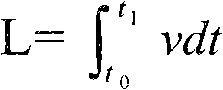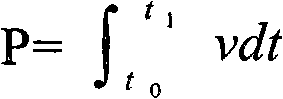Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Process diagnostics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process diagnostics
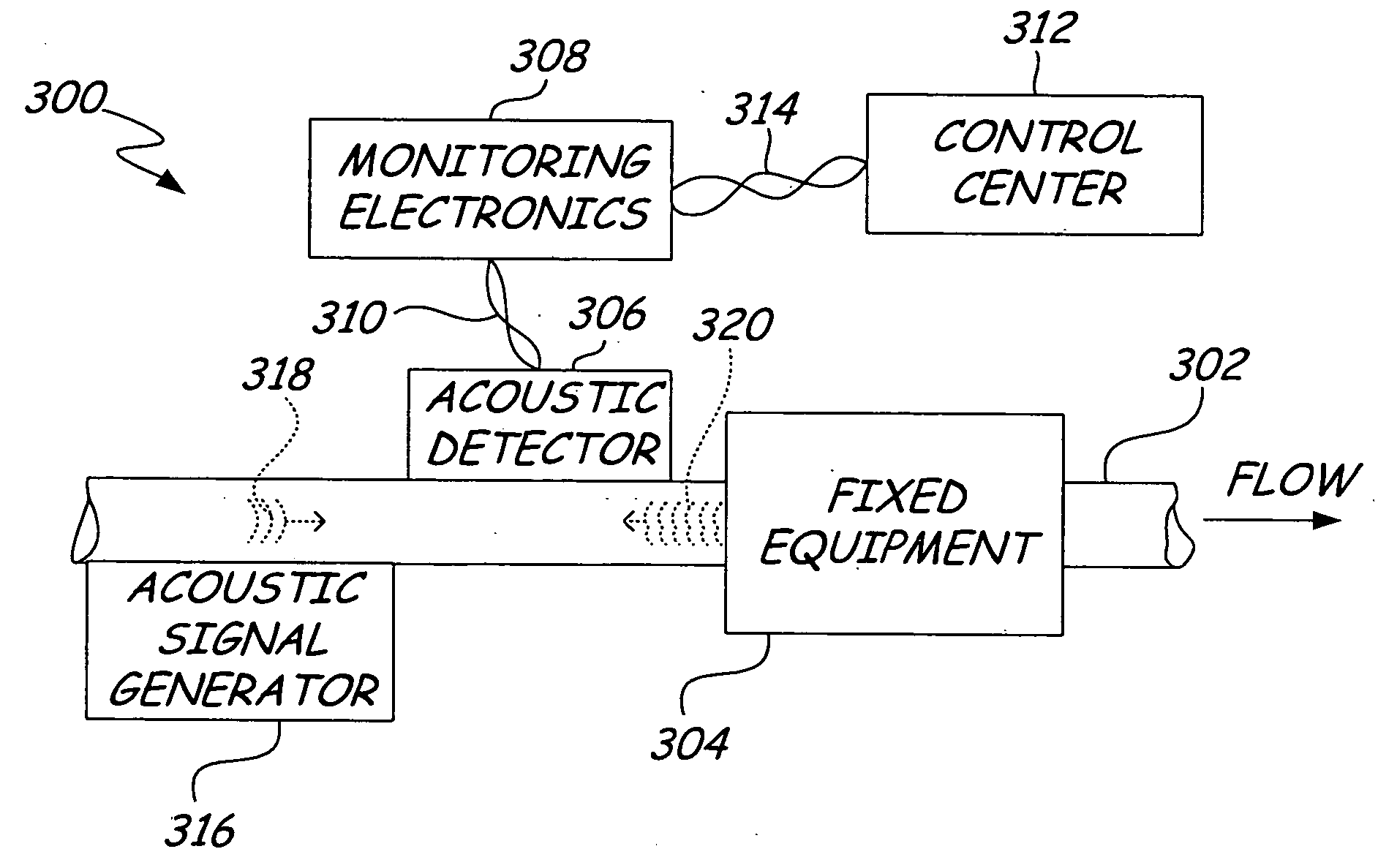
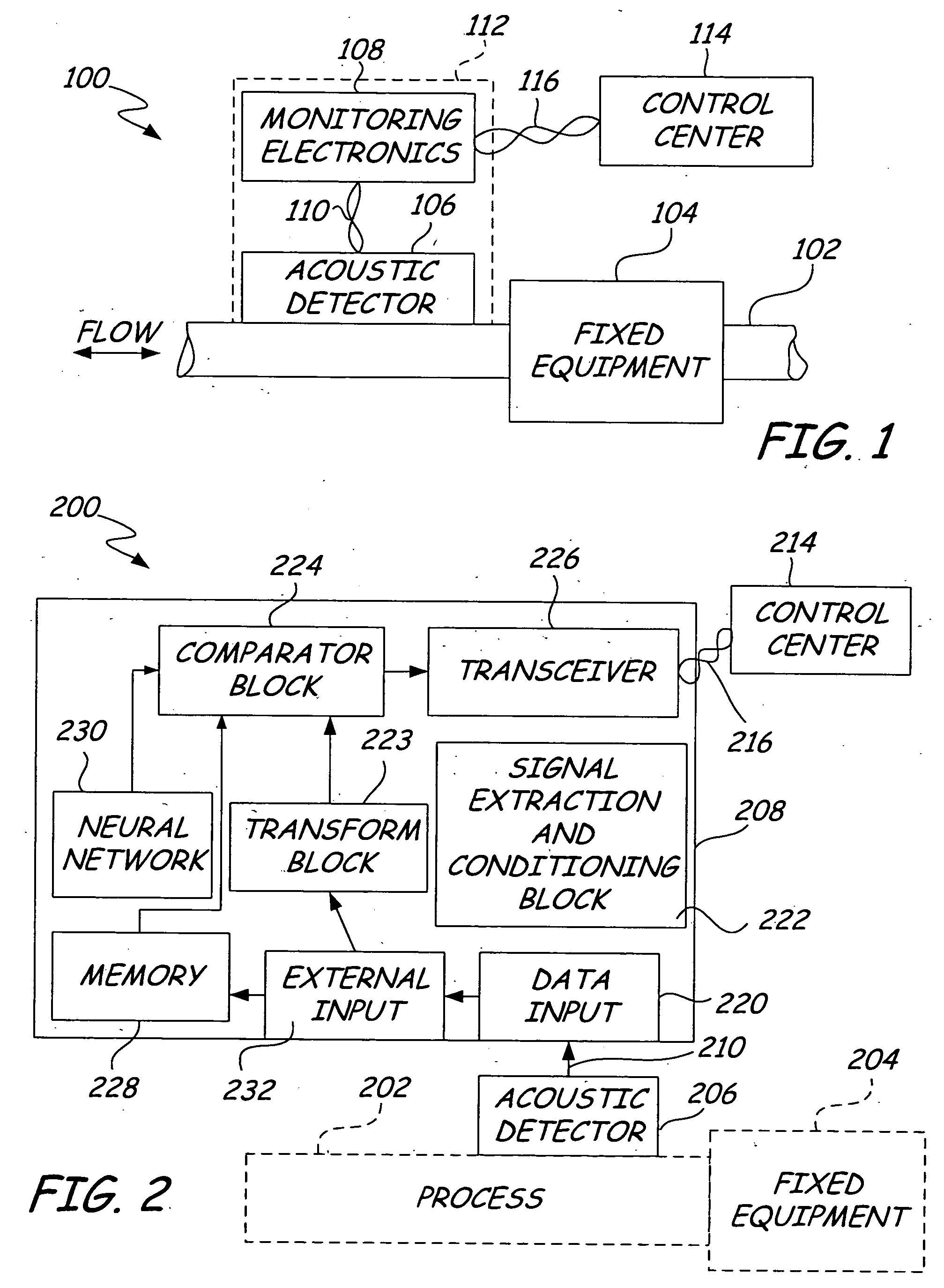
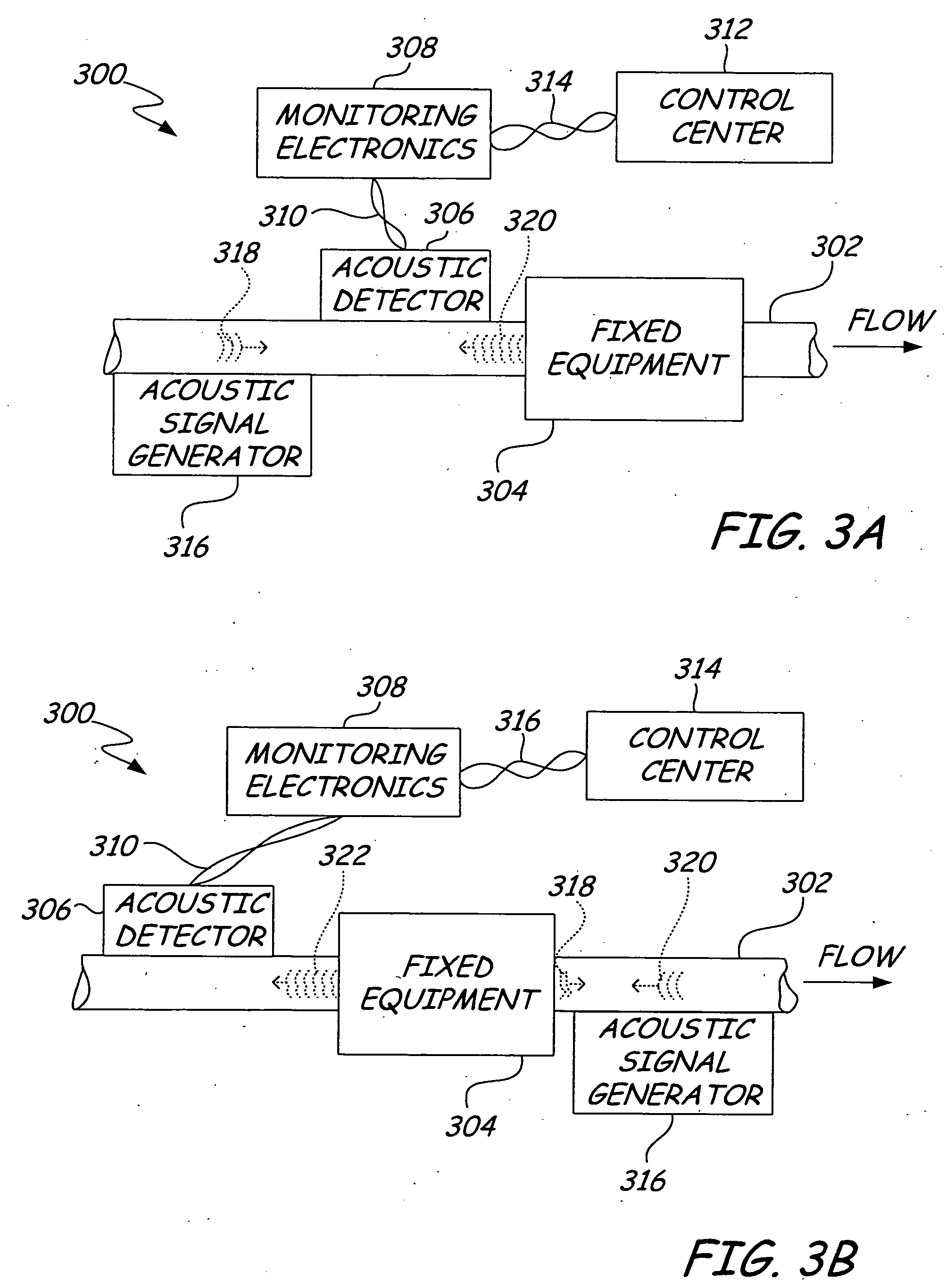
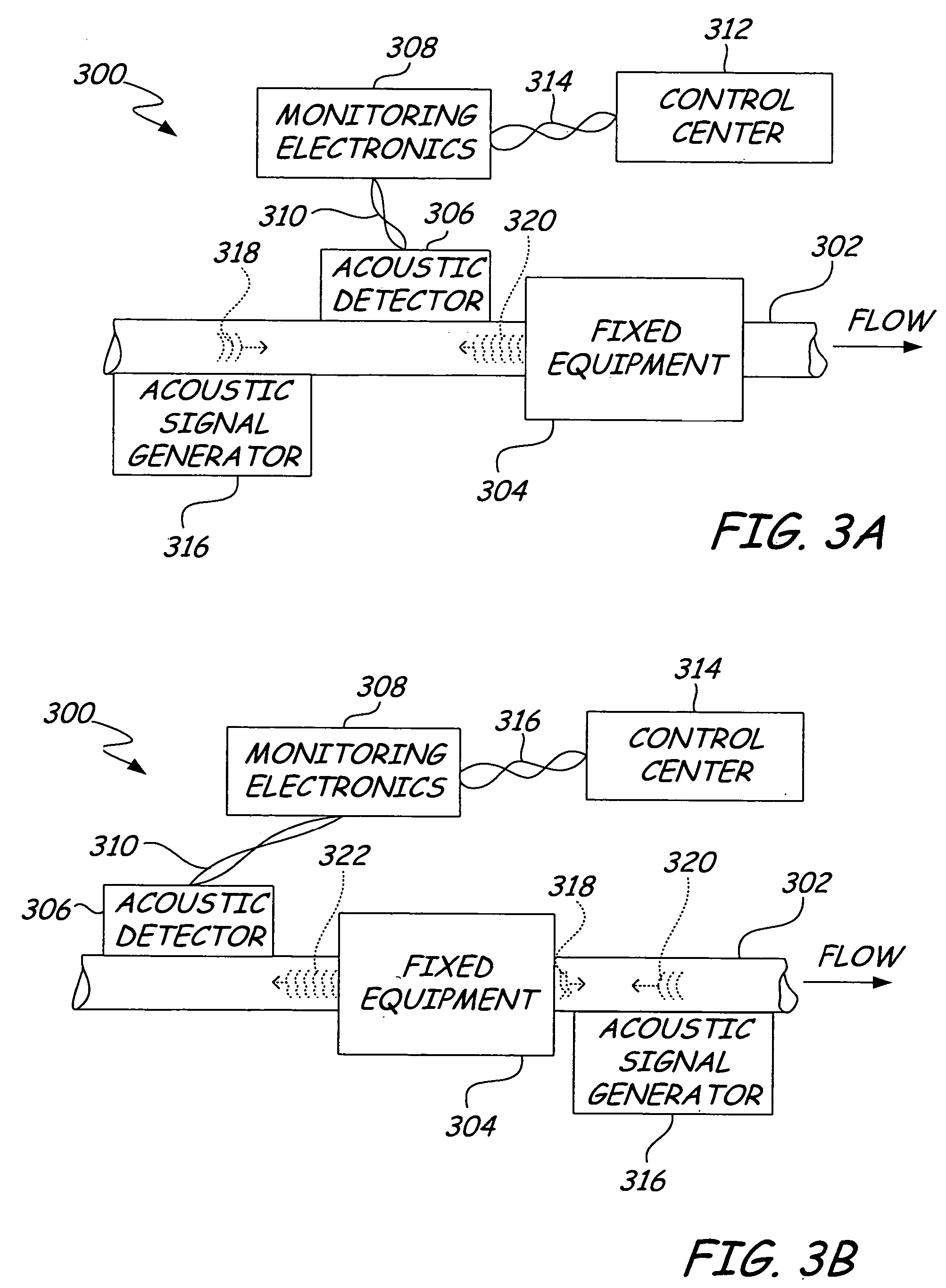
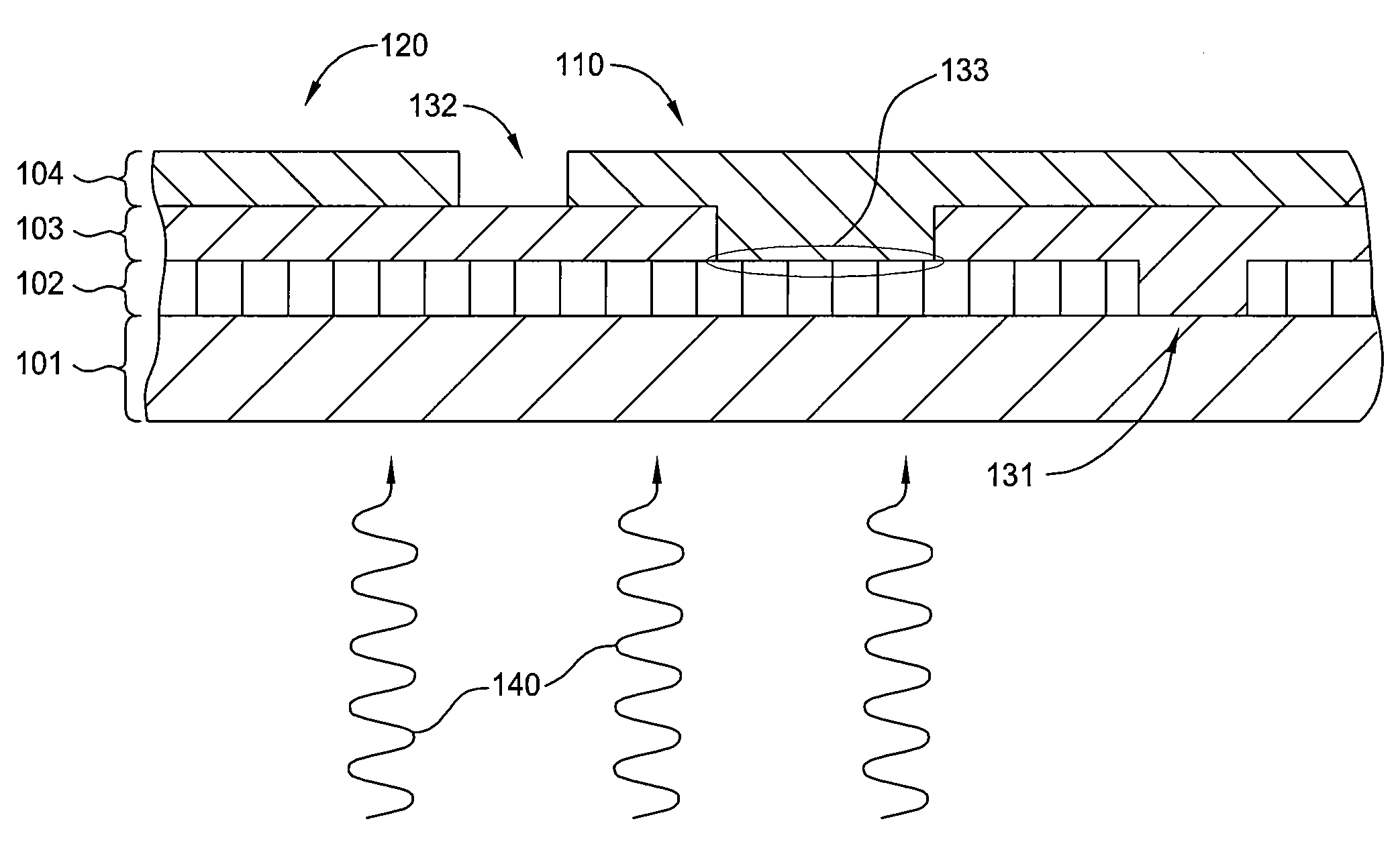
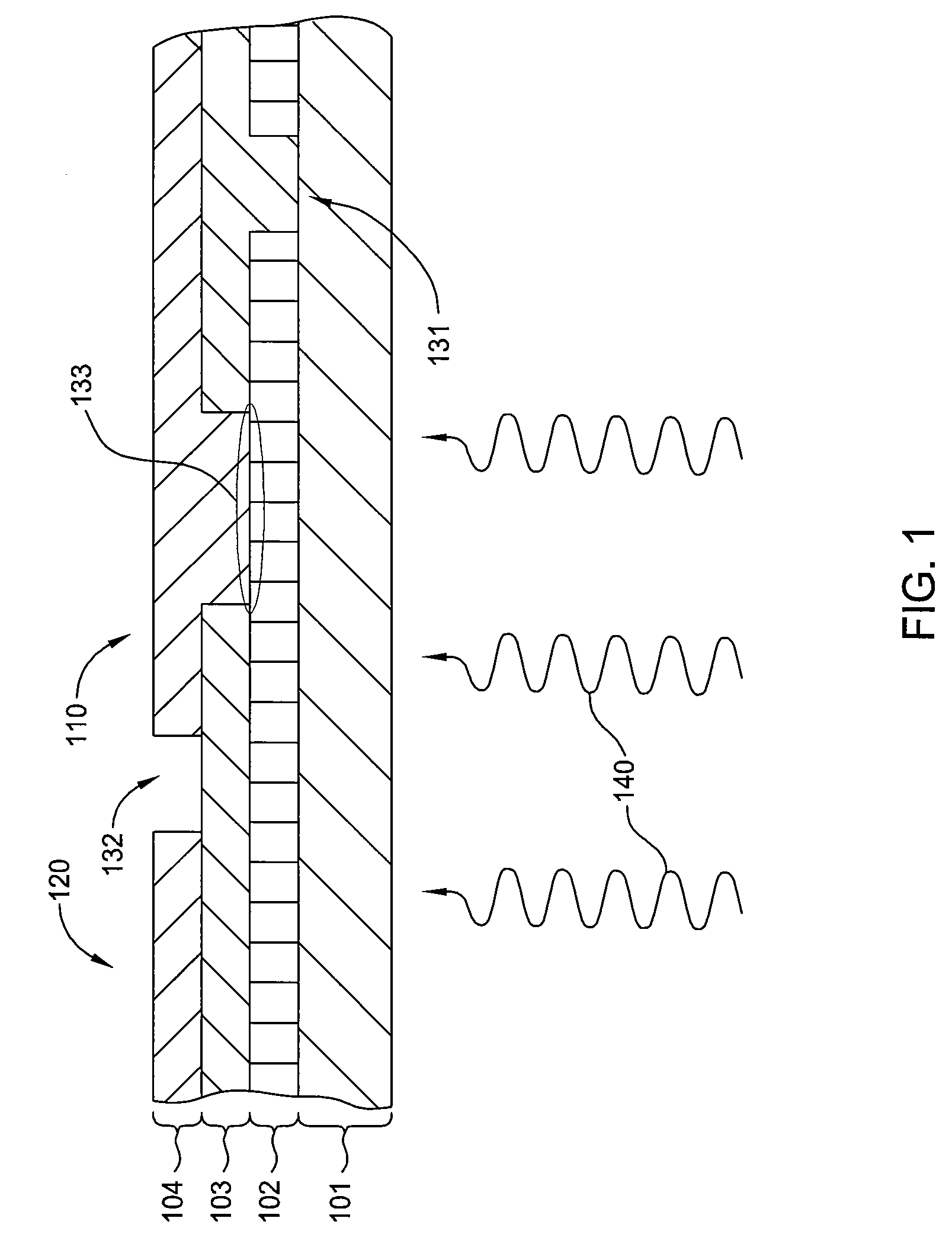
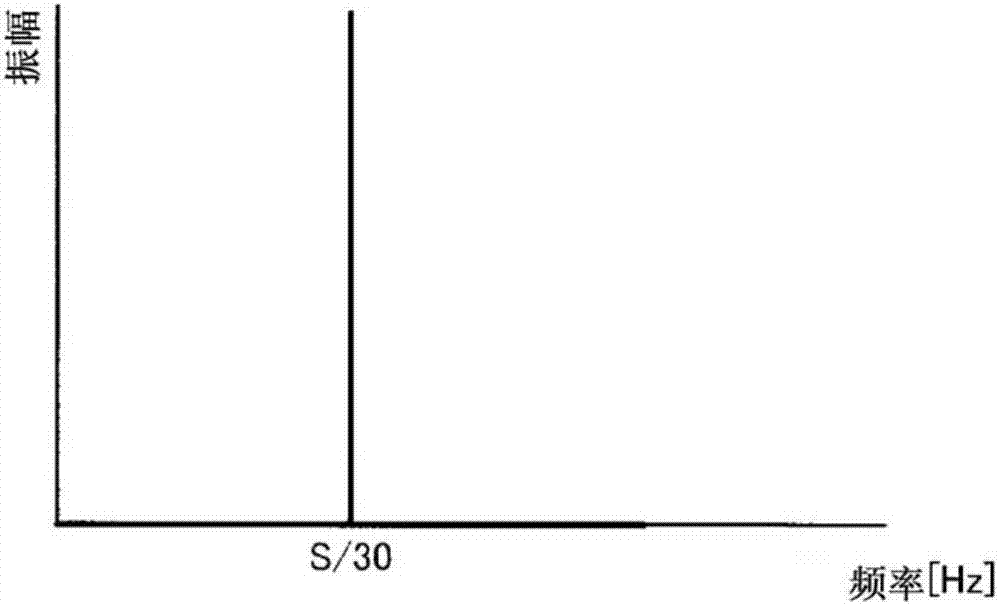
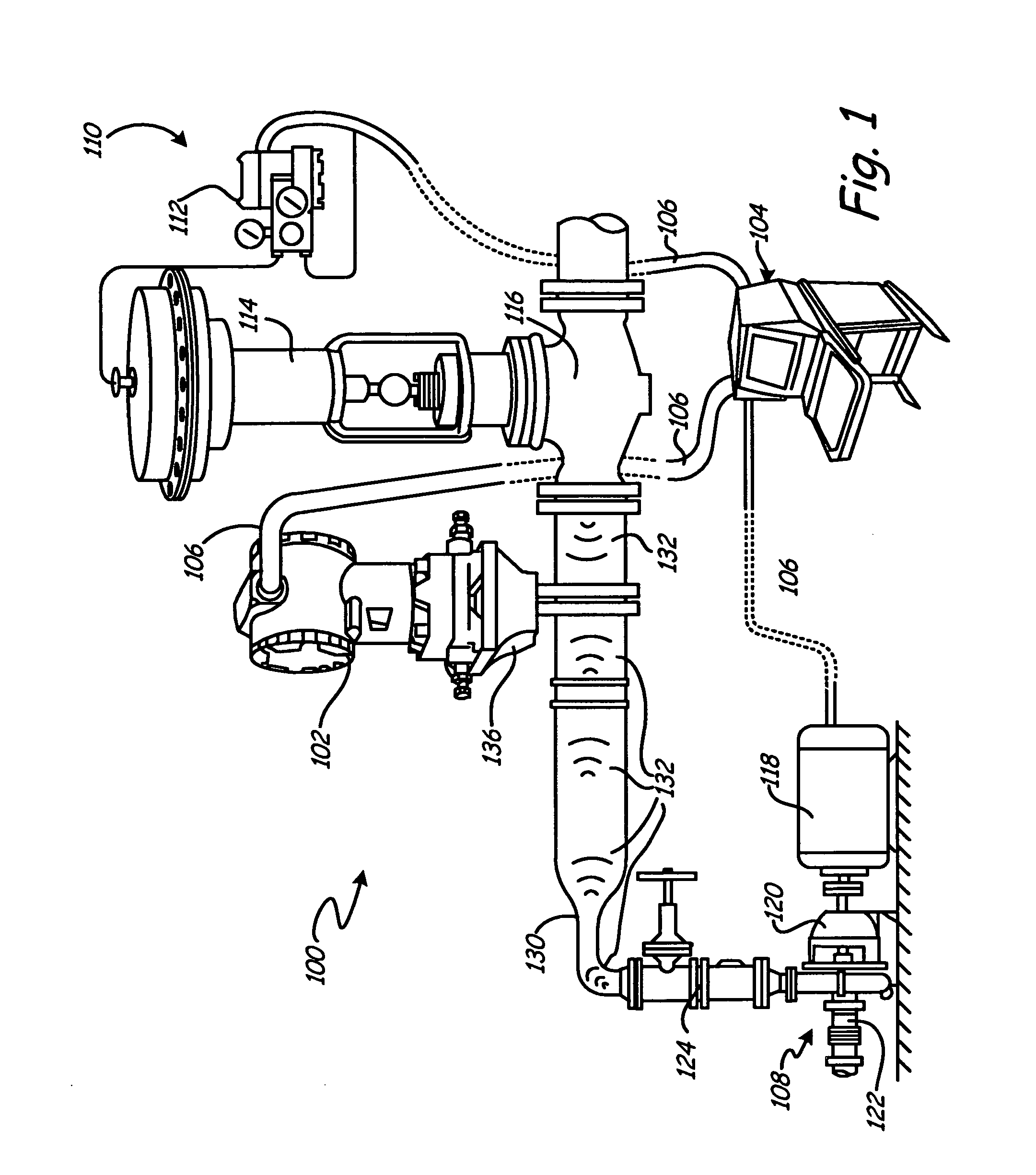
ActiveUS20050011278A1Improve abilitiesEasy to useVibration measurement in solidsAnalysing fluids using sonic/ultrasonic/infrasonic wavesTransducerEngineering
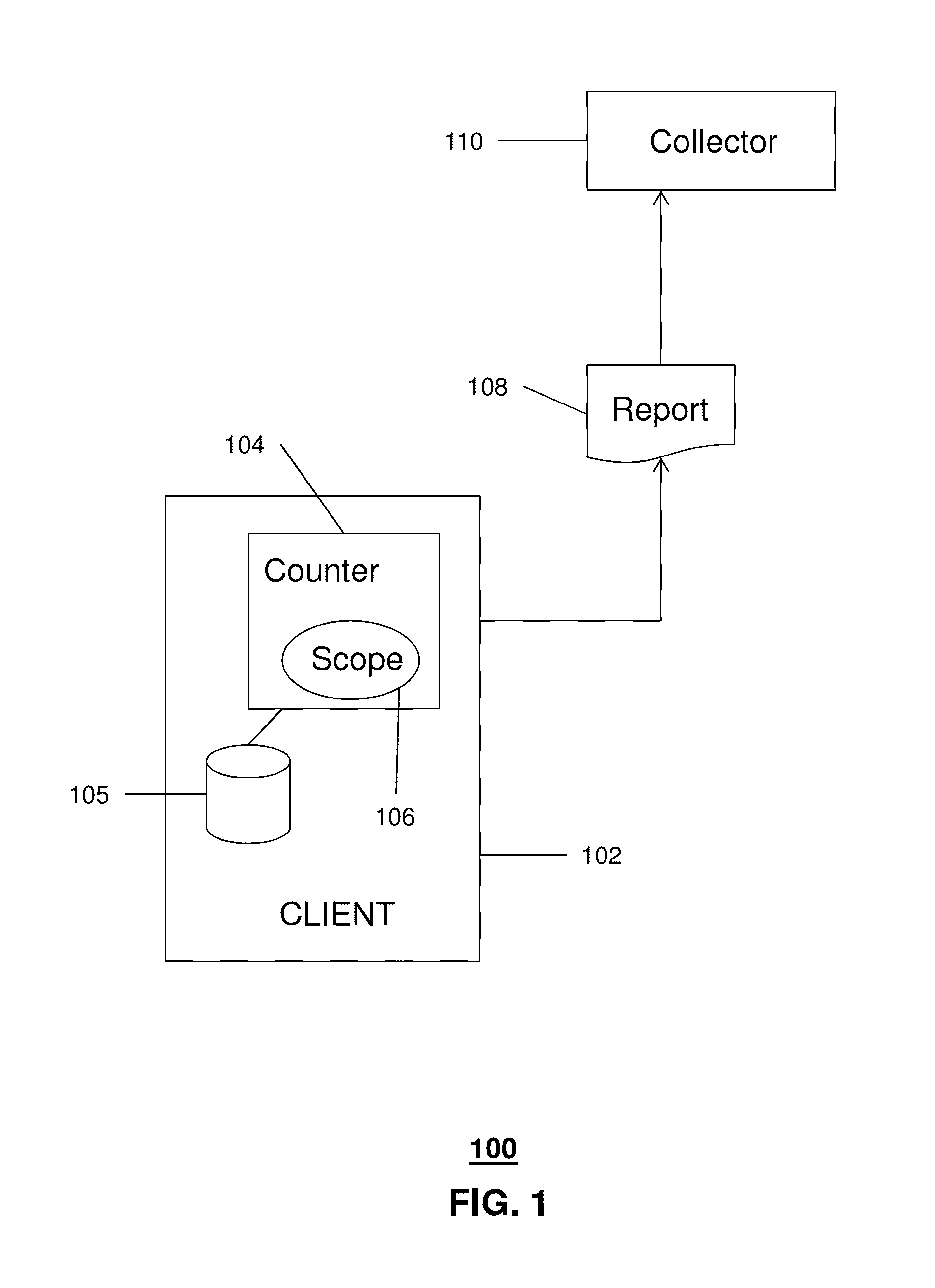
A diagnostic device for use in a industrial process includes monitoring electronics or diagnostic circuitry configured to diagnose or identify a condition or other occurrence in the industrial process. The system can be implemented in a process device such as a flowmeter, and in one example an acoustic flowmeter. A transducer can also be used and a frequency response, such as resonant frequency, can be observed.
