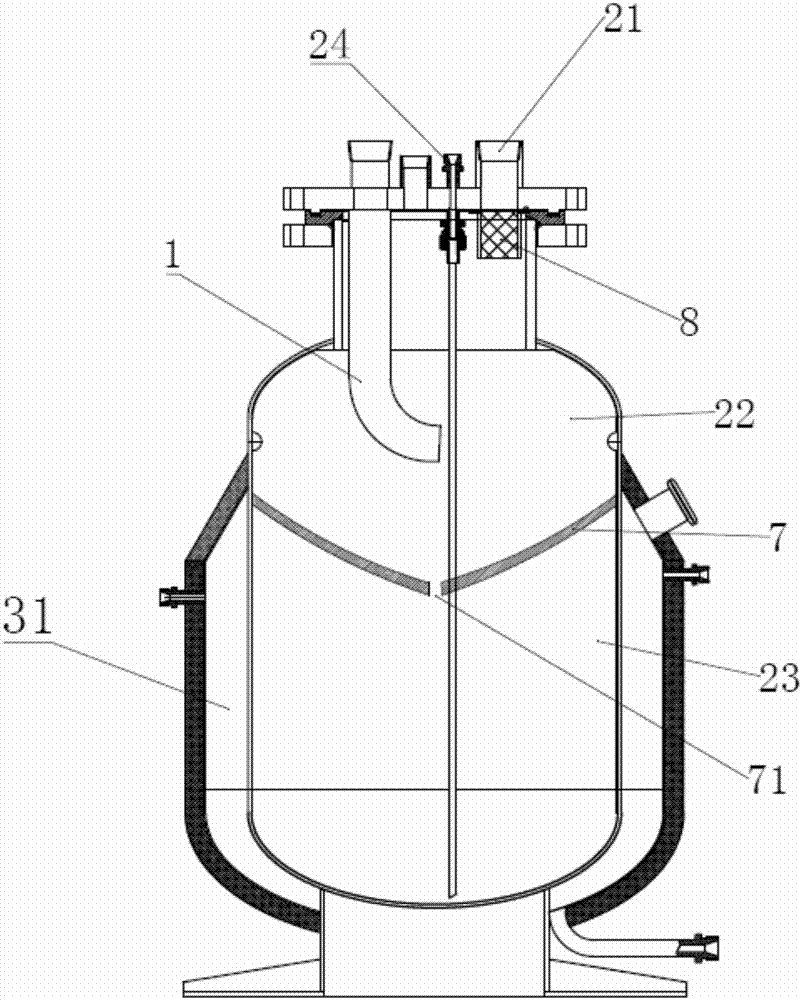
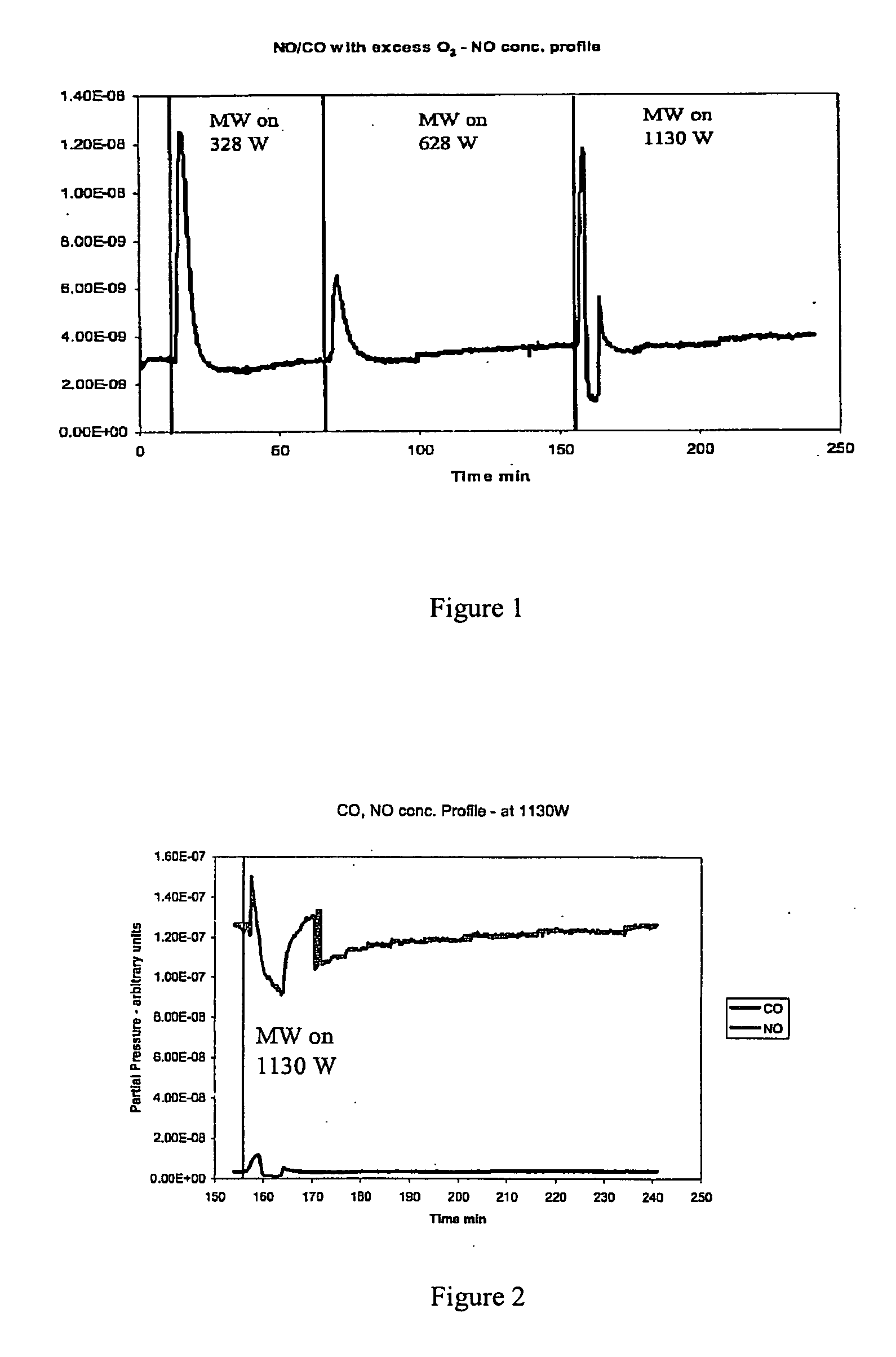
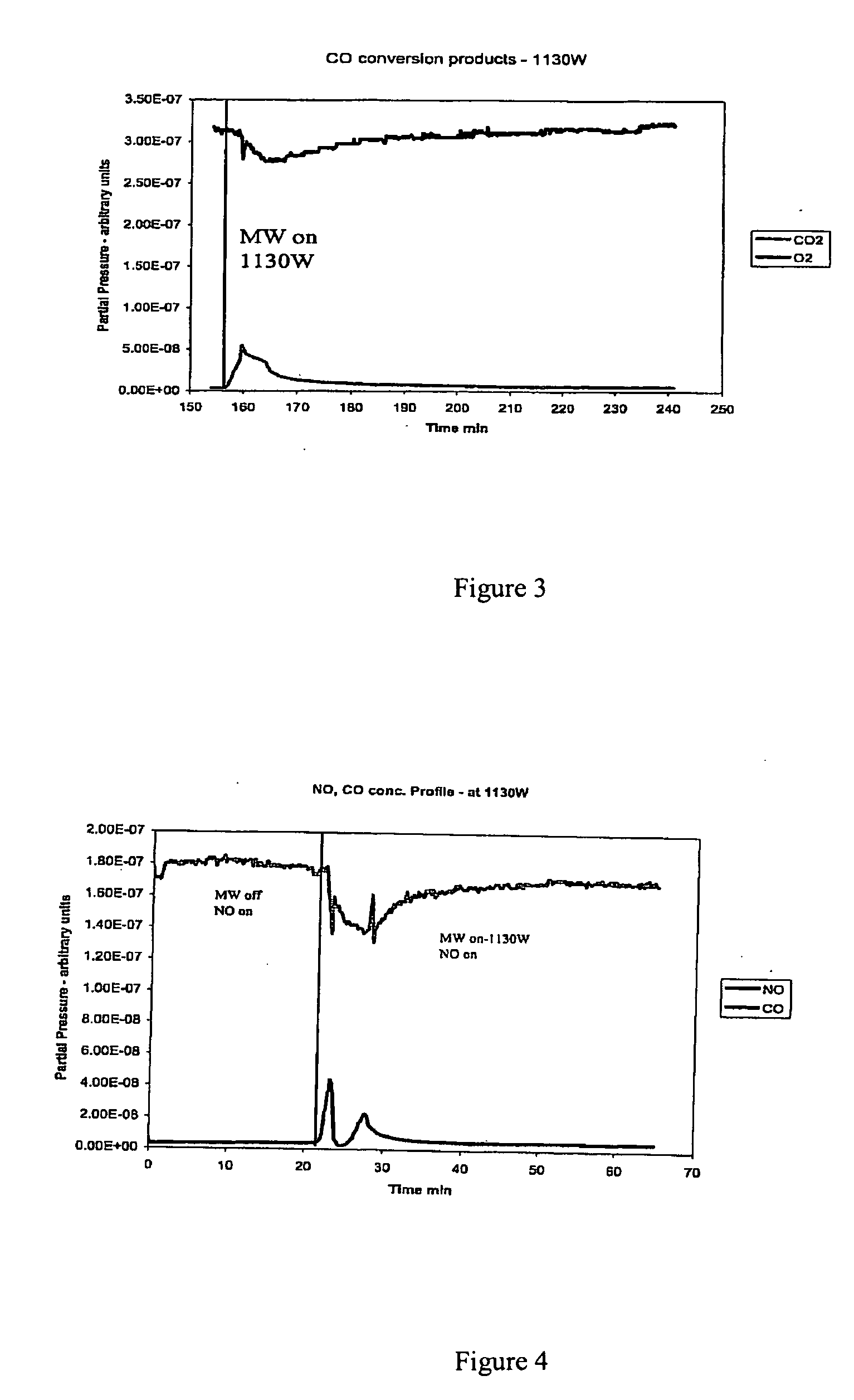
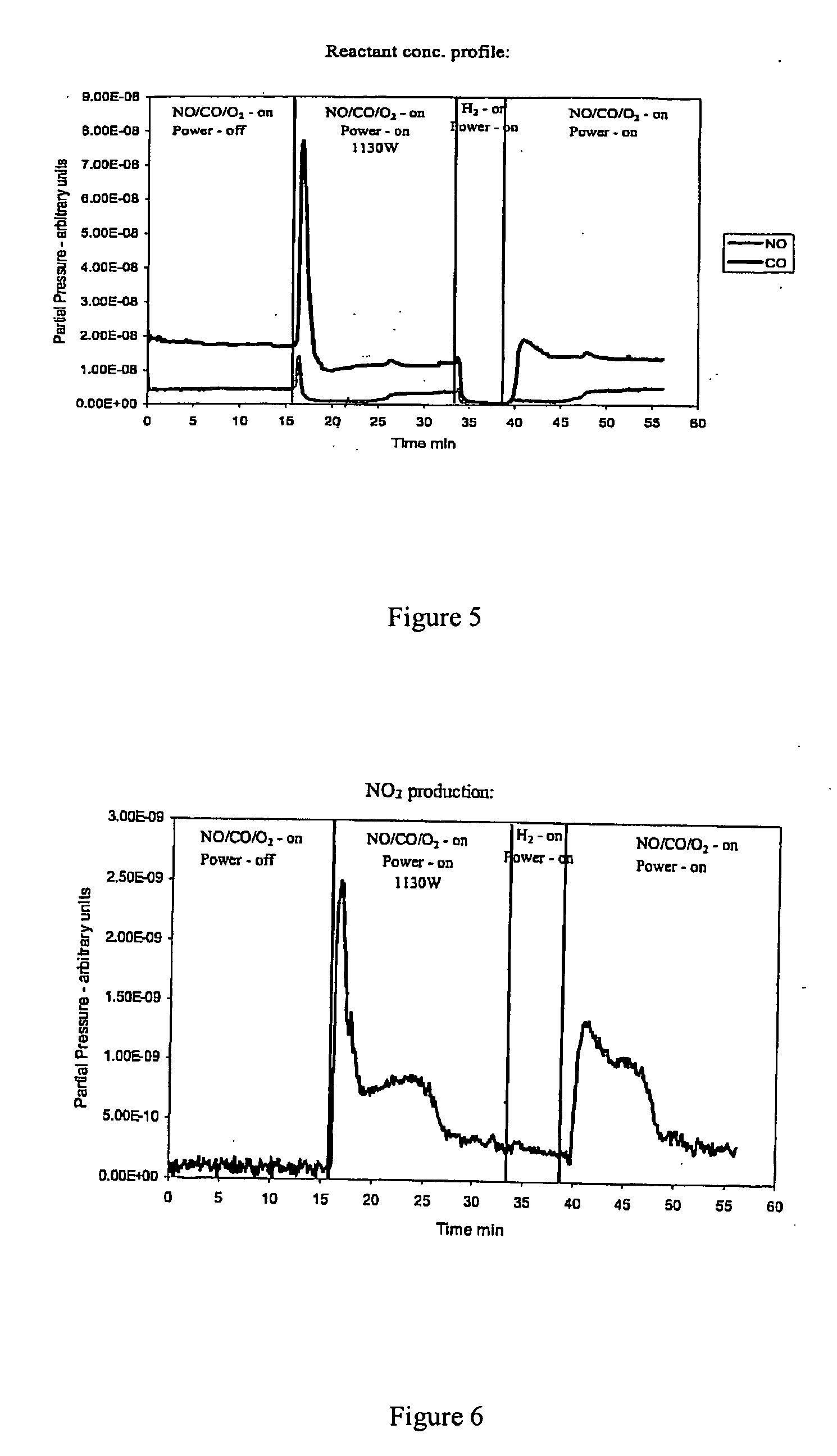
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67results about "Nitrogen dioxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
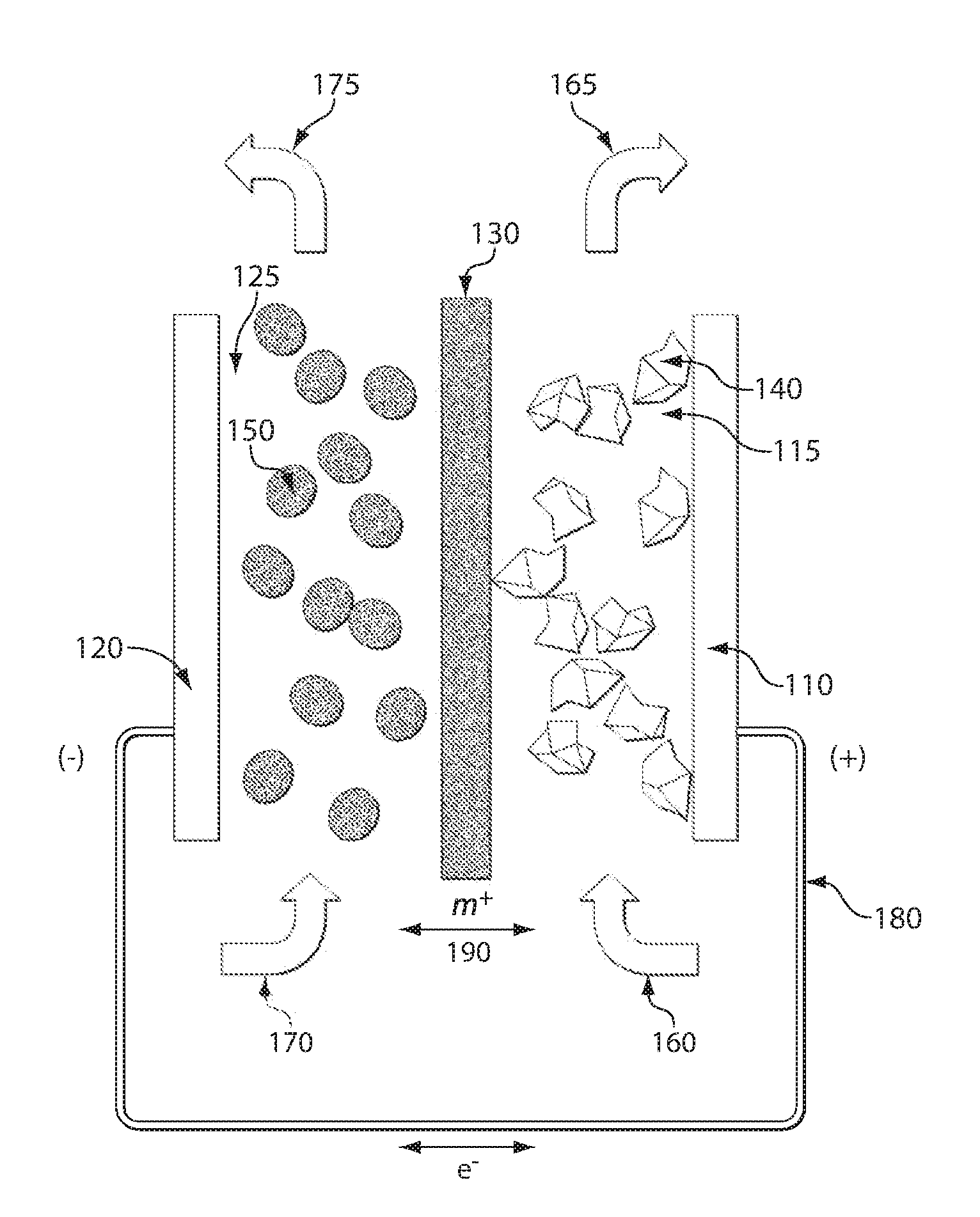
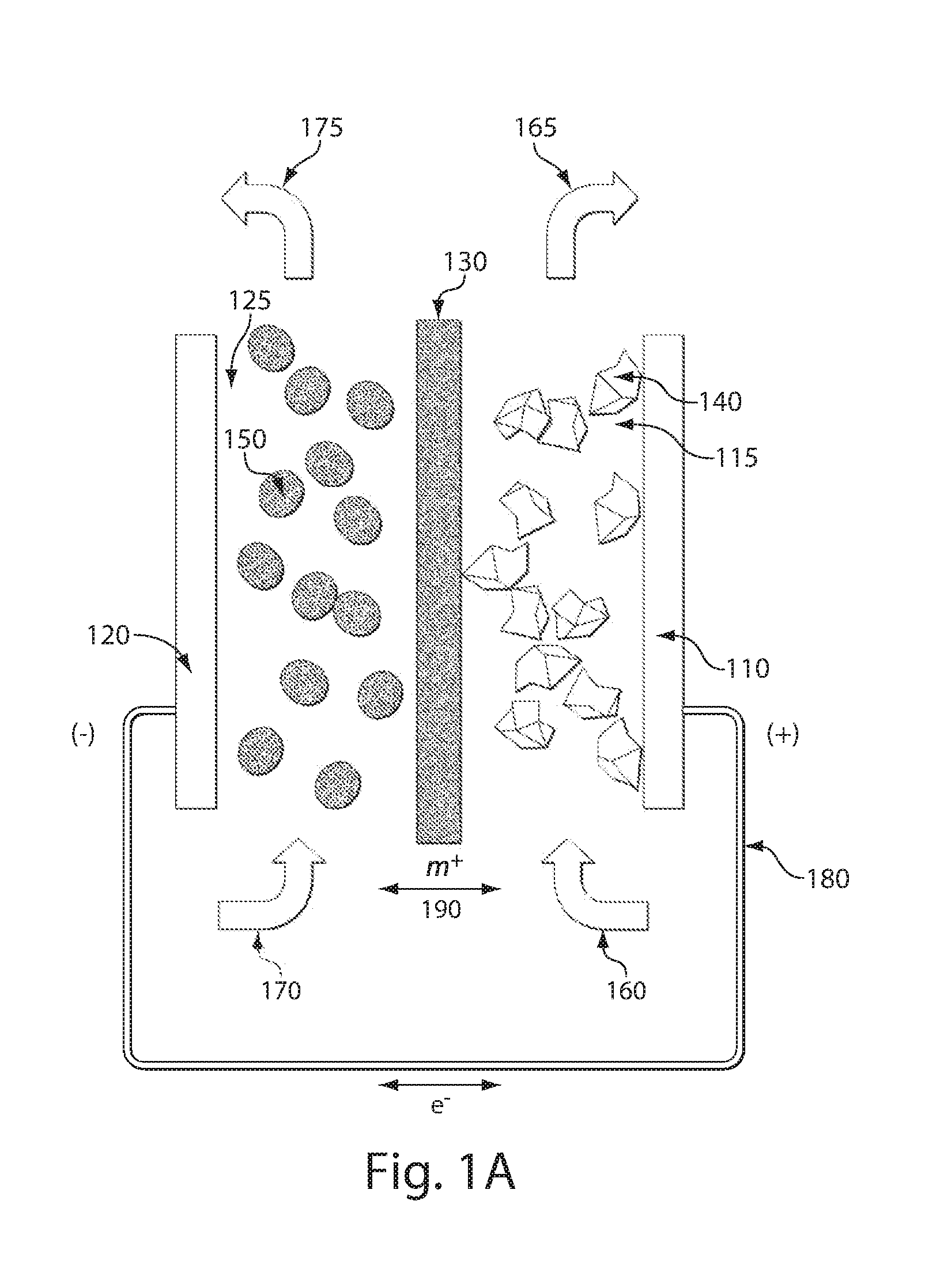
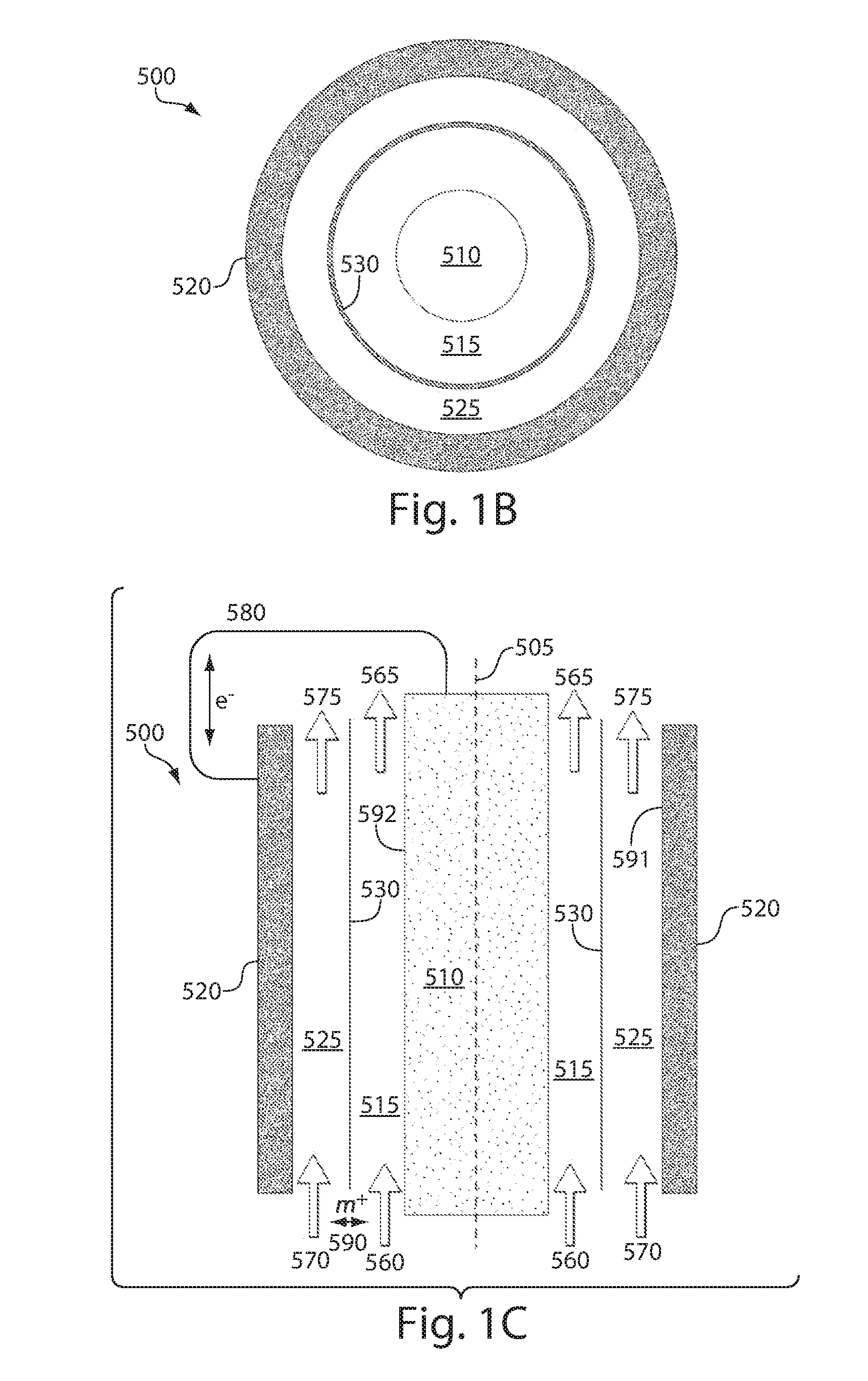
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
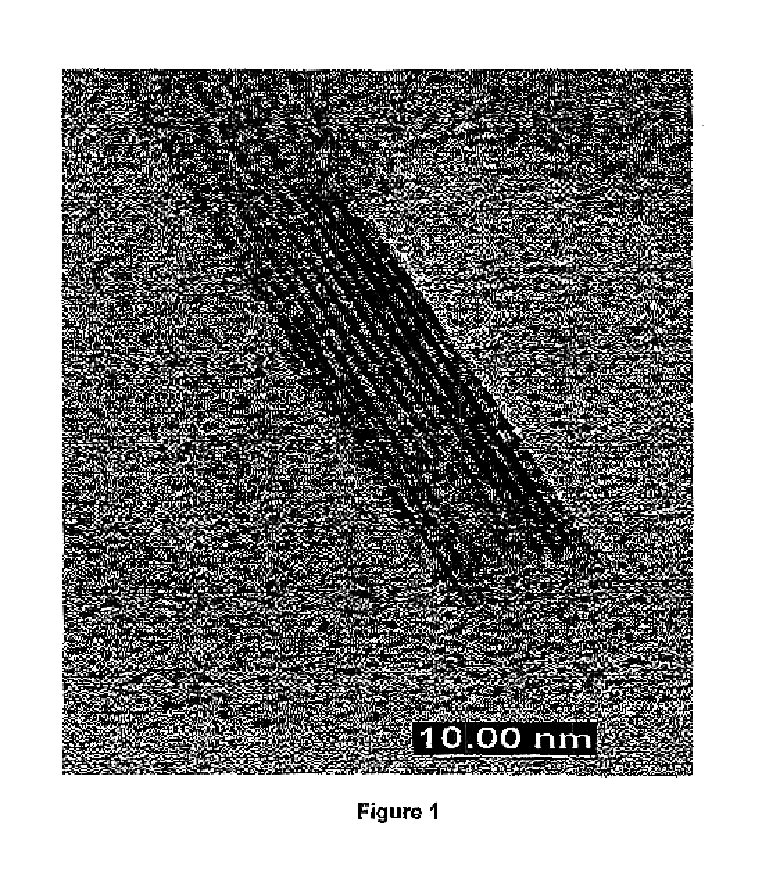
Synthesis method for producing carbon clusters and structured carbon clusters produced thereby
InactiveUS6855301B1Increase ratingsOptimizationMaterial nanotechnologyElectrolysis componentsSynthesis methodsMolecular cluster
The present invention includes carbon synthesis devices and systems. The invention also includes machines and instruments using those aspects of the invention. The present invention also includes methods of carbon synthesis. The present invention includes an array of carbon nanotubes, each nanotube having a longitudinal axis. The nanotubes are placed into an array such that the longitudinal axes of all nanotubes in the array are substantially parallel. The array may be a two-dimensional array or a three-dimensional array. The present invention also includes methods of preparing such carbon molecular clusters and arrays thereof.
