Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Sterility assurance level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
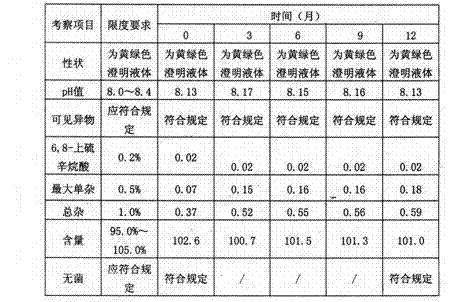
In microbiology, sterility assurance level (SAL) is the probability that a single unit that has been subjected to sterilization nevertheless remains nonsterile. It is never possible to prove that all organisms have been destroyed, as the likelihood of survival of an individual microorganism is never zero. So SAL is used to express the probability of the survival. For example, medical device manufacturers design their sterilization processes for an extremely low SAL, such as 10⁻⁶, which is a 1 in 1,000,000 chance of a non-sterile unit. SAL also describes the killing efficacy of a sterilization process. A very effective sterilization process has a very low SAL.
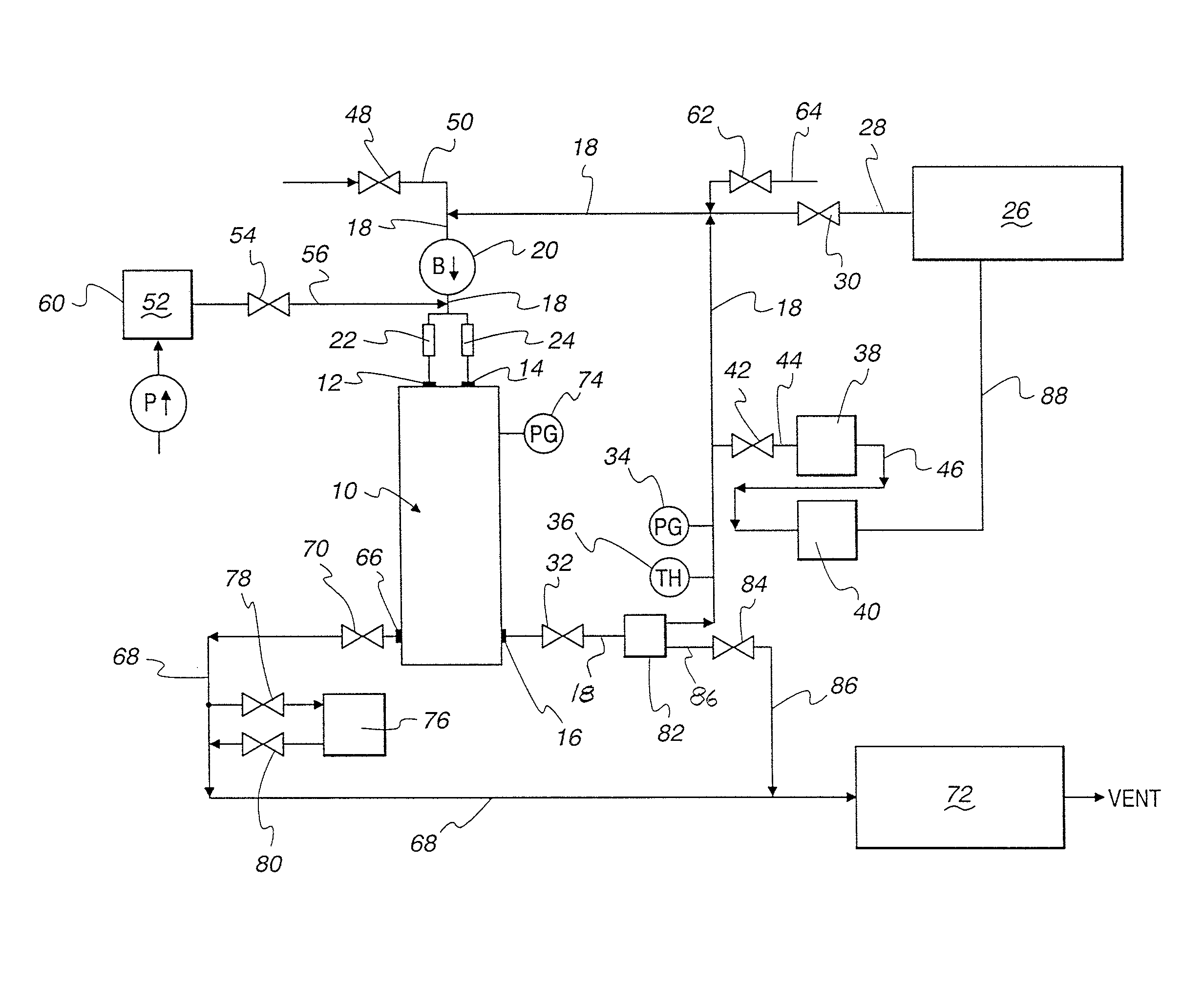
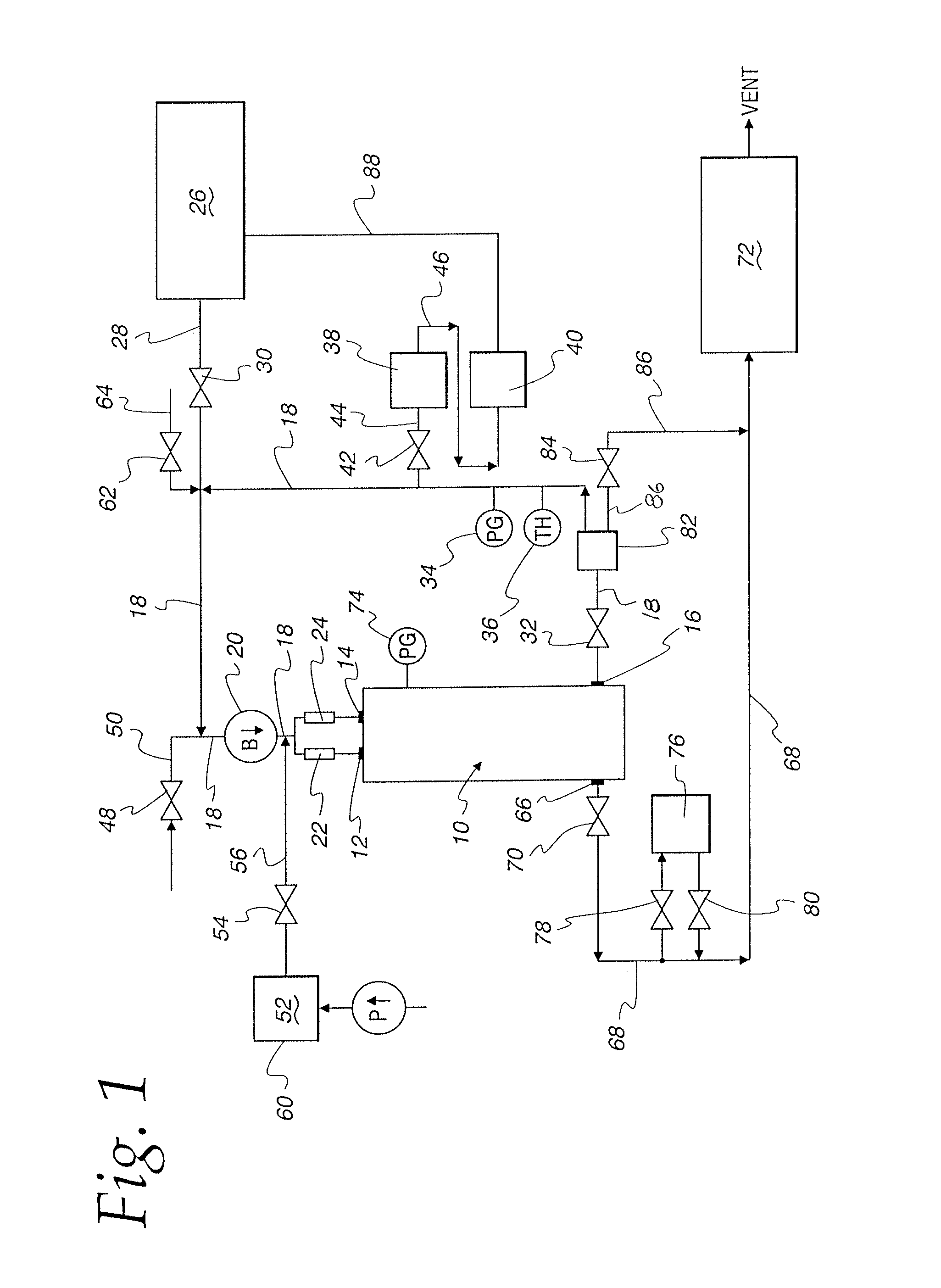
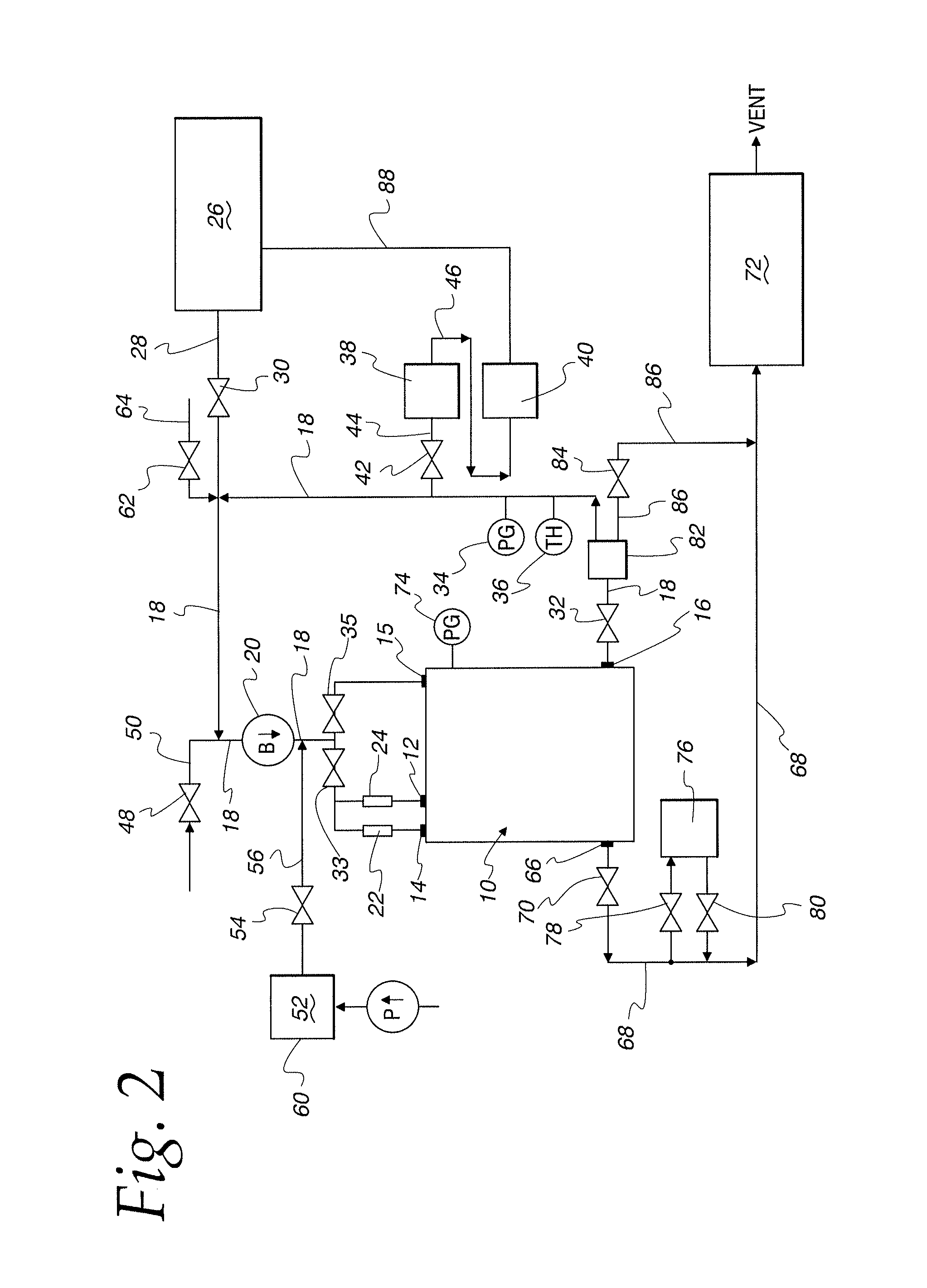