Owner:ROSEMOUNT INC
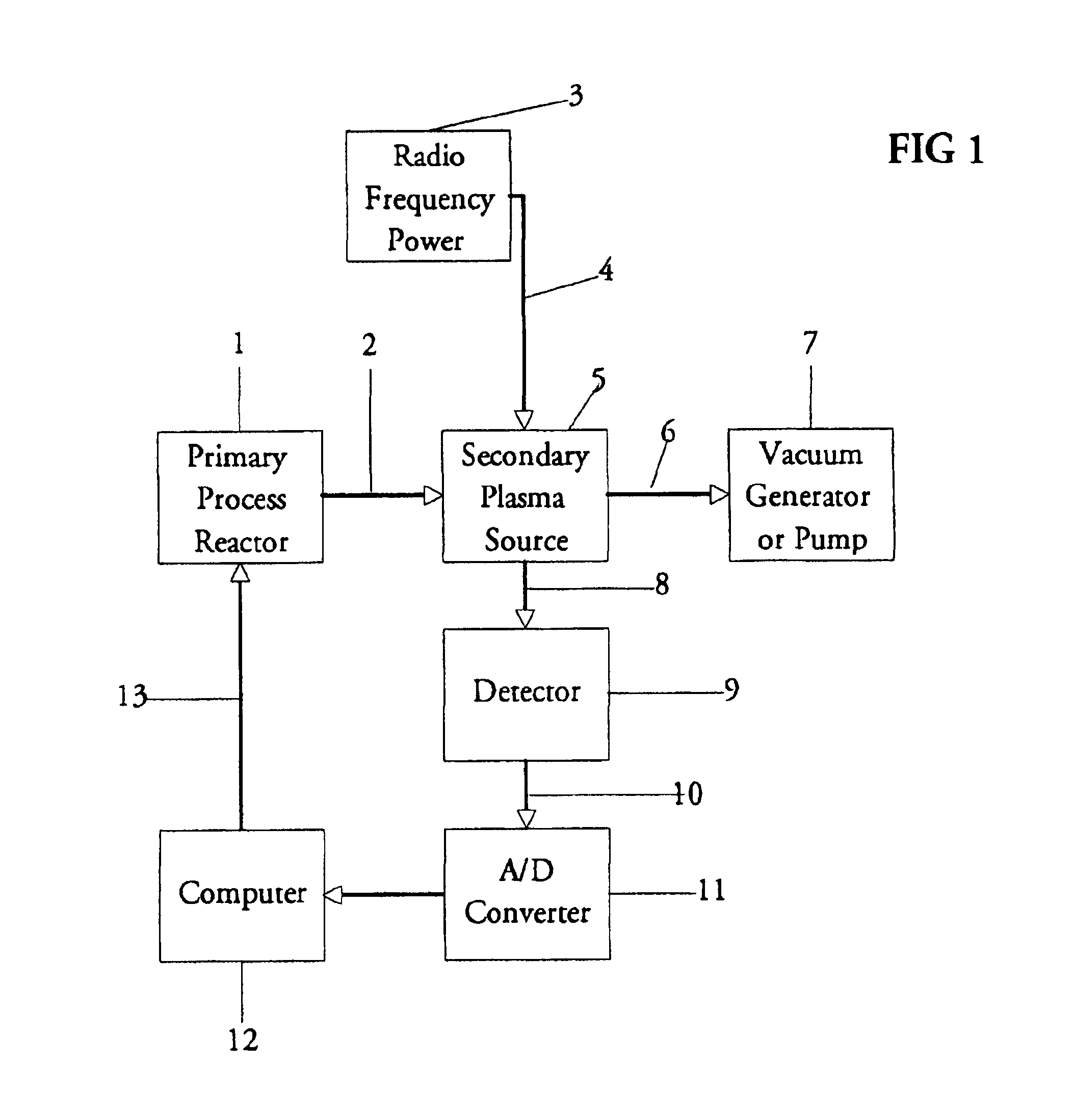
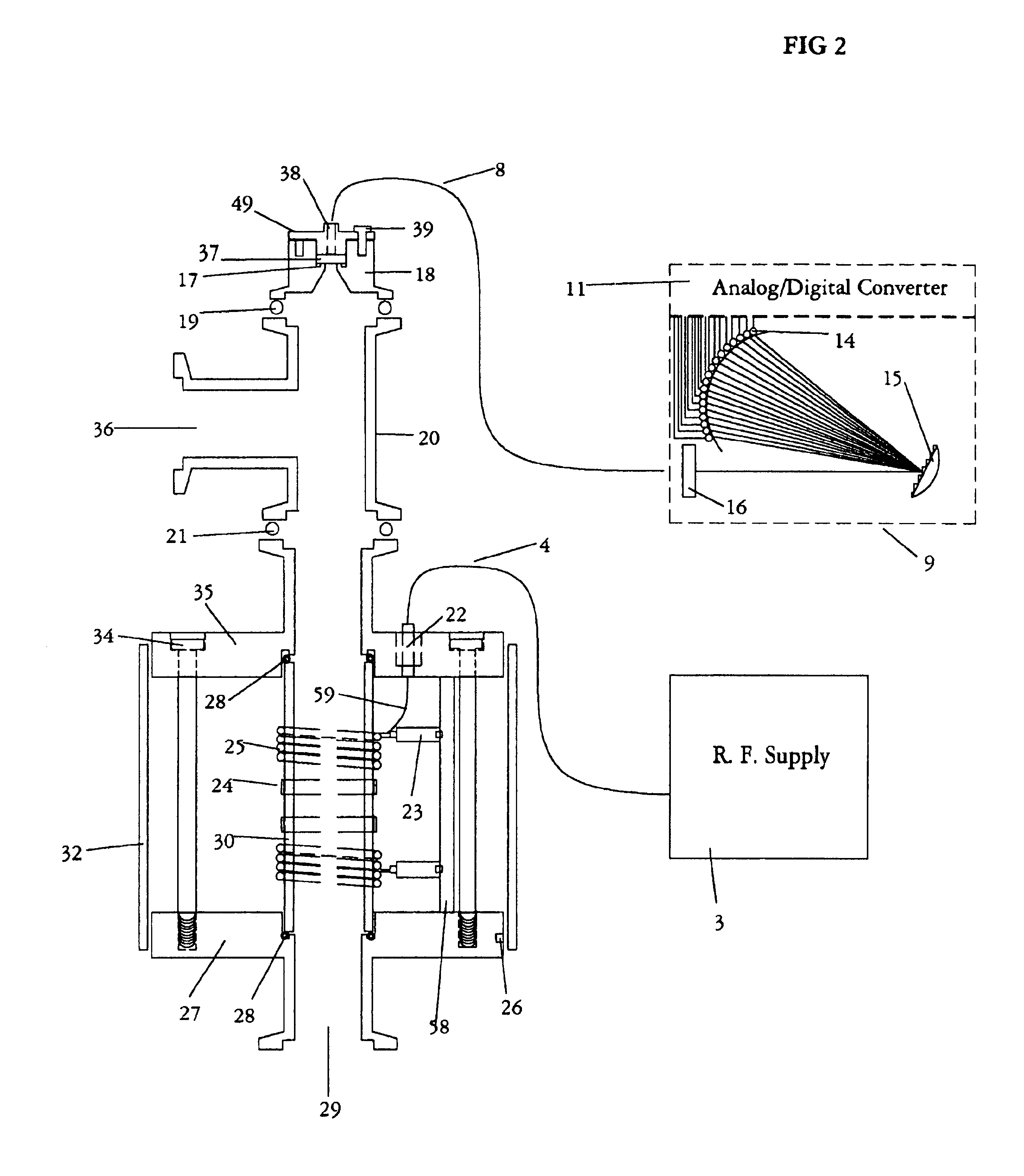
Inductively coupled plasma spectrometer for process diagnostics and control
InactiveUS6867859B1High sensitivitySimple reactor designEmission spectroscopyRadiation pyrometryOptical radiationInductively coupled plasma
The present invention relates to an apparatus and method for forming a plasma in the exhaust line of a primary process reactor. The plasma is generated in an inductive source (5) to examine the chemical concentrations of the waste or exhaust gas in vacuum lines that are below atmospheric pressure. The optical radiation emitted by the plasma is analyzed by an optical spectrometer (9) and the resulting information is used to diagnose, monitor, or control operating states in the main vacuum vessel.
Owner:LIGHTWIND CORP
Process diagnostics
ActiveUS7290450B2Improve abilitiesEasy to useVibration measurement in solidsAnalysing fluids using sonic/ultrasonic/infrasonic wavesTransducerEngineering
A diagnostic device for use in a industrial process includes monitoring electronics or diagnostic circuitry configured to diagnose or identify a condition or other occurrence in the industrial process. The system can be implemented in a process device such as a flowmeter, and in one example an acoustic flowmeter. A transducer can also be used and a frequency response, such as resonant frequency, can be observed.
Owner:ROSEMOUNT INC
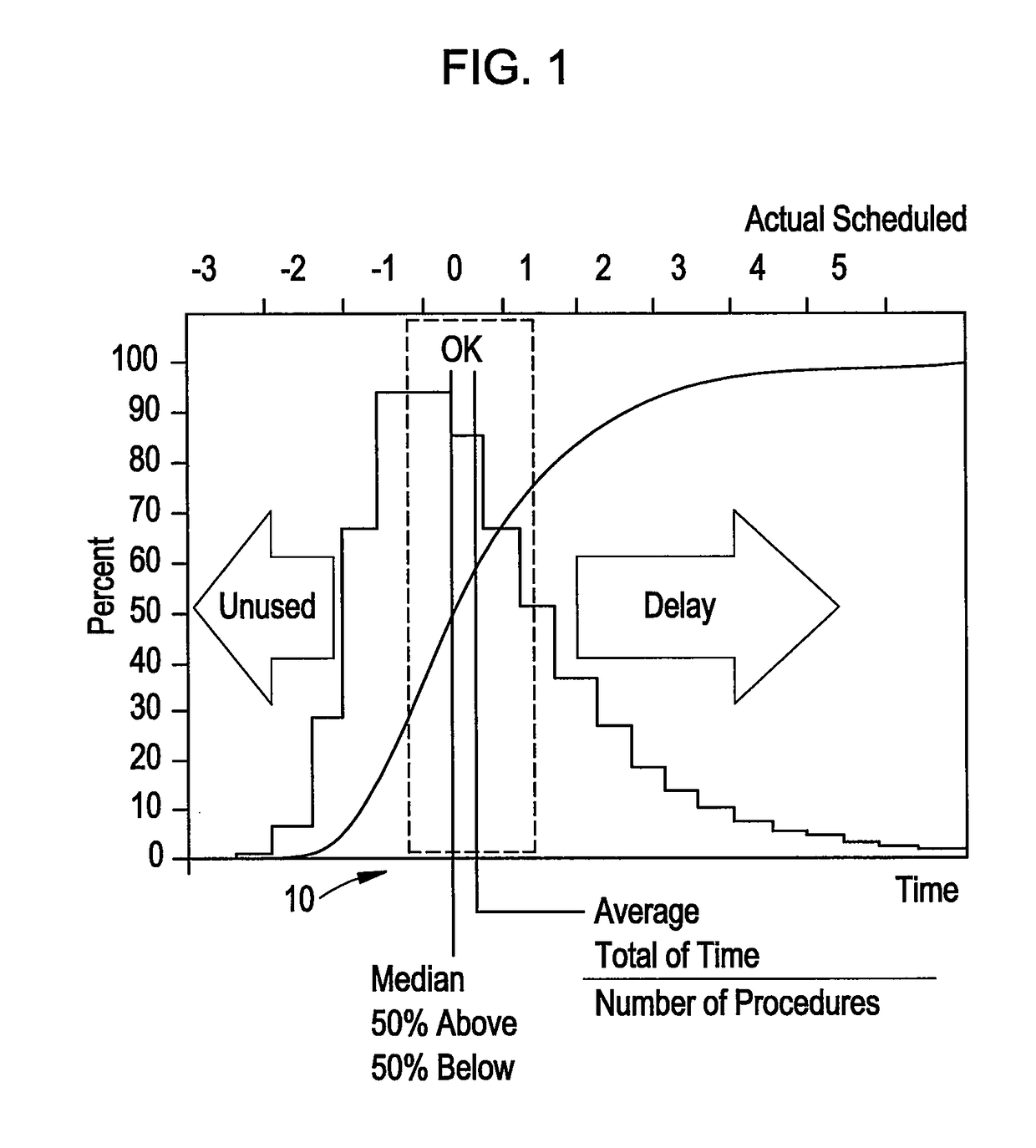
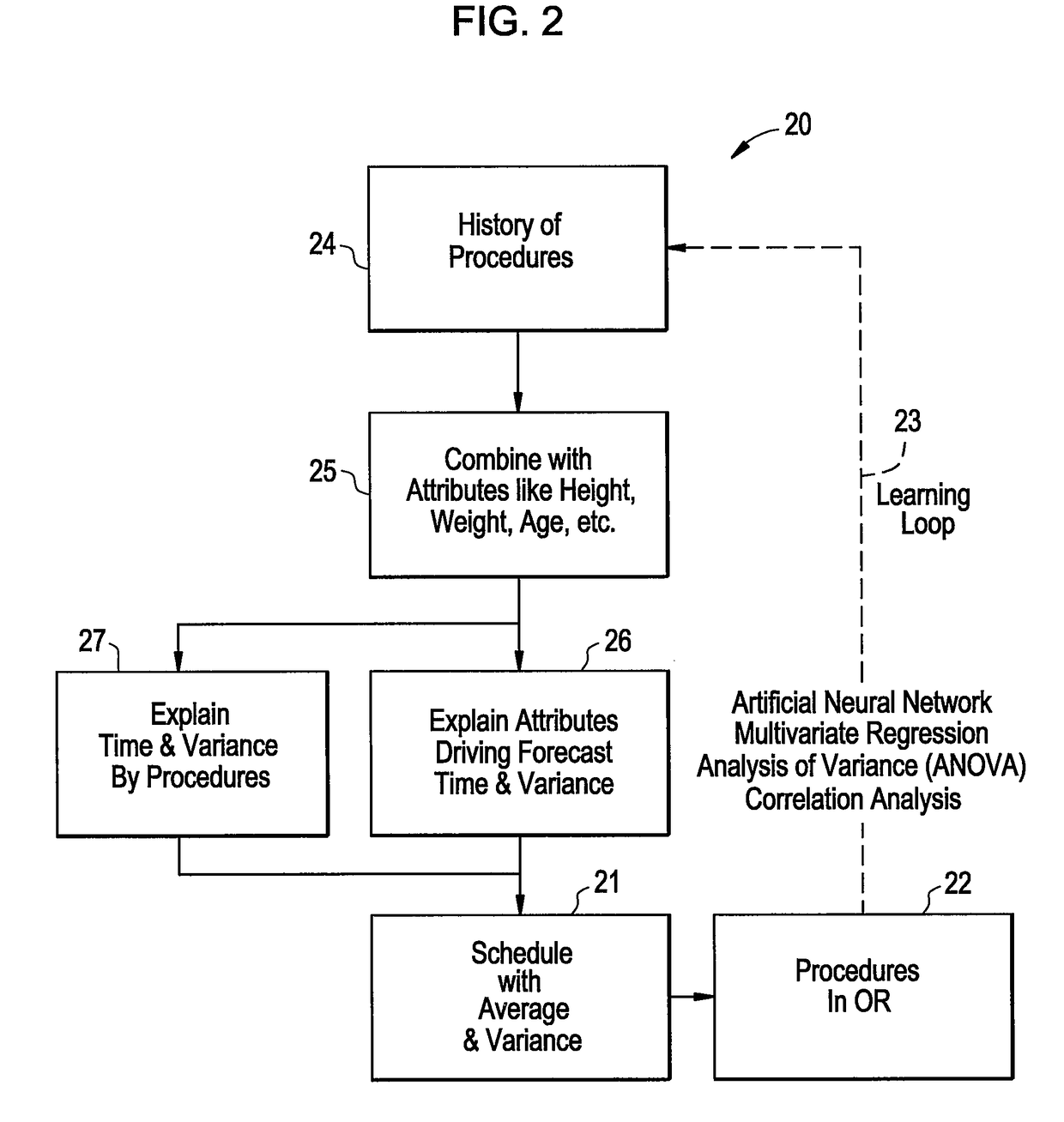
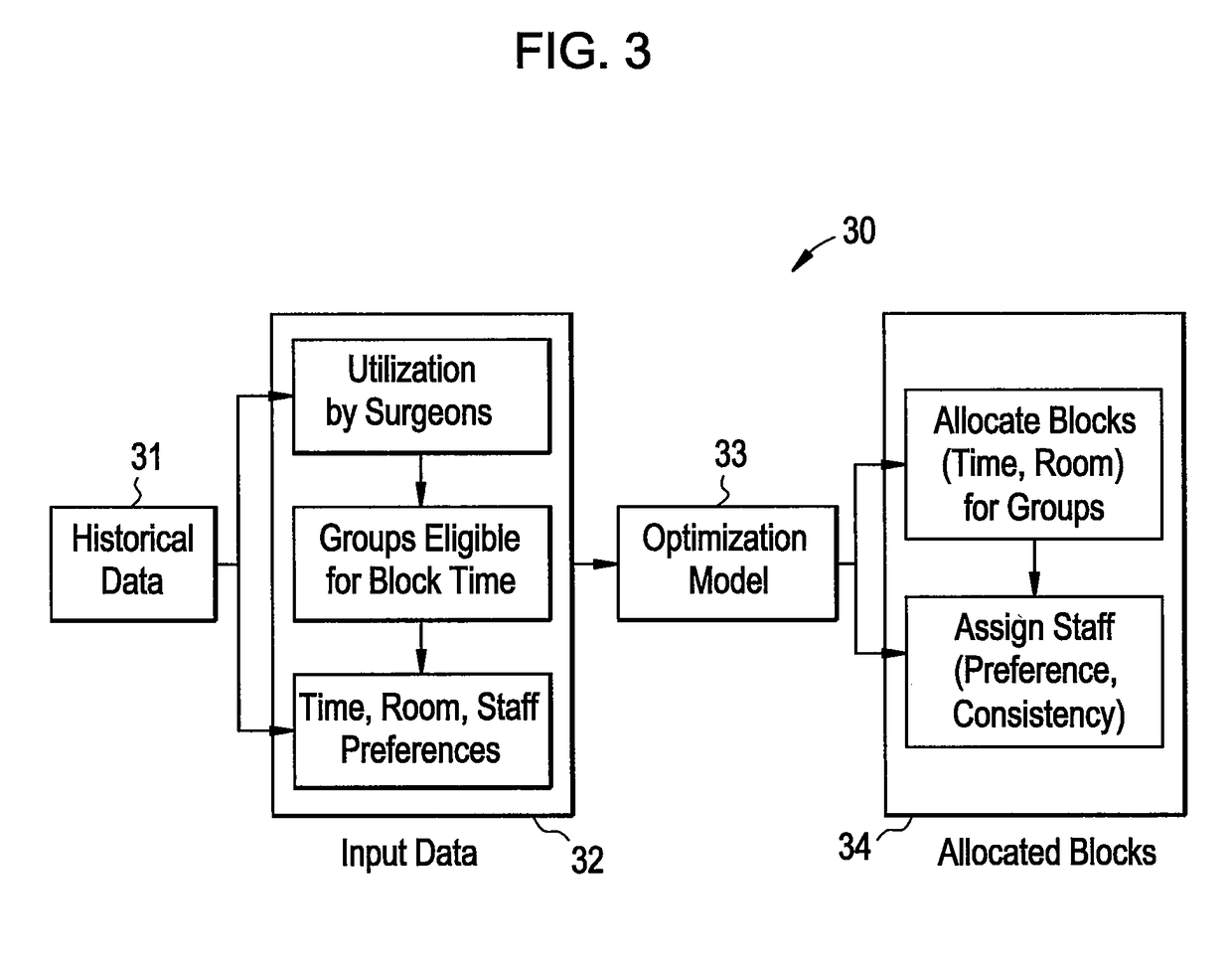
Method to view schedule interdependencies and provide proactive clinical process decision support in day view form
ActiveUS20090119126A1Quick scanGreat advantageHospital data managementMedical imagesREMS StakeholderComputer science
A system and method for use in managing and preparing for scheduled procedures that are characterized as being interdependent and variable. The disclosed method enables schedule risk management and provides a look-ahead capability along with process diagnostics to isolate specific assets and tasks that can be managed to reduce schedule risk. The method facilitates review of upcoming tasks by the process stakeholders for education as to where the schedule risks reside and in an emulation mode for review and improved scheduling going forward. Clinical workflow is integrated such that process stakeholders and assets are directed in such a way as to keep on, reduce delay risk or recover the schedule.
Owner:GENERAL ELECTRIC CO
Method to view schedule interdependencies and provide proactive clinical process decision support in day view form
ActiveUS10157355B2Quick scanGreat advantageOffice automationMedical imagesProgram planningREMS Stakeholder
A system and method for use in managing and preparing for scheduled procedures that are characterized as being interdependent and variable. The disclosed method enables schedule risk management and provides a look-ahead capability along with process diagnostics to isolate specific assets and tasks that can be managed to reduce schedule risk. The method facilitates review of upcoming tasks by the process stakeholders for education as to where the schedule risks reside and in an emulation mode for review and improved scheduling going forward. Clinical workflow is integrated such that process stakeholders and assets are directed in such a way as to keep on, reduce delay risk or recover the schedule.
