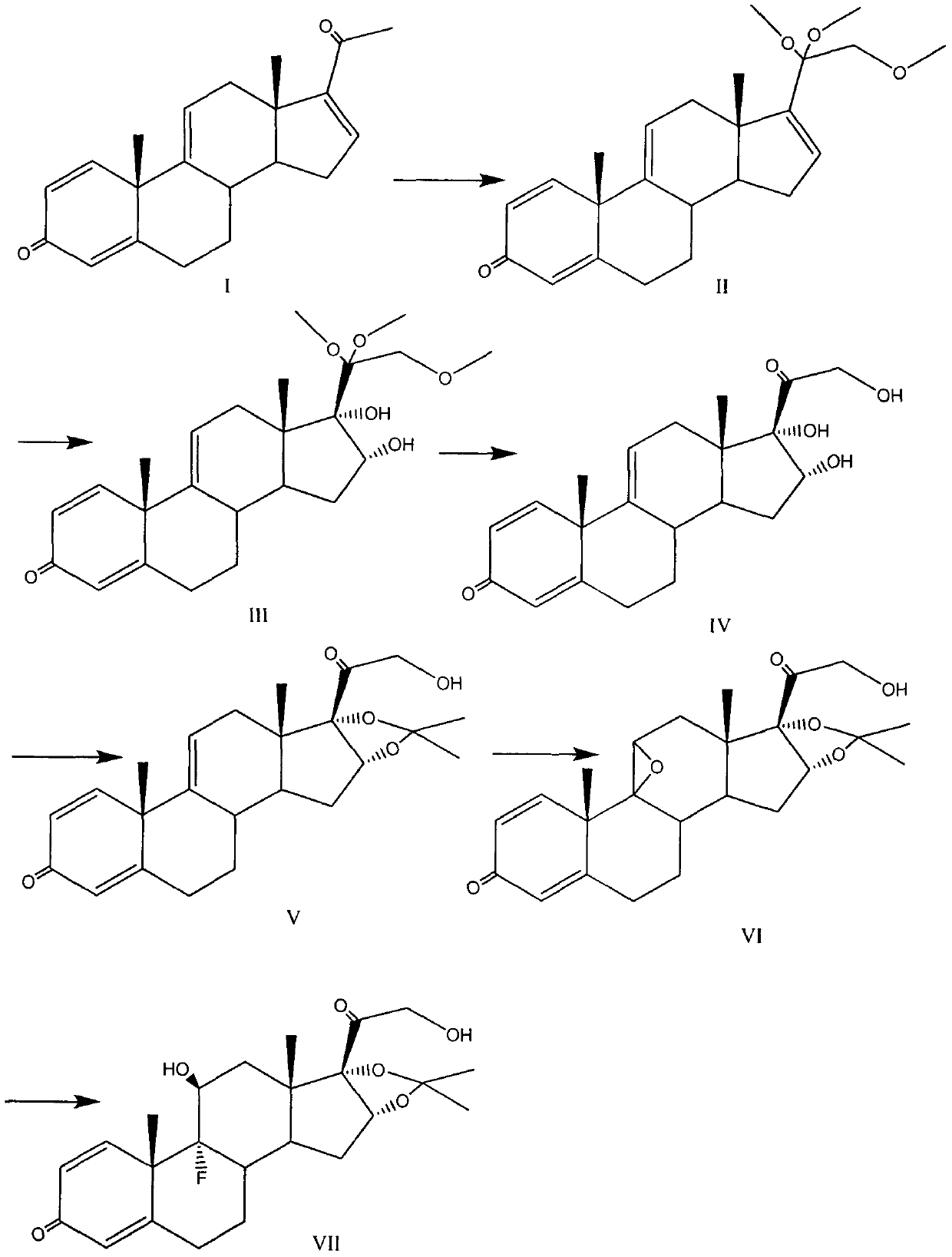
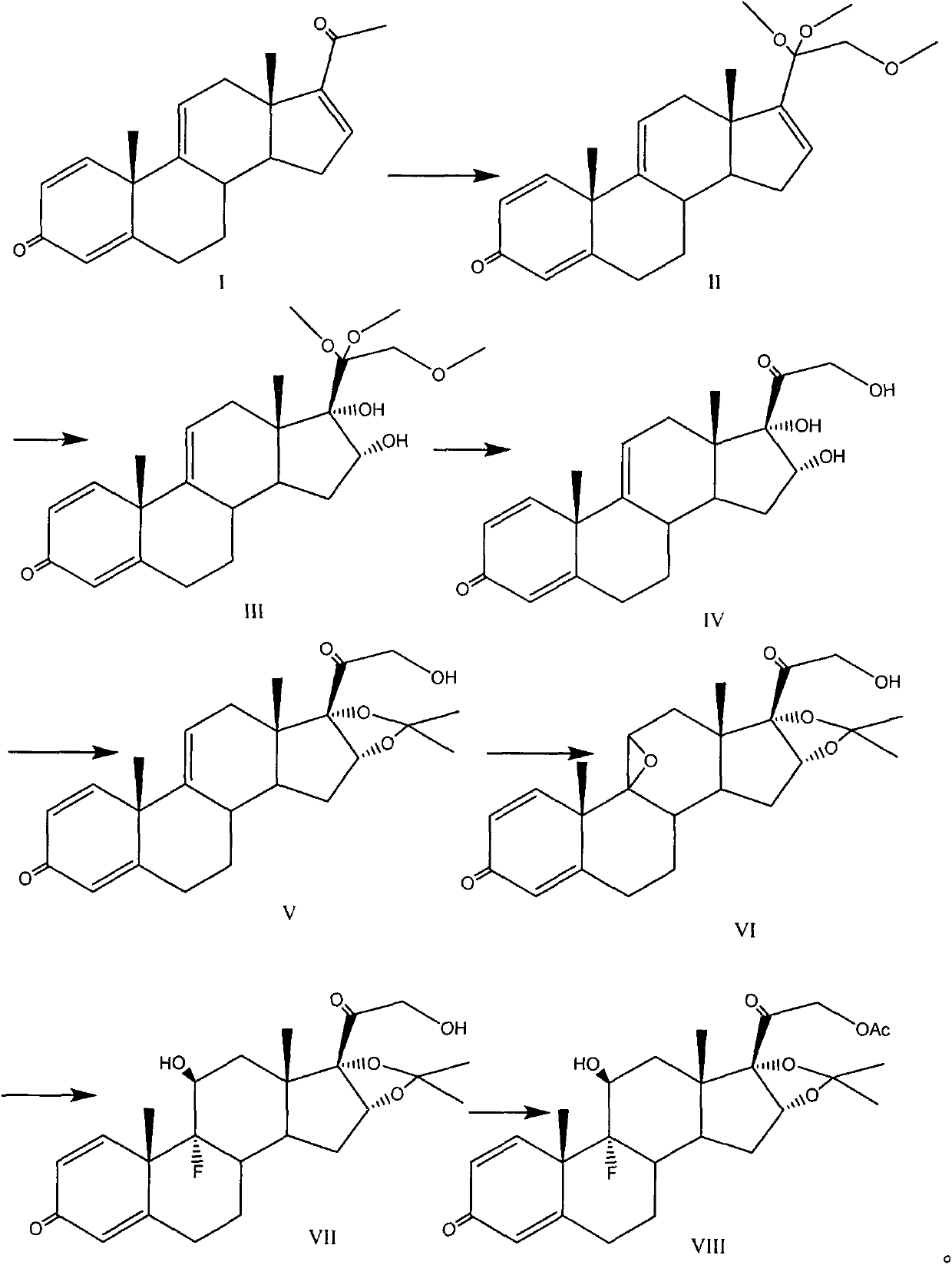
The invention provides a full-novel synthesizing route for preparing triamcinolone acetonide acetate. According to the full-novel synthetic route, utilized raw materials have lower cost and more easiness in obtaining. The synthetic route comprises the steps of hydroxylating reaction raw materials and protecting, then selectively oxidizing five-membered ring double bonds, removing a protecting group, then protecting oxidized di-hydroxyl, then epoxidizing six-membered ring double bonds, performing open-ring fluorination and esterfying hydroxyl to obtain a triamcinolone acetonide product. A reaction process has easiness in operation, yields of all the steps are higher, obtained products have higher purities, byproduct generation is effectively avoided, production cost is reduced, and industrial production is facilitated.
Owner:TIANJIN PACIFIC PHARMA
Ointment for treating skin disease and its production method
InactiveCN1709266AGood anti-inflammatory effectHigh cure rateOrganic active ingredientsAllergic dermatitisDisease
The present invention relates to an ointment for external application for curing dermatosises of psoriasis, neurodermatitis, allergic dermatitis, cutaneous pruritus, beriberi, mosquito bite and infantile eczema, etc. with obvious therapeutic effect. Said ointment is made up by using (by wt%) 30-60% of tramcinolone acetonide acetate urea cream, 15-30% of compound sulfamethoxazole, 10-20% of prednisone acetate and 10-20% of chlorpheniramine maleate. Besides, said invention also provides its production method and concrete steps.