Method and apparatus for validation of sterilization process
InactiveUS20050135965A1Analysis using chemical indicatorsMaterial analysis using wave/particle radiationDosimeterEngineering
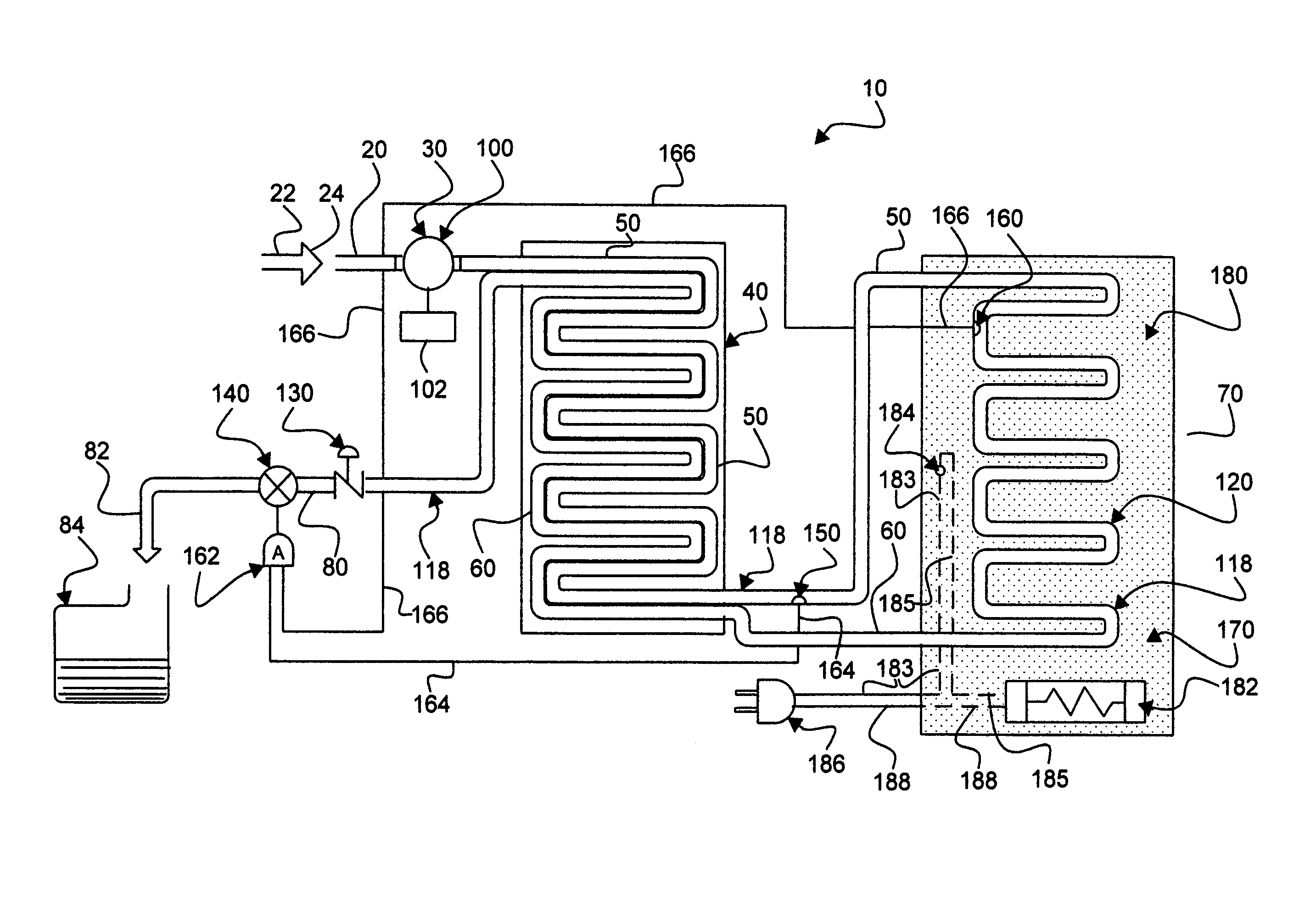
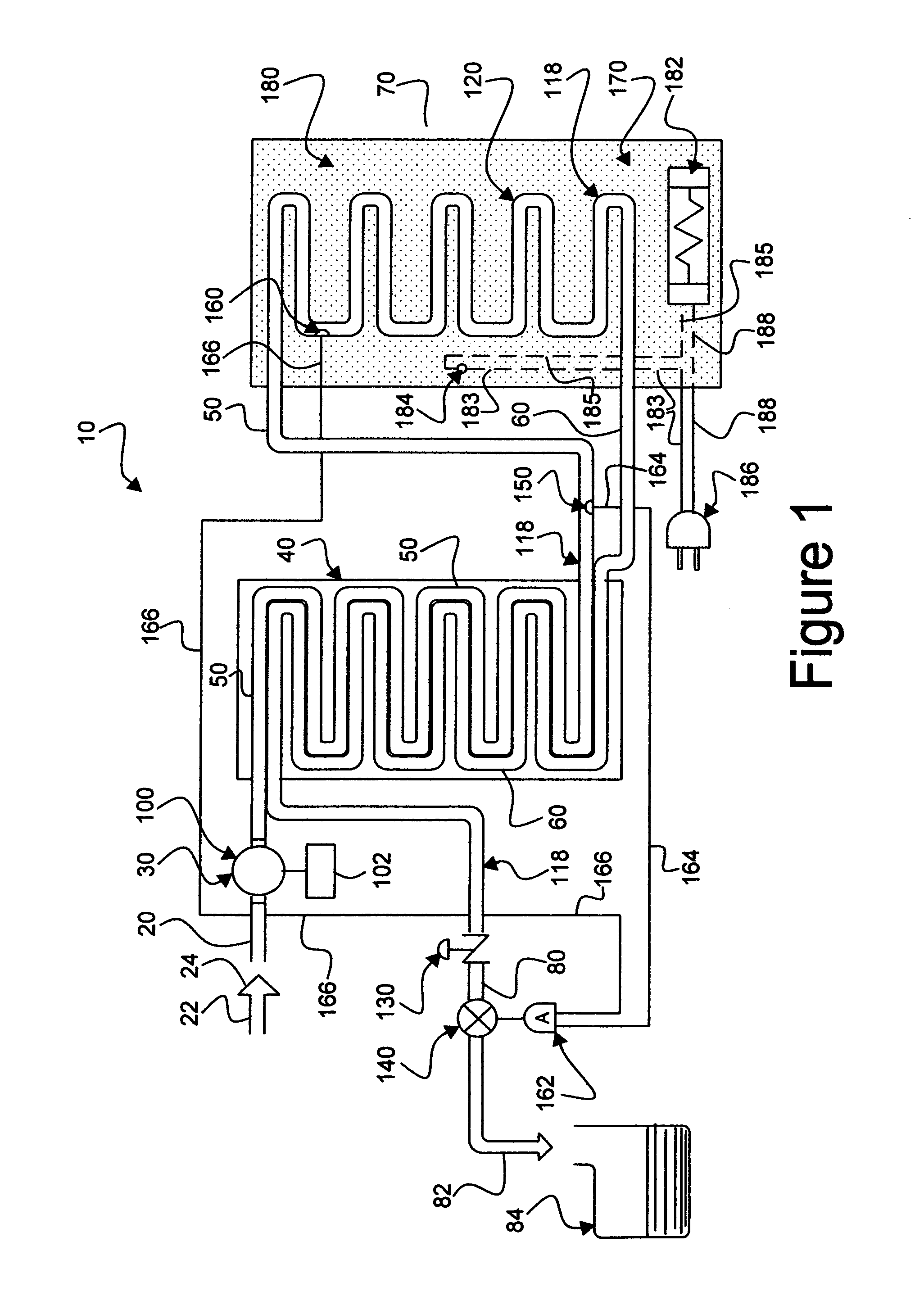
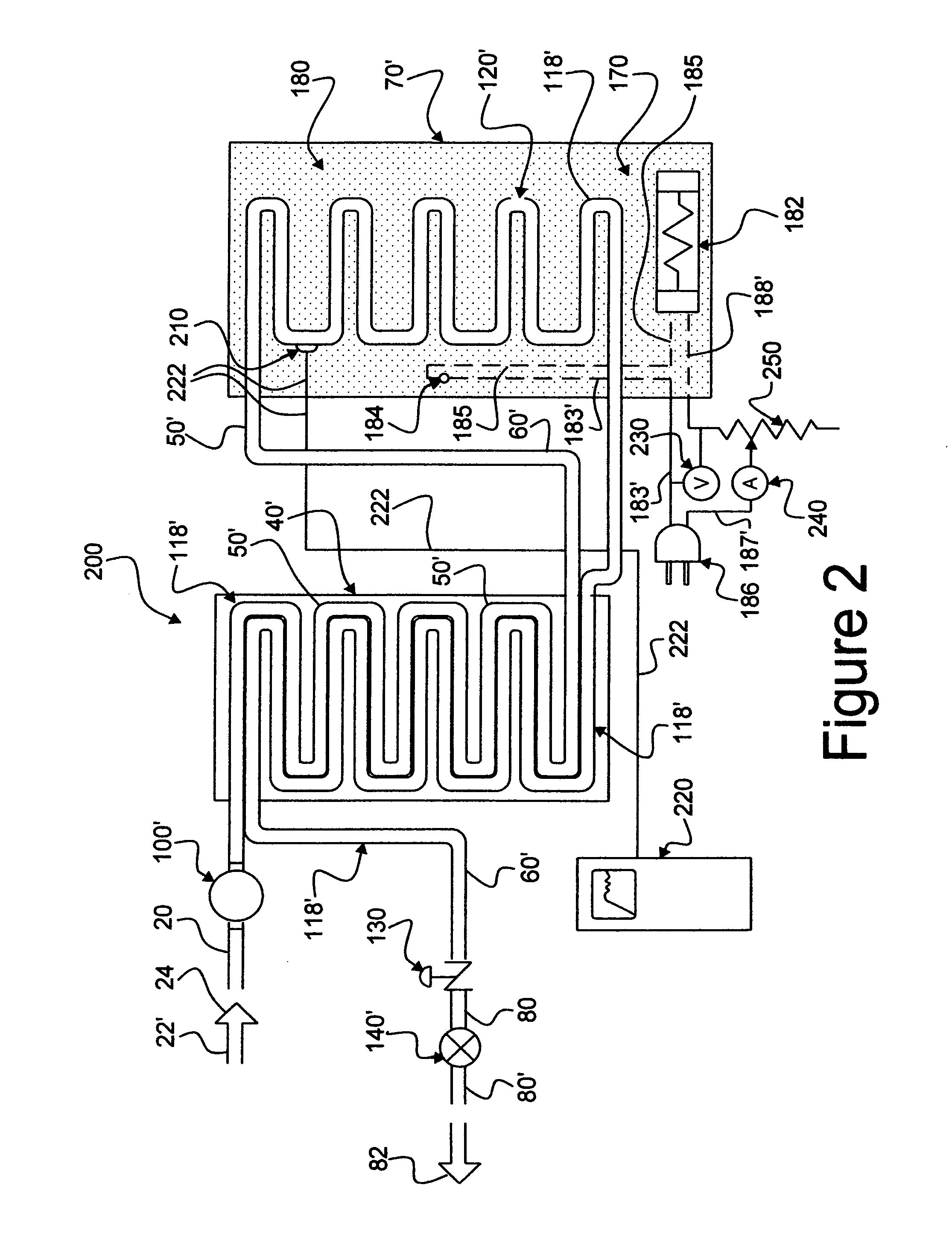
An apparatus, system and method for verifying the achievement of a desired sterility assurance level (SAL) for components manipulated within a low-energy electron beam sterilization chamber. The components are preferably pre-sterilized and connected together in an assembly fashion which creates and maintains the sterility of the connection by subjecting the components to low-energy (less than 300 KeV) electron beam radiation. The verification is completed by measuring the sterilization dose delivered to a sensor, also known as a dosimeter, positioned within the sterilization process to simulate the components.