Owner:GENERAL ELECTRIC CO
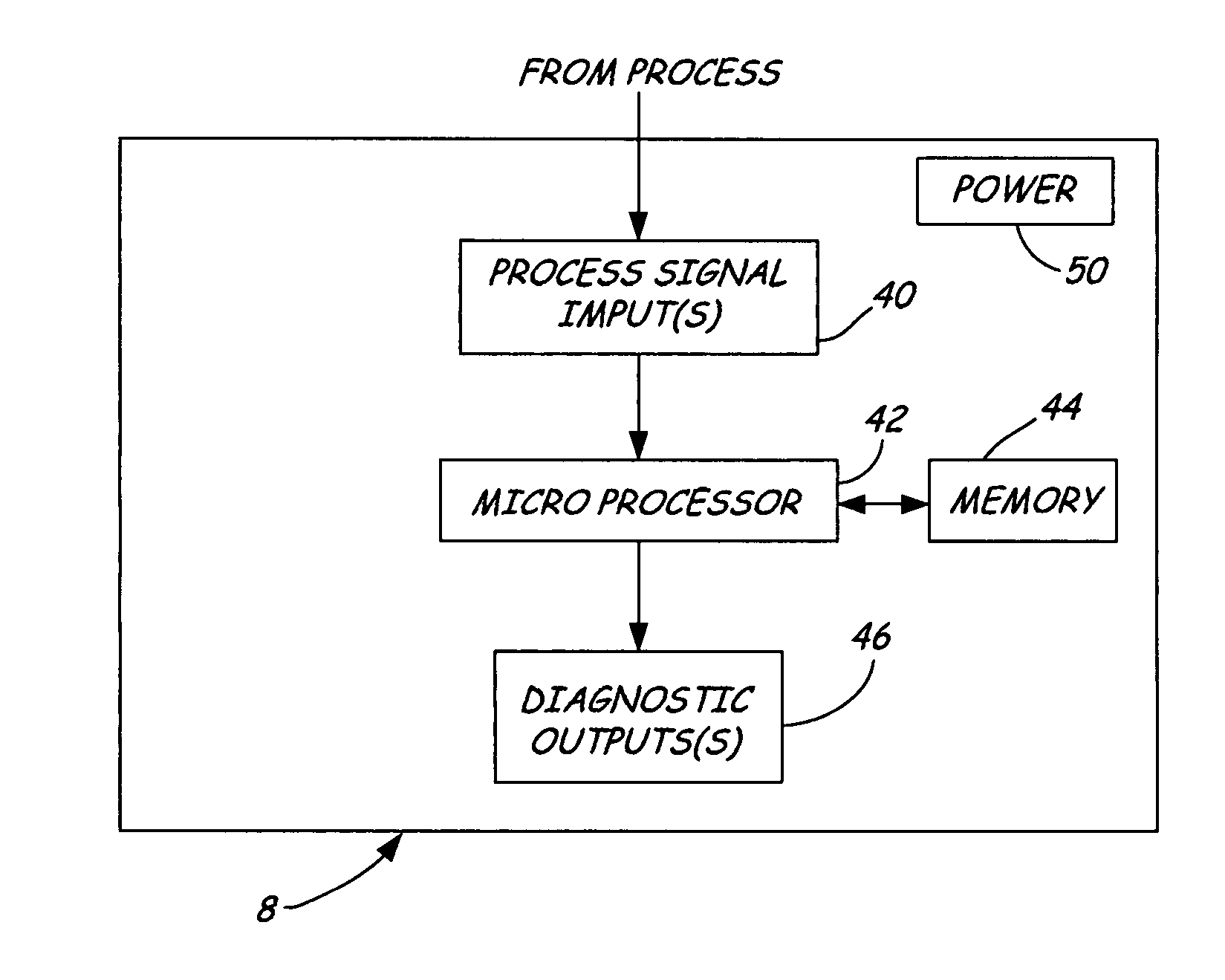
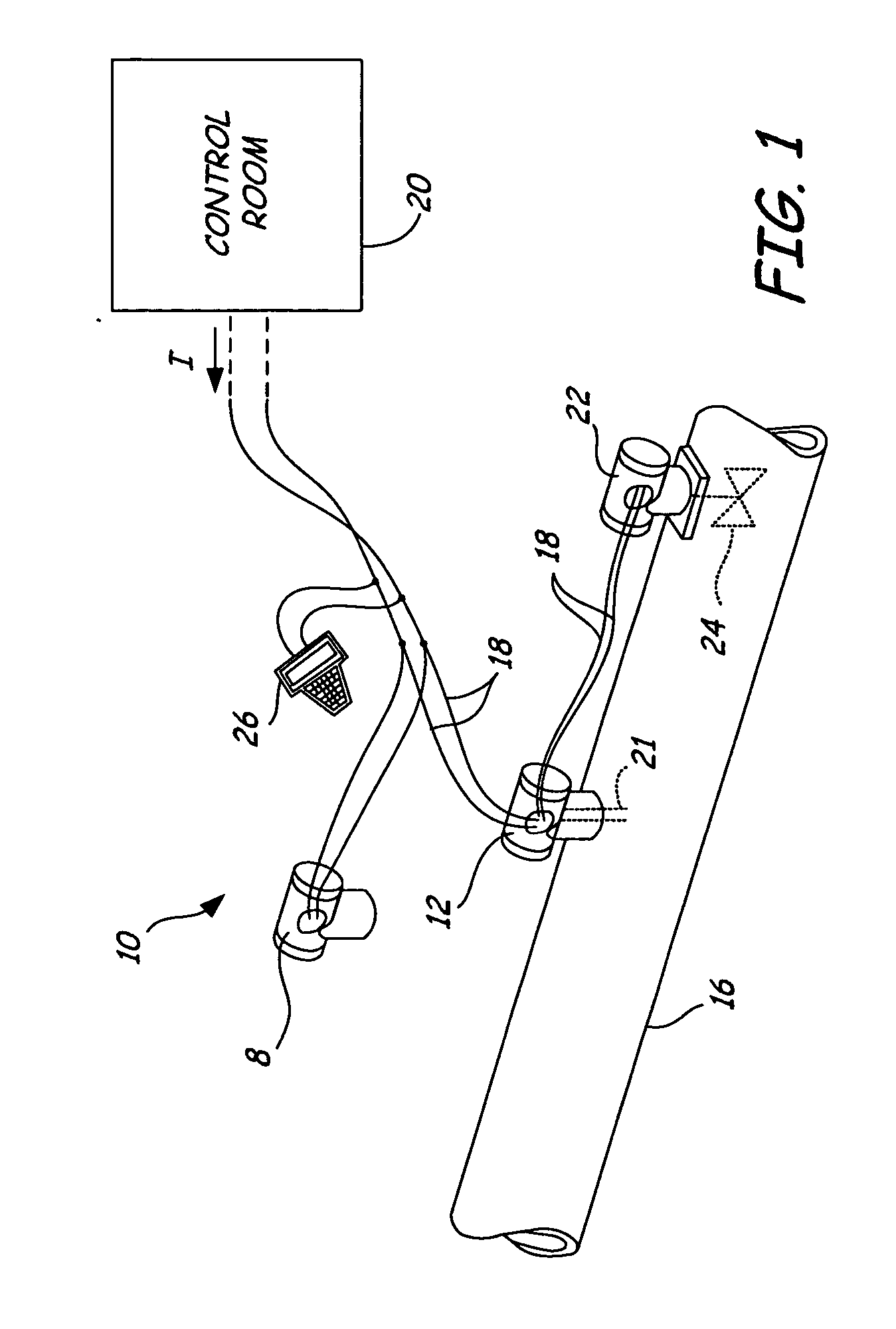
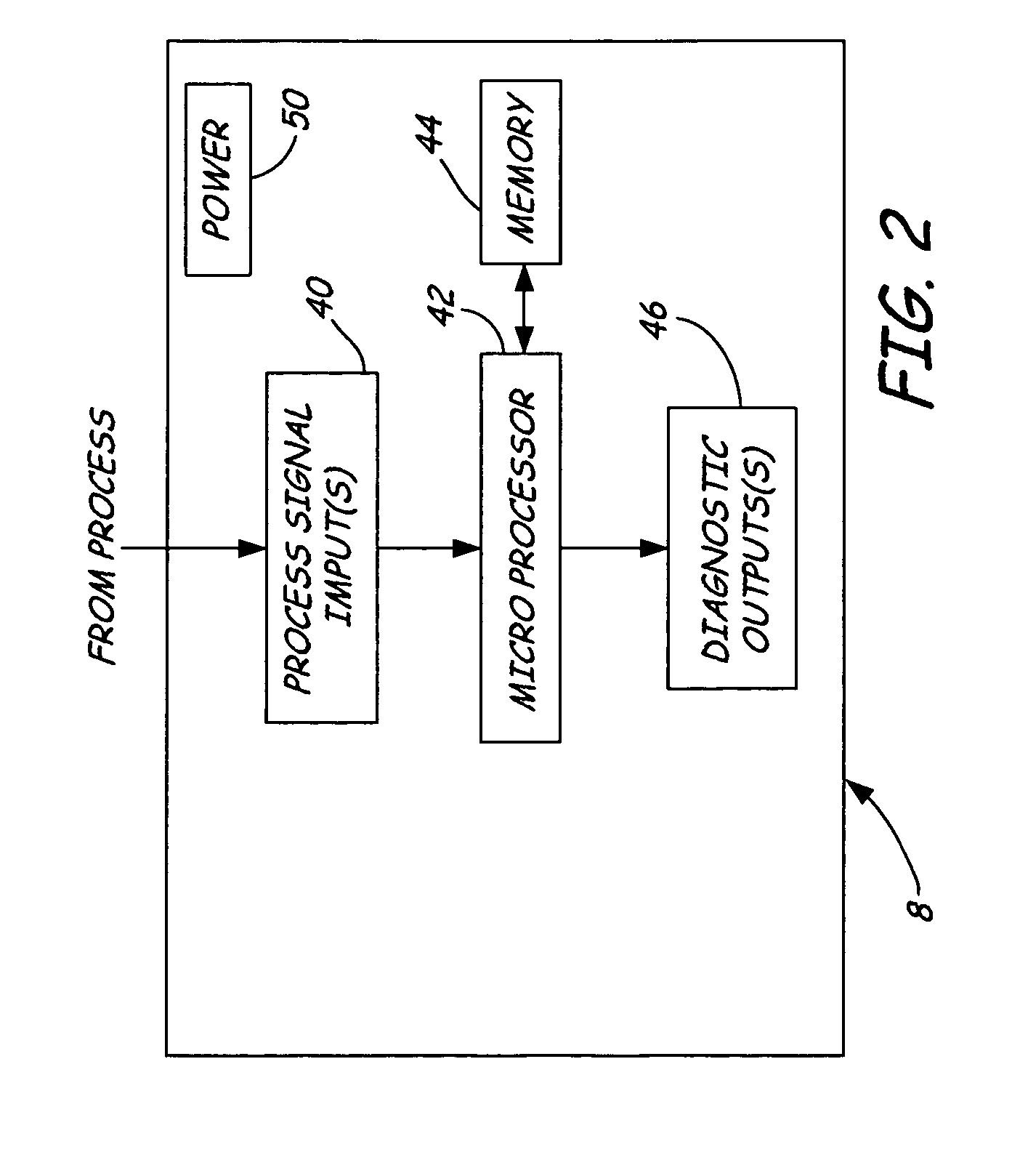
Dedicated process diagnostic device
InactiveUS20070010968A1Testing/calibration apparatusInflated body pressure measurementMicrocontrollerDiagnostic program
A field mountable dedicated process diagnostic device is used for diagnosing operation of an industrial control or monitoring system. An input is configured to receive at least one process signal related to operation of the industrial process. A memory contains diagnostic program instructions configured to implement a diagnostic algorithm using the process signal. The diagnostic algorithm is specific to the industrial process. A microcontroller performs the diagnostic program instructions and responsively diagnoses operation of the process based upon the process signal.
Owner:ROSEMOUNT INC
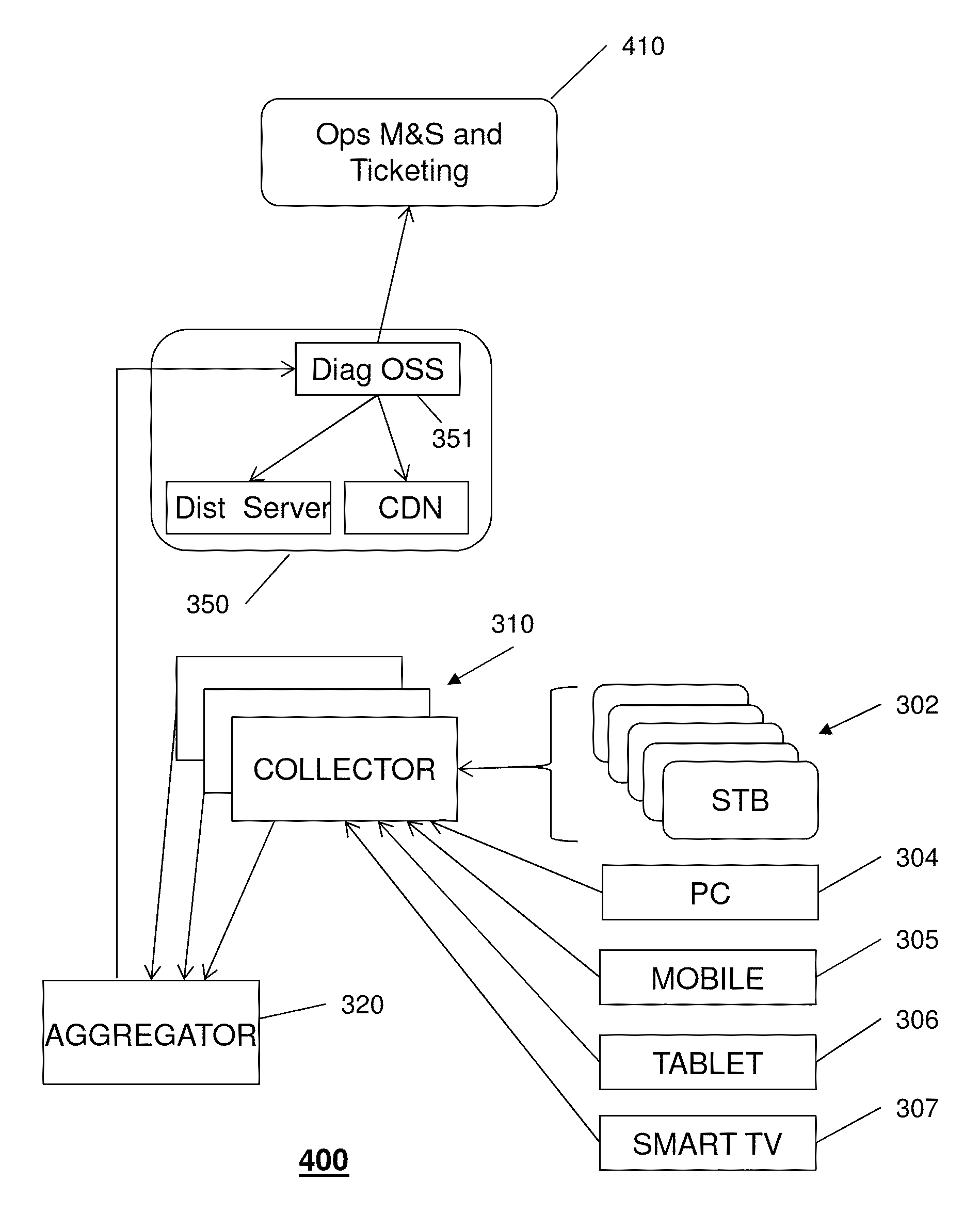
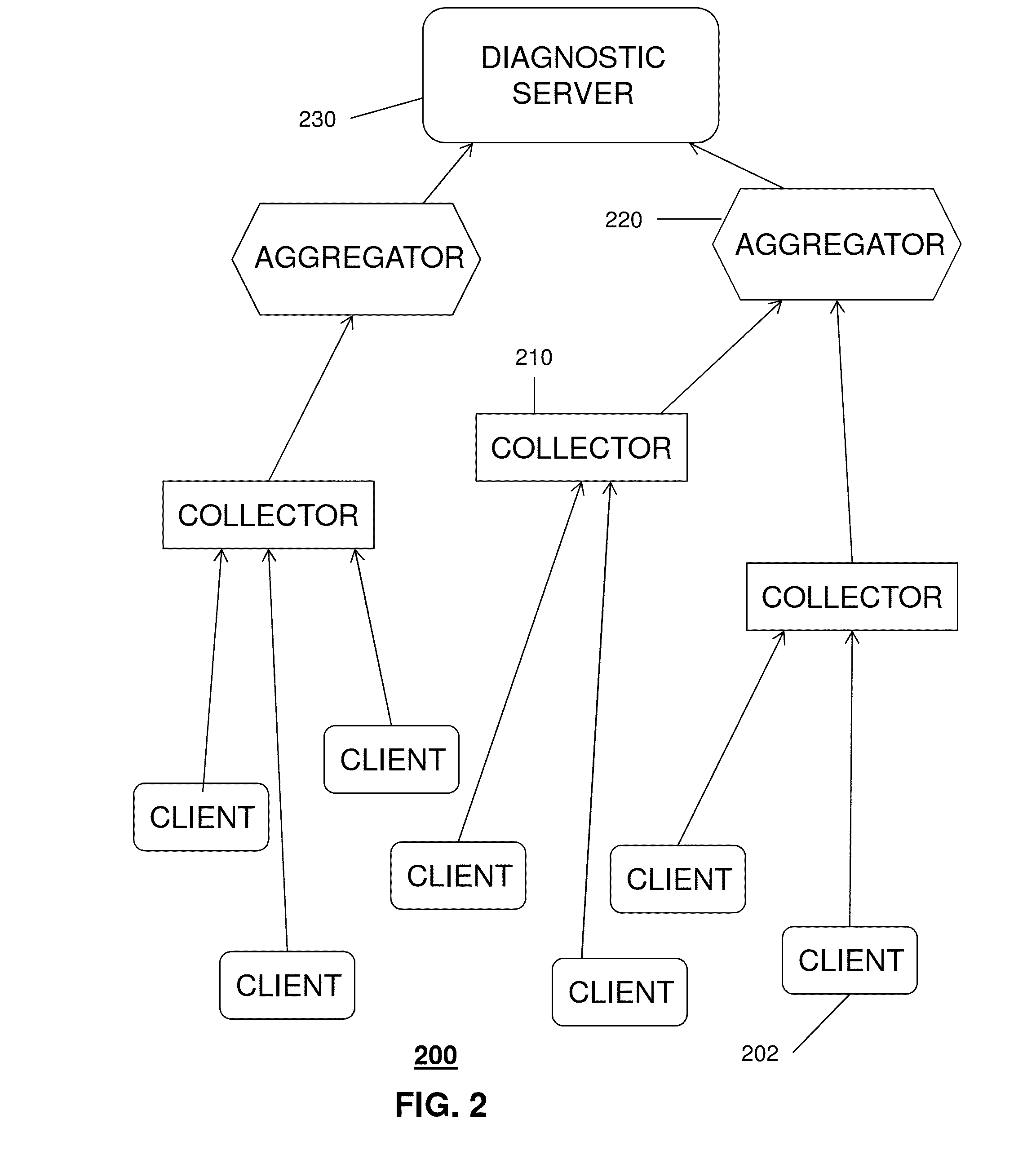
Processing diagnostics of media services
ActiveUS20150095704A1Hardware monitoringNon-redundant fault processingData compressionQuality of service
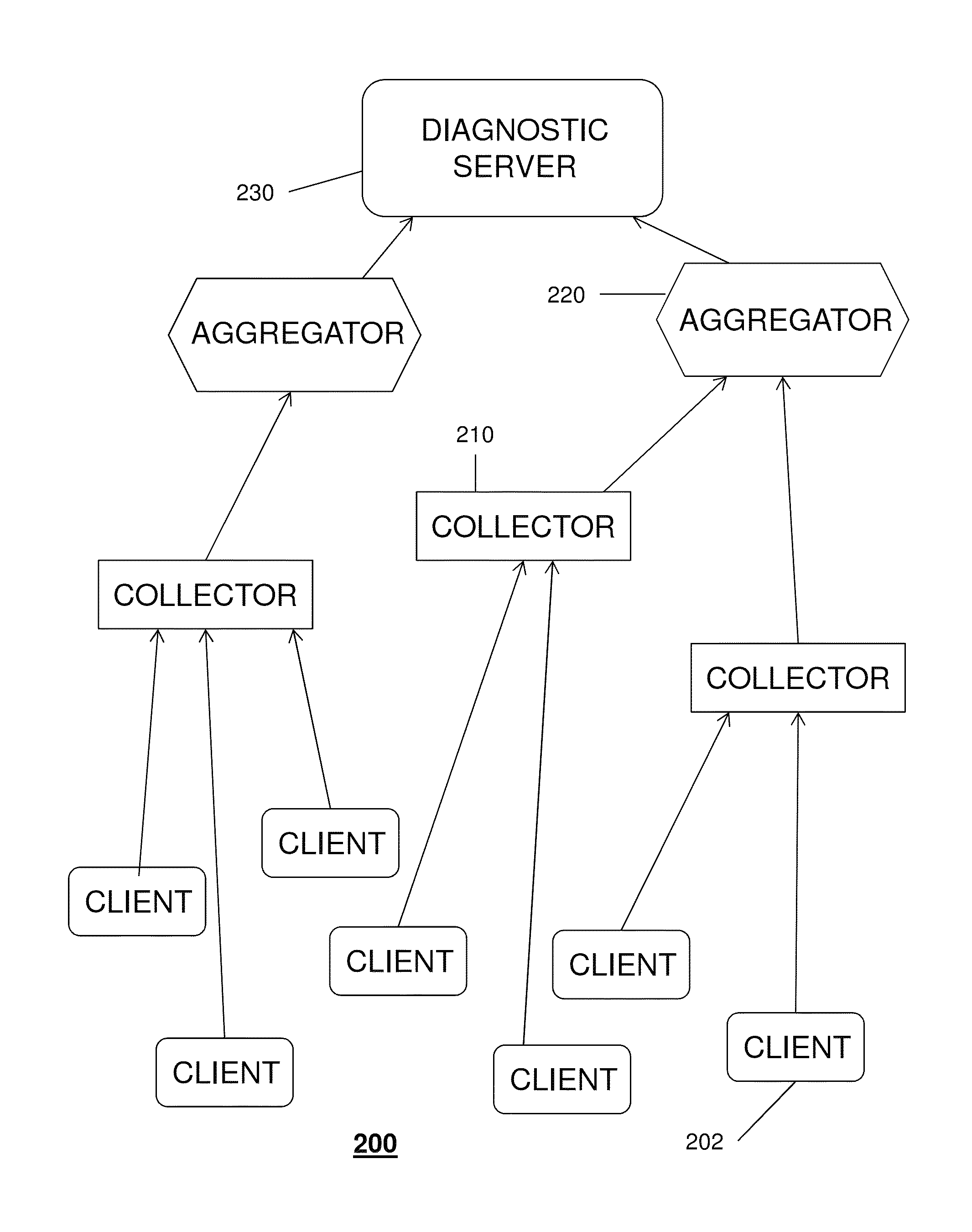
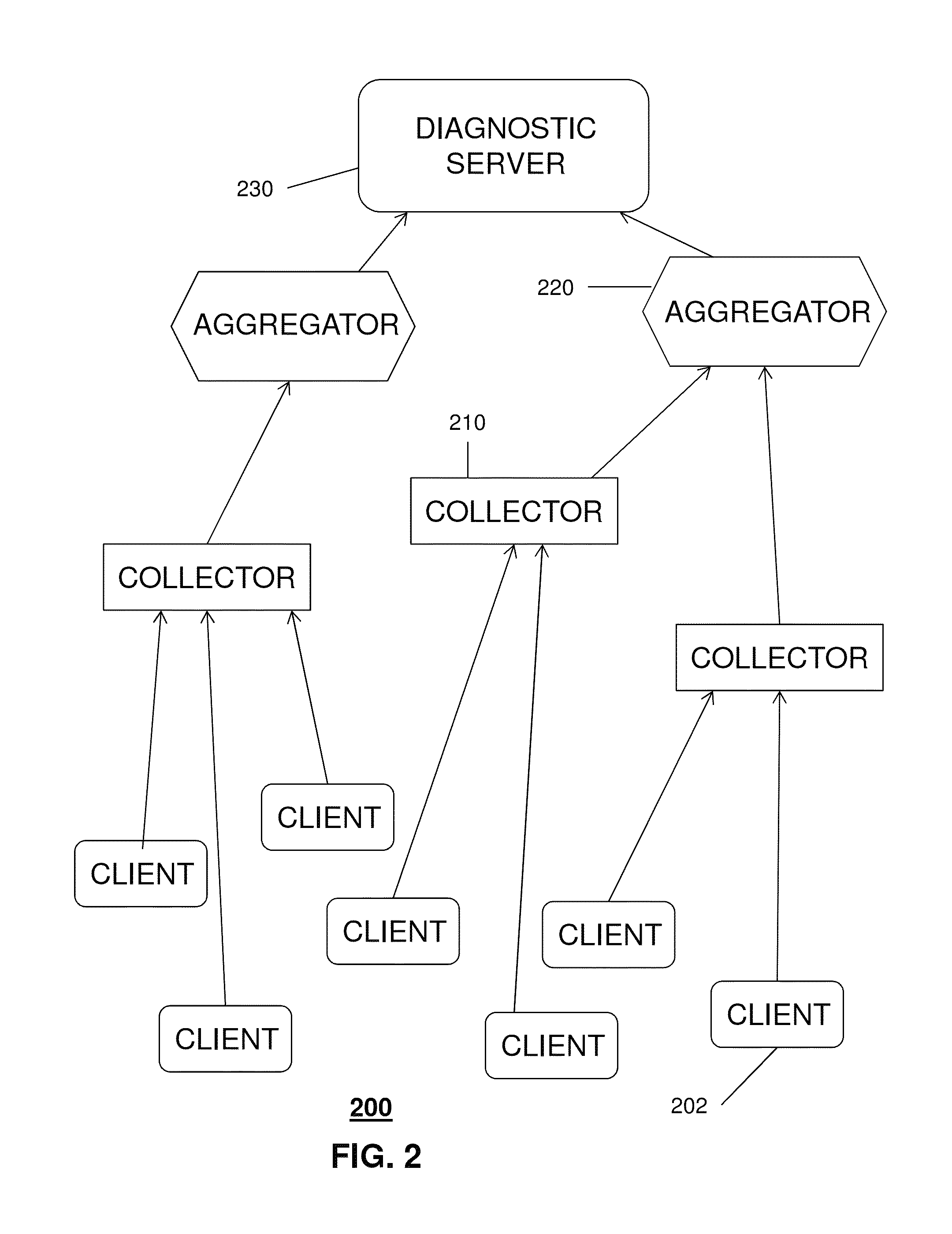
A system that incorporates the subject disclosure may include, for example, a device receiving diagnostic information from a plurality of client devices delivering media content, wherein the diagnostic information relates to a media delivery service quality and wherein the diagnostic information is sent automatically by the client devices; performing a data compression procedure for the diagnostic information; transmitting compressed data comprising the diagnostic information to an aggregator device; and sending to the client devices a message to delay or prevent transmission of additional diagnostic information in accordance with an instruction received from the aggregator device. Other embodiments are disclosed.