Owner:THE OHIO STATES UNIV
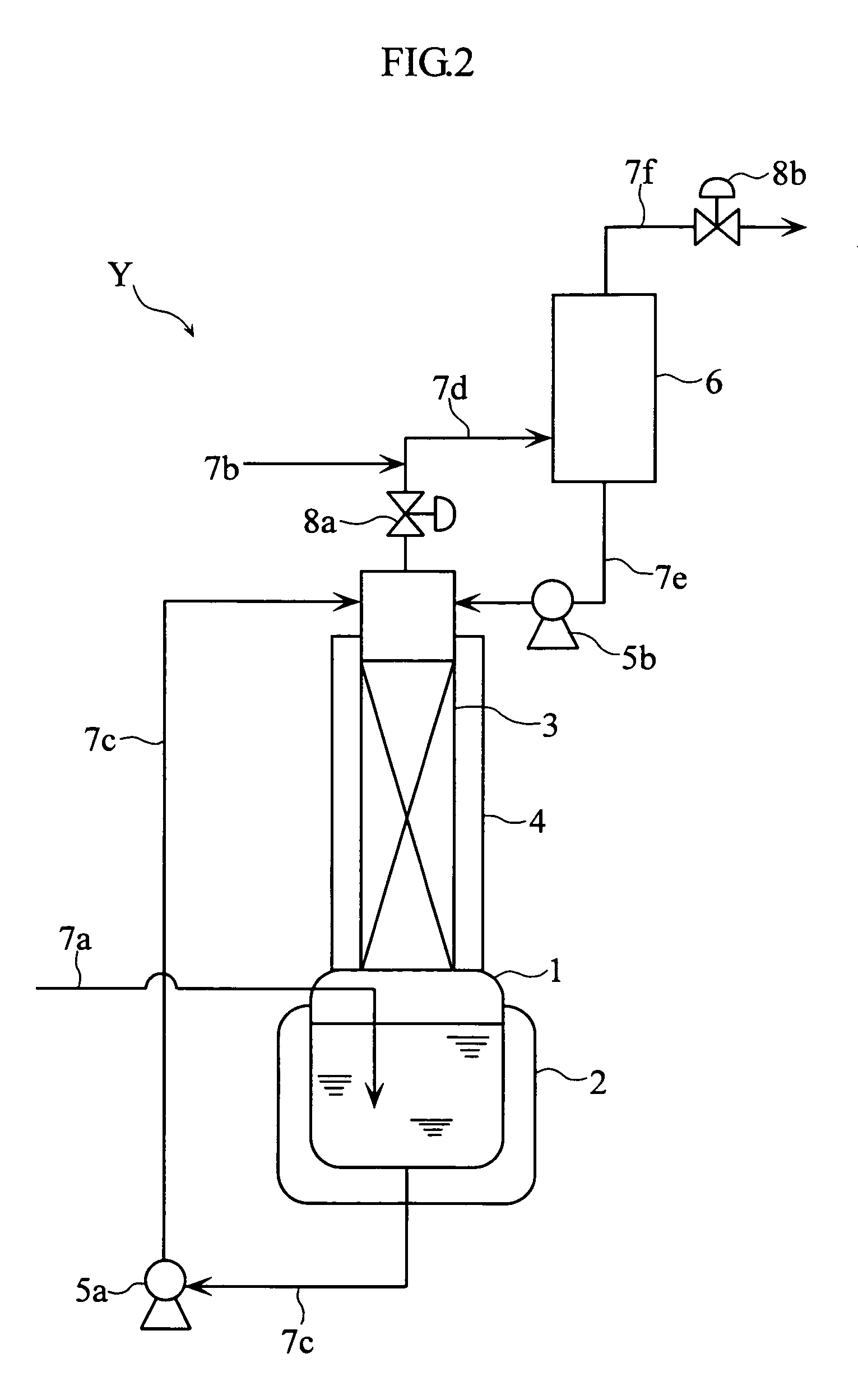
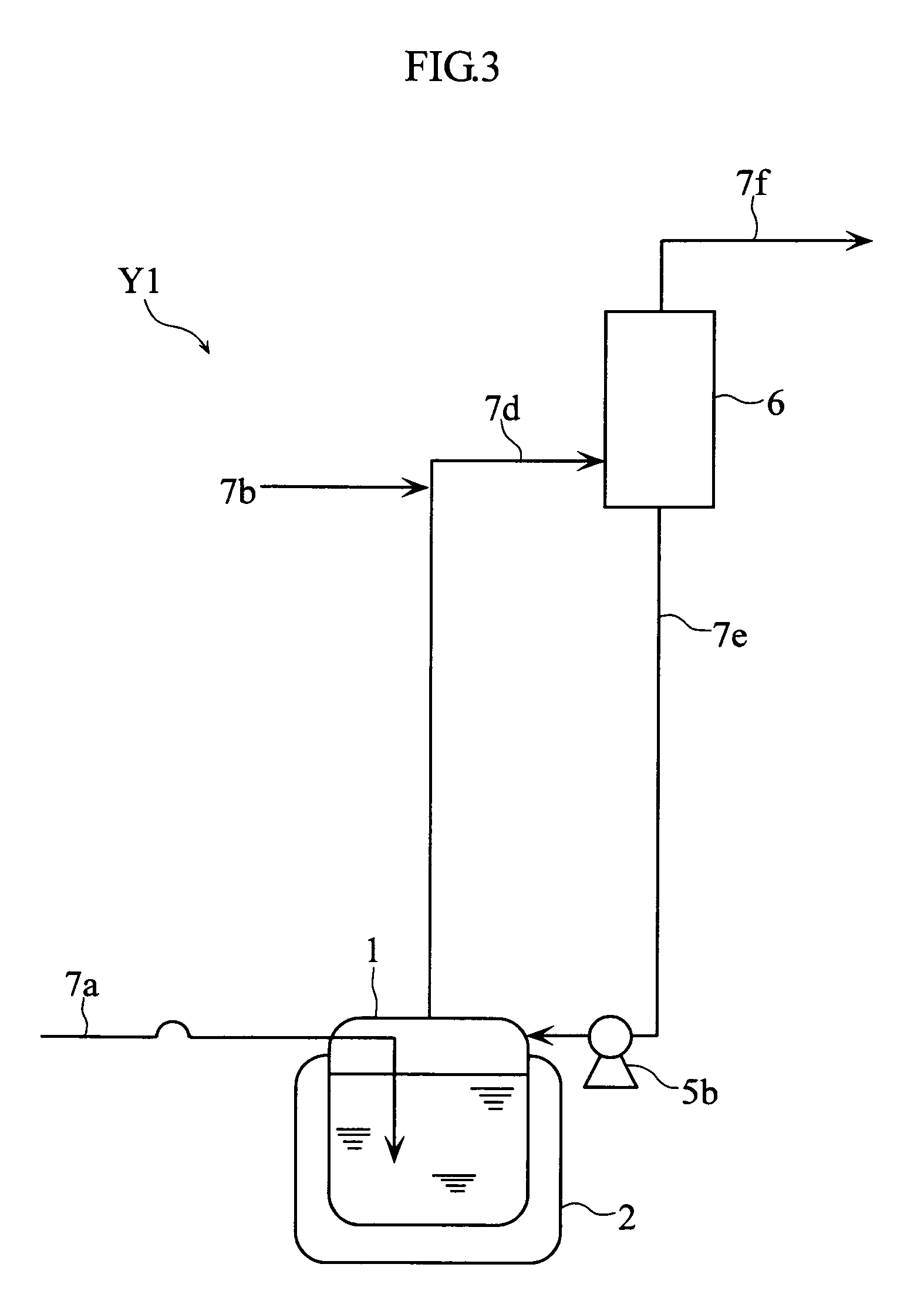
Method for purification of nitrogen oxide and apparatus for purification of nitrogen oxide
ActiveUS7776305B2Efficient purificationImprove securityNitric oxideNitrous oxidesNitrogen oxidesRadiochemistry
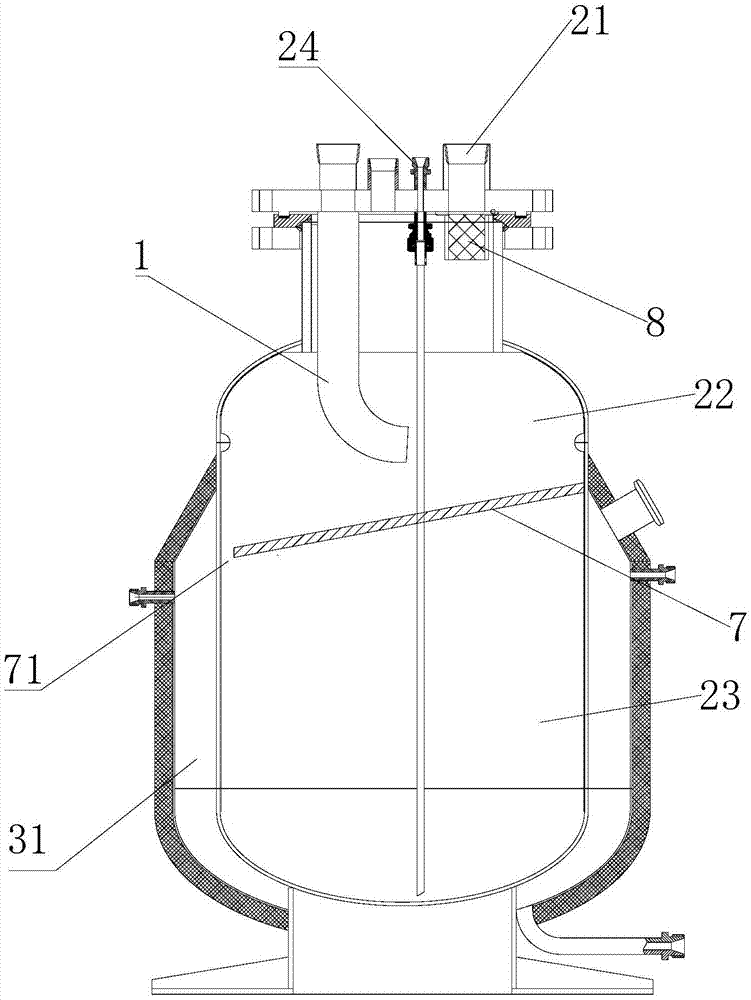
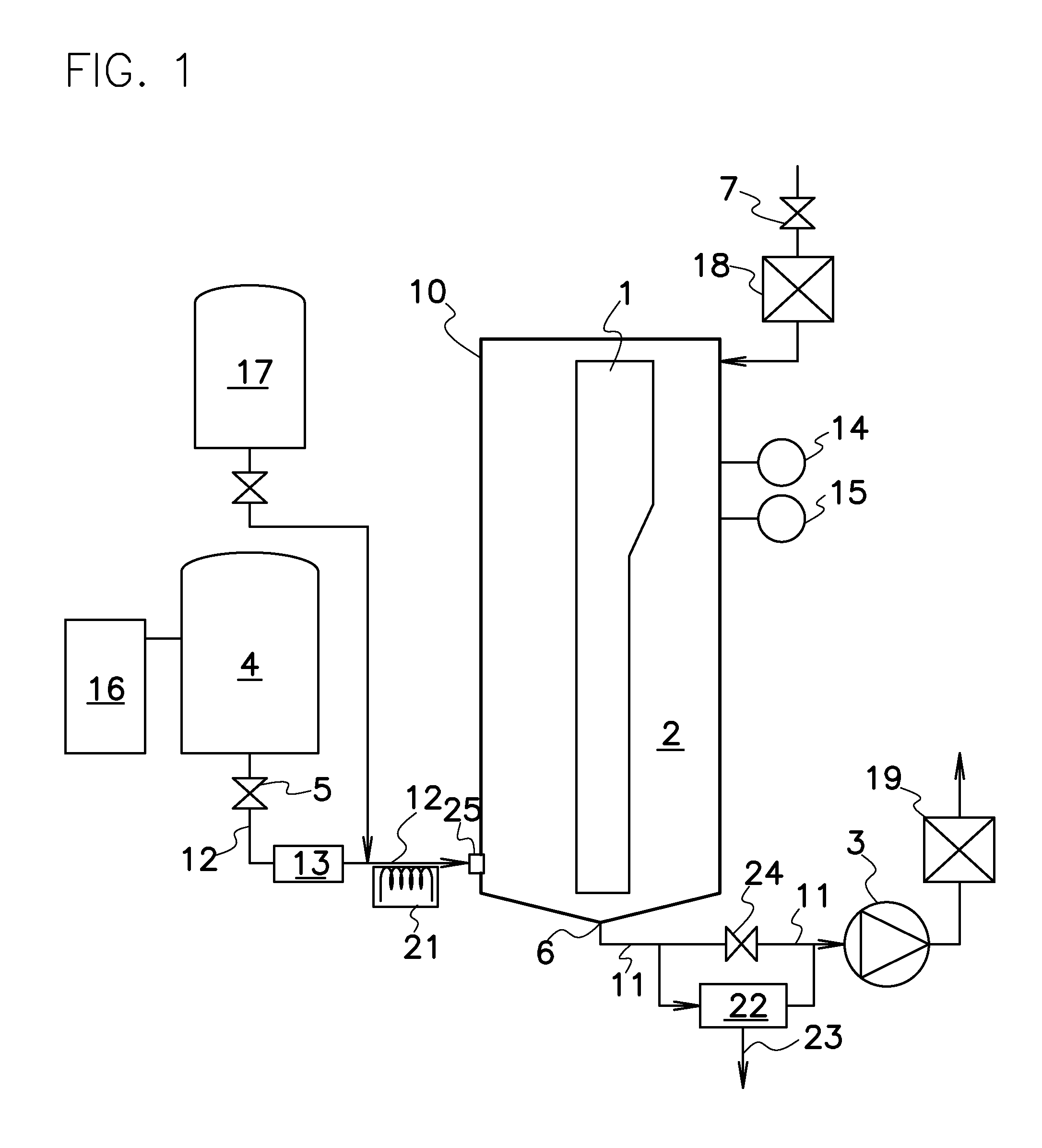
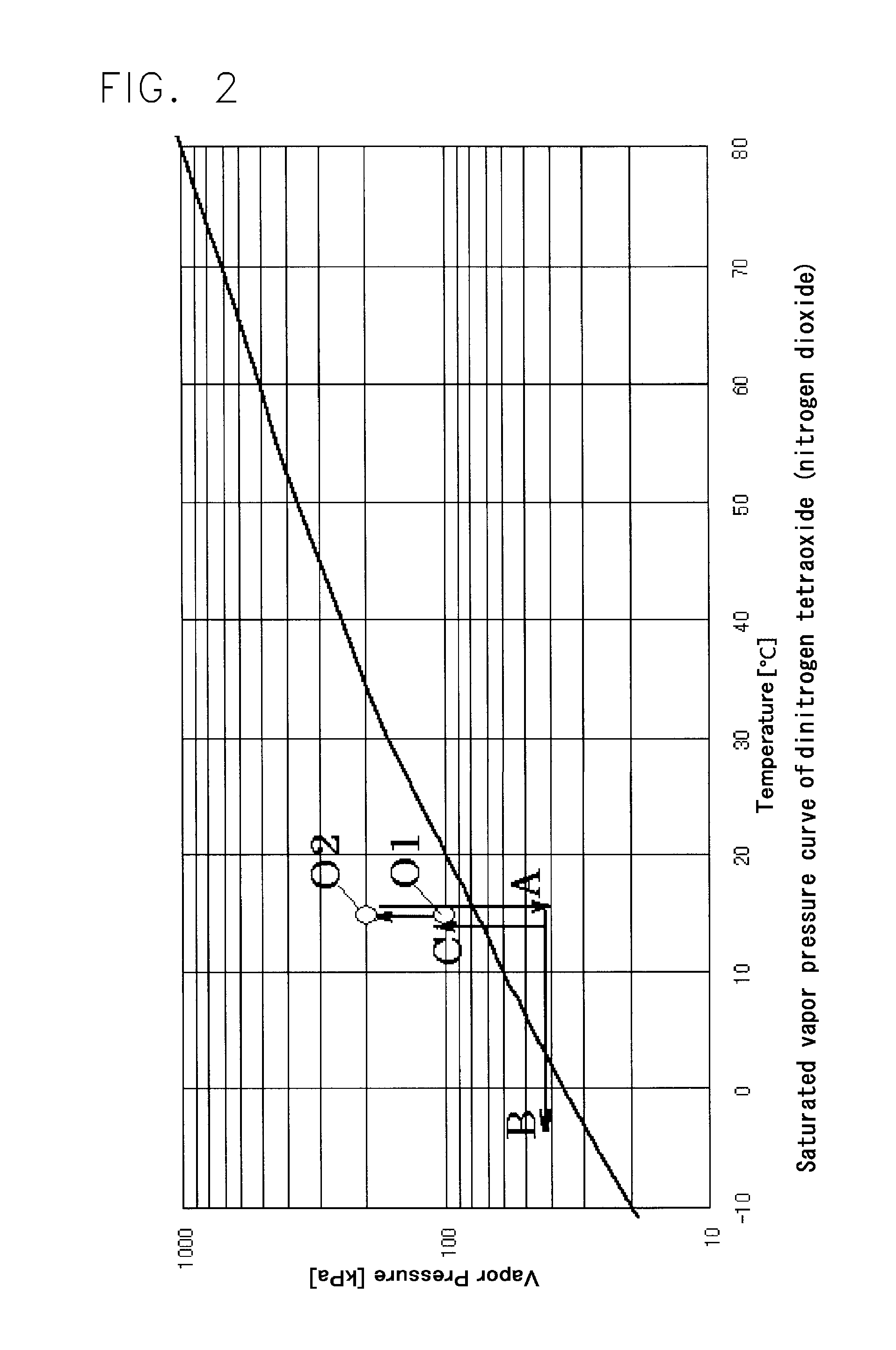
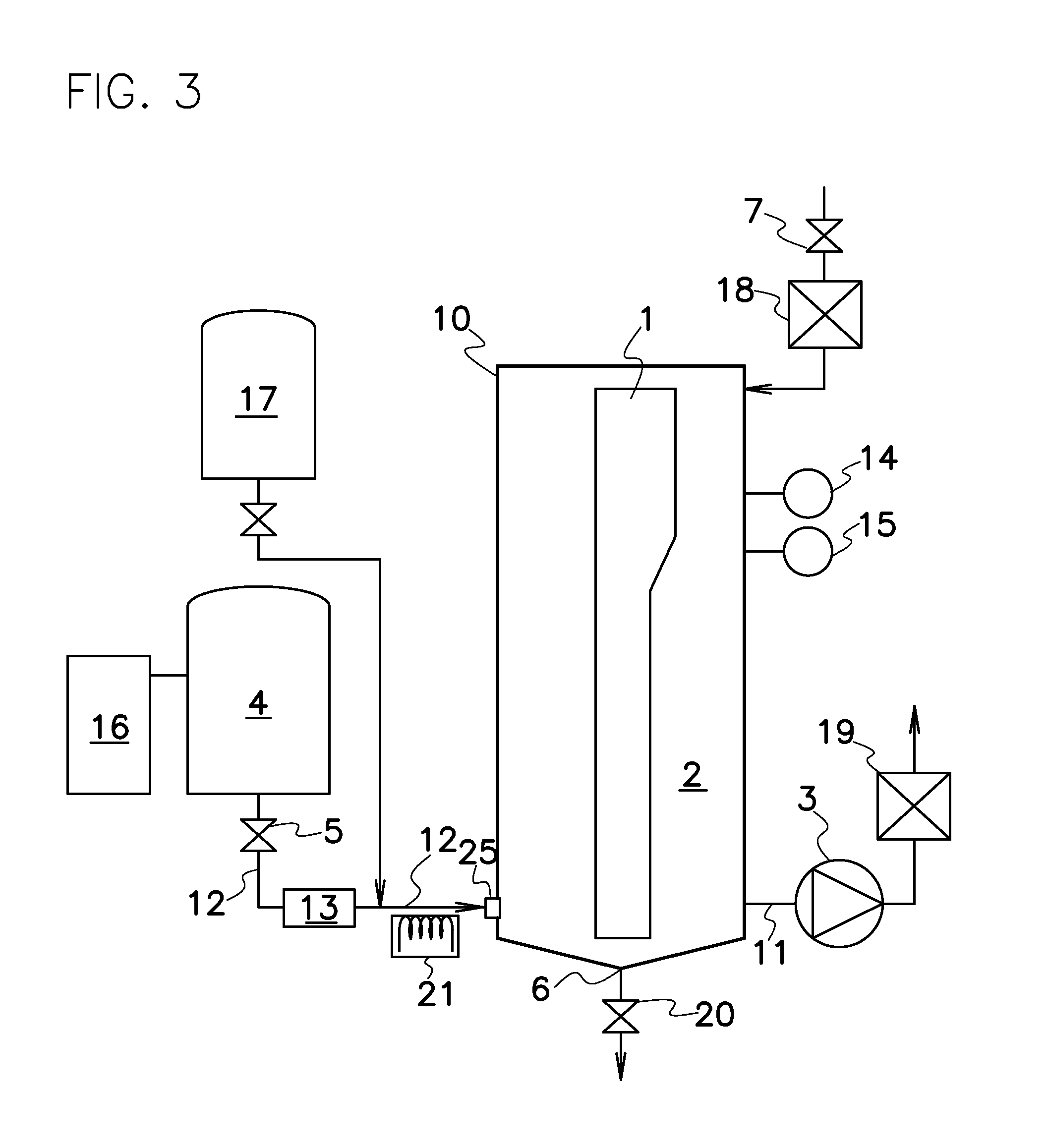
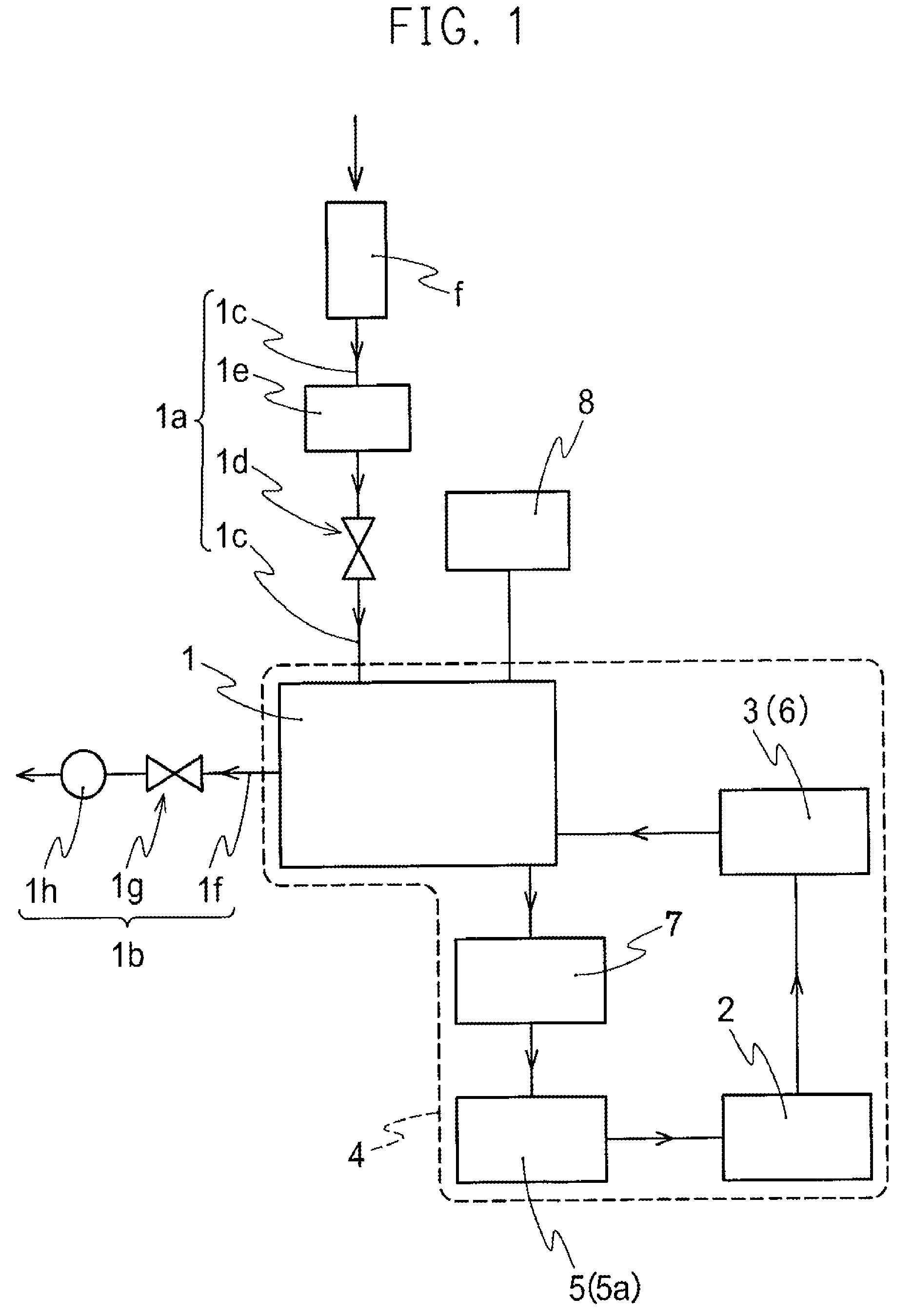
A nitrogen oxide purifying apparatus includes a gas absorption vessel (1) and a condenser (6), where the vessel receives an absorption solution containing liquefied N2O4 for absorbing NO and also receives a source gas to vary the temperature and / or pressure of the source gas and the absorption solution, while the condenser receives a gas from the gas absorption vessel (1) to vary the temperature and / or pressure of the gas. In the gas absorption vessel (1), the absorption solution containing liquefied N2O4 may be applied to the source gas containing NO, so that NO is absorbed in the absorption solution. Then the absorption-solution is heated and / or depressurized to generate an intermediate gas containing a relatively large amount of NO and a smaller amount of NO2 from the absorption solution. In the condenser (6), the intermediate gas is cooled and / or pressurized to give condensed N2O3 and / or condensed N2O4.
