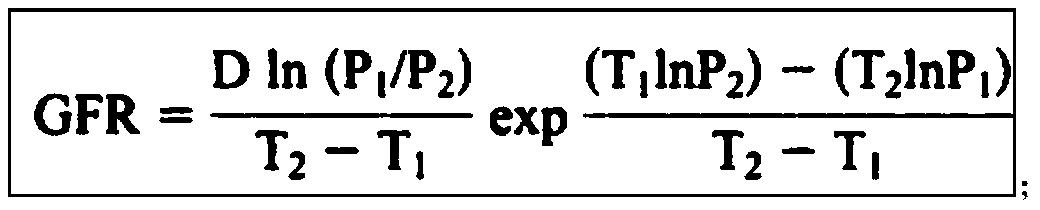Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Plasma clearance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
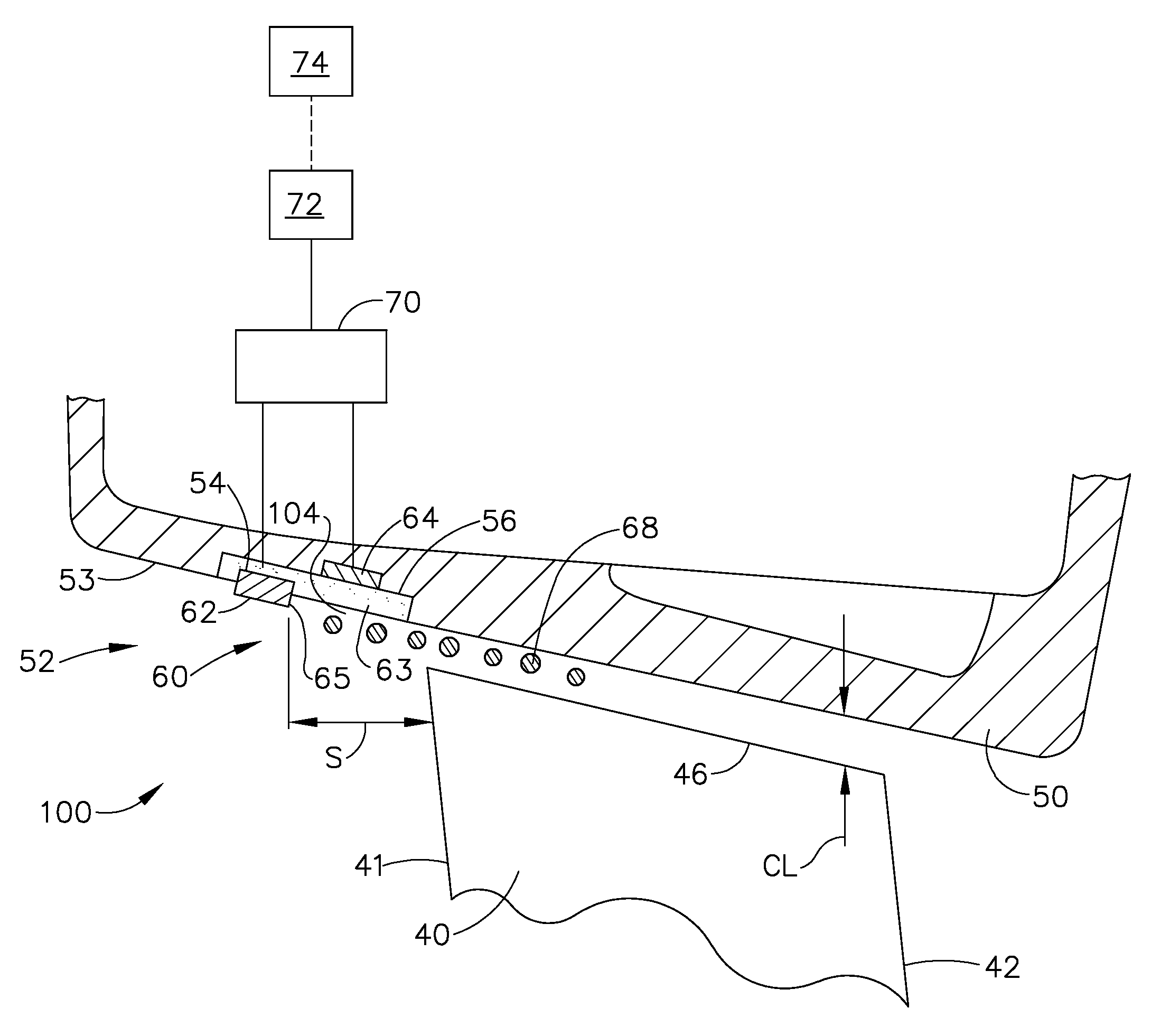
Plasma Clearance Controlled Compressor
InactiveUS20100284795A1Improving stable flow rangeReduce leakage flowPump componentsBlade accessoriesElectricityControl system
A plasma leakage flow control system for a compressor is disclosed, comprising a circumferential row of compressor blades, an annular casing surrounding the tips of the blades, located radially apart from the tips of the blades and at least one annular plasma generator located on the annular casing. The annular plasma generator comprises an inner electrode and an outer electrode separated by a dielectric material. A gas turbine engine having a plasma leakage flow control system further comprises an engine control system which controls the operation of the annular plasma generator such that the blade tip leakage flow can be changed.
Owner:GENERAL ELECTRIC CO
Oligopolynucleotide of inhibiting activity of necrosin in human tumor
InactiveCN1550501AInhibition of killingInhibit bindingOrganic active ingredientsSugar derivativesDiseaseL929 cell
Oligonucleotide sequence i.e. adaptor including DNA sequence and RNA sequence can combine with TNF alpha in specificity so as to restrain damaging effect on L929 cells from TNF alpha. The invented adaptor can be utilized to test TNF alpha and cure disease caused by raised level of TNF alpha. Comparing with current antagon of TNF alpha, the invented adaptor possesses advantages of high specificity, high affinity, fast reaching target tissue and quick plasma clearance etc. The oligonucleotide sequence possesses affinity and specificity of monoclonal antibody as well as permeability and pharmacokinetics characters of small molecule polypeptide. The invention is also related to derivation sequence and modified sequence of oligonucleotide, as well as its usage, preparation and diagnosis in curing disease relevant to TNF alpha.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Oligonucleotide antagonist for human tumor necrosis factor alpha (TNF-alpha)
InactiveUS7309786B2Strong specificityHigh affinitySugar derivativesPeptide/protein ingredientsL929 cellHigh concentration
The present invention relates to a group of new oligonucleotides sequences with human tumor necrosis factor α (TNF-α) inhibiting activity, which includes DNA sequences and RNA sequences. These oligonucleotides or aptamer can specifically be bound to TNF-α and inhibit the cytoxicity of TNF-α to L929 cells. Therefore, the aptamer of the present invention may be used to detect TNF-α and provide a therapeutic method for diseases related to the increasing level of TNF-α. Compared with other TNF antagonists such as monoclonal antibody and soluble receptor, the present invention has high specificity, high affinity, quick penetration to target tissue, rapid plasma clearance, and lower immunogenecity. Turthermore, it can be used repeatedly and keeps high concentration in target tissue and the like. It has the advantages of affinity and specificity similar to monoclonal antibodies and also has permeability and pharmacokinetics characteristics similar to small molecular polypeptide. The present invention also refers to derivative of the oligonucleotides sequence, including modified sequence. The present invention may further be manufactured as medicine for therapy and diagnosis of TNF-α related diseases.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
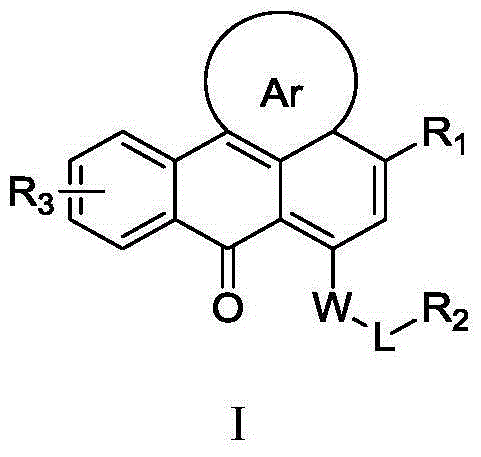
Heterocyclic anthracene ketone histone methyltransferase inhibitor and medical application thereof
The invention relates to the field of medicinal chemistry, in particular to a heterocyclic anthracene ketone histone methyltransferase inhibitor and a preparation method and application of the heterocyclic anthracene ketone histone methyltransferase inhibitor. It is proved by pharmacology experiments that the compound has a good inhibiting effect on histone methyltransferase from the molecular level to the cell level, has good antiproliferative activity on tumor cell strains and has reasonable medicinal parameters, such as a proper dissociation constant (pKa), a lipid-aqueous partition coefficient (LogD, pH=7.4), water solubility and film transmittance. It is testified by research on pharmacokinetics that the compound is wide in distribution in a body of a rat, the plasma clearance can be within an acceptable range, and good bioavailability is achieved. The compound and the pharmaceutic preparation of the compound can be used for treating a series of diseases caused by disorder of enzyme activity of the histone methyltransferase, such as solid tumor, leucocythemia, malaria and neurodegenerative diseases.
Owner:CHINA PHARM UNIV
Polyglycol modified recombinant human interferon
InactiveCN1375502AMaintain antiviral activitySimple manufacturing methodPeptide/protein ingredientsAntiviralsMedicineHalf-life
Owner:CHINA PHARM UNIV
Recombinant porcine interferon beta1-Fc fusion protein as well as encoding gene and expressing method thereof
ActiveCN103570836AMaintain biological activityExtended half-lifeBacteriaMicroorganism based processesEscherichia coliInclusion bodies
The invention provides a recombinant porcine interferon IFN beta1-Fc fusion protein as well as an encoding gene and expression, purification and inclusion body renaturation methods thereof, belonging to the biological genetic engineering field. The IFNbeta can enhance the immunity of pig and has a good application prospect in the veterinary medicine industry. However, natural porcine IFNbeta1 is less in expression quantity and is insufficient for research and development and application and has the deficiency of quick plasma clearance rate. The invention provides the recombinant porcine interferon IFN beta1-Fc fusion protein applicable to a coliform bacteria prokaryotic expression system. Part of the porcine IFNbeta1 is all sequences in an ectoenzyme area of the porcine IFNbeta1. The Fc section comprises a hinge region, a CH2 region and a CH3 region of an antibody. The porcine IFNbeta1 and the Fc section are directly fused. The fusion protein provided by the invention not only maintains the biological activity of the original protein IFNbeta1 to a great extent, but also extremely prolongs the half-life period of the original protein IFNbeta1, thereby providing the opportunity of industrialized development of the fusion protein.
Owner:GENSUN INST OF BIOMEDICINE
Human interferon alpha derivatives and preparation and use of pegylated products thereof
The invention relates to human interferon alpha derivatives and preparation and use of pegylated products thereof. The human interferon alpha derivatives are formed by bonding three amino acids to N terminals of human interferon alpha and have a structural formula of R3-R2-R1-interferon, wherein the interferon is the human interferon alpha, and a first position amino acid R1 bonded to the N terminals of the human interferon alpha is a sculpture amino acid or glycine; the second position amino R2 is an aromatic amino acid or glycine; and the third position amino acid R3 is an acidic amino acidor glycine. The invention also provides pegylated derivatives of the human interferon alpha derivatives, a preparation method thereof and use thereof in the preparation of medicaments for treating andpreventing viral infection or lung cancer. The human interferon alpha derivatives have the advantages of high specific activity and high pegylation rate. The pegylated derivatives of the human interferon alpha derivatives have the characteristics of high biological activity and no non-N terminal modified isomers and have the advantages of prolonging in-vivo half-life, reducing plasma clearance and the like.
Owner:BEIJING SIHUAN PHARMA +1
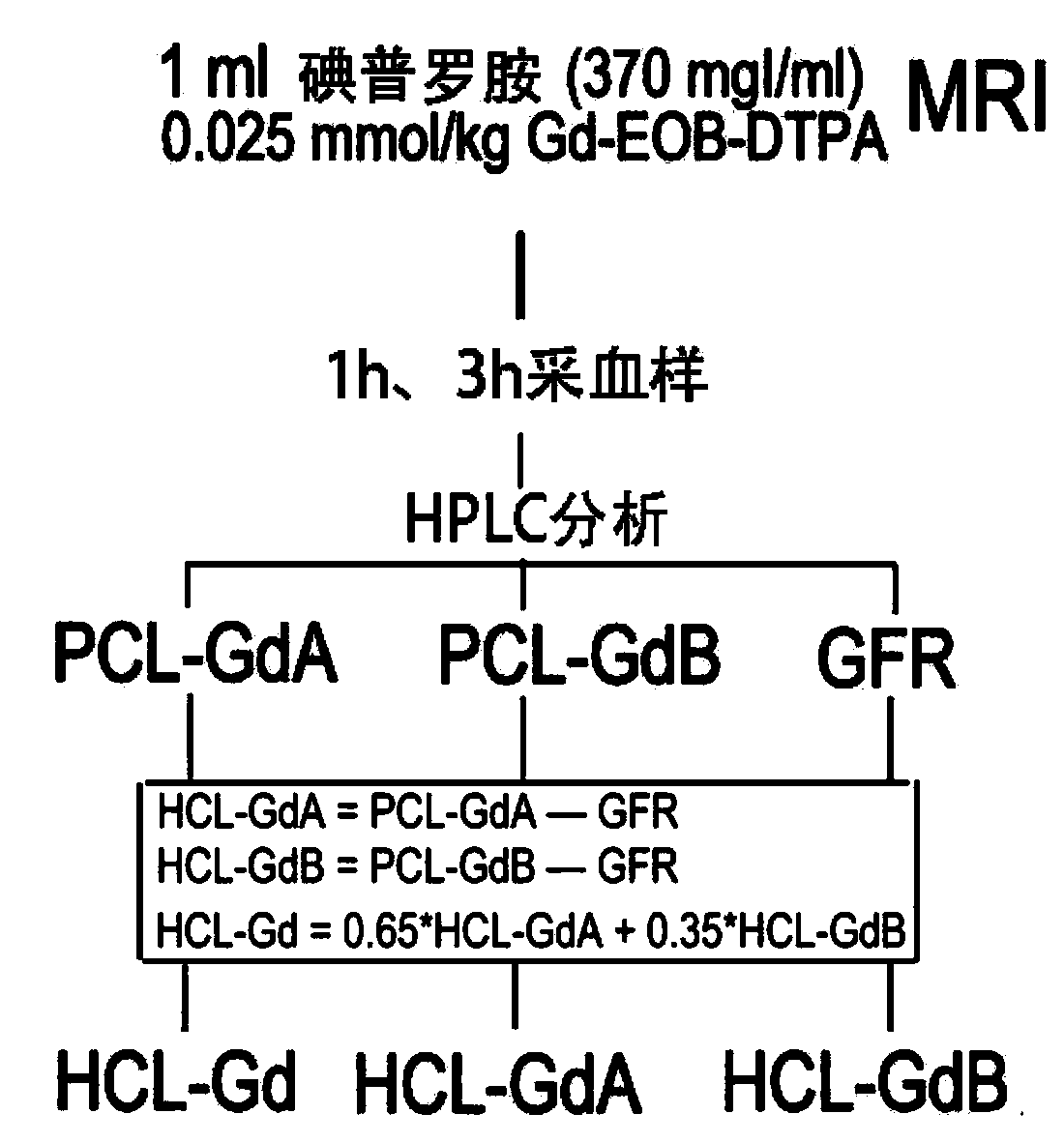
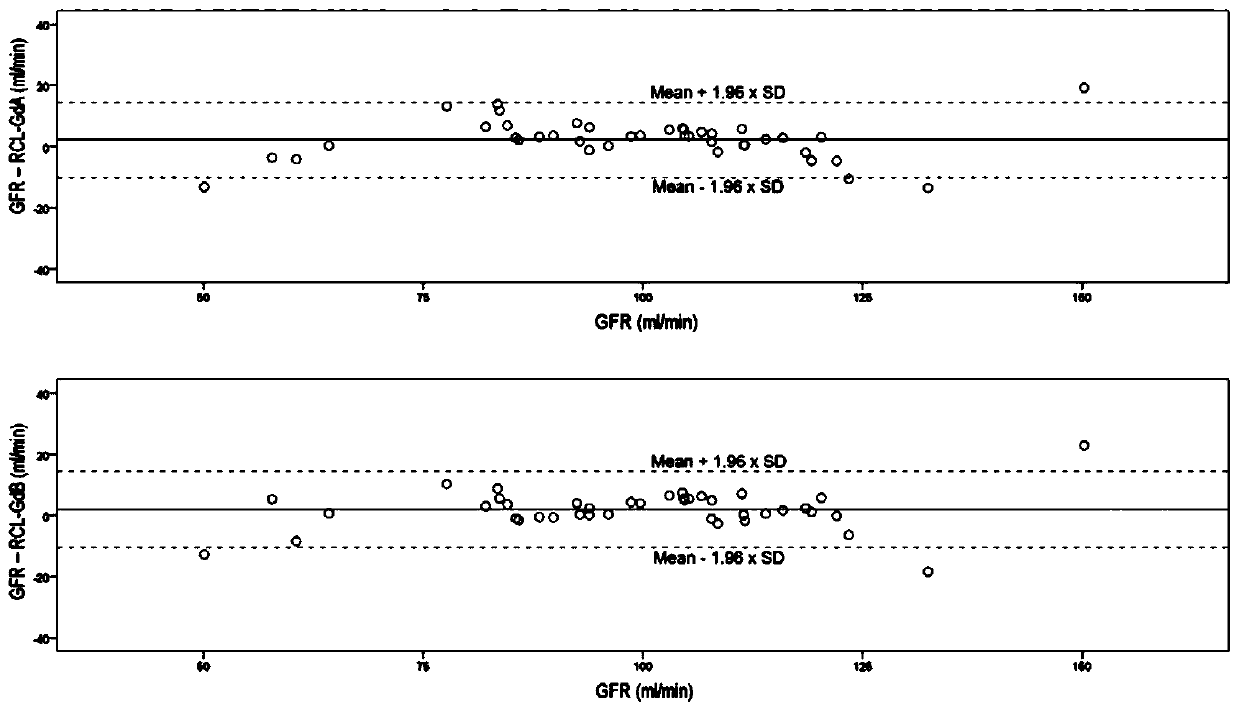
Method for detecting liver and kidney clearance rates of two isomers of Primovist
ActiveCN110702888AEasy and accurate measurementSimple and fast operationBiological testingLiver and kidneyFunctional imaging
The invention relates to a method for detecting liver and kidney clearance rates of two diastereoisomers Gd-A and Gd-B of Primovist. The method is characterized in that during regular dose administration of Primovist ( for example, intravenous administration during magnetic resonance enhanced imaging), a low dose of iodinated contrast agent (about 1% of the conventional dose) is given at the sametime, a small amount of blood sample is extracted to obtain the plasma clearance rates of the iodinated contrast agent, the Gd-A and the Gd-B, the plasma clearance rate of the iodinated contrast agentis GFR, it is discovered in the method provided by the invention for the first time that the GFR can approximately replace the kidney clearance rates of the Gd-A and the Gd-B, and the liver clearancerates of the Gd-A and the Gd-B can be obtained by subtracting the GRF from the respective plasma clearance rates. By adopting the detection method provided by the invention, the liver and kidney clearance rates of the two isomers of Gd-A and Gd-B of the Primovist can be measured conveniently and accurately, a "gold standard" and individualized pharmacokinetic indicators are provided for the magnetic resonance liver function imaging of the Primovist, and the method plays guidance and reference roles for rational drug use and evaluation of liver and kidney functions.