Owner:AT&T INTPROP I L P
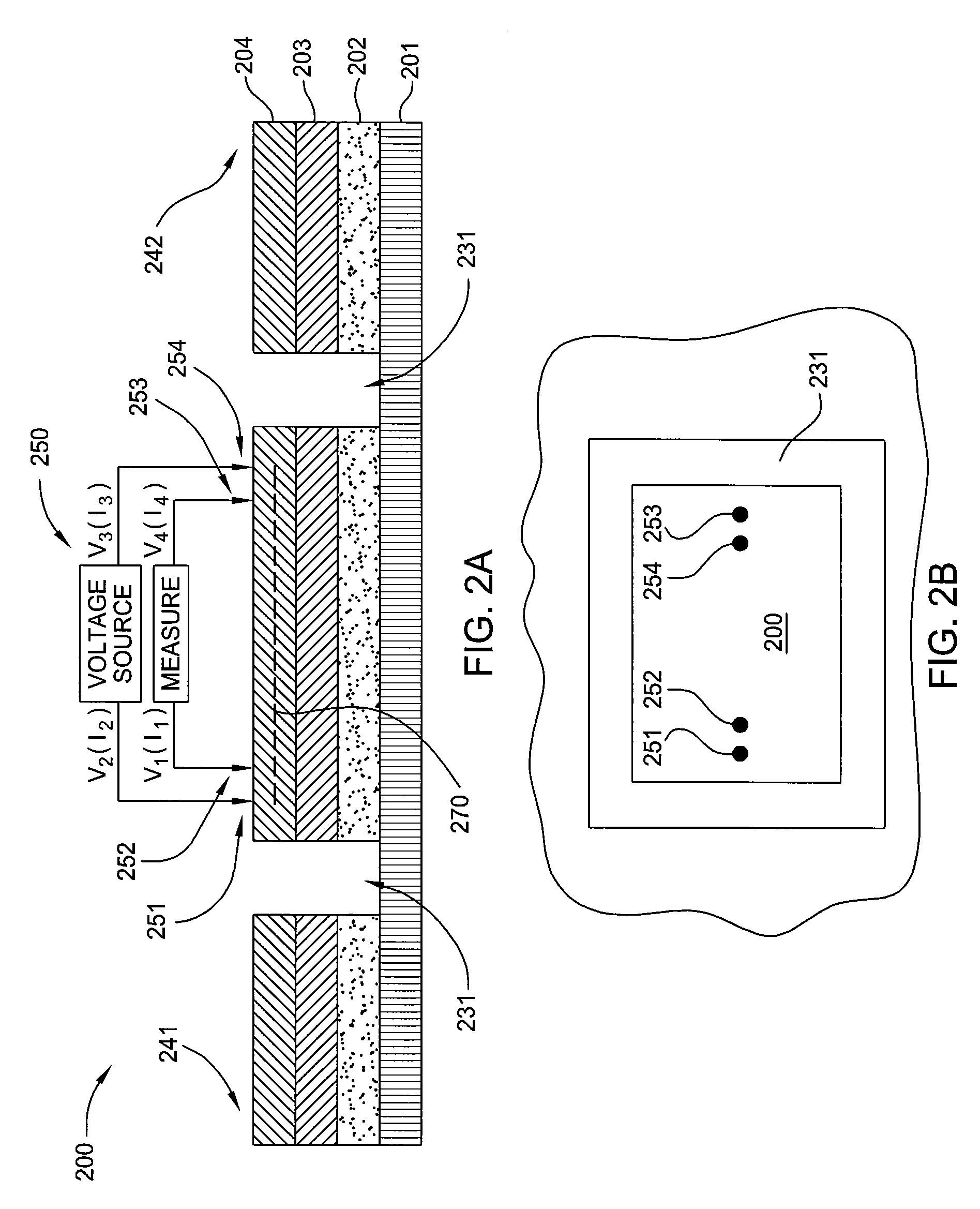
Electrochemical system for analyzing performance and properties of electrolytic solutions
InactiveUS6884333B2Data is very largeAvoid deposit build-upCellsWeather/light/corrosion resistanceElectrochemical responseAlloy
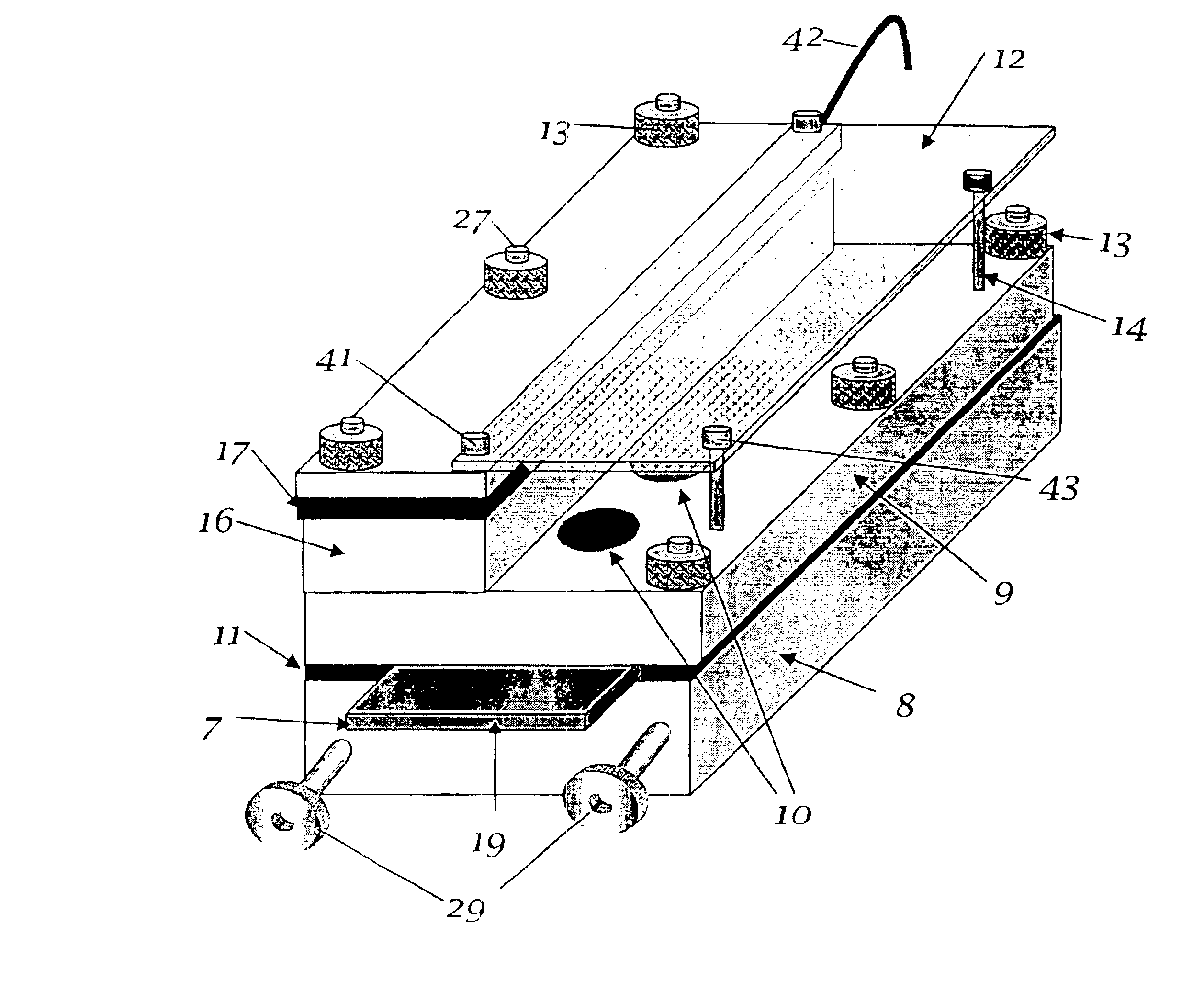
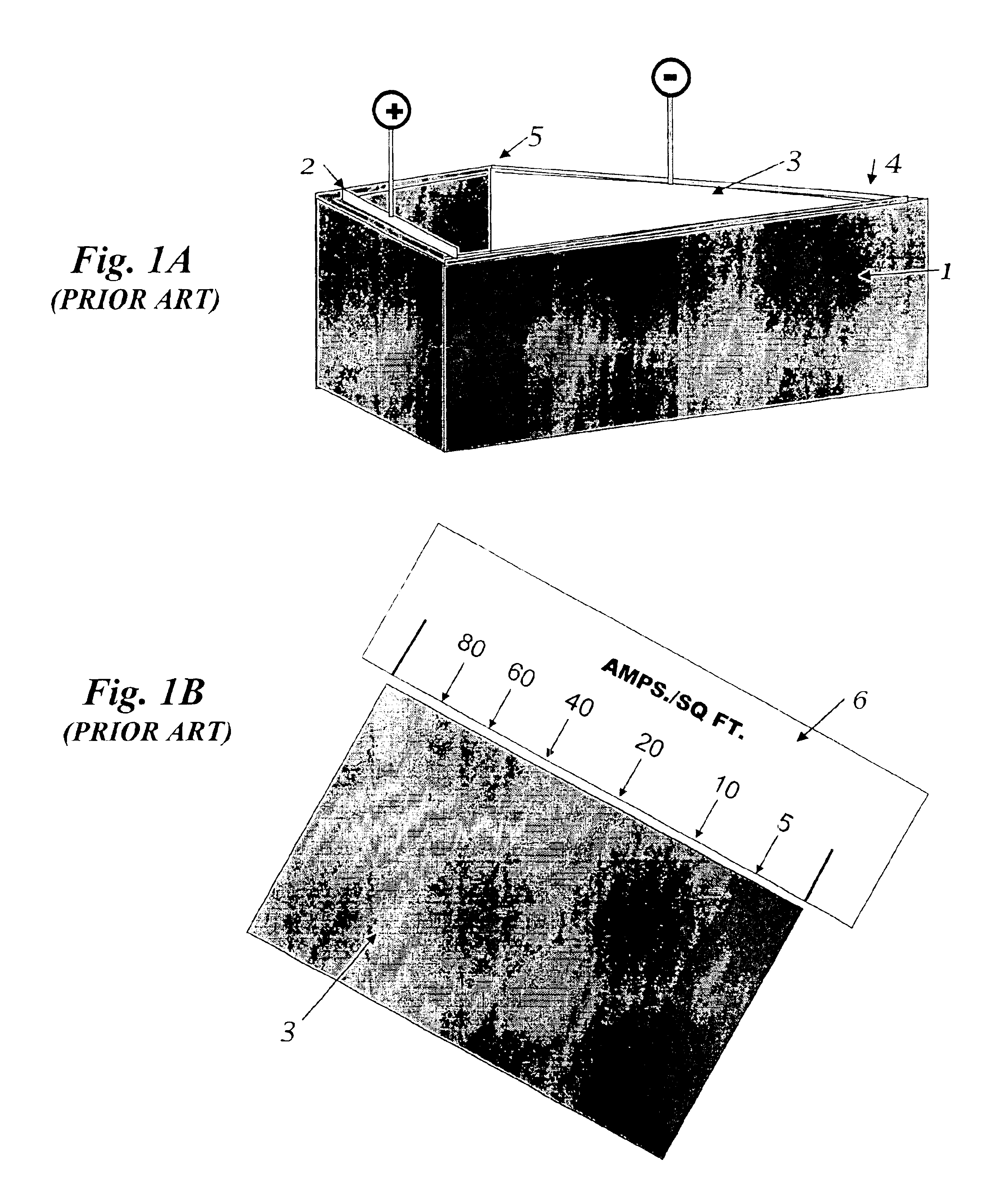
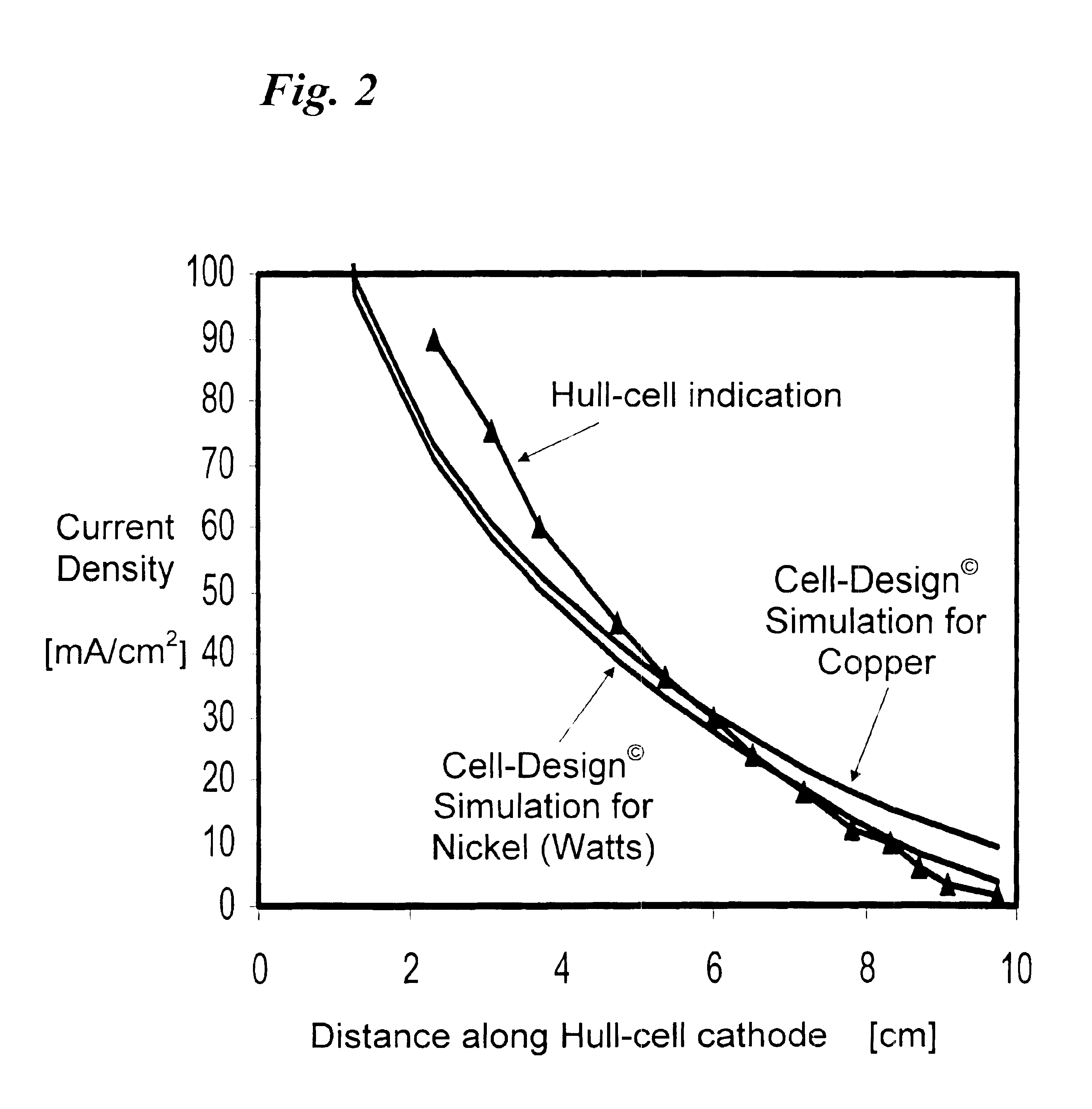
The invention relates to the analysis of the performance and properties of electrochemical processes, and specifically, to electrolytic solutions and electrode processes. The invention discloses a device and a method for obtaining qualitative and quantitative information for the kinetics of the electrode reactions, the transport processes, the thermodynamic properties of the electrochemical processes taking place in the cell. When a deposition reaction takes place, the device provides also valuable information about the relationship between the current density and deposit properties including but not limited to the deposit color, luster, and other aspects of its appearance. The device disclosed herein typically is comprised of a multiplicity of cathodic or anodic regions where one or more electrochemical reactions take place simultaneously, but at a different rate. From the precisely measured segmental currents one can obtain among other process properties: (1) An accurate relationship between the deposit appearance and the current density. This relationship can be used for process diagnostics, troubleshooting, control of concentrations, pH, and additives and contaminants and for optimizing the operating conditions, including the voltage, current, and circulation rate. (2) Quantitative determination of important process parameters including but not limited to, kinetics (e.g., exchange current density, cathodic and anodic transfer coefficients), transport (e.g. conductivity), and thermodynamics (e.g., standard potential). A particularly attractive application of the process is for the quantitative and qualitative processes of alloys plating and for the determination of the relationship between the current efficiency and the applied current density.
Owner:LANDAU UZIEL
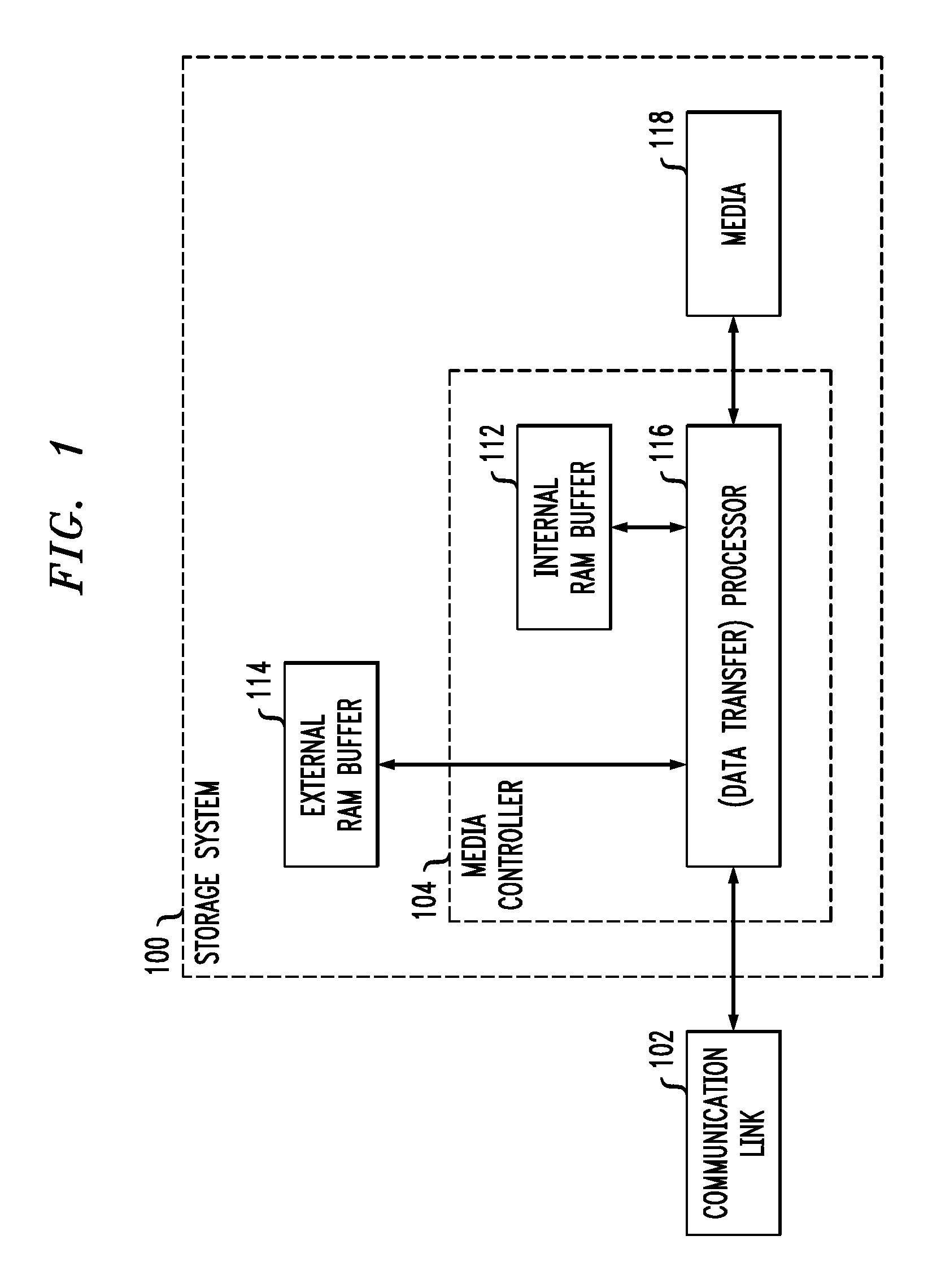
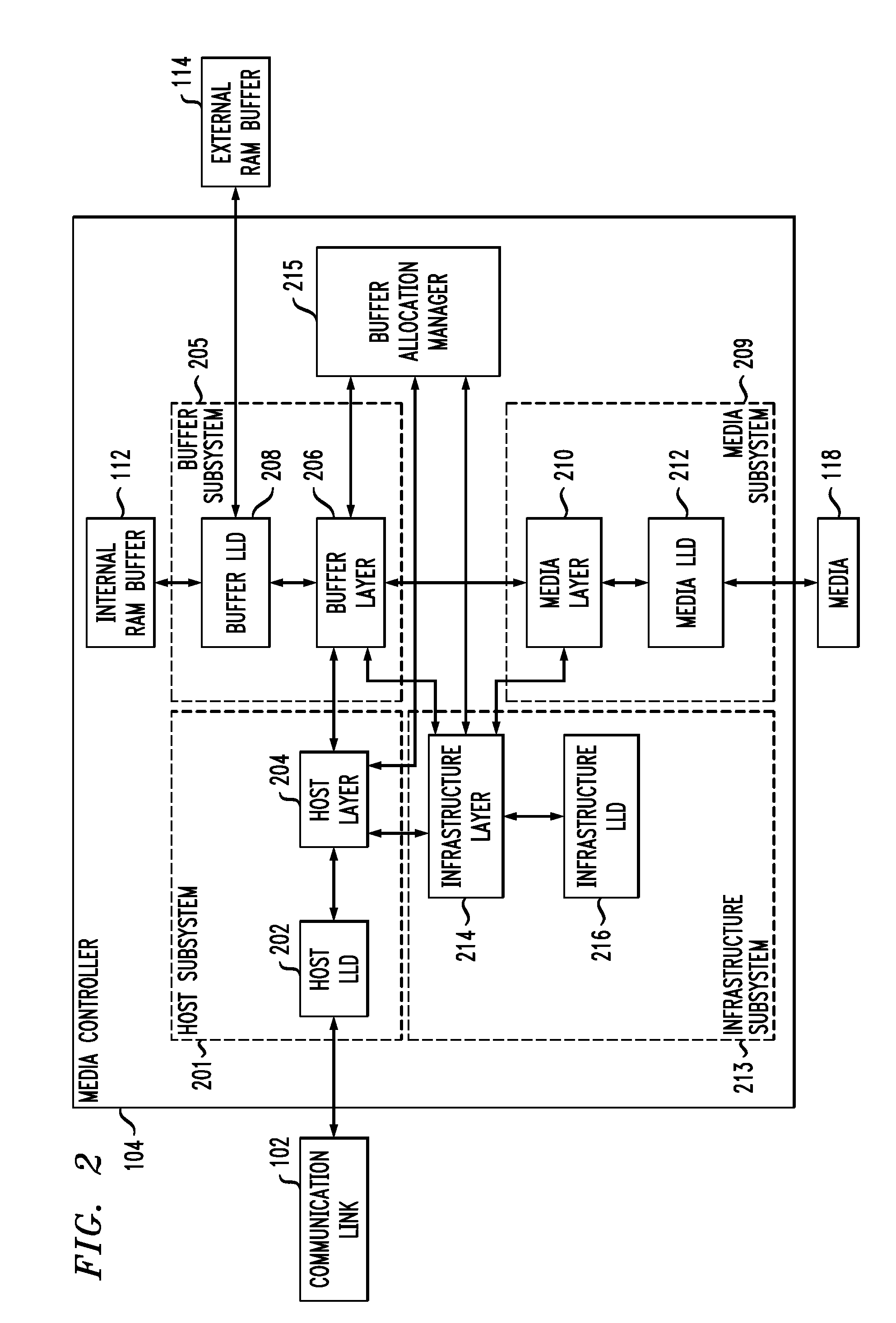
Processing Diagnostic Requests for Direct Block Access Storage Devices
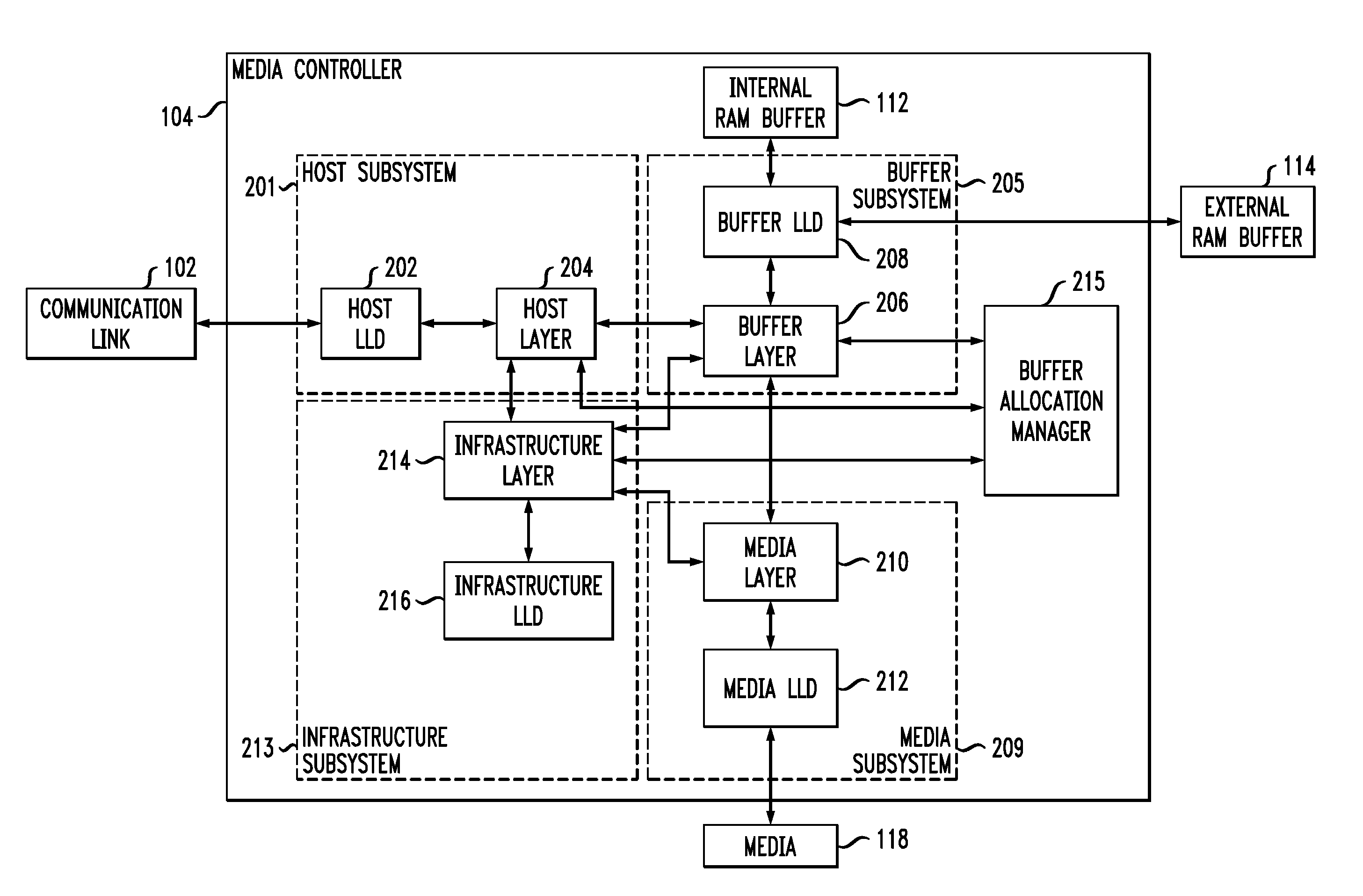
ActiveUS20110072209A1Well formedMemory architecture accessing/allocationError detection/correctionSource typeComputer module
Described embodiments provide a media controller for processing a diagnostic request received from a diagnostic source. The received diagnostic request is parsed by a corresponding request handling module of the media controller, where each diagnostic source type has a corresponding request handling module. If the received diagnostic request requires allocation of buffer space, a common diagnostic handling module of the media controller allocates buffer space in a buffer for the received diagnostic request. The common diagnostic handling module is common for all diagnostic source types. The common diagnostic handling module provides the received diagnostic request to a corresponding one of a plurality of end diagnostic handling modules. The end diagnostic handling module performs the diagnostic tasks. If the received diagnostic request requires a transfer of data to the diagnostic source, the common diagnostic handling module performs the data transfer between the media controller and the diagnostic source.
Owner:AVAGO TECH INT SALES PTE LTD
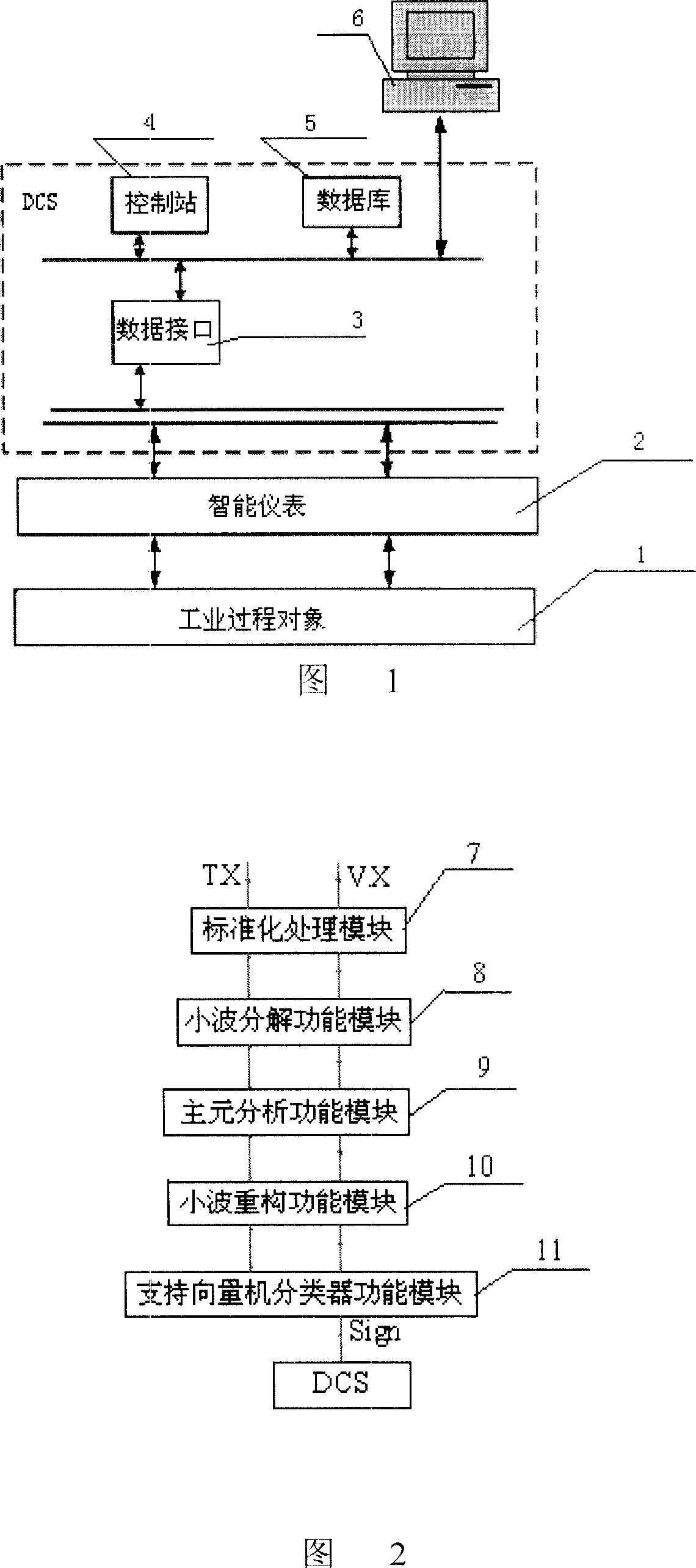
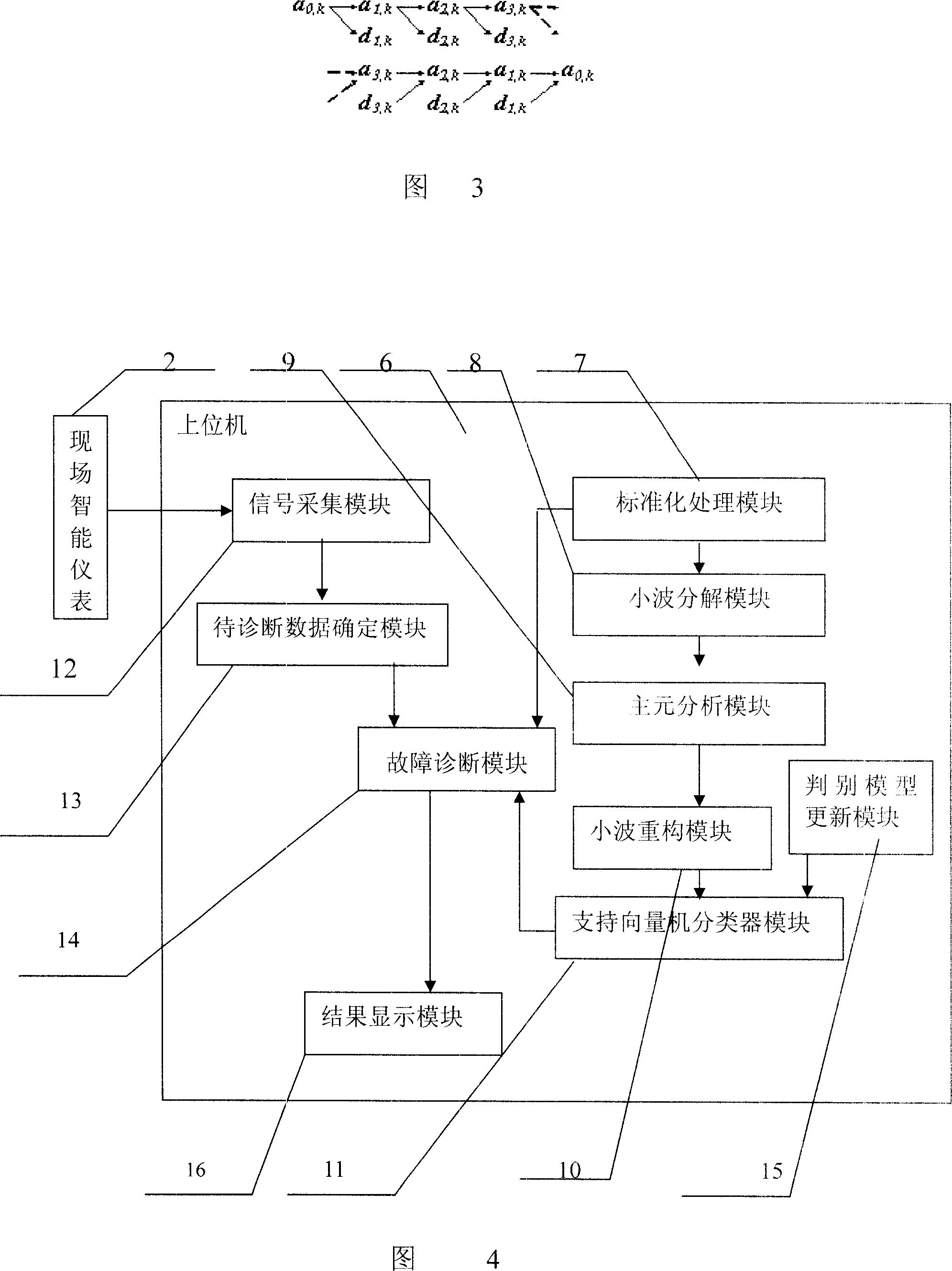
Industrial process fault diagnosis system and method based on wavelet analysis
InactiveCN1996190ASolving nonlinear classification problemsGuide production wellTotal factory controlProgramme total factory controlSupport vector machine classifierProcess failure
An industrial production process diagnostic system based on small wave analysis comprises industrial process object connected on site intelligent meter, DCS system and its upper position control machine with the DCS system made of data interface, control station, data base, the intelligent meter, DCS system and the upper position control machine connected sequentially, with the said upper position control machine composed of standardized handling module, small wave dissolving module, pivot element analysis function module, small wave restructuring module, support vector machine classifier module and diagnostic judging module. It also puts forward a failure diagnostic method. It provides an industrial production process failure diagnostic system and method with good diagnostic effect based on small wave analysis.