Owner:BAXTER INT INC
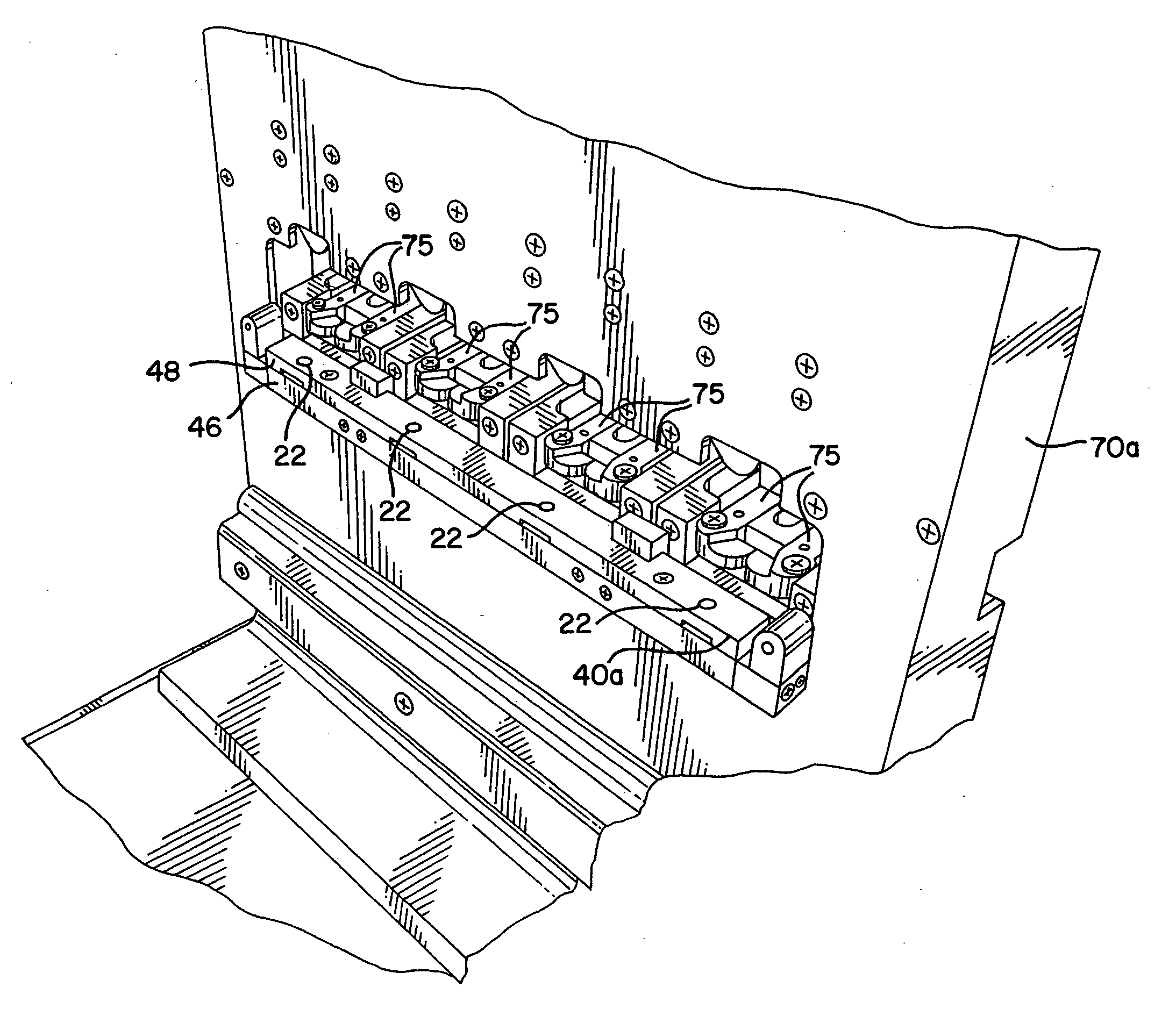
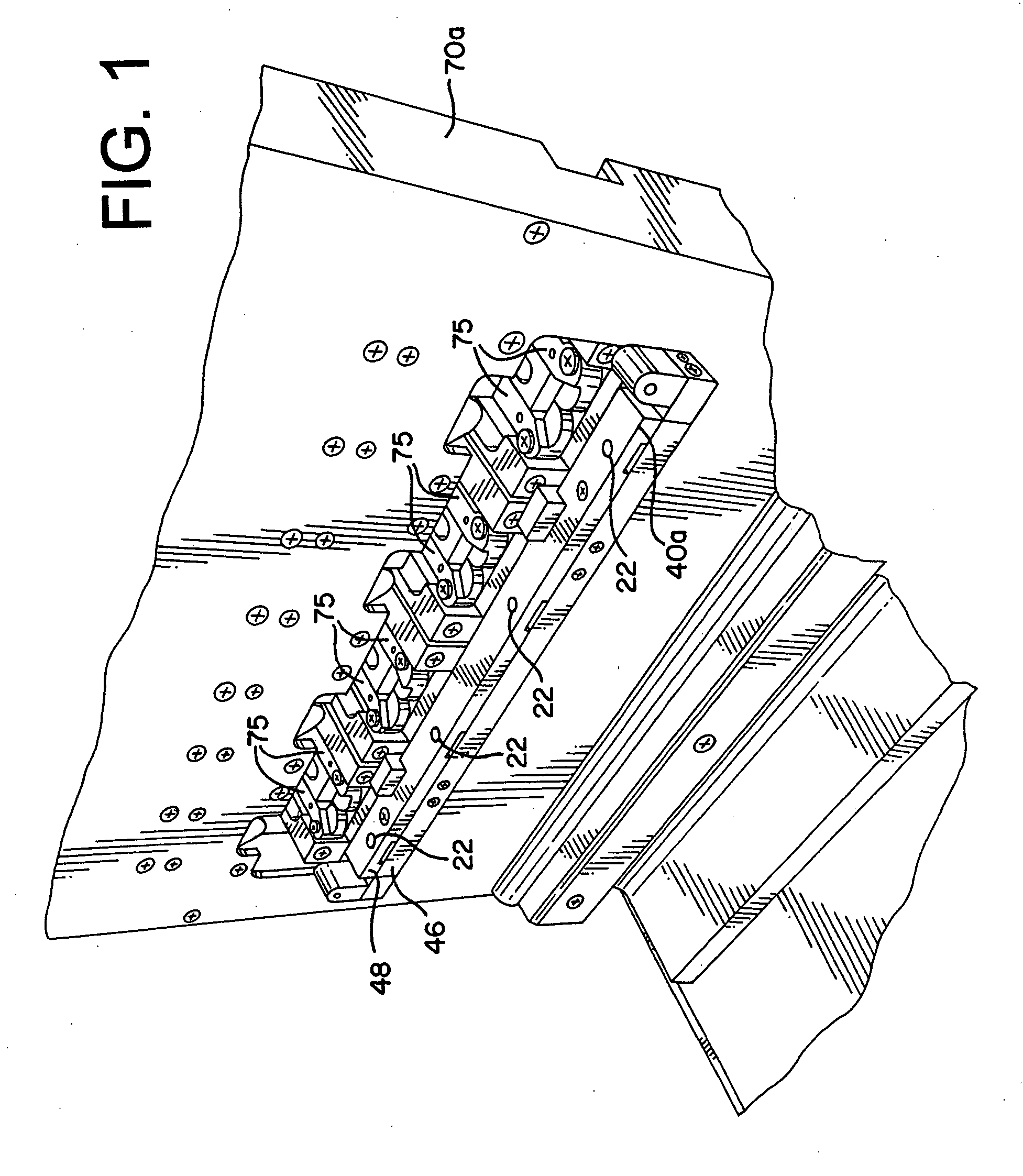
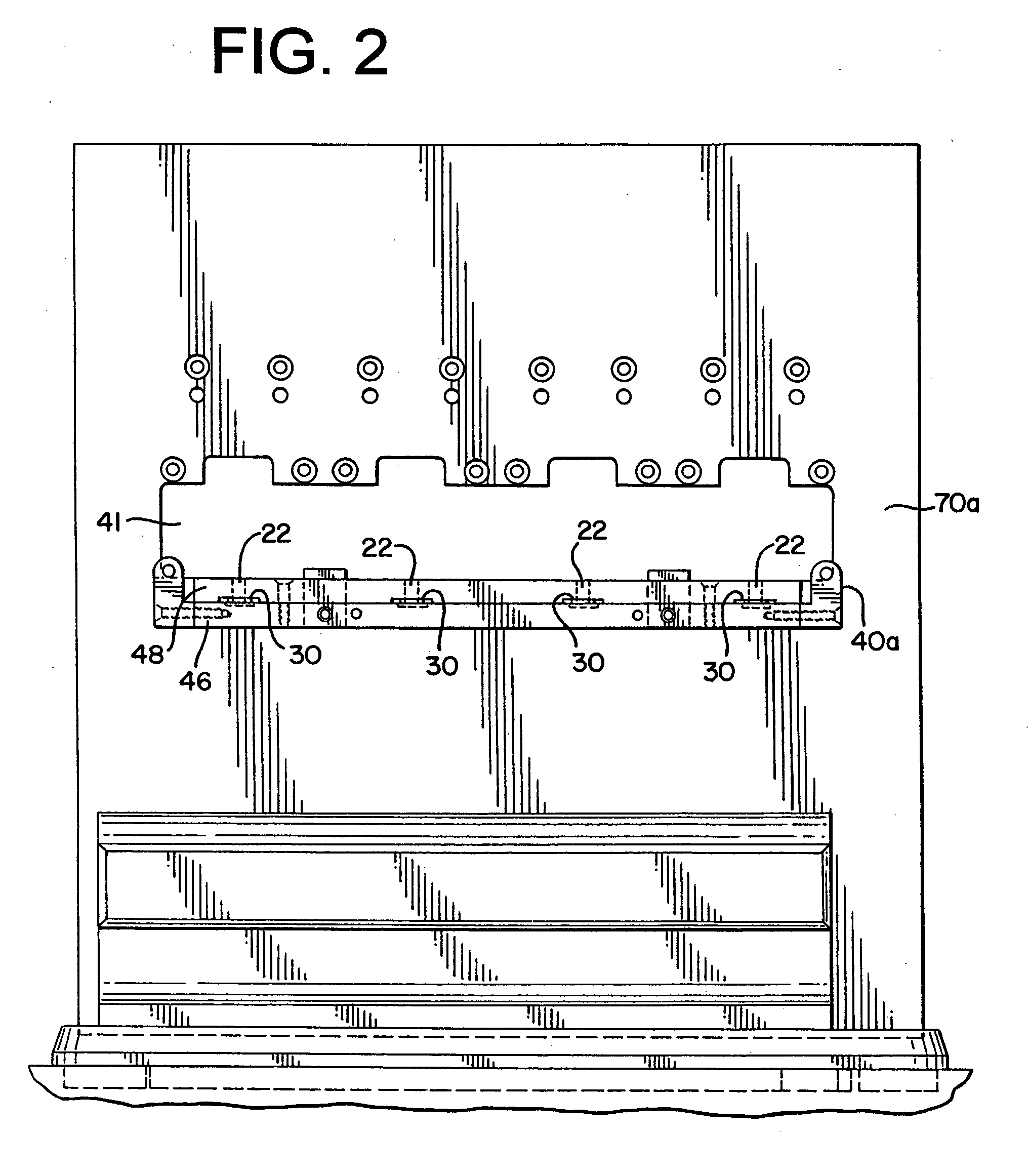
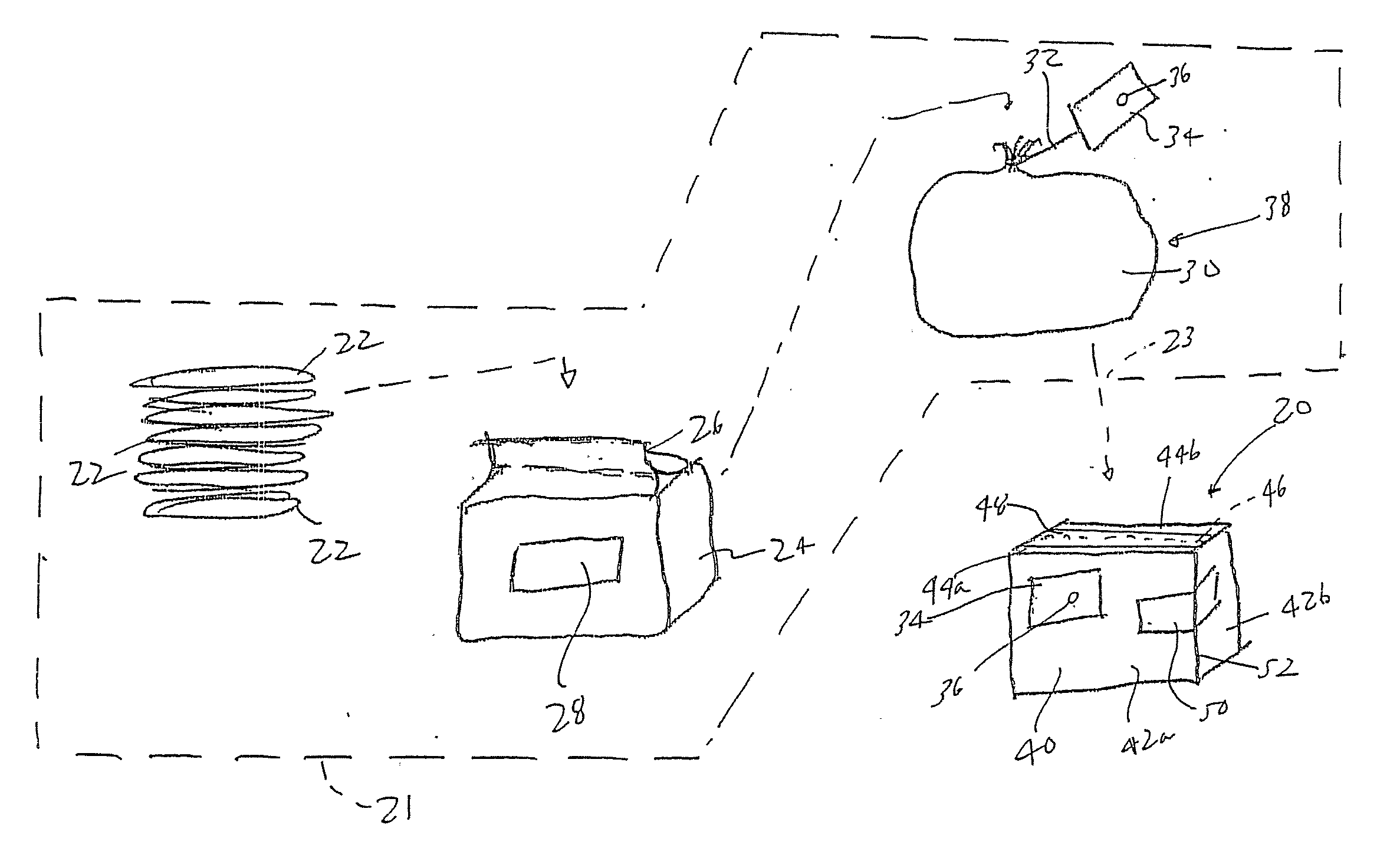
Process and packaging for a garment having a desired sterility assurance level
InactiveUS20070084145A1Reduces and limit bioburdenReduces and limit and diversityPackage sterilisationLavatory sanitoryCartonSterility assurance level
A packaging for a garment and the process for forming same is disclosed. The process reduces or limits bioburden and diversity of genome on a garment. At least one non-sterile garment is placed in a heat sealable bag, a vacuum is formed in the bag and thereafter, the bag is sealed by heat sealing. The bag is then placed in a carton liner and the carton liner is closed. This defines an assembly. The assembly is placed in a carton, and the carton is closed. Thereafter, the carton containing said assembly therein is irradiated to a desired Sterility Assurance Level. All of the steps may be performed in a clean room, or the steps prior to forming the assembly are performed in the clean room and the steps after assembly are performed outside of the clean room.
