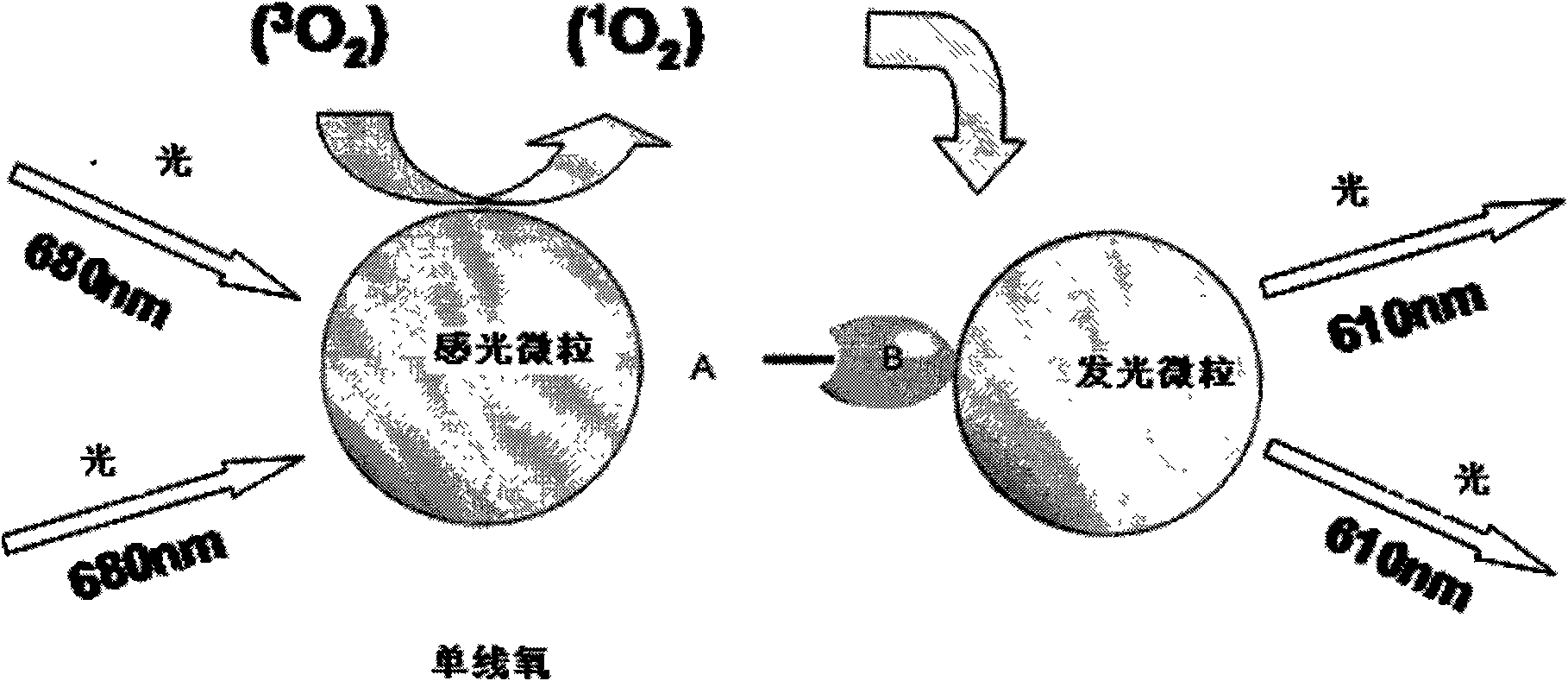
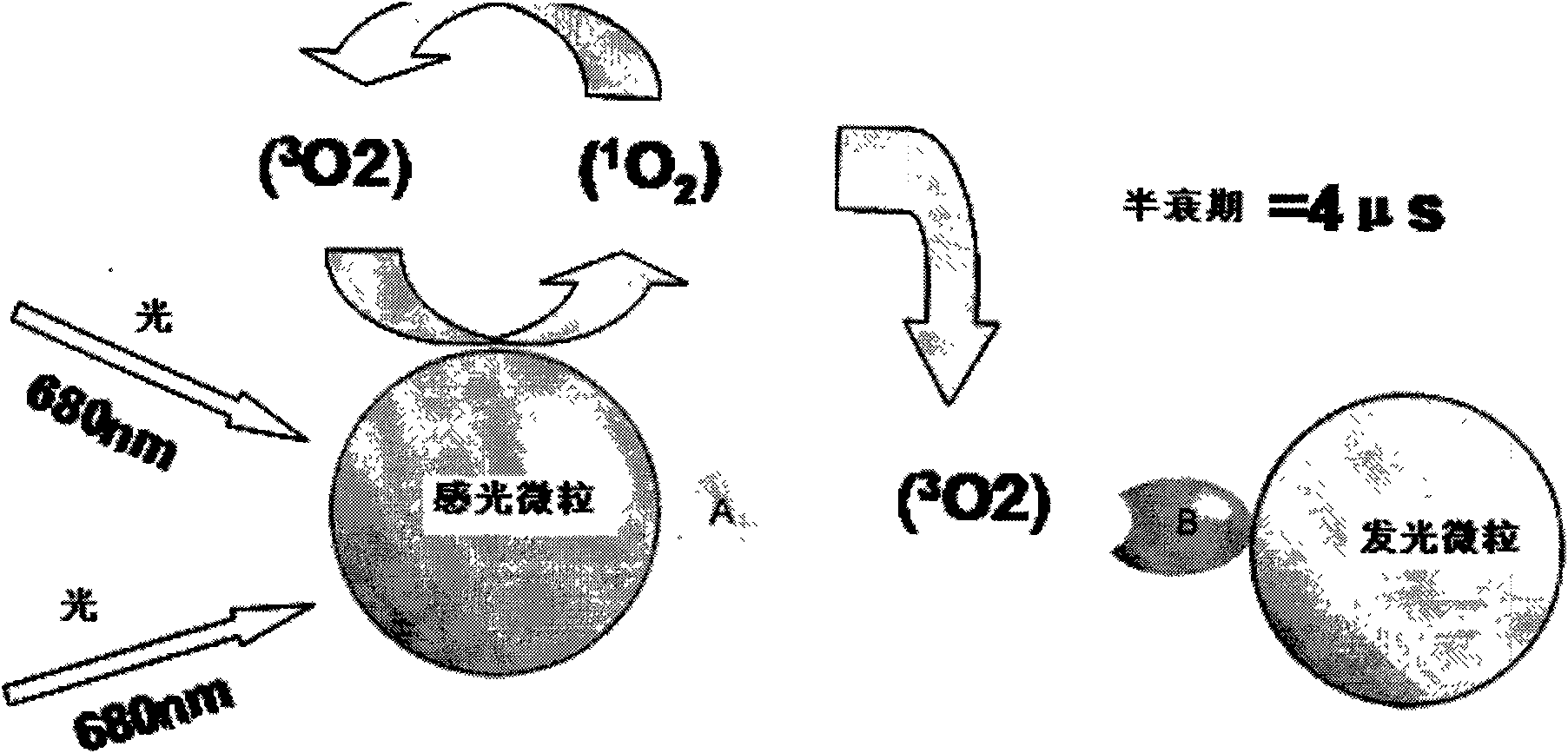
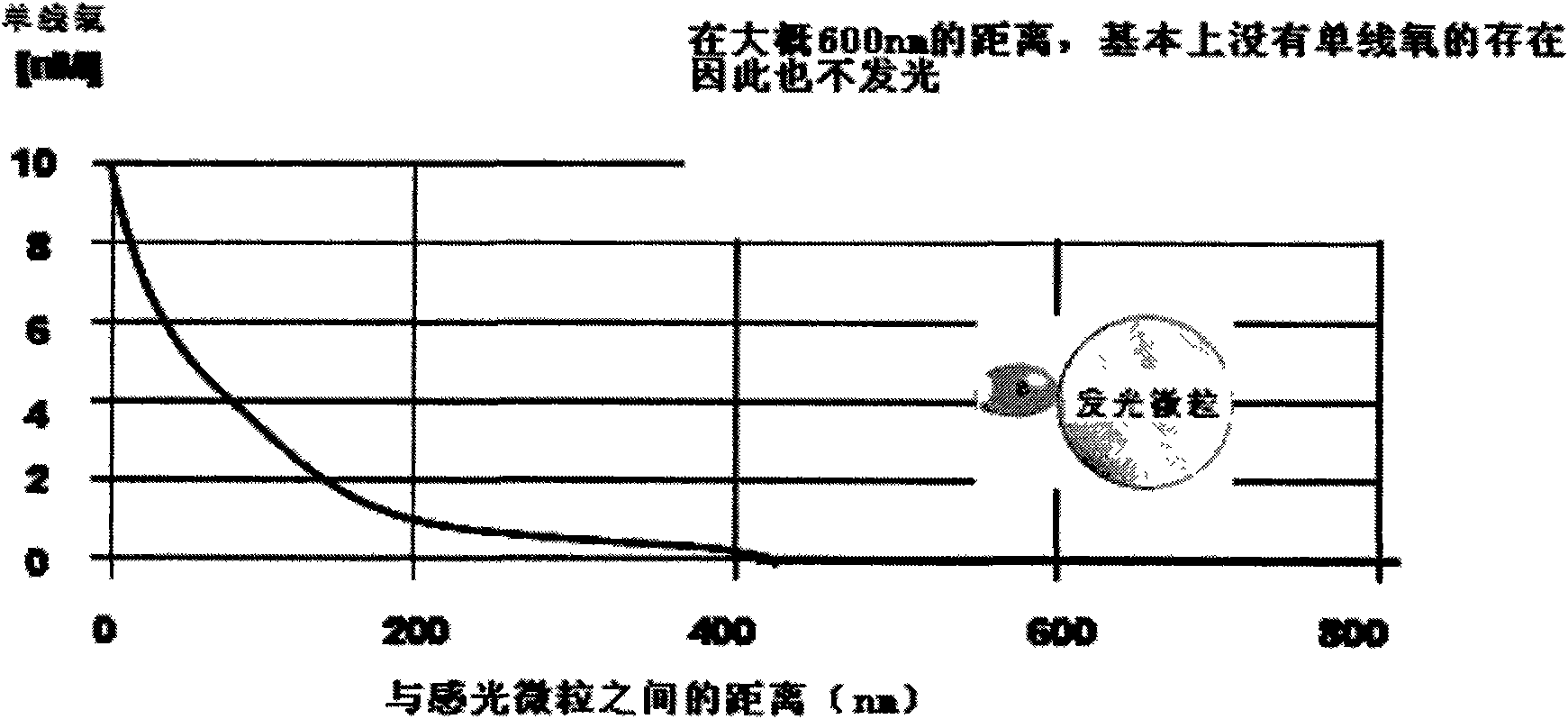
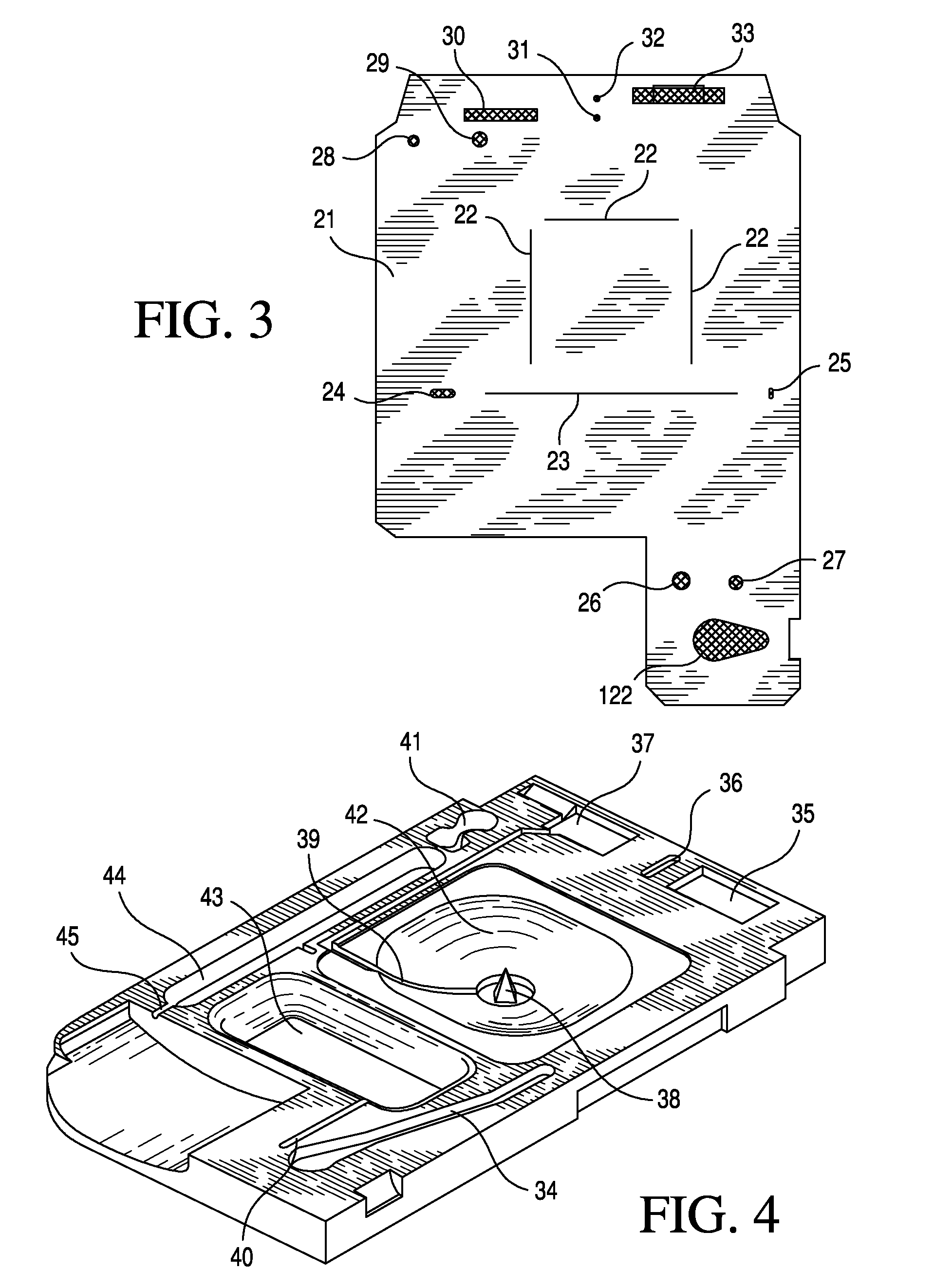
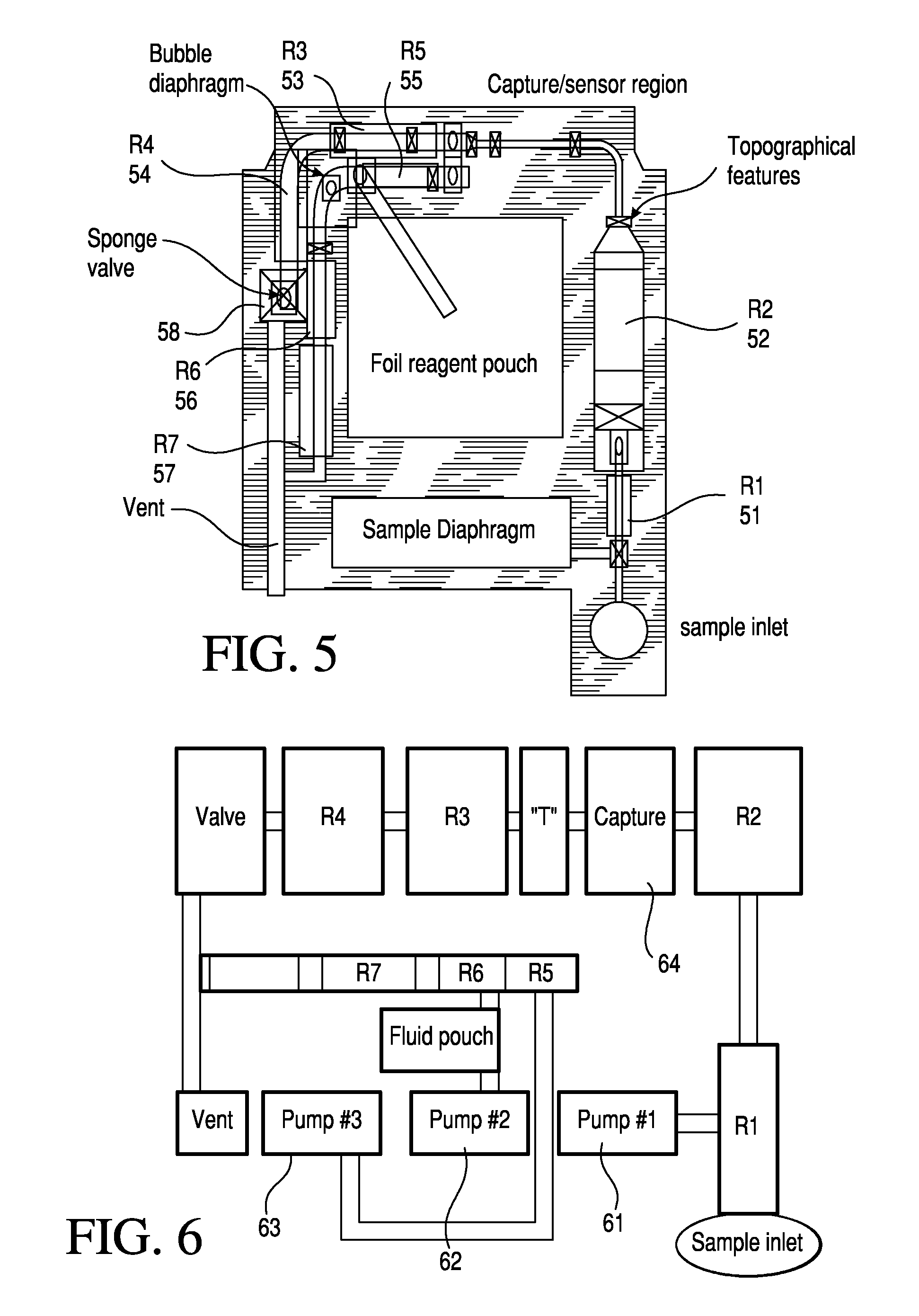
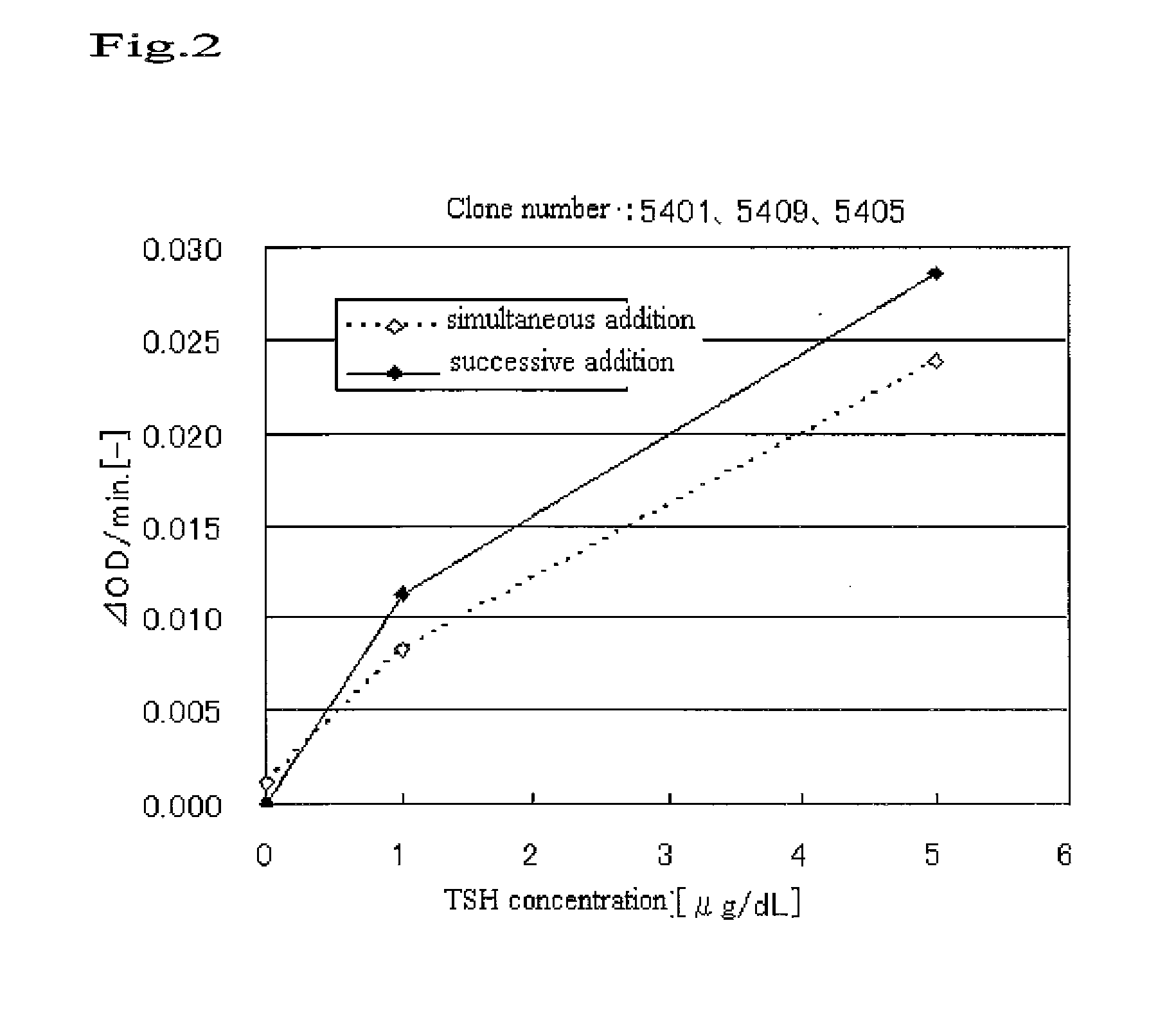
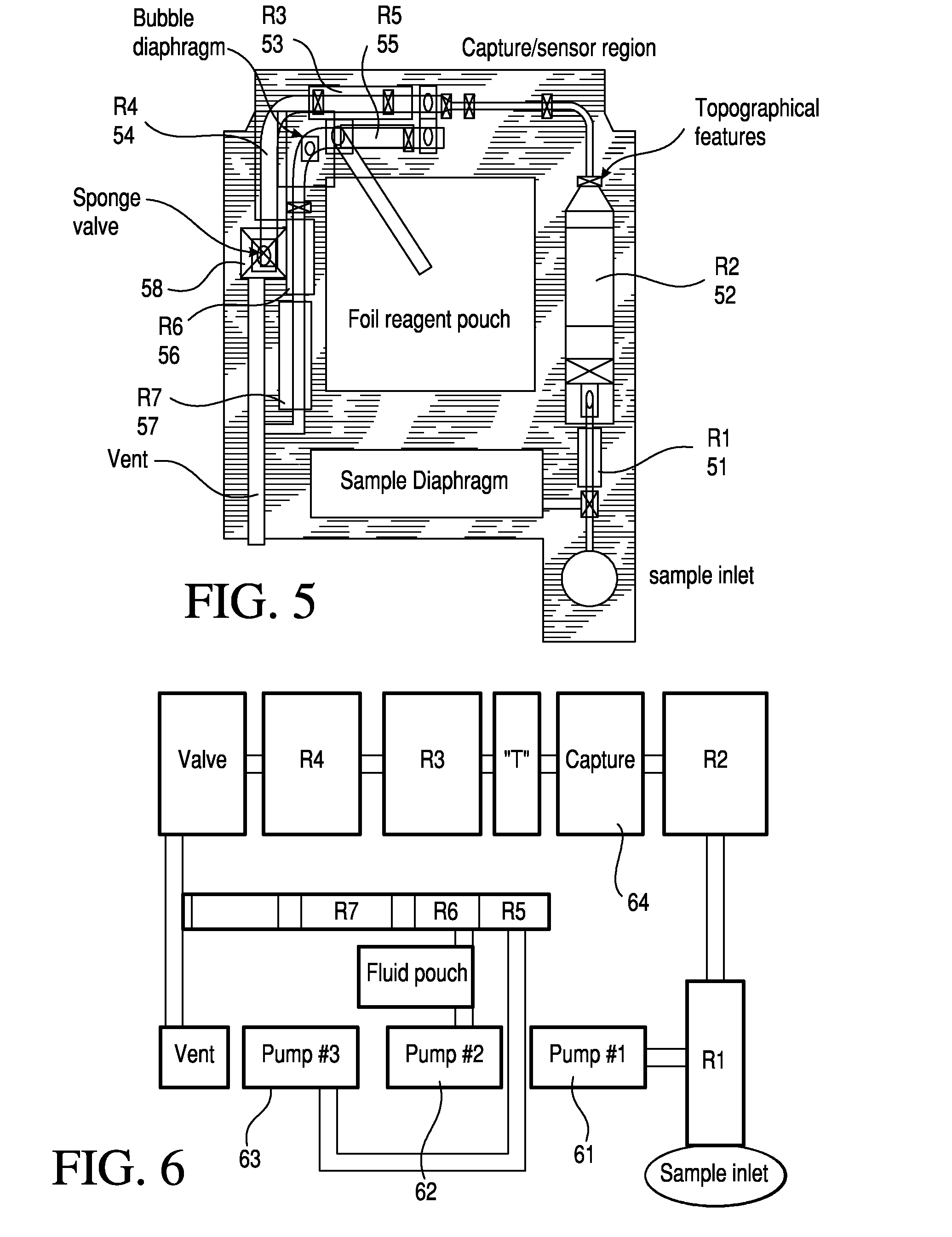
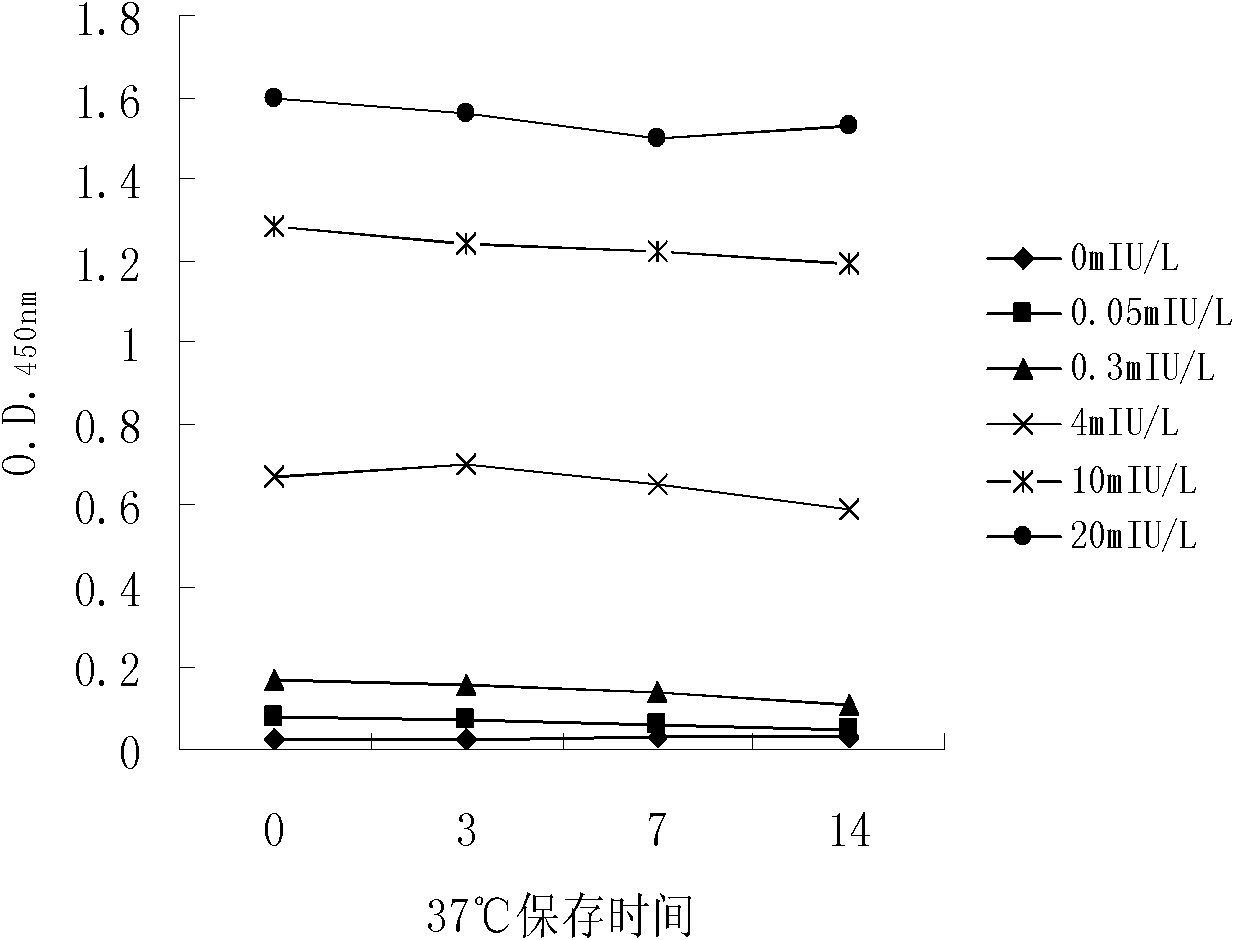Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Thyroid-stimulating hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thyroid-stimulating hormone (also known as thyrotropin, thyrotropic hormone, or abbreviated TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroxine (T₄), and then triiodothyronine (T₃) which stimulates the metabolism of almost every tissue in the body. It is a glycoprotein hormone produced by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid. In 1916, Bennett M. Allen and Philip E. Smith found that the pituitary contained a thyrotropic substance.