Owner:ZHEJIANG UNIV
Method and application of metrology and process diagnostic information for improved overlay control
InactiveUS6868301B1ConfidenceFavorable for determinationSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingMetrologyComputer science
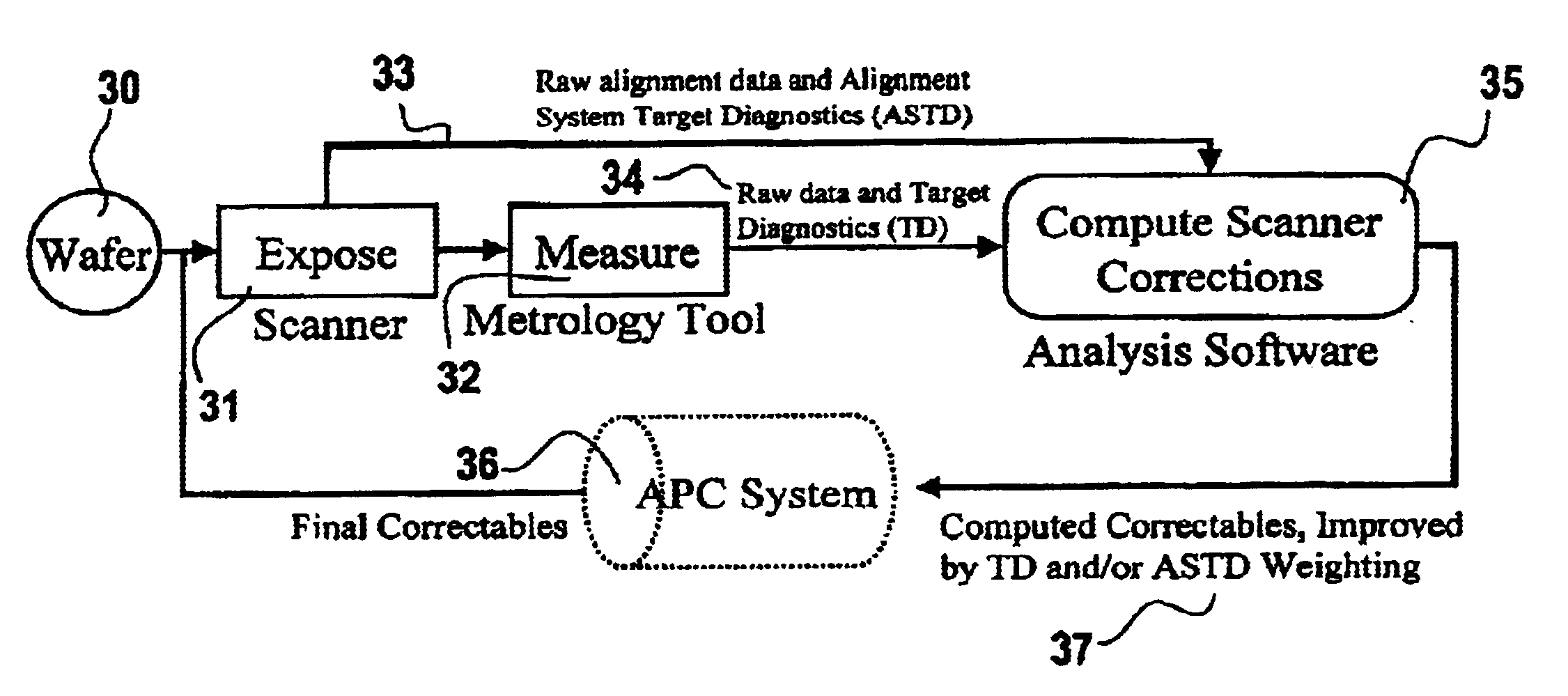
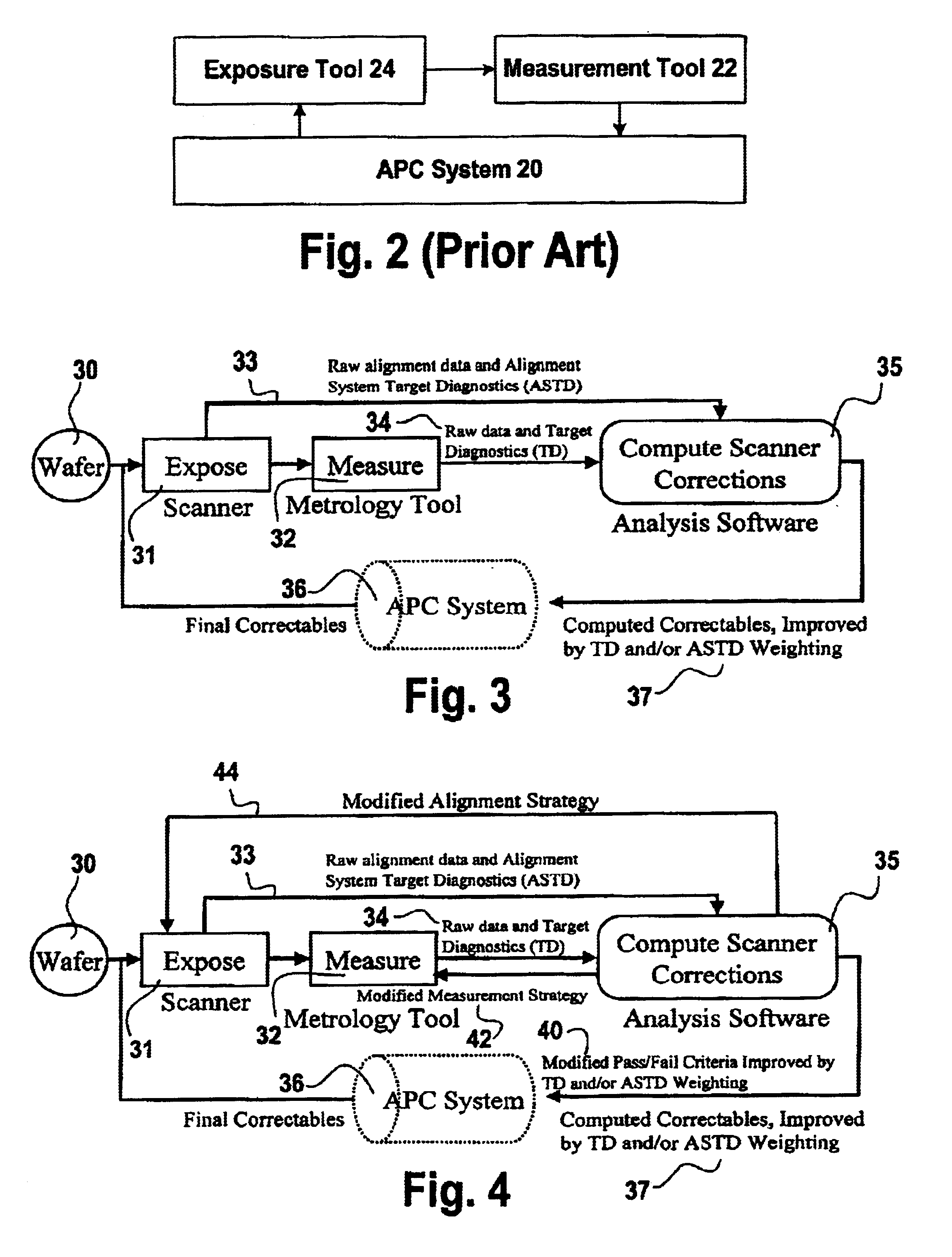
A method for operation of an exposure tool in the fabrication of an integrated circuit to control registration between a preceding layer of and a succeeding layer. The preceding layer having a first alignment mark and a first registration mark. The succeeding layer is aligned to the preceding layer using an exposure tool. The succeeding layer has a second alignment mark and a second registration mark. The exposure tool measures alignment of the first alignment mark relative to the second alignment mark. After additional process steps are performed, a measurement tool measures registration relating to relative positions of the first registration mark and the second registration mark. Both the alignment information from the exposure tool and the registration information from the measurement tool is analyzed to determine corrections to improve registration between the layers, and the operation of the exposure tool is altered to improve registration.
Owner:KLA TENCOR TECH CORP
Dedicated process diagnostic device
InactiveUS7630861B2Testing/calibration apparatusInflated body pressure measurementDiagnostic programProgram instruction
A field mountable dedicated process diagnostic device is used for diagnosing operation of an industrial control or monitoring system. An input is configured to receive at least one process signal related to operation of the industrial process. A memory contains diagnostic program instructions configured to implement a diagnostic algorithm using the process signal. The diagnostic algorithm is specific to the industrial process. A microprocessor performs the diagnostic program instructions and responsively diagnoses operation of the process based upon the process signal.
Owner:ROSEMOUNT INC
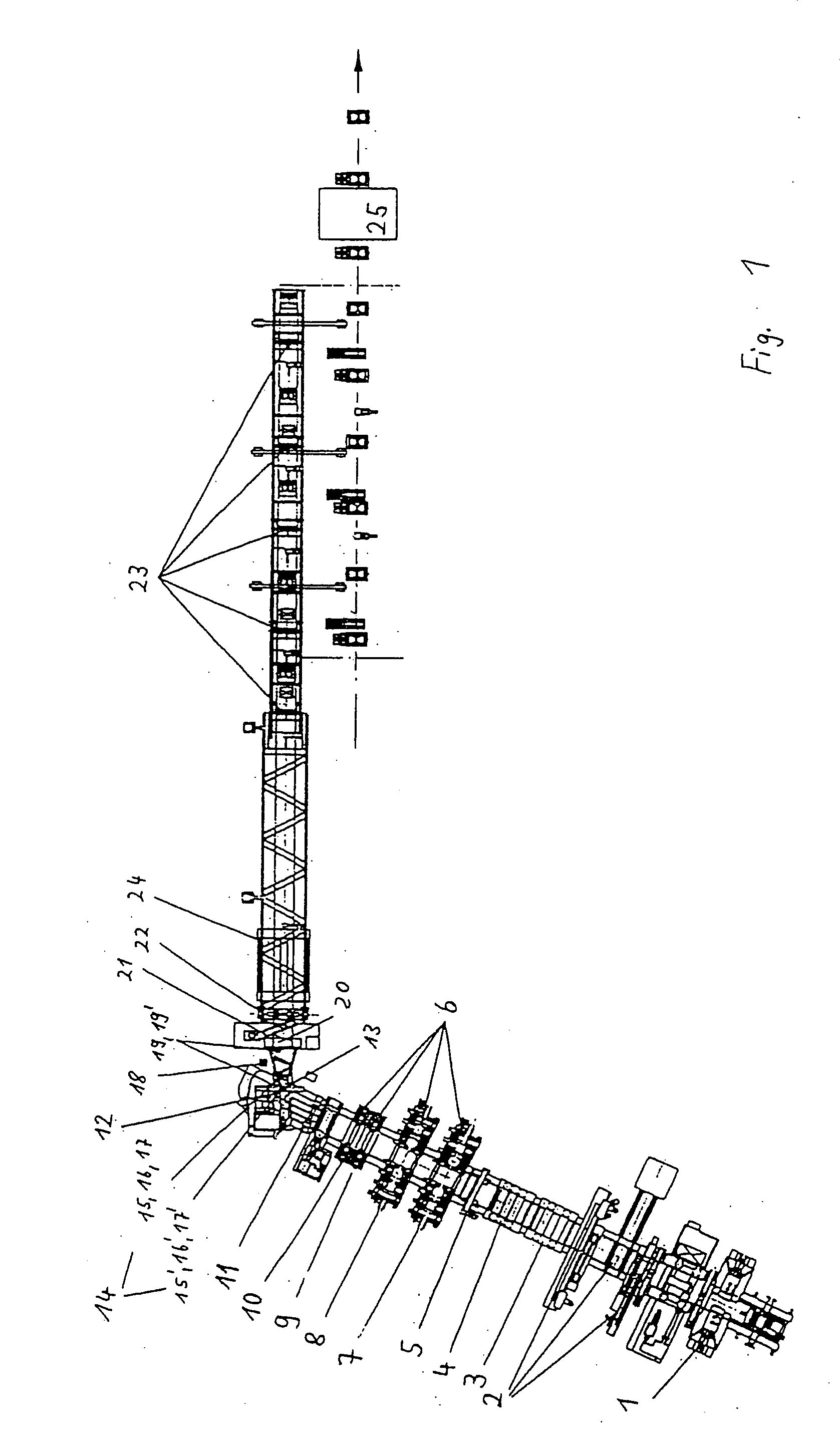
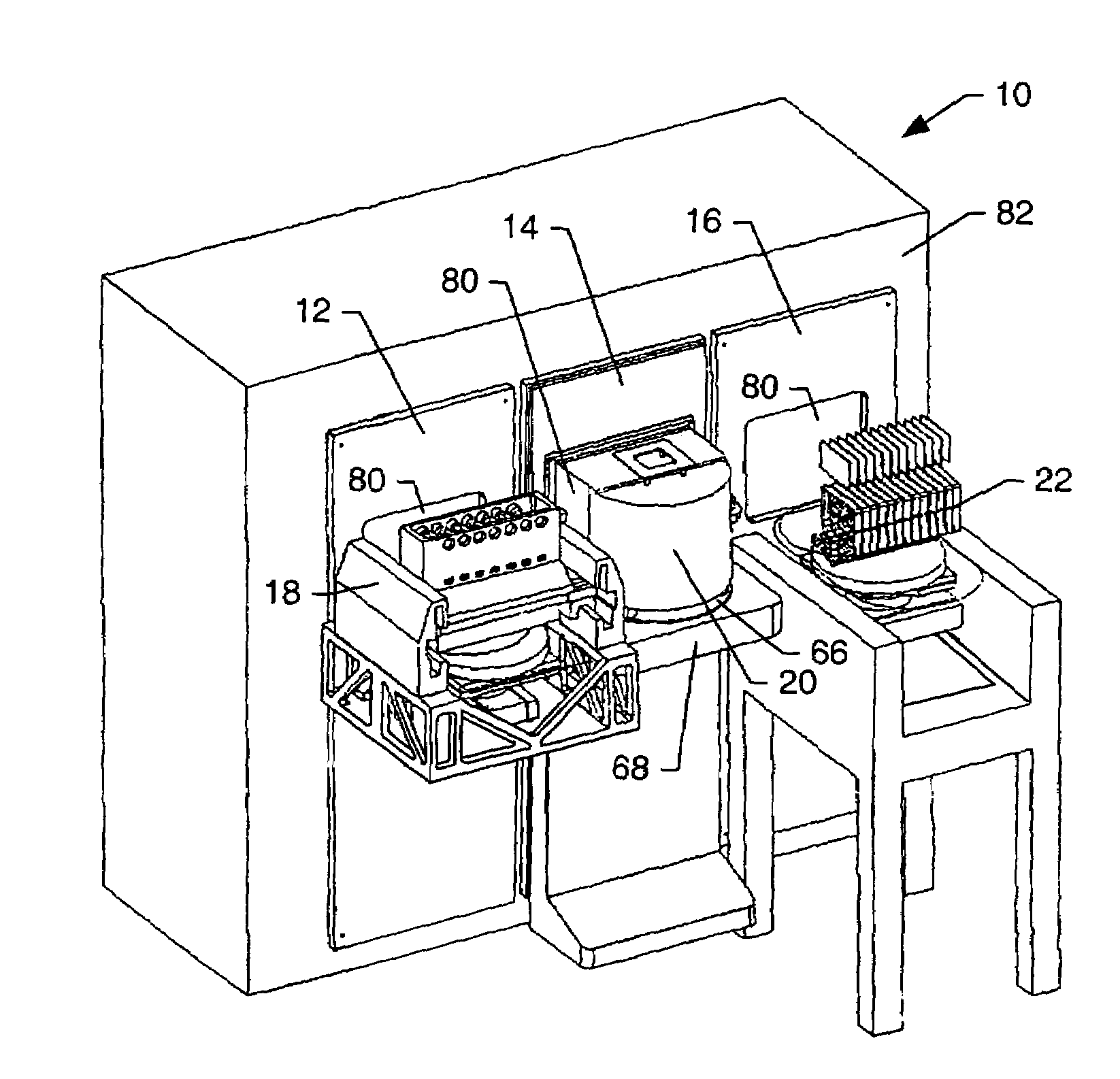
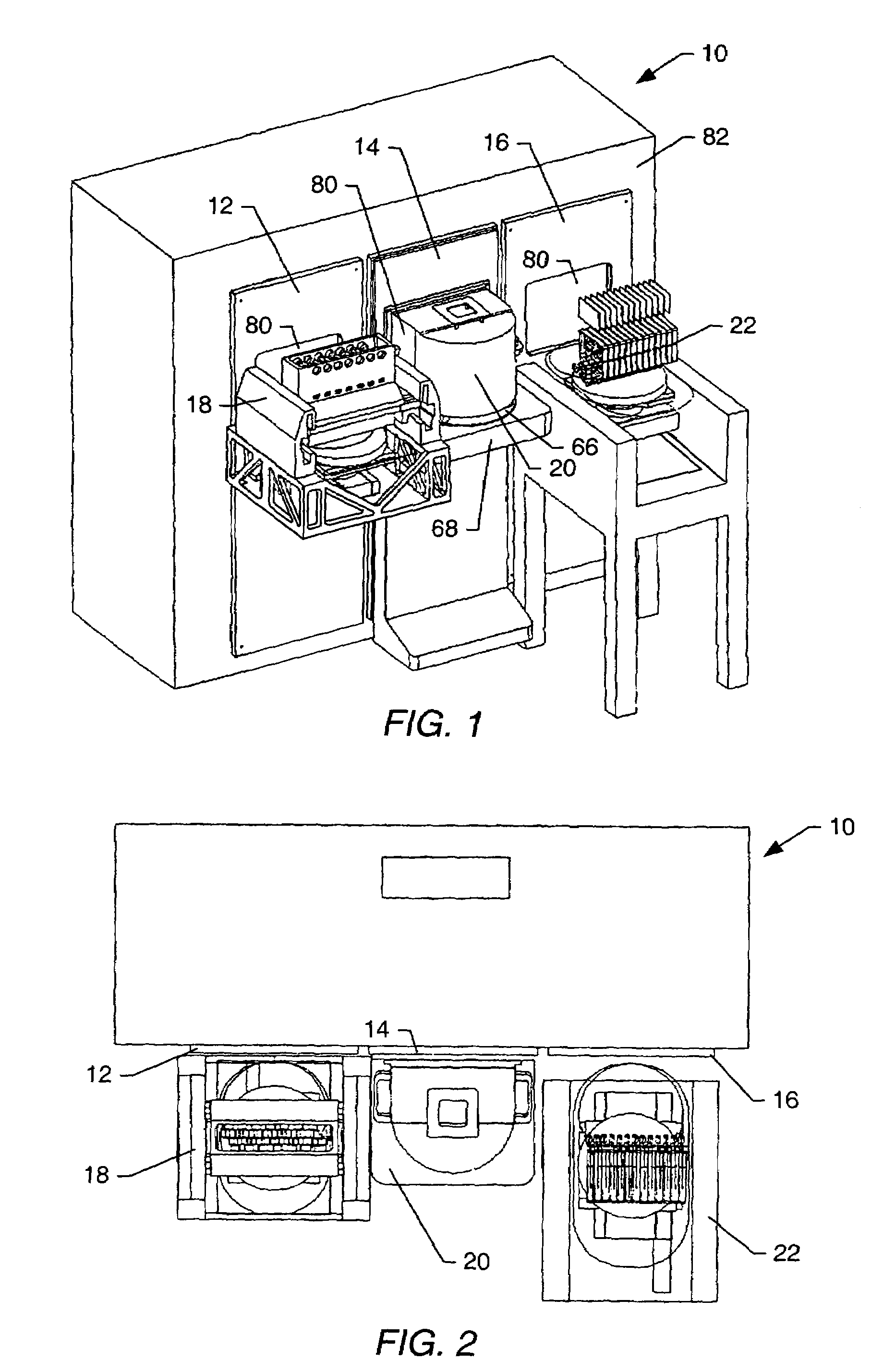
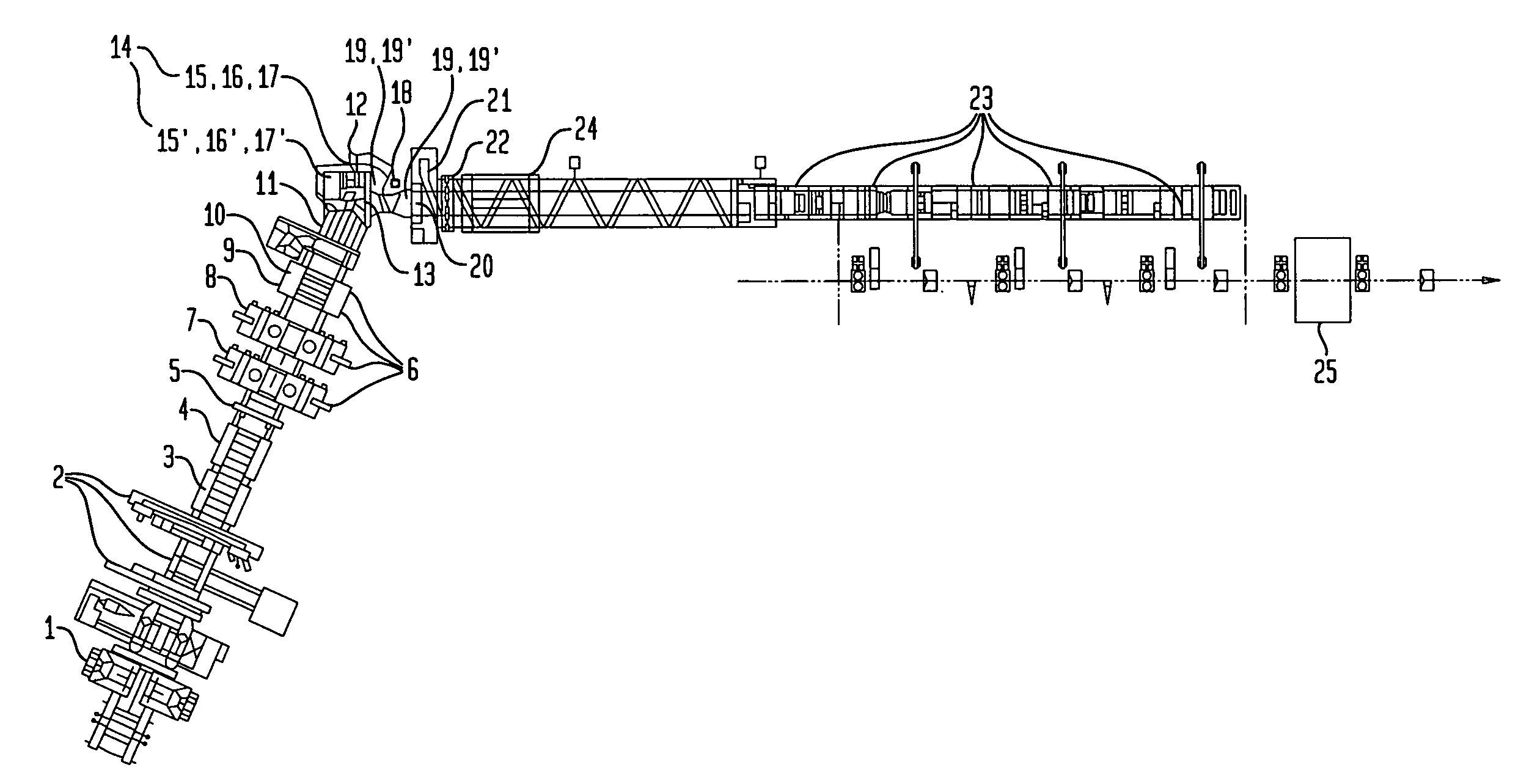
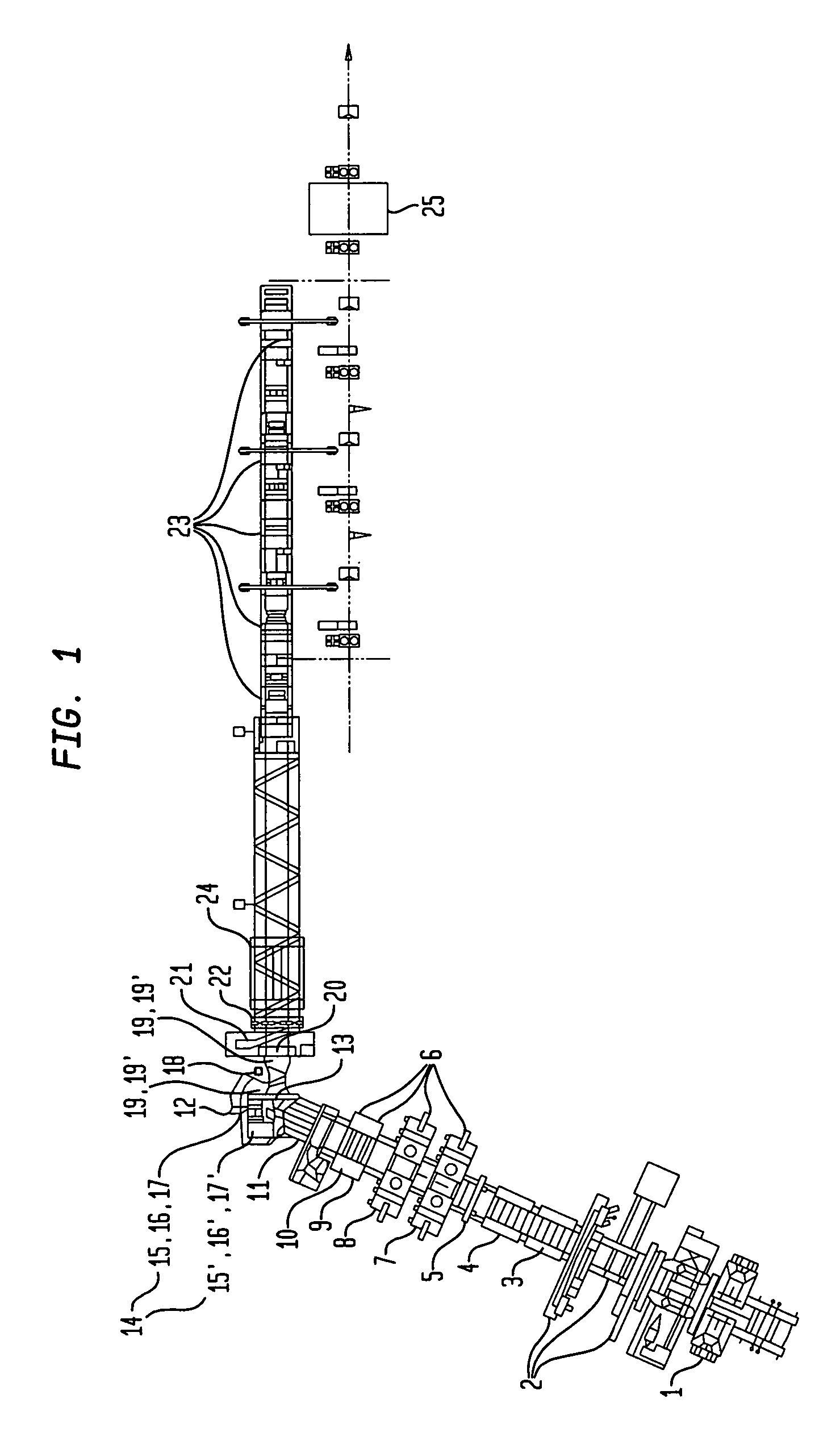
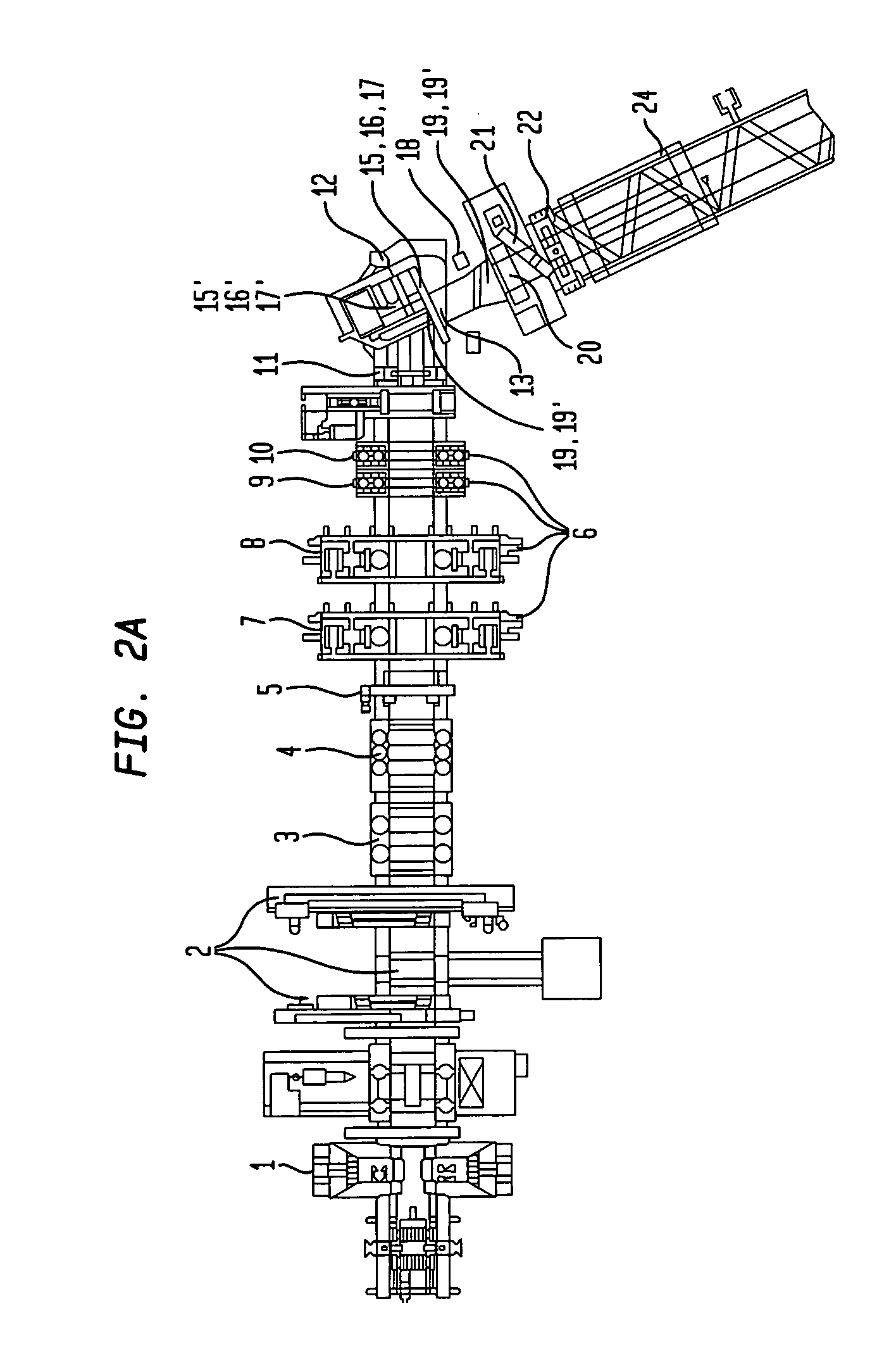
Method and apparatus for making welded large pipes
InactiveUS20050035094A1Inhibition formationPrecise bendingPlasma welding apparatusWelding/soldering/cutting articlesEngineeringWeld seam
In a method and apparatus for making welded large pipes, a leading end of a hot strip is connected to a trailing end of a leader strip and then subjected to a two-stage leveling for strip flatness in transverse direction and strip flatness in longitudinal direction. The entire surface of the hot strip including strip edges thereof is inspected by ultrasound and the strip edges are prepared in four stages before being pre-bent. The hot strip is then shaped into a slotted tube and the strip edges are welded along the inner and outer sides by laser to produce the pipe. Online process diagnostic is provided for monitoring the welding step and the finished welded pipe diameter is measured through online measurement. Online determination of a profile of a welded seam configuration and computer tomographic determination and evaluation of flaws inside the pipe, as well as ultrasonic inspection of the welded seam are further provided.