Owner:SUMITOMO SEIKA CHEM CO LTD
Reusable apparatus for gas generation
InactiveUS20060120945A1Low costSave resourcesCarbon compoundsSpecific water treatment objectivesRefill KitEngineering
Owner:SELECTIVE MICRO TECH
Method and device system for recovering nitric acid through pyrolyzing nitrate
ActiveCN109721038AImprove recycling ratesFull atomization heating decompositionOxide/hydroxide preparationChemical industryNitrateInternal temperature
The invention discloses a method for recovering nitric acid by pyrolyzing nitrate. The method comprises the following steps: (1) conveying the nitrate into at least two preheaters, heating the nitrateto firstly liquefy the nitrate, and then heating the nitrate to a temperature less than decomposition temperature in order to obtain a nitrate hot fluid; (2) conveying the nitrate hot fluid into a decomposer, and heating the nitrate hot fluid with a high temperature gas to maintain the internal temperature of the decomposer at 500-800 DEG C in order to decompose the nitrate into a mixed gas and asolid powder; and (3) separating mixed gas and the solid powder, conveying a part of the mixed gas into a nitric acid recovery tank, heating the remaining mixed gas to 500-800 DEG C, and then returning the heated remaining mixed gas into the decomposer to heat the nitrate hot fluid in order to thermally decompose the nitrate hot fluid. The method for recovering nitric acid has the advantages of less corrosion damages to devices, no introduction of other impurity components and no interference in the heating process, good decomposition speed and decomposition rate of the nitrate, and high recycling rate of the nitric acid.
Owner:MEISHAN SHUNYING POWER BATTERY MATERIALS CO LTD
High temperature decomposition of hydrogen peroxide
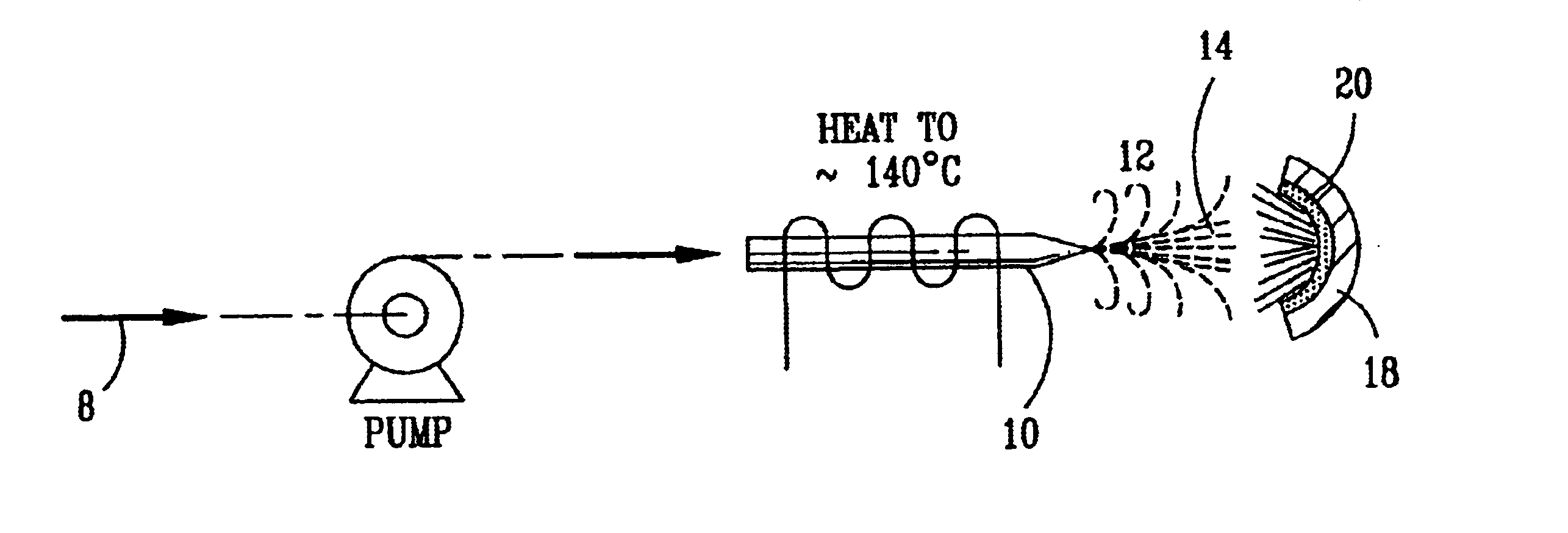
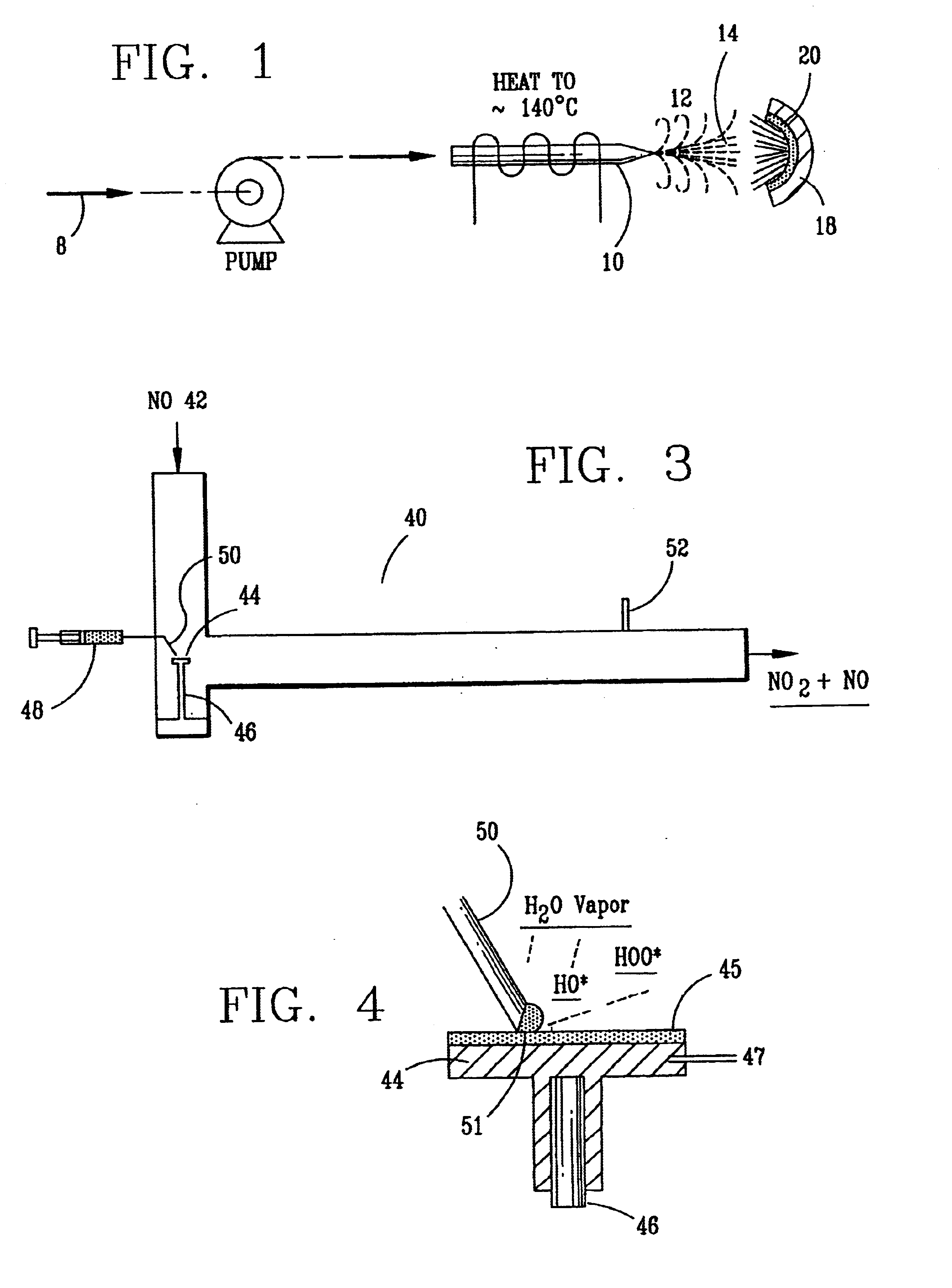
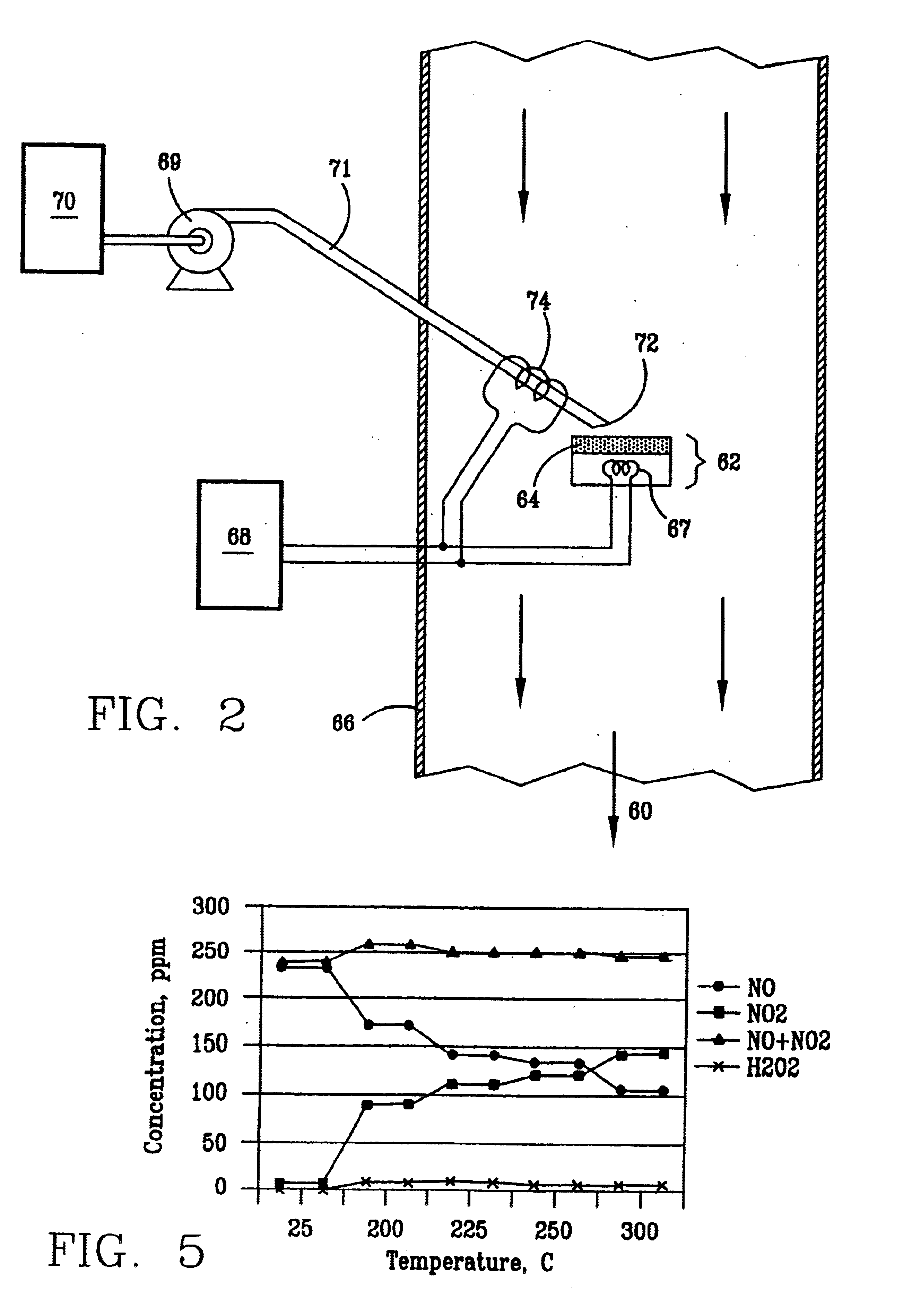
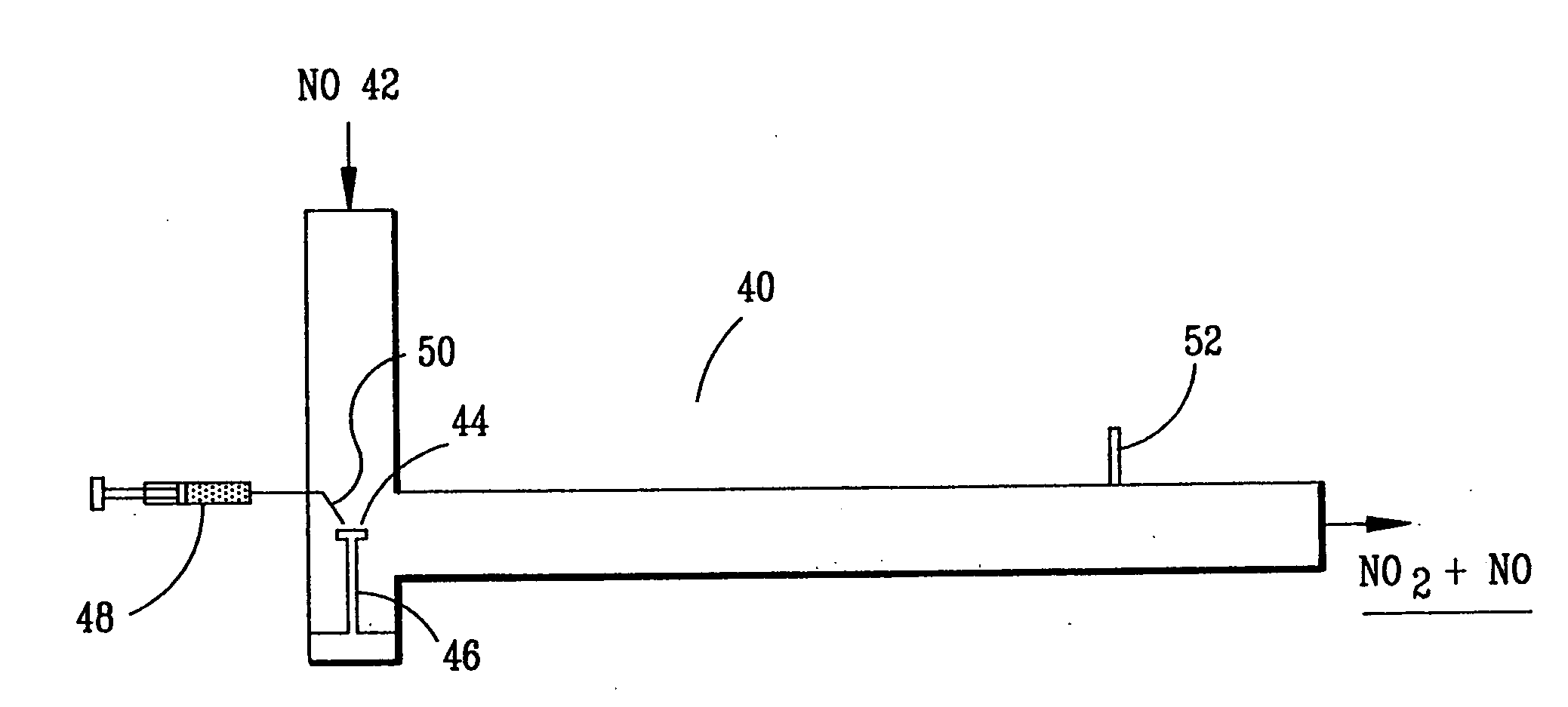
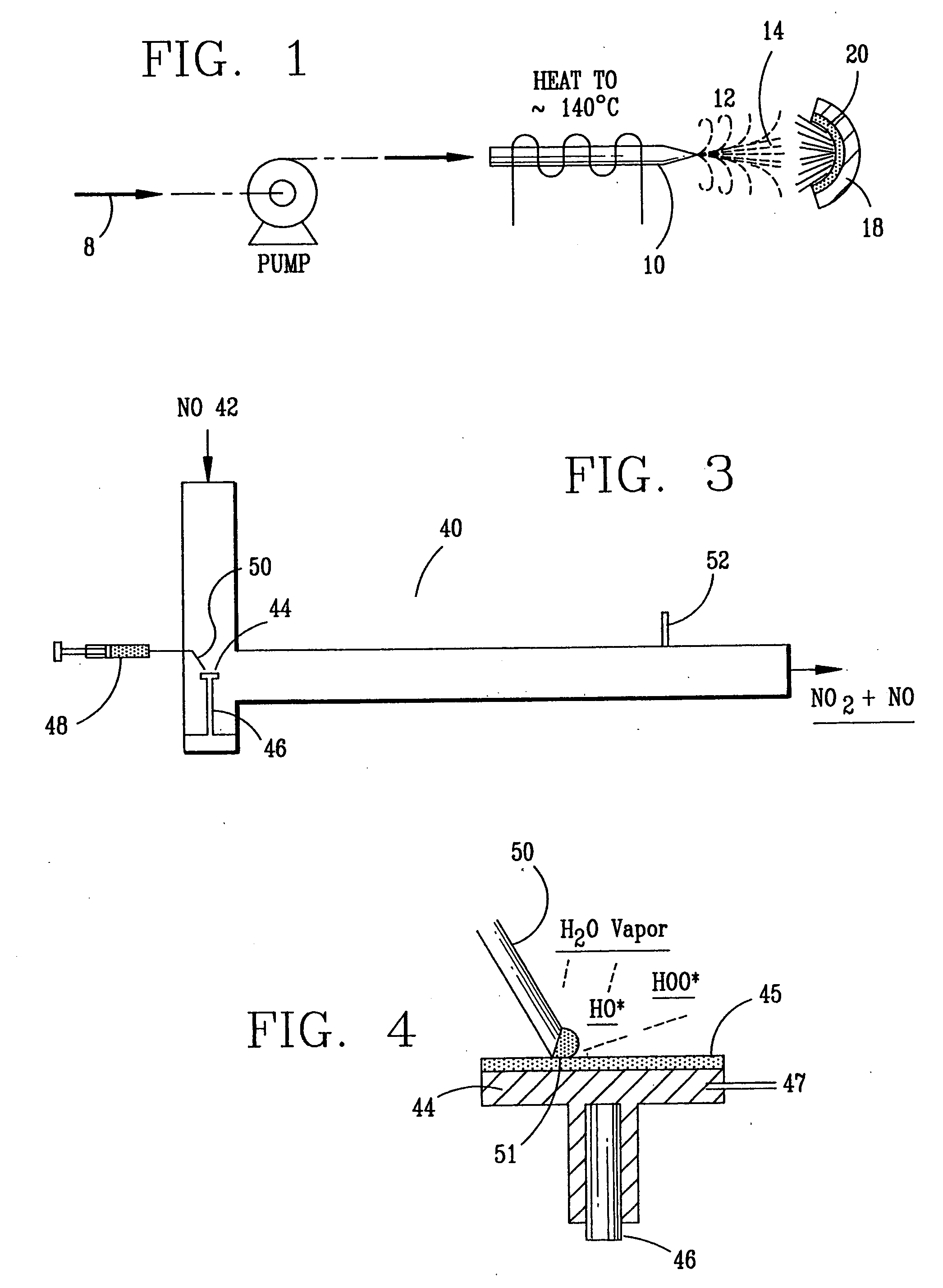
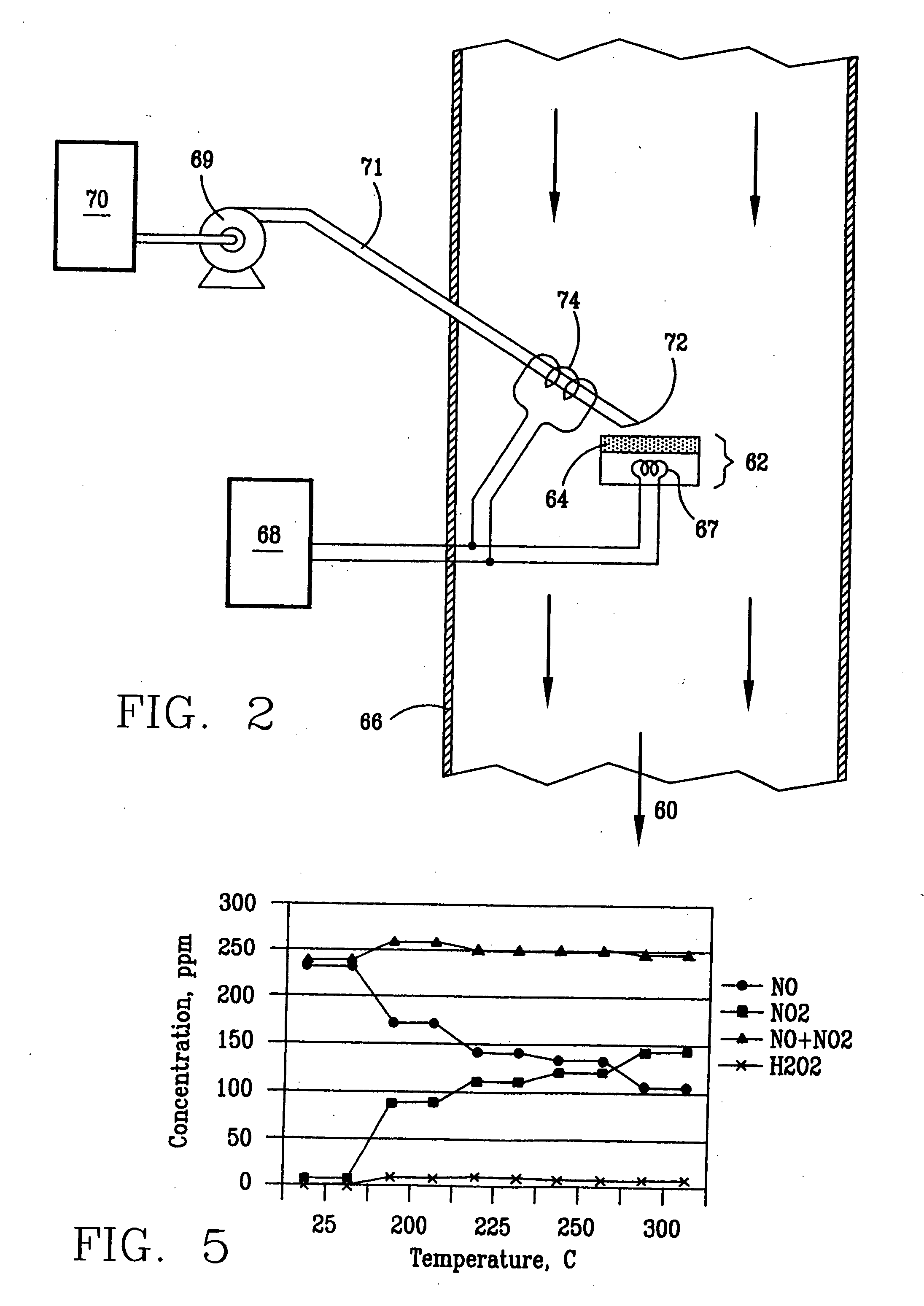
InactiveUS6793903B1Easy to disassembleReduce nitrogen oxide emissionsDispersed particle separationNitric oxideNitrogen dioxideGas phase
Nitric oxide (NO) is oxidized into nitrogen dioxide (NO2) by the high temperature decomposition of a hydrogen peroxide solution to produce the oxidative free radicals, hydroxyl and hydroperoxyl. The hydrogen peroxide solution is impinged upon a heated surface in a stream of nitric oxide where it decomposes to produce the oxidative free radicals. Because the decomposition of the hydrogen peroxide solution occurs within the stream of the nitric oxide, rapid gas-phase oxidation of nitric oxide into nitrogen dioxide occurs.
