Method for preparing sodium-potassium-magnesium-calcium glucose injection
A glucose injection and calcium gluconate technology, which is applied in the field of medicine, can solve the problems of 5-hydroxymethylfurfural degradation, unreported sterilization, and reduction of 5-hydroxymethylfurfural content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Injection composition (calculated per 1000ml injection)
[0021] calcium gluconate 0.672g Sodium chloride 6.372g potassium chloride 0.30g magnesium chloride 0.204g anhydrous glucose 10g Sodium acetate (as NaC 2 h 3 o 2 count) 2.052g sodium citrate 0.588g Water for Injection up to 1000ml
[0022] Preparation
[0023] a. Add water for injection into the preparation tank, and then add calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, anhydrous glucose, sodium acetate, and sodium citrate in sequence. After dissolving, adjust the pH to 4.5 with hydrochloric acid.
[0024] b. Filter through a 0.2 micron folded filter element and fill.
[0025] c. Sterilize at 121°C for 12 minutes, inspect by light, and pack.
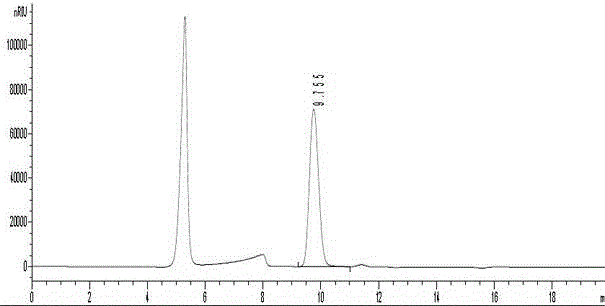
[0026] For the finished product, the glucose content and 5-hydroxymethylfurfural were detected according to the method of the second appendix of the "Chinese Pharmacopoeia" in 2...
Embodiment 2
[0028] Injection composition (calculated per 1000ml injection)
[0029] calcium gluconate 0.672g Sodium chloride 6.372g potassium chloride 0.30g magnesium chloride 0.204g anhydrous glucose 10g Sodium acetate (as NaC 2 h 3 o 2 count) 2.052g sodium citrate 0.588g Water for Injection up to 1000ml
[0030] Preparation
[0031] a. Add water for injection into the preparation tank, and then add calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, anhydrous glucose, sodium acetate, and sodium citrate in sequence. After dissolving, adjust the pH to 5.2 with hydrochloric acid.
[0032] b. Filter through a 0.2 micron folded filter element and fill.
[0033] c. Sterilize at 121°C for 12 minutes, inspect by light, and pack.
[0034] For the finished product, the glucose content and 5-hydroxymethylfurfural were detected according to the method of the second appendix of the "Chinese Pharmacopoeia" in 201...
Embodiment 3
[0036] Injection composition (calculated per 1000ml injection)
[0037] calcium gluconate 0.672g Sodium chloride 6.372g potassium chloride 0.30g magnesium chloride 0.204g anhydrous glucose 10g Sodium acetate (as NaC 2 h 3 o 2 count) 2.052g sodium citrate 0.588g Water for Injection up to 1000ml
[0038] Preparation
[0039] a. Add water for injection into the preparation tank, add calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, anhydrous glucose, sodium acetate, and sodium citrate in turn, and after dissolving, adjust the pH to 5.5 with hydrochloric acid.
[0040] b. Filter through a 0.2 micron folded filter element and fill.
[0041] c. Sterilize at 121°C for 12 minutes, inspect by light, and pack.
[0042] For the finished product, the glucose content and 5-hydroxymethylfurfural were detected according to the method of the second appendix of the "Chinese Pharmacopoeia" in 2010, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com