Owner:ALPHA PRO TECH
Terminal sterilization of injectable collagen products
ActiveUS20060280769A1Lower Level RequirementsAvoid radiationBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineVolumetric Mass Density
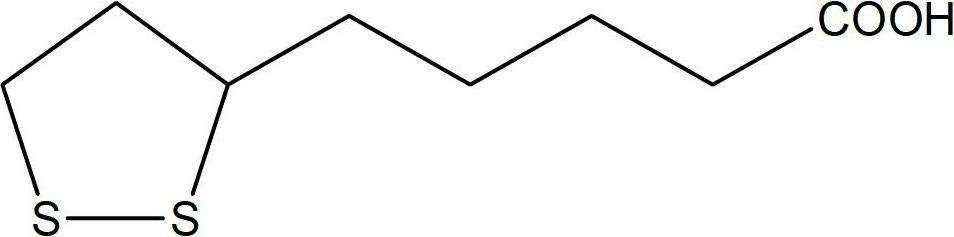
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5kGy and 12kGy and more preferably between 6kGy and 8kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
Terminal sterilization of injectable collagen products
ActiveUS7902145B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluenceVolumetric Mass Density
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5 kGy and 12 kGy and more preferably between 6 kGy and 8 kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
Method and apparatus for validation of sterilization process
An apparatus, system and method for verifying the achievement of a desired sterility assurance level (SAL) for components manipulated within a low-energy electron beam sterilization chamber. The components are preferably pre-sterilized and connected together in an assembly fashion which creates and maintains the sterility of the connection by subjecting the components to low-energy (less than 300 KeV) electron beam radiation. The verification is completed by measuring the sterilization dose delivered to a sensor, also known as a dosimeter, positioned within the sterilization process to simulate the components.
Owner:BAXTER INT INC
Method for preparing phloroglucinol injection
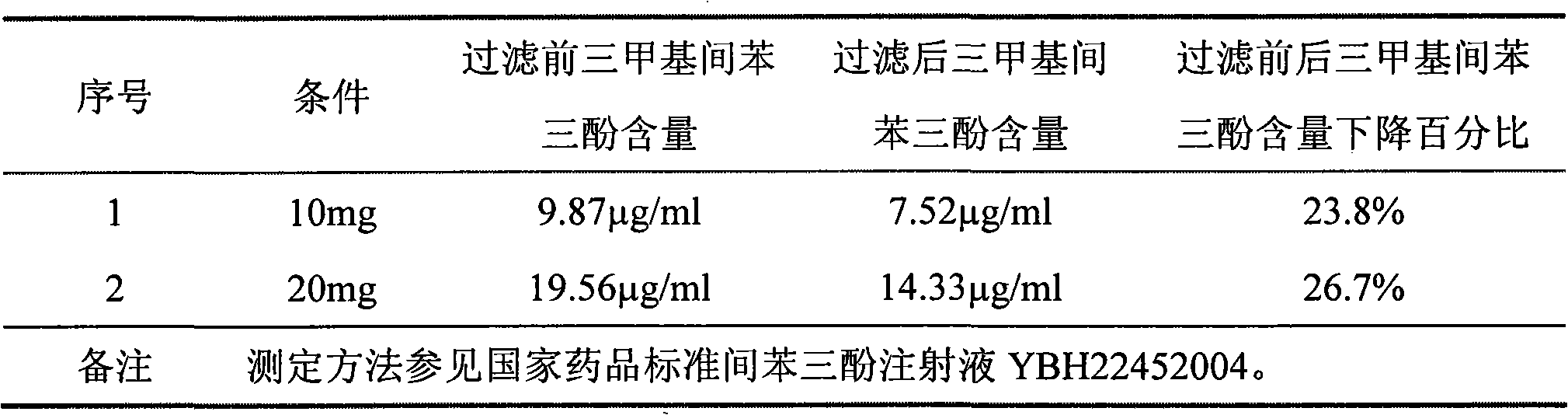
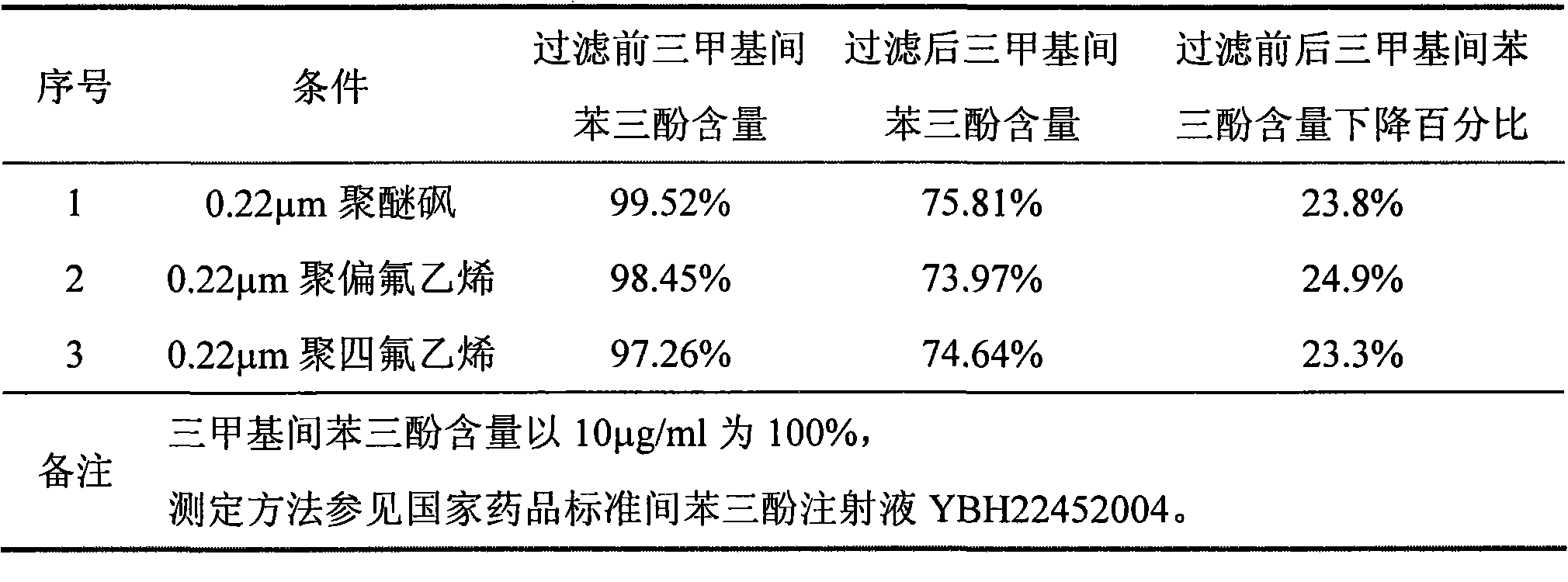
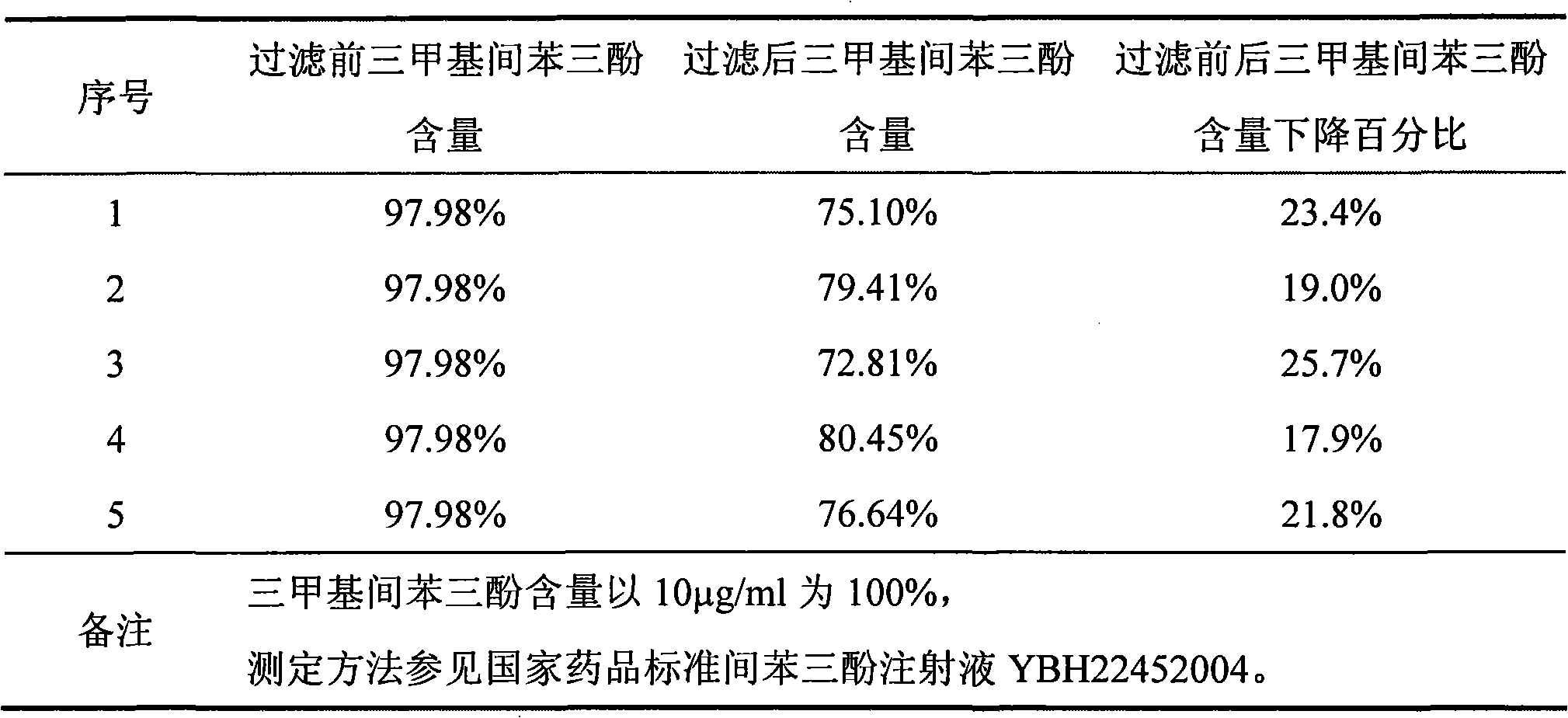
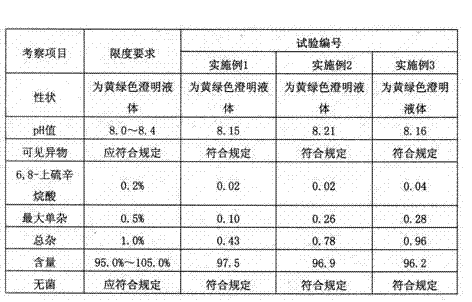
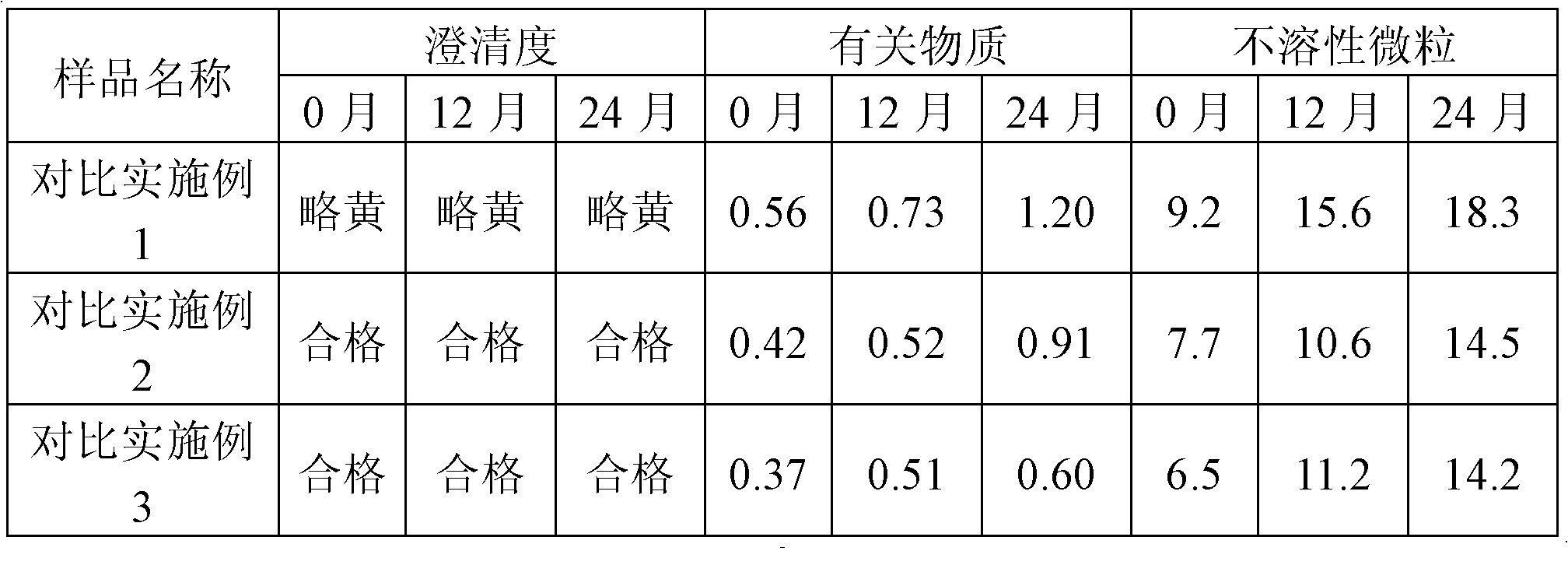
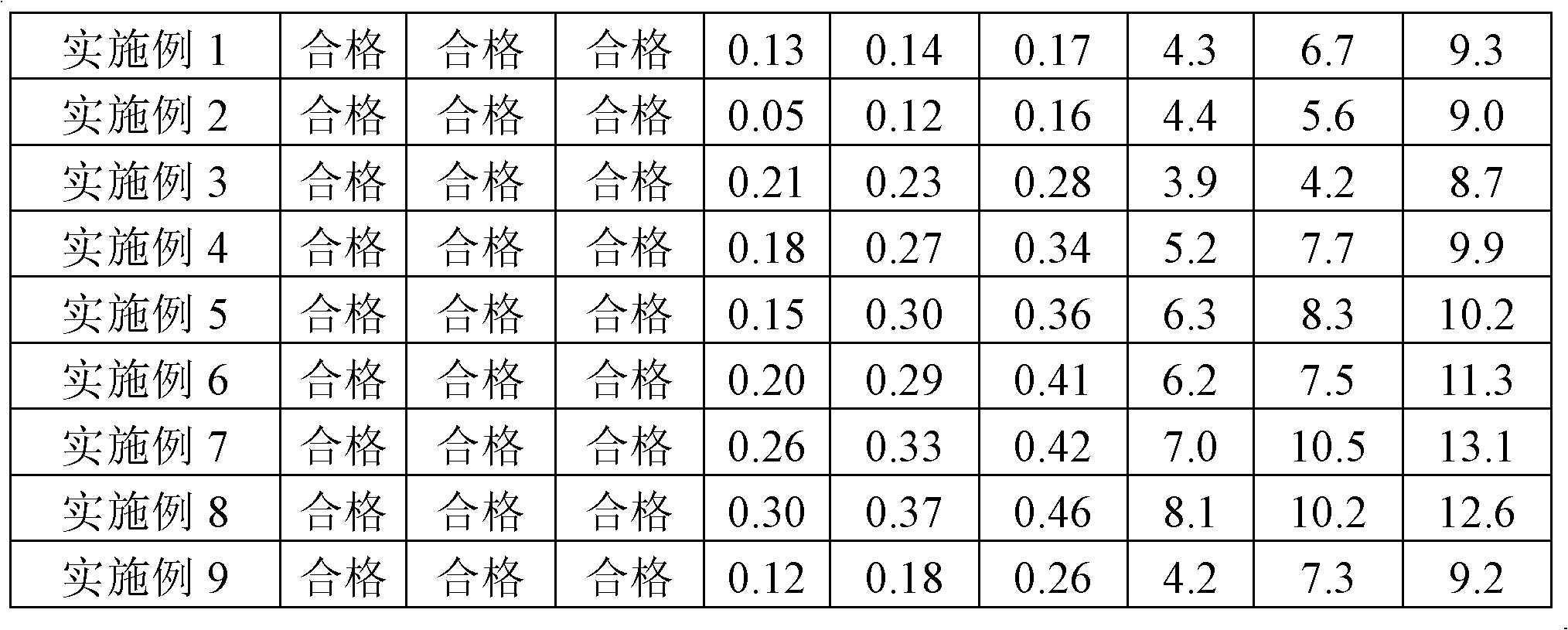
ActiveCN101856324AEnsure sterility assurance levelThe content of glucinol is stableHydroxy compound active ingredientsDigestive systemFiltrationPharmaceutical formulation