Owner:中国人民解放军总医院第八医学中心
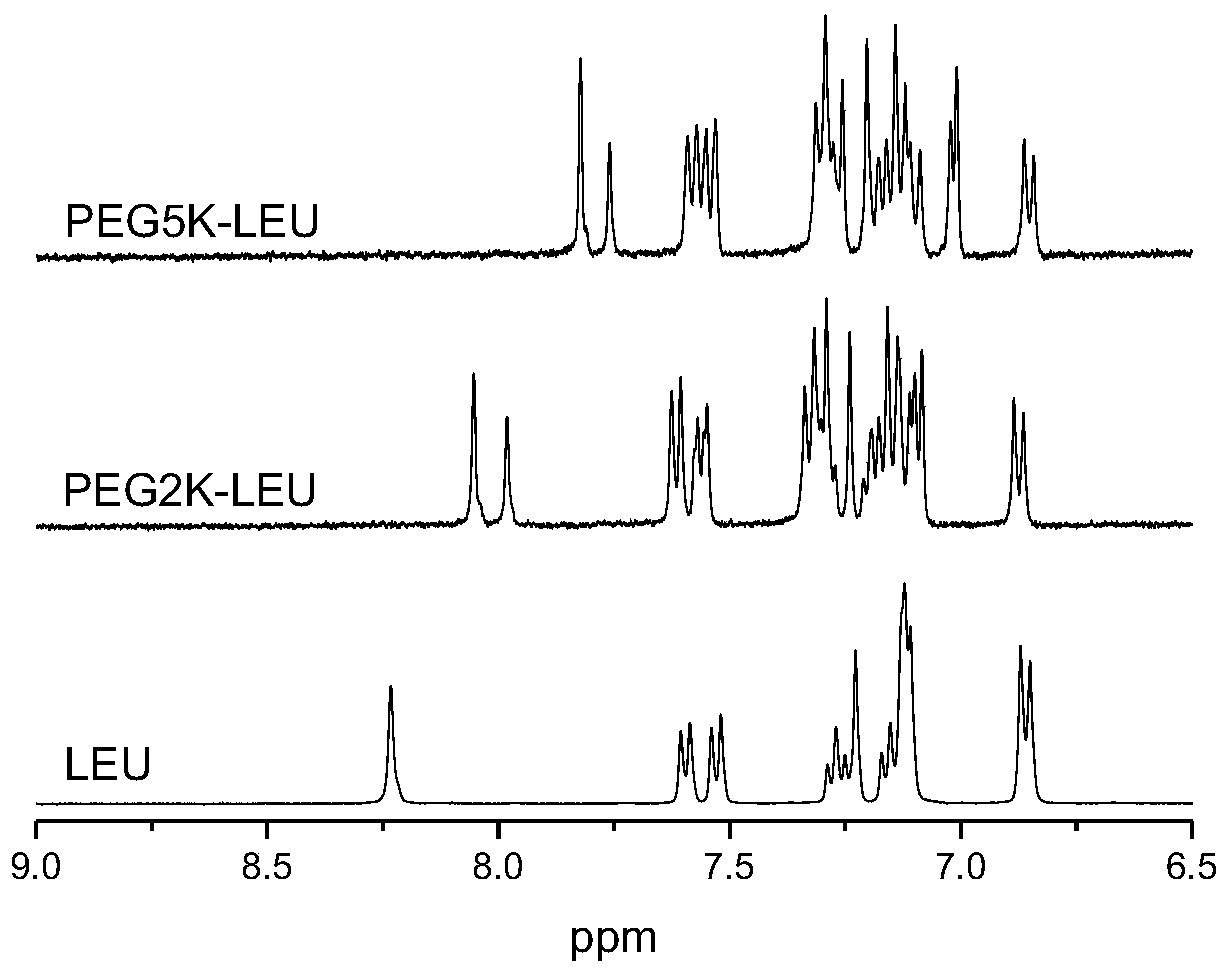
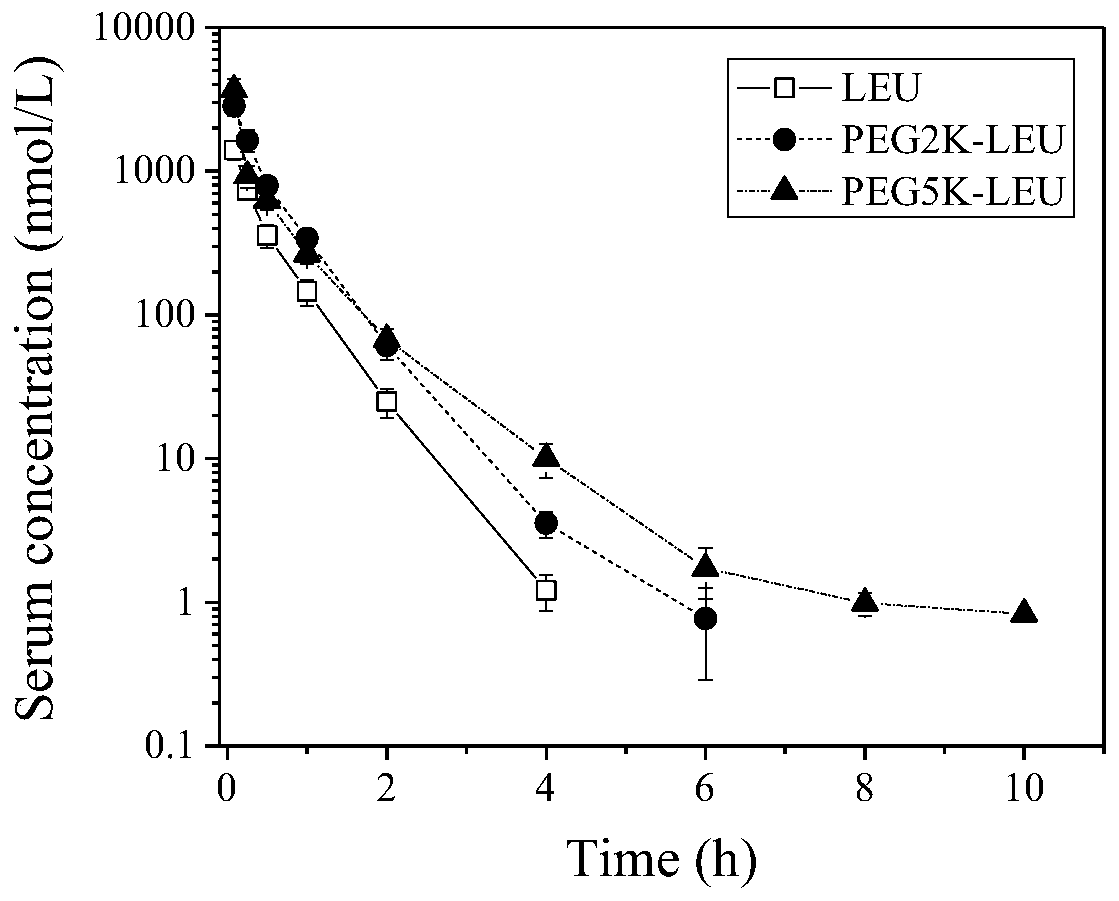
PEG-leuprolide conjugate and preparation method thereof
InactiveCN110639022AShort synthetic routeReduce lossesPeptide/protein ingredientsPharmaceutical non-active ingredientsImideMonomethoxypolyethylene glycol
The invention provides a novel PEG-leuprolide conjugate and a preparation method thereof. The preparation method comprises the steps that a methoxy-polyethylene glycol succinimide active ester directly reacts with unprotected leuprolide to obtain imidazolyl-modified PEG-leuprolide conjugate on the histidine 2-D-histidine. The conjugate retains leuprolide activity, a half-life period is extended, plasma clearance is reduced, and pharmacokinetic properties are improved.
Owner:NANKAI UNIV
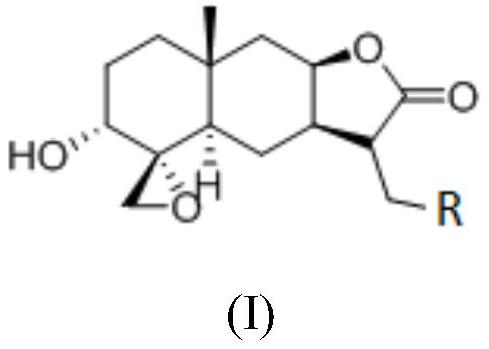
Sesquiterpene derivative as well as pharmaceutical composition, preparation method and application thereof
PendingCN114736214AStable structureExtended half-lifeOrganic active ingredientsOrganic chemistryPharmaceutical medicineSesquiterpene
The invention discloses a sesquiterpene derivative, and a pharmaceutical composition, a preparation method and application thereof. The invention relates to a sesquiterpenoid derivative shown in a formula (I) or a pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the sesquiterpenoid derivative, and a preparation method and application of the sesquiterpenoid derivative. The sesquiterpenoid derivative or the pharmaceutically acceptable salt thereof has a stable structure, a long half-life period and a slow plasma clearance rate, shows long-acting and stable drug release time, shows excellent anti-tumor activity, can be used as a candidate of a long-acting anti-tumor drug, and has wide application prospects. The potential clinical application value and the wide clinical application prospect are realized.
Owner:TIANJIN JIKUN MEDICAL TECH CO LTD
