Preparation method and apparatus of nitrogen oxides
A technology for preparing equipment for nitrogen oxides, applied in the direction of nitrogen oxides/oxyacids, nitrogen dioxide, etc., can solve serious problems, corrosion of equipment and pipelines, difficult long-term stable operation, etc., to improve the oxidation effect, The effect of avoiding corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
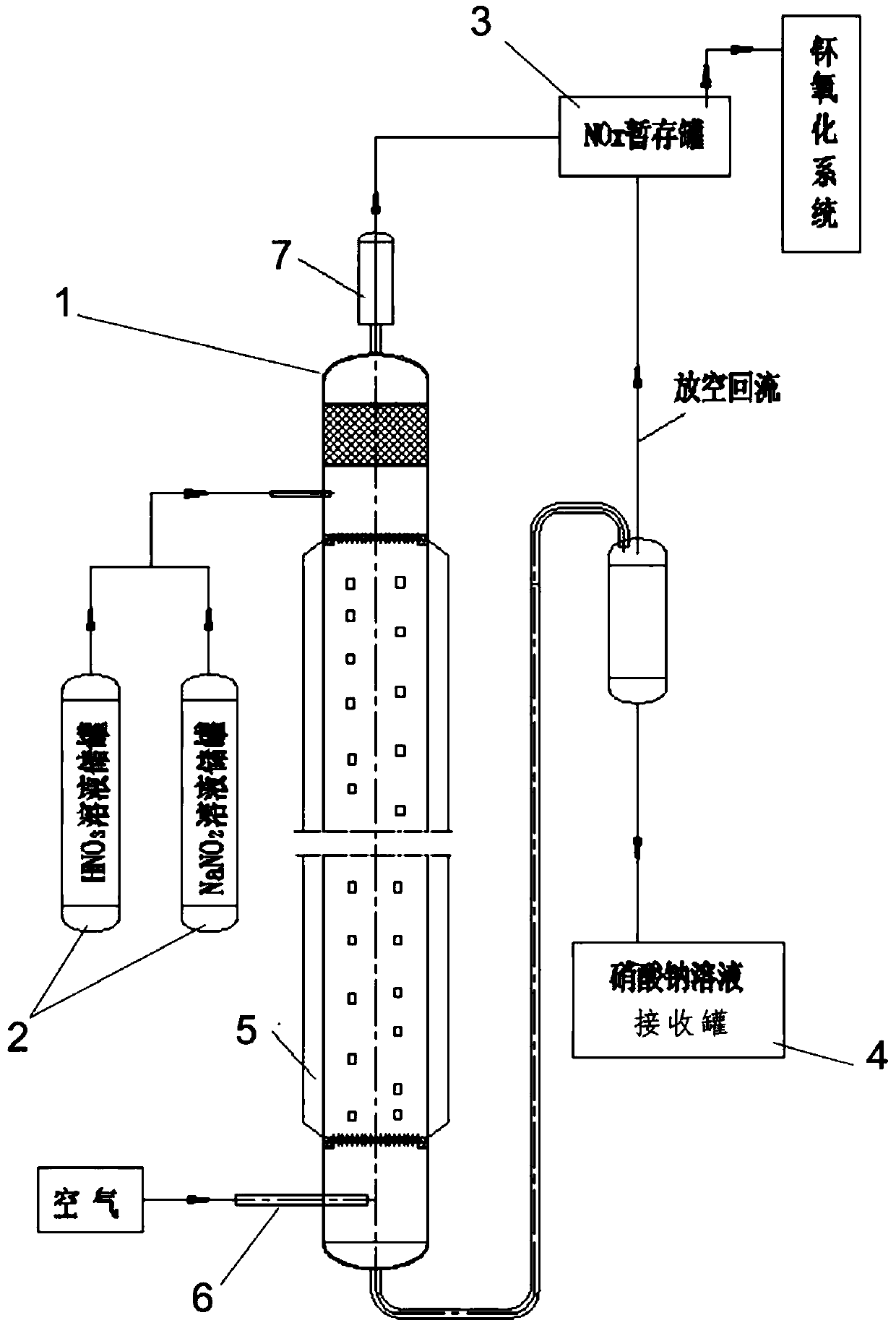
[0031] like figure 1 The size parameters of the nitrogen oxide preparation device shown are as follows: the total height of the packing column is 1900mm, the diameter is 60mm, the packing is made of theta ring (stainless steel), Φ5*5mm, and the upper part of the packing column is kept in a circulating water jacket.
[0032] Nitrogen oxide preparation experiments were carried out based on the established nitrogen oxide preparation device, and the generated nitrogen oxides were absorbed and treated with hydrazine nitrate-hydroxylamine solution.
[0033] Reaction feed liquid: nitric acid concentration 10mo / L, sodium nitrite concentration 4mol / L.
[0034] Operating process conditions:
[0035] The flow rate of nitric acid liquid is 2.5L / h, the flow rate of sodium nitrite solution is 5L / h, the air flow rate at the bottom of the packed column is 650L / h, and the temperature of heat preservation circulating water is 80°C; the nitrogen oxides produced are made of hydrazine nitrate-hyd...
Embodiment 2
[0037] like figure 1 The size parameters of the nitrogen oxide preparation device shown are as follows: the total height of the packing column is 1900mm, the diameter is 60mm, the packing is made of theta ring (stainless steel), Φ5*5mm, and the upper part of the packing column is kept in a circulating water jacket.
[0038] Nitrogen oxide preparation experiments were carried out based on the established nitrogen oxide preparation device, and the generated nitrogen oxides were absorbed and treated with hydrazine nitrate-hydroxylamine solution.
[0039] Reaction feed liquid: nitric acid concentration 10mo / L, sodium nitrite concentration 4mol / L.
[0040] Operating process conditions:
[0041] The flow rate of nitric acid feed solution is 2L / h, the flow rate of sodium nitrite solution is 4L / h, the air flow rate at the bottom of the packed column is 500L / h, and the temperature of heat preservation circulating water is 75°C; the nitrogen oxides produced use hydrazine nitrate-hydrox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com