The invention belongs to the field of medicinal preparations, and discloses a method for preparing phloroglucinol injection. The method for preparing the phloroglucinol injection comprises the following steps of: (1) preparing solution containing phloroglucinol and trimethyl phloroglucinol; (2) stirring the solution uniformly and then filtering the solution for sterilization; (3) encapsulating the filtered solution by a conventional method and sterilizing the encapsulated solution, wherein the temperature for the sterilizing filtration in the step (2) is 5 to 40 DEG C, and preferably 10 to 35 DEG C. The technical scheme can ensure stable content of the trimethyl phloroglucinol during the preparation of the phloroglucinol injection. Meanwhile, the method for preparing the phloroglucinol injection ensures the sterility assurance level of the injection by using the sterilization process with over sterilization effect.
Owner:NANJING LIFENERGY R & D +1
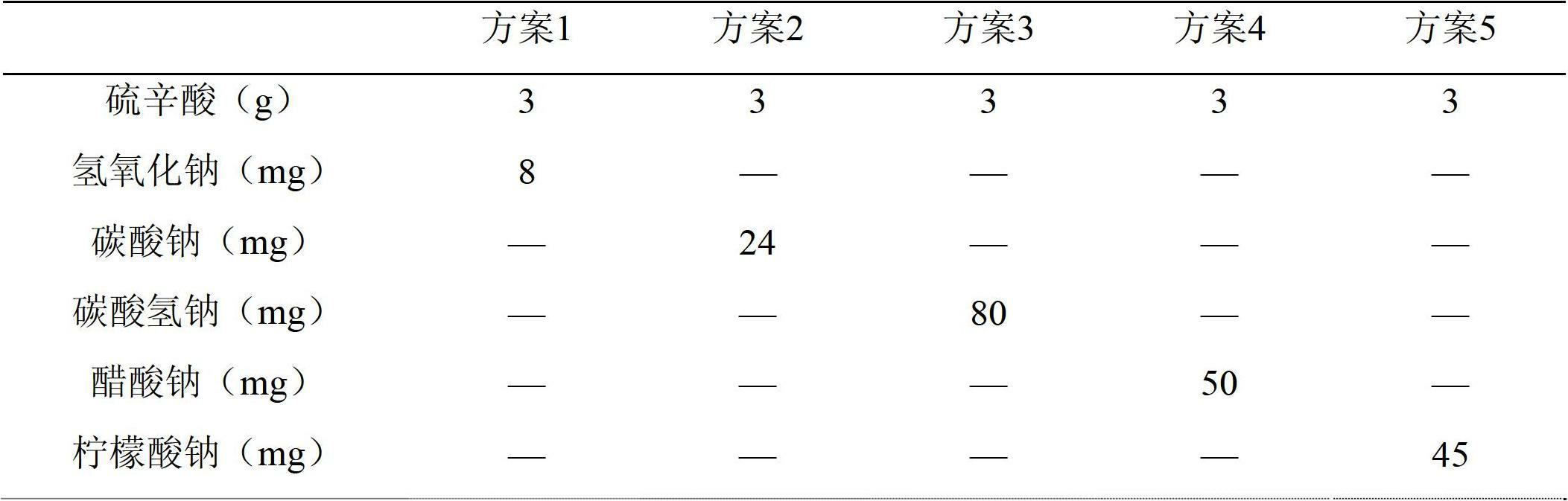
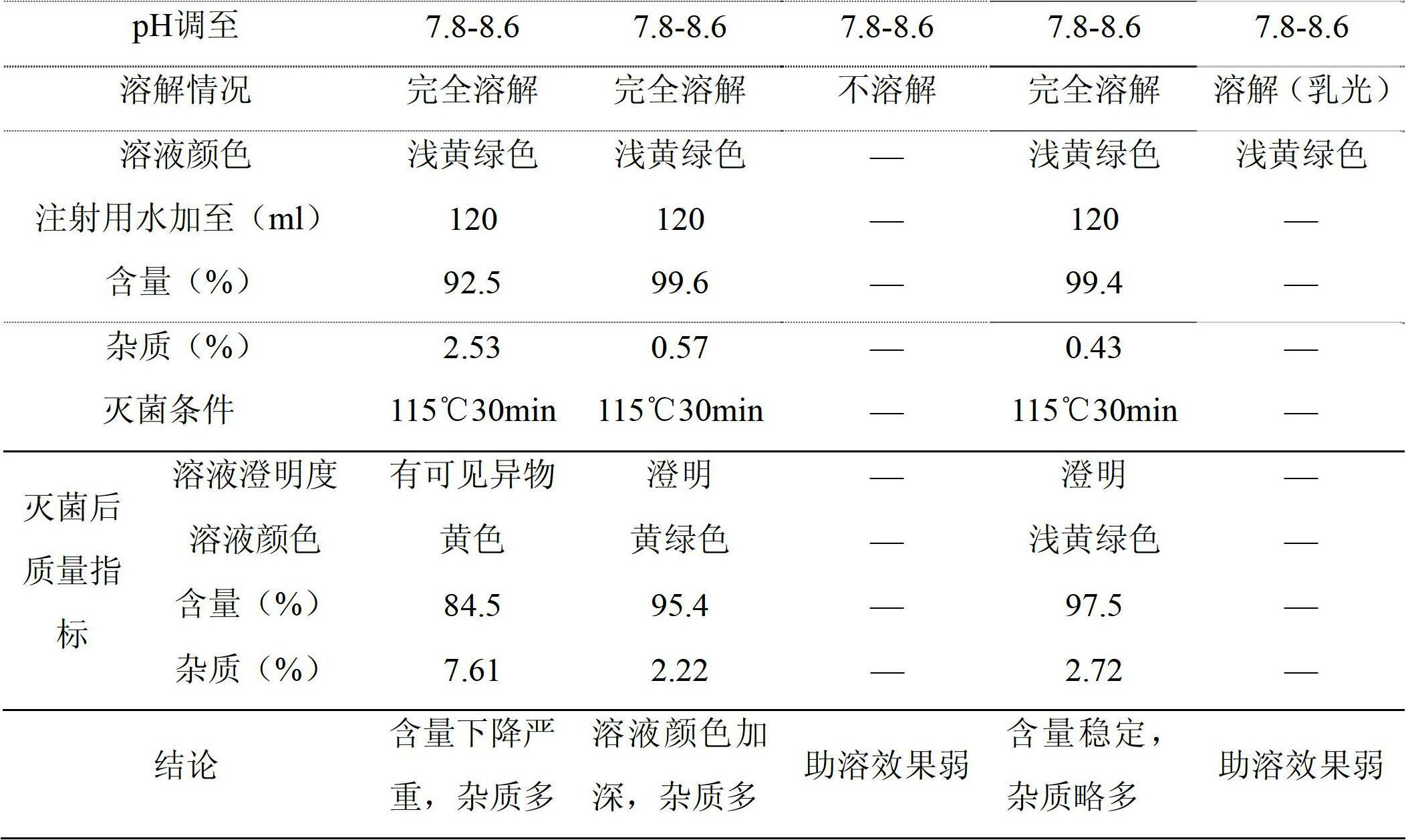
Prescription and preparation technology of lipoic acid injection combination
ActiveCN103655469AImprove stabilityOrganic active ingredientsMetabolism disorderBiochemistryPharmacology
The invention discloses a prescription and a preparation technology of a lipoic acid injection combination. Each 1000 ml of injection comprises the components as follows: 25 g of lipoic acid, 5-8 g of a cosolvent ethanediamine and the balance of water for injection. The disclosed lipoic acid injection is stable in quality and resistant to hot pressure for sterilization, and the sterility level is high. The invention further provides the prescription and the preparation technology of the lipoic acid injection. The technology is simple, convenient and efficient, and the quality can be controlled.