Construction method of tree shrew model for evaluating pharmacokinetics and pharmacodynamics of anti-influenza drugs
InactiveCN109453155AGood drug treatment predictabilityDetox InhibitionOrganic active ingredientsAntiviralsHalf-lifeBlood plasma
The invention provides a construction method of a tree shrew model for evaluating pharmacokinetics and pharmacodynamics of anti-infuenza drugs. The method adopts tree shrew as a platform to evaluate the drug effect of oseltamivir for treating the tree shrew infected by different influenza viruses. The drug plasma clearance half-time period and time to peak of the tree shrew after taking the oseltamivir are similar to that of mice, and the detoxification and corresponding inflammation response of H9N2 influenza viruses in the tree shrew body can be reduced by virtue of the treatment with the oseltamivir, so that the tree shrew can be used as an influenza animal model with the drug treatment foreseeability.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +3
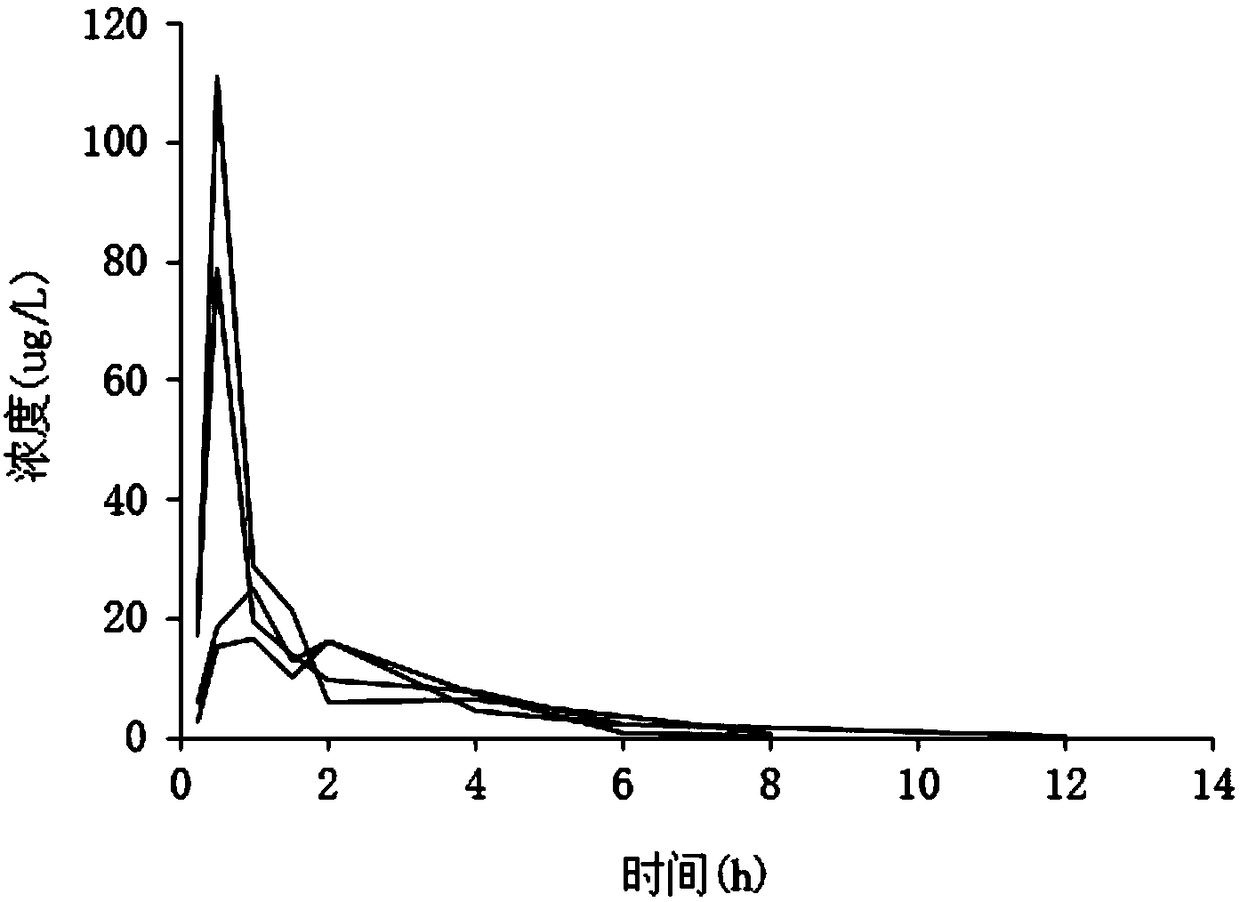
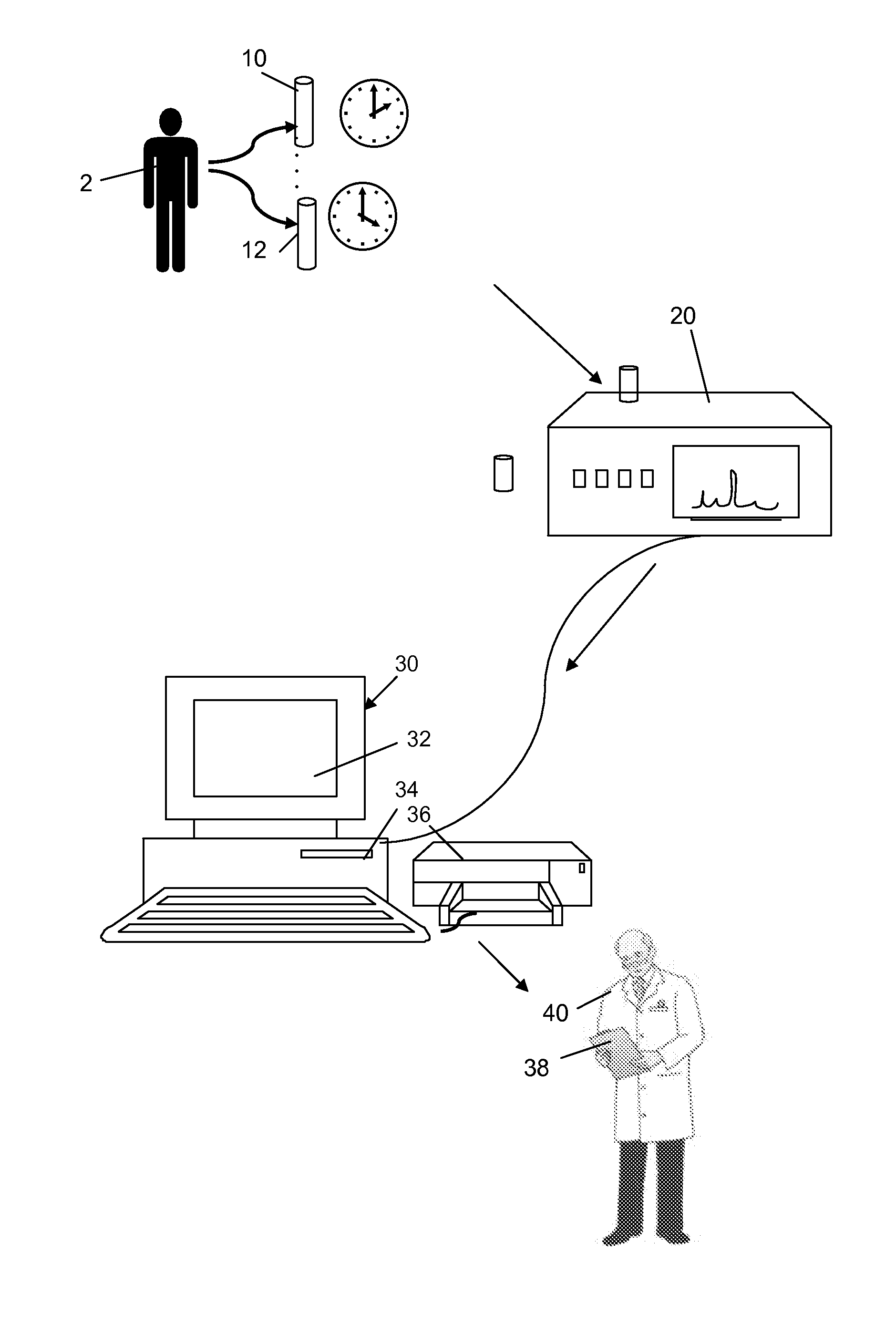
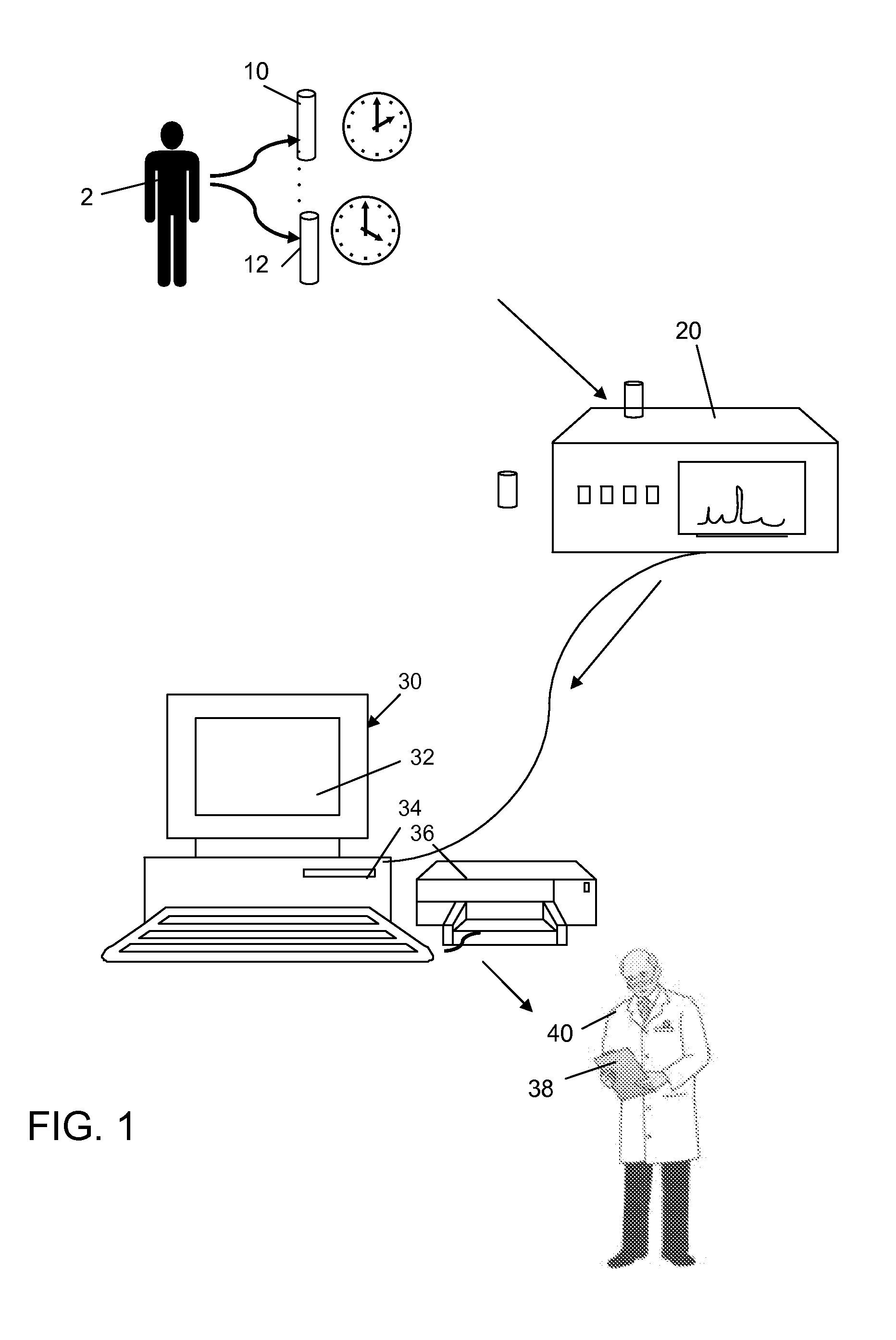
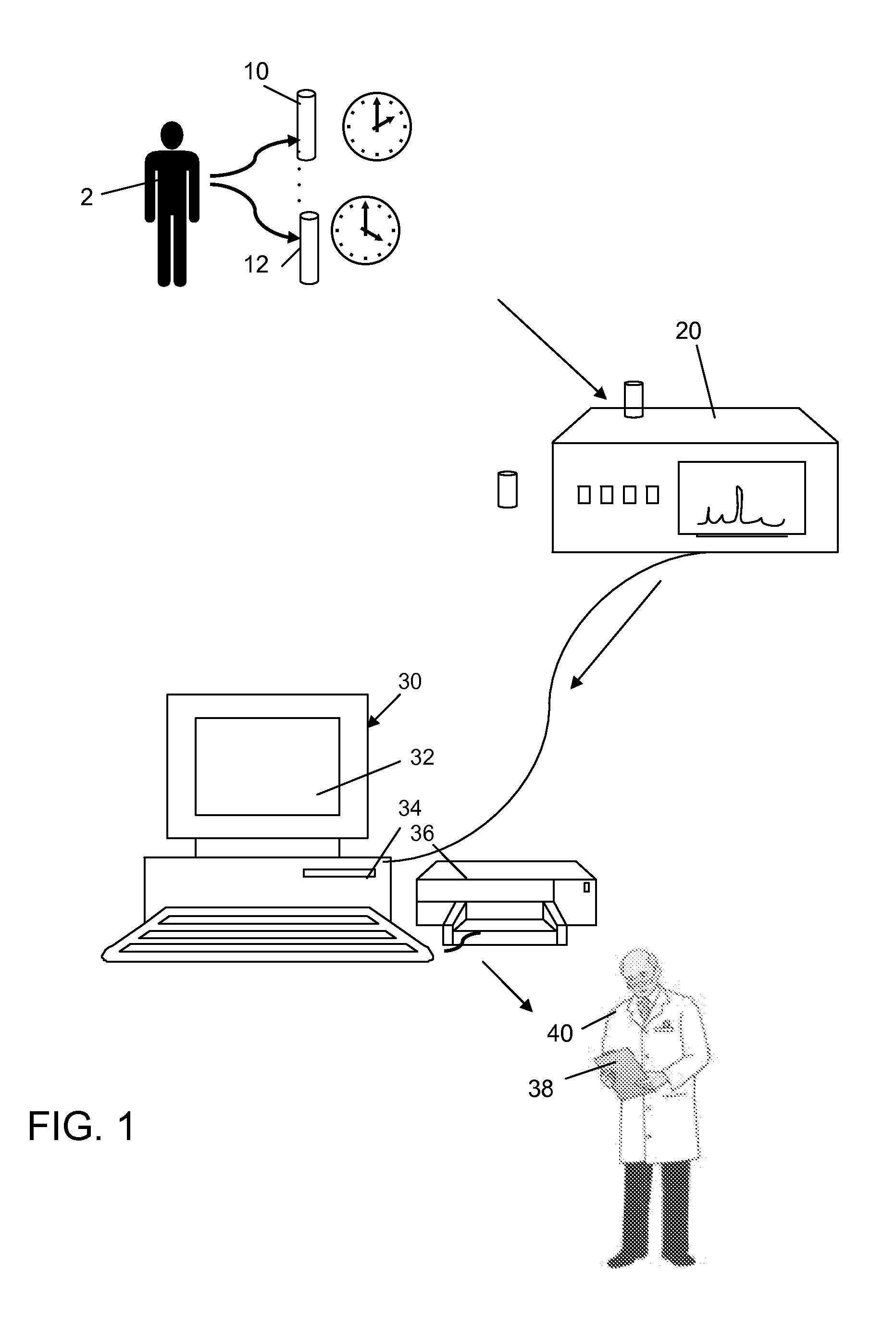
Method for Evaluating Renal Function
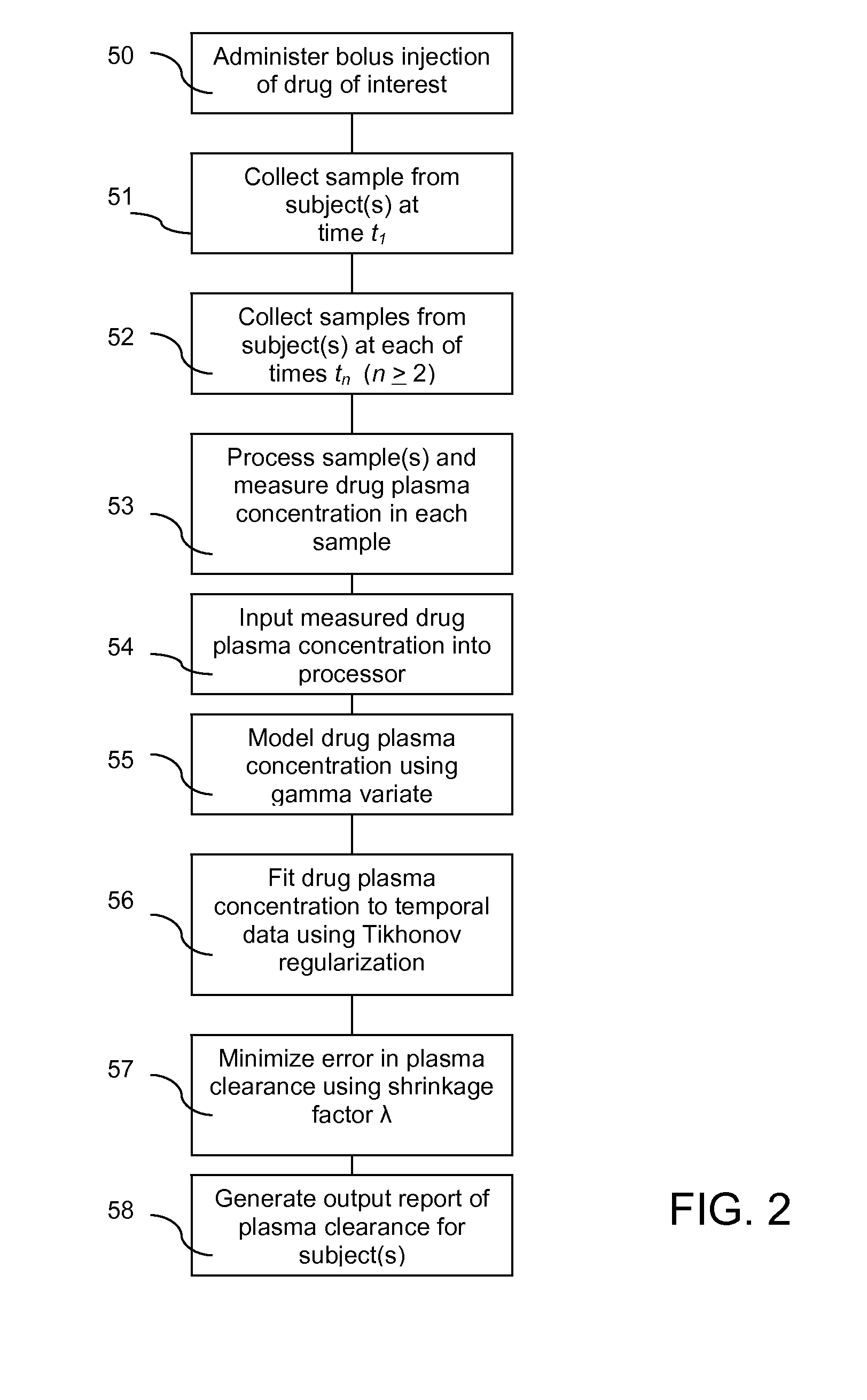
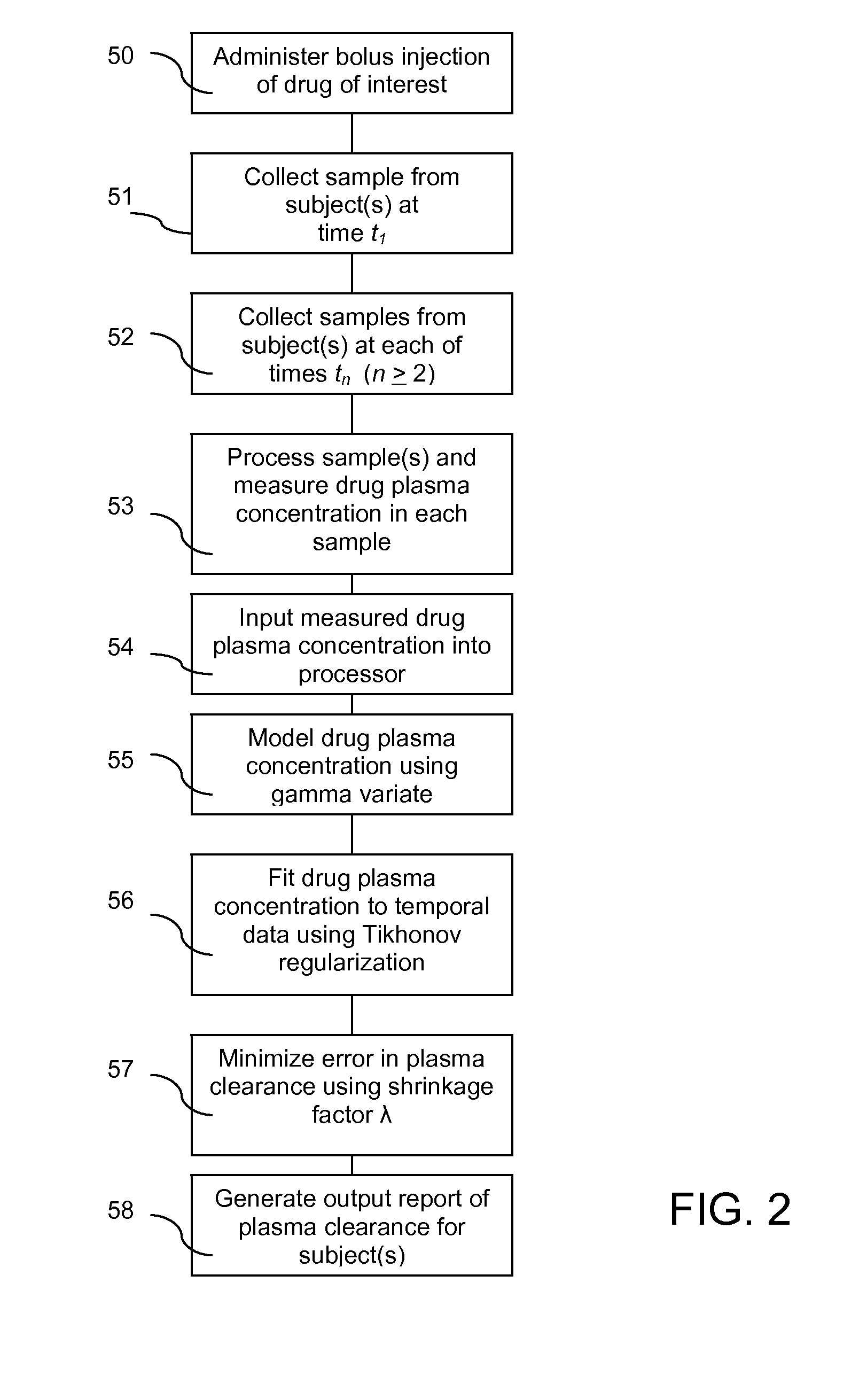
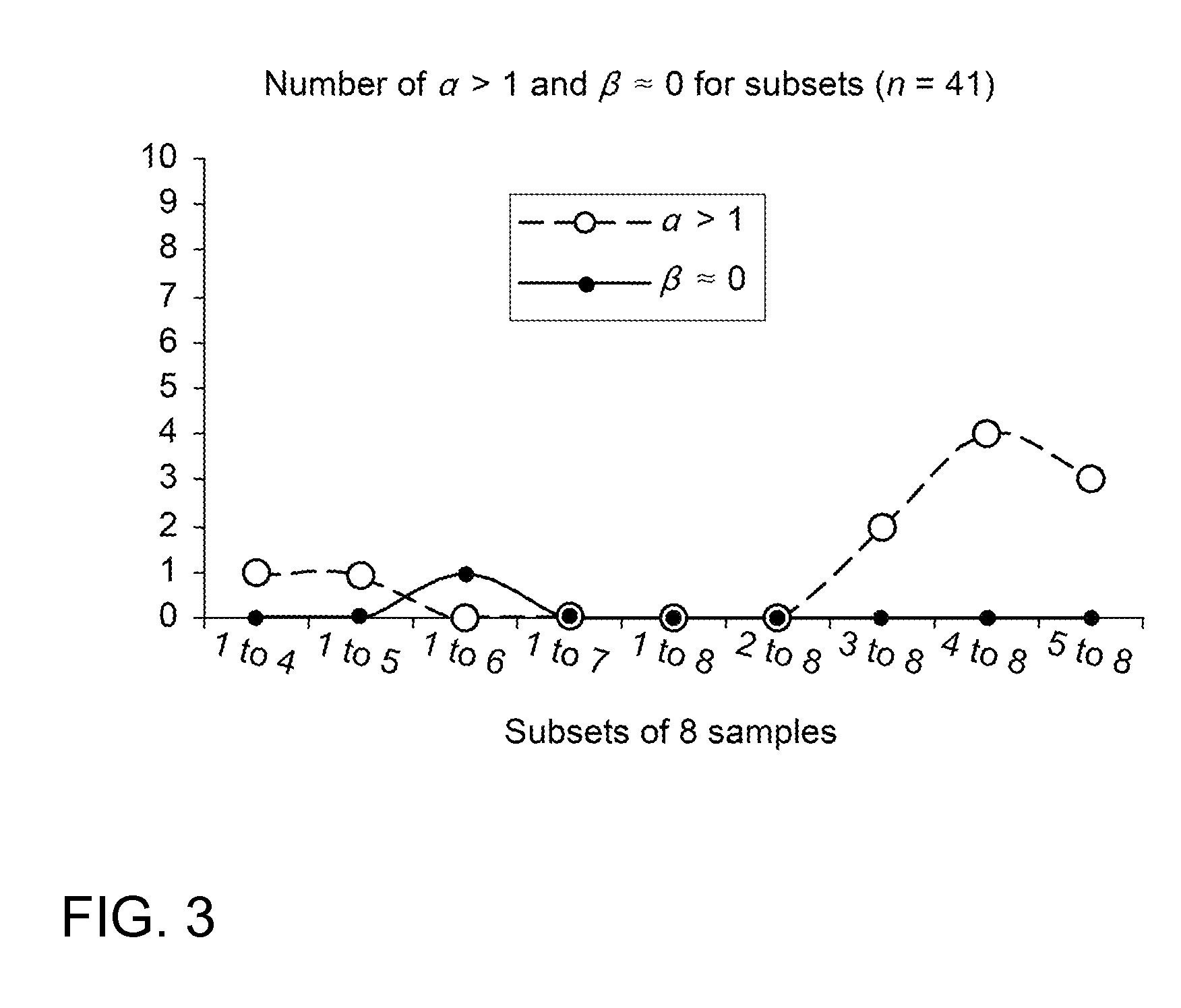
ActiveUS20120123694A1Error minimizationEasy to useSamplingImage data processing detailsMedicineErrors and residuals
Plasma concentration of a compound of interest is measured in two or ideally 4 or more blood samples taken from a patient over a period of time following bolus injection. The measured values are input into a computer processor programmed to execute a computer program comprising an algorithm that uses the gamma variate (GV) function to model drug plasma concentration, then uses Tikhonov regularization to perform the fit, selecting a regularization constant so that the relative error in the plasma clearance is minimized. One or more output values representative of renal function are generated.