Owner:MANNESMANN ROHRENWERKE
Information acquisition device of machine tool
InactiveCN106406228ARealize regular recordsReduce capacityProgramme controlComputer controlInstruction unitMachine diagnostics
The invention provides an information acquisition device of a machine tool that can preferably acquire machine information required for detailed process diagnostics and machine diagnostics. An NC device (10) has a machine information acquisition unit (13), a machine information clipping unit (18) correspondingly outputting time-series data and event data of machine information together, and a monitor. The machine information acquisition unit (13) includes a time series information recording unit (13A) acquiring machine information such as a main spindle load, loads of the respective feed axes, command values of the main spindle and the feed axes, or similar information from the machine operation instruction unit (14) at every any time, and an event information recording unit (13B) acquiring event information such as a program name, a tool number, and an override value through the operator's operation, or similar information from the machine operation instruction unit (14) at a time of change together with the change time.
Owner:OKUMA CORP
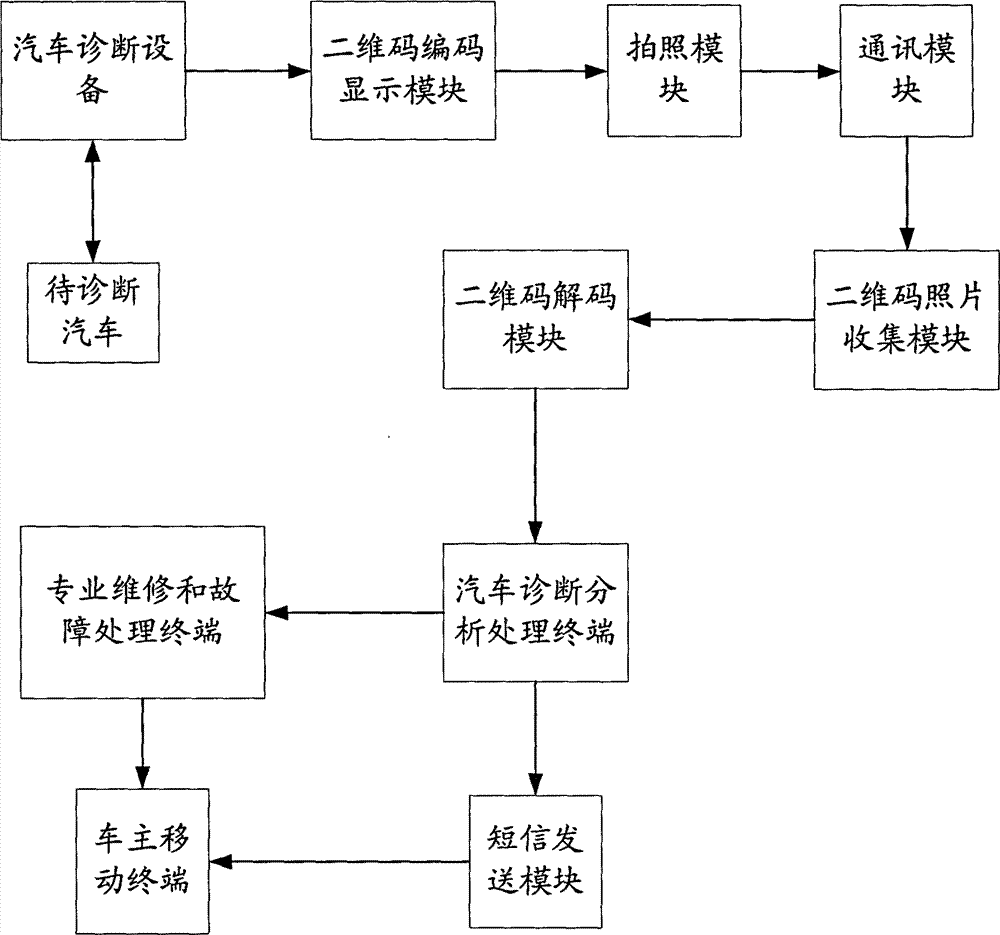
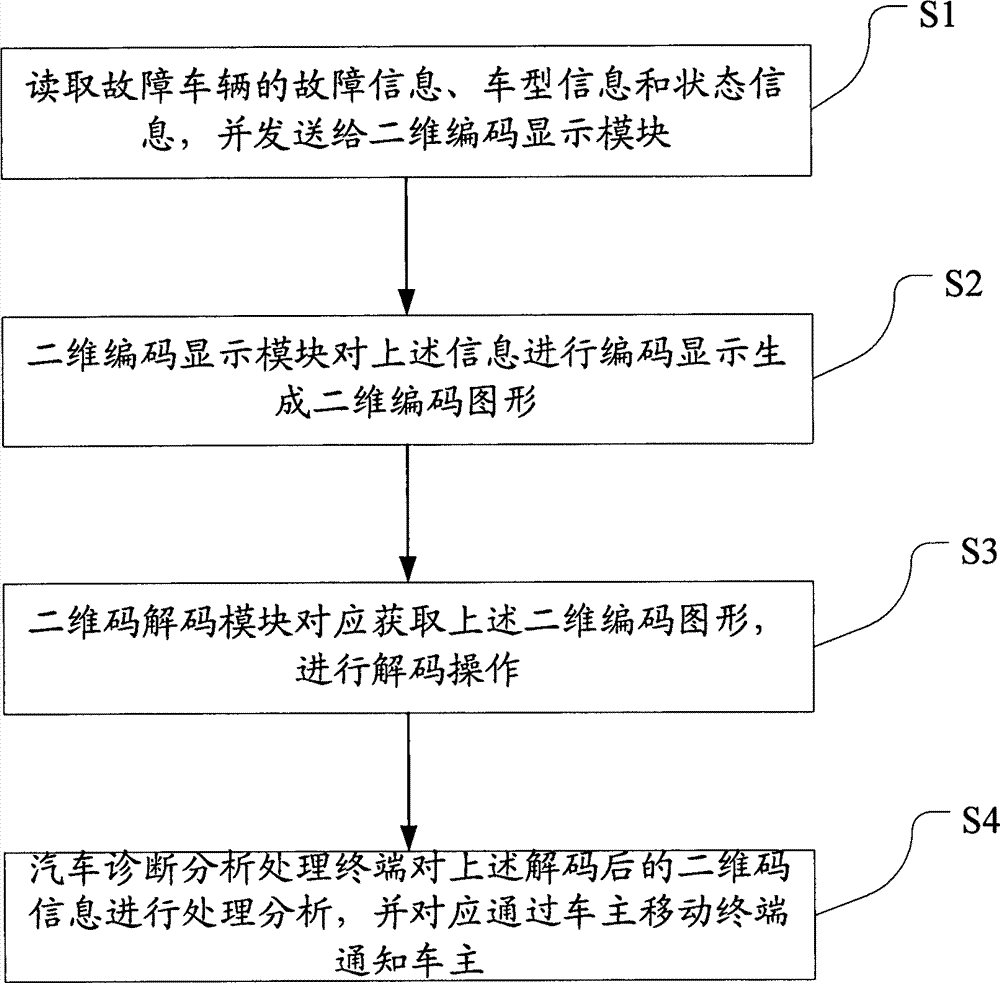
Vehicle diagnostic device and method based on two-dimensional code technology
InactiveCN103164724AImprove the ability to handle faultsImprove troubleshooting efficiencyMessaging/mailboxes/announcementsRecord carriers used with machinesCommunication deviceInformation handling
The invention discloses a vehicle diagnostic device and a method based on two-dimensional code technology. The method comprises the steps of processing diagnostic information obtained from vehicle diagnostic equipment to form a two-dimensional image, sending to a decoding module to conduct decoding treatment after shooting, sending and collecting, being obtained by a vehicle diagnostic analysis treatment terminal, informing a vehicle owner through a message or conducting treatment through a professional maintenance or fault treatment terminal, and putting forward a plan correspondingly. Technical methods of the two-dimensional code, shooting, communication equipment and the like are adopted to enable the vehicle owner to transmit professional vehicle diagnostic fault and state information to fault maintenance and treatment persons, capacity of vehicle fault treatment is greatly improved, and at the same time, fault treatment efficiency is improved.
Owner:LAUNCH SOFTWARE DEV
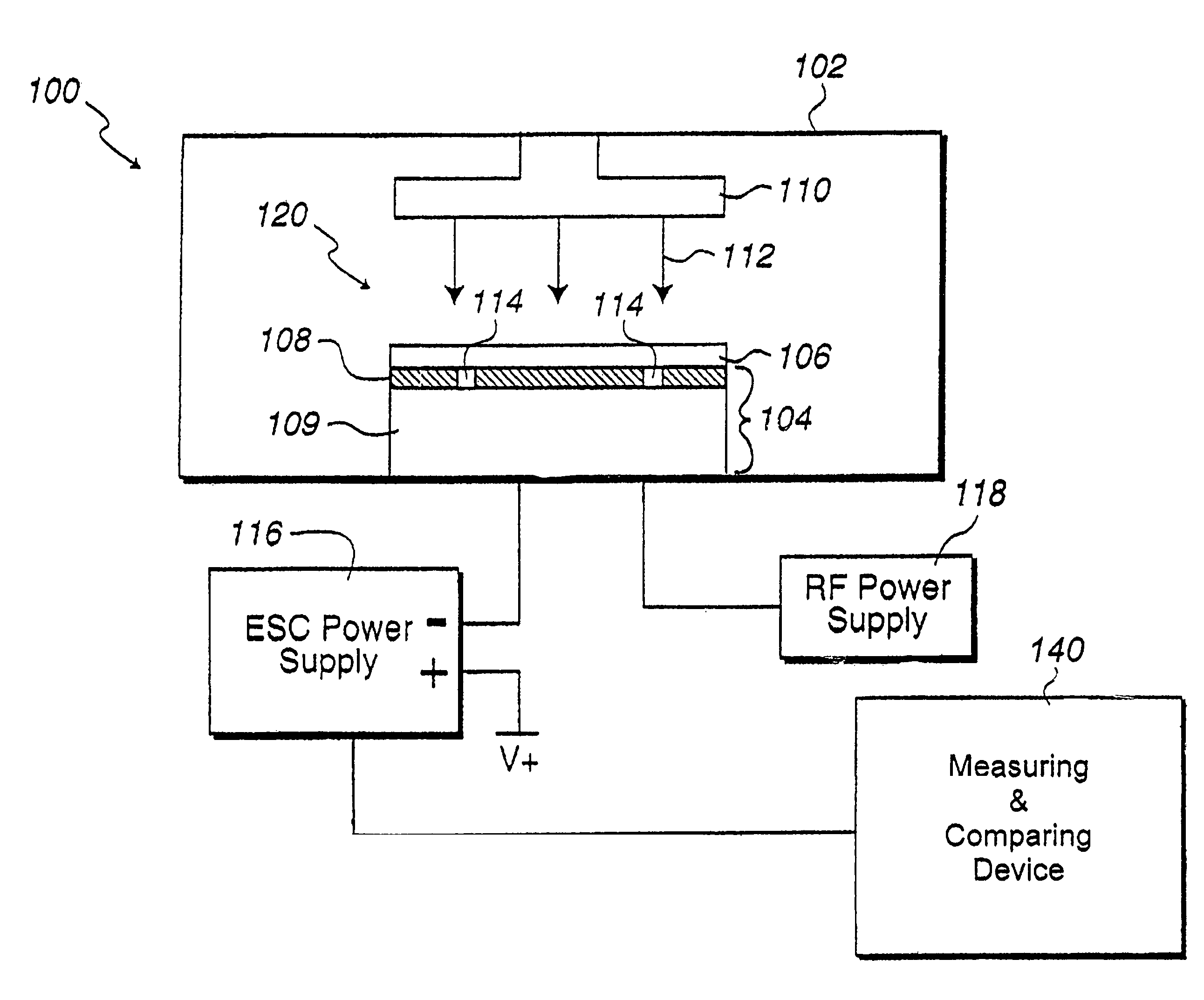
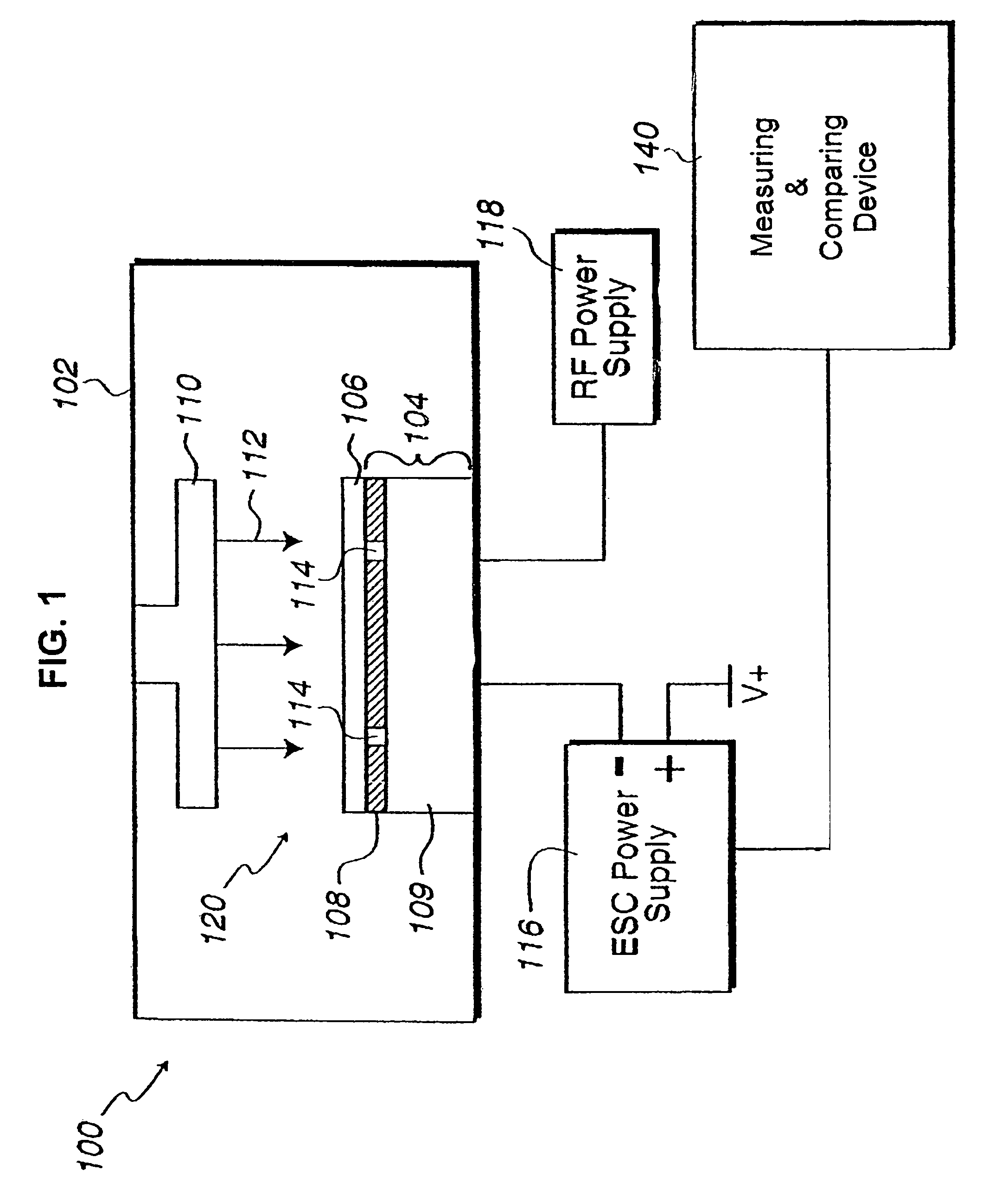
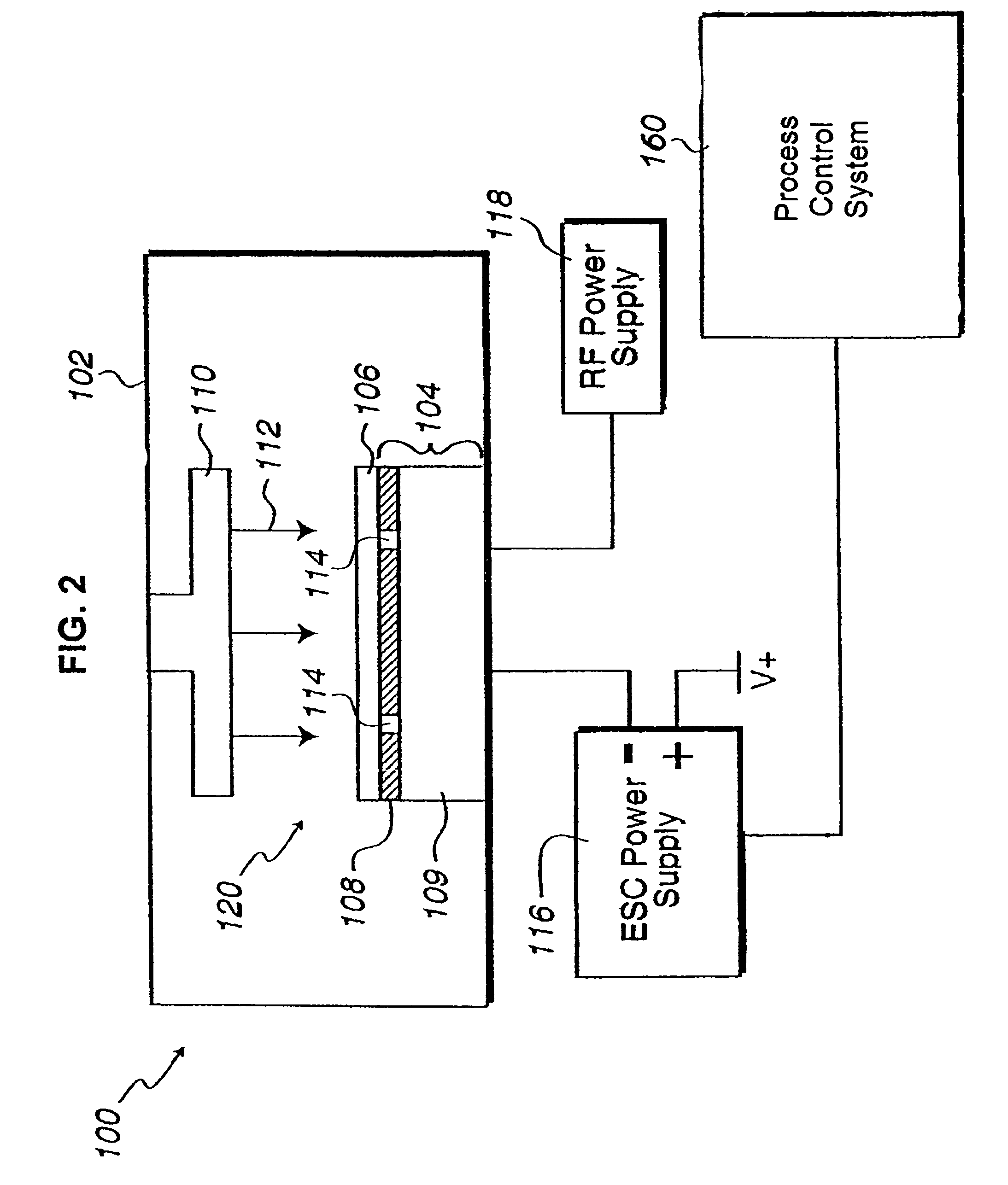
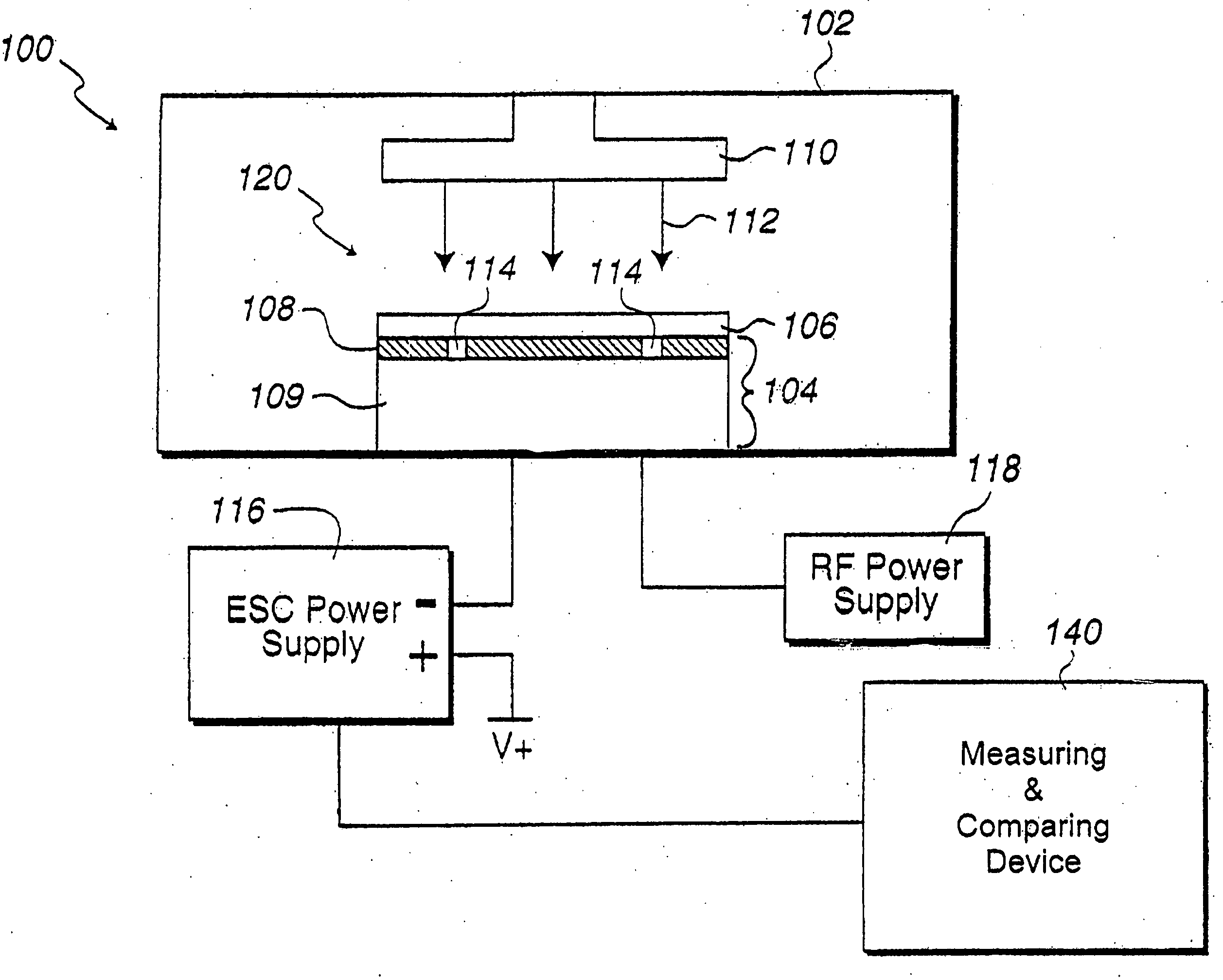
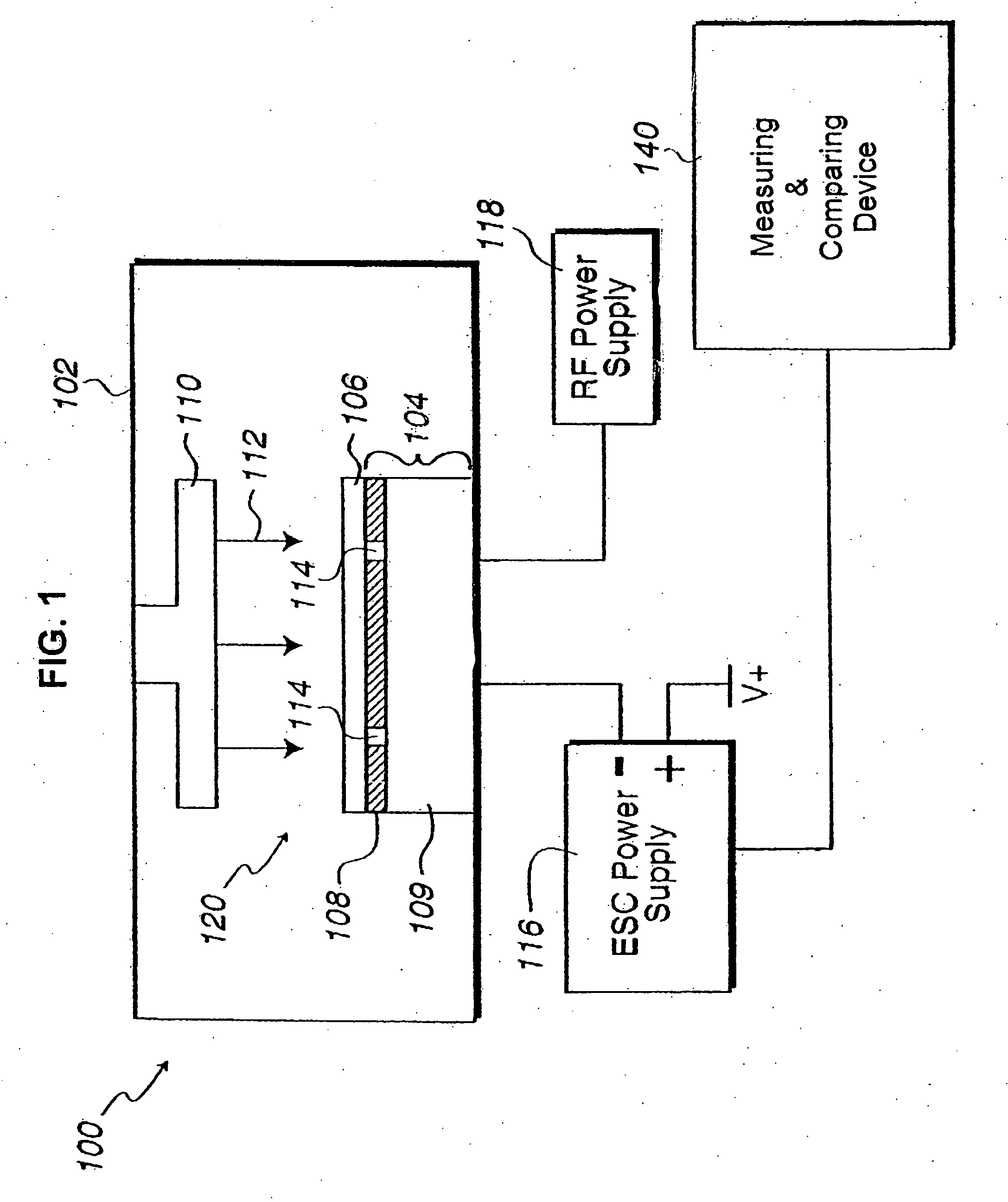
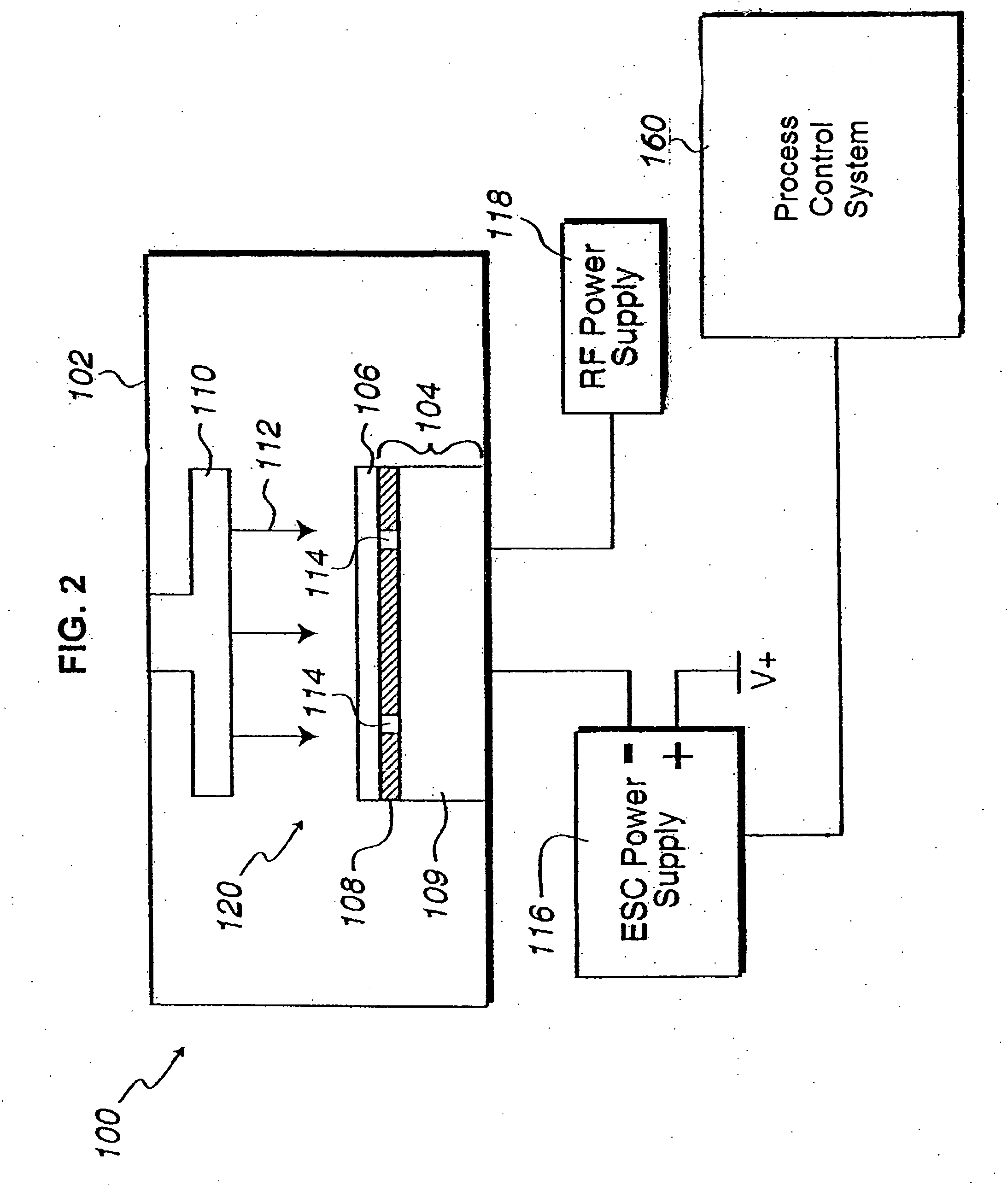
In situ monitoring of wafer charge distribution in plasma processing
A processing system and method. The processing system includes a processing tool, an electrostatic chuck (ESC) arranged within the processing tool, and a system that at least one of detects at least one of an ESC bias spike and an ESC current spike of the ESC and determines when an ESC bias voltage is zero or exceeds a threshold value. The method includes at least one of detecting at least one of an ESC bias spike and an ESC current spike of the ESC, and determining when an ESC bias voltage is zero or exceeds a threshold value. The system and method can be used in real time ESC and plasma processing diagnostics to minimize yield loss and wafer scrap.