Owner:YUANSHENG SUYUANG BIOLOGICAL SCI TECH BEIJING
Cyclic adenosine meglumine injection and preparation method thereof
InactiveCN102283804AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityFiltration
The invention relates to an adenosine monophosphate meglumine injection and a preparation method thereof. The preparation process is to take an appropriate amount of water for injection, add sodium chloride, cyclic adenosine monophosphate, and meglumine, stir to dissolve completely, add 0.05-0.2% (W / V) activated carbon for needles by volume, and stir for 15-30 minutes. Filter to remove carbon, add water for injection to nearly full amount, use phosphate buffer to adjust the pH value to between 6.0 and 6.5, add water for injection to the full amount, and check that the semi-finished product is qualified, filter, potting (full nitrogen gas in the whole process), and extinguish Bacteria, light inspection, packaging. The method has the advantages of selecting appropriate solvents and additives, improving the solubility and stability of meglumine cyclic adenosine monophosphate, and adopting a terminal sterilization preparation method to effectively ensure the sterility assurance level of the drug. The invention has the characteristics of formula composition, simple process, low production cost, strong drug stability and safety, and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Netilmicin sulfate injection and preparation method thereof
InactiveCN102451154AGuaranteed stabilityImproving Sterility Assurance LevelsAntibacterial agentsOrganic active ingredientsAntioxidantOxygen
The invention relates to a netilmicin sulfate injection and a preparation method thereof. The netilmicin sulfate injection is characterized in that: every 1,000 ml of injection contains 50,000,000 units of netilmicin sulfate, 0.5-2g of an antioxidant and 0.1-1g of a complexing agent, and the pH value of the netilmicin sulfate injection is 5.3-5.7. A preparation process of the netilmicin sulfate injection comprises the following steps of: cooling an appropriate amount of water for injection to below 30 DEG C under the condition that the air cleanliness is in a hundred level; introducing nitrogen to remove oxygen; adding a prescription dose of the antioxidant and the complexing agent into the water for injection for completely dissolving; adding a prescription dose of the netilmicin sulfate into a solution; stirring for completely dissolving; adsorbing with 0.05-0.2 percent (g / ml) needle active carbon; primarily filtering with one set of 1mum filter for removing carbon; adjusting the pH to 5.3-5.7 with a sodium citrate solution; stirring uniformly; fixing the volume to a full dose by using the water for injection; finely filtering with one set of 0.22 / 0.22mum polypropylene foldable filter core filter and two sets of 0.22 / 0.22mum polyether sulfone microporous membrane filter core filters for removing bacteria; embedding under a hundred-level condition (introducing nitrogen in the entire process); assisting in sterilizing at the temperature of 100 DEG C; and performing lamp inspection and packaging. Due to the adoption of a scientific formula and process, the stability of the netilmicin sulfate injection is ensured; and due to the adoption of a sterile assistant sterilization preparation method, the sterile guarantee level of a medicament is raised.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Production method for dopamine hydrochloride injection
InactiveCN101953782AImprove stabilityDefinite curative effectOrganic active ingredientsInorganic non-active ingredientsDecompositionCurative effect
The invention relates to a production method for dopamine hydrochloride injection, which comprises the following steps of: taking 50 to 80 percent of water for injection in a preparation amount, and introducing CO2 for 15 to 30 minutes to make the water saturated; adding 1 weight volume percent of dopamine hydrochloride and 0.15 to 0.25 weight volume percent of sodium hydrogensulfite into the saturated water into which the CO2 is introduced, and stirring for dissolution; and adjusting the pH value of the injection to be between 3.2 and 4.3 by using sodium hydroxide or hydrochloric acid, adding the water for injection to the full amount, and uniformly stirring; and filtering, filling and sealing, and sterilizing at the temperature of between 115 and 121 DEG C for 12 to 30 minutes. The production method has the advantages of high product stability, exact curative effect, difficult decomposition and precipitation, high sterility assurance level (F0 is more than or equal to 12), and suitability for large-scale industrial production.
Owner:上海禾丰制药有限公司
Lidocaine hydrochloride mucilage preparation method
InactiveCN101385702AOpen alternatelyEasy to closeOrganic active ingredientsAerosol deliveryDrug contentGlycerol
A method for preparing a lidocaine hydrochloride mucilage comprises the following steps: a formula amount of ethyl p-hydroxybenzoate is put into purified water which accounts for 25% of the total volume of the ethyl p-hydroxybenzoate, heated and stirred until complete dissolution, added with a formula amount of lidocaine hydrochloride and stirred for dissolution, added with a formula amount of glycerol and stirred evenly; additionally, a formula amount of sodium carboxymethyl cellulose is added to purified water which accounts for 50% of the total volume of the sodium carboxymethyl cellulose, and stirred for complete swelling; the two solutions obtained are mixed evenly, and added with 0.1N sodium hydroxide to adjust the pH value to 6.0-7.0; the monarch drug content of the obtained mucilage is determined; the mucilage is added with water to sufficient amount, mixed evenly and filled, and sterilized by 110 DEG C saturated vapor for 30 minutes; inspected under a lamp and packaged to obtain the finished product. Compared with the prior art, the lidocaine hydrochloride mucilage has the advantages of matching anaesthesia and inspection time, good lubricating property and sterility assurance level.
Owner:浙江康德药业集团股份有限公司
Sterilization system for a blow/fill/seal machine
A fill assembly sterilization system for a blow / fill / seal machine utilizes a closed loop circulation of sterilant containing gas. A typical sterilant is nitrogen dioxide. The closed loop includes a shroud that defines a plenum and encloses the fill system. Optionally, at least one high efficiency particulate absorption (HEPA) filter is provided in the closed loop. Sterility assurance level of 10−6 can be achieved by subjecting the fill system to the sterilizing gas for at least 20 minutes at a temperature in the range of about 18° C. to about 30° C.
Owner:WEILER ENG
Method for preparing sodium-potassium-magnesium-calcium glucose injection
InactiveCN104622896AQuality assuranceImproving Sterility Assurance LevelsOrganic active ingredientsMetabolism disorderSodium acetatePotassium
The invention discloses a method for preparing a sodium-potassium-magnesium-calcium glucose injection. The method comprises the following steps: adding water for injection into a preparation tank; sequentially adding calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, glucosum anhydricum, sodium acetate and sodium citrate; dissolving, regulating the pH value to 4.5-5.5 with hydrochloric acid, filtering and filling; sterilizing for 12 minutes at 121 DEG C, inspecting with a lamp, and packaging. According to the method, after the pH value is regulated to 4.5-5.5, a durable overkilling method is carried out at 121 DEG C for 12 minutes (F0 is more than or equal to 12) for sterilizing, the content of glucose is not remarkably reduced, 5-hydroxymethylfurfural and other glucose degrading impurities are in a relatively low level, and the sterility assurance level of the sodium-potassium-magnesium-calcium glucose injection is improved.
Owner:SHANDONG QIDU PHARMA
Methods and Apparatus for Preparing Autologous Blood Eye Drops
ActiveUS20200337946A1Improve sterilitySufficient materialSurgical furniturePharmaceutical containersKit deviceAutologous blood
Methods and apparatus for preparing autologous blood serum eye drops within a potentially contaminating environment are disclosed. Key convenience kit apparatus providing novel methodology includes a plastic bag in which an innovative tray securely holds a plurality of empty eye drop bottles and associated caps disposed and sealed therein. Providing the only access pathway into the bag is a sterilizing filter appliance which filters all fluid entering the bag and provides a nozzle for dispensing into bottles disposed in the tray. The key convenience kit apparatus is sterilized prior to use, assuring all components disposed within the bag are within a desired sterility assurance level. The sterilized state of items associated with the key apparatus is maintained by the single pathway for fluid. Capping and sealing the bottles before opening the bag permits delivery of sterile product for use outside the bag.
Owner:THORNE INTPROP HLDG LLC
Muscular amino acids and peptides and nucleosides injection and preparing method thereof
The present invention provides a muscular amino acids and peptides and nucleosides injection, which is extracted from the muscle and the cardiac muscle of healthy rabbit and is a freeze-drying agent which is prepared by the sterilized water solution containing the compositions of polypeptide, amino acids, nucleosides, nucleotides, etc. The muscular amino acids and peptides and nucleosides is used for the adjuvant therapy of brain hypofunction and peripheral nerve disease caused by cerebral apoplexy and cerehral circulation insufficiency. The present invention belongs to the technical field of medicine. The invention introduces a preparing method which comprises the steps of cleaning, cutting into blocks, homogenizing, deep freezing, heating to boiled, separating the supernatant, depositing with acid and the base, depositing with the base, hot pressing, deep freezing, ultra-filtering, detecting, adjusting the proportion between the polypeptide and the hypoxanthine, and ultra-filtering. The muscular amino acids and peptides and nucleosides injection is prepared through the steps of sterilizing filtering, filling into containers, and sterilizing. An appropriate amount of accessory is added and the steps of sterilizing filtering, filling into containers, and freeze drying are executed for preparing the muscular amino acids and peptides and nucleosides for injection. The muscular amino acids and peptides and nucleosides and the preparing method of the invention have the advantages of excellent convenience, excellent wholeness, ensured product which satisfies the national standard, guaranteed virus blanching, injection sterilization F0 larger than 8, and high level of sterilizing guaranteeing.
Owner:石海 +1
Methods for variably sterilizing aqueous liquids
ActiveUS9211351B2Improve sterilityCreating a hysteresis in each switching parameterWater treatment parameter controlSpecific water treatment objectivesSterility assurance levelTemperature and pressure
A method for flow-through aqueous liquid sterilization which employs apparatus operating at substantially fixed temperatures and pressures and variable flow rates through the apparatus for controllably processing aqueous liquids to achieve predetermined values of Sterility Assurance Levels (SAL's).
Owner:TOTH ANANISE +1
Lipoic acid injection for intravenous administration
ActiveCN102657606AGood thermal stability effectImprove solubilityOrganic active ingredientsMetabolism disorderMedicineSolvent
The invention discloses a lipoic acid injection for intravenous administration and a preparation method thereof. Per 1000ml of the injection comprises the following components: 25g of lipoic acid, 7.5 to 12.5g of cosolvent, and the balance of injection water. The lipoic acid injection has the advantages of stable quality, hot-press resistance, sterilization and high aseptic guarantee level. The invention also provides a prescription and a process preparation method of the injection, and the process has the advantages of convenience, high efficiency and controllable quality.