Owner:WESOLOWSKI CARL A
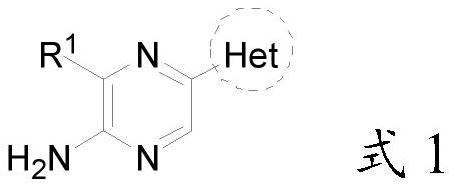
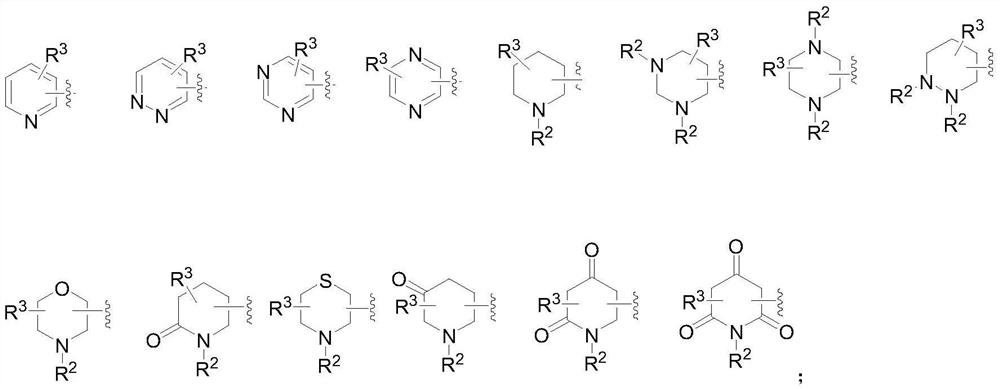
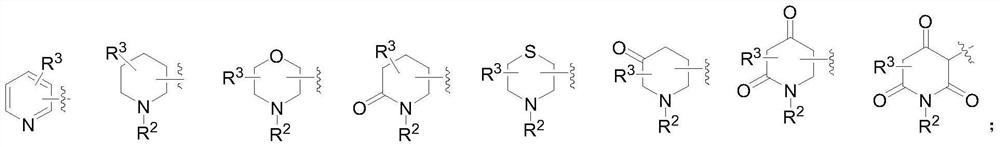
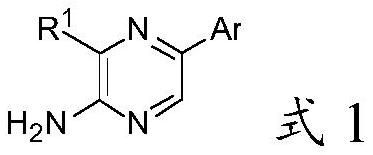
2-amino-5-heterocyclyl-substituted pyrazine derivative and application thereof
The invention provides a 2-amino-5-heterocyclyl-substituted pyrazine derivative with a chemical structure shown in a formula 1, a pharmaceutical preparation containing the 2-amino-5-heterocyclyl-substituted pyrazine derivative, and application of the 2-amino-5-heterocyclyl-substituted pyrazine derivative in medicines for treating or preventing malaria. The compound provided by the invention has effects significantly superior to those of the prior art against plasmodium falciparum proliferation and against plasmodium falciparum of different