Owner:王贵玉
Traditional Chinese medicine for treating psoriasis
InactiveCN101002833AHeal fastEasy to useOrganic active ingredientsInorganic active ingredientsVitamin CTraditional medicine
Owner:马学荣
Compound zinc undecylenate triamcinolone acetonide ointment and preparation method thereof
InactiveCN102366418AGood anti-inflammatory effectImprove anti-allergic effectOrganic active ingredientsAntimycoticsFormularyAllergy
The invention discloses a compound zinc undecylenate triamcinolone acetonide ointment and a preparation method thereof. The invention relates to the field of medicine. The compound zinc undecylenate triamcinolone acetonide ointment provided by the invention is composed of zinc undecylenate, undecylenic acid, triamcinolone acetonide acetate, and an emulsion substrate. Compared to existing technologies, the preparation method is advantaged in controllable temperature and simple formula, and the product is advantaged in good characters. With the added triamcinolone acetonide acetate, comprehensive inflammation resisting and allergy resisting effects of the medicine are stronger and more durable. The product and the method are especially suitable for industrial productions.
Owner:JILIN AODONG GROUP DALIAN PHARMACEUTICAL CO LTD
Medicinal composition for treating skin disease and its preparing method
InactiveCN1813771AObvious synergyPreserve anti-inflammatory effectsOrganic active ingredientsAntimycoticsDiseaseBetamethasone valerate
The present invention discloses a medicine composition for curing dermopathy and its preparation method. Said medicine composition contains the following components: (by weight portion) 0.3-3.0 portions of naftifine and 0.005-0.50 portion of one component selected from halcinonide, triamcinolone acetonide acetate, betamethasone (containing betamethasone propionate and betamethasone valerate) or clobetasol propionate. Said medicine composition can be made into various medicine preparations for external application.
Owner:YAKEXI PHARMA INST BEIJING
Preparation method for solution type triamcinolone acetonide acetate injection
ActiveCN105030663ASimple production processSmall local irritationOrganic active ingredientsAntipyreticCyclodextrinOrganosolv
The invention provides a preparation method for a solution type triamcinolone acetonide acetate injection. The preparation method comprises the following concrete steps: in the presence of an organic solvent, stirring triamcinolone acetonide acetate and sulfobutyl ether-beta-cyclodextrin until the obtained mixture is clear; then removing the organic solvent through evaporation; and adding an aqueous sodium hyaluronate solution, dilution water, etc. The preparation method for the solution type triamcinolone acetonide acetate injection is simple; the prepared injection has small local irritation in clinical application; and triamcinolone acetonide acetate can be easily absorbed especially when the injection is applied to intra-articular injection, and the problem of deposition of particles on the surface of periosteum caused by a suspension type triamcinolone acetonide acetate injection is avoided, so damage of the particles to the periosteum can be reduced.
Owner:上海正大通用药业股份有限公司
Triamcinolone acetonaide acetate nano eye drops and preparation method thereof
The invention relates to triamcinolone acetonaide acetate nano eye drops and a preparation method thereof, wherein the triamcinolone acetonaide acetate nano eye drops are prepared in the way that the triamcinolone acetonaide acetate and the chitosan nano carrier are prepared into nanoparticles according to the proportion of 1 / 2 and then are mixed with the distilled water. The invention provides the triamcinolone acetonaide acetate nano eye drops which have simple preparation process, can prevent pain and complication which are caused by local injection, facilitate clinical use and are easy to be accepted by patients and the preparation method of the triamcinolone acetonaide acetate nano eye drops.