Owner:JIANGSU SHENLONG PHARMA
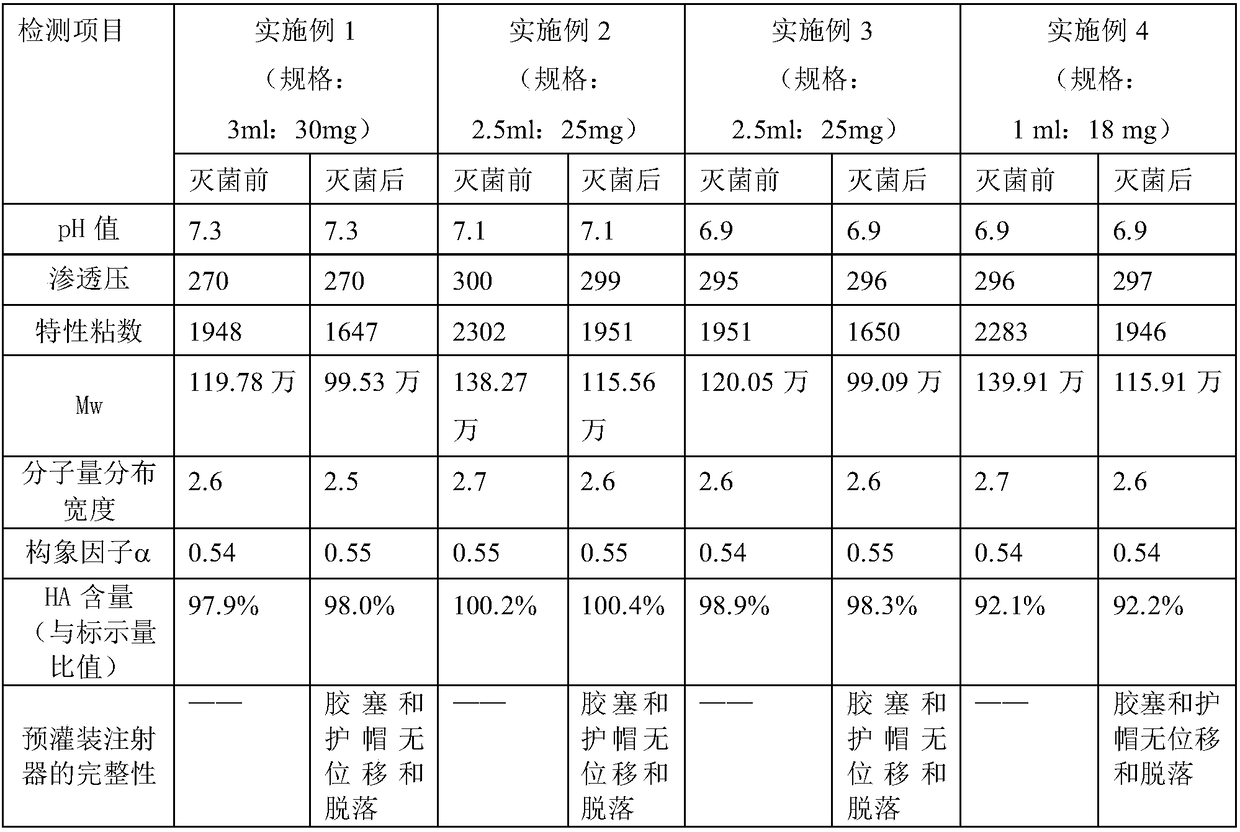
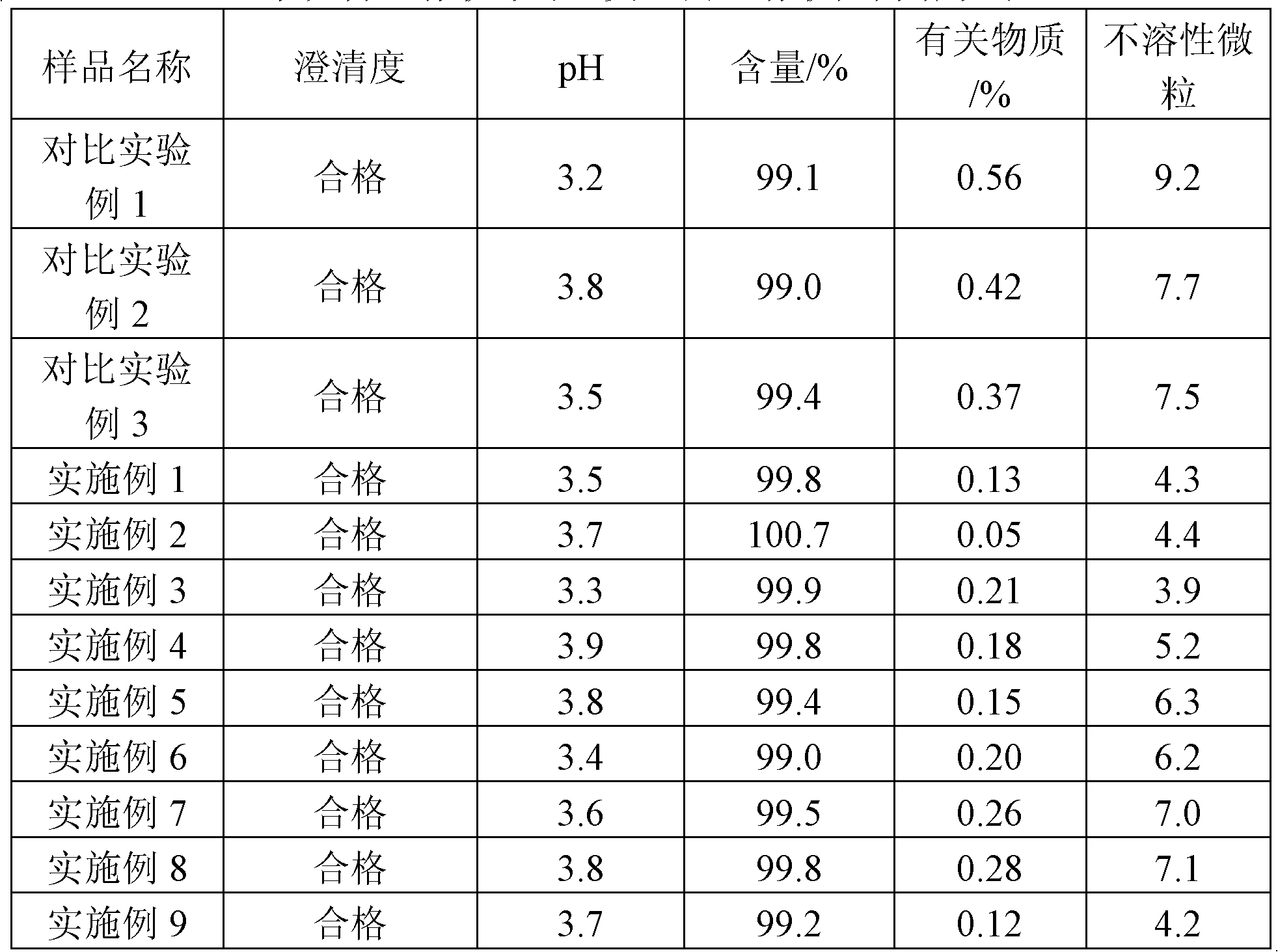
Method for large-scale preparation of mixed steam-air sterilized sodium hyaluronate injections
InactiveCN108261420AReduce degradationImprove product qualityOrganic active ingredientsInorganic non-active ingredientsDrug productSterility assurance level
The invention relates to the technical field of biomedicine, in particular to a method for large-scale preparation of mixed steam-air sterilized sodium hyaluronate injections. According to the method,sterile sodium hyaluronate dry powder is used as a raw material, prepared, intermittently stirred and dissolved, loaded into pre-filled syringes and finally sterilized by means of a mixed steam-air method. The sodium hyaluronate injections obtained by batch preparation are stable and uniform in product quality, and degradation of sodium hyaluronate after sterilization is less. According to the method, the sterility assurance level of the sodium hyaluronate injections is greatly increased, and the clinical requirements for effectiveness and safety of medicine are met.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
Method for establishing a sterilizing dose for radiation sensitive products
ActiveUS20100166599A1Adequate dosageEnsure sterilizationLavatory sanitoryMedical waste disposalMicroorganismDose level
A method provides for sterilizing with radiation objects which have a low bioburden and which are sensitive to radiation. A dosage of radiation sufficient to ensure sterilization without damaging the object is determined by determining the bioburden upon one or more samples of the objects, determining an estimate of the dose that results in a preselected probability (e.g., 0.1, 0.01, 0.001, 0.0003162 . . . etc. (i.e., SAL=10−1, 10−2, 10−3, 10−3.5 . . . etc.)) of a surviving microorganism on the objects preferably by testing a quantity of samples of the objects at varying dosage levels of radiation, confirming the estimate by testing a quantity of samples of the objects at the dose that was estimated; and calculating a dosage for the sterility assurance level of 10−6 by adding a factor to the dose that was confirmed to result in said preselected probability of a surviving microorganism on the objects and wherein the factor is proportional to the dose that yields said preselected probability of a surviving microorganism on the objects and inversely proportional to a log of the bioburden.
Owner:ETHICON INC
Vinpocetine injection and preparation method thereof
ActiveCN102716066AHelp dissolveImprove antioxidant capacityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantDissolution
The invention relates to a vinpocetine injection and a production method thereof. The vinpocetine injection comprises 10 to 20 parts of vinpocetine, 0.1 to 0.45 parts of ascorbic acid, 1 to 5 parts of one or more antioxidants, 6 to 15 parts of one or more cosolvents and 2000 parts of water. The preparation method comprises the following steps of 1, filtering 80% of injection water to remove oxygen, heating to a temperature of 40 to 50 DEG C, adding ascorbic acid, the one or more cosolvents and the one or more antioxidants into the injection water, and stirring for complete dissolution, 2, adding vinpocetine into the solution obtained by the step 1, stirring for full dissolution, supplying enough injection water, and carrying out stirring adsorption by 0.3% of active carbon, and 3, carrying out disinfection at a temperature of 121 DEG C for 15 minutes, and carrying out filling under nitrogen atmosphere. The vinpocetine injection obtained by the preparation method can be subjected to sterilization at a temperature of 121 DEG C for 15 minutes. A result of detection shows that impurity content is not improved obviously and thus the vinpocetine injection is stable and can be used for industrial production. Compared with the original vinpocetine injection, the vinpocetine injection obtained by the preparation method can be subjected to overkill sterilization at a temperature of 121 DEG C for 15 minutes so that a sterility assurance level of the vinpocetine injection can be ensured.
Owner:HENAN RUNHONG PHARMA
Filtration sterilization device and process for sterile medicines
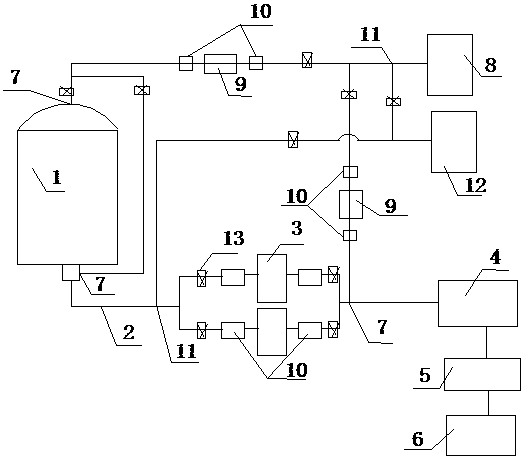
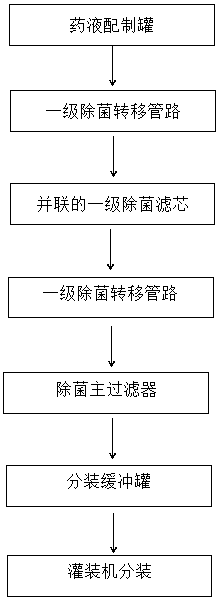
PendingCN109550297ASolve the problem of clogged filter elementReduce lossSemi-permeable membranesDispersed particle filtrationFiltrationBuffer tank
The invention relates to the technical field of sterile medicine production, and particularly relates to a filtration sterilization device and process for sterile medicines. The filtration sterilization device comprises a liquid medicine preparation tank, wherein a discharge port of the liquid medicine preparation tank is sequentially connected with first-stage sterilization filters and a main filter through pipelines; a discharge port of the main filter is sequentially connected with a sub-packaging buffer tank and a filling machine; a plurality of the first-stage sterilization filters are connected in parallel; a sterilizing and transferring pipeline behind the first-stage sterilization filters is provided with a compressed air inlet; and the compressed air inlet is connected with a compressed air source through a pipeline. According to the invention, the yield of product sterilization can be well improved, the liquid medicine transferring pipelines and the filters can be sterilizedwithout dead corners, risks in filtration and sterilization of medicines are reduced to the maximum extent, and the sterility assurance level of the medicines is greatly improved.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Iron sucrose injection and preparation method thereof
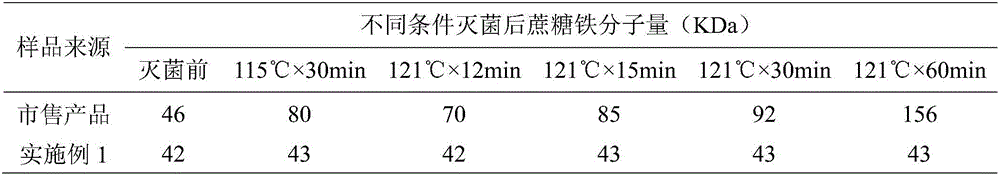
InactiveCN106137953AImprove thermal stabilityImprove stabilityOrganic active ingredientsHeavy metal active ingredientsActivated carbonSurvival probability
The invention provides an iron sucrose injection, comprising iron sucrose, a stabilizer, a bacteriostat, an acid-base regulator and water for injection; the thermal stability of the iron sucrose injection preparation has been significantly improved, and the overkill method can be adopted Compared with terminal sterilization by residual probability method, especially the preparation produced by aseptic production process, the sterility assurance level of the product has been significantly improved by carrying out terminal sterilization. On the other hand, the present invention provides a preparation method of iron sucrose injection, which has a simple preparation process, does not need to repeatedly add activated carbon for adsorption, is convenient for industrial production operation, has a short production cycle and low energy consumption.