Detection micro particle of thyroid stimulating hormone and preparation and application thereof
ActiveCN101769933AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingTreatment effectClinical information
The invention relates to an aided diagnostic reagent of thyroid diseases and discloses a detection micro particle of thyroid stimulating hormone, which is a luminous micro particle coated by an antithyrotropic hormone antibody. The invention also discloses preparation and application of the thyroid stimulating hormone. In addition, the invention further discloses a vitro diagnostic kit of the thyroid stimulating hormone in a determined sample. Meanwhile, the invention discloses a using method of the kit. The kit can be combined with other serums and clinical information for aided diagnosis of the thyroid diseases and the monitoring of treatment effect of related patients.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Methods and kits for the diagnosis of acute coronary syndrome
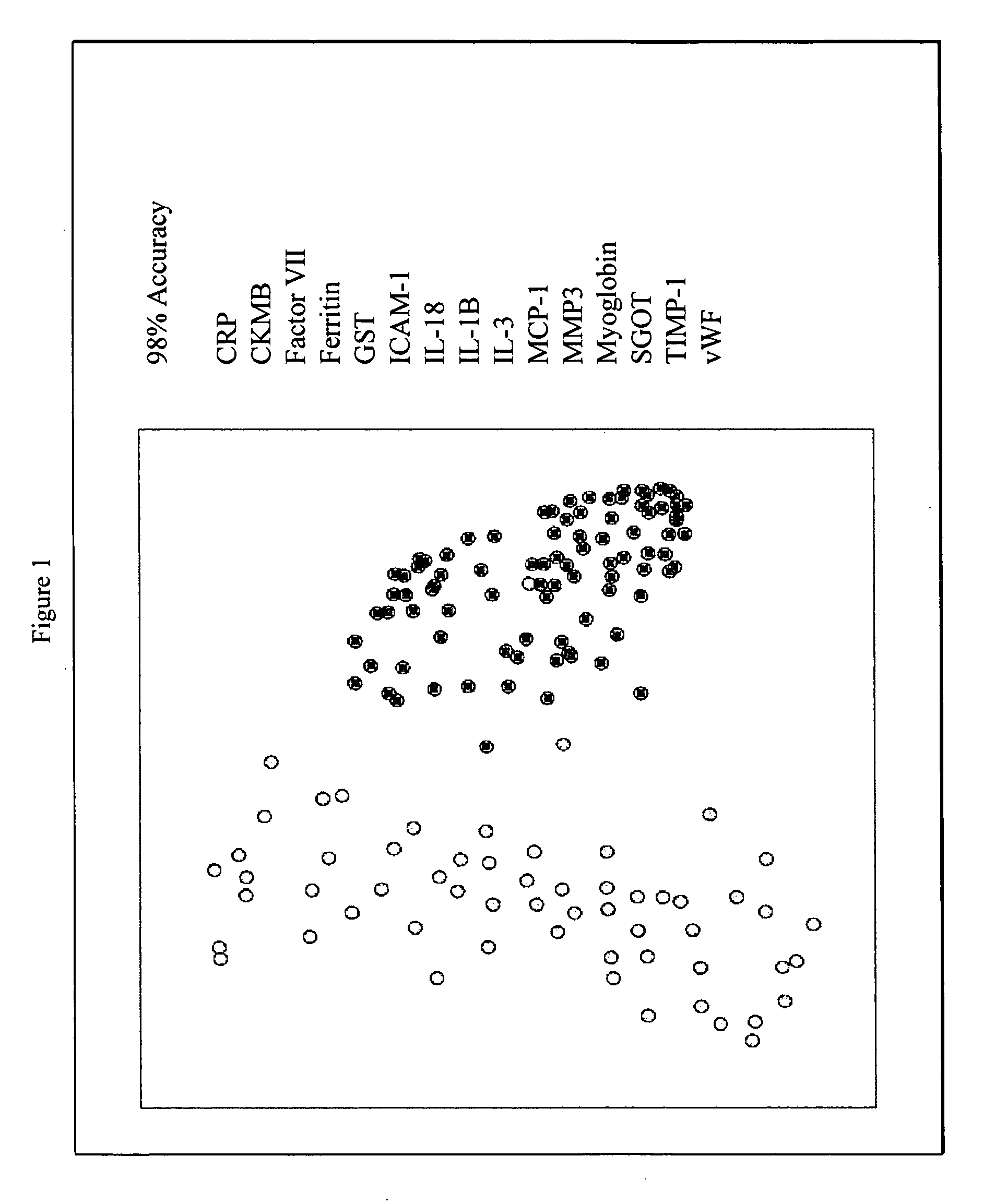
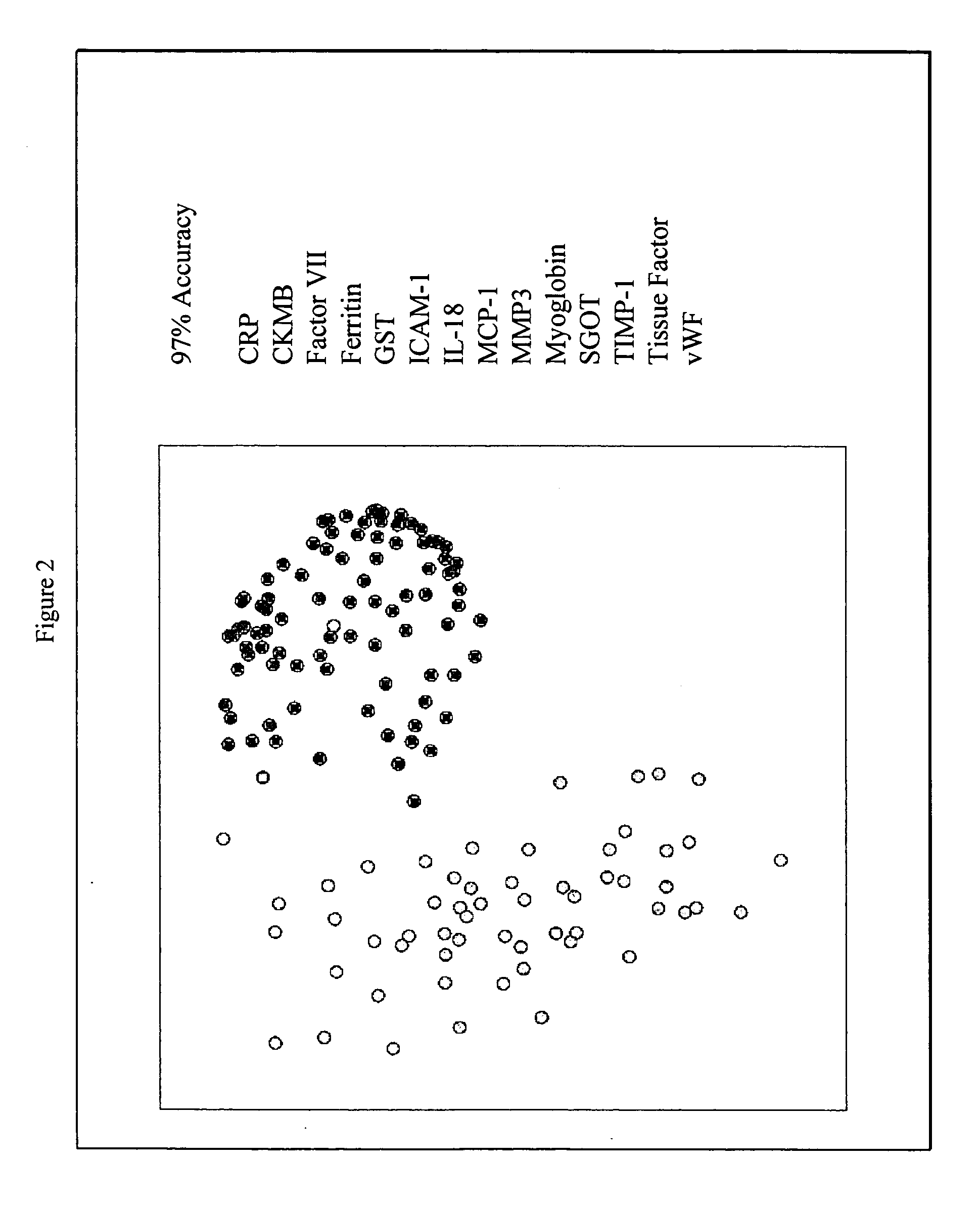
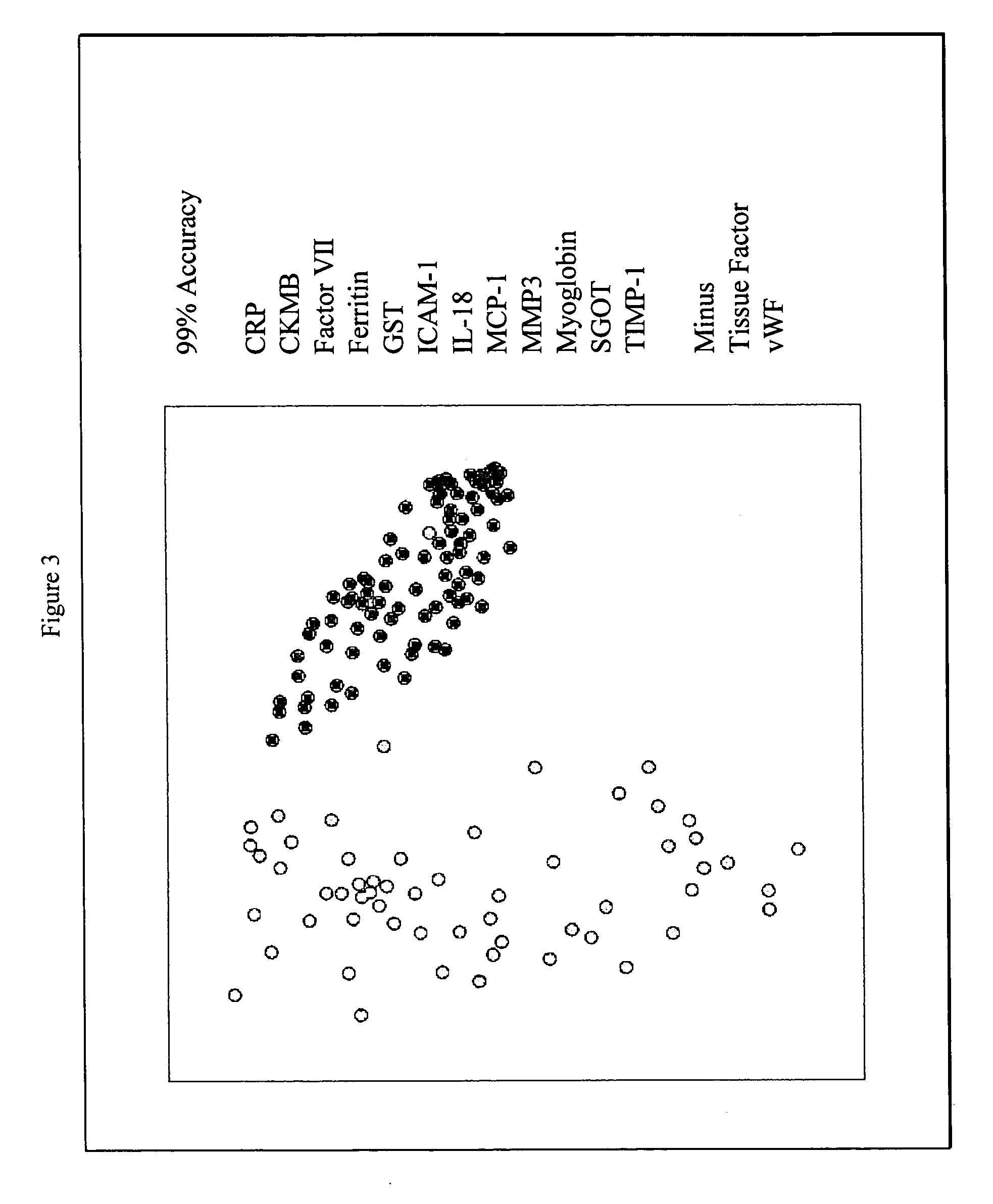
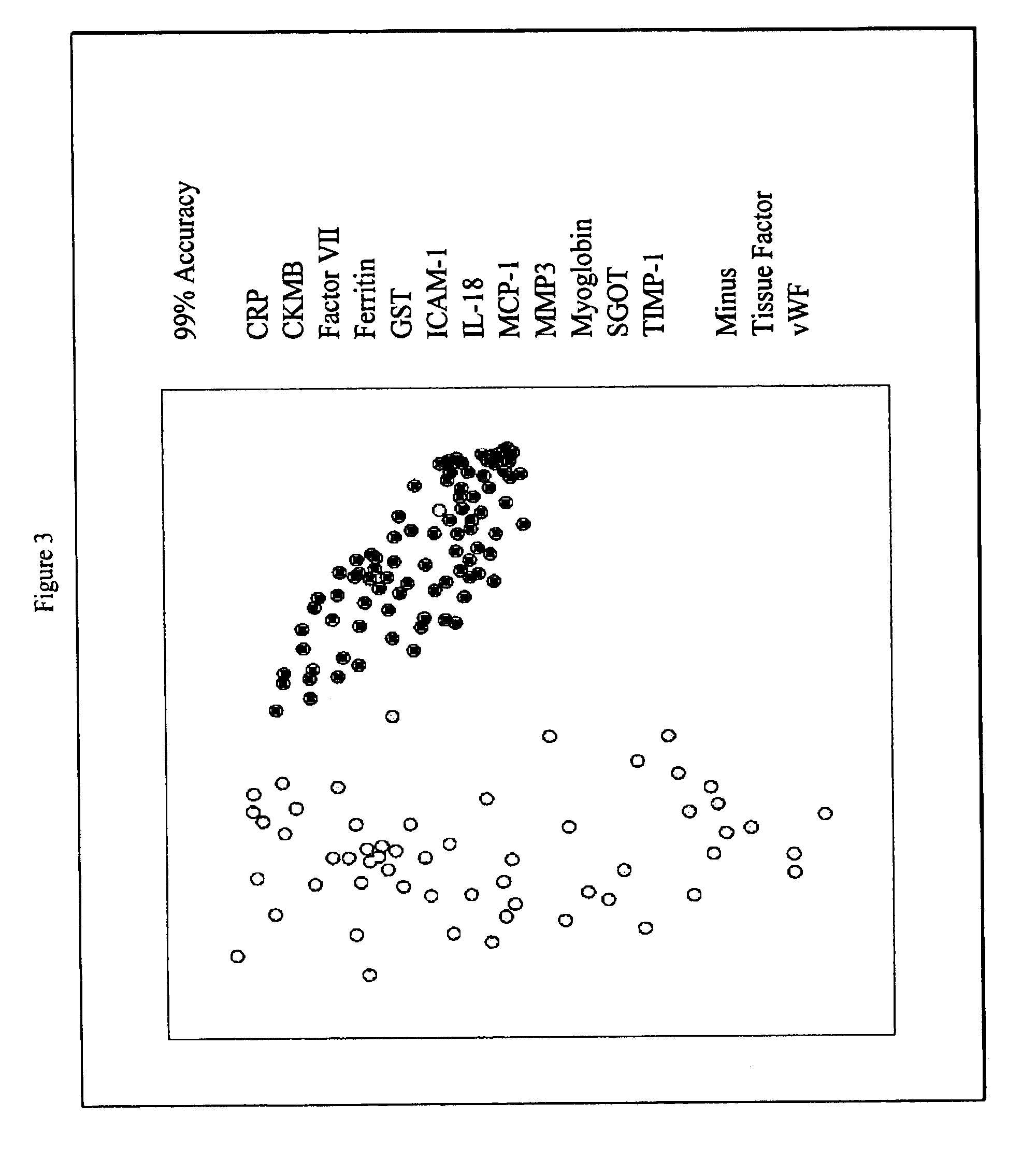
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Thyroid function detection protein chip and kit thereof
InactiveCN102095869AShorten the timeSave resourcesChemiluminescene/bioluminescenceBiological testingDiseaseProtein chip
The invention discloses a thyroid function detection protein chip and a kit thereof. The kit comprises the thyroid function detection protein chip, wherein the chip is coated with a free triiodothyronine (FT3) antibody, a free thyroxine (FT4) antibody, a triiodothyronine (T3) antibody, a thyroxine (T4) antibody and a thyroid stimulating hormone (TSH) antibody. The kit can quantitatively detect the content of FT3, FT4, T3, T4 and TSH in patient serum, and is used for the auxiliary diagnosis of thyroid dysfunction diseases. Compared with the conventional enzyme linked immunosorbent assay (ELISA) technology, the chemiluminescence immunoassay keeps high specificity of the ELISA technology, stability and reliability of a detection result, and convenience of operation, and can improve detection sensitivity; and various indexes can be detected simultaneously, so time and cost are greatly saved.
Owner:上海裕隆生物科技有限公司
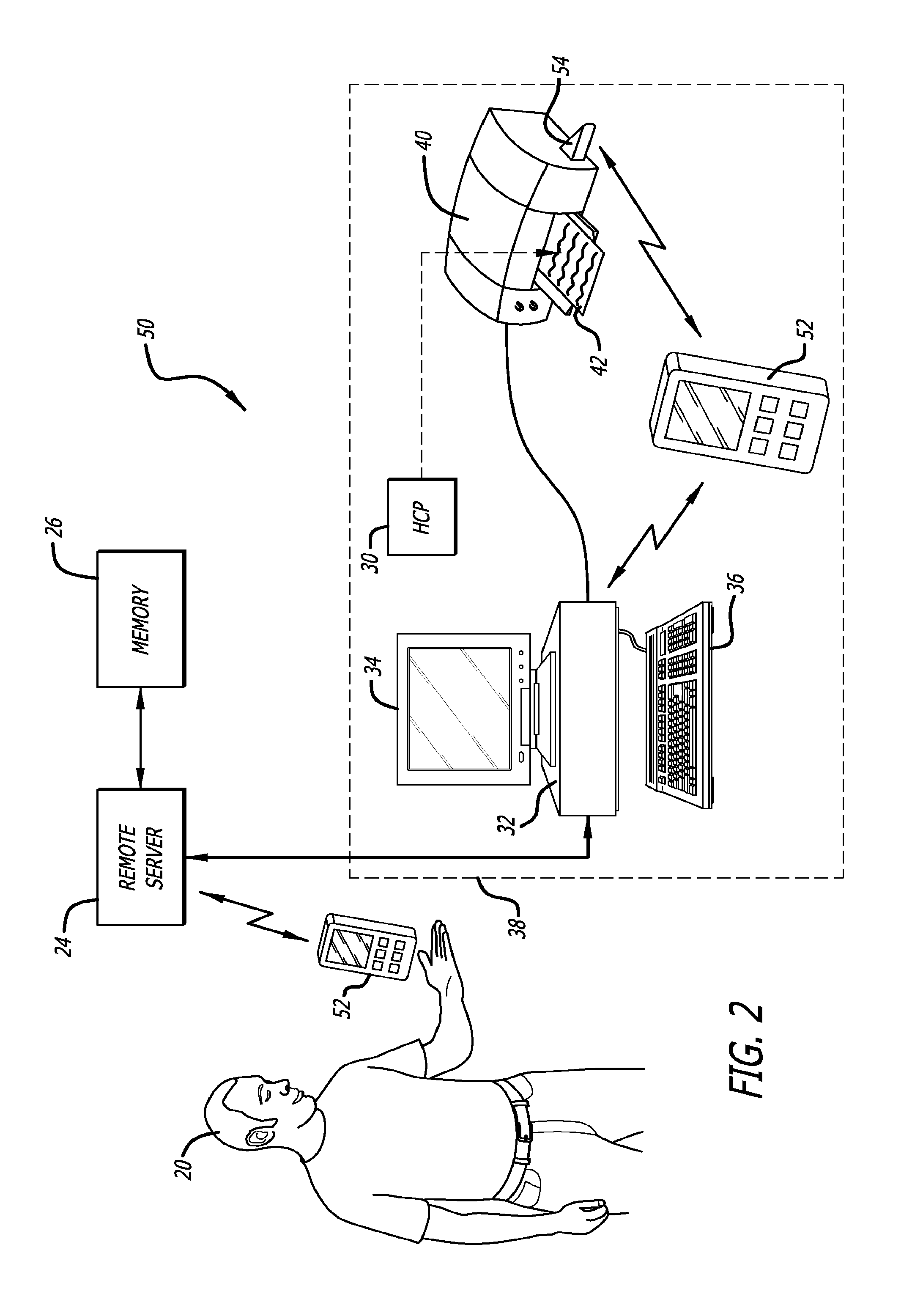
Infusion of insulin into a patient and diabetes mellitus medical methods based on the patients monitored analyte concentration
InactiveUS20170007762A1Reduce data transfer costsDrug and medicationsMedical devicesAmylaseCreatine kinase
Medical data provided by a physiological parameter sensor is used for management of the patient's medical condition. Analytes that may be monitored and managed include, but are not limited to, acetyl choline, amylase, bilirubin, cholesterol, chorionic gonadotropin, creatine kinase (e.g., CK-MB), creatine, glucose, glutamine, growth hormones, hormones, ketones, lactate, oxygen, peroxide, prostate-specific antigen, prothrombin, thyroid stimulating hormone, and troponin.