Owner:严宏 +1
Chitosan modified triamcinolone acetonide acetate lipidosome and preparation method thereof
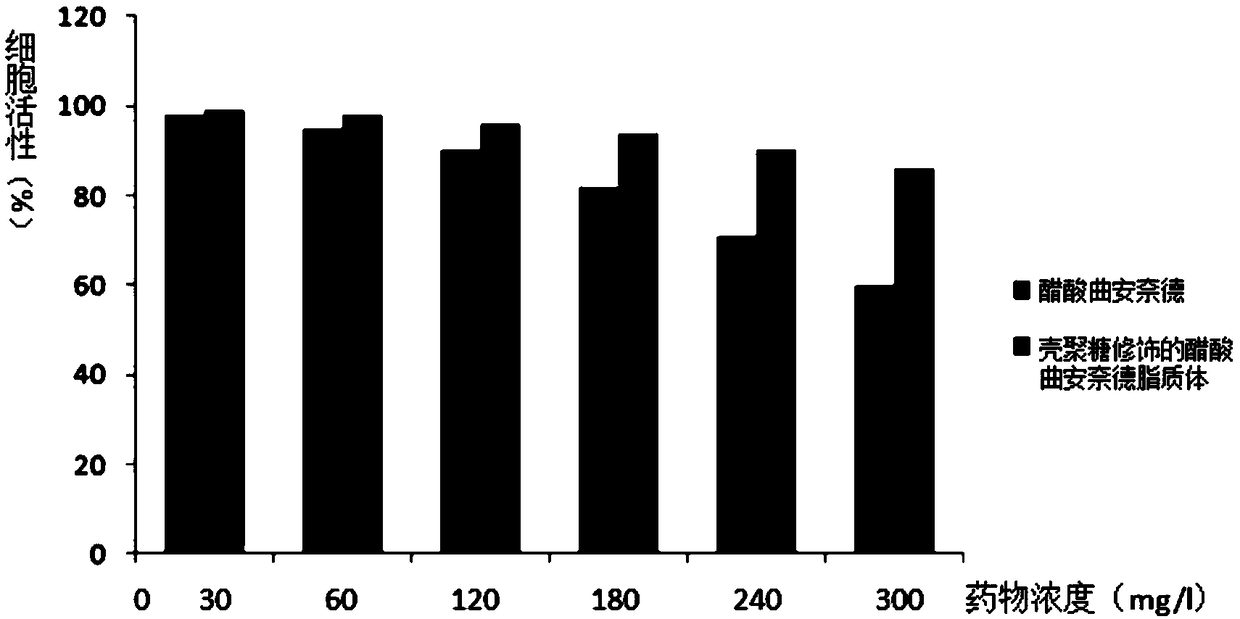
ActiveCN109481403AHigh encapsulation efficiencyConducive to active entryOrganic active ingredientsSenses disorderSolubilityCholesterol
The invention belongs to the field of pharmaceutical preparations and in particular relates to chitosan modified triamcinolone acetonide acetate lipidosome eye drops and a preparation method thereof.The invention provides chitosan modified triamcinolone acetonide acetate lipidosome prepared prepared by a calcium acetate gradient method. The preparation method comprises the following steps: preparing a lipid membrane from soya bean lecithin and cholesterol, adding a calcium acetate solution to prepare blank liposome, adding triamcinolone acetonide acetate to obtain triamcinolone acetonide acetate lipidosome, and performing chitosan coating, thereby obtaining the chitosan modified triamcinolone acetonide acetate lipidosome. The lipidosome has excellent pharmaceutical property, the liposomesurface presents positive charge, and the lipidosome and a sodium hyaluronate solution are prepared into the eye drops, so that the water solubility of the triamcinolone acetonide acetate and the adsorptivity to cornea can be improved, and the drug toxicity can be reduced. Compared with injection, the chitosan modified triamcinolone acetonide acetate lipidosome eye drops are simple and safe to use.
Owner:SHANDONG UNIV QILU HOSPITAL
External use medicine for treating dermatosis and its preparing method
InactiveCN100435801CEasy to useLow priceHydroxy compound active ingredientsAmide active ingredientsDiseaseMenthol
The invention is to disclose a medicine of external use for treating skin disease And its preparation method, said medicine includes materials according to part by weight as follows: egocort 6-9 ; camphor 80-120;menthol 80-120;triamcinolone acetonaide acetate 8-12;carbamide 800-1200;aspirin 440-4400.
Owner:刘亚军
Medicine for treating psoriasis
InactiveCN100551394CHeal fastEasy to useOrganic active ingredientsInorganic active ingredientsVitamin CCure rate
The invention discloses a medicine for treating psoriasis, which is made of realgar, indigo naturalis, salvia miltiorrhiza, triamcinolone acetonide acetate, vitamin E, vitamin B6, vitamin C and urea. The invention has good effect in treating psoriasis, the cure rate reaches 91%, and the total effective rate is 100%.
Owner:马学荣
Method for improving stability of triamcinolone acetonide preparation
InactiveCN106727614AReduce dosageEasy to makeSalicyclic acid active ingredientsNervous disorderPolyethylene glycolSalicylic acid
The invention relates to a method for improving the stability of a triamcinolone acetonide preparation. The triamcinolone acetonide preparation is prepared from the following components: 100-150g of triamcinolone acetonide acetate, 0.2-0.5g of 15-hydroxyl stearic acid polyethylene glycol ester, 30-50g of sodium carboxymethyl cellulose, 2-5g of salicylic acid, 70-100L of 2-propylene glycol, 40-60L of 5% ethanol and the balance of water for injection; the pH value of the triamcinolone acetonide preparation is 4-7. Sodium chloride is added into a triamcinolone acetonide acetate solution, and the amount of the sodium chloride is selected to be limited, so that the problem that the content of the triamcinolone acetonide acetate is reduced in a standing process of a compound triamcinolone acetonide solution is solved; furthermore, the sodium chloride is cheap and less in consumption, so that the method is lower in production cost and suitable for industrial production and application; in addition, the sodium chloride does not affect the cleanliness of the active ingredients in the solution, and does not affect the safety and effectiveness of the solution.