Owner:HARVEST PHARMA HUNAN CO LTD
Disinfection preparation containing levorotatory folinic acid sodium
InactiveCN102743331AOrganic active ingredientsPharmaceutical delivery mechanismSterility assurance levelLevorotatory
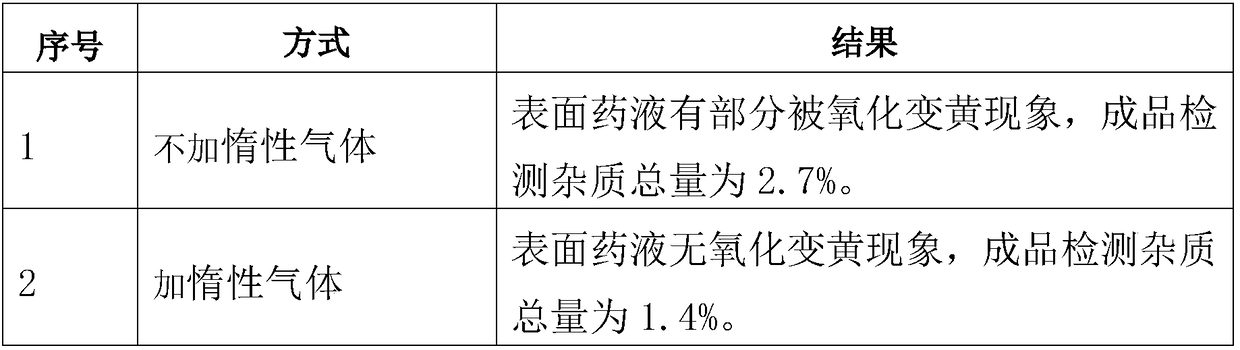
The present invention relates to a disinfection preparation containing levorotatory folinic acid sodium and a preparation method thereof. The levorotatory folinic acid sodium is a pharmaceutical composition, which is prepared from a main component of levorotatory folinic acid sodium and pharmaceutically acceptable excipients. The disinfection preparation has advantage that an injection disinfection preparation with high level of sterility assurance is prepared by addition of pharmaceutically acceptable stabilizer and inert gas protection and through terminal disinfection process.
Owner:NANJING HEALTHNICE MEDICAL TECH
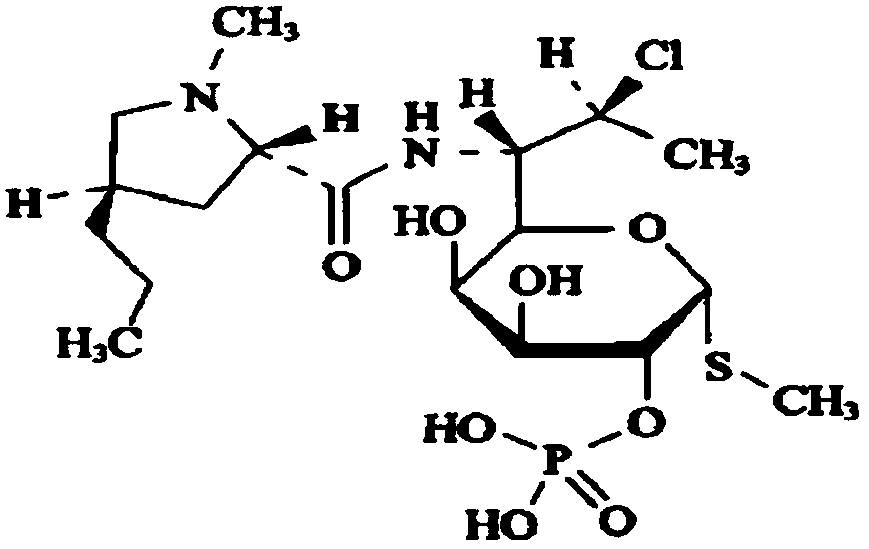
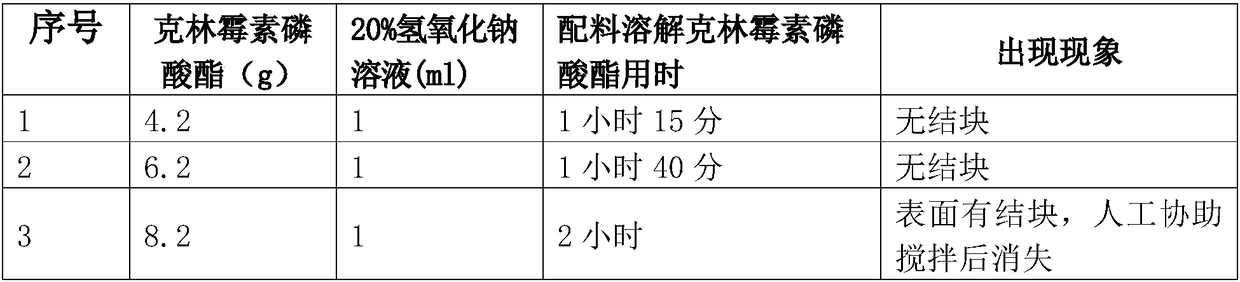
Clindamycin phosphate compound liquid and preparation method thereof
ActiveCN109498564AIncrease temperatureShorten the dissolution timeAntibacterial agentsOrganic active ingredientsActivated carbonFiltration
The invention discloses a clindamycin phosphate compound liquid and a preparation method thereof. The clindamycin phosphate compound liquid is prepared from the following components in proportions: 500-700 parts of clindamycin phosphate, 35-55 parts of sodium hydroxide, 1700-3000 parts of water for injection and 2-10 parts of activated carbon; the clindamycin phosphate dose is prepared by adding raw materials and a PH regulator gradually according to the proportions, dissolving and other processes. By adopting the preparation method, the uniformity of medicinal liquid dissolution is greatly improved, the oxidation process of a product is eliminated, the sterility assurance level in the production process of the product is improved, and the amount of air bubbles in an injection is reduced;in addition, the dissolution time and the filtration time in the liquid preparation process are greatly shortened and the production cost is reduced; therefore, the preparation method is especially suitable for industrial production; the dissolution method is not only applicable to the liquid preparation process of the clindamycin phosphate for injection, but also applicable to the liquid preparation process of other products using the clindamycin phosphate as a raw material and sodium hydroxide as the pH regulator.
Owner:GUIZHOU JINGFENG INJECTION
Sterilization method of protein A immuno-absorbent column
ActiveCN105126131AGuaranteed stabilityHigh activitySolid sorbent liquid separationHeatAlcoholImmunosorbents
The invention relates to a sterilization method of a protein A immuno-absorbent column. The sterilization method comprises the following steps: adding a protein A immuno-absorbent to a buffer solution which is 4-7 in pH value, packing a column and sterilizing with steam. The method further comprises a step of adding a stabilizer to the buffer solution, wherein the stabilizer consists of the following components: an antioxidant, polyhydric alcohol, polysaccharide, a chelating agent, a thickening agent, polypeptide or protein. By virtue of the sterilization process, the activity of the absorbent can be protected after sterilization, and the final sterilization of the protein A immuno-absorbent column can be achieved; and the obtained protein A immuno-absorbent column product, compared with products obtained from a sterile process, is higher in sterility assurance level and is lower in production cost.
Owner:GUANGZHOU KONCEN BIOSCI
Kanamycin sulfate injection and preparation method thereof
ActiveCN102552118AImproving Sterility Assurance LevelsImprove the level ofAntibacterial agentsOrganic active ingredientsAntioxidantSterility assurance level
The invention discloses a kanamycin sulfate injection and a preparation method thereof. The injection is prepared from kanamycin sulfate, an antioxidant, a metal ion complexing agent, a pH value stabilizing agent and water for injection. After the antioxidant, the metal ion complexing agent and the pH value stabilizing agent are added into a prescription of the kanamycin sulfate, the kanamycin sulfate can withstand high-temperature sterilization at the temperature of 115 DEG C for 30 minutes under the synergistic actions of the antioxidant, the metal ion complexing agent and the pH value stabilizing agent, the sterility assurance level of the injection is raised, and clinical use safety is ensured.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Production process of unstable chemical injection
InactiveCN102552950AGood for sterility assuranceOrganic active ingredientsPharmaceutical delivery mechanismSodium Chloride InjectionInjection product
The invention relates to a sterilization process of injection products and in particular relates to a sterilization method of adenosine triphosphate disodium-sodium chloride injections. The method comprises the steps as follows: preparing a stock solution of injection in which the content of adenosine triphosphate salt is more than 1% (by weight) and performing wet-process heating on the stock solution of injection for an effective time period; diluting the stock solution of injection to desired concentration and filtering with a filter membrane; and sterilizing the filtered dilution under high pressure (250 MPa to 450 MPa). The method can not only guarantee the activity of adenosine triphosphate in the sterilized injection product but also control the sterility assurance level below 10-6.
Owner:刘小清
Composition and preparation method of deslanoside injection
InactiveCN112336687AImprove the level ofQuality improvementOrganic active ingredientsInorganic non-active ingredientsGlycerolSterility
The invention discloses a composition and preparation method of a deslanoside injection. According to formulation composition, the composition consists of deslanoside, ethanol, glycerin, sodium dihydrogen phosphate, disodium hydrogen phosphate and water for injection,wherein the use amount of the sodium dihydrogen phosphate is 0.02-0.8 g / ml, and the use amount of the disodium hydrogen phosphate is0.01-0.1 g / ml. The deslanoside injection disclosed by the invention is stable in quality, can tolerable hot pressurized sterilization, and F0 value is not less than 12; the sterility assurance levelis high, the process of the deslanoside injection is simple and efficient and the quality is controllable.
Owner:南京科宁检测科技有限公司
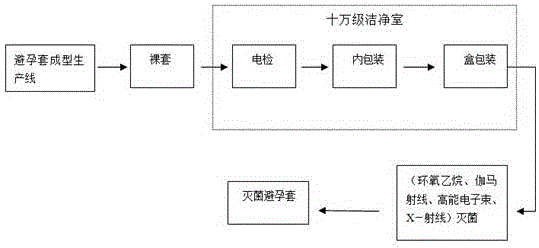
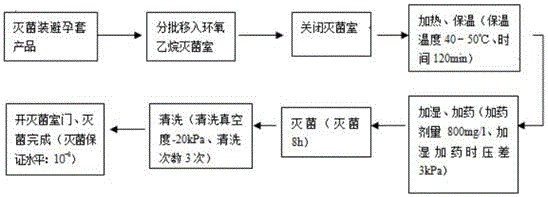
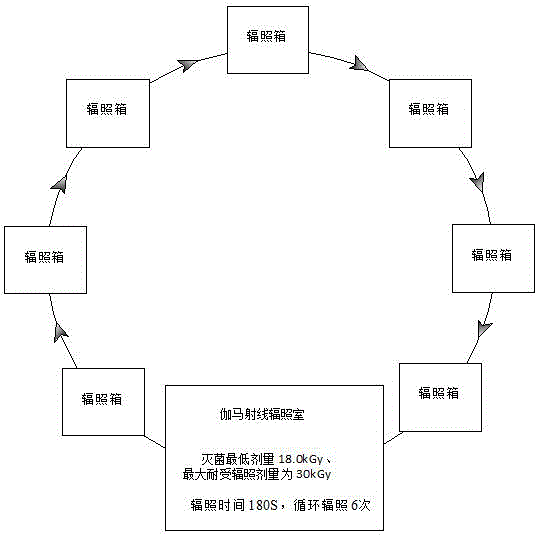
Production method for sterile condom
ActiveCN106395018ANo damage to physical and chemical propertiesPackage sterilisationPackaging machinesComposite filmHigh energy
The invention relates to a production method for a sterile condom, and belongs to the technical field of condom production. The main raw material of the sterile condom is natural latex rubber. A PE and PVC composite film is selected in a packaging material to serve as an inner packaging material of the condom, wherein a PE layer makes direct contact with the condom, a PVC layer is an outer printing layer, packaging is conducted in a hundred-thousand-level cleaning chamber, the initial contamination amount of a condom product is controlled, and sterilization can be conducted through an ethylene oxide gaseous sterilizing agent, gamma ray irradiation and high-energy electron beams or X-rays. According to the production method, the sterility assurance level is 10<-6>, meanwhile, the original physical and chemical properties of the condom are not destroyed, the sanitary condom product with the quality meeting the national standard requirement is provided for consumers, and the production method is particularly suitable for being used on occasions with the high sanitation standard requirement.
Owner:GUANGDONG FITONE LATEX PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com