Owner:NAT AERONAUTICS & SPACE ADMINISTATION U S GOVERNMENT AS REPRESENTED BY THE ADMINISTATION OF
Composition for controlled sustained release of a gas
InactiveUS20060178445A1High quantity of gasShort stayBiocideCyanogen compoundsBiological activationSodium chlorite
The invention relates to an improved composition for generating at least one gas comprising an energy-activated catalyst capable of being activated by electromagnetic energy, heat and / or moisture and anions capable of being oxidized or reacted to generate at least one gas, the composition, when exposed to electromagnetic energy, heat and / or moisture being capable of generating and releasing the gas after activation of the catalyst and oxidation or reaction of the anions. The process comprises: (a) metering a liquid composition comprising a source of the anions, specifically sodium chlorite, into a flow restrictor; (b) injecting a gas stream through the flow restrictor, concurrently with step (a) to create a zone of turbulence at the outlet of the flow restrictor, thereby atomizing the liquid composition; (c) heating the gas stream prior to injecting the gas stream through the flow restrictor; and (d) adding the energy-activated catalyst, specifically titanium dioxide, to the zone of turbulence concurrently with steps (a) and (b) to contact the energy-activated catalyst with the atomized liquid composition wherein the contacting at the zone of turbulence treats the energy-activated catalyst with the source of the anions. The titanium dioxide can be pigmentary or nano-sized. The composition can be useful in polymeric composition, specifically for making a body covering article.
Owner:EI DU PONT DE NEMOURS & CO
Method and device using plasmon- resonating nanoparticles
Disclosed herein are methods and articles that include a plasmon-resonating nanostructure that employ a photo-thermal mechanism to catalyze the reduction of an oxidant. As such, the plasmon-resonating nanostructure catalyzes a redox reaction at a temperature below a predetermined activation temperature. The method can be efficiently used to catalyze the reduction of an oxidant, for example in a catalytic reactor or in a fuel cell that includes a photon source.
Owner:RGT UNIV OF MICHIGAN
High temperature decomposition of hydrogen peroxide
InactiveUS20050019229A1Improve concentrationPromote decompositionCombination devicesExhaust apparatusGas phaseNitrogen dioxide
Owner:NASA
Method for preparing active carbon loaded with manganese oxide by hydrothermal method and device thereof
ActiveCN107352540AImprove conversion rateImprove loading efficiencyGas treatmentCarbon compoundsActivated charcoal powderActivated carbon
The invention relates to a method for preparing active carbon loaded with manganese oxide by a hydrothermal method and a device thereof. The method comprises the following steps: firstly carrying out equivalent-volume impregnation on vacuumdryingactive carbon and potassium permanganate solution, then putting the active carbon, the potassium permanganate solution and mixed solution of manganese nitrate and nitric acid together into a hydrothermal crystallization device for reaction, cooling and separating obtained solid-liquid mixture, washing and drying solid-phase products, calcining in inertial gas to obtain modified active carbon powder loaded with the manganese oxide; and the modified active carbon powder has good property in low-temperature NO catalytic oxidation, and the conversion rate is 48.5%-58.3%. The invention also discloses the device for implementing the method for preparing the active carbon loaded with the manganese oxide by the hydrothermal method. The equipment is high in automation degree, clean in production process, simple in process and low in cost.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI
Nitrogen tetroxide leakage collection device and method
InactiveCN107998806ARemove completelyImprove collection efficiencyNitrous oxide captureGas treatmentNitrogen tetroxideHazardous substance
The invention discloses a nitrogen tetroxide leakage collection device and method. The nitrogen tetroxide leakage collection device comprises a collection pipe, a collection container connected to thecollection pipe, an exhaust gas treatment module, a high negative pressure suction device and an electronic control module. The collection container and the exhaust gas treatment module are providedwith cooling systems. The upper part of the collection container is provided with a gas outlet. The gas outlet is connected to the negative pressure suction device through a gas suction pipe. The gassuction pipe between the collection container and the negative pressure suction device is provided with an absorption device for removing exhaust gas. Through being driven by the negative pressure suction device, the leaked nitrogen tetroxide liquid / gas and nitrogen dioxide are sucked into the inner collection container through the collection pipe, the gas-liquid mixture is subjected to gas-liquidseparation and cooling liquidation, the nitrogen tetroxide liquid is collected, and the tail gas is subjected to adsorption filtration so that toxic and hazardous substances in the tail gas are removed and thus collection efficiency is high and the environmental protection and conservation of resources are realized.
Owner:BEIJING INST OF AEROSPACE TESTING TECH +1
Process using microwave energy and a catalyst to decompose nitrogen oxides
A process for decomposing nitrogen oxides includes the following steps: providing a catalyst, passing a gaseous nitrogen oxide over the catalyst and exposing the catalyst to microwave energy. The gaseous nitrogen oxide is broken down into nitrogen and oxygen molecules.
Owner:TOYOTA MOTOR CO LTD +1
Method and apparatus for sterilization with nitrogen oxide
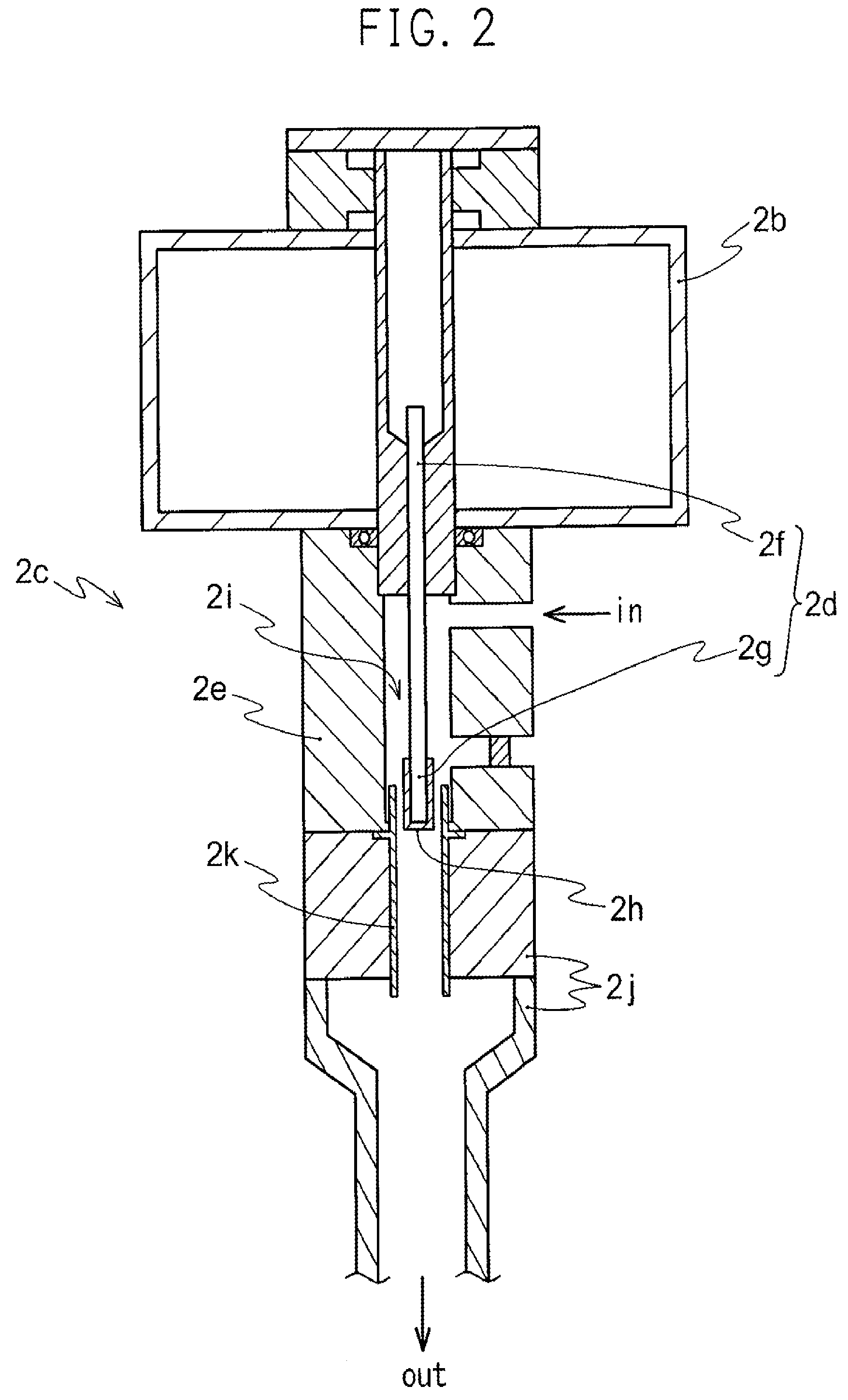
ActiveUS20150037206A1Efficiently and safely introducedAvoid infectionFood preservationLavatory sanitoryNitrogen oxideEngineering
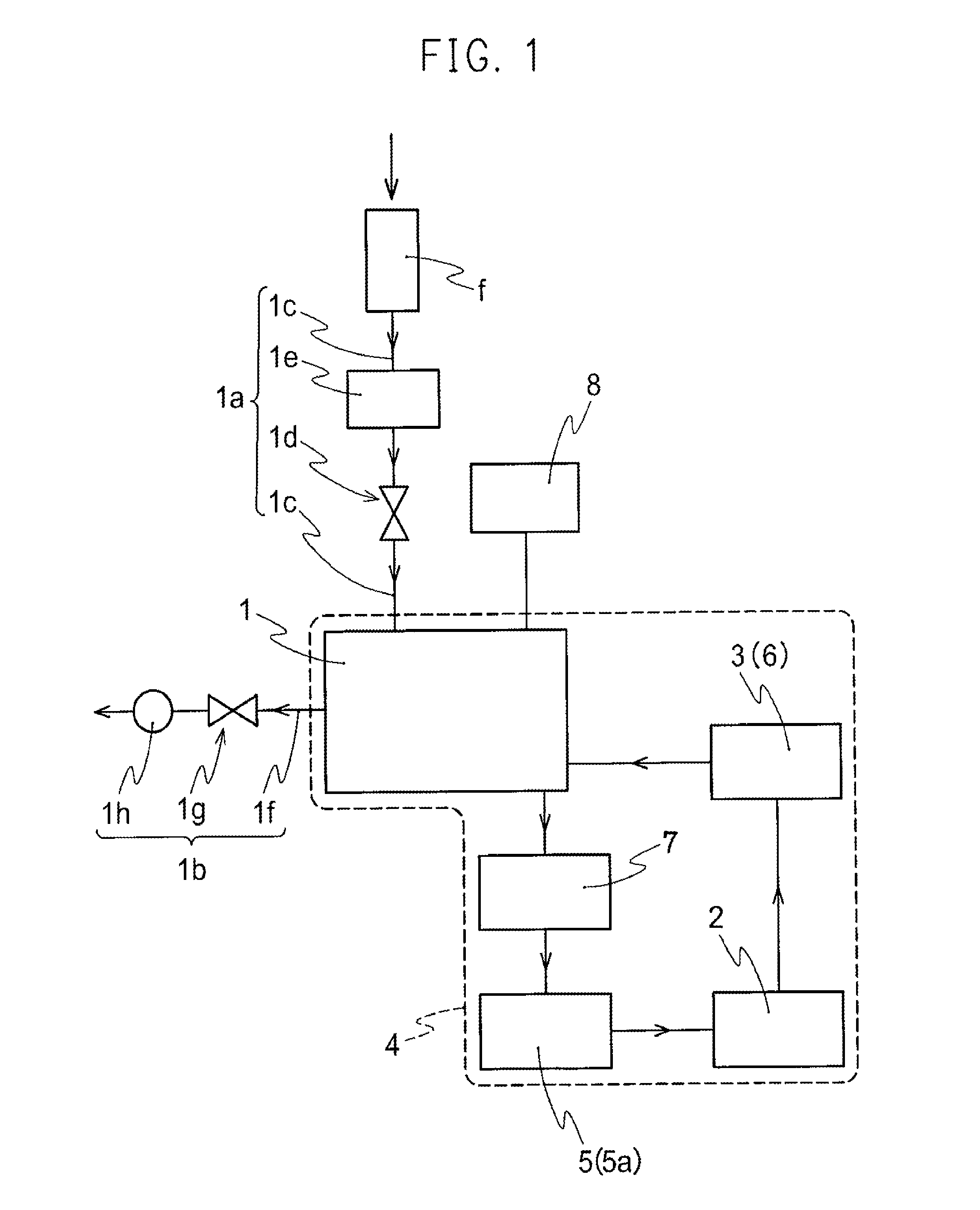
Nitrogen oxide is used to safely sterilize inside of a space area or an object arranged within the space area. A sterilization method is provided to comprise: introducing nitrogen oxide liquid stored in a vessel (4) into a space area (2, 2′, 2″) to gasify nitrogen oxide liquid, and sterilizing inside of space area (2, 2′, 2″) or object (1) arranged in space area (2, 2′, 2″) with nitrogen oxide gas evaporated from nitrogen oxide liquid to completely annihilate and destroy microorganisms inclusive of bacteria and virus with nitrogen oxide gas for preventing the infection by microorganisms to human body and damage to precision components.
Owner:ENA
High Concentration NO2 Generating System and Method for Generating High Concentration NO2 Using the Generating System
ActiveUS20110286908A1Efficient processMaterial nanotechnologyInorganic active ingredientsHigh concentrationPlasma generator
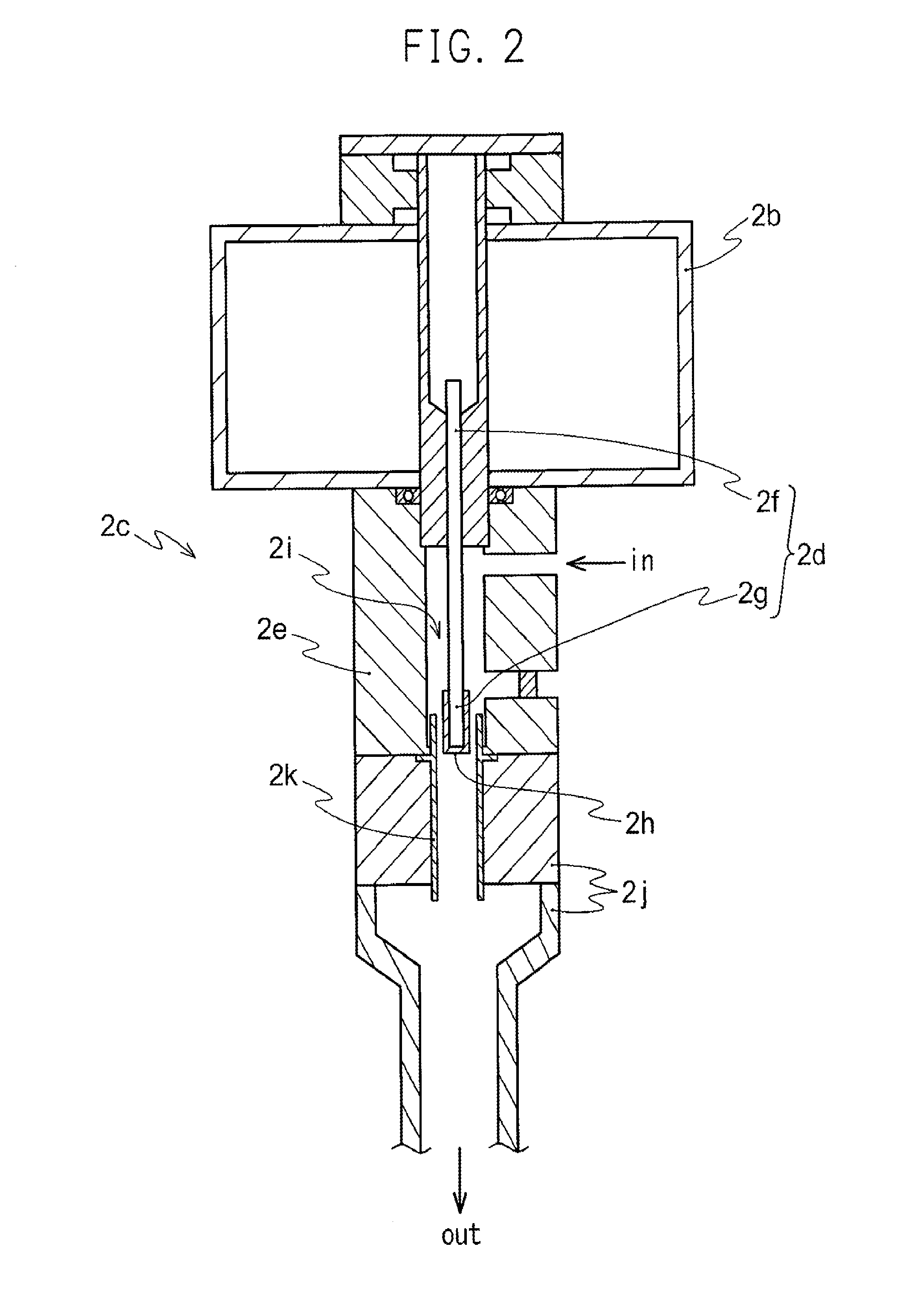
A high concentration NO2 gas generating system including a circulating path configured by connecting a chamber, a plasma generator, and a circulating means, wherein NO2 is generated by circulating a gas mixture including nitrogen and oxygen in the circulating path is provided. The high concentration NO2 gas generating system provides a high concentration NO2 generating system and the high concentration NO2 generating method using the generating system by which NO2 of high concentration (approximately 500 ppm or above) required for a high level of sterilization process in such as sterilization of medical instruments can be simply and selectively obtained. In addition, since indoor air is used as an ingredient, the management of ingredients is simple and highly safe, and the high concentration of NO2 can be simply and selectively prepared on demand.