Owner:TIANJIN JINHUI PHARMA
A compound preparation for treating psoriasis
ActiveCN106937955BEffective treatmentSave money on medicineOrganic active ingredientsDermatological disorderCurative effectEffective treatment
The invention discloses a compound preparation for treatment of psoriasis; the compound preparation includes econazole nitrate, triamcinolone acetonide acetate, ketoconazole and a plurality of accessories. With use of the formula of the compound preparation for preparing a drug, the charges for medicines can be reduced, the curative effect is improved, and effective treatment of psoriasis is achieved.
Owner:天津久益生物科技有限公司
Triamcinolone acetonide acetate particle, and preparation method and medicinal composition thereof
ActiveCN101618019BEffective control of crystal morphologyControl crystal morphologyPowder deliveryOrganic active ingredientsFree coolingMicroparticle
Owner:上海正大通用药业股份有限公司
Drug for treating dermatoses and preparation method and application thereof
InactiveCN106074572ASmall particle sizeLow viscosityOrganic active ingredientsAntimycoticsNeomycin SulfateRemove blood
The invention discloses a drug for treating dermatoses. The drug is prepared from, by weight, 55-120 parts of chloramphenicol, 120-200 parts of propylene glycol, 50-90 parts of water for injection, 0.5-1.5 parts of chlorpheniramine maleate, 200-280 parts of white vaseline, 160-220 parts of glycerol, 1.5-3 parts of miconazole nitrate, 0.2-0.5 part of triamcinolone acetonide acetate, 220-300 parts of natural fatty alcohol and 0.5-0.9 part of neomycin sulfate. The drug has the effects of clearing away heat and toxic materials, promoting blood circulation to remove blood stasis, purging fire and cooling blood, relieving swelling and pain and resisting bacteria and viruses, is used for treating the dermatoses such as acnes, pachulosis, sunburn, dermatophytosis and mosquito bites and has the advantages that the treatment effect is good and is quickly achieved, the cure rate is high, cost is low, no irritation is generated to skin, and scars are not prone to be left.
Owner:梁东荣
A kind of medical product containing solution type triamcinolone acetonide acetate
ActiveCN104971039BSimple production processSmall local irritationOrganic active ingredientsAerosol deliveryIrritationMedicine
The invention provides a medical product containing solution-type triamcinolone acetonide acetate. The medical product containing solution-type triamcinolone acetonide acetate comprises triamcinolone acetonide acetate, sulfobutyl ether-β-cyclodextrin and sodium hyaluronate. The invention provides a solution-type medical product containing triamcinolone acetonide acetate, which has less local irritation in clinical use; especially when it is used for intra-articular injection, the drug is easy to absorb, avoiding the suspension-type triamcinolone acetonide acetate medical product Particle deposition on the periosteum surface, thereby reducing periosteal damage caused by particle deposition.
Owner:上海正大通用药业股份有限公司
Compound triamcinolone acetonide salicylic acid liniment
PendingCN114504581AImprove transdermal absorption rateGood curative effectSalicyclic acid active ingredientsAntipyreticSalicylic acidAqueous ethanol
The invention relates to a compound triamcinolone acetonide salicylic acid liniment. Each 100ml of the liniment is prepared from the following components: 0.01 to 0.2 g of triamcinolone acetonide acetate, 1 to 5g of chloramphenicol, 1 to 5g of salicylic acid, 1 to 5ml of azone, 1 to 5ml of dimethyl sulfoxide, 5 to 20ml of propylene glycol and the balance of 95 percent ethanol aqueous solution. The compound triamcinolone acetonide salicylic acid liniment disclosed by the invention is remarkable in psoriasis treatment effect, stable and reliable in quality, simple in preparation process and capable of realizing industrial production.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL
Triamcinolone acetonide acetate injection and preparation method thereof
InactiveCN104414972AImprove securityReduce adverse reactionsOrganic active ingredientsAntipyreticTRIAMCINOLONE ACETONIDE INJECTIONCellulose
The invention discloses a triamcinolone acetonide acetate injection and a preparation method thereof. The injection is prepared from 10.0g of triamcinolone acetonide acetate, sodium chloride which is 5.00-20 percent of the injection in a weight-to-volume ratio, sodium merthiolate which is 0.005-0.01 percent of the injection in a volume ratio, HS-15 which is 1.00-15 percent of the injection in a volume ratio, sodium carboxymethylcellulose which is 10.00-30 percent of the injection in a volume ratio and the balance of water for injecting. The preparation method comprises the following steps: putting triamcinolone acetonide acetate, sodium merthiolate, sodium chloride and sodium carboxymethylcellulose in a beaker according to the prescription, adding partial water for injecting, uniformly stirring, adding activated carbon, uniformly stirring, and filtering to obtain a solution (1); weighing a proper amount of water for injecting at 30-40 DEG C, adding HS-15 which is 1.00-15 percent of the injection in a volume ratio, and uniformly stirring to obtain a solution (2); mixing the solution (1) and the solution (2), uniformly stirring, adding the water for injecting to total amount, regulating the pH value of the mixture to be 5.5-7.0 by using acetic acid, filtering and filling and sealing. By adopting a brand-new prescription and process, a produced product has stable quality, and the safety of clinical administration can be remarkably improved.