Owner:ABBOTT DIABETES CARE INC
Preparation of 131I-thyroid stimulating hormone (TSH) and application thereof
InactiveCN101787077ASimple preparation processStable markerRadioactive preparation carriersDepsipeptidesTyrosineUndifferentiated Thyroid Tumor
The invention relates to the preparation of 131I-thyroid stimulating hormone (TSH) and an application thereof, belonging to the field of radionuclide therapeutic drugs. The compound 131I-thyroid stimulating hormone is a radioiodine marker for thyroid stimulating hormone with pathoclisis in vivo acting on thyrocyte. A preparation method of the 131I-thyroid stimulating hormone comprises the following steps of: carrying out iodine 131 marking on the thyroid stimulating hormone by utilizing a chloramine-T method, a peroxide oxidation method or an iodogen method, and introducing radionuclide iodine 131 for treatment into tyrosine residues in thyroid stimulating hormone molecules. The marking rate of the prepared 131I-TSH is 85.9 percent, the radiochemical purity after purification achieves more than 90 percent, and the prepared 131I-TSH can be stored stably for more than one week at room temperature; the 131I-TSH is mainly metabolized through the liver and excreted through the kidneys; the percentage of ID / g of the 131I-TSH in the thyroid gland is not obviously reduced in four hours; and the 131I-TSH can be concentrated in thyroid gland issues sealed by an iodine solution. Concerning low / undifferentiated thyroid tumours incapable of iodine uptake, the 131I-TSH can increase the radioiodine uptake of thyroid gland cancer cells, and the radioiodine 131 can be chosen for treatment.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Tsh antibodies for point-of-care immunoassay formats
ActiveUS20120301896A1Interference minimizationBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careFollicle-stimulating hormone
The invention relates to antibody characteristics used to design a whole blood Point of Care Thyroid Stimulating Hormone (TSH) immunoassay using an ELISA sandwich assay lacking one or more wash steps between the antigen capture, detection antibody addition and substrate introduction steps. This invention exhibits low cross reactivity with biologically similar interfering cross reacting species, such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
Methods and kits for the diagnosis of hypothyroidism
InactiveUS20080020475A1Quick checkAccurate diagnosisGenetic modelsDigital computer detailsAntigenWhite blood cell
Provided are methods for the detection and diagnosis of Hypothyroidism. The methods are based on the discovery that altered levels of selected analytes in sample fluid, typically blood samples, of patients are supportive of a diagnosis of Hypothyroidism. At least twenty-four new biomarkers for hypothyroidism are thus disclosed (singly or in any combination), Thyroid Stimulating Hormone, Interleukin-12p40, Tumor Necrosis Factor Alpha, Tissue Factor, Interleukin-15, Insulin, Immunoglobulin E, Growth Stimulating Hormone, Calcitonin, Prostate-Specific Antigen, Interleukin-4, Granulocyte Macrophage Colony Stimulating Factor, Matrix Metalloproteinase 9, Lymphotactin, Fatty Acid Binding Protein, Alpha Fetoprotein, Alpha-2 Macroglobulin, Serum Glutamic Oxaloacetic Transaminase, Matrix Metalloproteinase 3, Cancer Antigen 125, Mumps Antibody, Double Stranded DNA Antibody, Proliferating Cell Nuclear Antigen Antibody, Smith Antibody, or Herpes Simplex Virus 1 Glycoprotein D Antibody. Altogether the concentrations of one or more of these analytes, as well as Thyroid Stimulating Hormone, or any combination thereof, provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering from Hypothyroidism. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:HEALTH RES INC +1
Parathyroid hormone determination kit and preparation method
InactiveCN106771128ASuitable for detectionSuitable for economical practicalityMaterial analysisLanthanideRare earth
The invention relates to the technical field of fluorescence immunochromatography in medical immunology and particularly relates to a parathyroid hormone determination kit and a preparation method. A test strip is arranged. The kit is characterized in that the test strip is sequentially provided with a PVC plate, a sample pad, a conjugate pad, a nitrocellulose membrane and an absorbent pad from bottom to top; a rare-earth Eu<3+> fluorescent microsphere-labelled thyroid parathyroid hormone monoclonal antibody is adsorbed to the conjugate pad; the diameters of rare-earth fluorescent microspheres are 200nm; the rare-earth fluorescent microspheres contain rare-earth lanthanide Eu<3+>, are stable in a ground state, and emit fluorescence with the wavelengths of 615nm under the action of an excitation light source of 337nm; and the monoclonal antibody is a purified and mixed monoclonal antibody, and is from monoclonal antibody cell lines of 2-6 different thyroid stimulating hormone epitopes. The kit has the advantages of being simple and convenient to operate, fast in reaction, high in sensitivity and high in specificity.
Owner:WEIHAI NEOPROBIO
Long-optical-path enzyme-linked immunoassay for testing thyroid stimulating hormone, and kit
ActiveCN102192888AHigh sensitivityLarge specific surface areaColor/spectral properties measurementsBiological testingOptical pathlengthEnzyme linked immunoassay
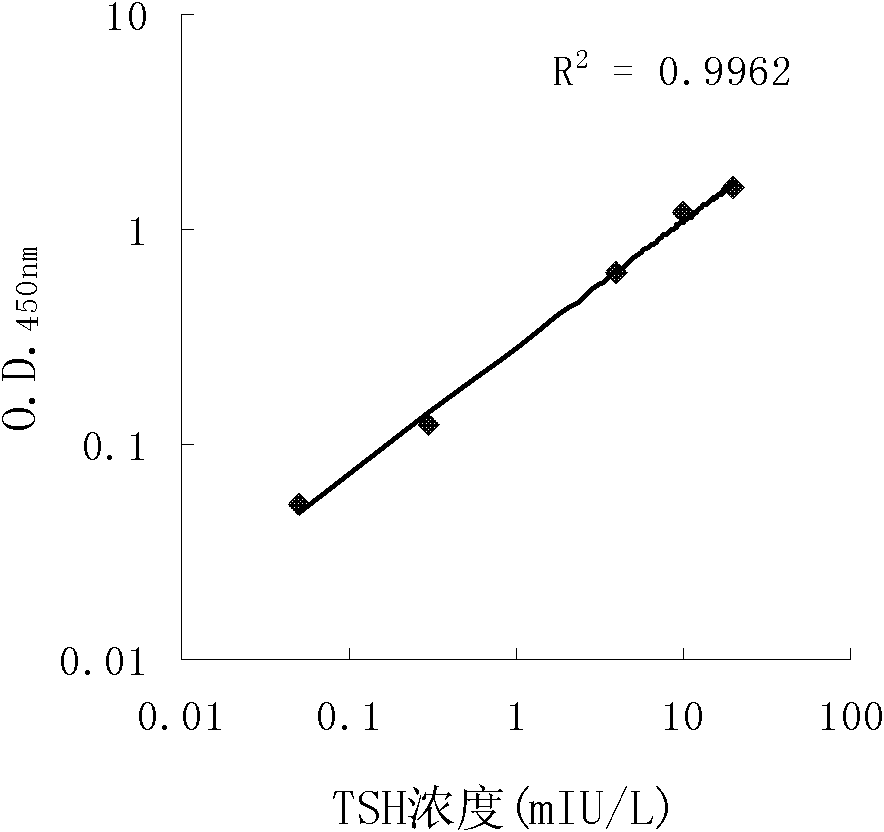
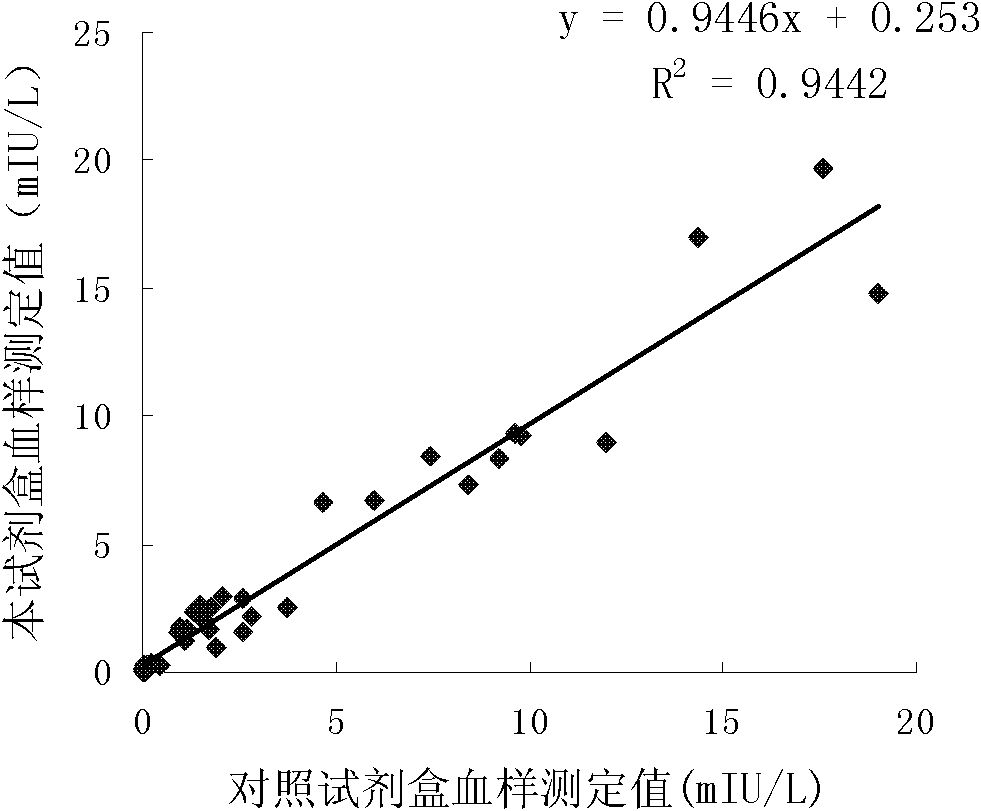
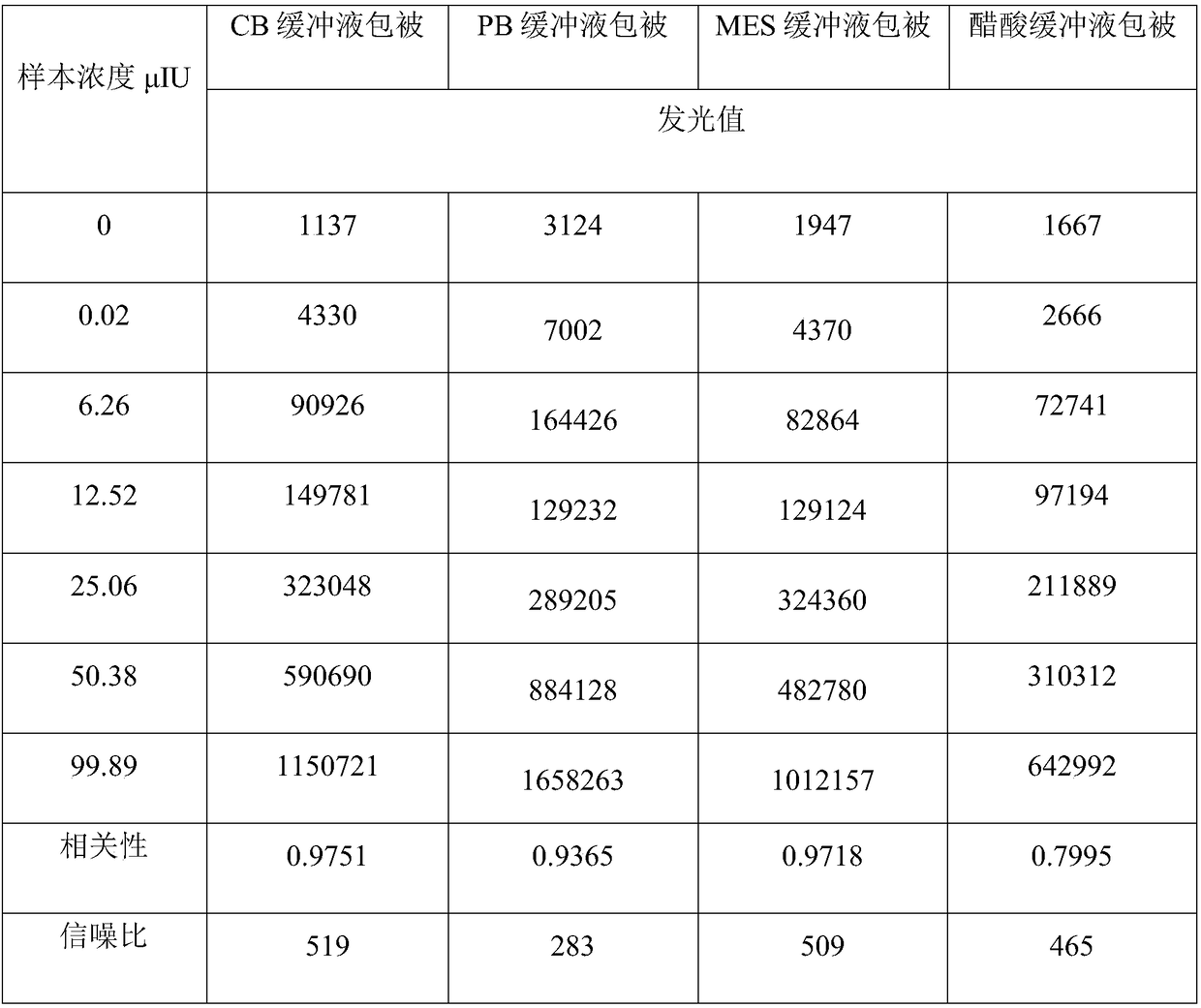
The invention discloses long-optical-path enzyme-linked immunoassay for testing thyroid stimulating hormone and provides a kit of the method. The long-optical-path enzyme-linked immunoassay comprises the following test steps of: performing immune reaction, separating, developing, measuring and processing data. A long-optical-path flowing sample pool and an optical fiber spectrometer are adopted for measurement; the detection sensitivity is 0.015mIU / L by increasing a measuring optical path, adopting a low-background substrate solution as well as optimizing a component preparation method; and compared with the sensitivity of the conventional optimized enzyme-linked immunoassay in which the same antibody is used, the sensitivity is enhanced by four times. The test linear range is 0.05 to 20mIU / L. The immune reaction is performed in a wrapped tube, and the reaction mode is a dual antibody sandwich one-step method. The kit comprises an antibody coated tube, a serial calibrator, an enzyme marker, a condensed cleaning solution, the low-background substrate solution, a terminating solution and an end-product diluent. The method is high in sensitivity and proper in test range, and the requirement of clinical detection can be met; meanwhile, the kit has low manufacturing cost and is favorable to popularization and application.
Owner:HTA CO LTD
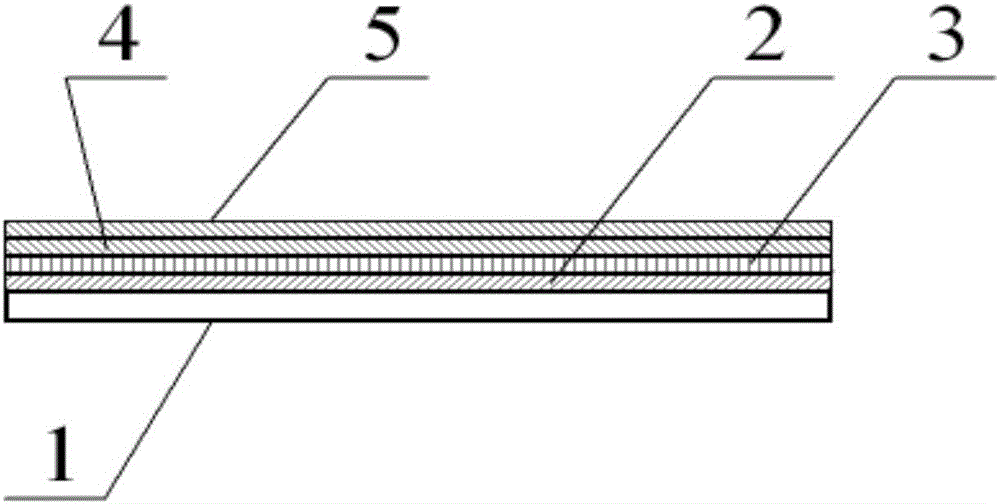
Thyroid stimulating hormone test paper and preparation method thereof
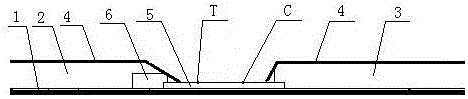
The invention discloses a thyroid-stimulating hormone detection test paper, which comprises a plastic substrate, a sample pad and a water-absorbing pad are arranged on the plastic substrate, a protective film is arranged on the sample-loading pad and the water-absorbing pad, and the bottom between the sample-loading pad and the water-absorbing pad is A nitrocellulose membrane is provided, and the connection end of the sample pad is provided with solid-phase colloidal gold, and one end of the solid-phase colloidal gold is overlapped with one end of the nitrocellulose membrane, and the nitrocellulose membrane is sequentially coated with detection Line and quality control line; the manufacturing method steps include: preparation of nitrocellulose membrane; marking and solid phase of polyester fiber strip; assembly and cutting. The present invention applies the principle of double-antibody sandwich method and immunochromatography to qualitatively detect human thyroid-stimulating hormone (TSH) in urine. The detection steps are relatively simple, and the results can be observed within 10 minutes. General users can perform the detection by themselves. The present invention judges whether there is a risk of hypothyroidism by comparing the color depth of the detection line with the matched color card.