Owner:NOXILIZER
Advanced dry head-end reprocessing of light water reactor spent nuclear fuel
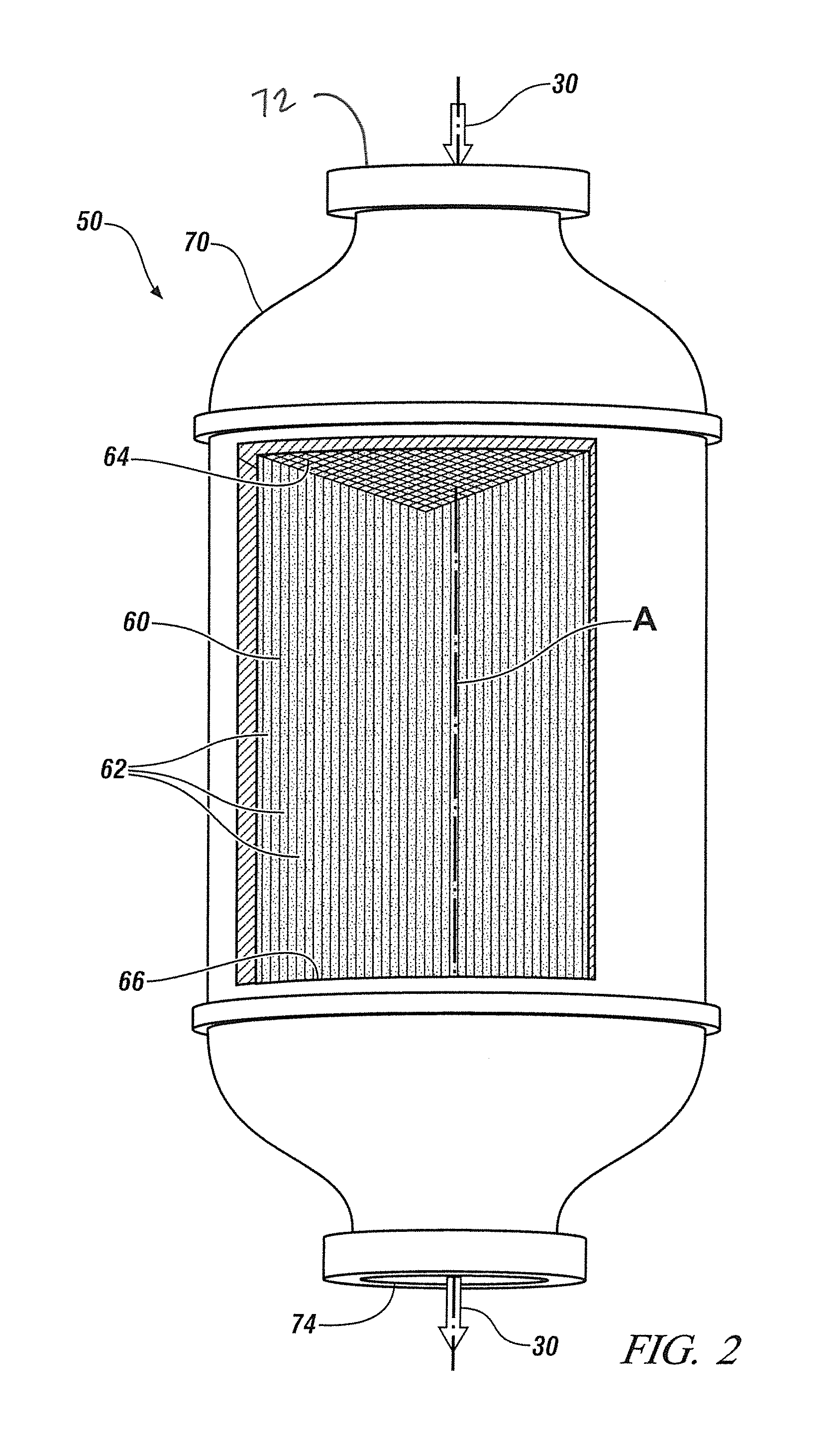
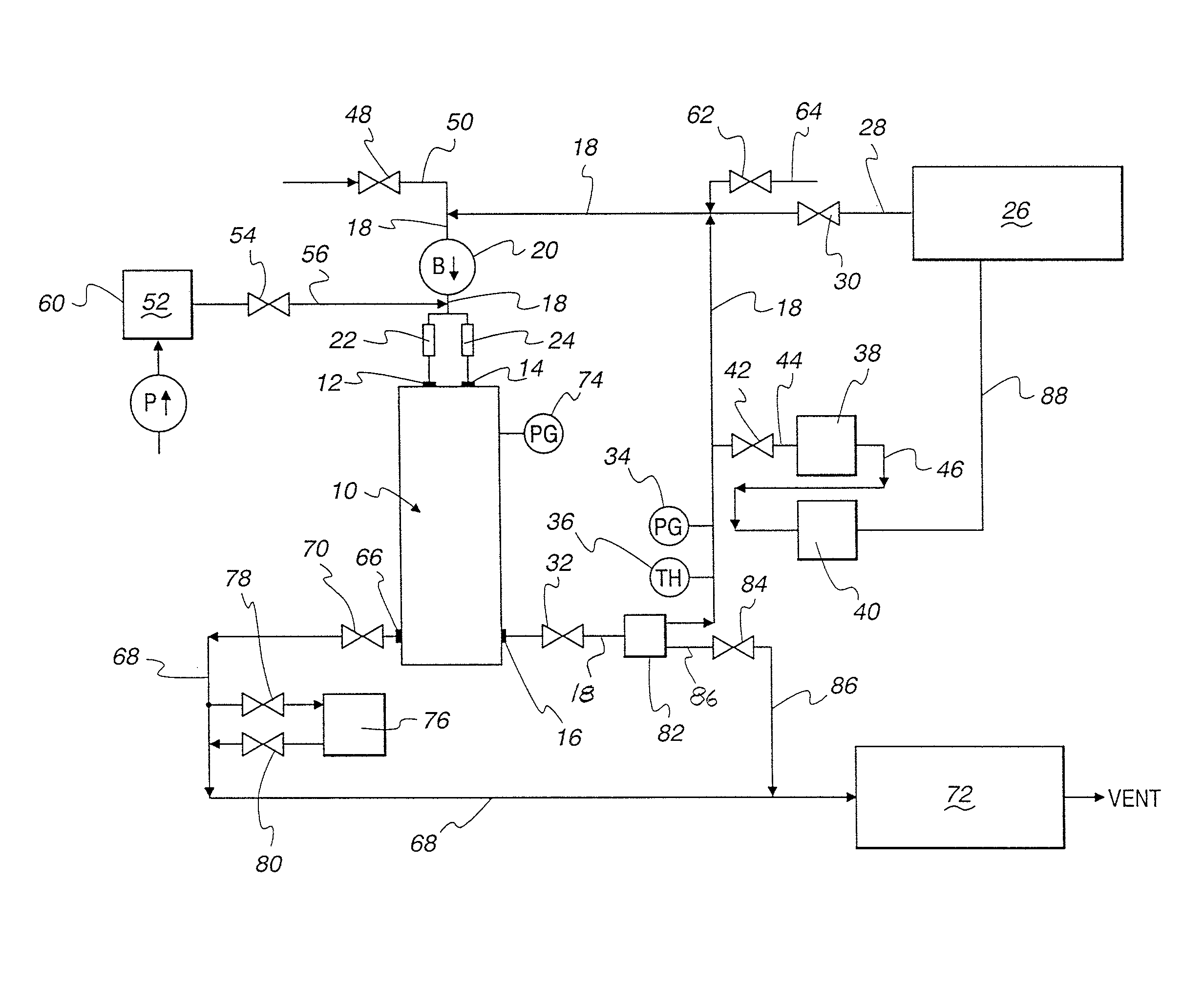
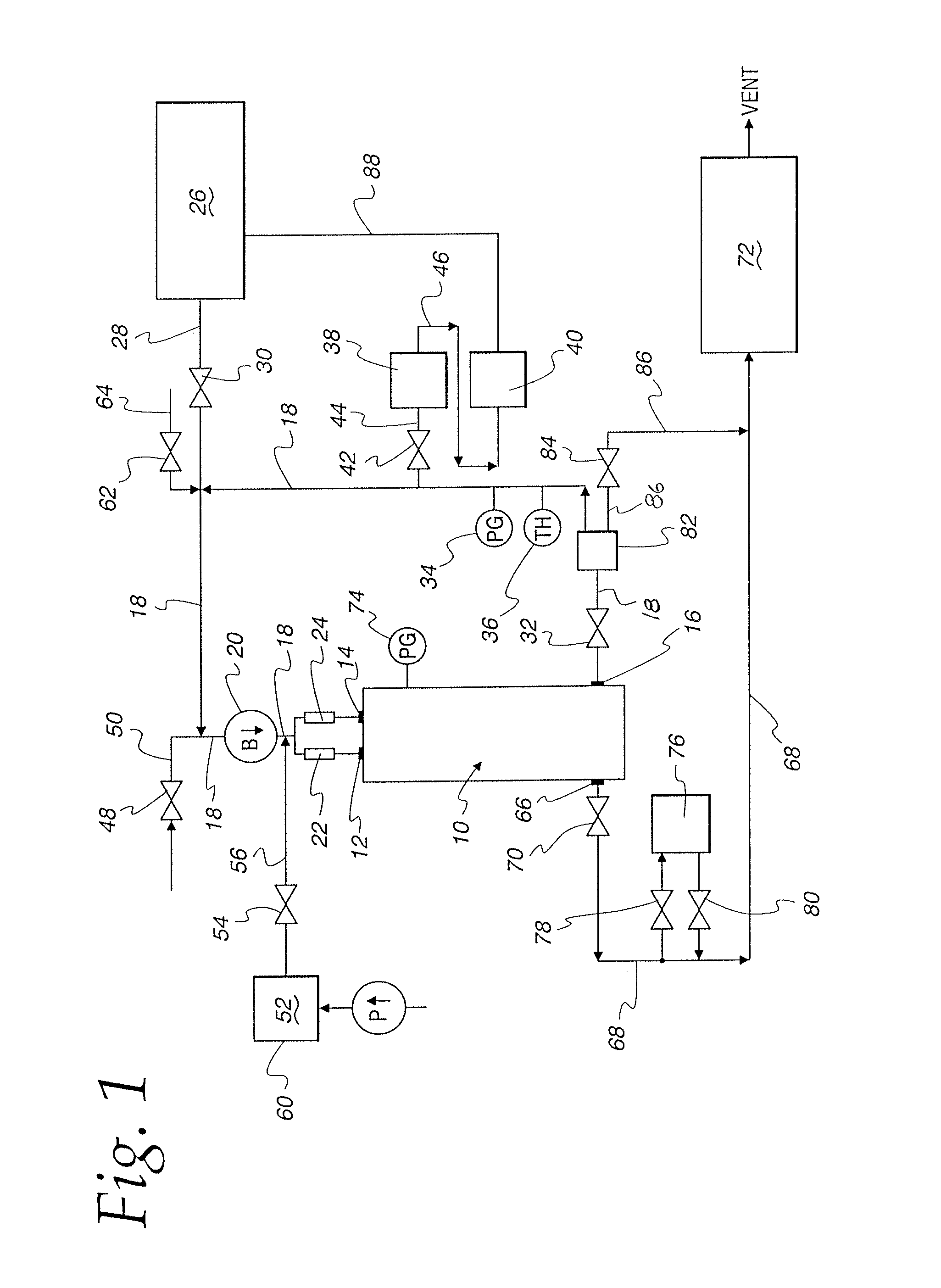
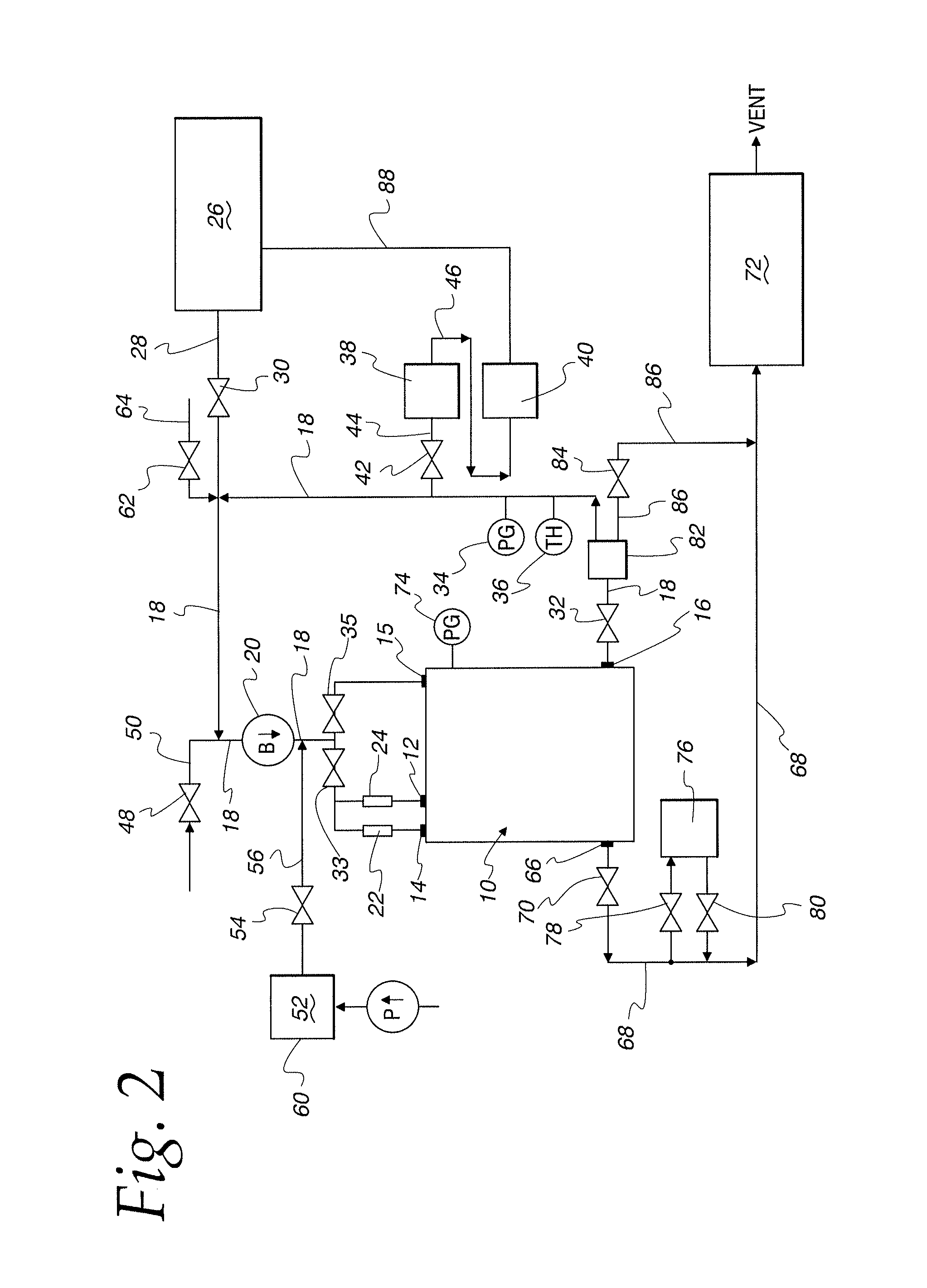
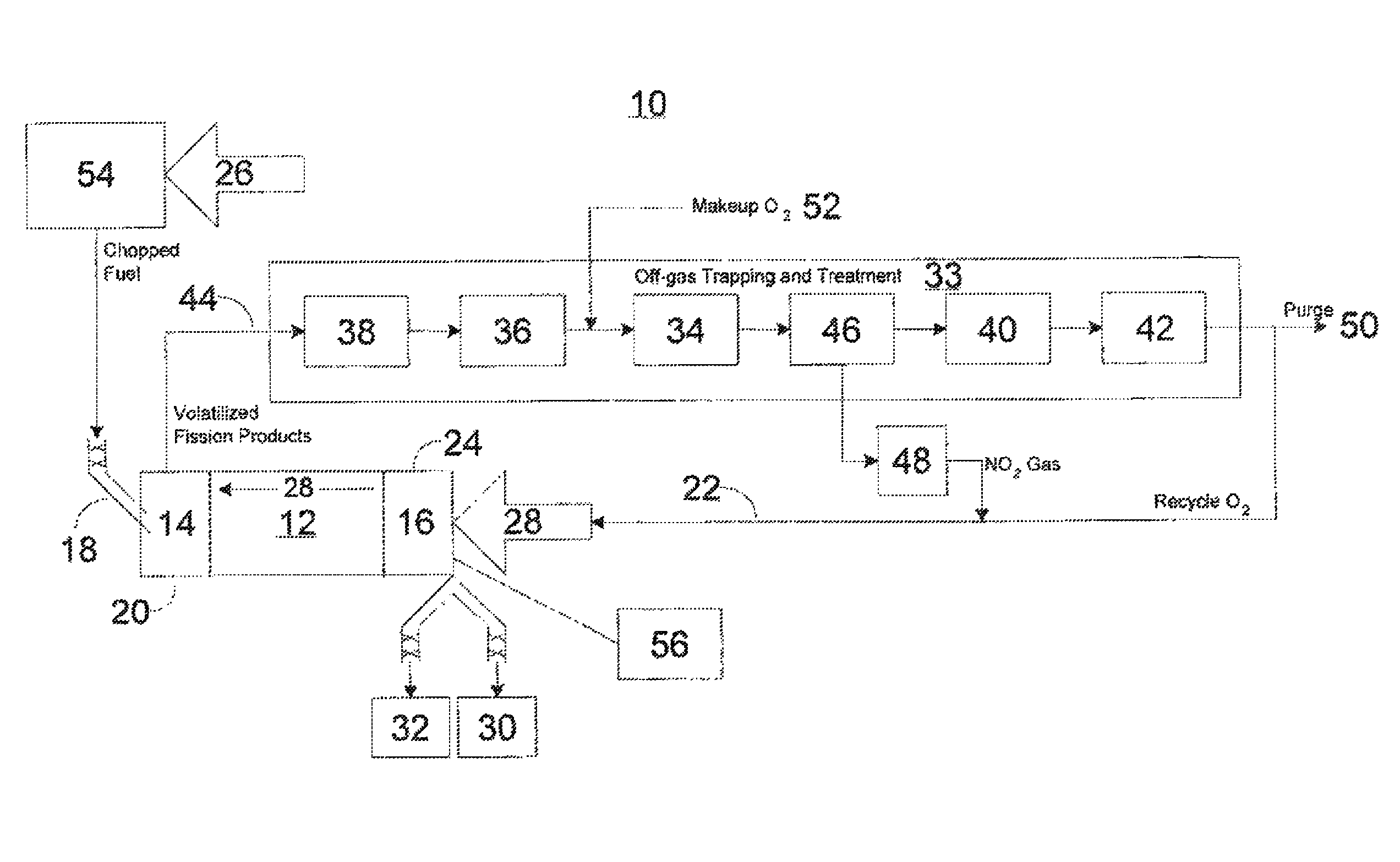
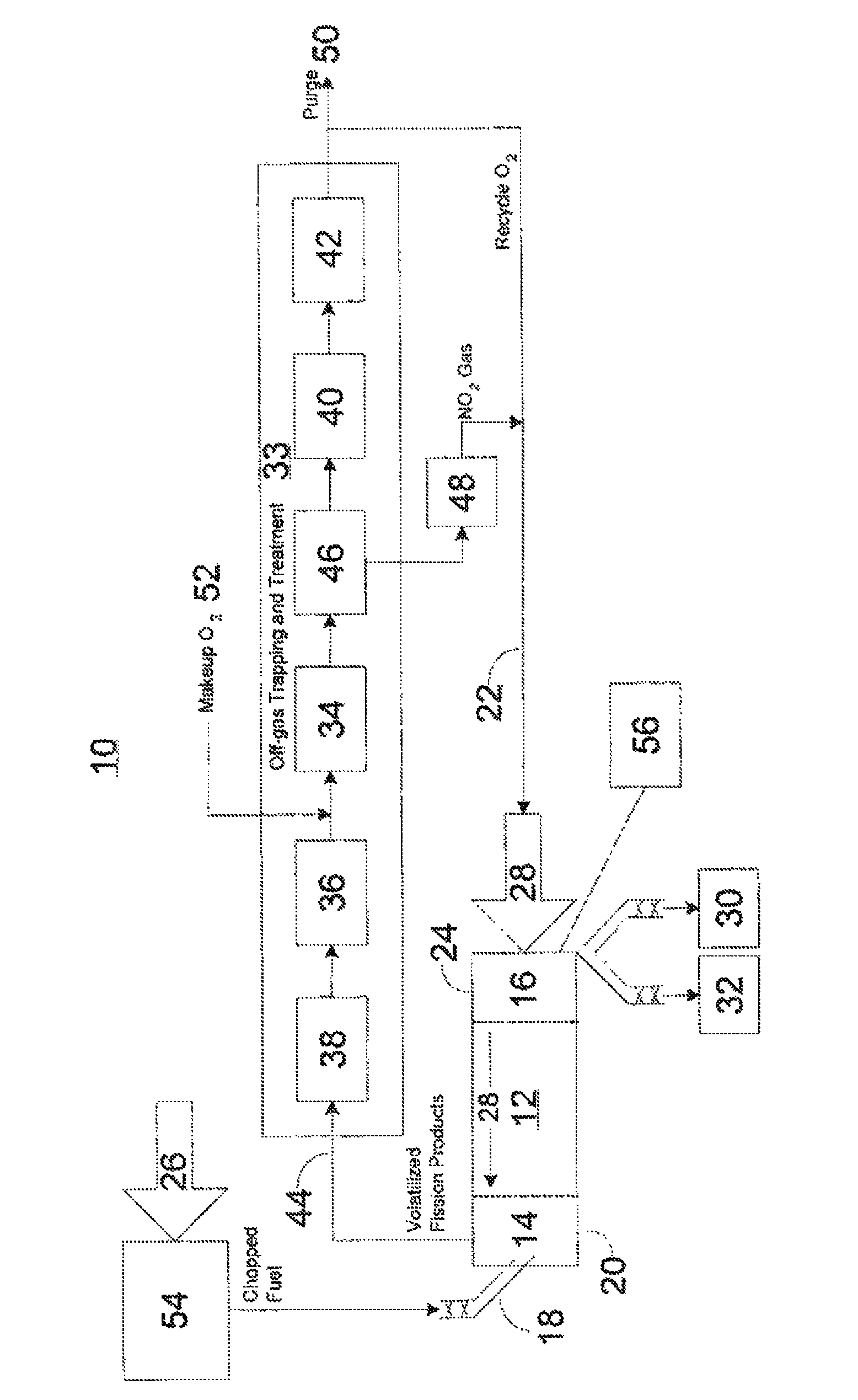
ActiveUS20110250108A1Solvent extractionTransuranic element compoundsNitrogen monooxideNitrogen dioxide
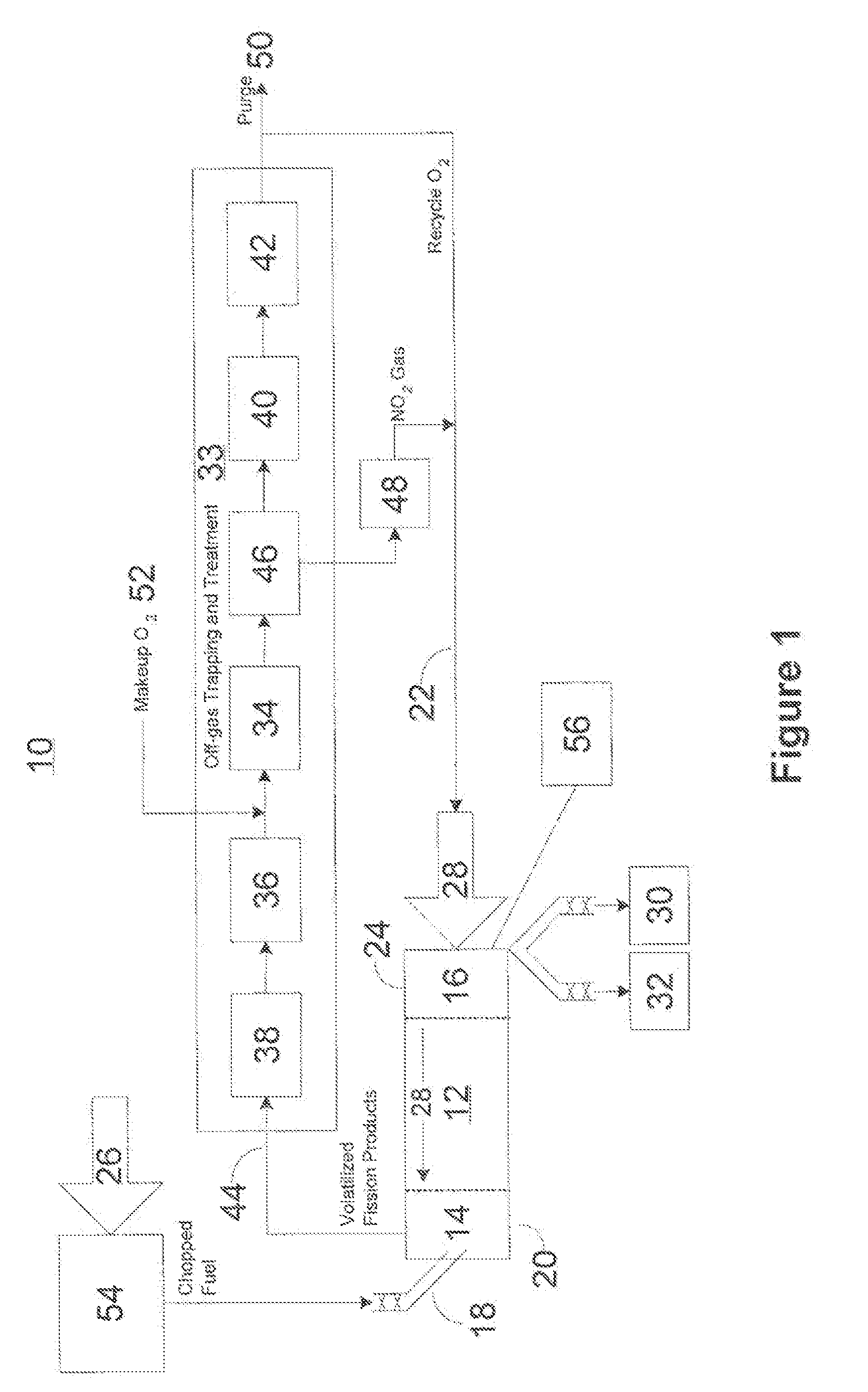
A method for reprocessing spent nuclear fuel from a light water reactor includes the step of reacting spent nuclear fuel in a voloxidation vessel with an oxidizing gas having nitrogen dioxide and oxygen for a period sufficient to generate a solid oxidation product of the spent nuclear fuel. The reacting step includes the step of reacting, in a first zone of the voloxidation vessel, spent nuclear fuel with the oxidizing gas at a temperature ranging from 200-450° C. to form an oxidized reaction product, and regenerating nitrogen dioxide, in a second zone of the voloxidation vessel, by reacting oxidizing gas comprising nitrogen monoxide and oxygen at a temperature ranging from 0-80° C. The first zone and the second zone can be separate. A voloxidation system is also disclosed.
