Method and device system for recovering nitric acid through pyrolyzing nitrate
A nitrate and preheating device technology, which is applied in the direction of nitric acid, nitrogen oxides/oxo acids, sustainable manufacturing/processing, etc., can solve the problems of high energy consumption and poor efficiency of the device, and achieve avoiding direct contact and recycling The effect of high utilization rate and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Recovery of Nitric Acid by Pyrolysis of Nitrate
[0046] The crystalline nitrate is heated using a two-stage preheating device. The crystalline nitrate is first heated to about 170°C in the first stage preheating device and converted into liquefied nitrate. Then it is transported to the second-stage preheating device and heated to about 330°C, which is 500°C lower than the decomposition temperature, to obtain a nitrate hot fluid with a temperature of about 330°C. The nitrate hot fluid is delivered to the decomposer, heated by high-temperature gas (temperature 550-800°C), and the internal temperature of the decomposer is maintained at 500-800°C, so that the nitrate is decomposed to produce mixed gas and solid powder. Separating the mixed gas and solid powder, part of the mixed gas is sent to the nitric acid recovery tank to be absorbed by water and converted into nitric acid, and the other part of the mixed gas is heated to 500-800°C, and then returned to the decompose...
Embodiment 2
[0048] Recovery of Nitric Acid by Pyrolysis of Nitrate
[0049] According to the thermal decomposition characteristics of nitrate: firstly, heat the nitrate once, raise the temperature to 150~200°C and keep the temperature, so that the nitrate can be transformed into a liquid state. Then, the liquid nitrate is pumped into the secondary heating tank through the transfer pump, and heated again to make the temperature of the nitrate reach 250-350°C to obtain the nitrate hot fluid.
[0050] Pump the nitrate hot fluid (liquid) into the nitrate decomposer, and at the inlet of the pump into the decomposer, there is a liquid mist distributor, so that the pumped nitrate liquid is distributed in mist form. Control the internal temperature of the decomposer to maintain between 500 and 800 °C, and the pumped nitrate liquid is decomposed into nitrogen dioxide, oxygen and oxides. The oxide powder is deposited at the bottom of the decomposer, and there is a sealing valve at the bottom of ...
Embodiment 3
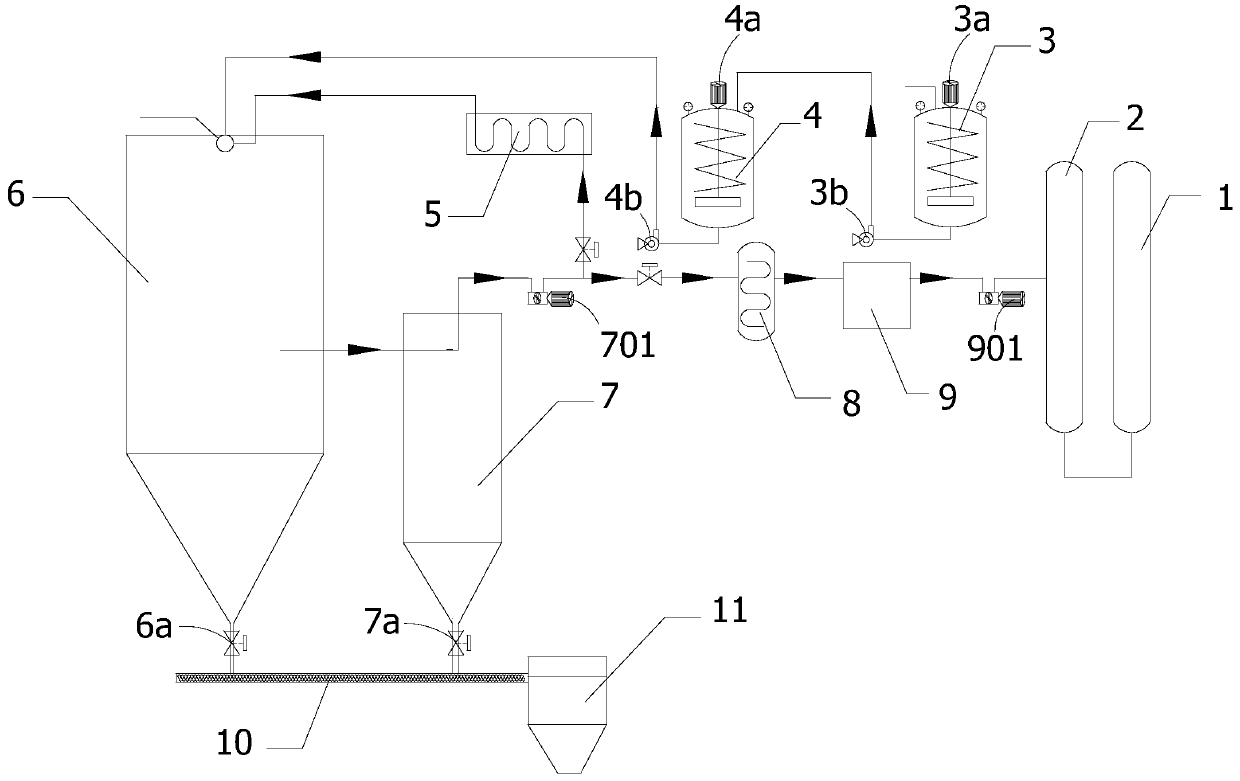
[0063] A device system for recovering nitric acid by pyrolysis of nitrate, including a heating tank, a decomposer, a dust collector, a heater, a heat exchanger, and a nitric acid recovery tank. The heating tank is used for heating and melting nitrate to obtain nitrate hot fluid. The decomposer is used to receive the nitrate hot fluid melted in the heating tank and the mixed gas heated by the heater; the nitrate is thermally decomposed inside the decomposer to produce oxides and mixed gas. The dust collector is used to receive the decomposed mixed gas discharged from the decomposer, and to separate the dust entrained in the mixed gas. The heater is used to receive the dust-separated mixed gas discharged from the dust collector, heat the mixed gas and send it to the feed port of the decomposer as a heat source. The heat exchanger is used to receive the dust-separated mixed gas discharged from the dust collector, cool it and transport it to the nitric acid recovery tank.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com