Owner:NANTONG EGENS BIOTECH
TSH immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues
ActiveUS8617826B2Interference minimizationBiological testingImmunoassaysFollicle-stimulating hormoneIntracrine
Thyroid Stimulating Hormone (TSH) immunoassays are performed using an ELISA sandwich assay that employs scavenging or sacrificial beads for reducing interference caused by cross-reacting endocrine glycoprotein hormone analogues such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
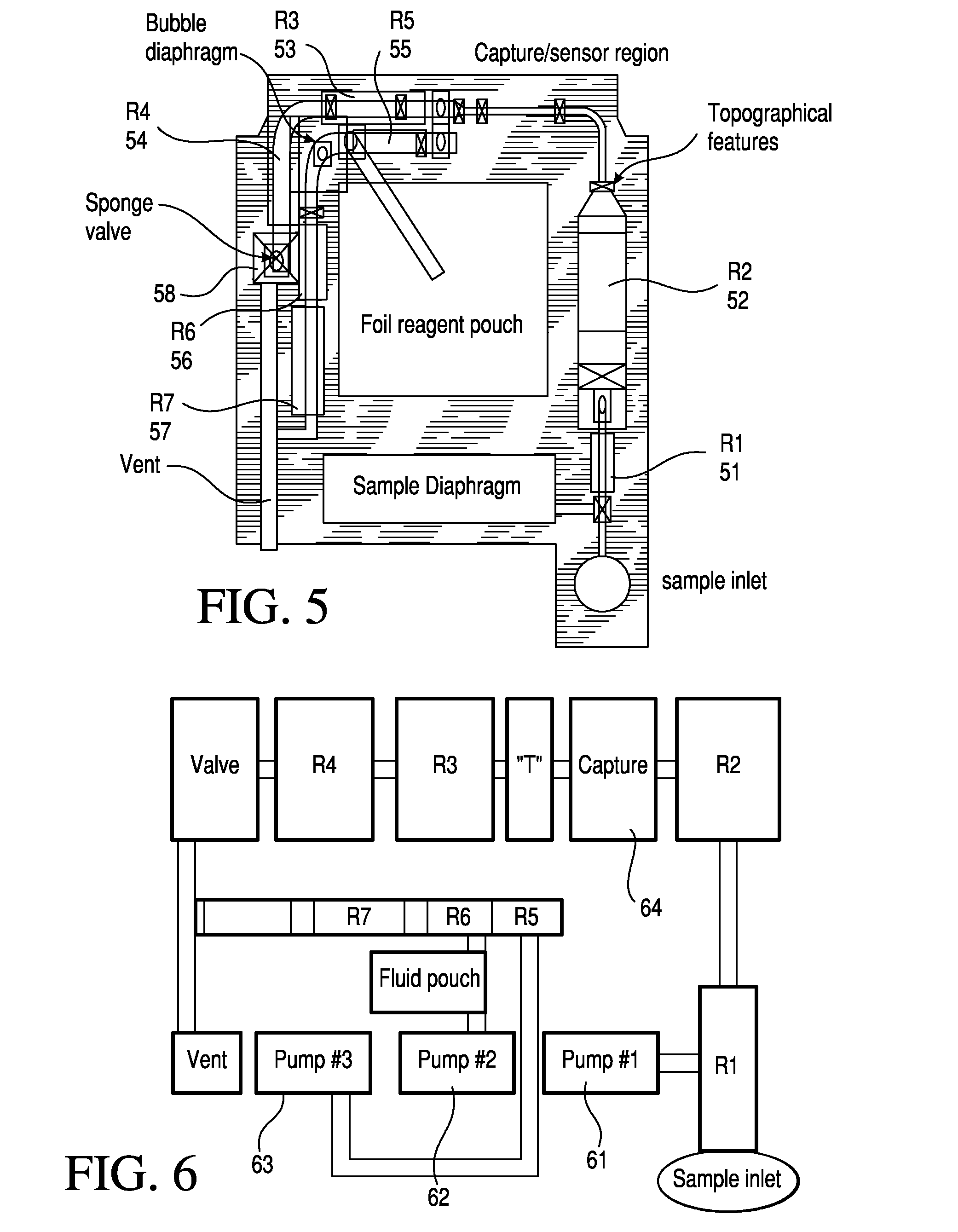
Micro-fluidic chemiluminescence detecting system for magnetic particles for detecting thyroid functions
ActiveCN107655878AAccurate quantitative detectionHigh sensitivityChemiluminescene/bioluminescenceMicrofluidicsEngineering
The invention discloses a micro-fluidic chemiluminescence detecting system for magnetic particles for detecting thyroid functions. The system comprises a bottom plate and an upper chip, and the upperchip comprises a sample adding area in the center of the upper chip and five micro-fluidic reaction detecting channels communicated with the sample adding area, wherein the five micro-fluidic reactiondetecting channels include a total triiodothyronine micro-fluidic reaction detecting channel, a total thyroxine micro-fluidic reaction detecting channel, a free triiodothyronine micro-fluidic reaction detecting channel, a free thyroxine micro-fluidic reaction detecting channel and a thyroid stimulating hormone micro-fluidic reaction detecting channel. In application of the system, the bottom plate is arranged under the upper chip, and magnets which are permanent magnets or electromagnets are arranged at positions, corresponding to magnetic particle coating areas of the micro-fluidic reactiondetecting channels, of the bottom plate. By ingenious combination of the micro-fluidic technology and the magnetic particle chemiluminescence technology, quickness, high sensitivity and accuracy in quantitative detection of target substances are realized.
Owner:BEIJING ELCOTEQ BIO TECH
TSH immunoassays and processes for performing TSH immunoassays in the presence of endogenous contaminants in restricted wash formats
ActiveUS9201078B2Interference minimizationEasy to washBiological material analysisLaboratory glasswaresFollicle-stimulating hormoneAntigen capture
The invention relates to low wash Thyroid Stimulating Hormone (TSH) immunoassays using an ELISA sandwich assay having limited or no wash step between the antigen capture, detection antibody addition and substrate introduction steps. This invention exhibits low cross reactivity with biologically similar interfering cross reacting species, such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
Preparation method of TSH (thyroid stimulating hormone) quantitative detection kit based on time-resolved fluoroimmunoassay
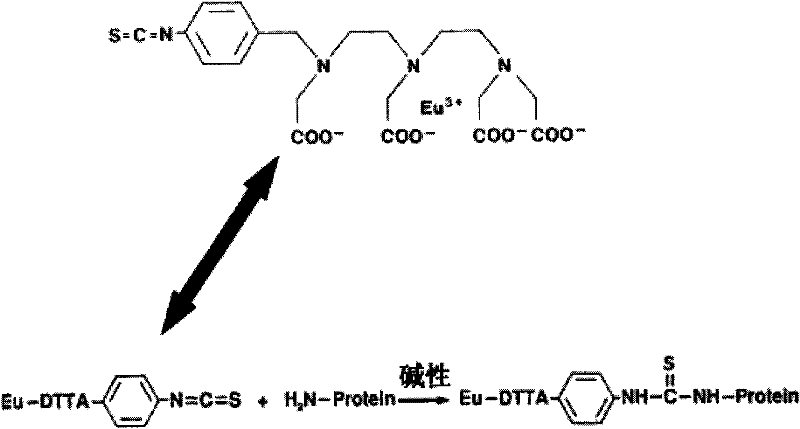