strains, and has a longer half-life period, a lower plasma clearance rate, a higher distribution volume and better oral bioavailability.
Owner:LUNAN PHARMA GROUP CORPORATION
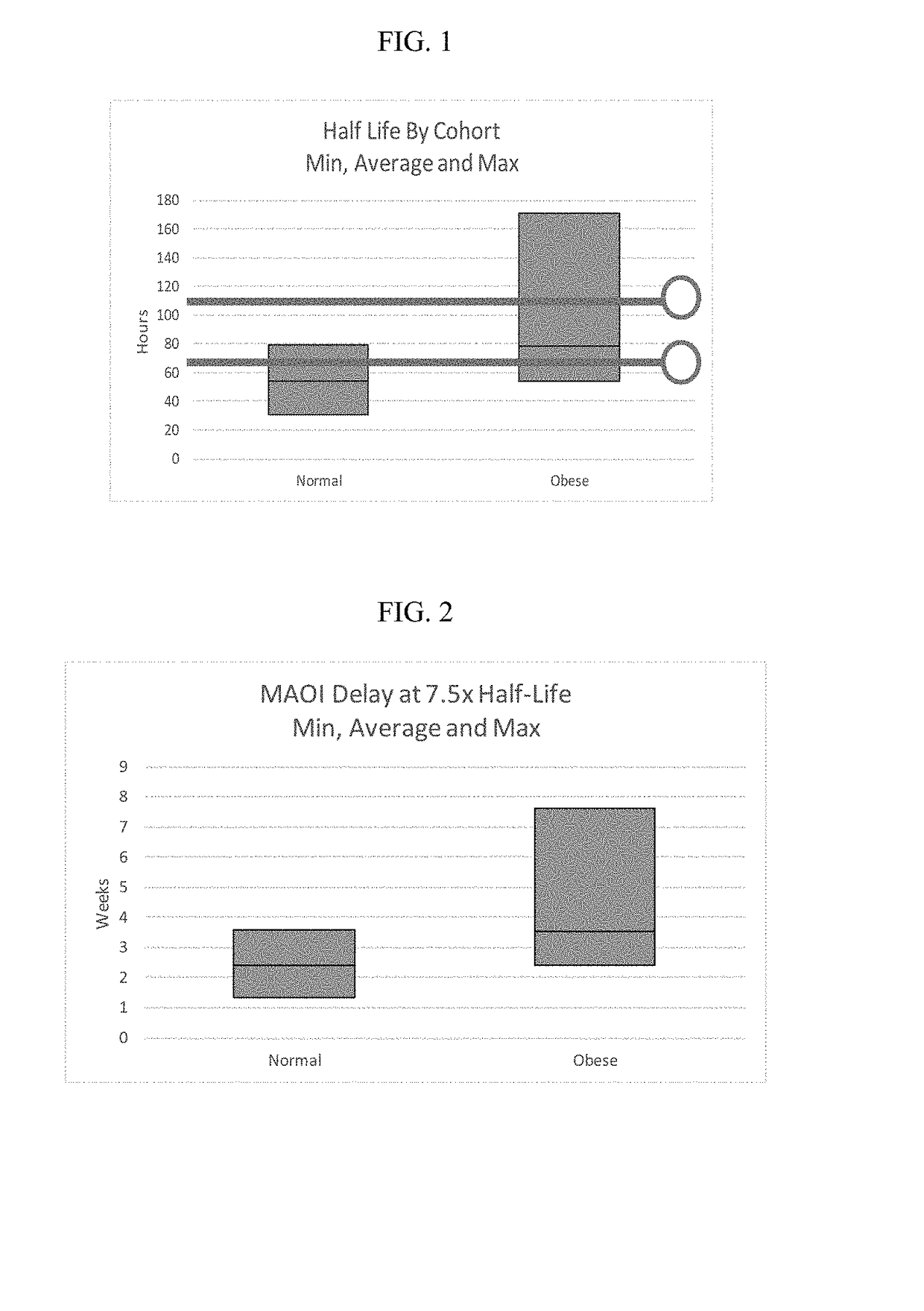
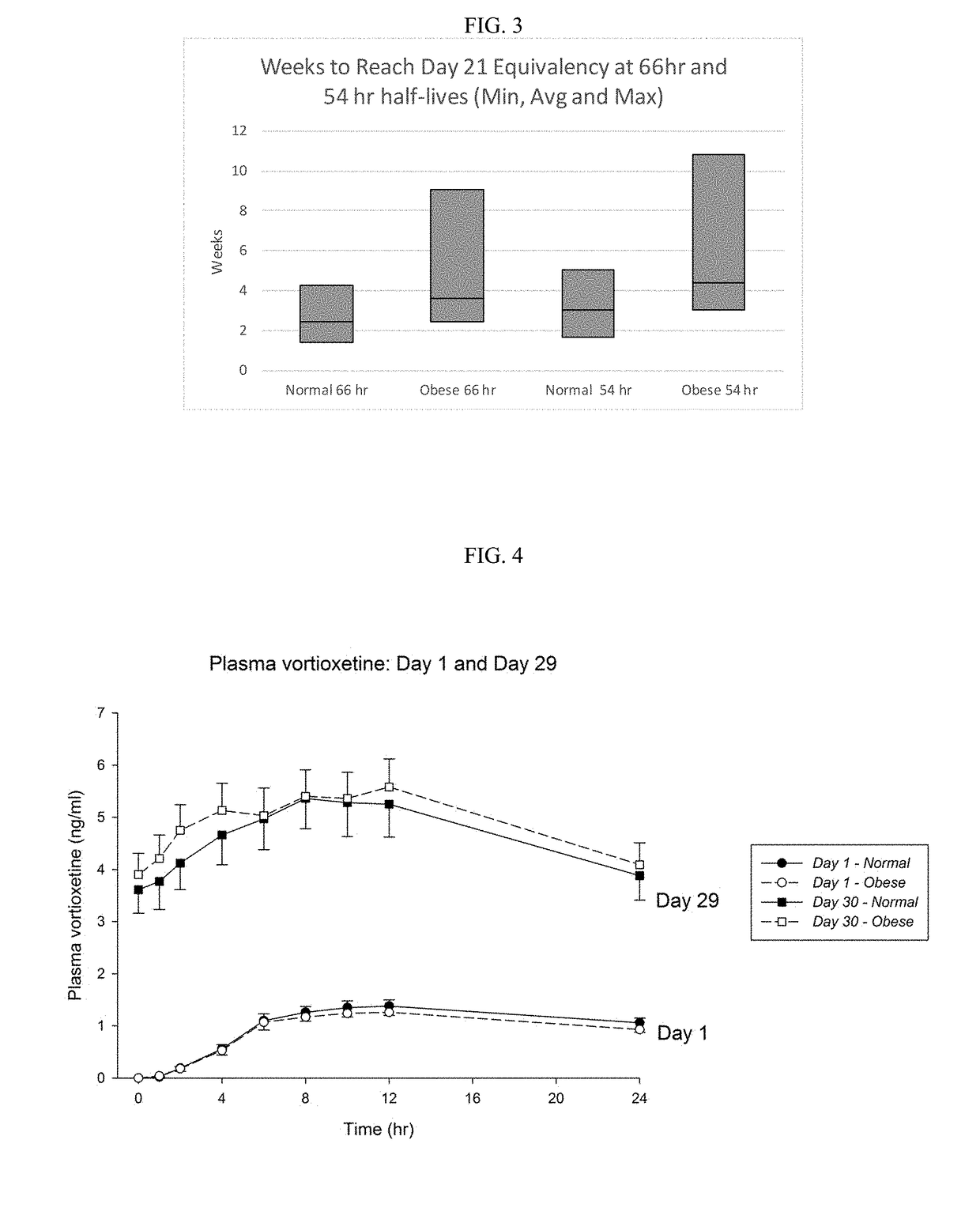
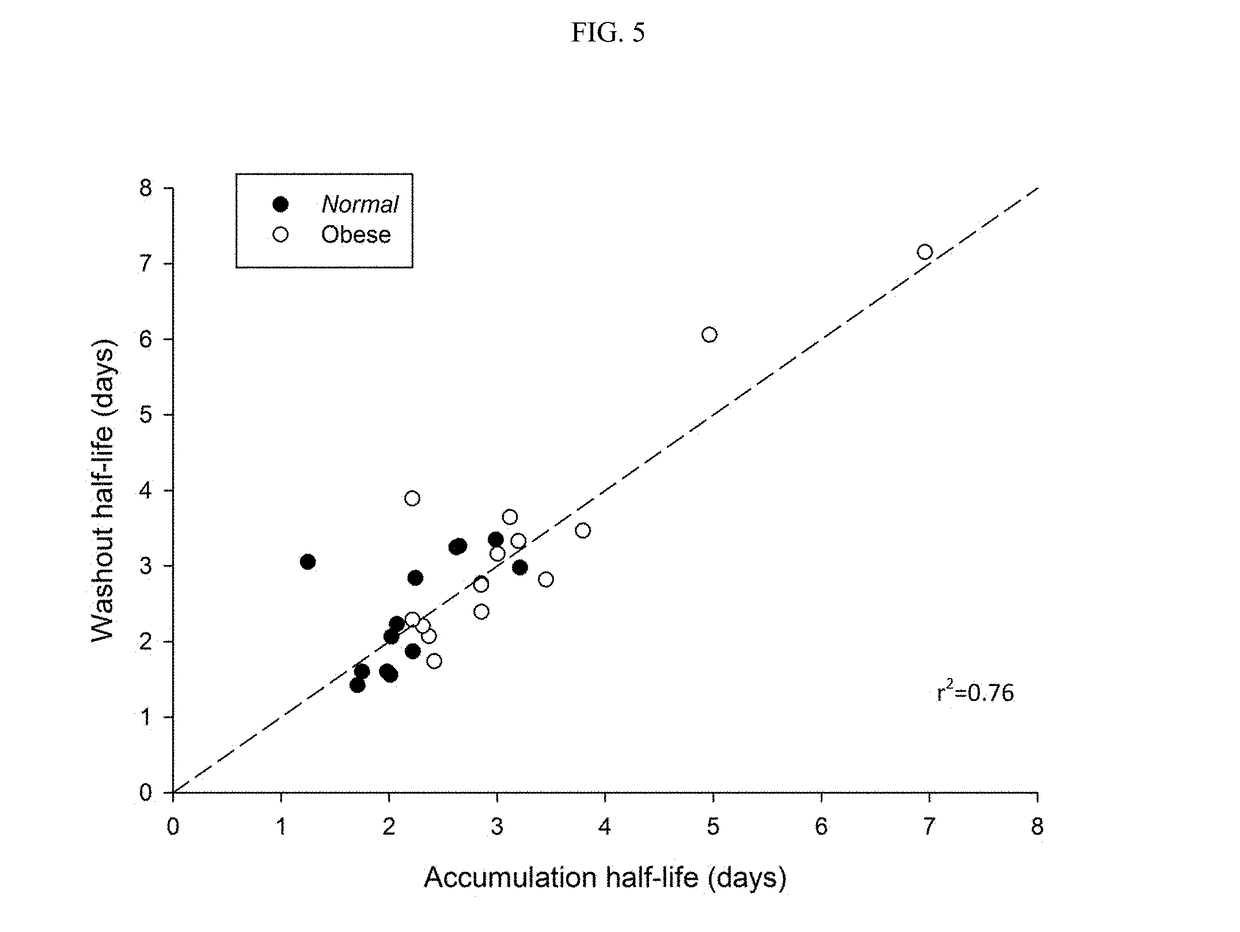
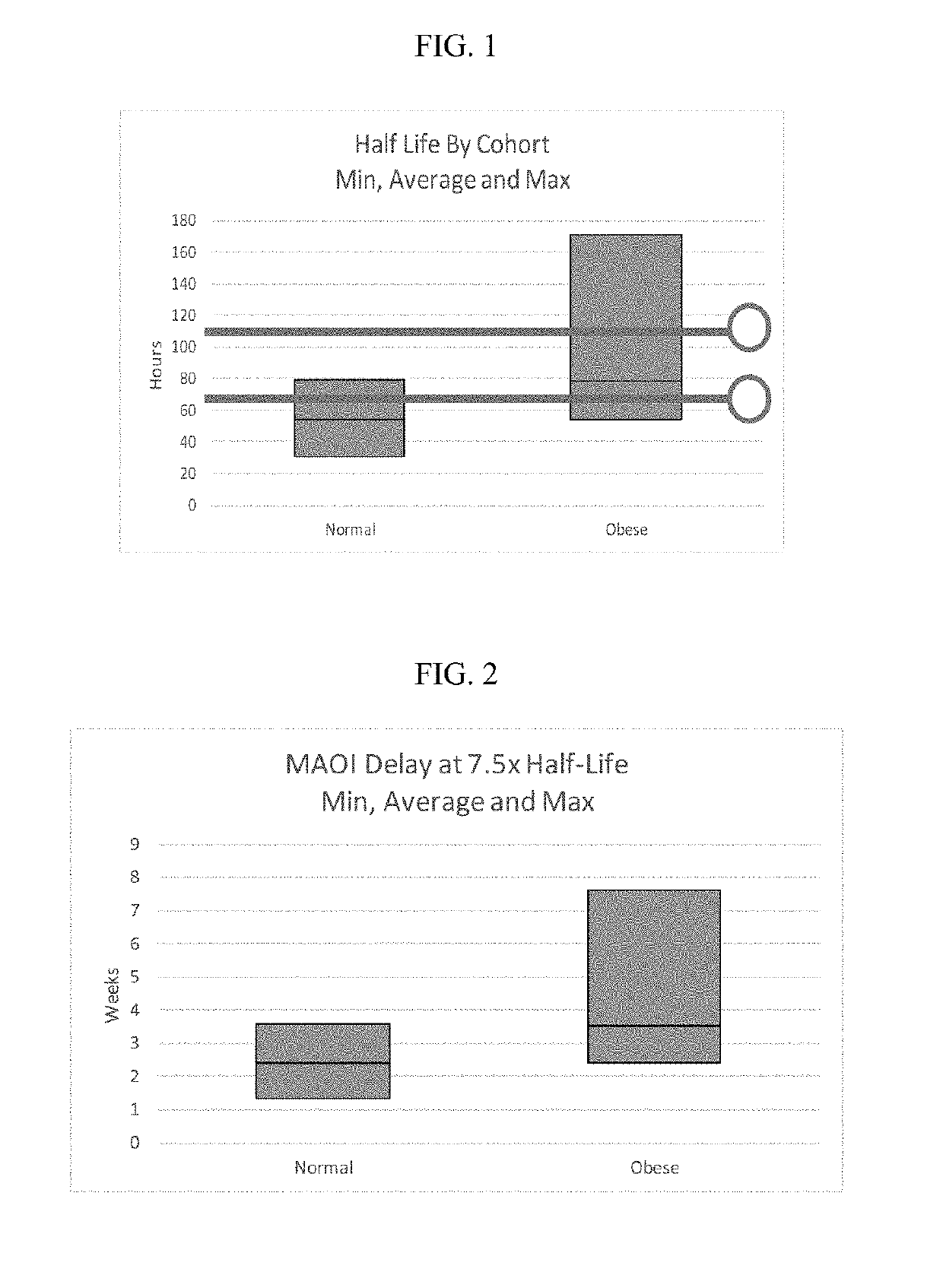
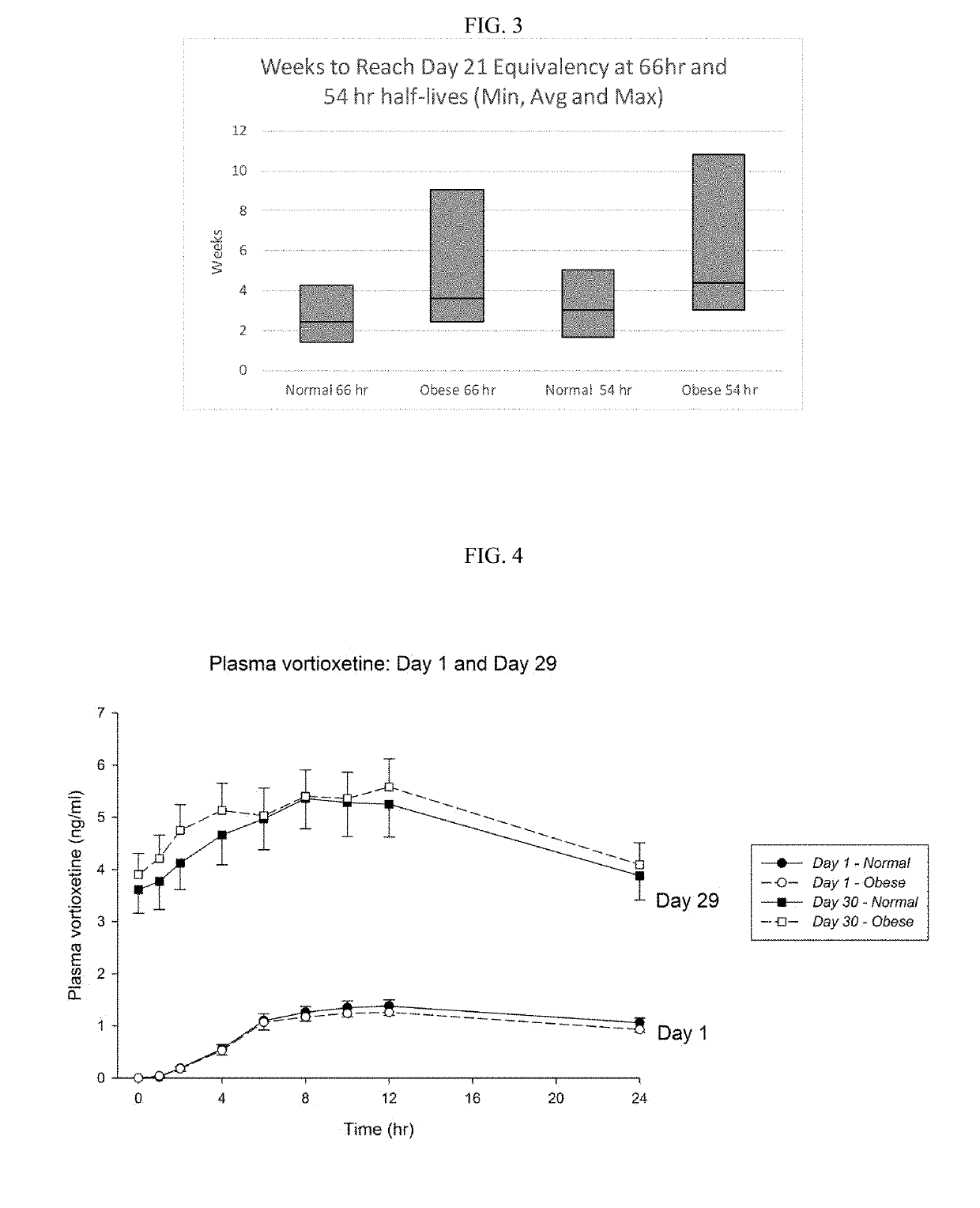
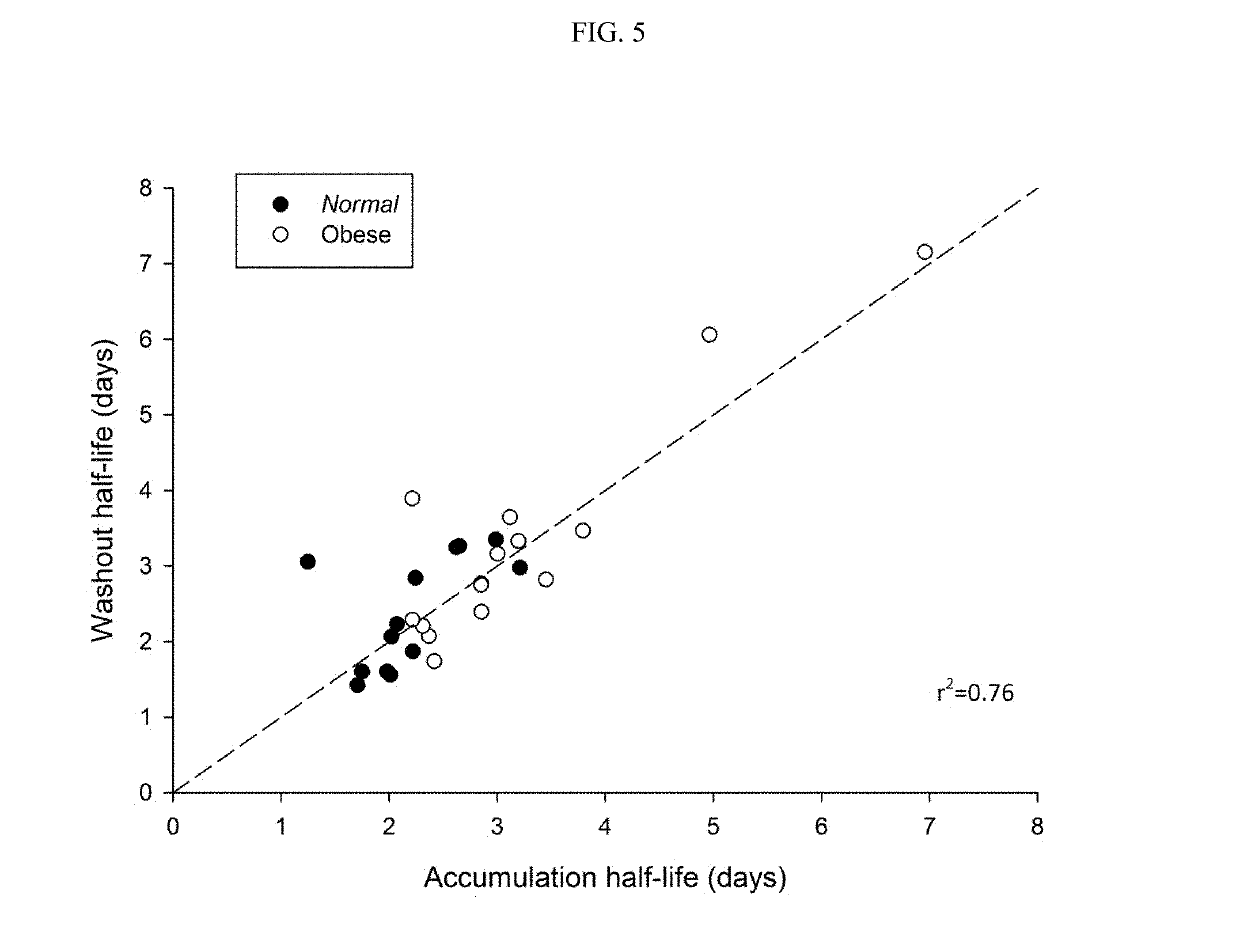
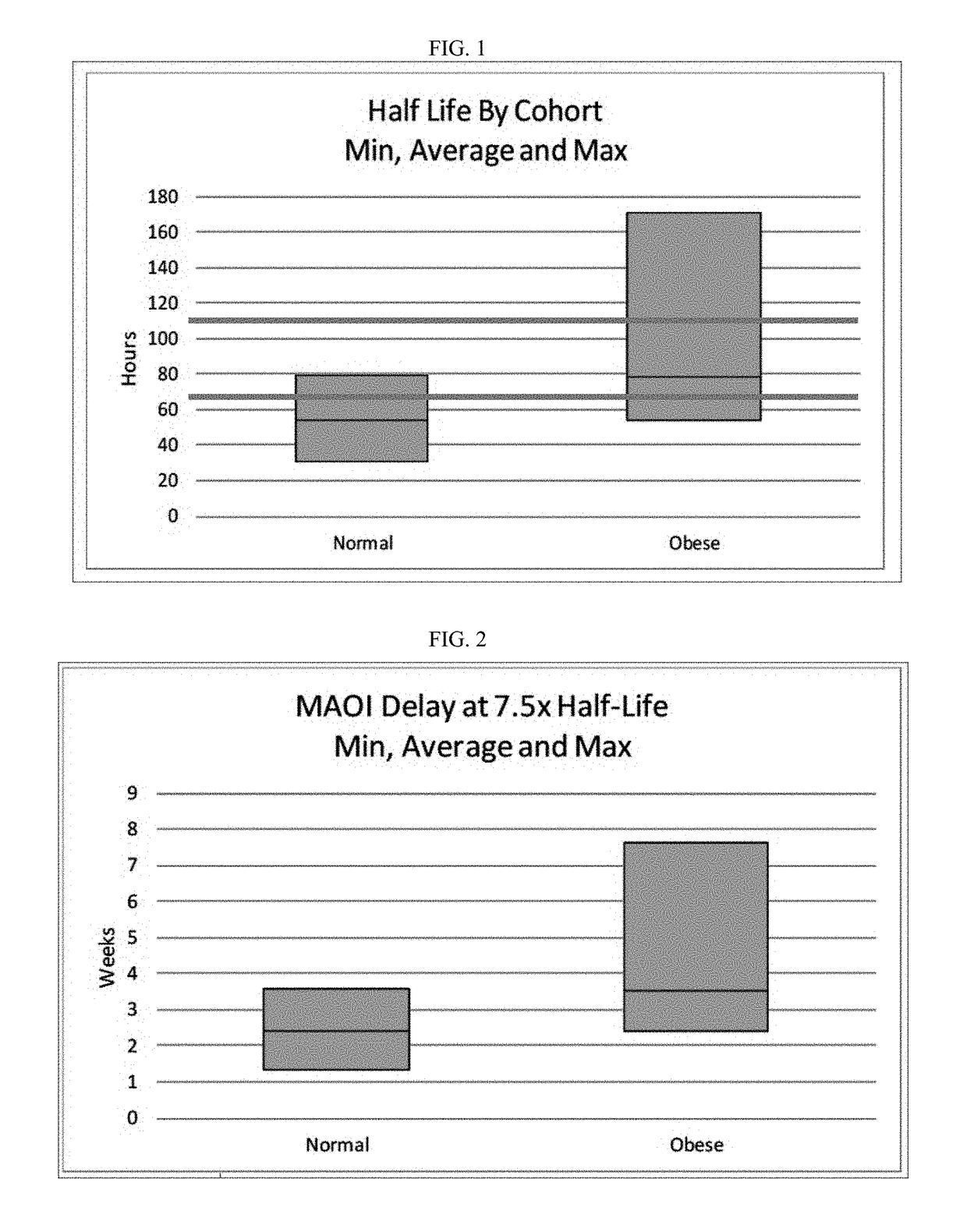
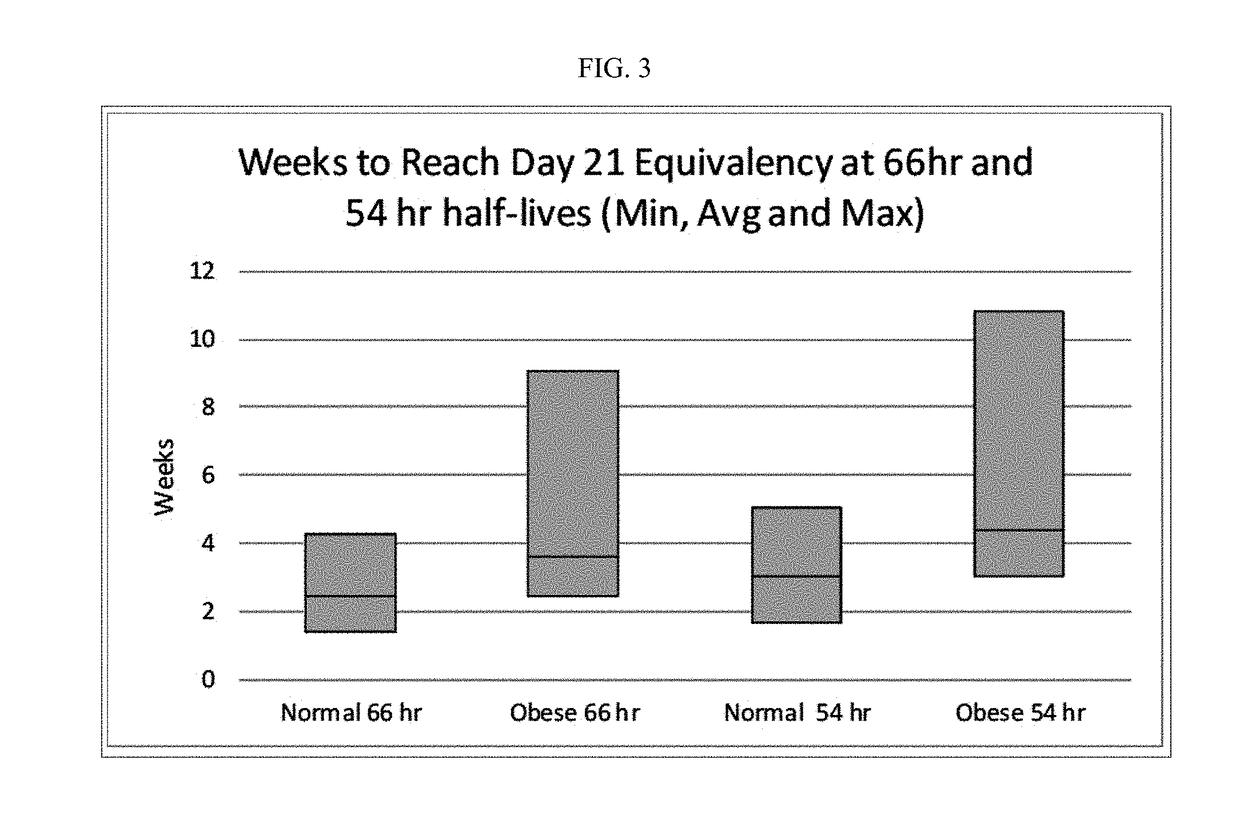
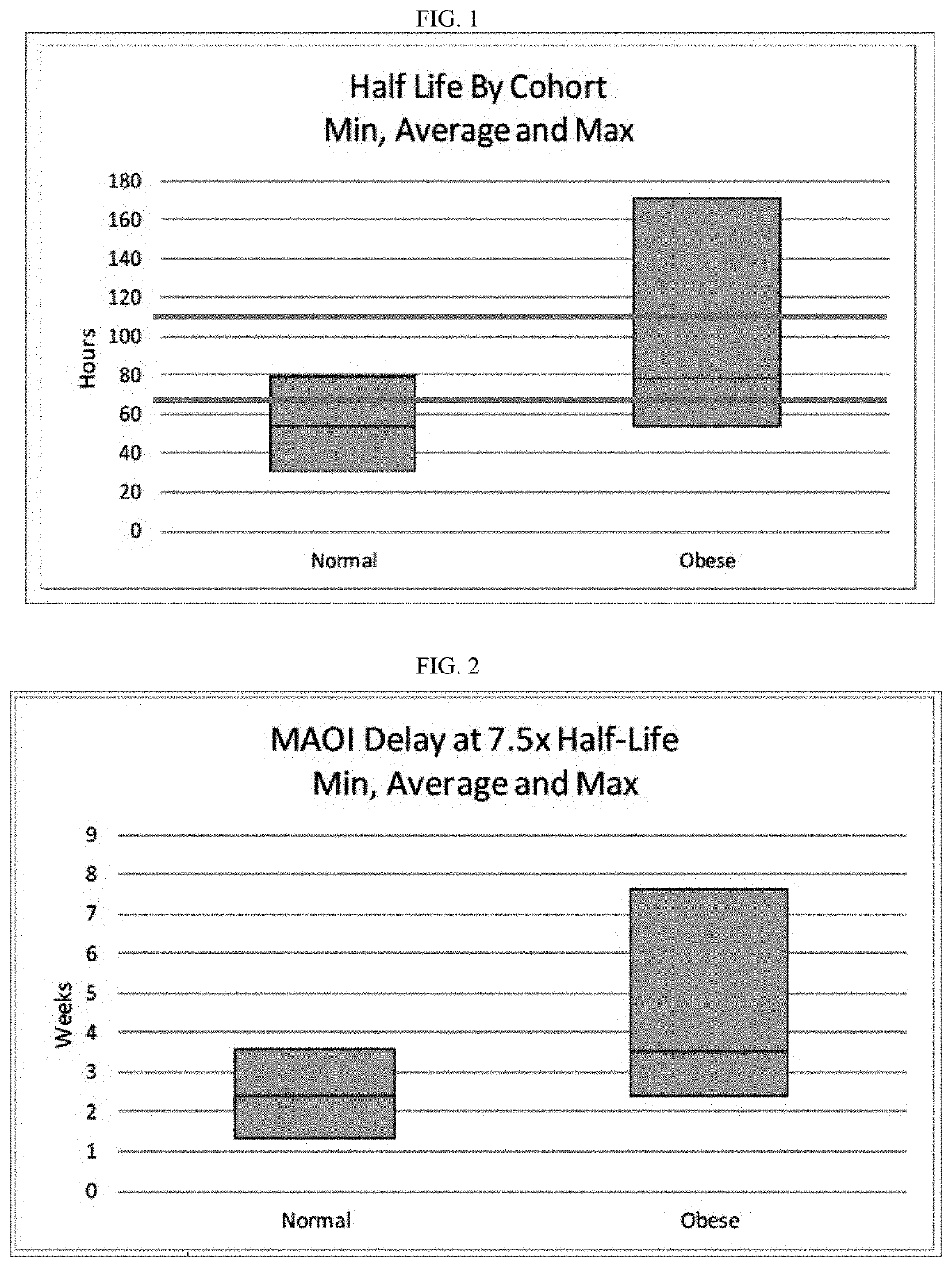
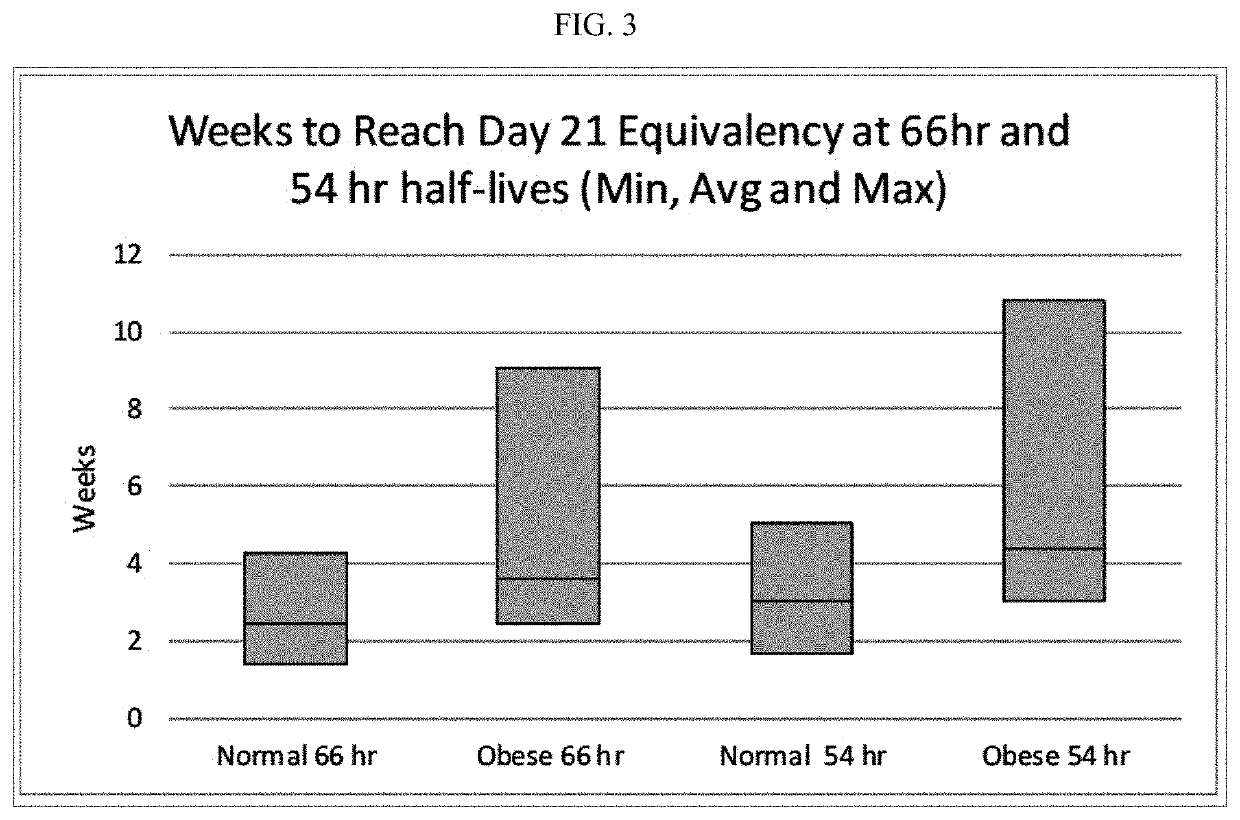
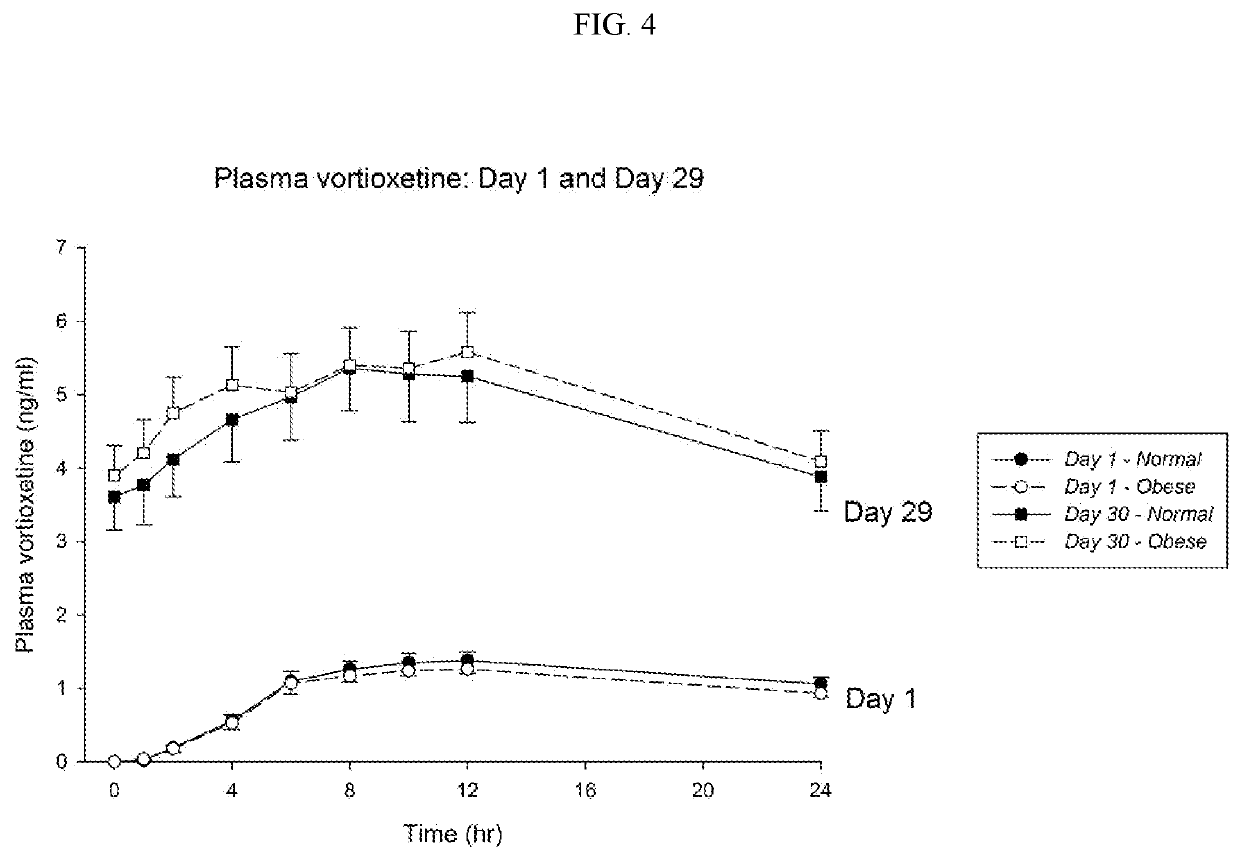
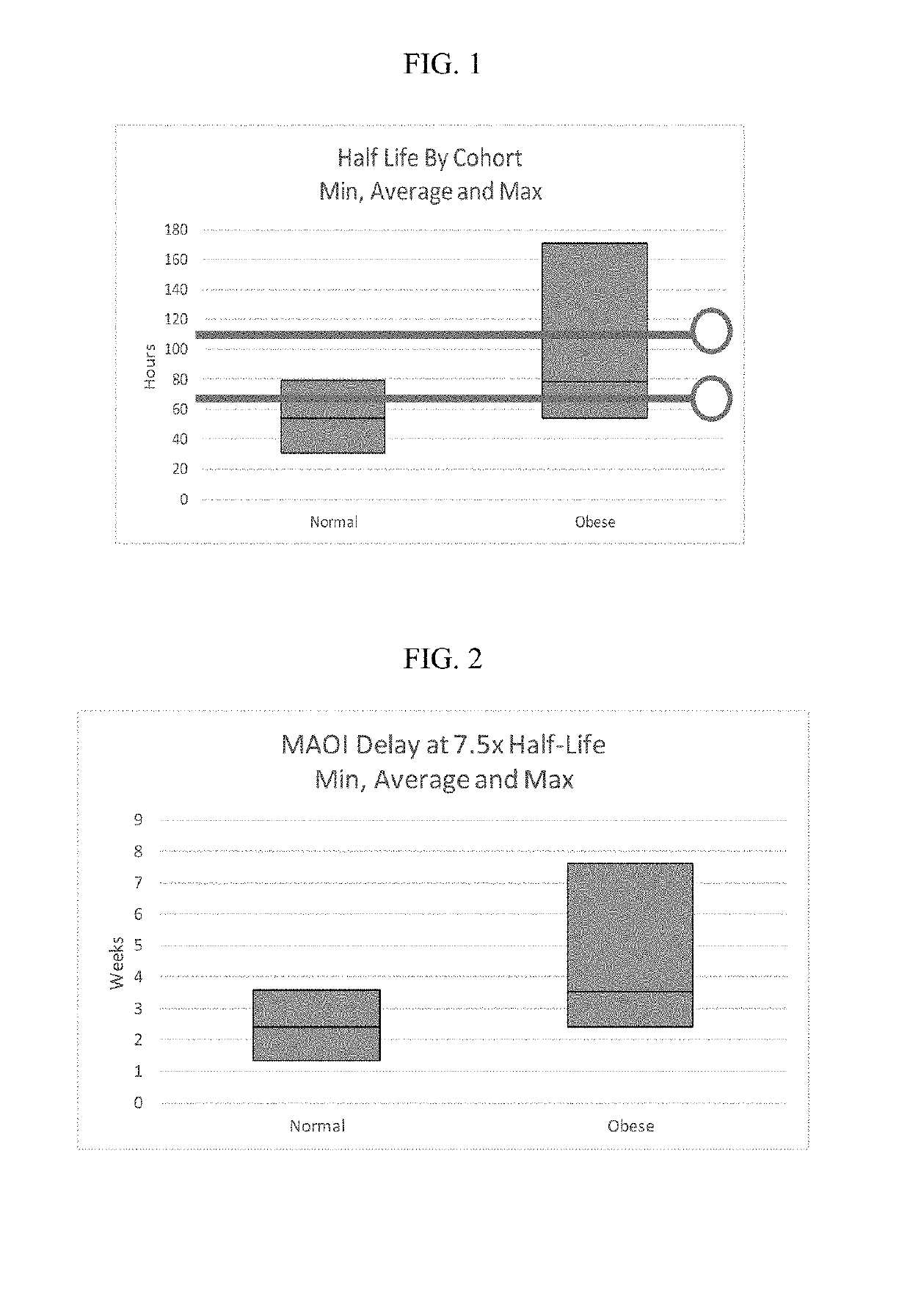
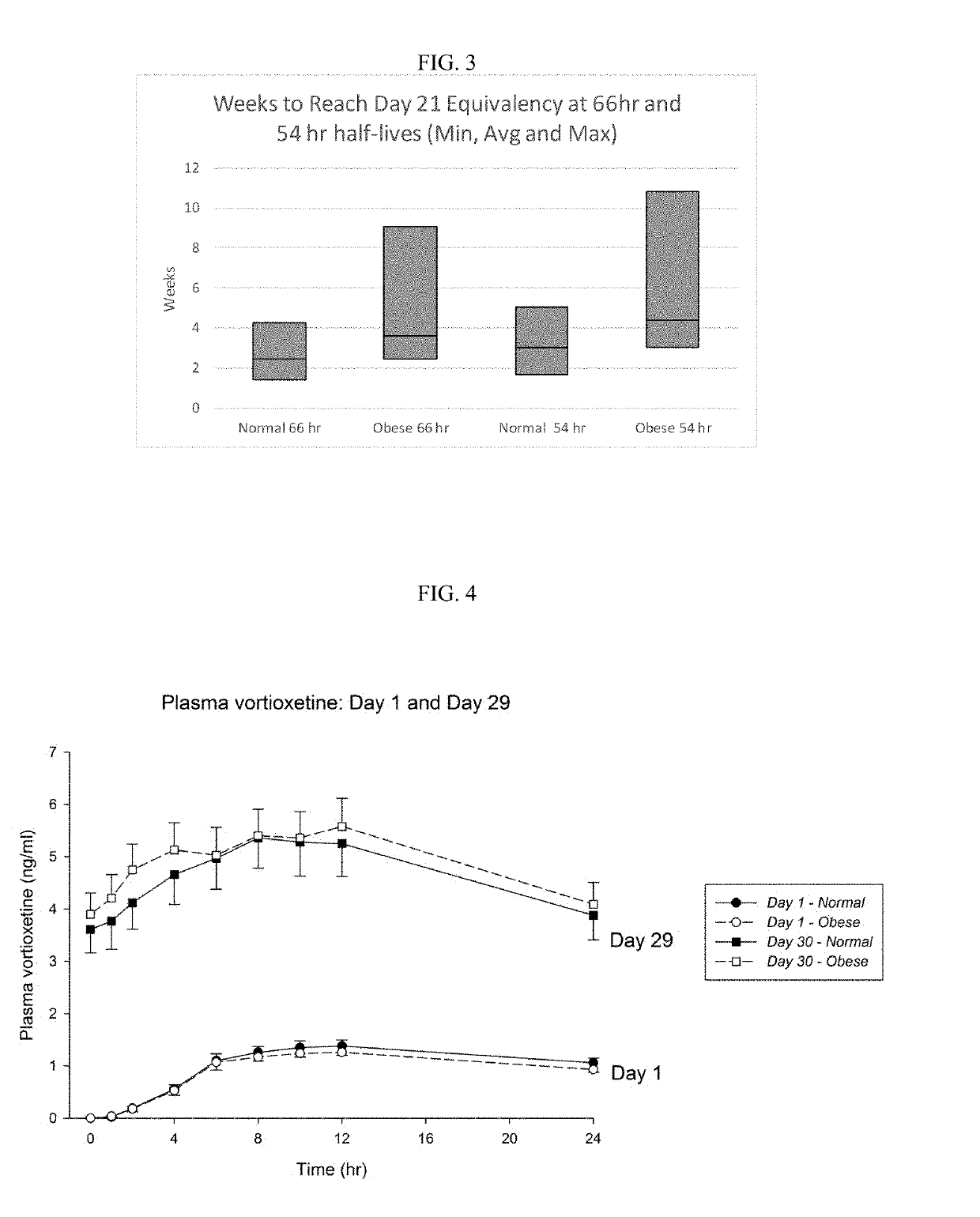
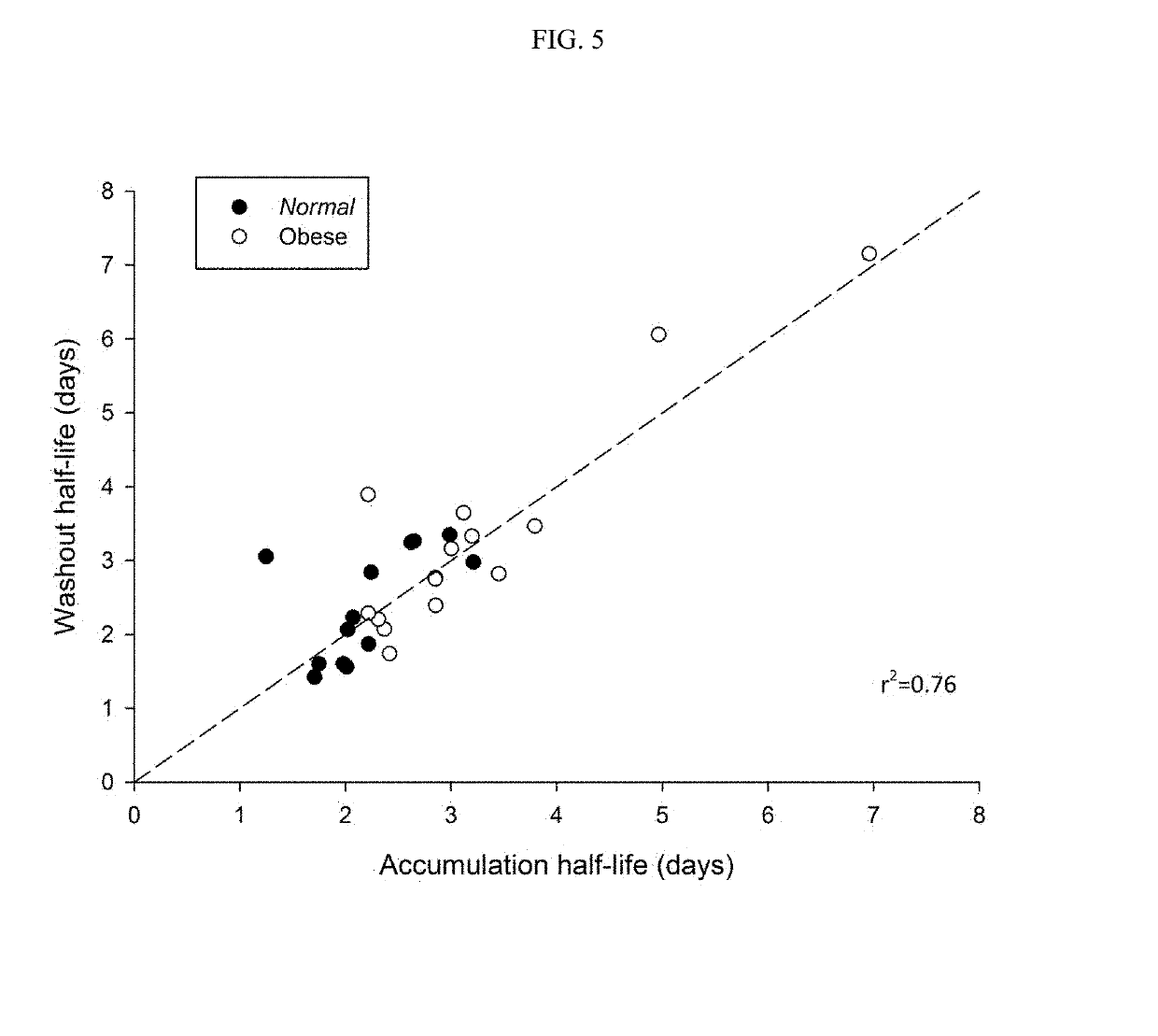
Methods of treating depression
ActiveUS20180280382A1Extended half-lifeReduce eliminateOrganic active ingredientsNervous disorderDiseasePsychiatry
The present disclosure relates to methods of transitioning patients or obese patients being treated with vortioxetine to treatment with a monoamine oxidase inhibitor (MAOI). The methods provided include delaying administration of the MAOI for certain time periods after stopping administration of vortioxetine. The patients or obese patients possess various capabilities of metabolizing vortioxetine. The current disclosure also includes methods of switching patients to a MAOI intended to treat psychiatric disorders while being treated with vortioxetine. The methods disclosed further comprise determining vortioxetine plasma clearance and washout time for patients with different body fat status and / or different CYP2D6 metabolizer status.