Owner:UT BATTELLE LLC
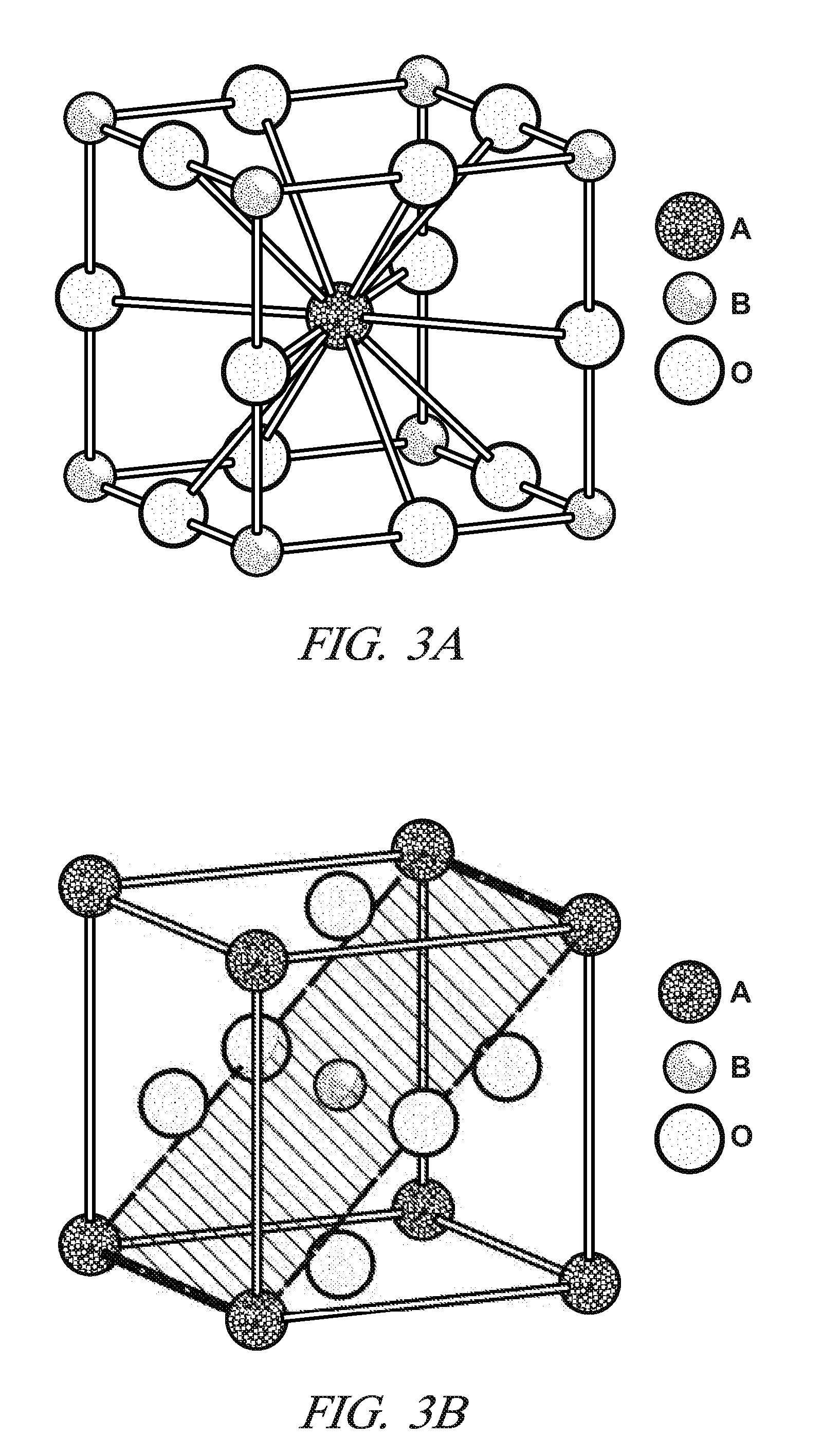
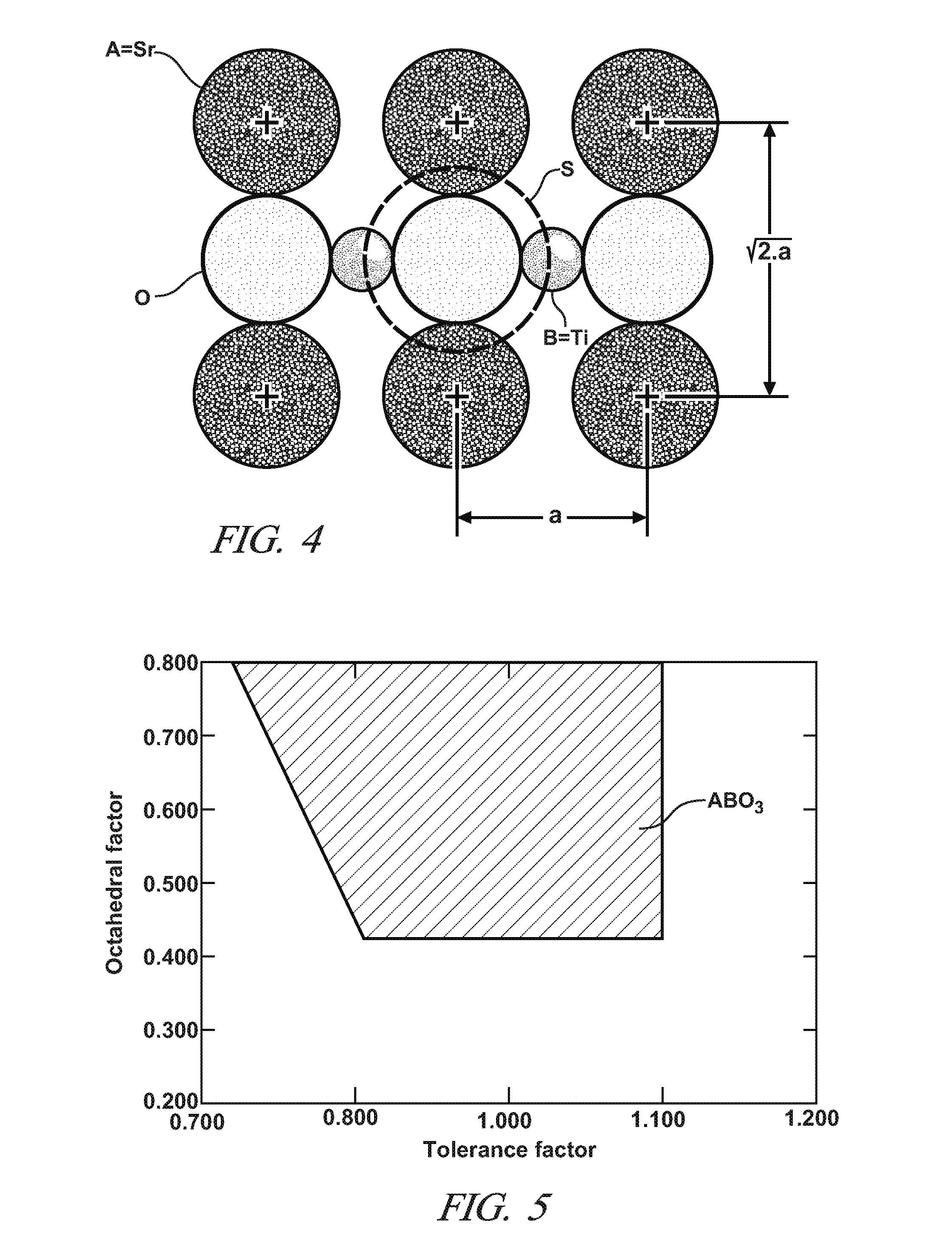
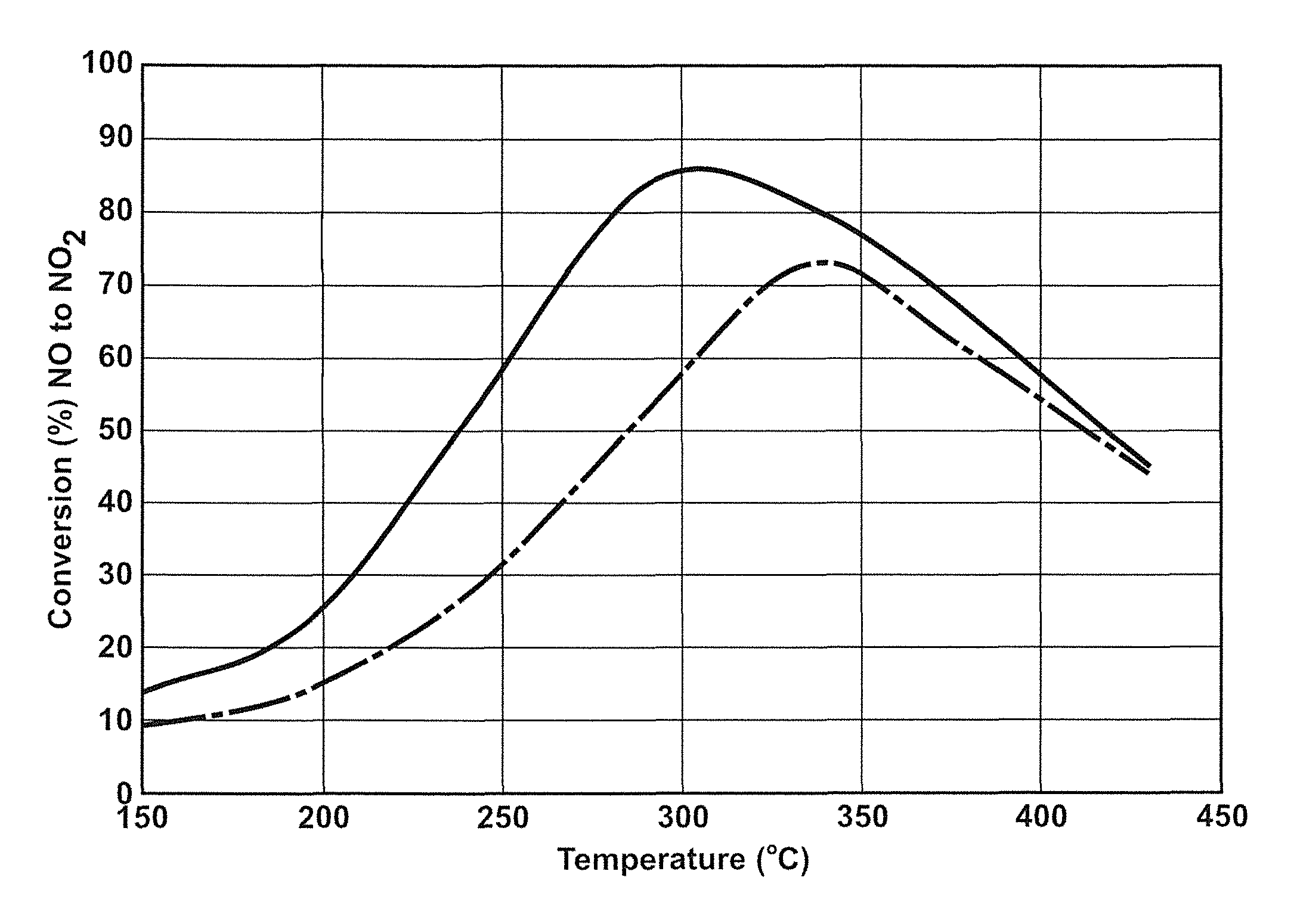
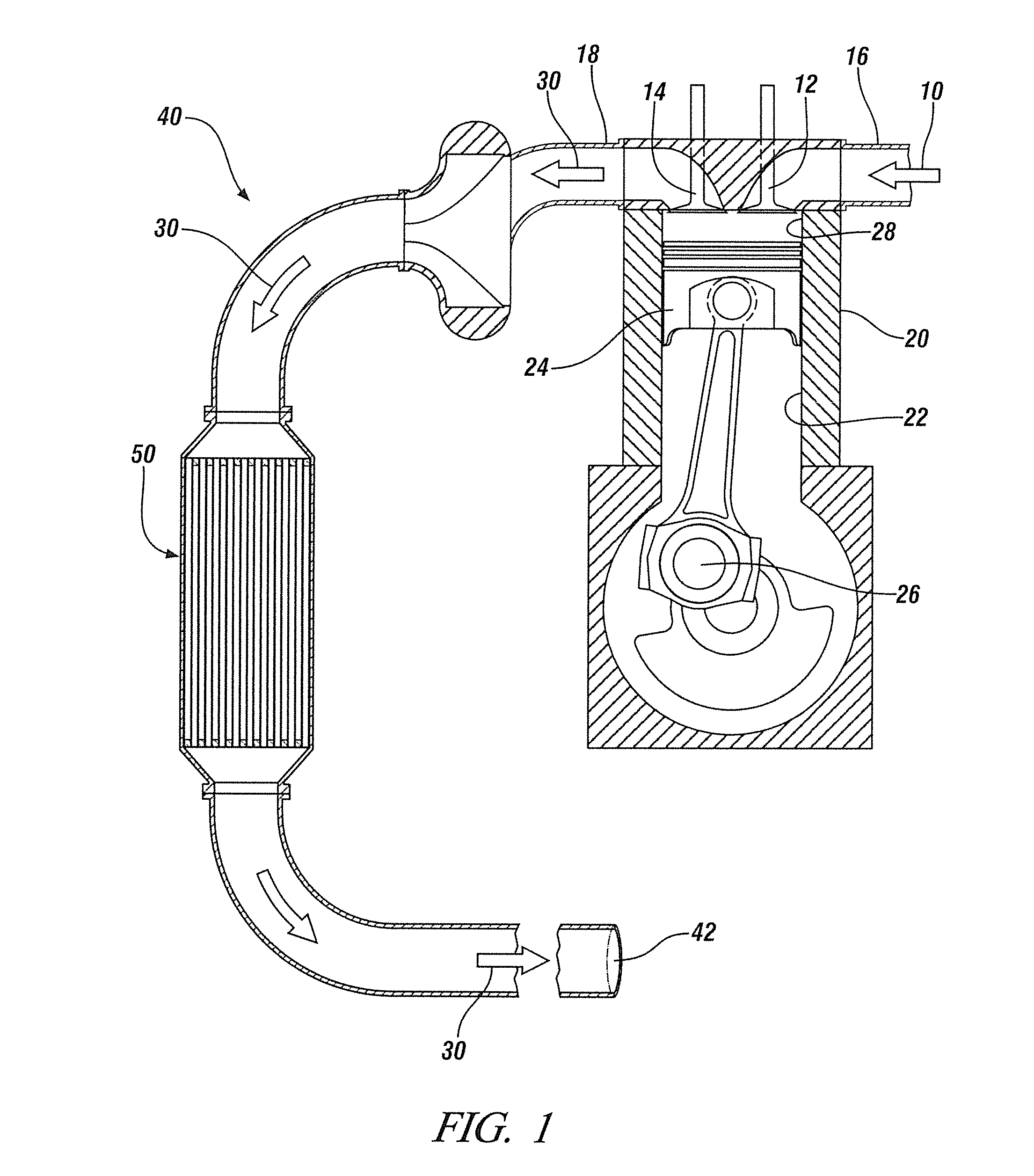
Sulfur-tolerant perovskite NOx oxidation catalysts
Owner:GM GLOBAL TECH OPERATIONS LLC
Mn, Ce and Zr mixed oxides oxidation catalyst
ActiveUS8404201B2Great proportionImprove performanceOrganic chemistryMachines/enginesMixed oxideSulfur
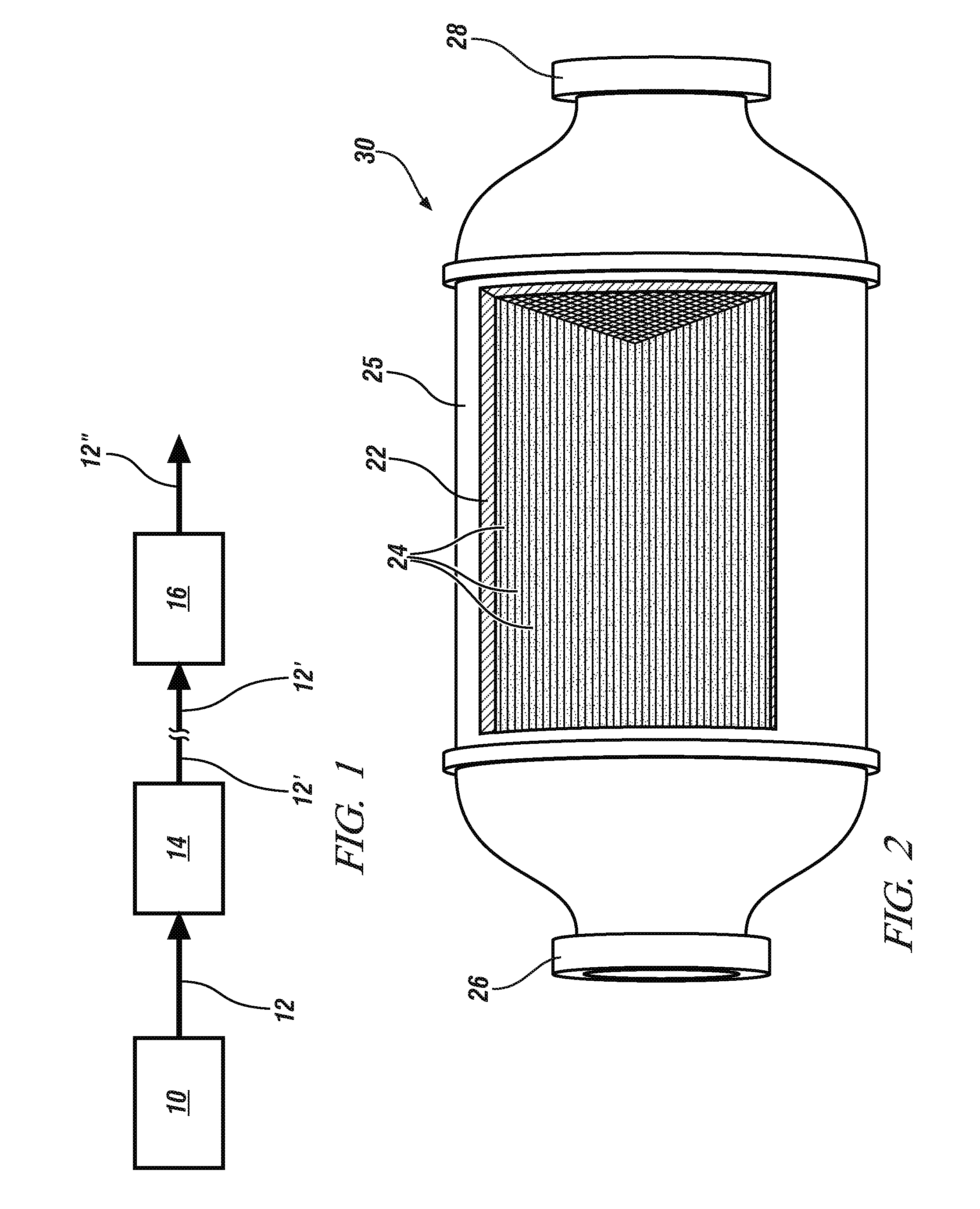
The oxidation of nitrogen oxide (NO) in an oxygen-containing exhaust gas flow from a diesel or other lean-burn engine may be catalyzed using particles of co-precipitated and calcined manganese (Mn), cerium (Ce) and zirconium (Zr) mixed oxides. In preferred embodiments, the molar ratios of Mn, Ce and Zr to the total amount of base metals in the ternary mixed oxide catalyst are in the range of 0.25-0.35, 0.40-0.50 and 0.20-0.25, respectively. Further, this ternary mixed oxide catalyst is less susceptible to sulfur poisoning than previously-disclosed binary mixed oxide catalysts. The ternary mixed oxide catalyst may also be regenerated—and the inhibiting effect of SO2 reversed—by briefly exposing the catalyst to a reducing exhaust gas environment.
Owner:GM GLOBAL TECH OPERATIONS LLC
Sterilization system for a blow/fill/seal machine
A fill assembly sterilization system for a blow / fill / seal machine utilizes a closed loop circulation of sterilant containing gas. A typical sterilant is nitrogen dioxide. The closed loop includes a shroud that defines a plenum and encloses the fill system. Optionally, at least one high efficiency particulate absorption (HEPA) filter is provided in the closed loop. Sterility assurance level of 10−6 can be achieved by subjecting the fill system to the sterilizing gas for at least 20 minutes at a temperature in the range of about 18° C. to about 30° C.
Owner:WEILER ENG
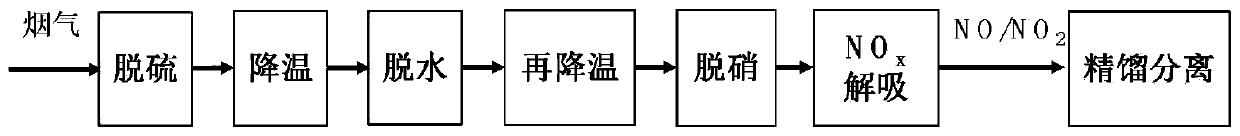
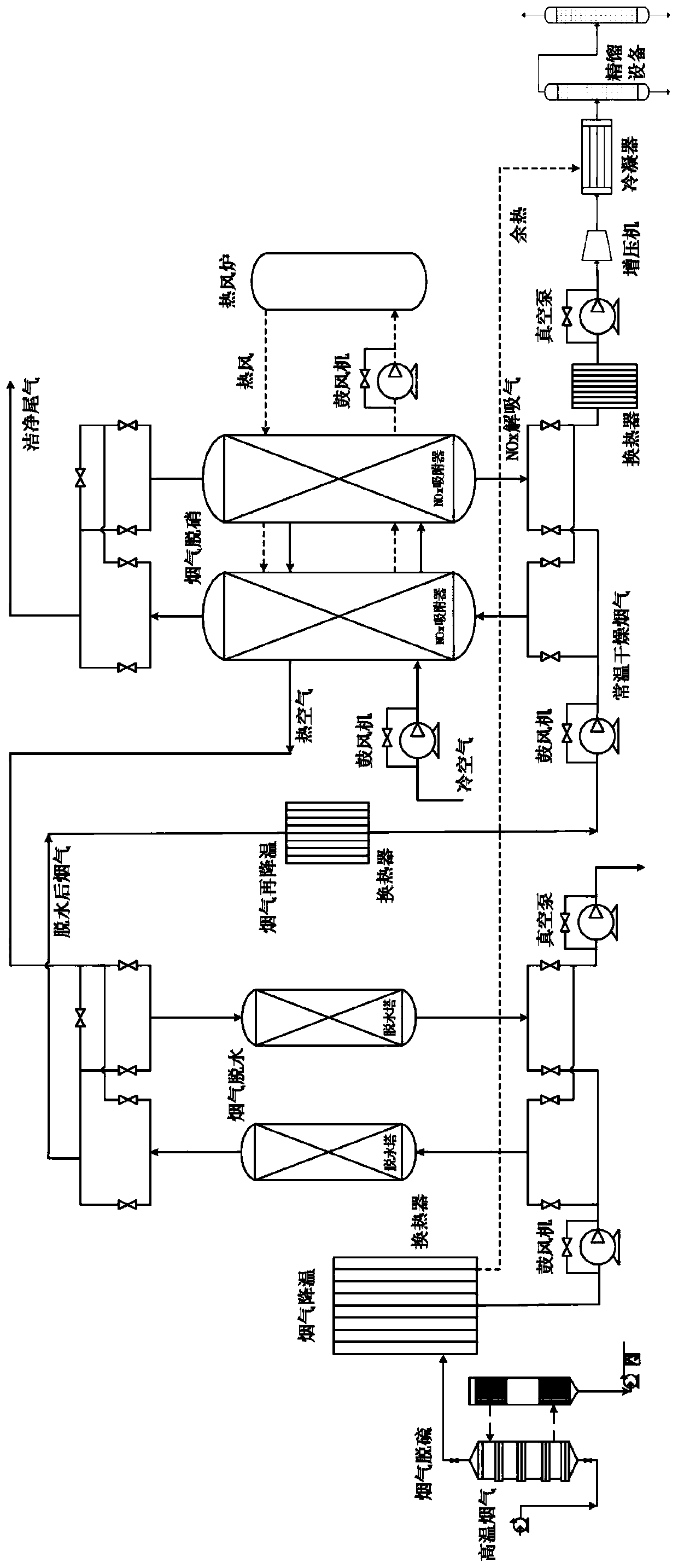
Method and system for adsorbing, purifying, enriching and recycling nitrogen oxides in flue gas
ActiveCN109794137AEfficient removalReduce consumptionDispersed particle separationEnergy inputHigh concentrationFlue gas
The invention belongs to the technical field of gas recycling in flue gas, and particularly relates to a method and system for adsorbing, purifying, enriching and recycling nitrogen oxides in flue gas. The method comprises the steps that firstly, the flue gas is desulfurated, cooled, dehydrated and re-cooled on the premise of ensuring that the flue gas does not form acid or dew in the transportation process, and the content of NOX components is not reduced; then sulfur-free and anhydrous flue gas at normal temperature is introduced into an adsorber to adsorb the NOX for desorption; after adsorption is saturated, an adsorbent is desorbed and regenerated in a heating and negative pressure mode to obtain mixed desorbed gas with high concentrations of NO2 and NO; finally, a rectification method is adopted for liquefying, separating and concentrating the desorbed gas to obtain high-purity NO2 liquid-state and NO gas-state products. The method overcomes the defects of existing methods for lossless enriching and recycling of NOX in the flue gas, and the NO and NO2 products with very high added values are formed while the NOX in the flue gas is efficiently desorbed; meanwhile, the waste heat resource is sufficiently utilized, and compared with the prior art, the method and system have more obvious economic benefits.