Owner:GLOBALFOUNDRIES U S INC
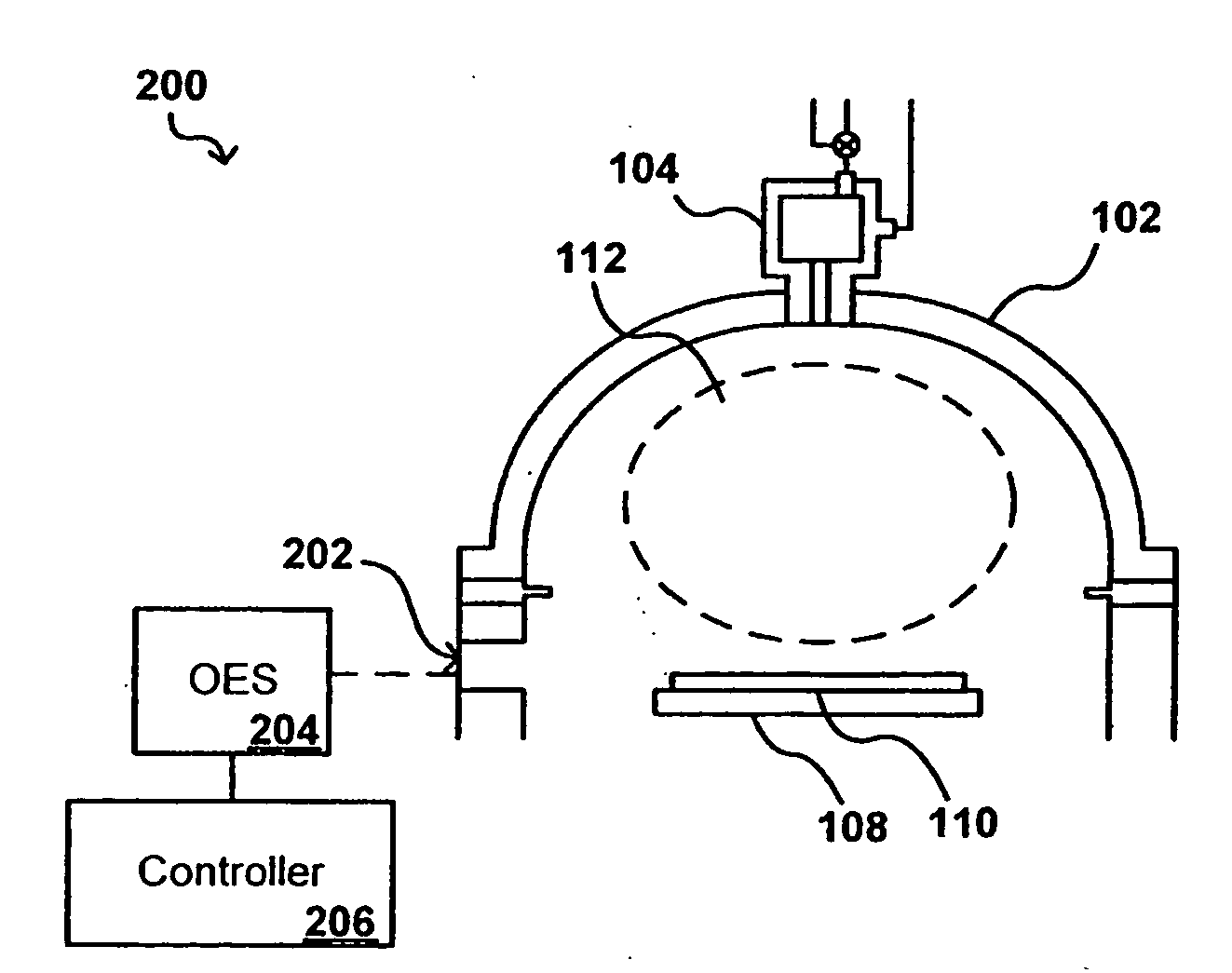
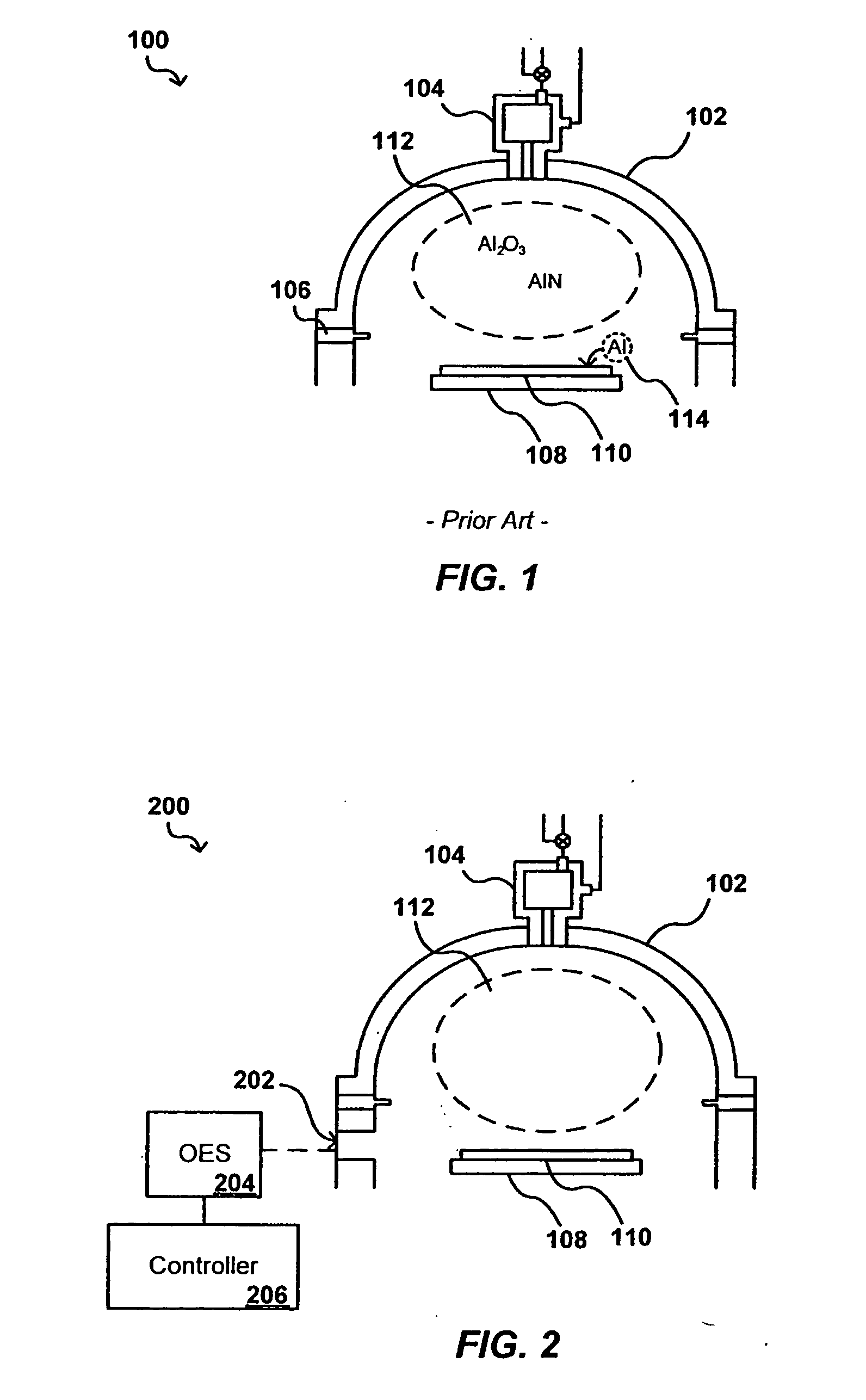
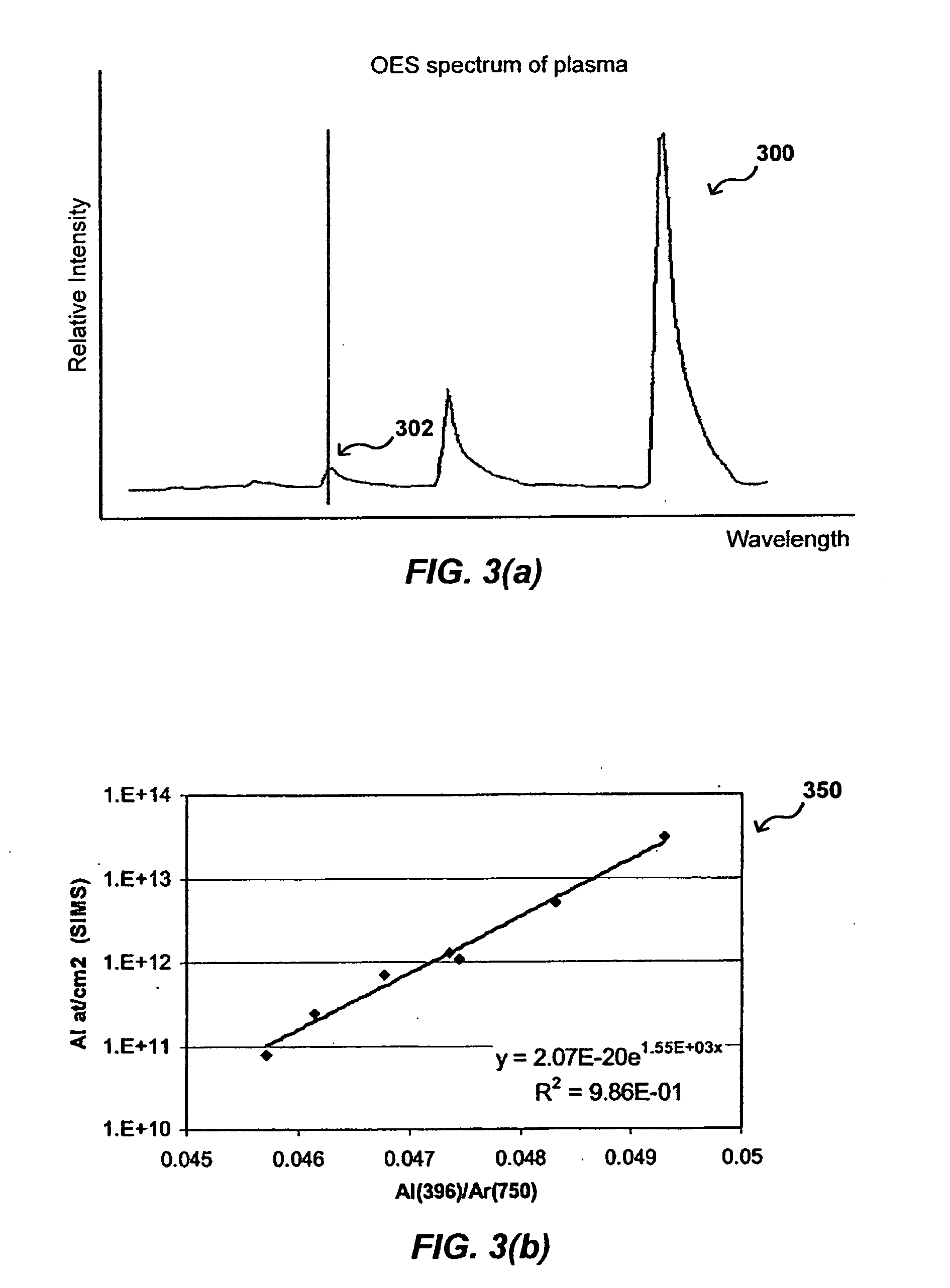
In-situ process diagnostics of in-film aluminum during plasma deposition
InactiveUS20080029484A1Less expensiveReduce the amount requiredElectric discharge tubesDecorative surface effectsOptical Emission SpectrometerPlasma deposition
The concentration of various contaminants in a plasma can be monitored during processing of a substrate such as a silicon wafer, in order to prevent an unacceptable amount of contamination from being deposited on the substrate. The radiation emitted from the plasma through a window in the processing chamber during processing can be detected and measured by a tool such as an optical emission spectrograph (OES) and the relative intensity of peaks in the spectrum corresponding to various contaminants can be analyzed in order to determine contaminant concentration. In one embodiment, the concentration of aluminum in a plasma is monitored during a plasma chemical vapor deposition (CVD) process in order to ensure that the amount of aluminum in the produced device is lower than a maximum threshold amount.
Owner:APPLIED MATERIALS INC
Self-contained network browser with diagnostic abilities
InactiveCN1391677AEasy accessDetecting faulty hardware by remote testDigital computer detailsOperational systemNetwork communication
A network browser with diagnostic abilities stored in a persistent memory, wherein the persistent memory is not a hard disk, is provided. The network browser is used to repair failures of peripheral devices in a networked computer, such as a hard drive, so as to avoid forcing a user to manually diagnose or solve the failure. Moreover, the network browser removes strict dependence on a traditional operating system, and thus the hard disk, to make such repairs. According an embodiment, the network browser comprises a plurality of software modules. The modules include: a device driver module (216), which is configured to initialize and test one or more peripheral devices; a real time kernel module (220), which is configured to detect and dispatch data to and from peripheral devices, though said device driver module (216), including processing diagnostic data corresponding to operation of said peripheral devices, and to perform memory management tasks; an internet protocol module (224), which is configured to handle network communications with remote devices; a graphics windowing module (232), which is configured to process visual display data and control; and a hypertext markup language module (248) configured to interpret hypertext markup language documents for display with said graphics windowing module (232).
Owner:ELEGENT TECH
Method of interfacing ancillary equipment to FIMS processing stations
ActiveUS7187994B1Efficient and flexible to useSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingEngineeringSemiconductor
This invention includes a method of integrating into a semiconductor specimen fabrication station a process diagnostic module that performs on the semiconductor specimen a processing operation that otherwise would not be performed by the processing components to thereby make the fabrication station more efficient and flexible to use. The process diagnostic module includes, for example, a specimen parameter measurement system or a specimen inspection system and is configured to mount on a front-opening interface mechanical standard (FIMS) load port and fits within the allowable spatial envelope. This invention further includes located external to the semiconductor specimen fabrication station a processor that receives and processes data acquired by the process diagnostic module during its operation.
Owner:KLA TENCOR TECH CORP
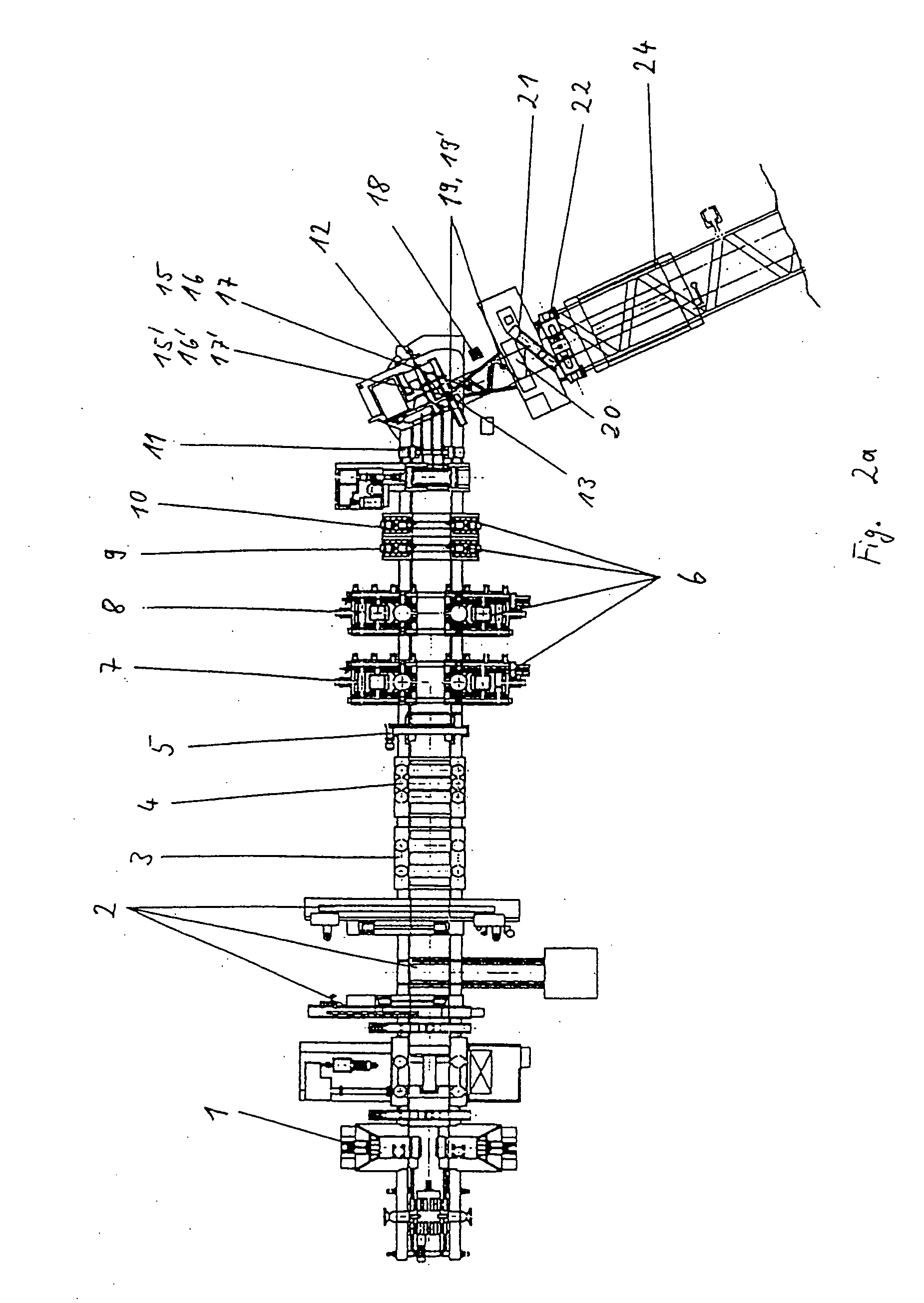
Method and apparatus for making welded large pipes
InactiveUS7012217B2Improve mobilityLower manufacturing requirementsPlasma welding apparatusWelding/soldering/cutting articlesWeld seamEngineering
In a method and apparatus for making welded large pipes, a leading end of a hot strip is connected to a trailing end of a leader strip and then subjected to a two-stage leveling for strip flatness in transverse direction and strip flatness in longitudinal direction. The entire surface of the hot strip including strip edges thereof is inspected by ultrasound and the strip edges are prepared in four stages before being pre-bent. The hot strip is then shaped into a slotted tube and the strip edges are welded along the inner and outer sides by laser to produce the pipe. Online process diagnostic is provided for monitoring the welding step and the finished welded pipe diameter is measured through online measurement. Online determination of a profile of a welded seam configuration and computer tomographic determination and evaluation of flaws inside the pipe, as well as ultrasonic inspection of the welded seam are further provided.
Owner:MANNESMANN ROHRENWERKE
System and method for diagnosing and intelligently optimizing grinding processes
ActiveCN103395001AQuality improvementImprove precision machining technologyGrinding feed controlPower sensorProcess engineering
The invention relates to the technical field of online monitoring on grinding processes, in particular to a system and a method for diagnosing and intelligently optimizing the grinding processes. The system comprises sensors, a data collecting card, a grinding process diagnosing system and an intelligent grinding technique optimizing system. The sensors include a power sensor and a displacement sensor, and are connected to the grinding process diagnosing system through the data collection card. The grinding process diagnosing system is further connected with the intelligent grinding technique optimizing system. The grinding processes are systematically thought, process data are analyzed on the basis of grinding science through monitoring on the grinding processes, and then optimization schemes are proposed. Therefore, optimization is not experimental but scientific.
Owner:INTELLIGENT GRINDOCTOR TECH SHENZHEN CO LTD
Photovoltaic fabrication process monitoring and control using diagnostic devices
InactiveUS20090104342A1Improve the immunitySemiconductor/solid-state device testing/measurementFinal product manufactureElectrical batteryCell fabrication
The formation of diagnostic devices on the same substrate used to fabricate a photovoltaic (PV) cell is described. Such devices, also referred to as process diagnostic vehicles (PDVs), are configured for in-line monitoring of electrical characteristics of PV cell features and are formed on the substrate using the same process steps for PV cell fabrication. The data collected via the PDVs can be used to tune or optimize subsequent PV cell fabrication, i.e., used as feedback for the fabrication process. Alternatively, the data collected via PDVs can be fed forward in the fabrication process, so that later process steps performed on a PV cell substrate can be modified to compensate for issues detected on the PV cell substrate via the PDVs.
Owner:APPLIED MATERIALS INC
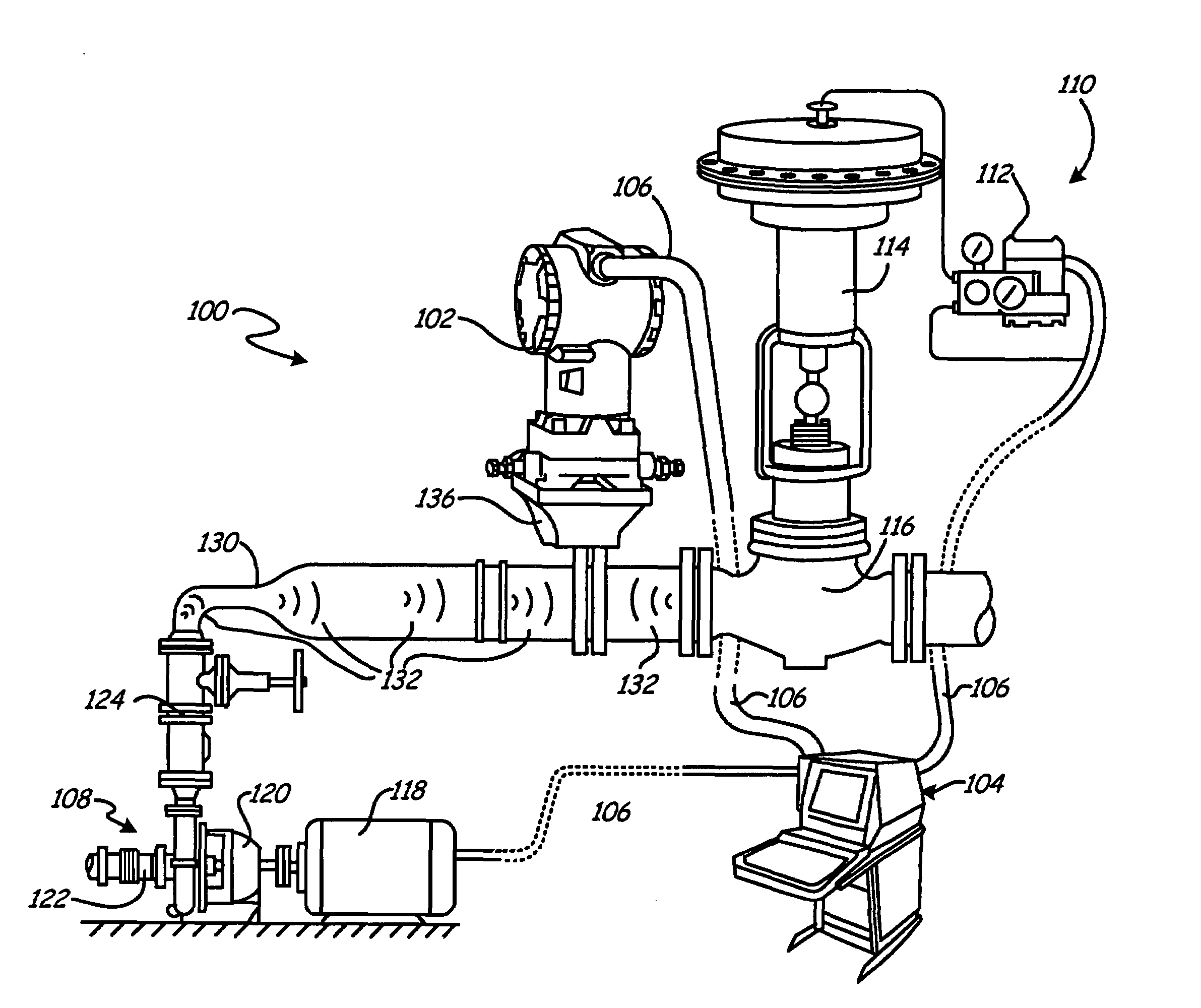
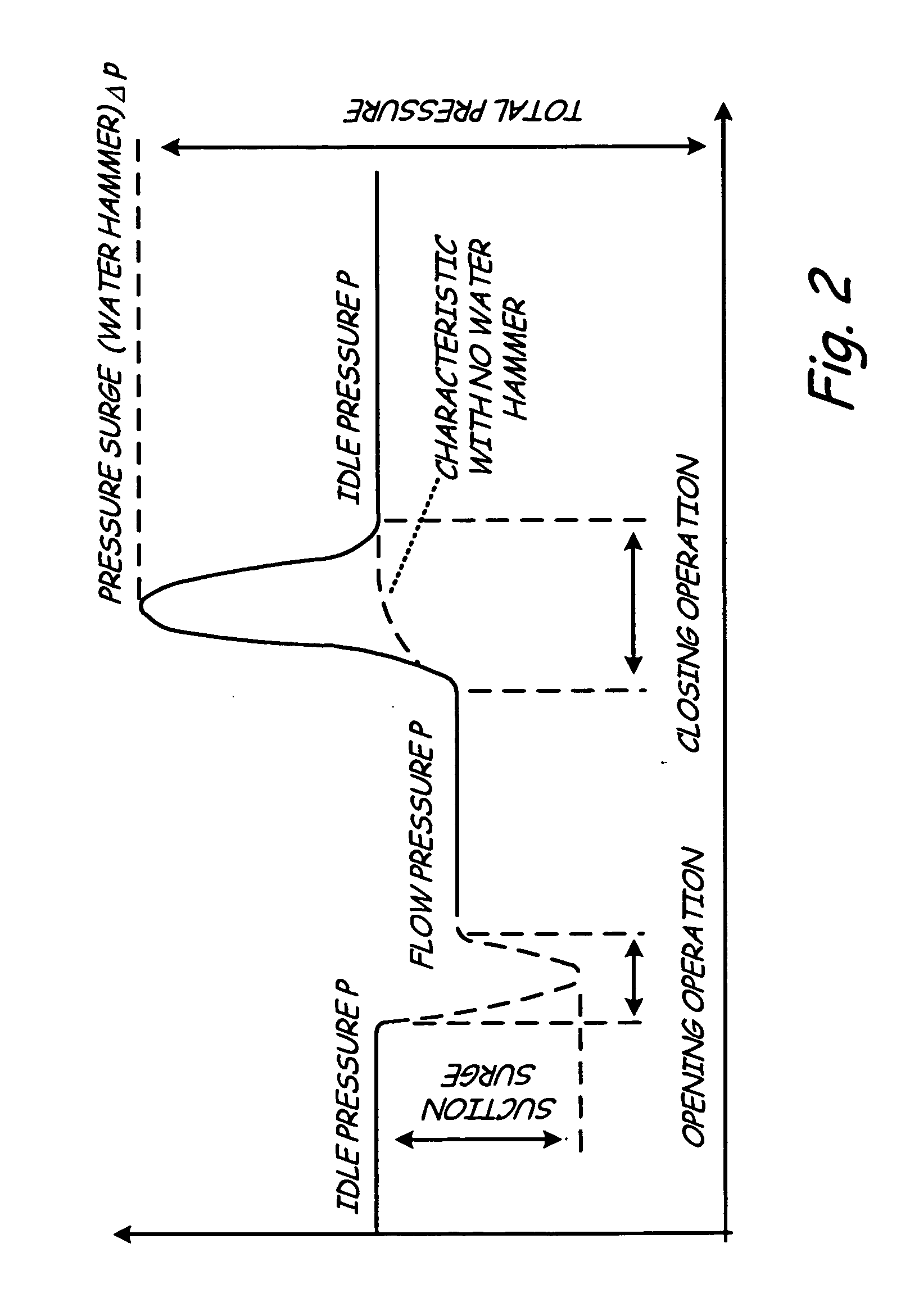
Process connection for process diagnostics
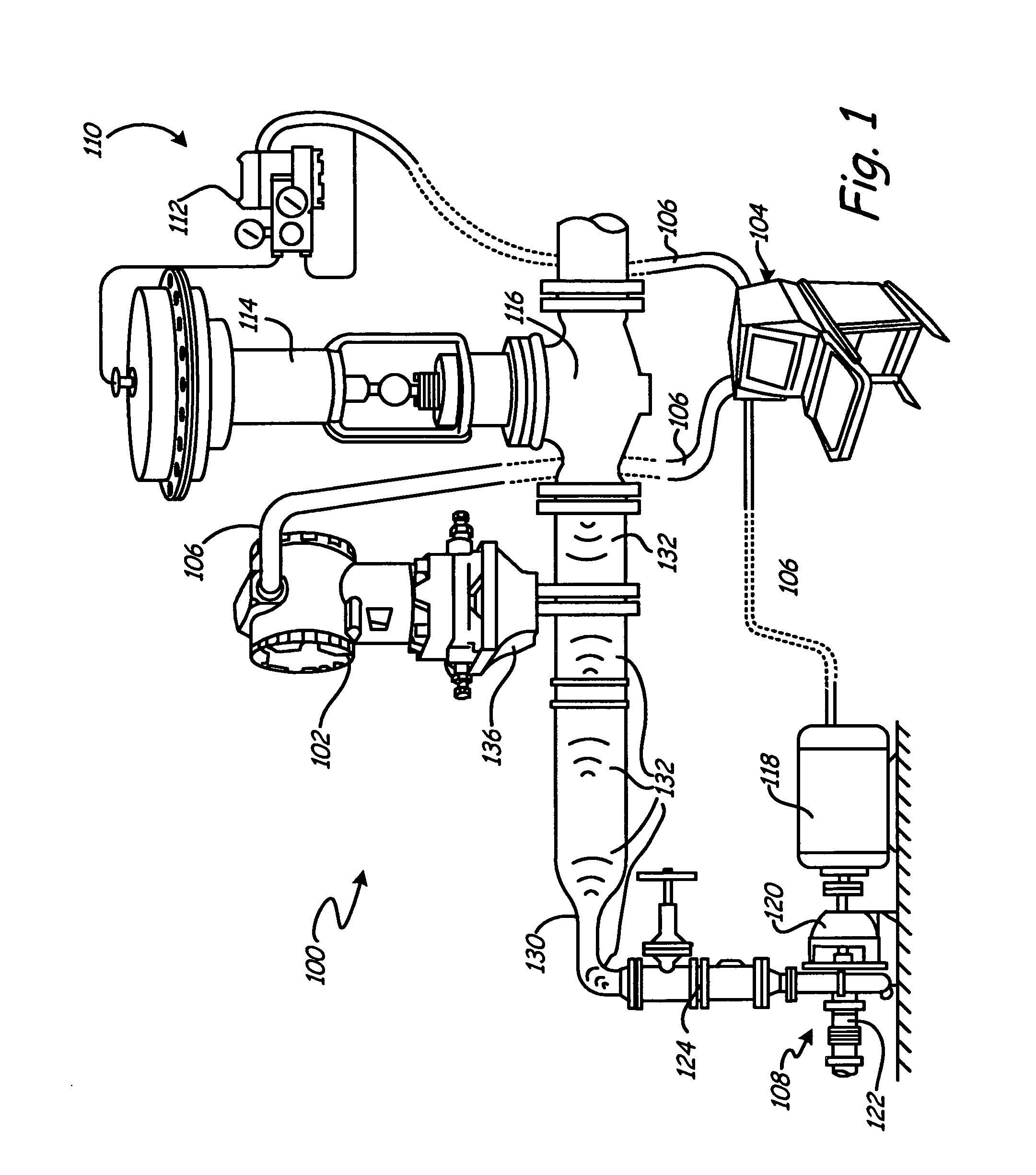
ActiveUS7702478B2Spread the wordTesting/calibration apparatusTesting/monitoring control systemsProcess noiseProcess engineering
A process coupling for coupling a diagnostic device to process fluid of an industrial process includes a process interface configured to physically couple to the process fluid. A fluid pathway extending from the process interface configured to couple a process interface element of the process device to the process fluid. The fluid pathway is configured to optimize transmission of process noise from the process fluid to the process device for use by the diagnostic device.