Owner:CHENGDU LIST PHARMA
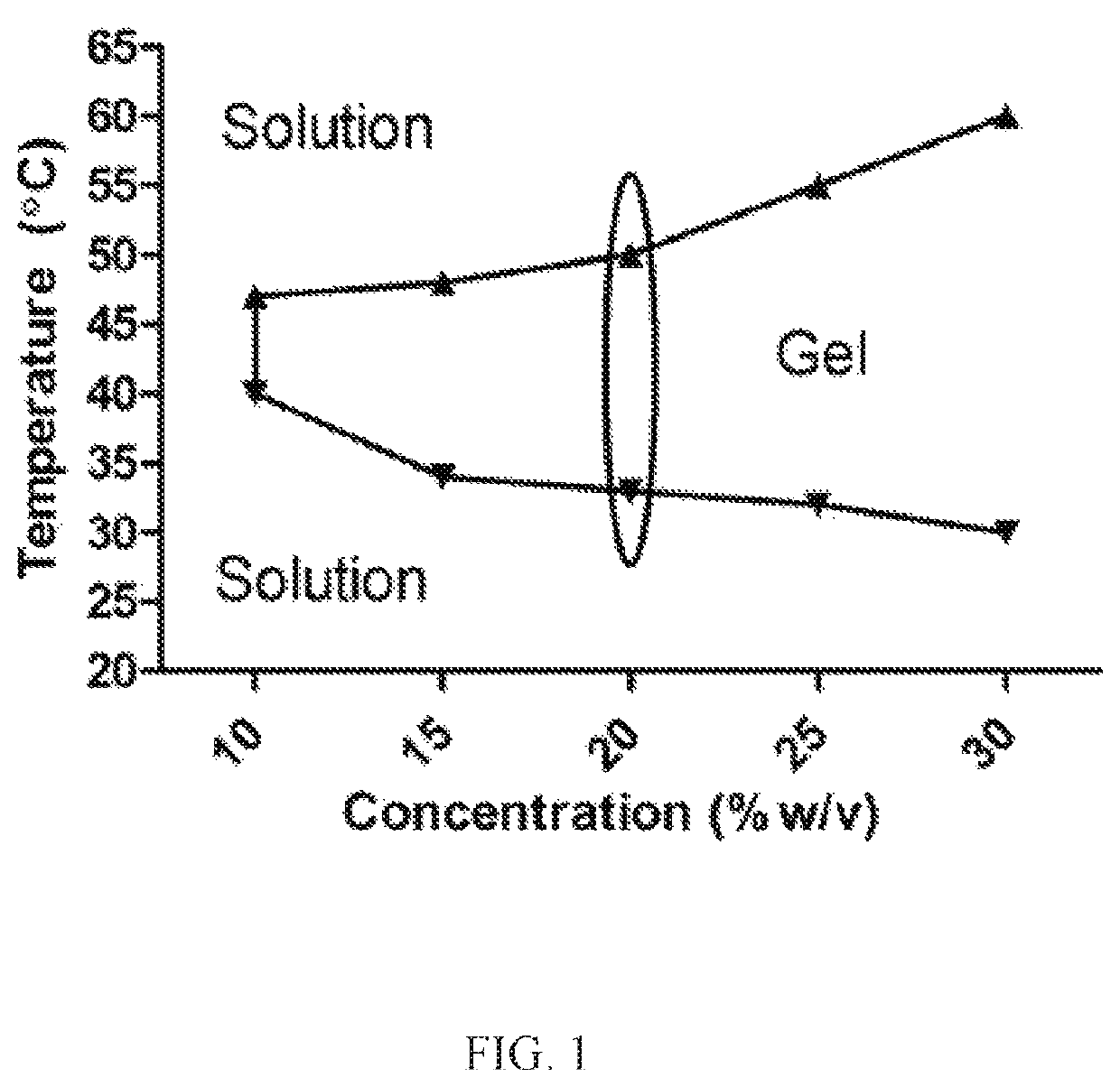
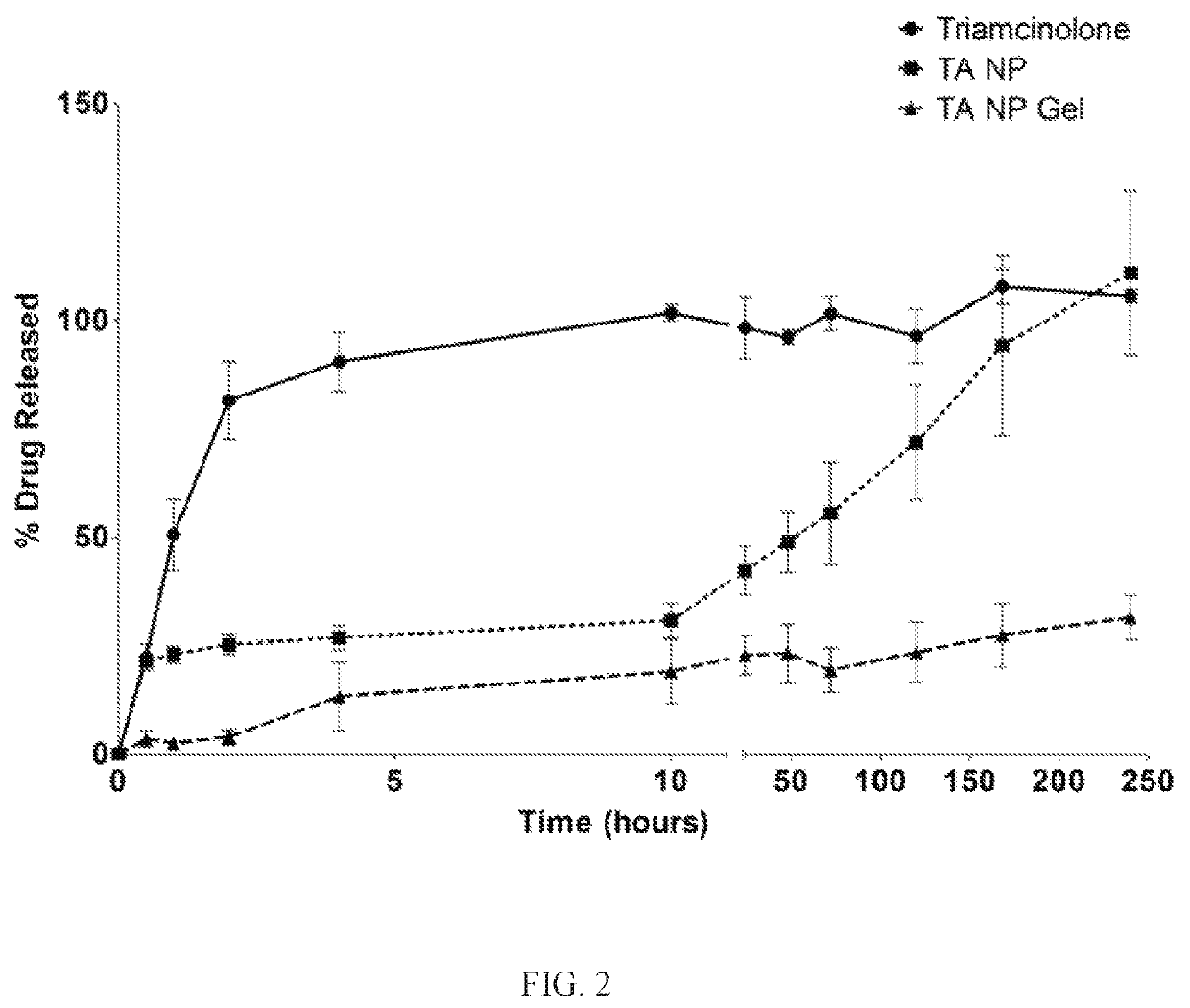
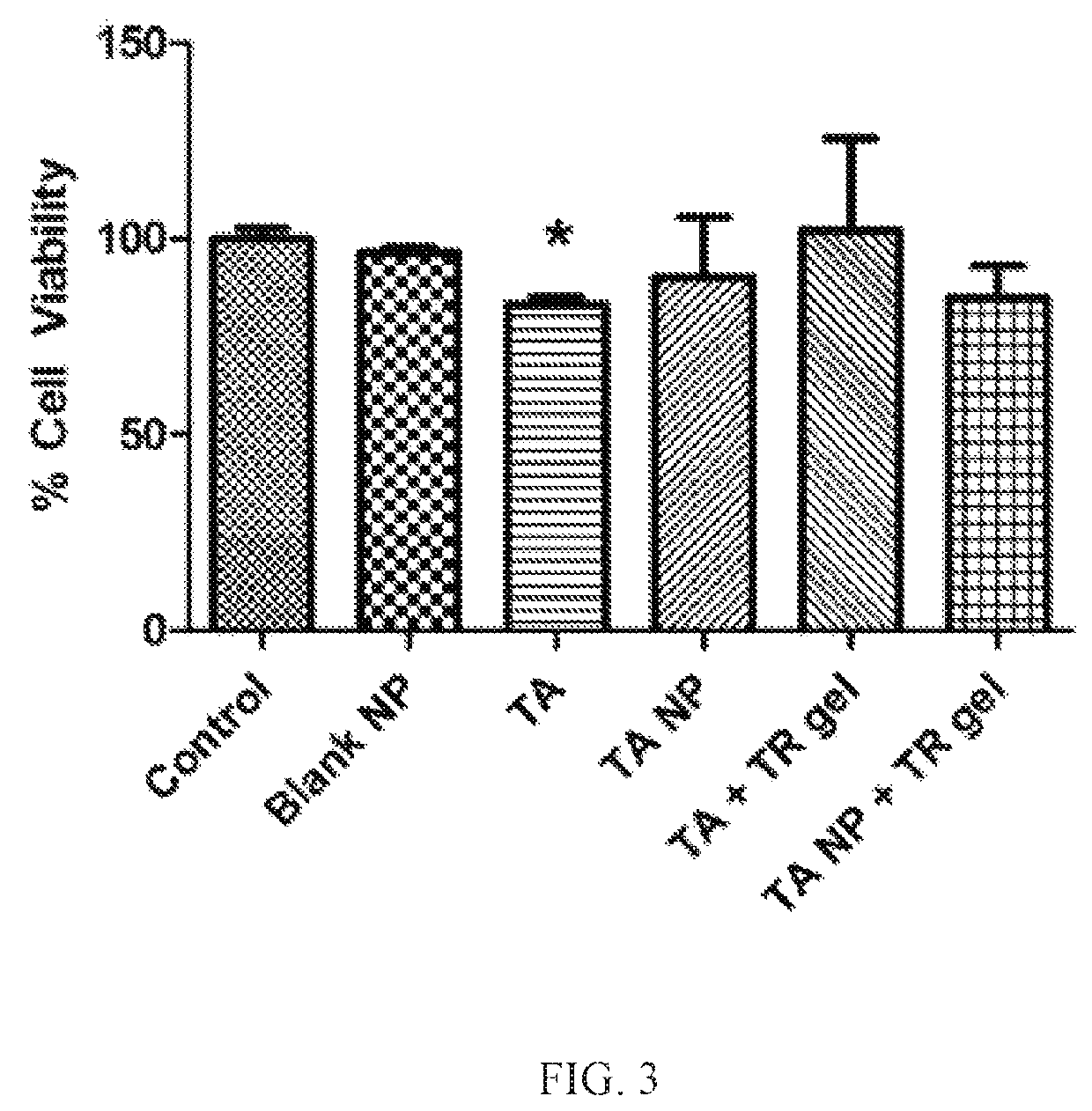
Nanoparticles in thermoreversible gels for enhanced therapeutics
ActiveUS10729663B1Reduce frequencyReduced VEGF expressionOrganic active ingredientsSenses disorderMacula lutea degenerationEye posterior segment
The present invention provides a sustained drug delivery system for the treatment of age-related macular degeneration (AMD), comprising corticosteroid encapsulated nanoparticles incorporated into a thermoreversible hydrogel. The corticosteroid may be triamcinolone acetate (TA), dexamethasone, or loteprednol etabonate (LE). The proposed drug delivery system is nontoxic to ARPE-19 (retinal pigment epithelium) cells and significantly reduces VEGF (vascular endothelial growth factor) expression as compared to solutions of the coticosteroids. The present invention provides sustained delivery of