Owner:RUNDLE RES LLC
Polyethylene glycol modified and recombined human interleukin and its preparation method
InactiveCN1651461AStrong antiviral activityLow plasma clearancePeptide/protein ingredientsAntiviralsPolyethylene glycolBlood plasma
A polyethanediol modified recombinant human interleukin used for preparing the antiviral and anticancer medicines features that one or more polyethanediol chains are linked to recombinant human interleukin for higher activity and stability.
Owner:EAST CHINA UNIV OF SCI & TECH
Human interferon alpha derivatives and preparation and use of pegylated products thereof
The invention relates to human interferon alpha derivatives and preparation and use of pegylated products thereof. The human interferon alpha derivatives are formed by bonding three amino acids to N terminals of human interferon alpha and have a structural formula of R3-R2-R1-interferon, wherein the interferon is the human interferon alpha, and a first position amino acid R1 bonded to the N terminals of the human interferon alpha is a sculpture amino acid or glycine; the second position amino R2 is an aromatic amino acid or glycine; and the third position amino acid R3 is an acidic amino acidor glycine. The invention also provides pegylated derivatives of the human interferon alpha derivatives, a preparation method thereof and use thereof in the preparation of medicaments for treating and preventing viral infection or lung cancer. The human interferon alpha derivatives have the advantages of high specific activity and high pegylation rate. The pegylated derivatives of the human interferon alpha derivatives have the characteristics of high biological activity and no non-N terminal modified isomers and have the advantages of prolonging in-vivo half-life, reducing plasma clearance and the like.
Owner:BEIJING SIHUAN PHARMA +1
2-amino-5-heteroaryl substituted pyrazine derivative and application thereof
The invention provides a 2-amino-5-heteroaryl substituted pyrazine derivative with a chemical structure as shown in a formula 1, a pharmaceutical preparation containing the 2-amino-5-heteroaryl substituted pyrazine derivative, and application of the 2-amino-5-heteroaryl substituted pyrazine derivative in drugs for treating or preventing malaria. Compared with the prior art, the compound has the effects of resisting plasmodium falciparum proliferation and resisting plasmodium falciparum of different strains, and has the advantages of longer half-life period, lower plasma clearance rate, higher distribution volume and better oral bioavailability.
Owner:徐新杰
Development for tanshinol-naringenin composite pellet
InactiveCN106474091APromote dissolutionGuaranteed uniformityOrganic active ingredientsPharmaceutical delivery mechanismEnterohepatic circulationSalvia miltiorrhiza
The invention, which belongs to the medicine field, relates to development for a tanshinol-naringenin composite pellet. Tanshinol being a water-soluble component of the root of red-rooted salvia being Chinese medicine is effective in treating angina pectoris. At present, most of tanshinol preparations being available in the market are immediate-release dosage forms that can not satisfy the demand of long-term therapy for angina pectoris; the bioavailability, being lower than 10%, of the preparation is low and the metabolism is fast, wherein t1 / 2 is equal to 0.5h approximately; and the pure sustained-release pellet can not keep enough blood concentration for long time and thus is not effective in treating angina pectoris. Besides, naringenin is capable of inhibiting activity of P450enzymeCYP3A4; and after taking of the naringenin with tanshinol, the metabolic process of the tanshinol in vivo can be inhibited, so that the bioavailability of tanshinol is enhanced and the plasma clearance is reduced. Therefore, according to development provided by the invention, tanshinol and naringenin are prepared into a composite pellet. In addition, enterohepatic circulation exists during oral naringenin taking and thus a long-acting effect can be realized by an immediate-release dosage form, so that the naringenin is prepared into an immediate-release pellet and the tanshinol is prepared into a sustained-release pellet.
Owner:CHINA PHARM UNIV
Methods of treating depression
Owner:RUNDLE RES LLC
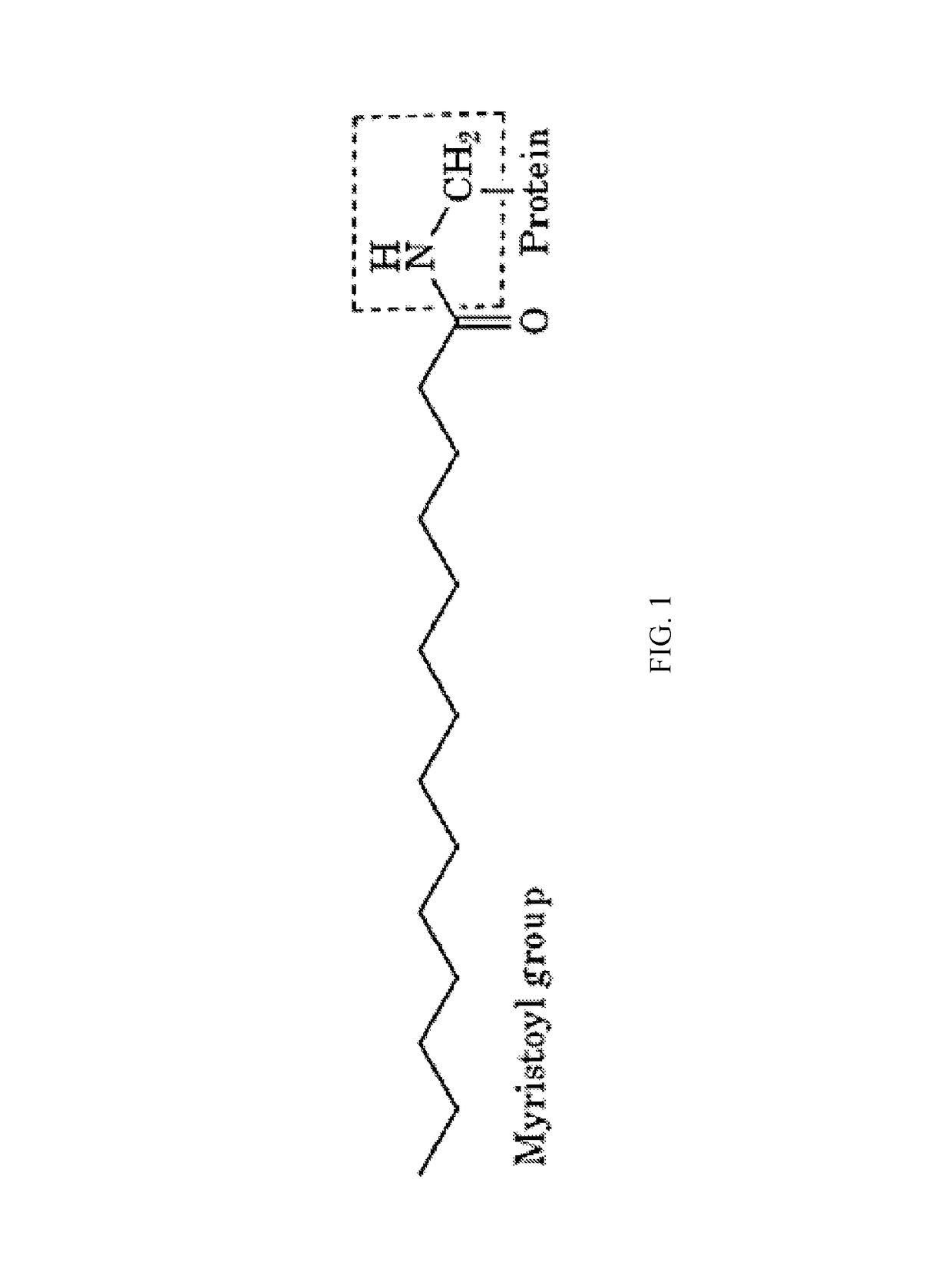
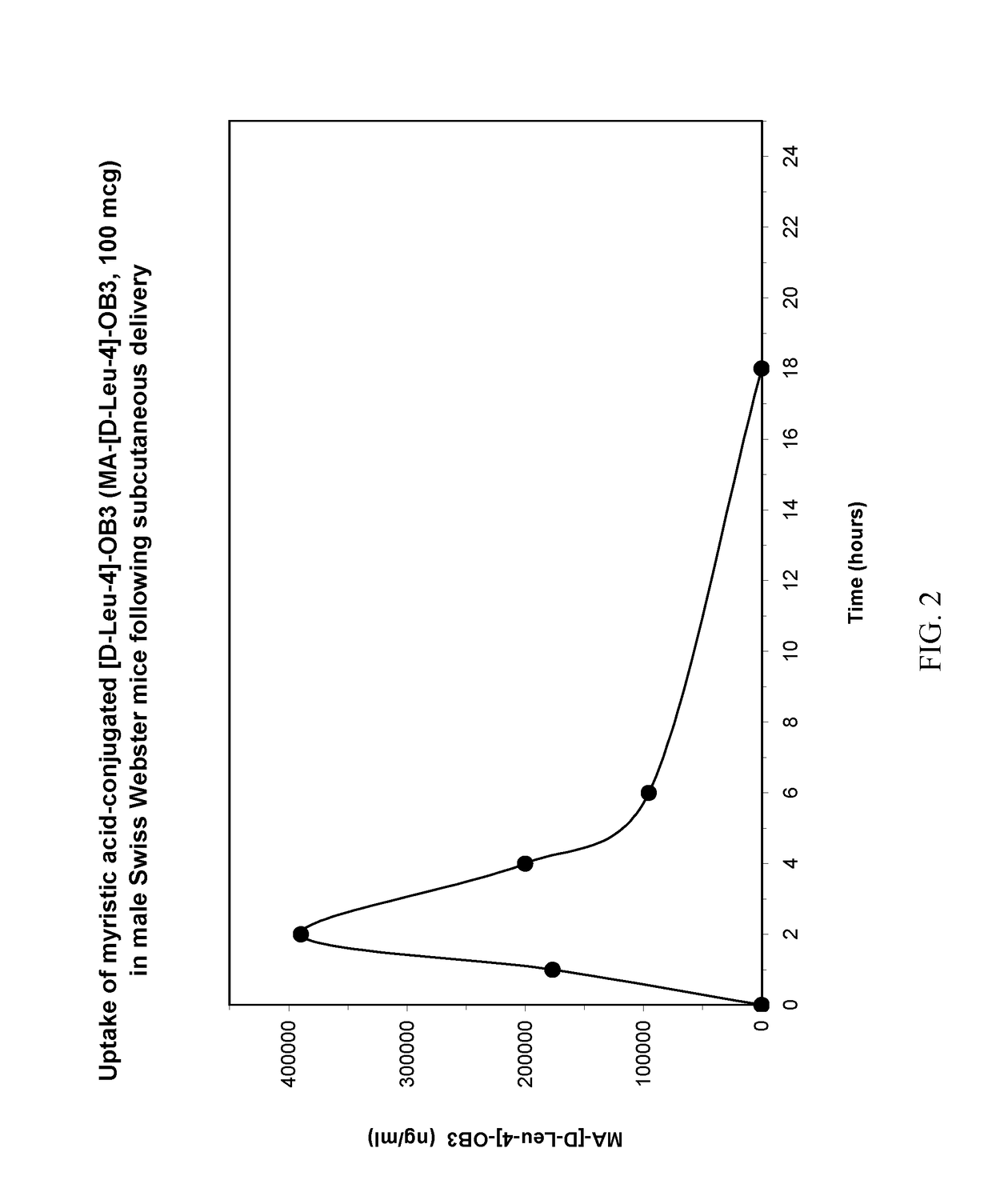
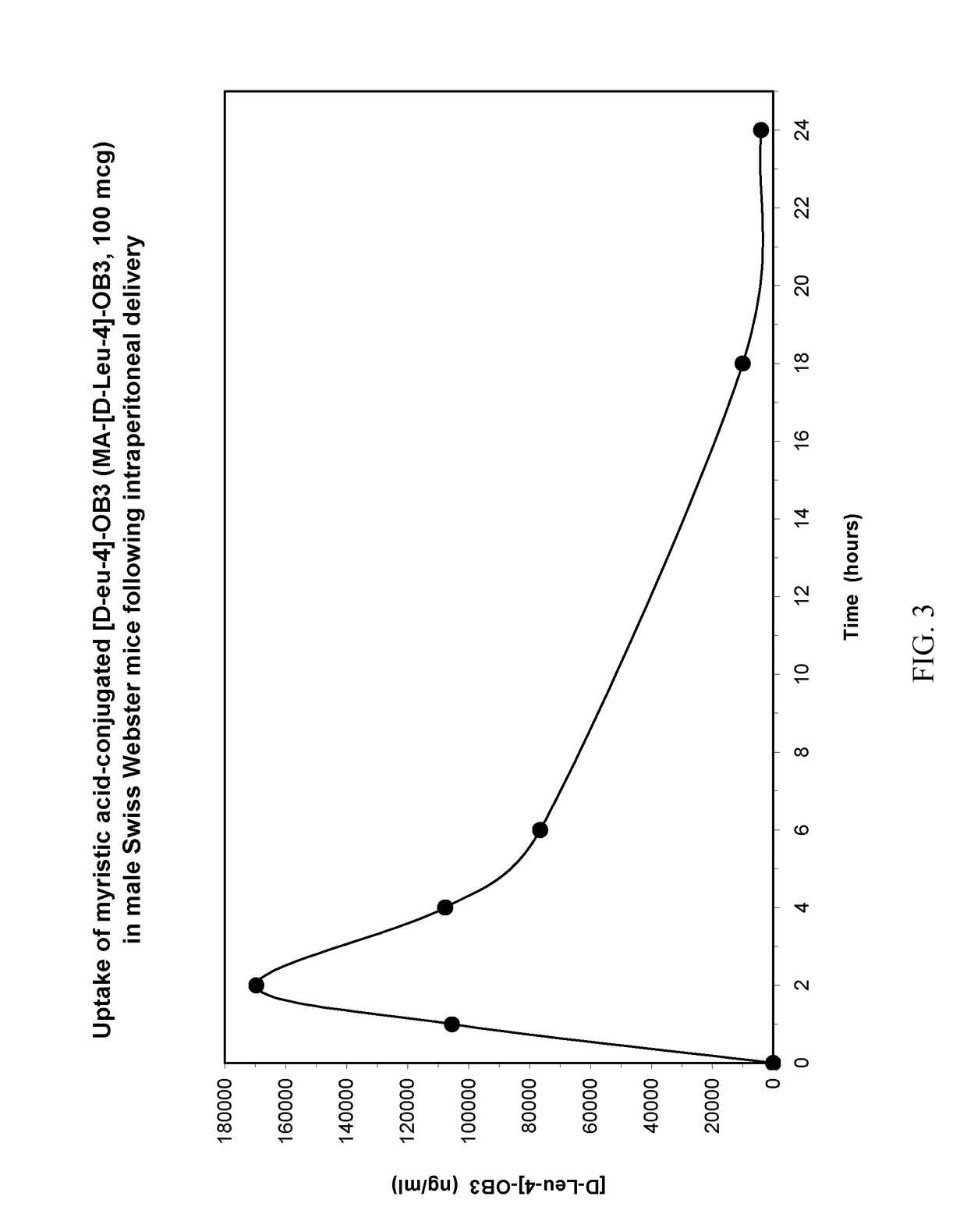