Owner:UNIV OF SCI & TECH BEIJING
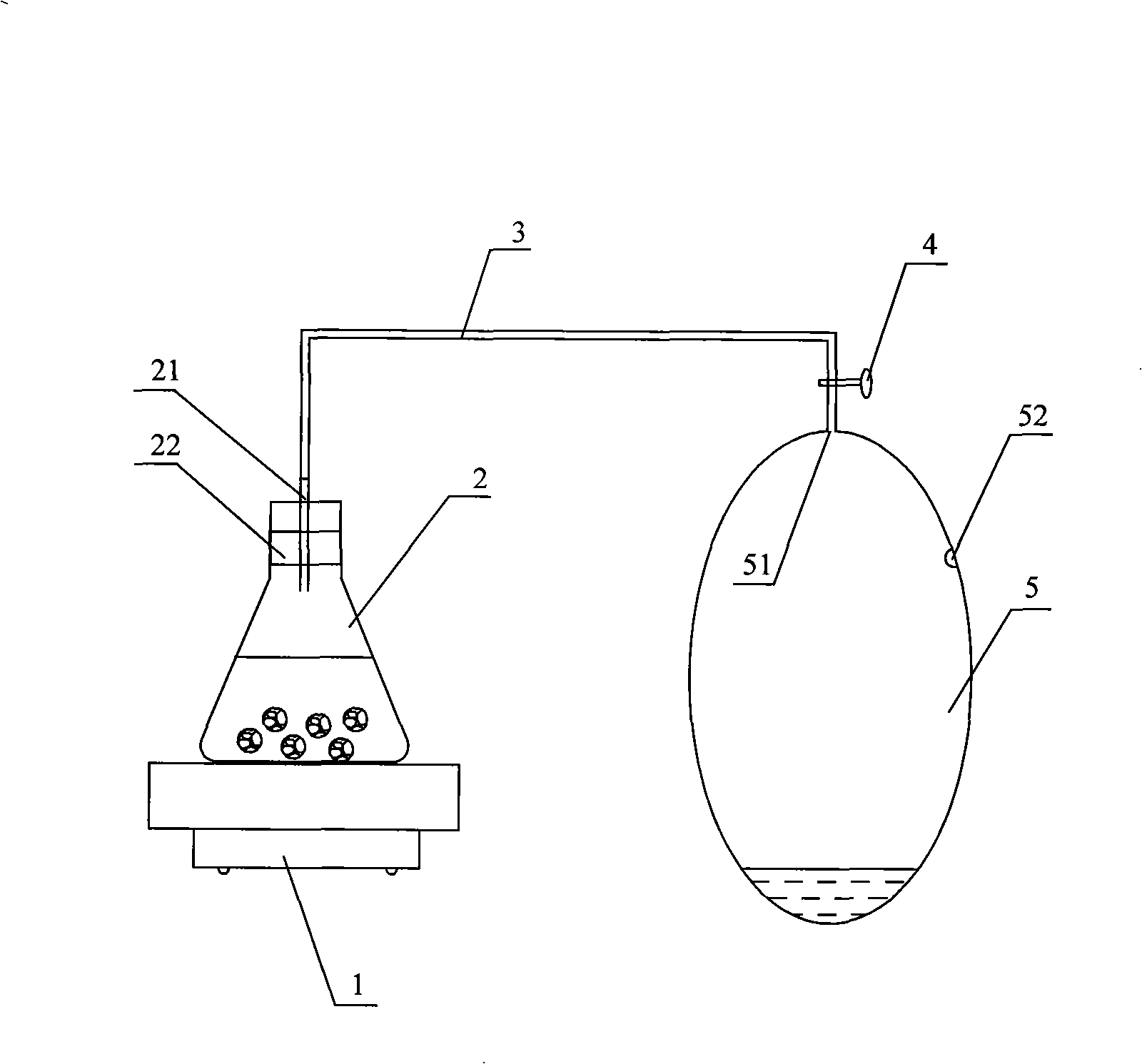
Method for improving yield and quality of greenhouse vegetable and using device
InactiveCN101258801APromote growthIncrease productionFertilising methodsNitric oxideHarvest timeGreenhouse
The invention discloses a method for increasing the yield and quality of greenhouse vegetables. Nitric Oxide gas is fertilized in a greenhouse once a day from three to four weeks before the harvest time of the greenhouse vegetables till the day before the harvest time according to routine estimation, the amount of fertilizer applied each time is 0.5 to 2 mL / m<3>. The invention also provides an acquisition device of NOx gas for realizing the method, which comprises a gas collection bag which is provided with an inlet and a gas extracting port. A reaction vessel is arranged on a heating device, one end of a connecting tube is connected with the outlet of the reaction vessel in a sealing way and the other end is connected with the inlet of the gas collection bag in a sealing way, and the connecting tube is provided with a regulator. The method of the invention can not only reduce the nitrate content in the vegetables but also increase the yield of the vegetables.
Owner:ZHEJIANG UNIV
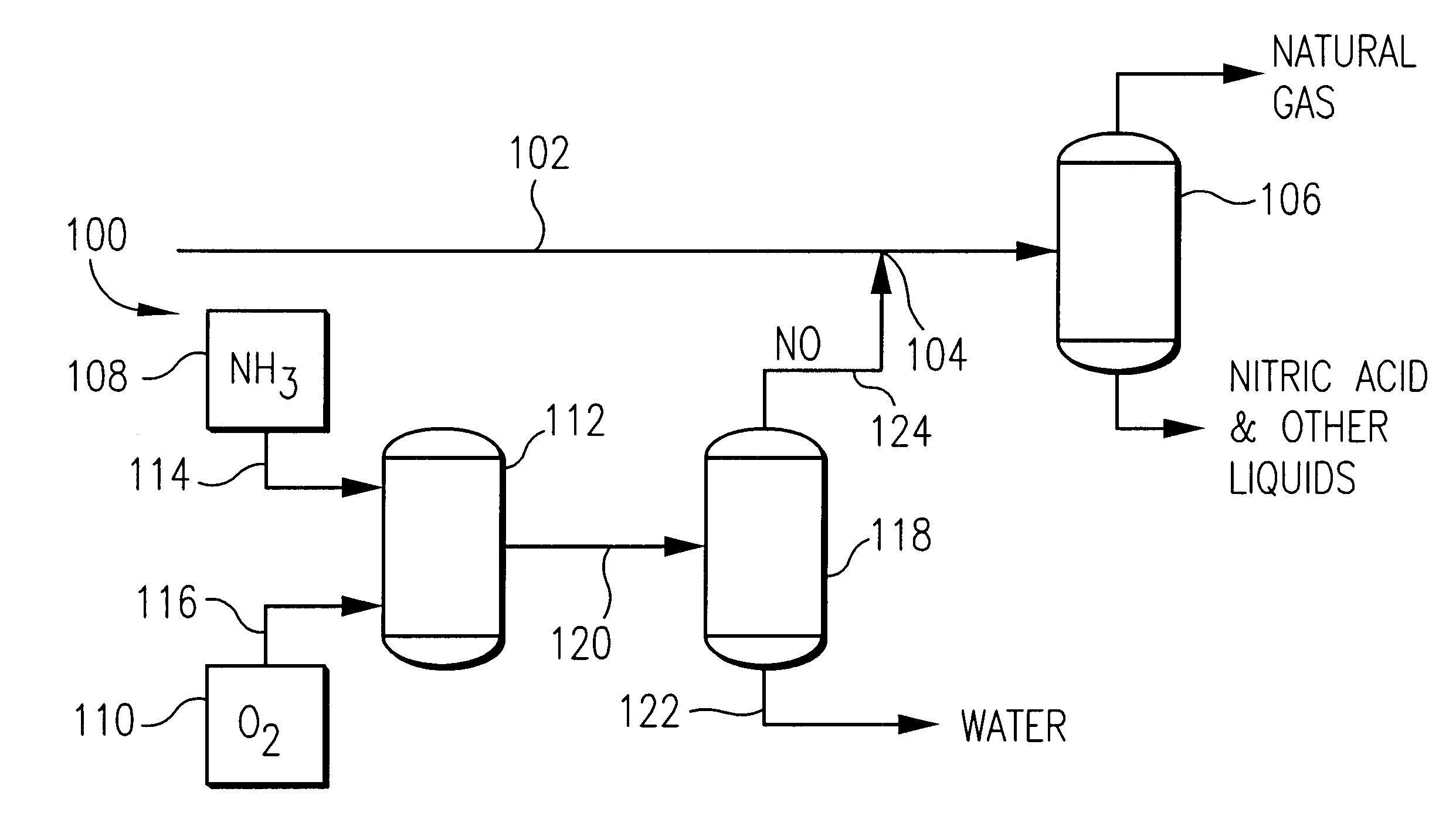
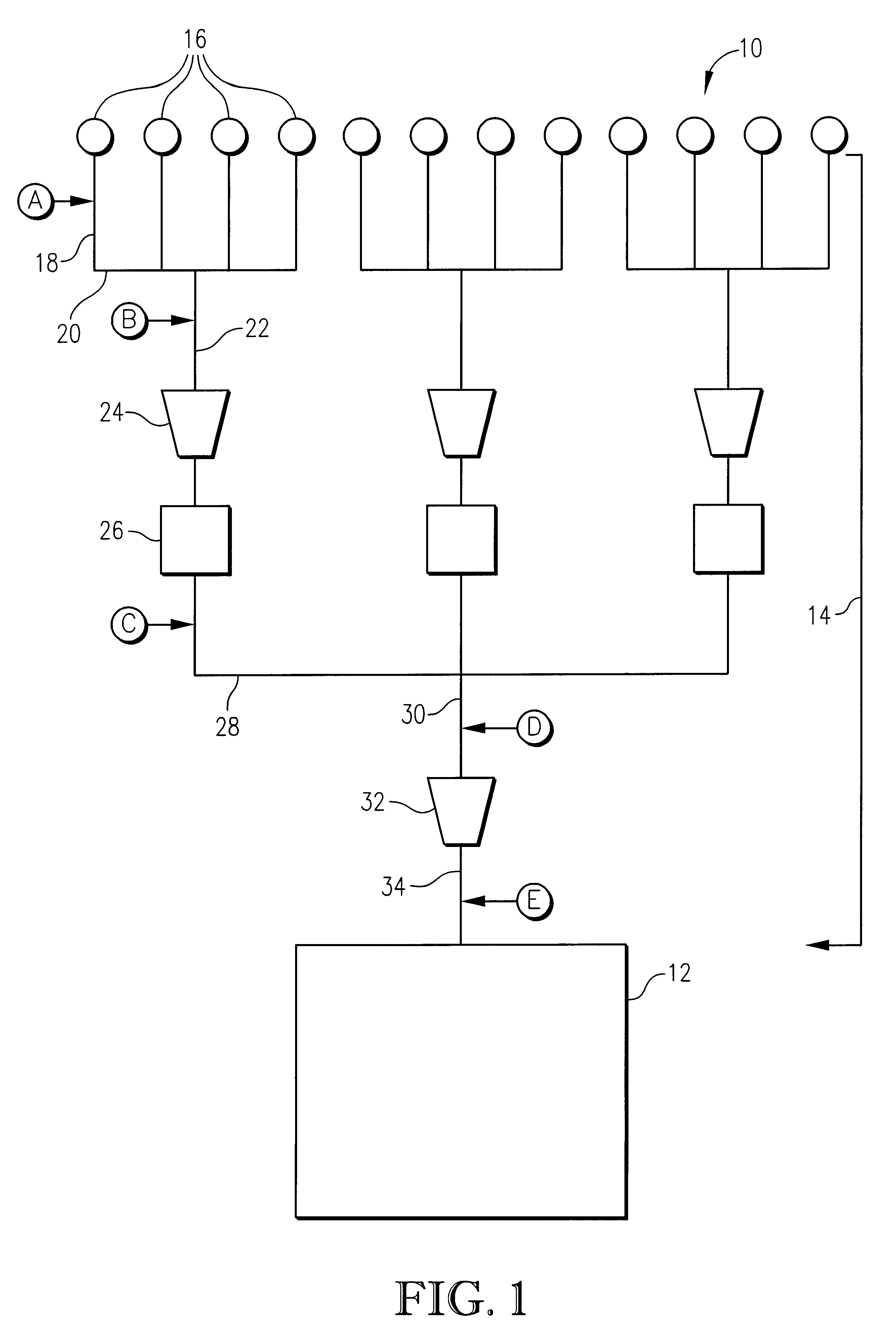
Method and apparatus for removing oxygen from natural gas
InactiveUS6495112B2Easy to separatePressurized chemical processCyanogen compoundsNitrogen dioxideNitric oxide
Oxygen is removed from natural gas by contacting oxygen-containing natural gas with nitric oxide under conditions sufficient to produce nitrogen dioxide.
Owner:PHILLIPS PETROLEUM CO
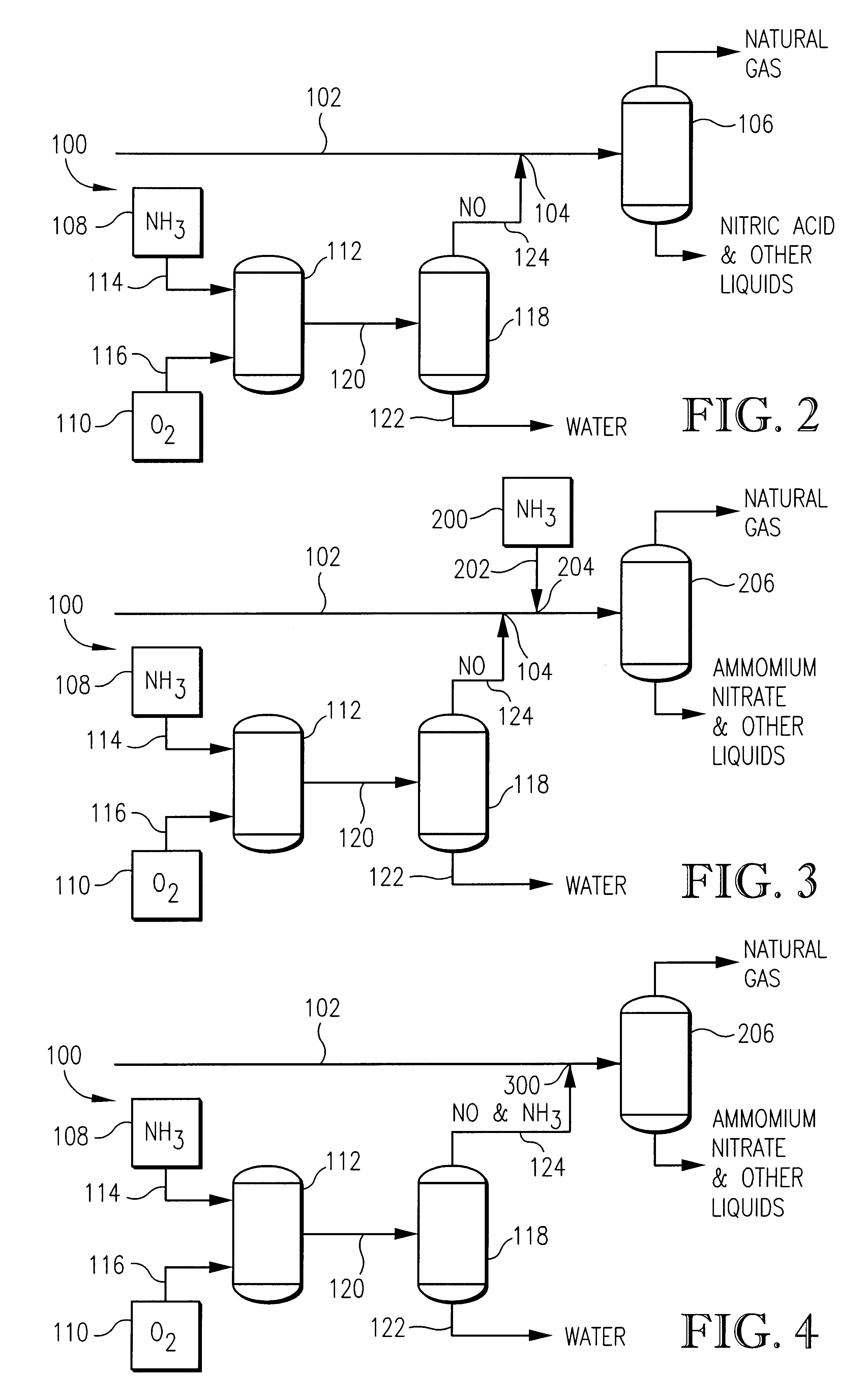
Method for producing N2O4 (nitrogen tetroxide) through combining normal pressure diluted nitric acid, pressurized diluted nitric acid and concentrated nitric acid devices
InactiveCN102774819ALess investmentShort construction periodEnergy inputNitrogen dioxideNitrogen dioxideDinitrogen tetroxide
The invention relates to a method for producing N2O4 (nitrogen tetroxide) through combining normal pressure diluted nitric acid, pressurized diluted nitric acid and concentrated nitric acid devices. The method comprises the following steps that the normal pressure diluted nitric acid, pressurized diluted nitric acid and concentrated nitric acid devices are combined for preparing NO2 (nitrogen dioxide) gas; liquid-stage N2O4 is prepared; and the discharged tail gas is treated. The public engineering conditions of the existing device and idle equipment can be sufficiently utilized, the investment is reduced, and the building construction period is shortened. The combination of the normal pressure diluted nitric acid, pressurized diluted nitric acid and concentrated nitric acid devices is realized, and the industrial level N2O4 is produced for reaching the goals of revitalizing the existing assets, increasing the product varieties and improving the risk-resistance capability of enterprises.
Owner:魏作胜 +2
High concentration NO2 generating system and method for generating high concentration NO2 using the generating system
ActiveUS8425852B2Efficient processMaterial nanotechnologyInorganic active ingredientsHigh concentrationPlasma generator
A high concentration NO2 gas generating system including a circulating path configured by connecting a chamber, a plasma generator, and a circulating means, wherein NO2 is generated by circulating a gas mixture including nitrogen and oxygen in the circulating path is provided. The high concentration NO2 gas generating system provides a high concentration NO2 generating system and the high concentration NO2 generating method using the generating system by which NO2 of high concentration (approximately 500 ppm or above) required for a high level of sterilization process in such as sterilization of medical instruments can be simply and selectively obtained. In addition, since indoor air is used as an ingredient, the management of ingredients is simple and highly safe, and the high concentration of NO2 can be simply and selectively prepared on demand.
Owner:NOXILIZER
Non-stoichiometric perovskite oxide oxidation catalyst for oxidizing NO to NO2
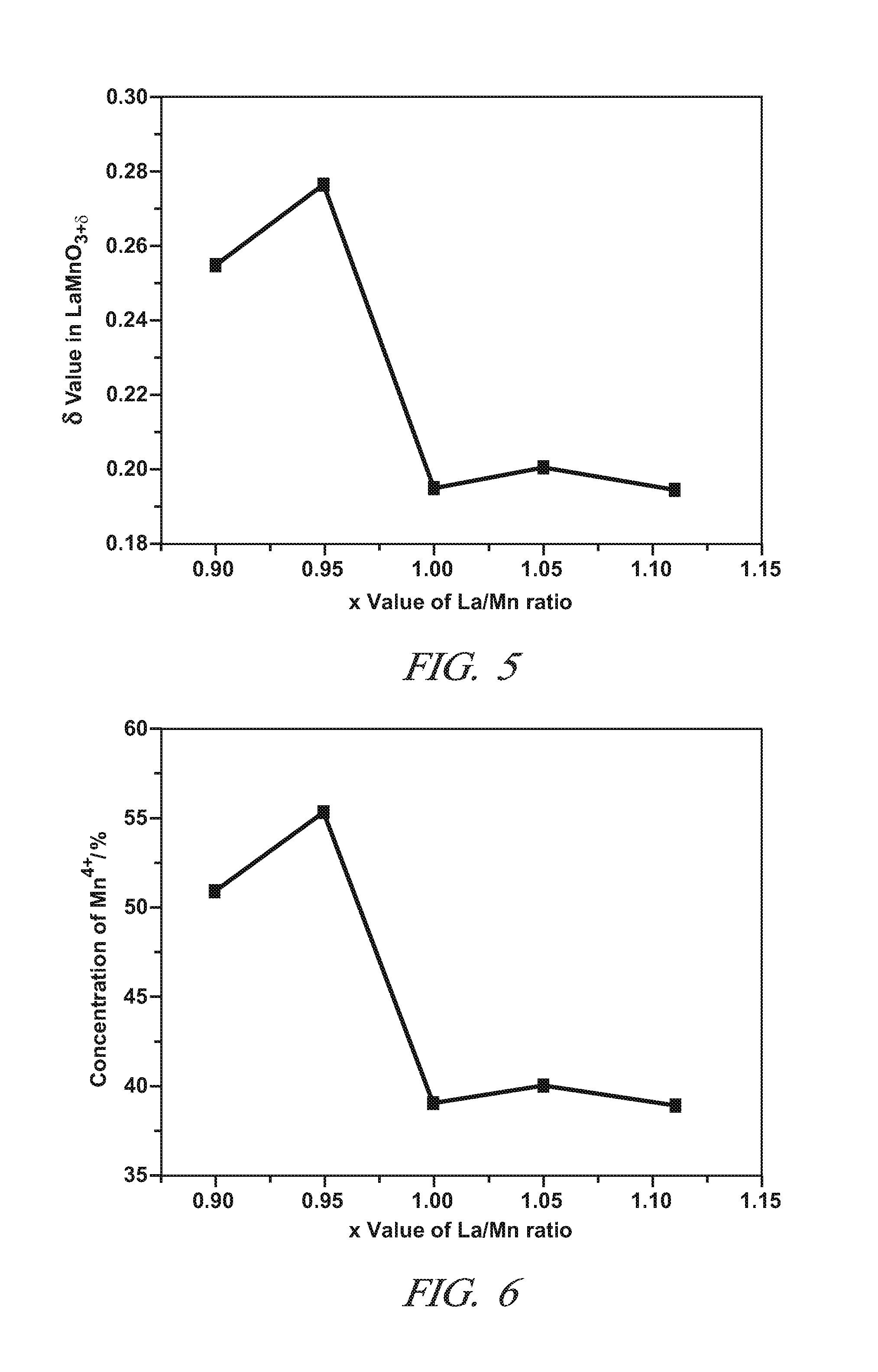
A non-stoichiometric perovskite oxide having the general chemical formula LaXMnOY, in which the molar ratio of lanthanum to manganese (“X”) ranges from 0.85 to 0.95, can be used in particle form as an oxidation catalyst to oxidize NO to NO2 in an exhaust aftertreatment system for a hydrocarbon-fueled engine. The oxygen content (“Y”) fluctuates with variations in the molar ratio of lanthanum to manganese but generally falls somewhere in the range of 3.0 to 3.30. The crystal lattice adjustments spurred by the non-stoichiometric molar ratio of lanthanum to manganese are believed responsible for an enhanced NO oxidative activity relative to similar perovskite oxides with a higher molar ratio of lanthanum and manganese.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
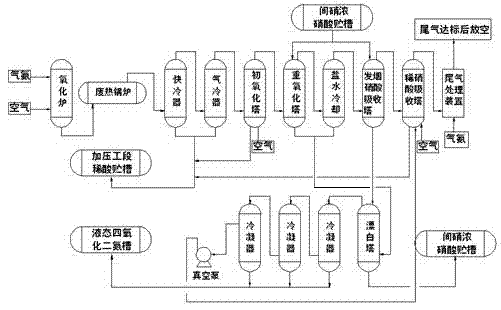
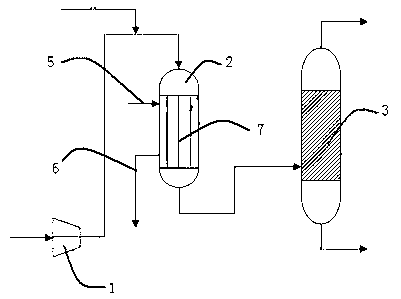
Preparation method and device of nitrogen oxide
The invention discloses a preparation method and device of nitrogen oxide. The preparation method includes following steps: preheating and mixing a nitric acid solution and a nitrite solution for reaction to generate nitrogen oxide gas, wherein nitrogen oxide is NOx, and x is greater than 1 and smaller than 2. The preparation method is easy-to-get in raw material, simple in process, easy in reaction, conducive to continuity of production operation and capable of ensuring continuous and stable supply and use of nitrogen oxide serving as an oxidant in a nuclear fuel aftertreatment plant.