Owner:ROSEMOUNT INC
In situ monitoring of wafer charge distribution in plasma processing
A processing system and method. The processing system includes a processing tool, an electrostatic chuck (ESC) arranged within the processing tool, and a system that at least one of detects at least one of an ESC bias spike and an ESC current spike of the ESC and determines when an ESC bias voltage is zero or exceeds a threshold value. The method includes at least one of detecting at least one of an ESC bias spike and an ESC current spike of the ESC, and determining when an ESC bias voltage is zero or exceeds a threshold value. The system and method can be used in real time ESC and plasma processing diagnostics to minimize yield loss and wafer scrap.
Owner:GLOBALFOUNDRIES US INC
Structure for diagnosis system of reaction process
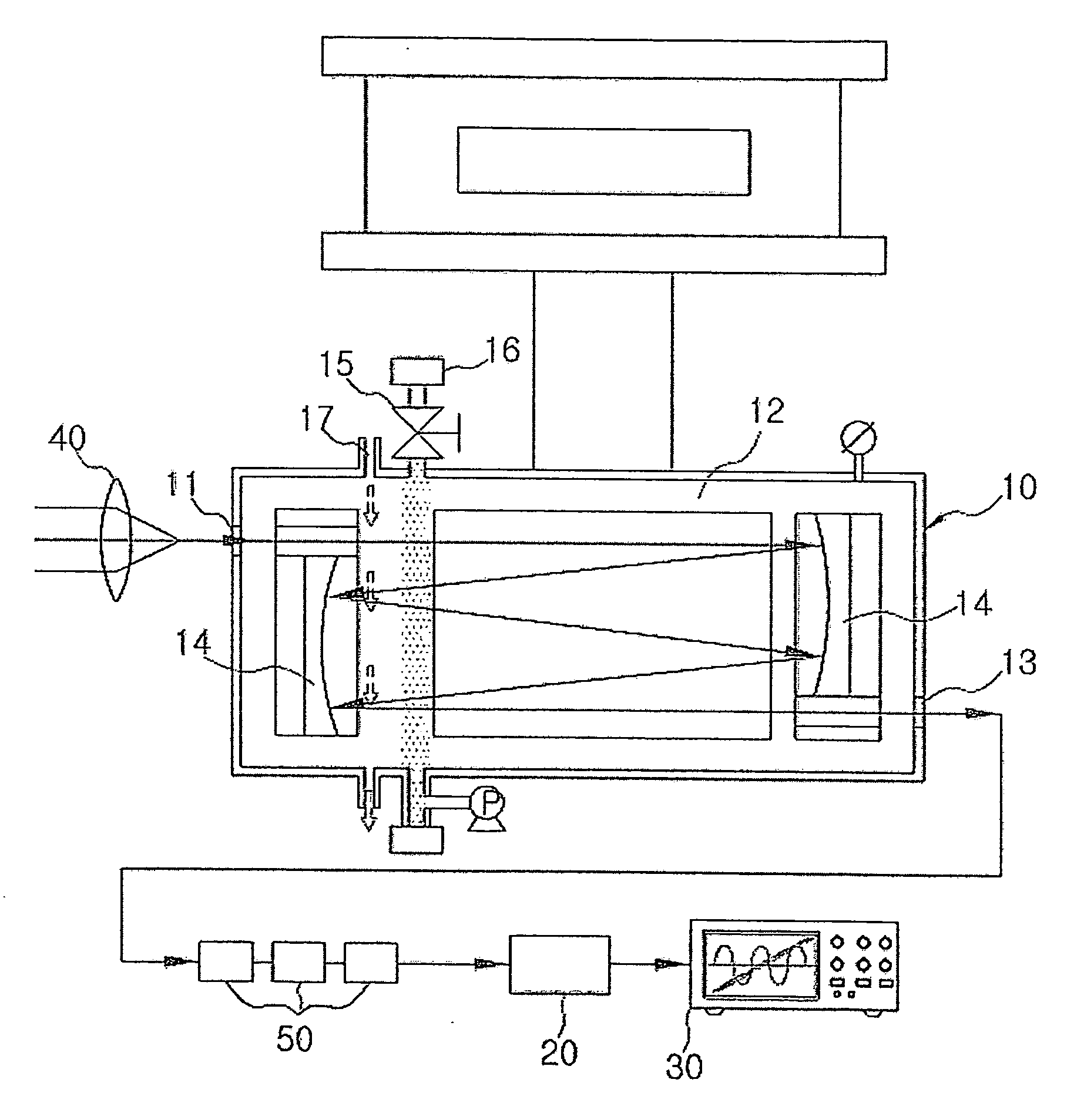
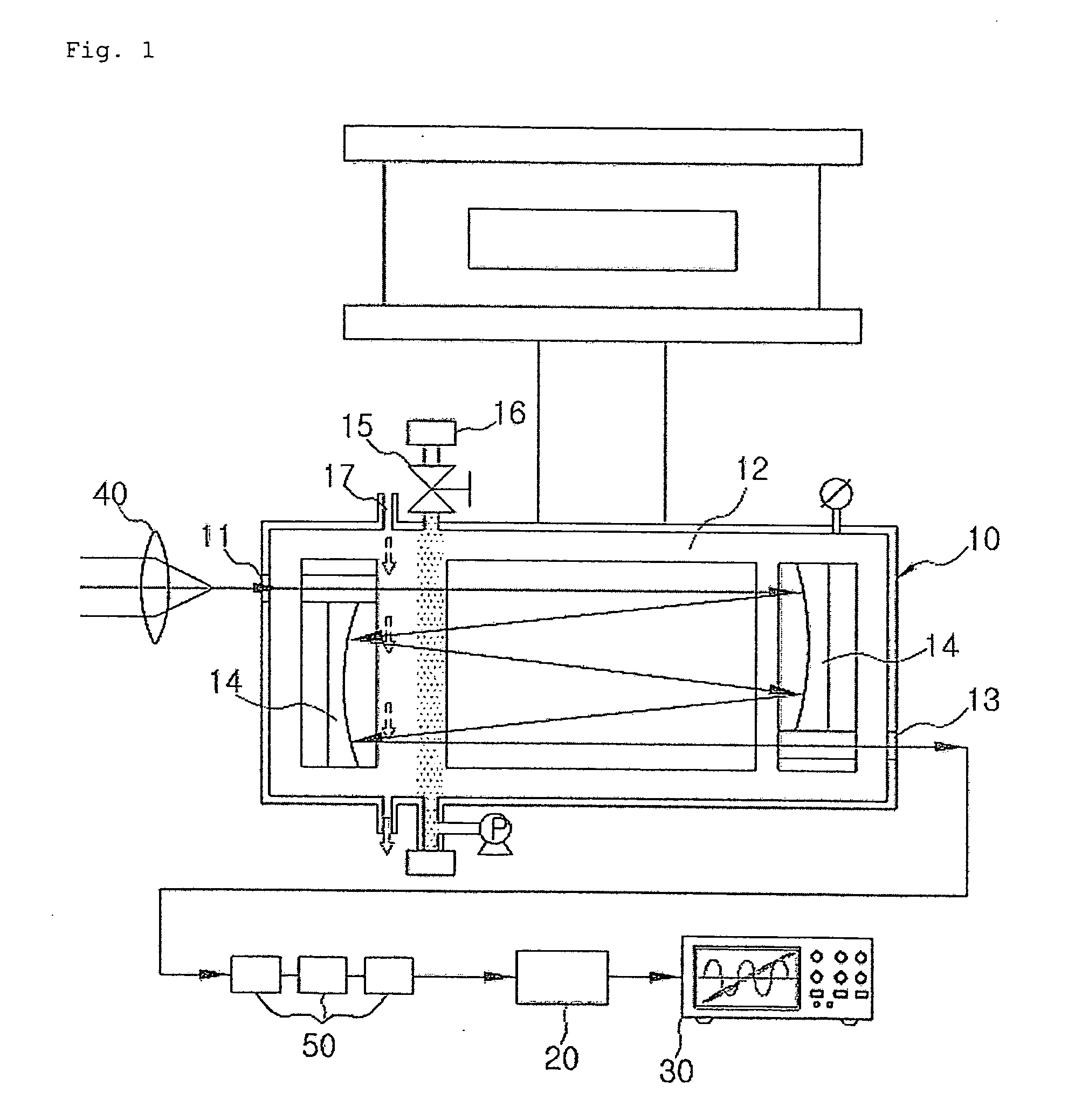
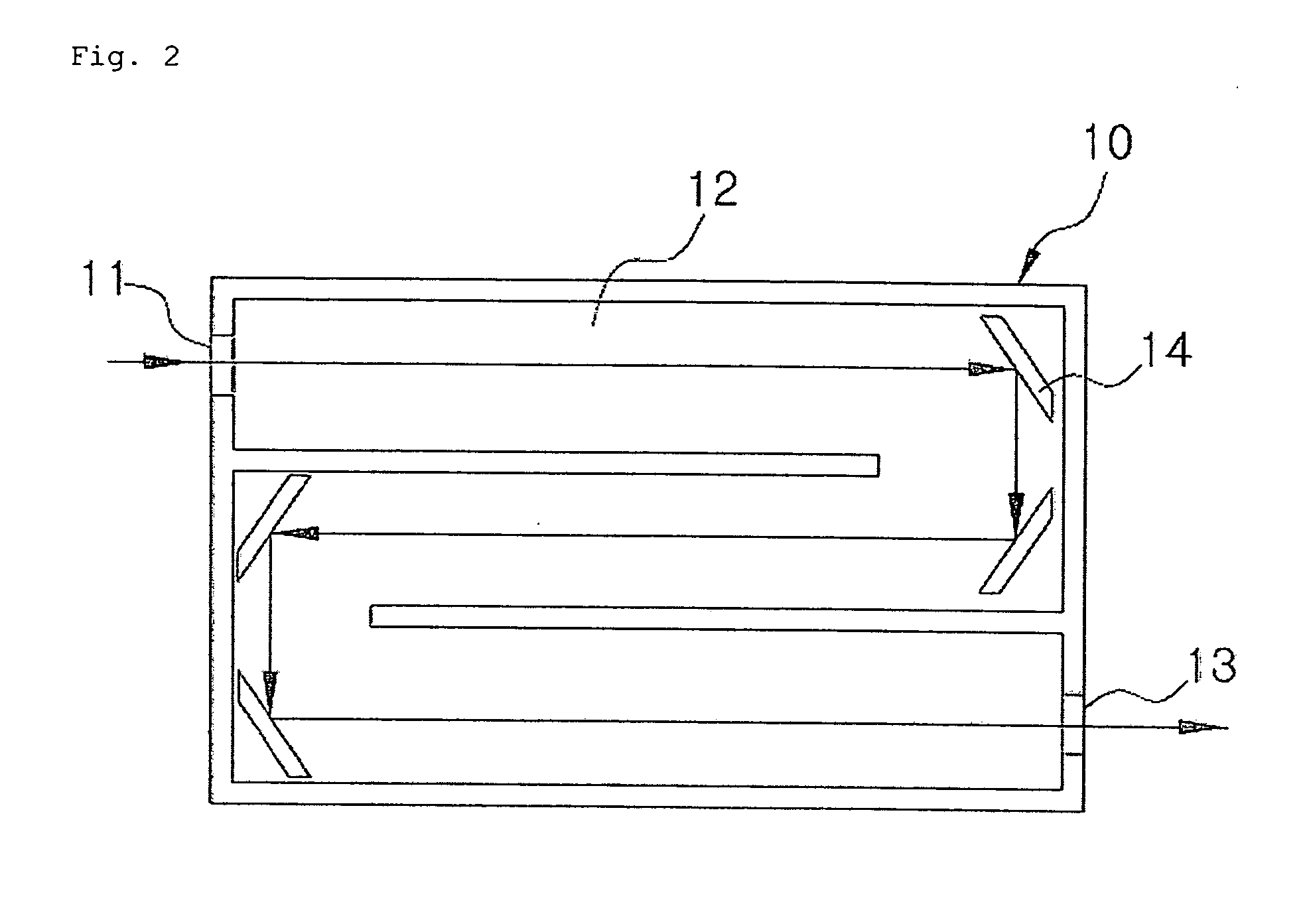
ActiveUS20090046285A1Maintenance periodEnsure correct executionEmission spectroscopyRadiation pyrometryQualitative analysisReagent
The present invention relates to a spectroscopy analyzer for real-time diagnostics of process, and more particularly, to a spectroscopy analyzer for real-time diagnostics of process, in which a beam is injected to a reaction byproduct or a reactant and then an output beam is measured, thereby performing quantitative and qualitative analysis of the reaction byproduct or the reactant.
Owner:KOREA RES INST OF STANDARDS & SCI
Relevance method of continuous annealing machine set detection signals and band steel positions
ActiveCN102486811AImprove quality and efficiencyIncrease productivitySpecial data processing applicationsProcess engineeringContinuous annealing
The invention relates to a control method for a continuous annealing machine set, in particular to a method for judging detection signals generation positions of continuous annealing machine set. A relevance method between continuous annealing machine set detection signals and band steel positions comprises the following steps of: step 1, setting reference points; step 2, circulating the length of an inlet coil; step 3, obtaining the positions of all detection points and the reference points; step 4, obtaining the relevance conversion results of the reference points; and step 5, obtaining the relevance conversion results of the detection points. The method disclosed by the invention has the advantages that the relevance integration of space, time, various kinds of equipment information, process information and marking information data in the steel coil production process is built so that the comprehensive analysis of the whole flow data is carried out, in addition, a quality model and a process diagnosis model are built for the goals of production guide, fault early warning, accident tracing, quality defect analysis and the like, and the product quality and the production efficiency are further improved.
Owner:SHANGHAI BAOSTEEL IND TECHNOLOGICAL SERVICE
Vibration cutting process diagnostic device
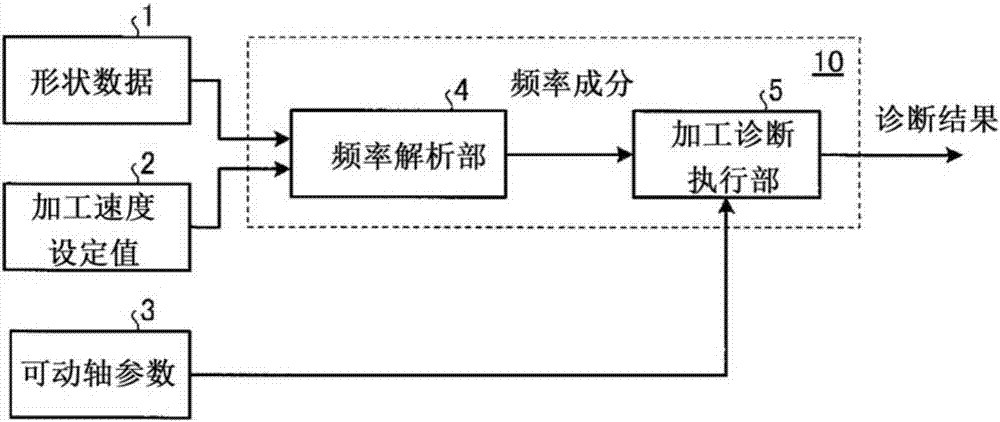
The purpose of the present invention is to obtain a vibration cutting process diagnostic device such that, when performing a vibration cutting process for processing the cross sectional shape of an object to be processed into a non-perfect circular shape by reciprocating a tool, whether the process can be performed under a speed condition designating a vibration cutting can be diagnosed prior to the process. A vibration cutting process diagnostic device that makes a diagnosis as to whether a vibration cutting process for processing the cross sectional shape of an object to be processed into a non-perfect circular shape can be performed by a reciprocating motion of a movable axis is provided with: a frequency analysis unit (4) that computes, on the basis of shape data (1) which are processing shape data of a work as the object to be processed and a processing speed set value (2), a frequency component included in a movable axis position command signal; and a processing diagnosis implementation unit (5) that makes a diagnosis as to the possibility of processing the shape data (1) with the processing speed set value (2), on the basis of the frequency component and a movable axis parameter (3) of the movable axis.
Owner:MITSUBISHI ELECTRIC CORP
Process connection for process diagnostics
ActiveUS20060212139A1Spread the wordTesting/calibration apparatusTesting/monitoring control systemsProcess noiseEngineering
A process coupling for coupling a diagnostic device to process fluid of an industrial process includes a process interface configured to physically couple to the process fluid. A fluid pathway extending from the process interface configured to couple a process interface element of the process device to the process fluid. The fluid pathway is configured to optimize transmission of process noise from the process fluid to the process device for use by the diagnostic device.
Owner:ROSEMOUNT INC
Processing diagnostics of media services
Owner:AT&T INTPROP I LP
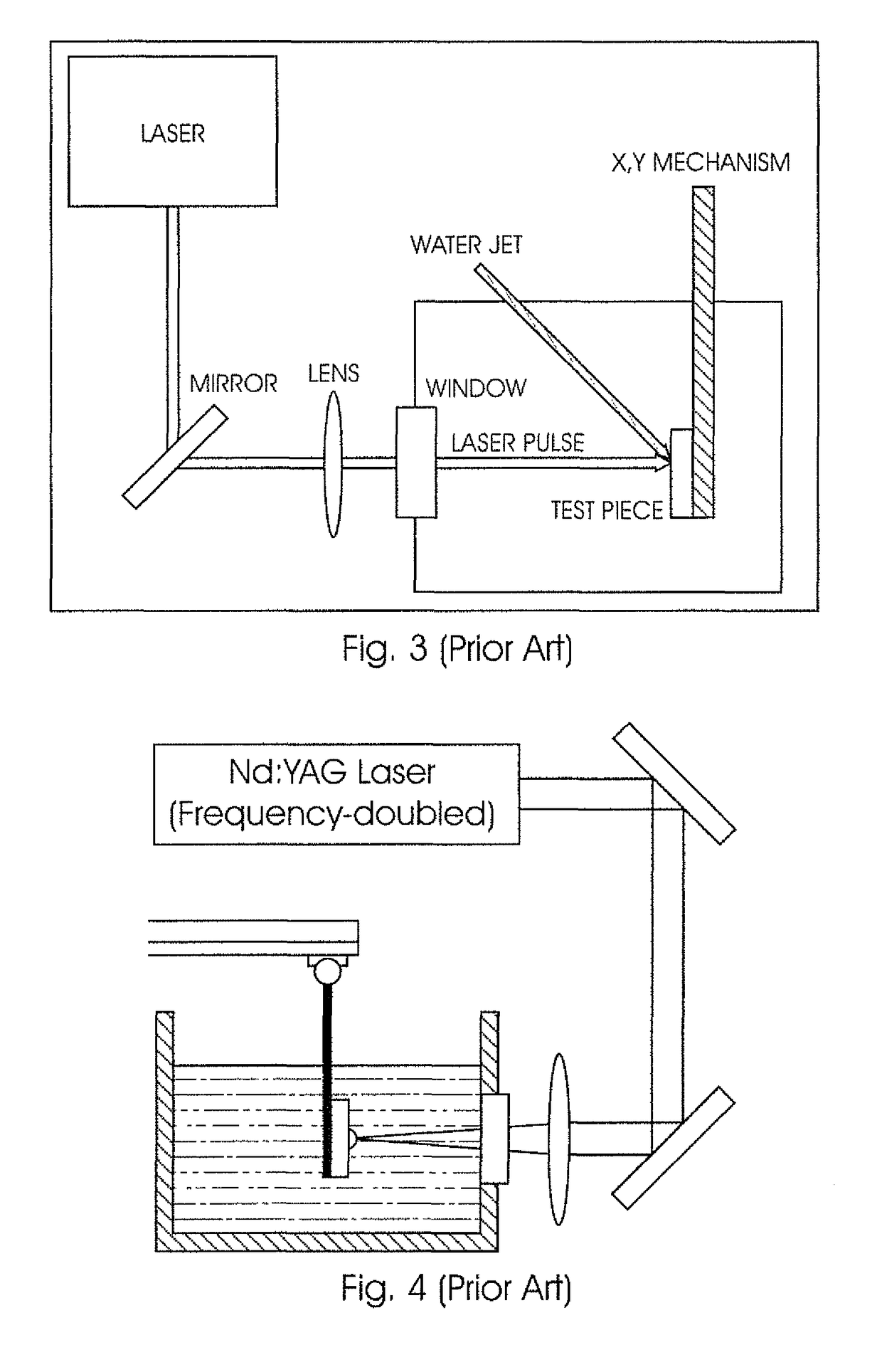
System for and method of performing Laser Shock Peening on a target with a fluid flow path sandwiched between a transparent to laser light solid medium and the target
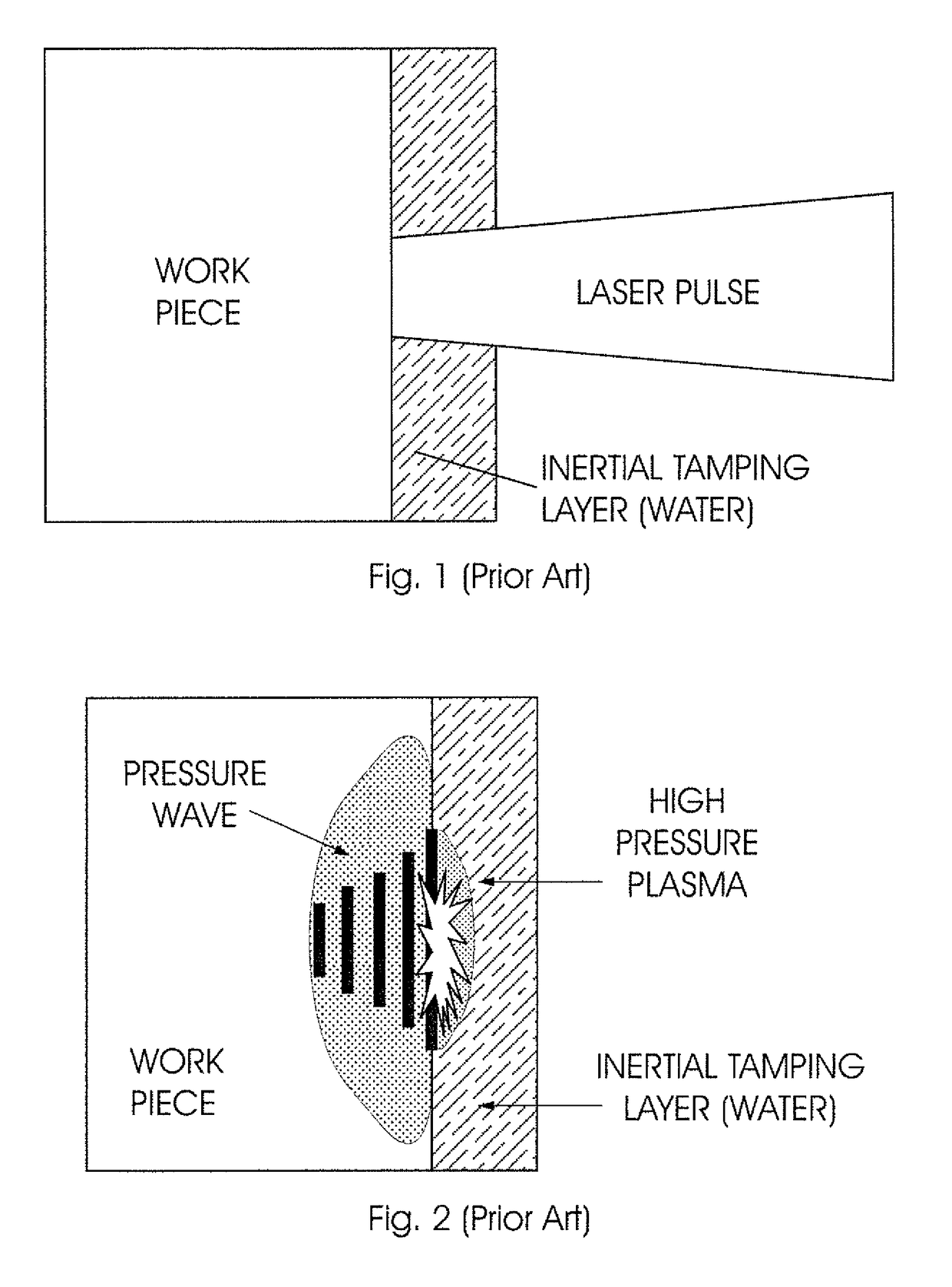
The invention is concerned with a system for performing Laser Shock Peeing on a target (100). The system includes a device (10) for generating and transmitting a laser pulse to the target (100) and a fluid source for supplying a fluid into a fluid flow path arranged between an inlet (20) and an outlet (22.1, 22.2). A solid medium (14), which is transparent to incident laser light (12), is located in the laser path so as to allow the laser pulse to pass through it. In use, the fluid flow path is sandwiched between the solid medium (14) and the target (100) during the laser shock peening process so that the fluid is in direct contact with the solid medium (14) and the target (100), thereby eliminating any air-fluid interface in the travel path of the laser pulse. The fluid is also supplied into the fluid flow path having a constant thickness such that a second shock event through cavitation in the fluid layer occurs upon the collapse of a plasmalvapor bubble generated after the laser pulse striking the target. The invention also concerns a method of performing Laser Shock Peeing using the system in accordance with the invention and, in particular, the use of the first bubble oscillation period to determine the amount of energy being delivered to the target (100). The monitoring of the energy being delivered to the target (100) provides for process diagnostics during the LSP procedure.
Owner:UNIVERSITY OF THE WITWATERSRAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com