The invention provides a TSH (thyroid stimulating hormone) quantitative detection kit based on time-resolved fluoroimmunoassay, applicable to clinical screening of neonatal hypothyroidism. The preparation method of the kit is characterized by comprising the following steps of: 1, separating an antibody conjugate (Eu-Anti-TSH) from free rare earth ions (Eu<3+>) by using a specific chromatograph column; (2) using a bifunctional chelator (DTTA) for marking, wherein one end of the bifunctional chelator is chelated with rare earth ions (Eu) and the other end of the bifunctional chelator is connected with -NH2 of protein; adding beta-NTA to reinforcing liquid and regulating the concentration so that the fluorescence intensity of a finally obtained reagent is improved; and (4) adding a substitute Proclin300 for sodium azide to an analysis buffer system. Based on the characteristics of the steps in the preparation method, the TSH quantitative detection kit based on time-resolved fluoroimmunoassay can be produced and other in vitro diagnosis detection reagents can be developed.
Owner:北京协和洛克生物技术有限责任公司
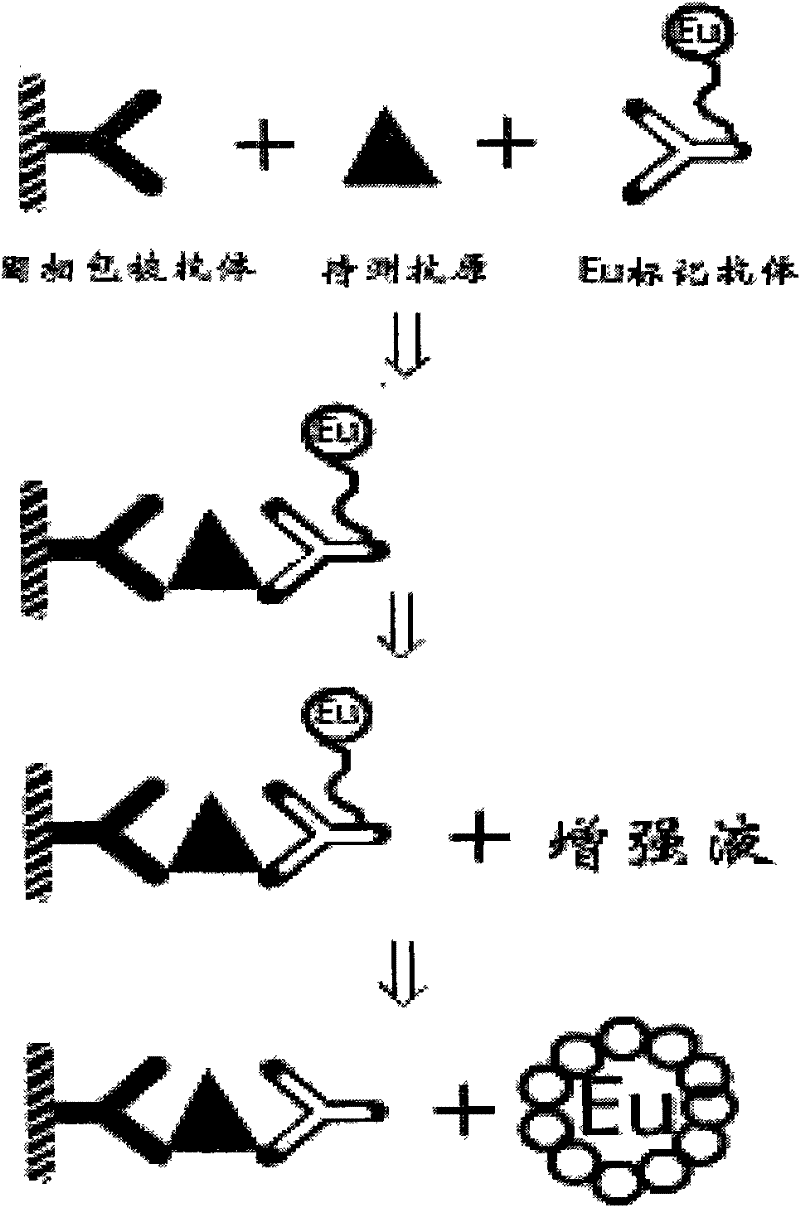
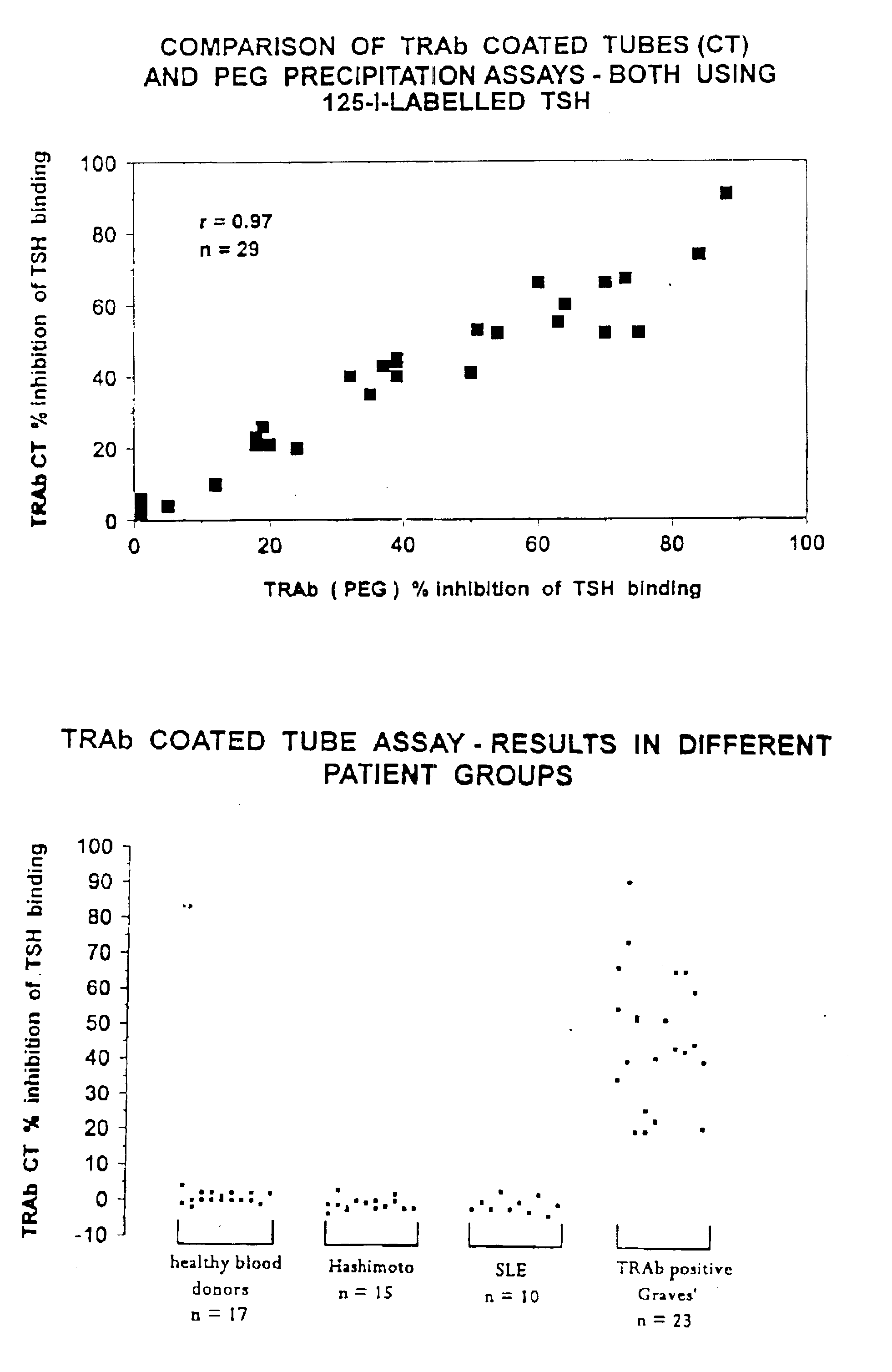
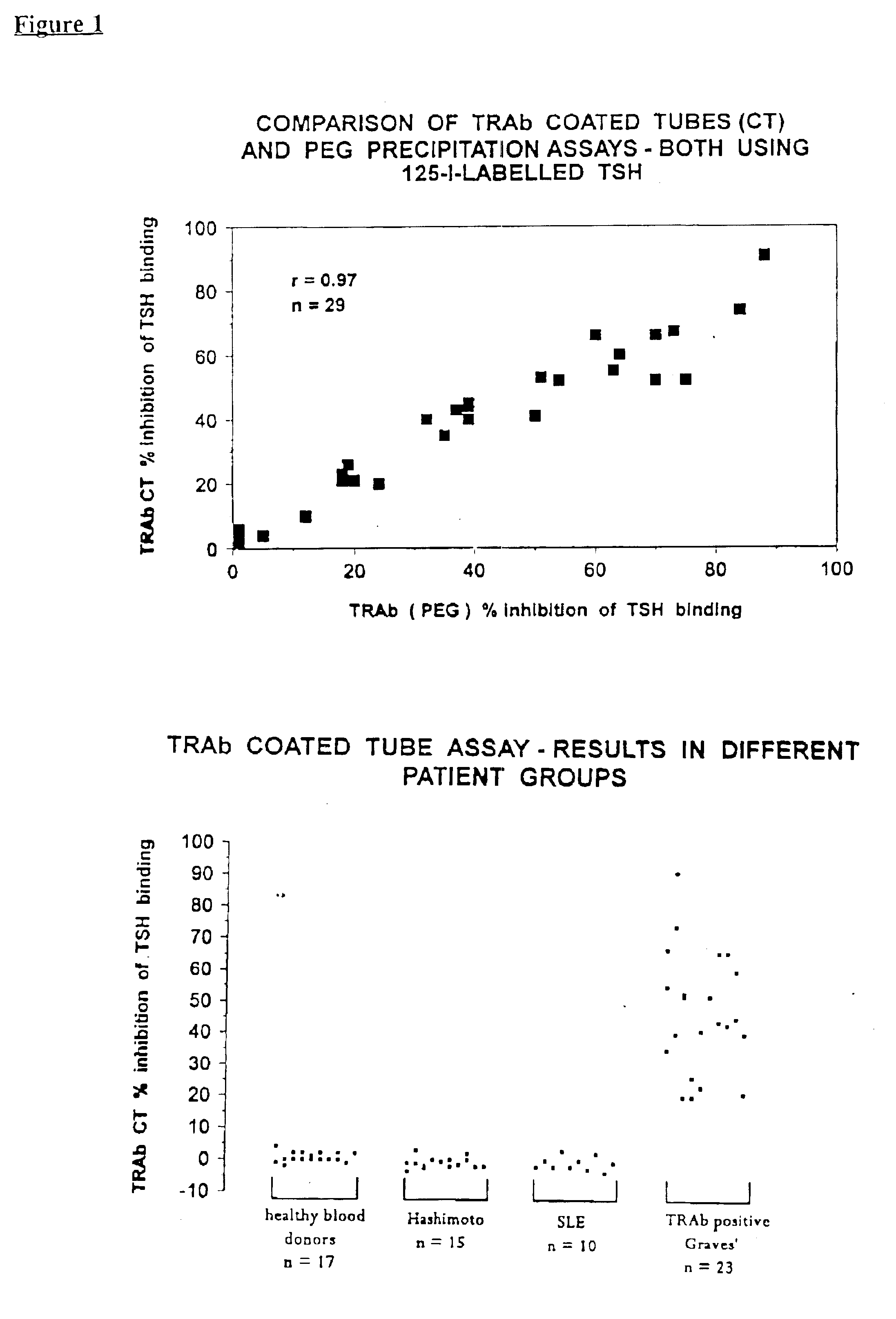
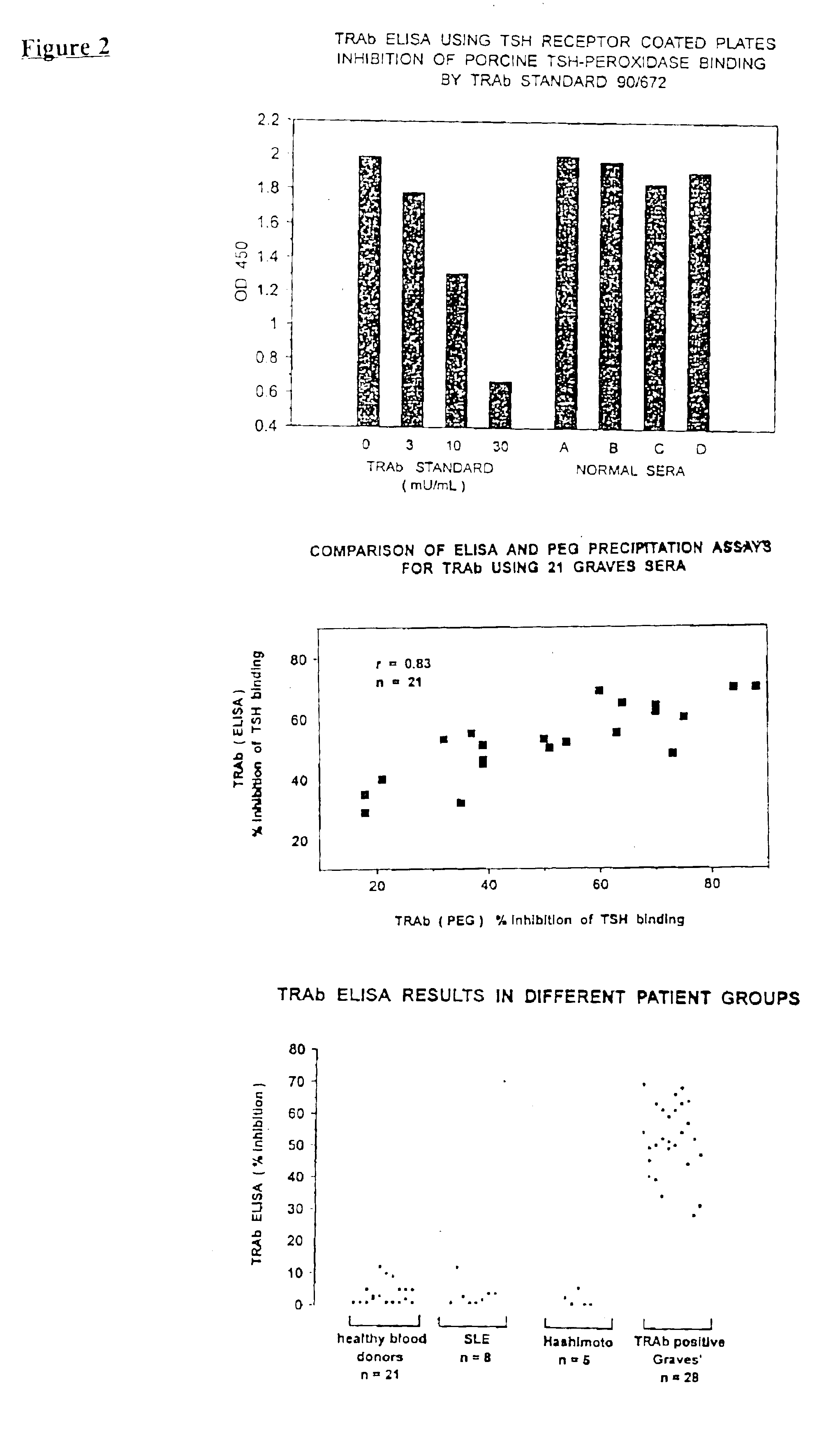
Assays for TSH receptor autoantibodies
A method and kit for monitoring autoantibodies to thyroid stimulating hormone (TSH) receptor in a sample of body fluid, which employs the steps of:(i) incubating TSH receptor with a sample of body fluid;(ii) reacting the incubated sample of body fluid with at least one binding agent which is capable of binding to the TSH receptor in competitive reaction with TSH receptor autoantibodies (TRAb), or in a case where TSH receptor is complexed to labelled antibody, reacting the sample of body fluid with at least one binding agent which can bind to TRAb in such a way as not substantially to interfere with binding of the TRAb to the TSH receptor; and(iii) detecting bound TRAb in the reacted incubated sample of body fluid.
Owner:R S R
TSH antibodies for point-of-care immunoassay formats
ActiveUS9199234B2Interference minimizationBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careFollicle-stimulating hormone
The invention relates to antibody characteristics used to design a whole blood Point of Care Thyroid Stimulating Hormone (TSH) immunoassay using an ELISA sandwich assay lacking one or more wash steps between the antigen capture, detection antibody addition and substrate introduction steps. This invention exhibits low cross reactivity with biologically similar interfering cross reacting species, such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
Method of high sensitive immunoassay
InactiveUS20090087923A1Enhancing agglutination reactionBiological material analysisBiological testingEpitopeAnalyte
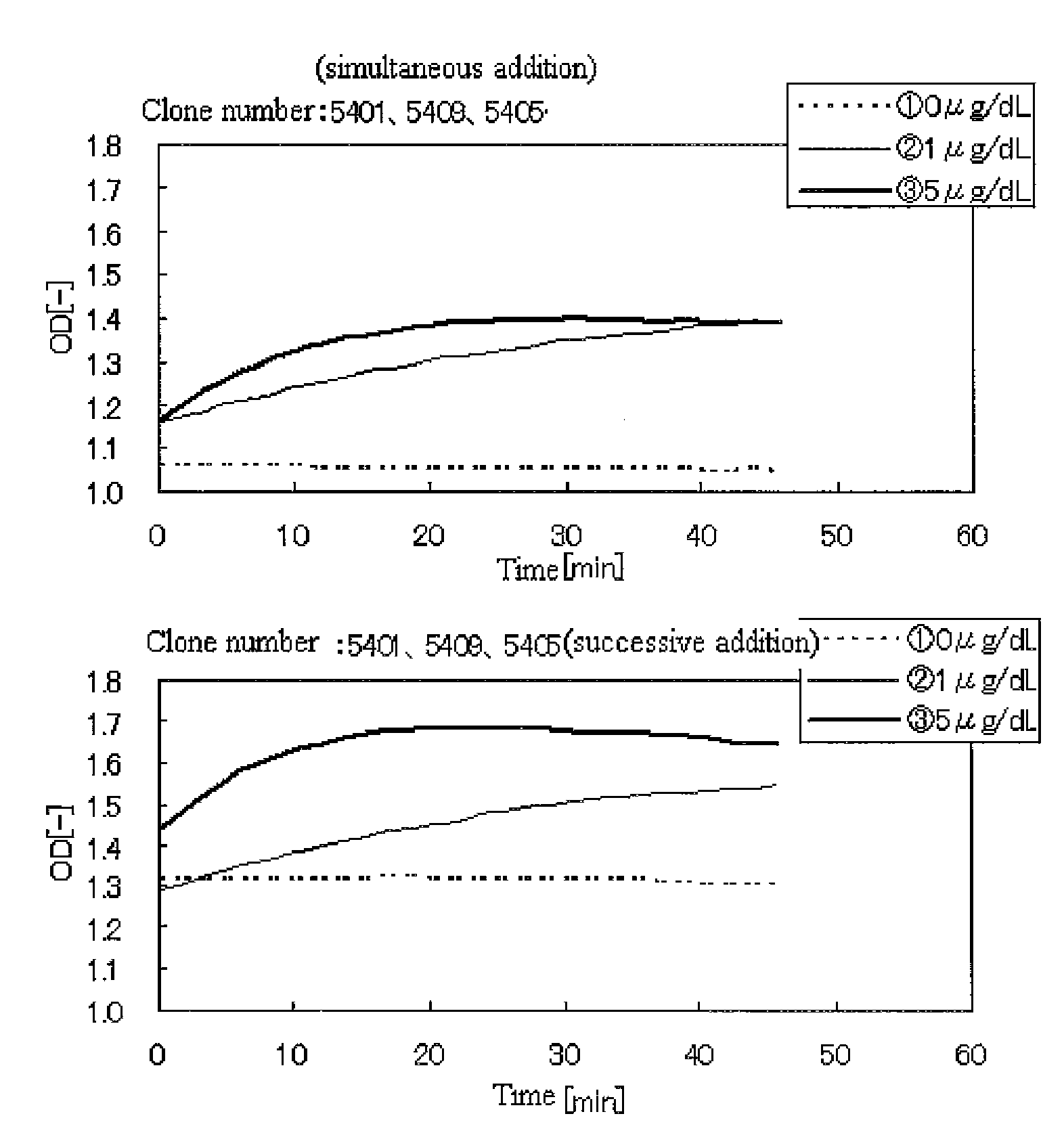
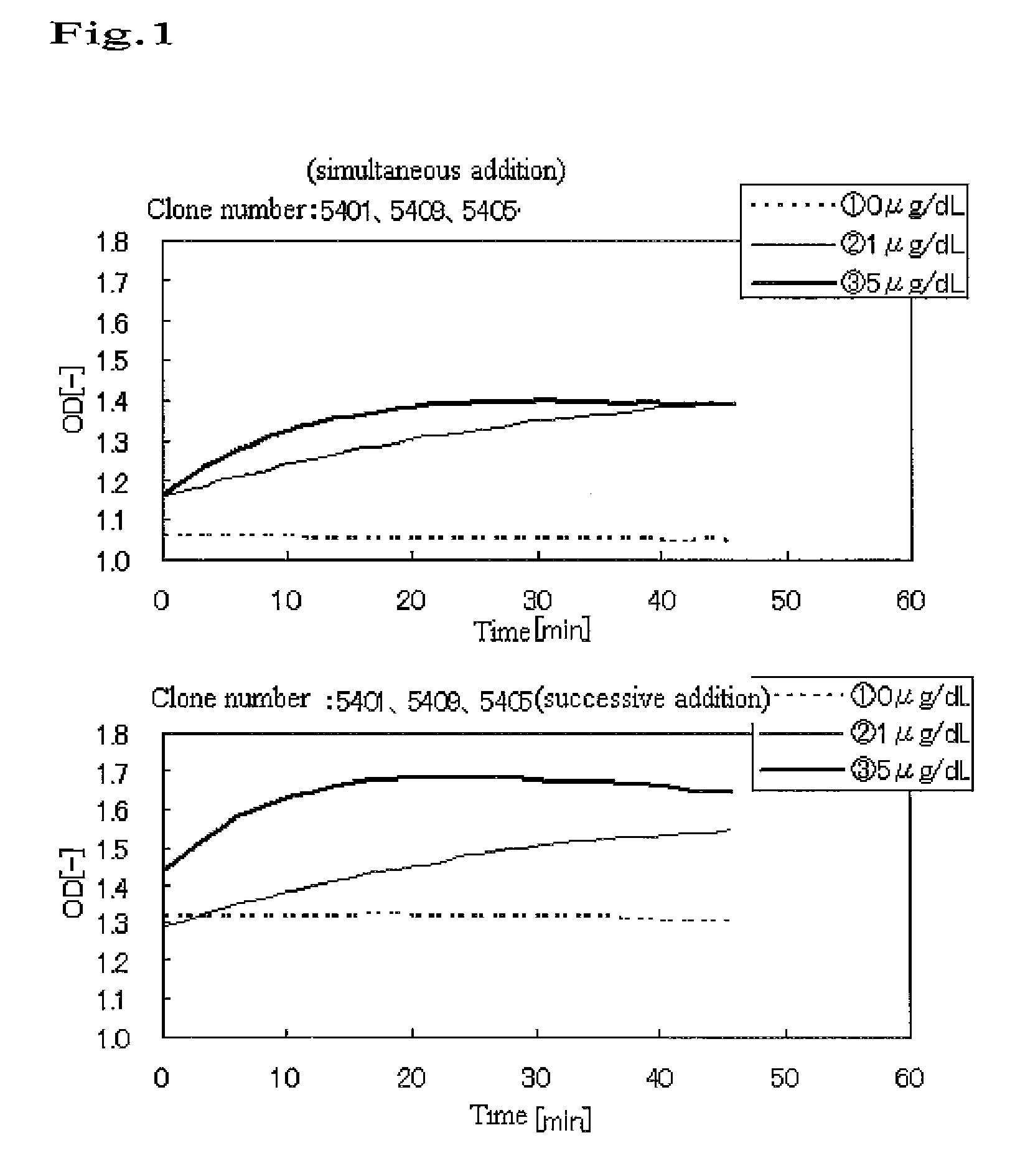
An object of the present invention is to provide a method of high sensitive immunoassay using immunoagglutination reaction by antigen-antibody reaction for quantification of thyroid stimulating hormone (TSH). The present invention provides a method of assaying a thyroid stimulating hormone (TSH) comprising assaying agglutination which is generated by contacting TSH with a carrier to which an anti-TSH antibody has been bound, wherein a plurality of types of anti-TSH antibodies that recognize different epitopes of TSH are independently supported on separate carriers, and each carrier on which a TSH antibody has been supported is brought into contact with a TSH-containing analyte with time intervals.