Owner:CHINA NUCLEAR POWER ENG CO LTD
NO2 pre-reaction device system used in industrial production of ethylene glycol
ActiveCN103182283AImprove responseQuick responseChemical/physical/physico-chemical processesNitrogen dioxideExothermic reactionEngineering
The invention discloses an NO2 pre-reaction device system used in industrial production of ethylene glycol, which comprises an NO2 prereactor connected with an NO compressor through a pipeline, wherein an outlet of the NO2 prereactor is connected to an esterification column, the NO2 prereactor is a vertical reactor, and a cooling device is arranged inside or outside the NO2 prereactor. The original NO2 prereactor (empty barrel type reactor) is modified into a tubular NO2 prereactor, so that the NO2 pre-reaction device system has the advantages that the oxidation reaction is exothermic reaction, the adopted tubes are favorable for heat exchange, the reaction speed is accelerated by circulating water condensation, the liquid accumulation also can be avoided, the potential safety hazard is eliminated, and the reaction can be fully carried out.
Owner:PUJING CHEM IND SHA
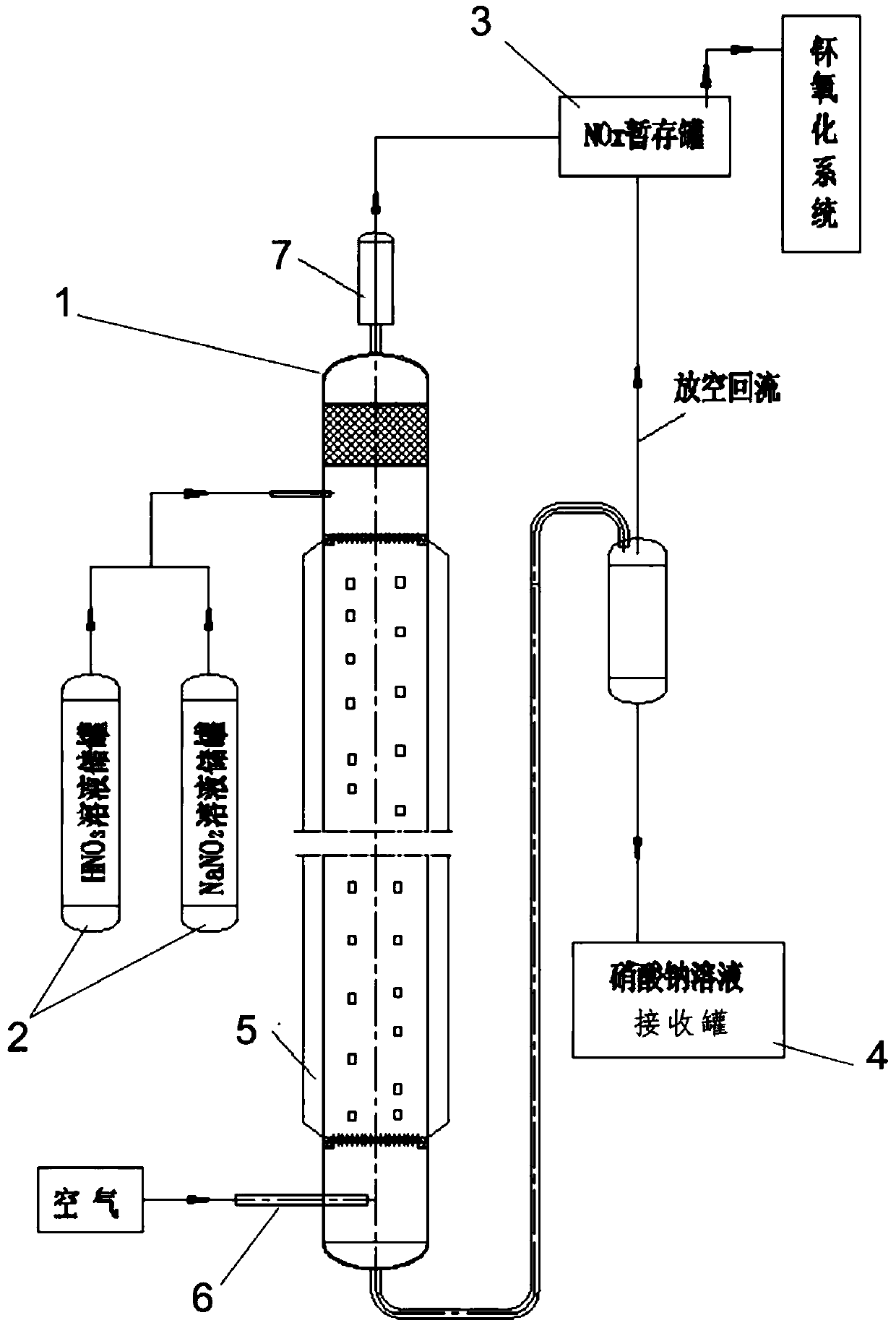
Preparation method and apparatus of nitrogen oxides
PendingCN109665503AAvoid corrosionImprove oxidation capacityNitrogen dioxideNitrogen dioxideLiquid storage tank
The invention relates to a preparation method and apparatus of nitrogen oxides. The method includes using concentrated nitric acid and sodium nitrite as reaction liquids, and using air flow to performstripping the reaction liquids to obtain nitrogen oxides. The apparatus herein comprises a filler column, a reaction liquid storage tank, a nitrogen oxide pending tank and a receiving tank; all the reaction liquid storage tank, the nitrogen oxide pending tank and the receiving tank are connected with the filler column; the reaction liquid storage tank comprises a nitric solution storage tank anda sodium nitrite solution storage tank; the lower portion of the filler column is also provided with an intake line. The preparation method has the advantages that concentrated nitric acid and sodiumnitrite are matched to replace concentrated nitric acid, so that the corrosive effect of the concentrated nitric acid upon equipment and lines is avoided; air is utilized to perform contact strippingon the reaction flows, the blown-in air helps oxidize nitrogen monoxide in a system into nitrogen dioxide, so that oxidation effect of nitrogen oxides is improved; an insulation system helps hold theinterior temperature of the filler column to a high level, so that the nitrous acid can be carried out in nitrogen oxide form.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
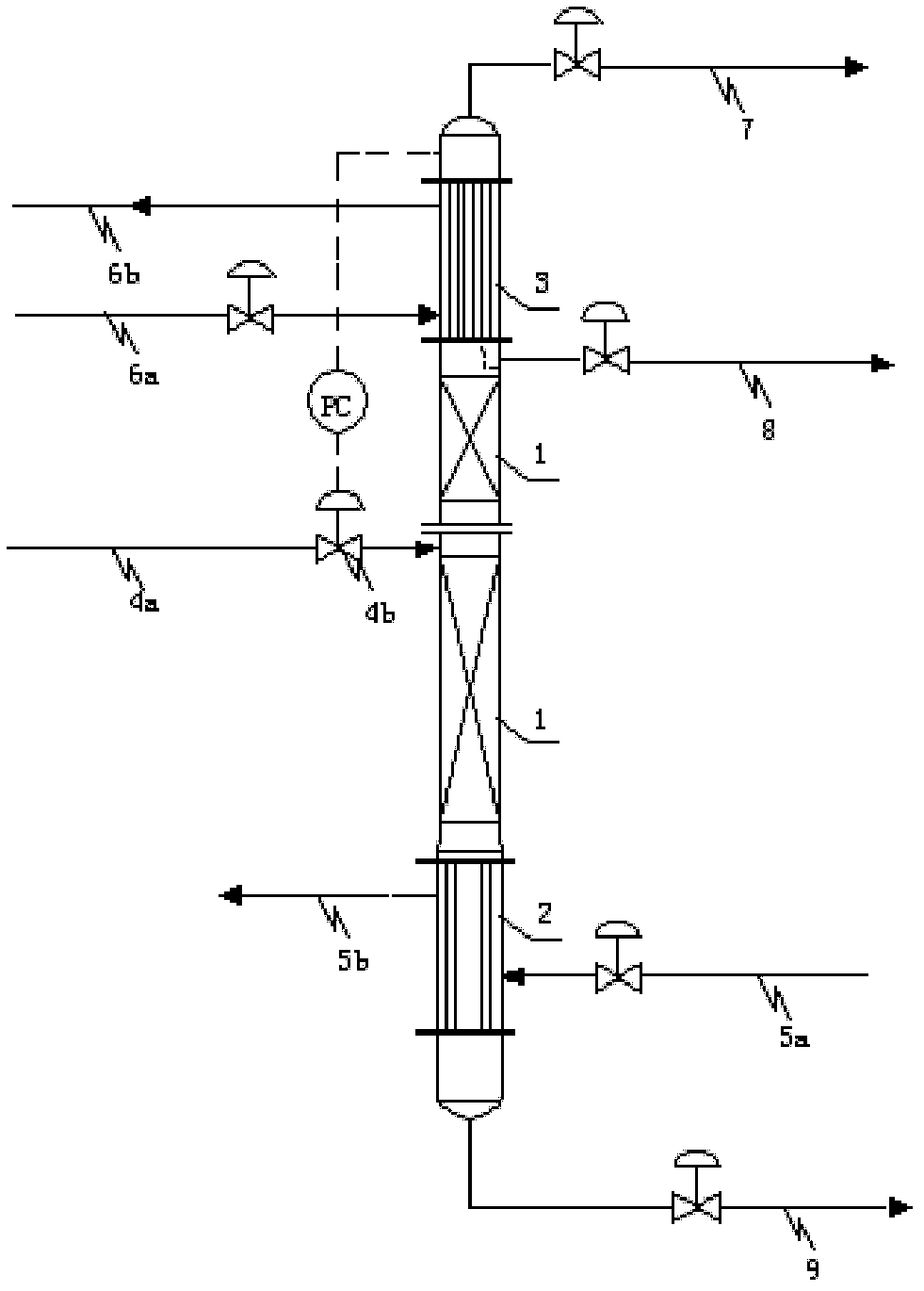
Rectification dehydration tower and dehydration method for nitrogen dioxide
ActiveCN103318859AImprove dehydration efficiencyBreakthrough dehydration efficiencySolidificationLiquefactionRefluxNitrogen dioxide
The invention discloses a rectification dehydration tower and a dehydration method for nitrogen dioxide. According to the rectification dehydration tower, a condensation section, a rectification section and a re-boiling section are arranged inside the rectification dehydration tower from top to bottom, wherein the re-boiling section is provided with a heat medium introduction pipeline and a heat medium eduction pipeline, the condensation section is provided with a cold medium introduction pipeline and a cold medium eduction pipeline, a nitrogen dioxide feeding pipeline is arranged on the middle portion of the rectification dehydration tower, the upper portion of the rectification dehydration tower is provided with a product outlet pipeline, the bottom of the rectification dehydration tower is provided with a raffinate outlet pipeline, and the nitrogen dioxide feeding pipeline is provided with a regulation valve. The method comprises that a nitrogen dioxide raw material enters and is subjected to rectification, wherein gas pressure on the top pf the rectification dehydration tower is 0.06-0.46 MaPG, the theoretical column plate number of the rectification dehydration tower is 3-26, and a reflux ratio is 0.46-16. With the present invention, water in the raw material dinitrogen tetroxide or nitrogen dioxide can be effectively removed, and the high purity nitrogen dioxide (or dinitrogen tetroxide) product containing a trace amount of water can be prepared.
Owner:CHINA PETROCHEMICAL CORP +1
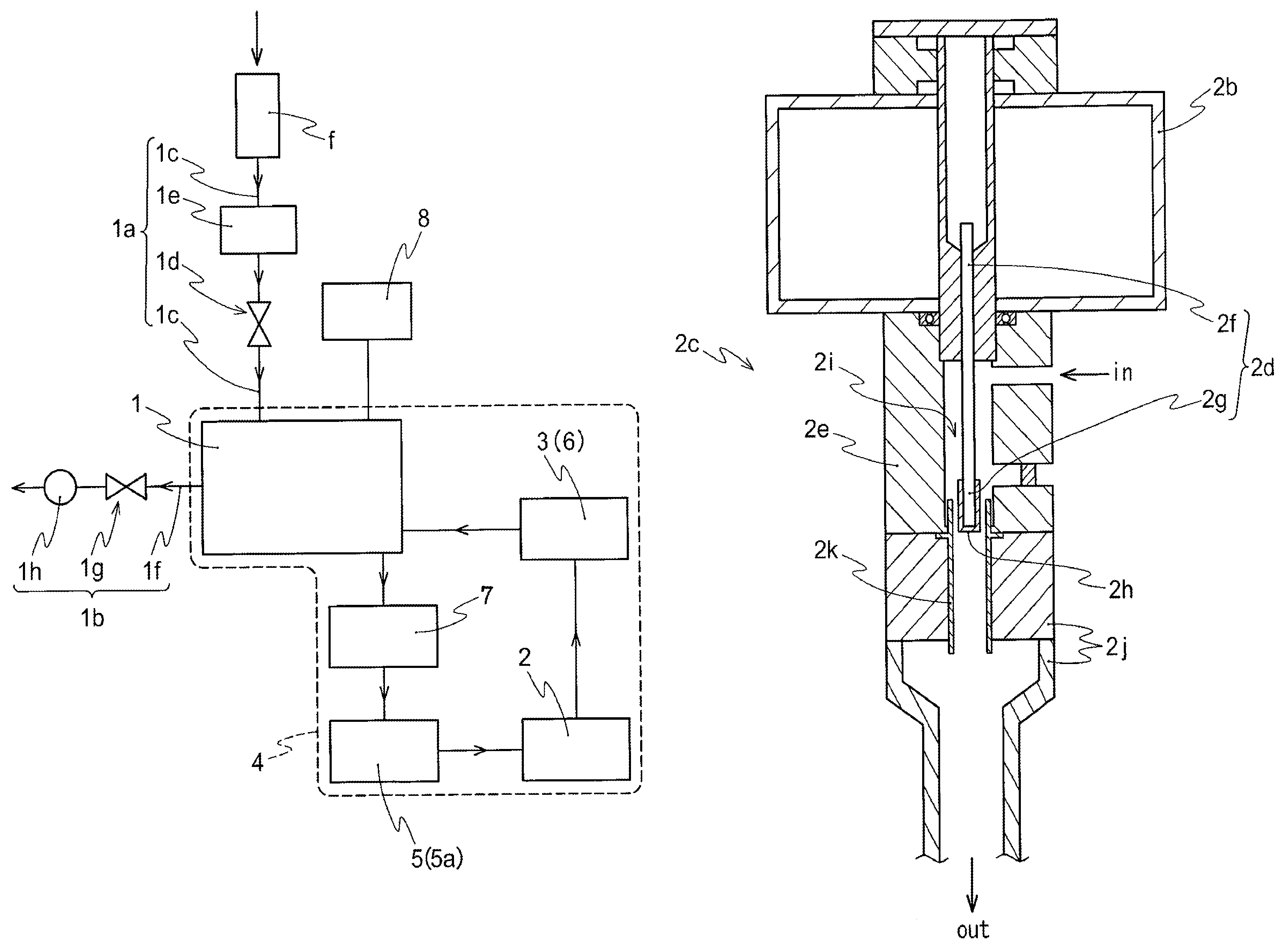
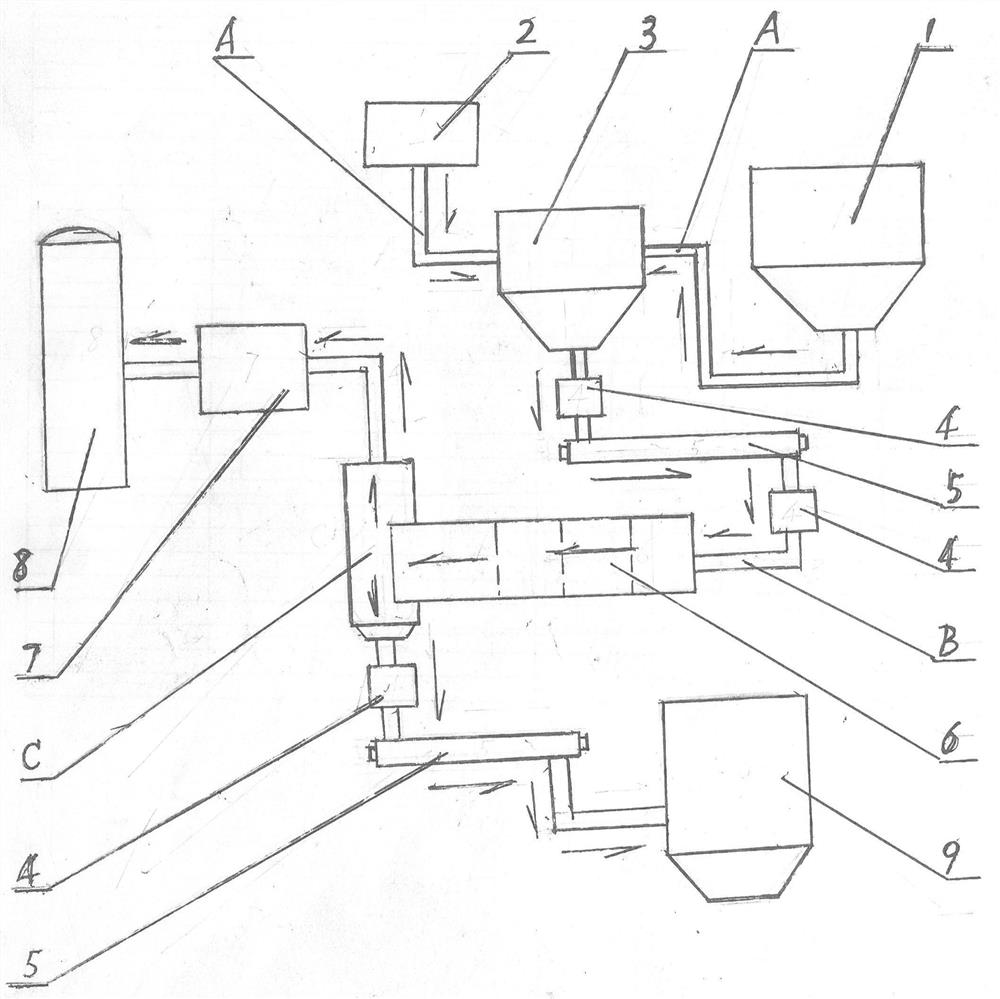
Method for preparing NO2 gas oxidizing agent through metal nitrate pyrolysis
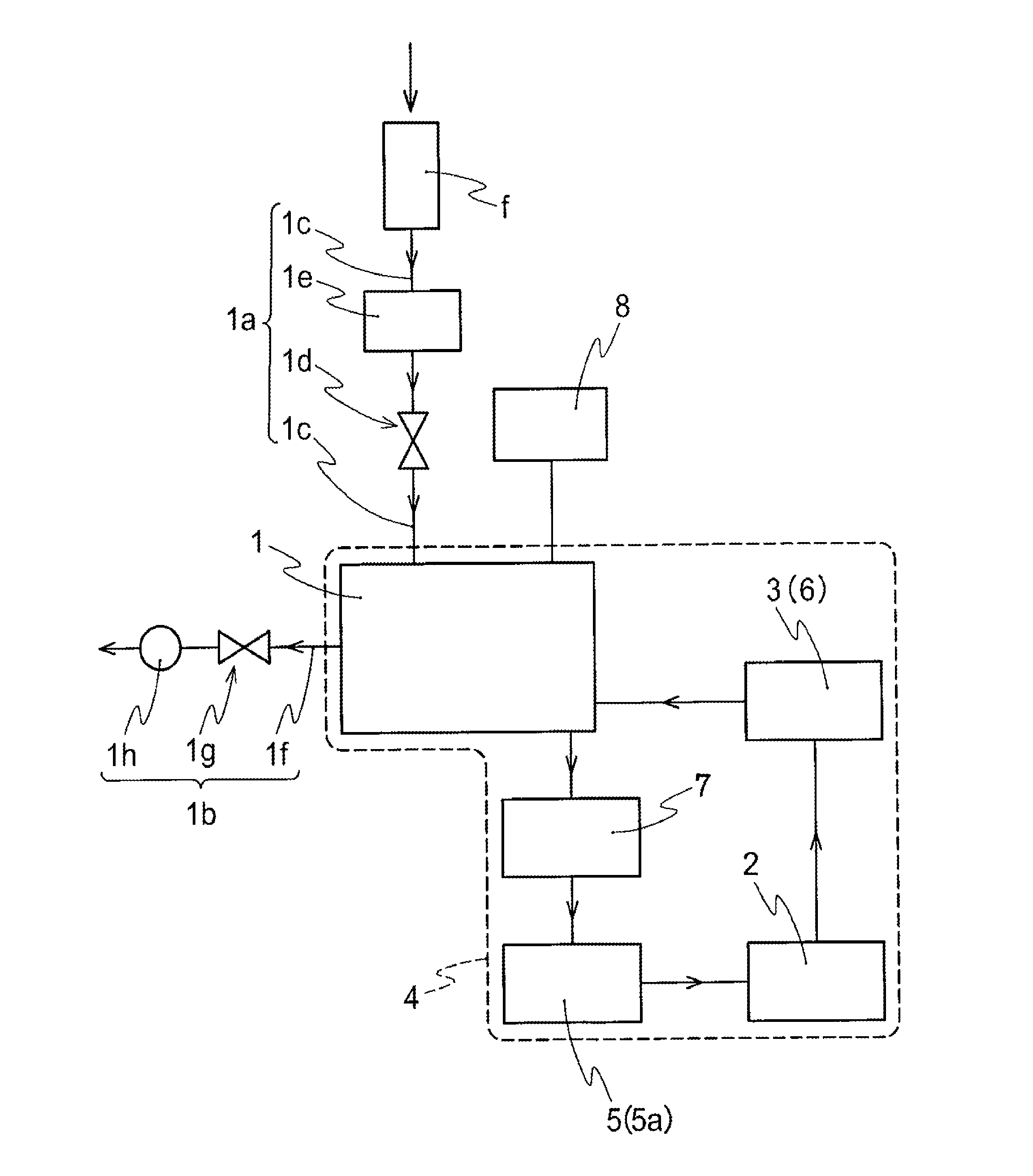
The invention provides a method for preparing an NO2 gas oxidizing agent through metal nitrate pyrolysis. An air compressor (2) and a metal nitrate storage bin (1) are connected with a nitrate bin (3), the air compressor (2) conveys nitrate powder to the nitrate bin (3) by conveying air, the nitrate bin (3) is connected with a conveyor (5) through an air blocking valve (4), an outlet of the conveyor (5) is connected with the air blocking valve (4) on the lower side, the air blocking valve (4) is connected with an articulated chute (B), and the nitrate powder is conveyed into a tubular microwave pyrolyzer (6). The upper end of the discharging cover (C) of the tubular microwave pyrolyzer (6) is connected with a gas conveying pipe (A) and a spiral vacuum pump (7), O2 and NO2 are conveyed to an NO2 storage tank (8) for producing high-purity NO2 or nitric acid, the lower end of the discharging cover (C) is connected with the air blocking valve (4) and the conveyor (5), and metal oxide powder is conveyed to a metal oxide storage bin (9) for recycling.
Owner:山东省辉煌环保科技有限责任公司
Advanced dry head-end reprocessing of light water reactor spent nuclear fuel
A method for reprocessing spent nuclear fuel from a light water reactor includes the step of reacting spent nuclear fuel in a voloxidation vessel with an oxidizing gas having nitrogen dioxide and oxygen for a period sufficient to generate a solid oxidation product of the spent nuclear fuel. The reacting step includes the step of reacting, in a first zone of the voloxidation vessel, spent nuclear fuel with the oxidizing gas at a temperature ranging from 200-450° C. to form an oxidized reaction product, and regenerating nitrogen dioxide, in a second zone of the voloxidation vessel, by reacting oxidizing gas comprising nitrogen monoxide and oxygen at a temperature ranging from 0-80° C. The first zone and the second zone can be separate. A voloxidation system is also disclosed.
Owner:UT BATTELLE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com