the corticosteroid to the posterior segment of the eye, reducing the frequency of intraocular injections necessary to maintain therapeutic concentrations.
Owner:UNIV OF SOUTH FLORIDA
Compound preparation for treatment of psoriasis
ActiveCN106937955AEffective treatmentSave money on medicineOrganic active ingredientsDermatological disorderCurative effectEffective treatment
The invention discloses a compound preparation for treatment of psoriasis; the compound preparation includes econazole nitrate, triamcinolone acetonide acetate, ketoconazole and a plurality of accessories. With use of the formula of the compound preparation for preparing a drug, the charges for medicines can be reduced, the curative effect is improved, and effective treatment of psoriasis is achieved.
Owner:天津久益生物科技有限公司
A kind of preparation method of solution type triamcinolone acetonide injection
The invention provides a preparation method for a solution type triamcinolone acetonide acetate injection. The preparation method comprises the following concrete steps: in the presence of an organic solvent, stirring triamcinolone acetonide acetate and sulfobutyl ether-beta-cyclodextrin until the obtained mixture is clear; then removing the organic solvent through evaporation; and adding an aqueous sodium hyaluronate solution, dilution water, etc. The preparation method for the solution type triamcinolone acetonide acetate injection is simple; the prepared injection has small local irritation in clinical application; and triamcinolone acetonide acetate can be easily absorbed especially when the injection is applied to intra-articular injection, and the problem of deposition of particles on the surface of periosteum caused by a suspension type triamcinolone acetonide acetate injection is avoided, so damage of the particles to the periosteum can be reduced.
Owner:上海正大通用药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com