Owner:FUJIFILM CORP
Tsh immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues
ActiveUS20120301906A1Interference minimizationBiological testingFluorescence/phosphorescenceFollicle-stimulating hormoneIntracrine
The invention relates to Thyroid Stimulating Hormone (TSH) immunoassays using an ELISA sandwich assay that employs scavenging or sacrificial beads for reducing interference caused by cross-reacting endocrine glycoprotein hormone analogues such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
Methods for diagnosis of acute coronary syndrome
InactiveUS20090215077A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Owner:RULES BASED MEDICINE
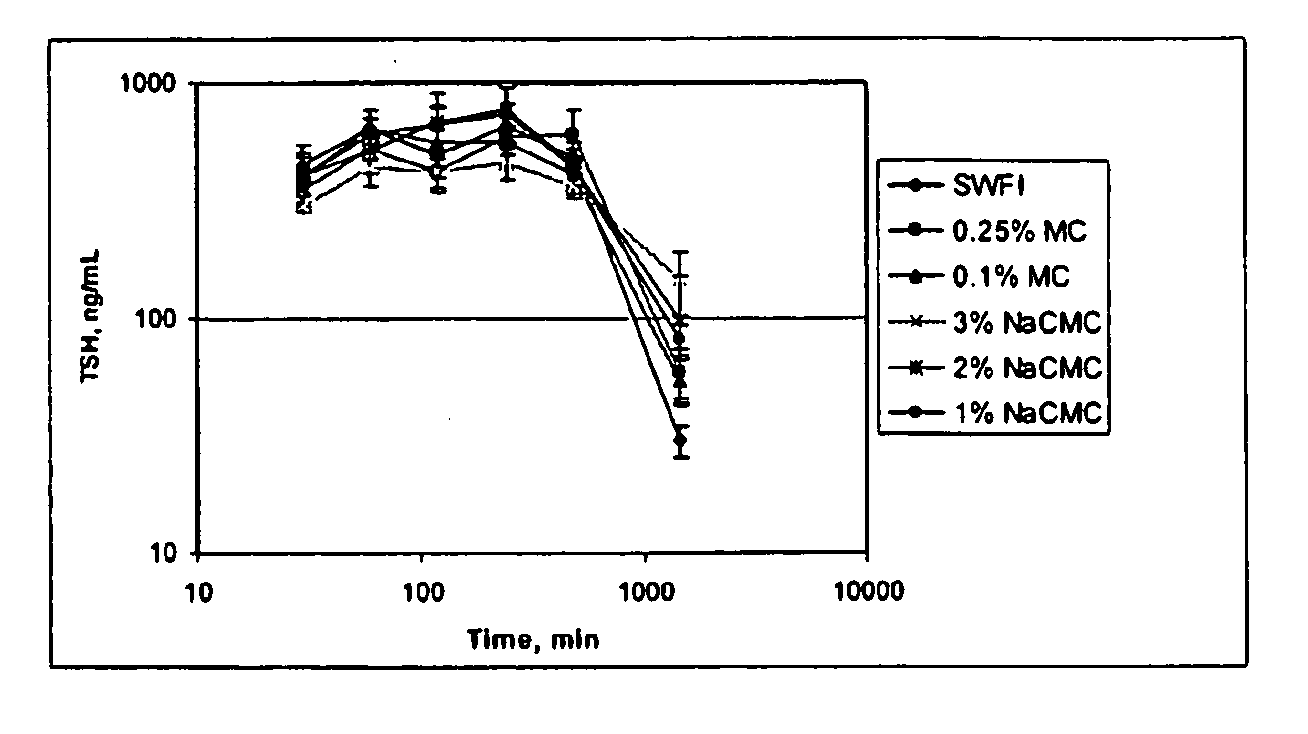
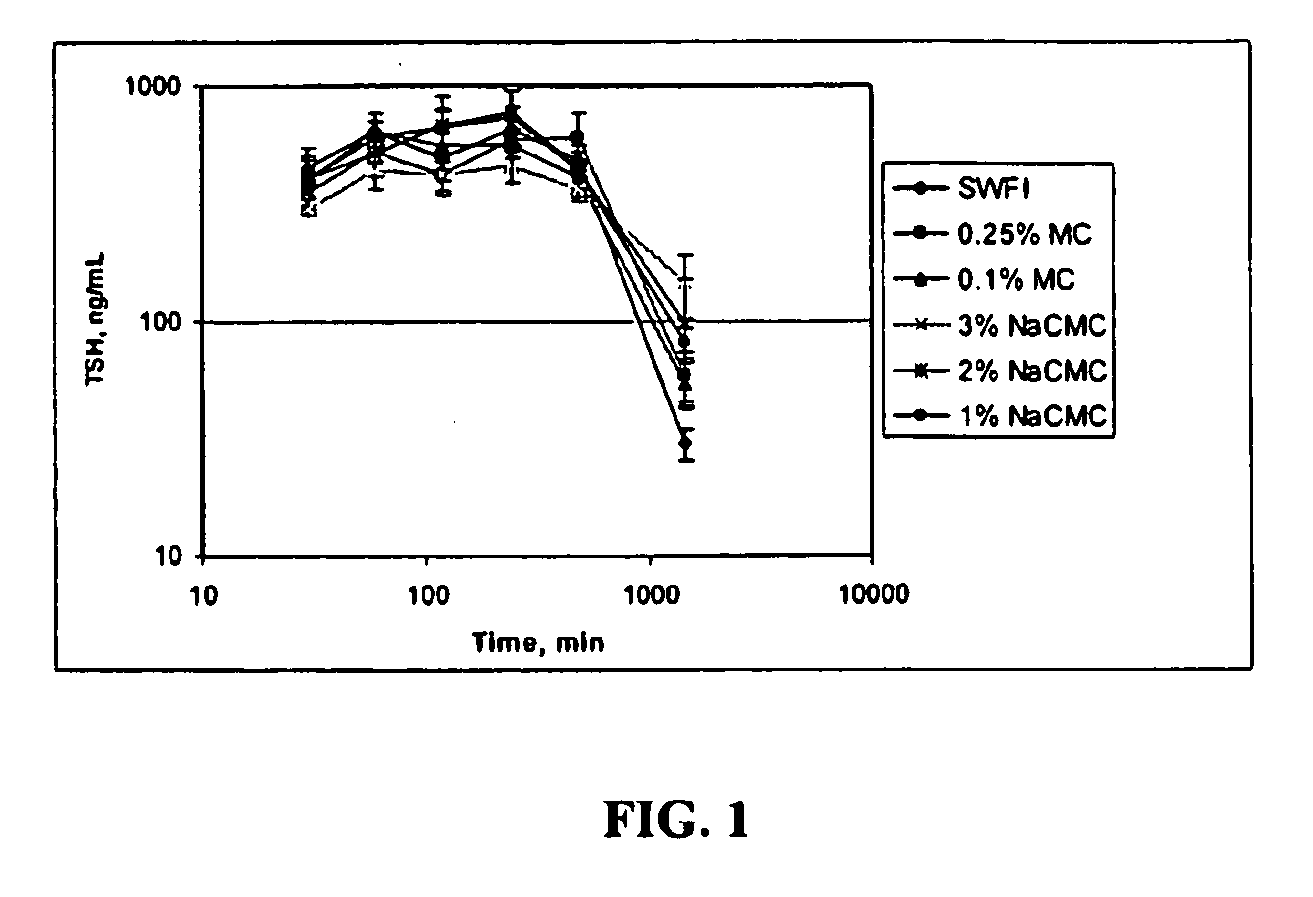
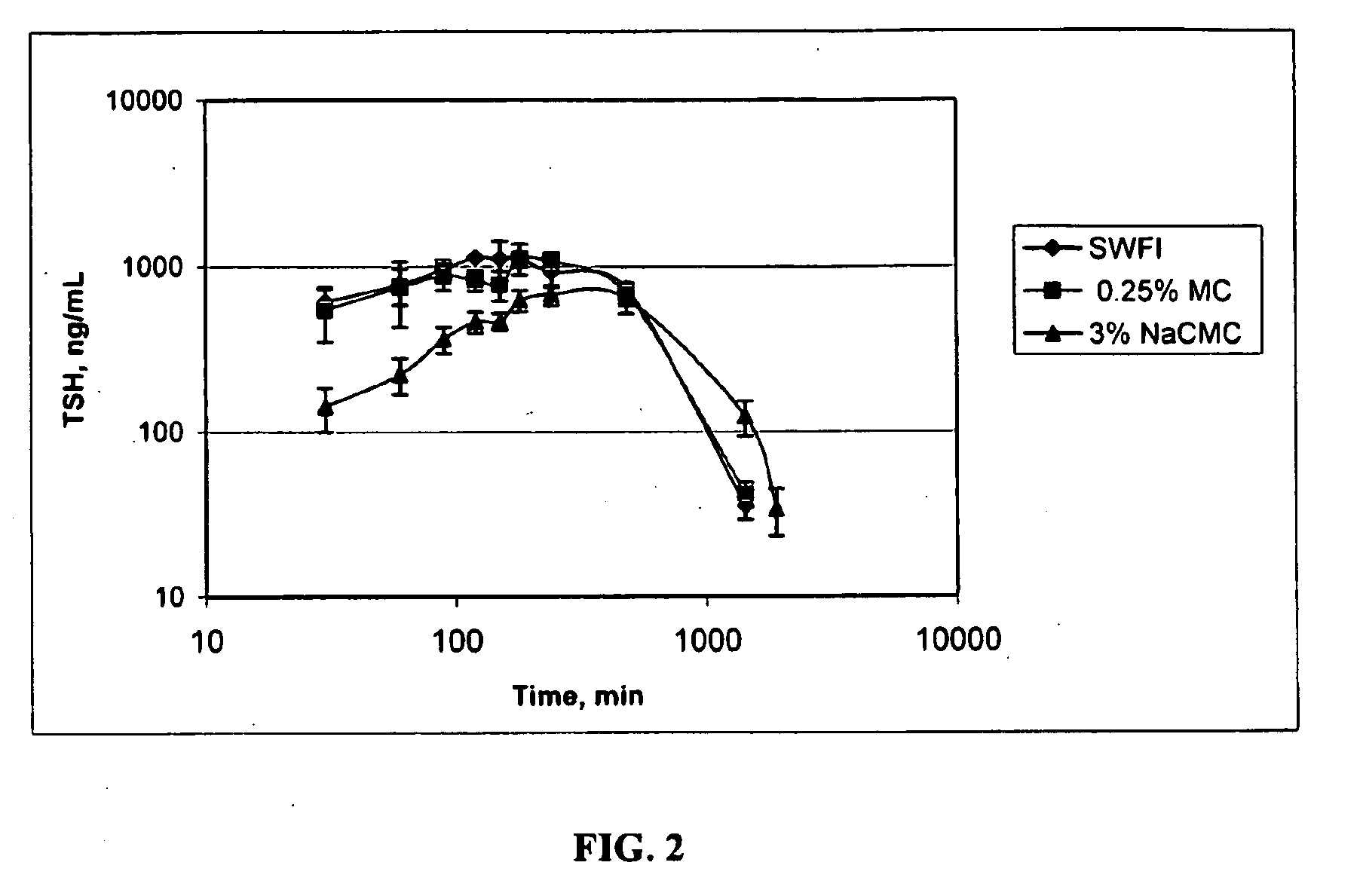
Formulations For Therapeutic Administration Of Thyroid Stimulating Hormone (TSH)
InactiveUS20100048455A1Nervous disorderPeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
This disclosure generally relates to novel formulations containing the active ingredient thyroid stimulating hormone (TSH) having modified pharmacokinetic profiles as compared to prior art formulations.
Owner:GENZYME CORP
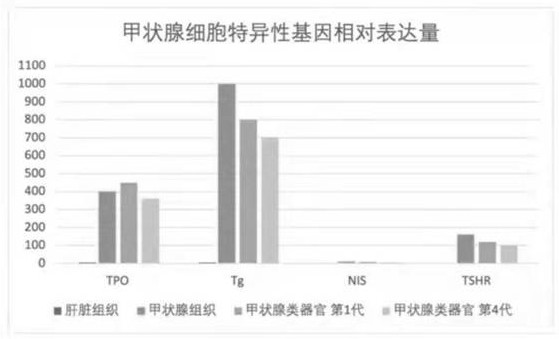
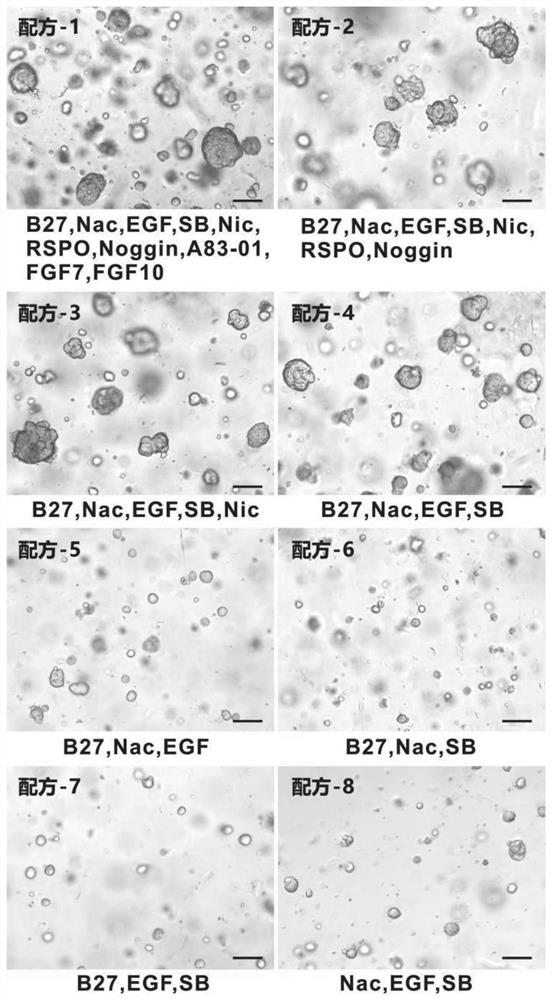
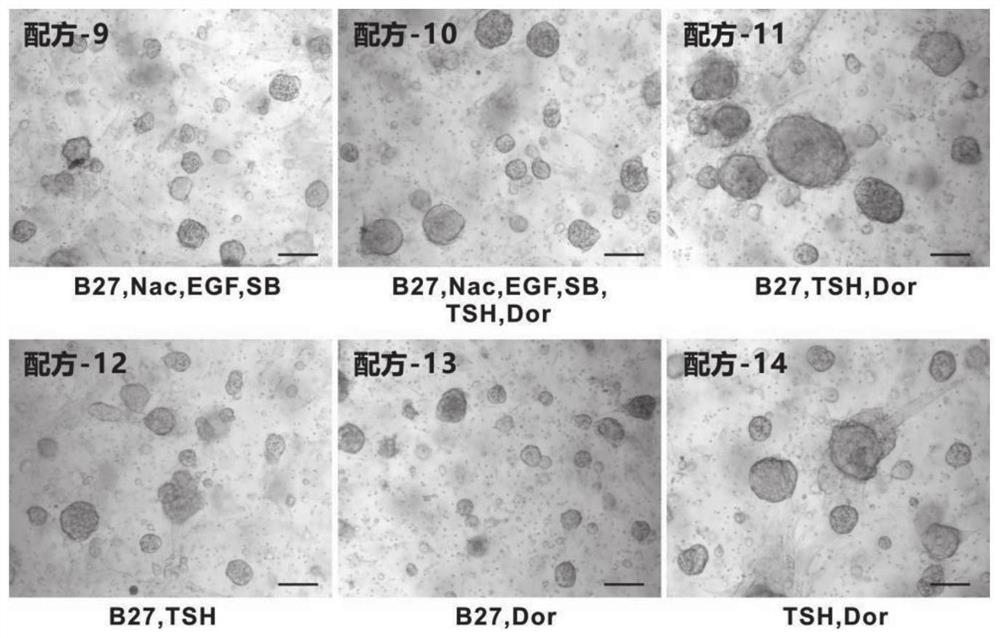
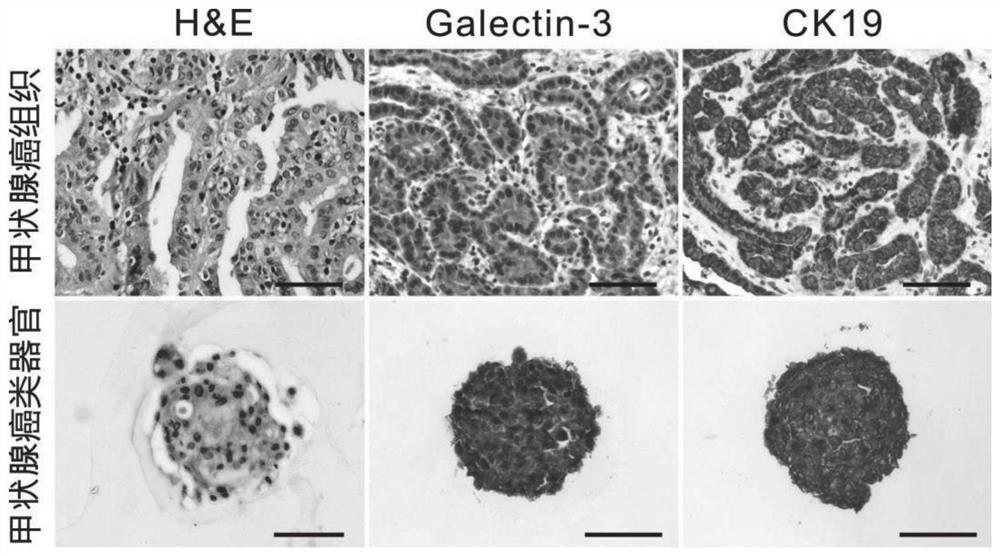
Thyroid organoid culture medium and thyroid organoid culture and passage method
PendingCN114317399AEasy to trainPromote formationVertebrate cellsArtificial cell constructsHydrocortisonePancreatic hormone
The invention discloses a thyroid organoid culture medium and a thyroid organoid culture and passage method. The thyroid organ culture medium comprises an Advanced DMEM (Dulbecco's Modified Eagle Medium) / F12 culture medium and the following components: B-27, N-acetylcysteine, nicotinamide, R-spondin1 recombinant protein, head protein, Wnt-3a, an epidermal growth factor, A83-01, a thyroid stimulating hormone, hydrocortisone, insulin, a fibroblast growth factor-10 and Y27632. The thyroid stimulating hormone, the hydrocortisone and the insulin are innovatively added into the thyroid organ culture medium, the thyroid organ formation can be remarkably promoted by adding the thyroid stimulating hormone, the cell proliferation and growth effect can be effectively promoted by adding the hydrocortisone, the clone formation rate can be increased, and the culture medium has the advantages that the cost is reduced; by adding insulin, the dryness of cells can be maintained, the passage efficiency is improved, and the thyroid organ culture medium can be used for culturing thyroid organs very well.
Owner:川北医学院
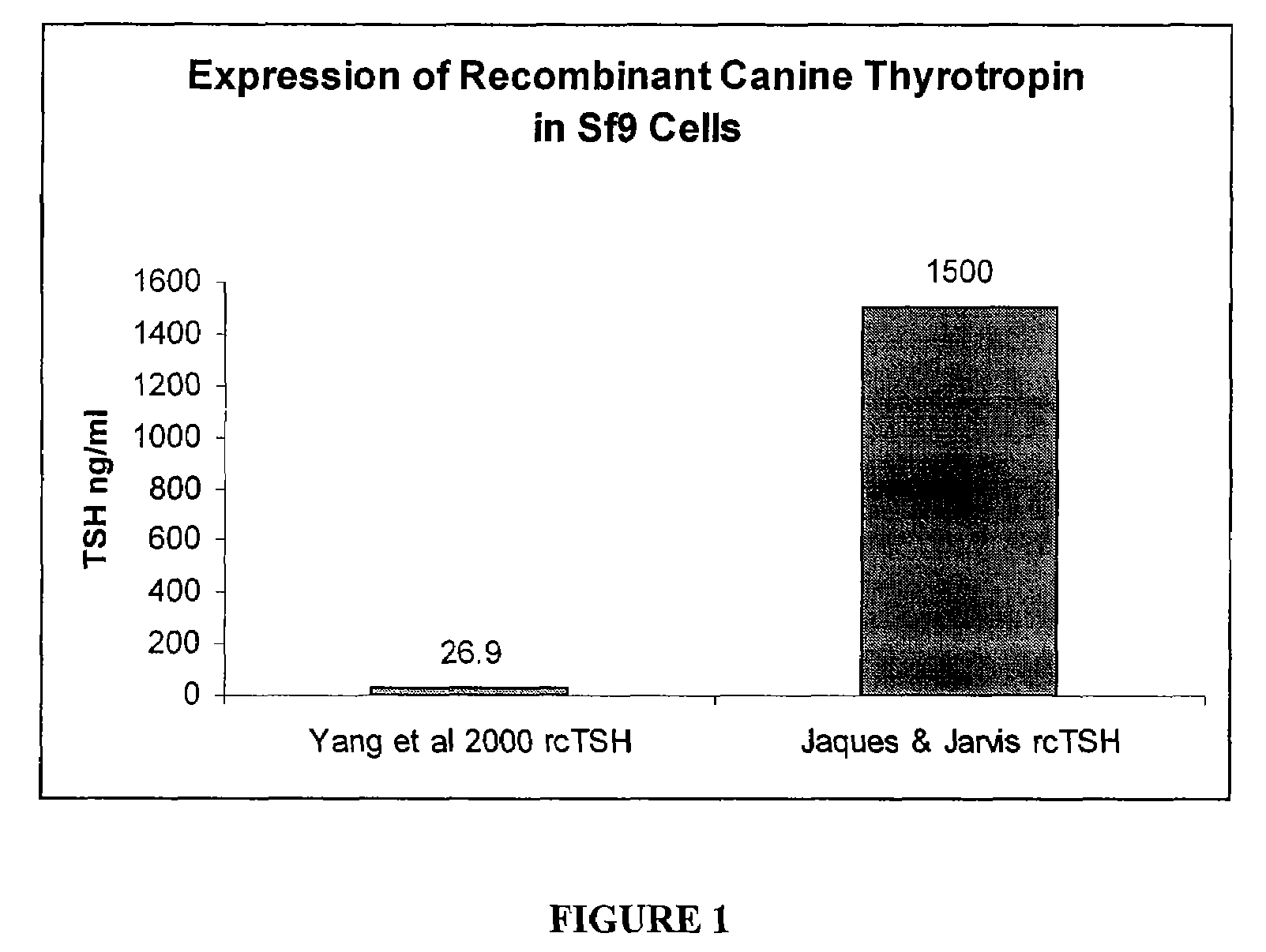
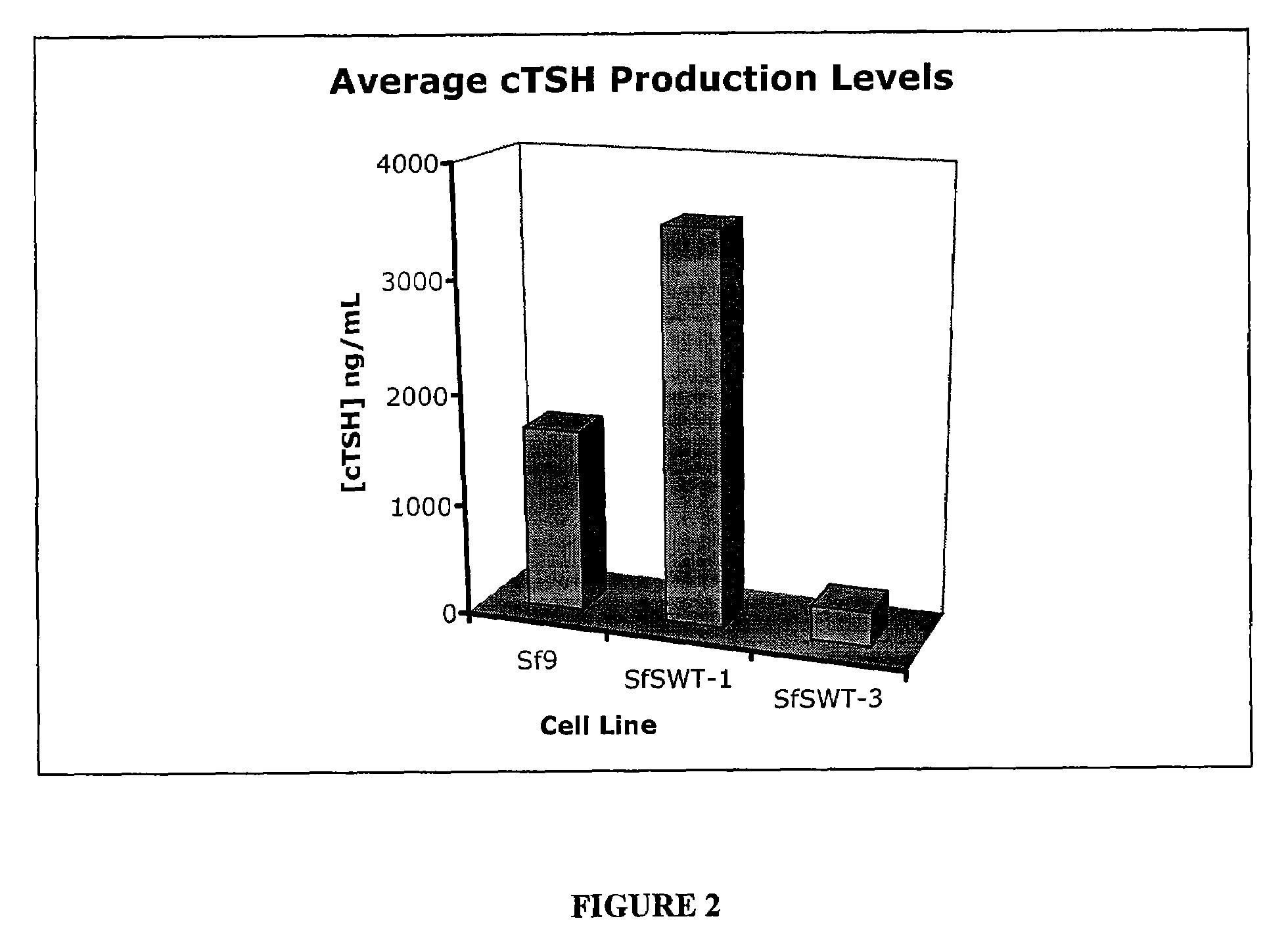
Recombinant canine thyroid stimulating hormone and methods of production and use thereof
The invention includes a nucleic acid having a sequence at least 98% homologous to SEQ ID NO: 1, which encodes the α subunit of canine thyroid stimulating hormone (TSH). The invention also includes a nucleic acid having a sequence at least 98% homologous to SEQ ID NO: 2, which encodes the β subunit of canine TSH. The invention also includes a method of producing an recombinant canine thyroid stimulating hormone (rcTSH) subunit by expressing a nucleic acid having a sequence of SEQ ID NO: 1 and a nucleic acid having a sequence of SEQ ID NO: 2 in a transgenic insect cell modified to sialylate proteins and producing a sialylated rcTSH subunit. The insect cell may be a lepidopteran cell. The rcTSH may be used for diagnosis and treatment. It may be used to diagnose canine hypothyroidism.
Owner:JAQUES JOHN SCOTT T +1
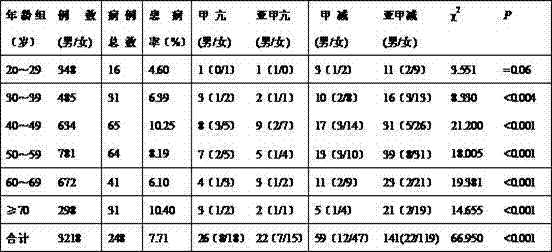
Investigation and analysis method of thyroid function abnormalities of healthy people in water-source-originated high-iodine region
InactiveCN106934751AExpedited screeningEasy to monitorData processing applicationsDisease diagnosisDiseaseStatistical analysis
The invention discloses an investigation and analysis method for abnormal thyroid function of healthy people in water-sourced high-iodine areas. 3218 healthy people were taken for physical examination, and fasting venous blood was collected to separate serum, and serum thyroid-stimulating hormone (TSH) was measured with an immunochemiluminescent instrument. , total triiodothyronine (TT3), total thyroxine (TT4), free triiodothyronine (FT3), and free thyroxine (FT4), and statistically analyzed the test results. The detection rate of thyroid disease is affected by many factors such as gender, age, race, and region. In areas with high iodine in water sources, the detection rate of abnormal thyroid function in healthy people increases year by year, mainly subclinical hypothyroidism, and is related to gender and Age-related, especially the higher incidence of subclinical hypothyroidism. Through the method of the invention, the thyroid function in an area can be screened and analyzed conveniently, the monitoring of thyroid diseases is strengthened, the early diagnosis of thyroid diseases is beneficial, and the health of the people is ensured.
Owner:CANGZHOU MEDICAL COLLEGE
Tsh immunoassays and processes for performing tsh immunoassays in the presence of endogenous contaminants in restricted wash formats
ActiveUS20120301905A1Interference minimizationEasy to washBiological material analysisLaboratory glasswaresFollicle-stimulating hormoneAntigen capture
The invention relates to low wash Thyroid Stimulating Hormone (TSH) immunoassays using an ELISA sandwich assay having limited or no wash step between the antigen capture, detection antibody addition and substrate introduction steps. This invention exhibits low cross reactivity with biologically similar interfering cross reacting species, such as Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH) and Chorionic Gonadotropin (CG).
Owner:ABBOTT POINT CARE
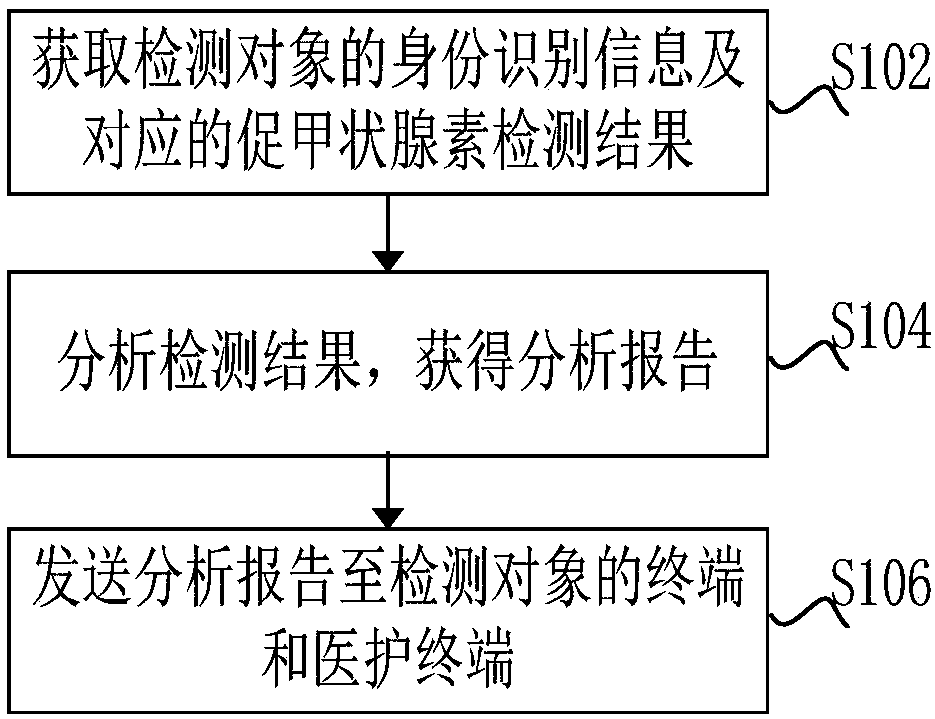
Intelligent detection method and system for thyroid stimulating hormone
InactiveCN109324196AEnsure consistencyImprove convenienceBiological testingIdentity recognitionComputer terminal
The invention discloses an intelligent detection method and system for thyroid stimulating hormone. The method comprises the following steps of acquiring identity recognition information of a detectedobject and a corresponding thyroid stimulating hormone detection result; analyzing the detection result to obtain an analysis report; and sending the analysis report to a terminal of the detected object and a medical care terminal. According to the intelligent detection method and system, voice information and identifier information containing identity information of the detected object are read;the identity information contained in a voice and the identity information contained in an identifier are compared; if the identity information contained in the voice is consistent with the identityinformation contained in the identifier, the thyroid stimulating hormone is detected; the detection result is analyzed; a detection value is compared with a preset threshold value to judge whether thedetection result is normal or not; and the detection result and abnormal alarm information are pushed to the terminal of the detected object and the medical care terminal to remind attention of a patient and medical care personnel, so that the consistency between the identity information of the detected object and a sample is ensured, the working efficiency is improved, and the convenience of theto-be-detected object is improved.
Owner:芜湖森爱驰生物科技有限公司
Thyroid stimulating hormone TSH kit based on microfluidic chip and preparation and detection methods
The invention discloses a thyroid stimulating hormone TSH kit based on a microfluidic chip and preparation and detection methods. The preparation method of the thyroid stimulating hormone TSH kit comprises the following steps: (1) coating the microfluidic chip; (2) labeling fluorescent microspheres; (3) assembling the microfluidic chip; (4) preparing a scaling product; (5) obtaining the thyroid stimulating hormone TSH kit based on the microfluidic chip. The prepared thyroid stimulating hormone TSH kit adopts a double-antibody sandwich method to carry out measurement, selects a time-resolutionfluorescent substance with high sensitivity as a labeling substance, carries out antibody labeling on the fluorescent microspheres and utilizes immunoreaction of antibody pairs to carry out analysis and detection, and the property of the prepared reagent can reach the level of an equivalent chemiluminescence reagent. After immunoreaction, cleaning solution is used for completely removing redundantcomponents, detection reading is carried out on the fluorescent microspheres combined by the immunoreaction, so that the problem that developing solution is introduced into the chip to cause incomplete reaction or untimely reading after developing is avoided.
Owner:NANJING LANSION BIOTECH CO LTD
Method for examining irregular menstruation
InactiveCN113030496AProtection from radiation damageShort detection timeDisease diagnosisBiological testingPhysiologyProgestogen
The invention discloses a method for examining irregular menstruation. The method comprises the following steps of: taking menstrual blood; and detecting estradiol, prolactin, testosterone, luteinizing hormone, progestational hormone, follicle-stimulating hormone, thyroxine (T4), triiodothyronine (T3), thyroid stimulating hormone (TSH), free T3, free T4, thyroid stimulating hormone (TSH) and epithelial cells in the menstrual blood; and prompting the reasons of the irregular menstruation. The method has no radiation damage to human bodies, and is short in detection time and low in cost. As the menstrual blood can be obtained very conveniently and noninvasively , women can detect the menstrual blood once a month, so that the women can know the reason of irregular menstruation as soon as possible and take prevention and treatment measures.
Owner:广州往圣智能科技有限公司
Trophic hormone fusion protein, preparation method and application thereof
ActiveUS9969786B2Improve fertilityStimulating folliculogenesisPeptide/protein ingredientsAntibody mimetics/scaffoldsΒ subunitHalf-life
Owner:SUZHOU ALPHAMAB
Culture medium and culture method for thyroid cancer organoid
PendingCN113278586AImprove the success rate of cultivationExtended passage timesCell culture active agentsTumor/cancer cellsOncologyThyroid cancer
The invention provides a culture medium and a culture method for a thyroid cancer organoid. The culture medium contains a basic culture medium, B27, a p38MAPK inhibitor and thyroid stimulating hormone; and the culture medium does not contain at least one of Wnt3a, R-spondin-1, noggin, a fibroblast growth factor and A83-01. When the culture medium is used for organoid culture, the cost is low, the operation difficulty is low, the culture success rate is high, and the passage number is large.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Ovulation detection test strip and detection method
PendingCN112730854AHigh detection sensitivityStrong specificityBiological testingAntiendomysial antibodiesCotinine
The invention discloses an ovulation detection test strip. The ovulation detection test strip comprises a PVC bottom plate, a sample pad, a gold label pad, a nitrocellulose film and a water absorption pad, wherein the nitrocellulose film is provided with a detection line T1, a detection line T2 and a quality control line, the detection line T1 is coated with a mouse anti-human beta LH monoclonal antibody 1A4, the detection line T2 is coated with a cotinine antigen, and the quality control line is coated with a goat anti-chicken IgY polyclonal antibody; and a colloidal gold mouse anti-human beta LH monoclonal antibody 6B8 conjugate, a colloidal gold chicken IgY antibody conjugate and a colloidal gold cotinine monoclonal antibody conjugate are cured on the gold label pad. The test strip is high in detection sensitivity and good in specificity, abnormal interpretation results caused by crossing of LH with follicle stimulating hormone (FSH) and thyroid stimulating hormone (TSH) can be avoided, detection results are more accurate, and monitoring of the smoking metabolite cotinine in urine is combined. The invention further discloses an ovulation detection method, the steps are simple, and the test result is accurate.
Owner:杭州新脉生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com