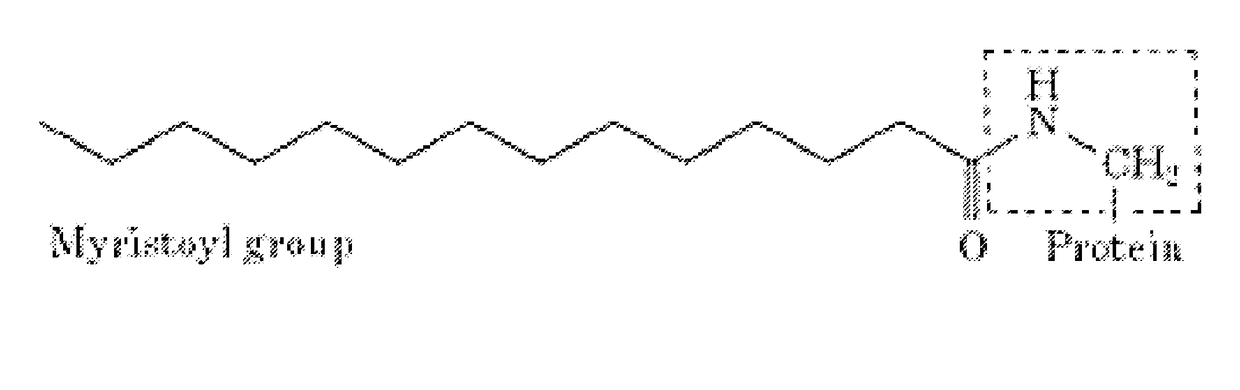
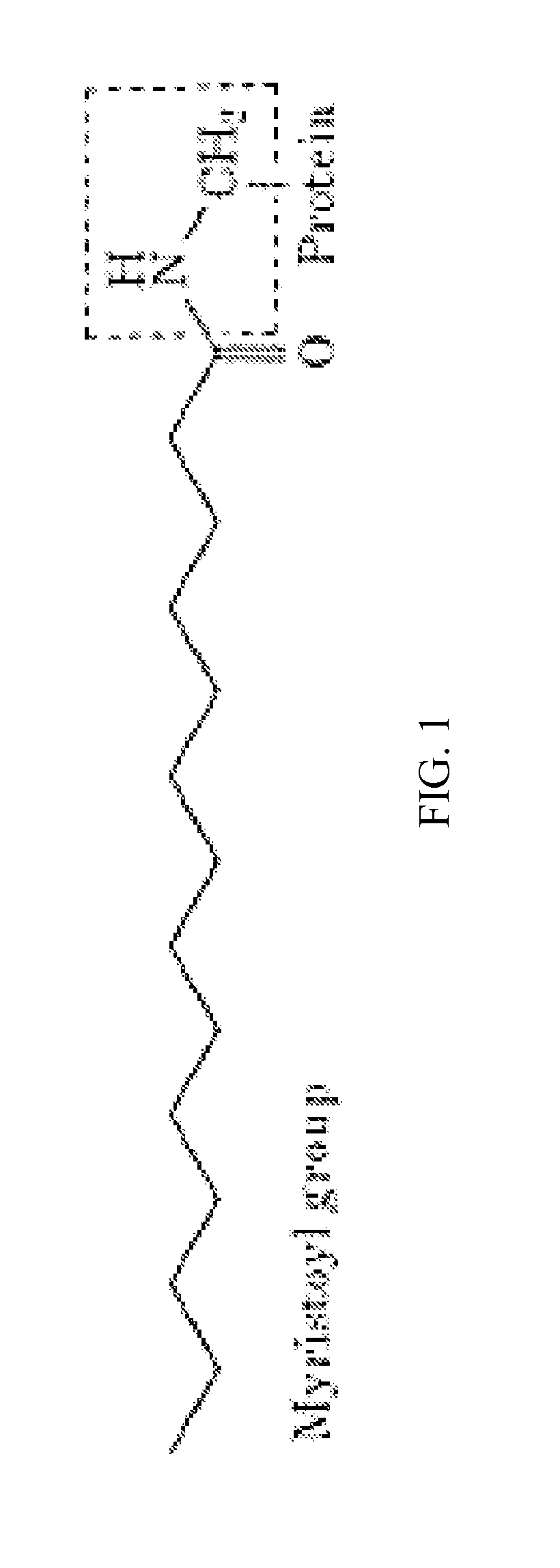
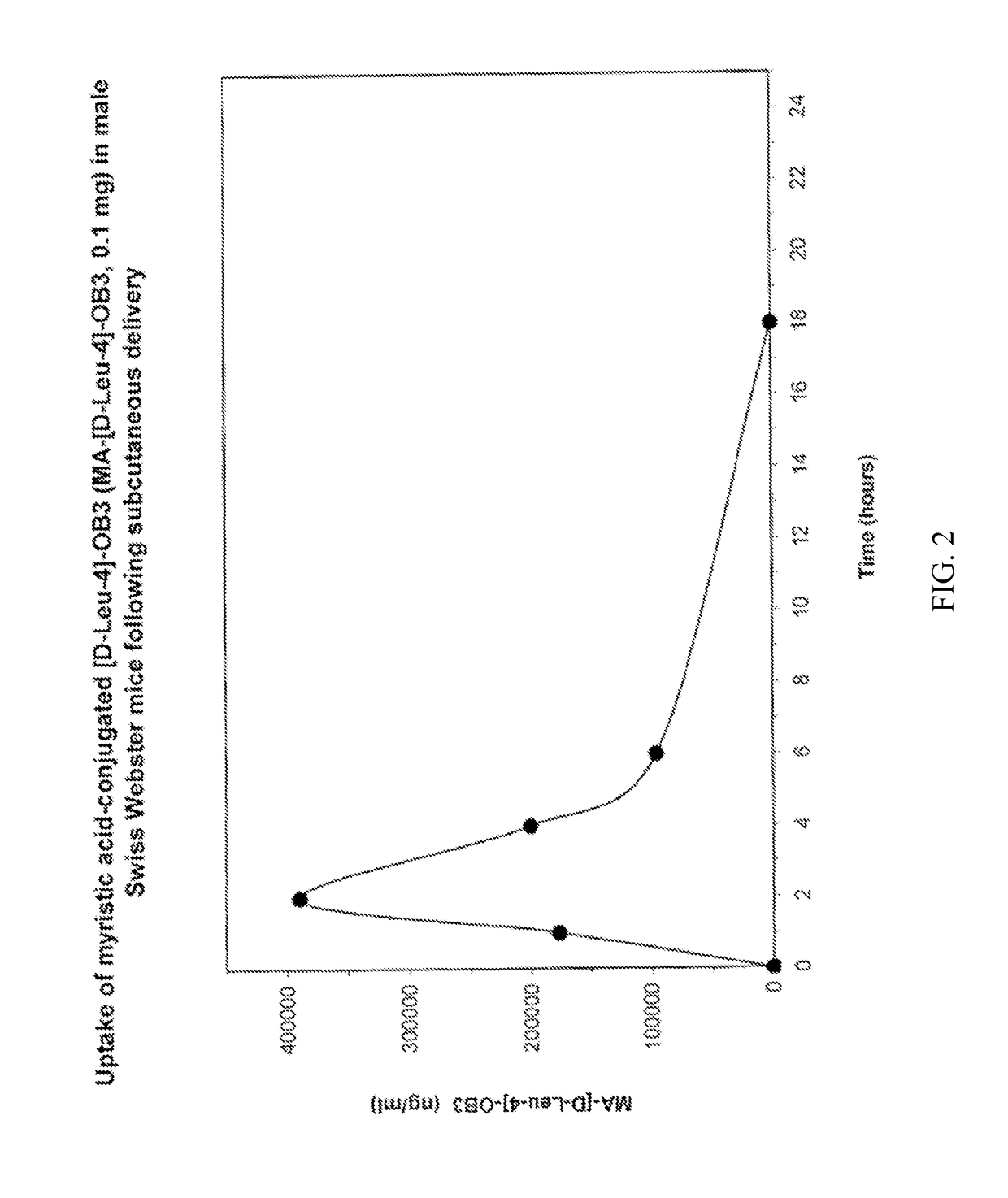
Myristoylated leptin-related peptides and uses thereof
ActiveUS10195254B2Good effectModulate body massAntibacterial agentsNervous disorderMedicineDrug compound
Owner:ALBANY MEDICAL COLLEGE
Methods of treating depression
The present disclosure relates to methods of transitioning patients or obese patients being treated with vortioxetine to treatment with a monoamine oxidase inhibitor (MAOI). The methods provided include delaying administration of the MAOI for certain time periods after stopping administration of vortioxetine. The patients or obese patients possess various capabilities of metabolizing vortioxetine. The current disclosure also includes methods of switching patients to a MAOI intended to treat psychiatric disorders while being treated with vortioxetine. The methods disclosed further comprise determining vortioxetine plasma clearance and washout time for patients with different body fat status and / or different CYP2D6 metabolizer status.
Owner:RUNDLE RES LLC
Portable liver reserve function detection device and detection method thereof
PendingCN114259228AImprove detection accuracyLow costSensorsMeasuring/recording heart/pulse rateComputer hardwareMedicine
The invention provides a portable liver reserve function detection device. The portable liver reserve function detection device comprises a computer, a detector and fingers of a patient to be detected, the computer is provided with a Bluetooth receiving module, and the detector comprises a first circuit board and a second circuit board; the first circuit board and the second circuit board are connected through a data line and are symmetrically mounted in the upper supporting part and the lower supporting part of the detector respectively; a light source is arranged below the first circuit board, and a Bluetooth transmitting module and a photoelectric receiver are respectively arranged above the second circuit board; a finger of a patient to be detected is placed in the detector, the first circuit board controls the light source to emit a light signal, the Bluetooth emission module transmits a digital signal to the Bluetooth receiving module, and the plasma clearance rate and the ICG retention rate are calculated through the computer. According to the invention, non-invasive measurement is realized, the detection precision is improved, the stability is enhanced, the cost is low, and large-area popularization of the liver reserve function detection instrument in clinical use is facilitated.
Owner:吉林亚泰中科医疗器械工程技术研究院股份有限公司
Methods of treating depression with vortioxetine
InactiveUS20220202809A1Reduce eliminateReduce riskOrganic active ingredientsNervous disorderDepressantBlood plasma
The present disclosure relates to methods of transitioning patients or obese patients being treated with vortioxetine to treatment with a monoamine oxidase inhibitor (MAOI). The methods provided include delaying administration of the MAOI for certain time periods after stopping administration of vortioxetine. The patients or obese patients possess various capabilities of metabolizing vortioxetine. The current disclosure also includes methods of switching patients to a MAOI intended to treat psychiatric disorders while being treated with vortioxetine. The methods disclosed further comprise determining vortioxetine plasma clearance and washout time for patients with different body fat status and / or different CYP2D6 metabolizer status.
Owner:RUNDLE RES LLC
Myristoylated leptin-related peptides and uses thereof
ActiveUS20170312340A1Enhance pharmacological effectsModulate body massAntibacterial agentsNervous disorderDrug compoundHalf-life
A pharmaceutical compound for the treatment of obesity related disorder that is a conjugate of myristic acid and a leptin-related peptide. Preferably, the leptin-related peptide is OB3 that has been D-substituted at Leu-4. The resulting conjugate significantly improved the pharmacokinetic profile of the leptin-related peptide by extending its half-life from less than one hour to as long as twenty-eight hours, depending on the route of delivery, increasing uptake, reducing the rate of plasma clearance, and enabling the minimal effective dose to be reduced several fold.
Owner:ALBANY MEDICAL COLLEGE
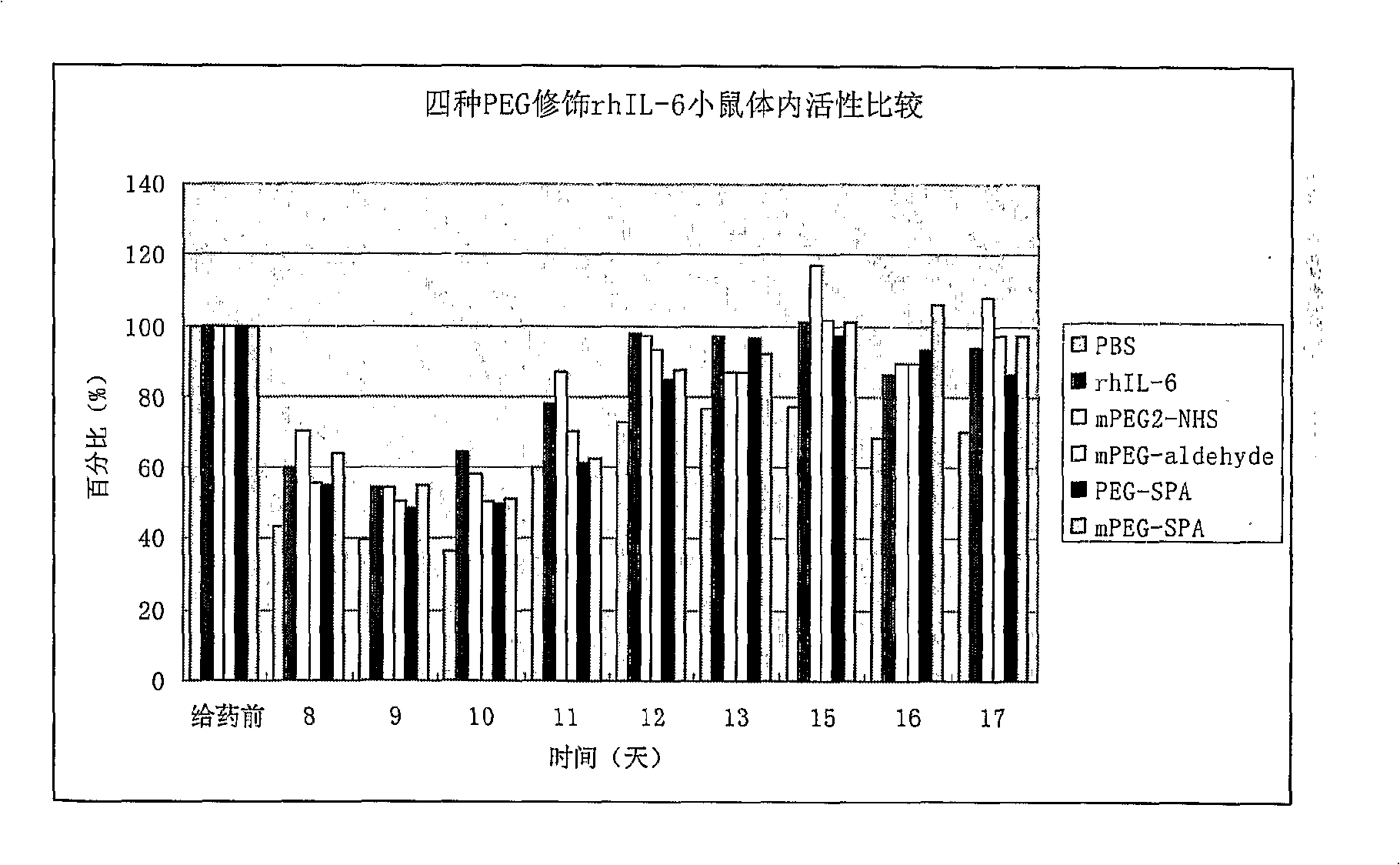
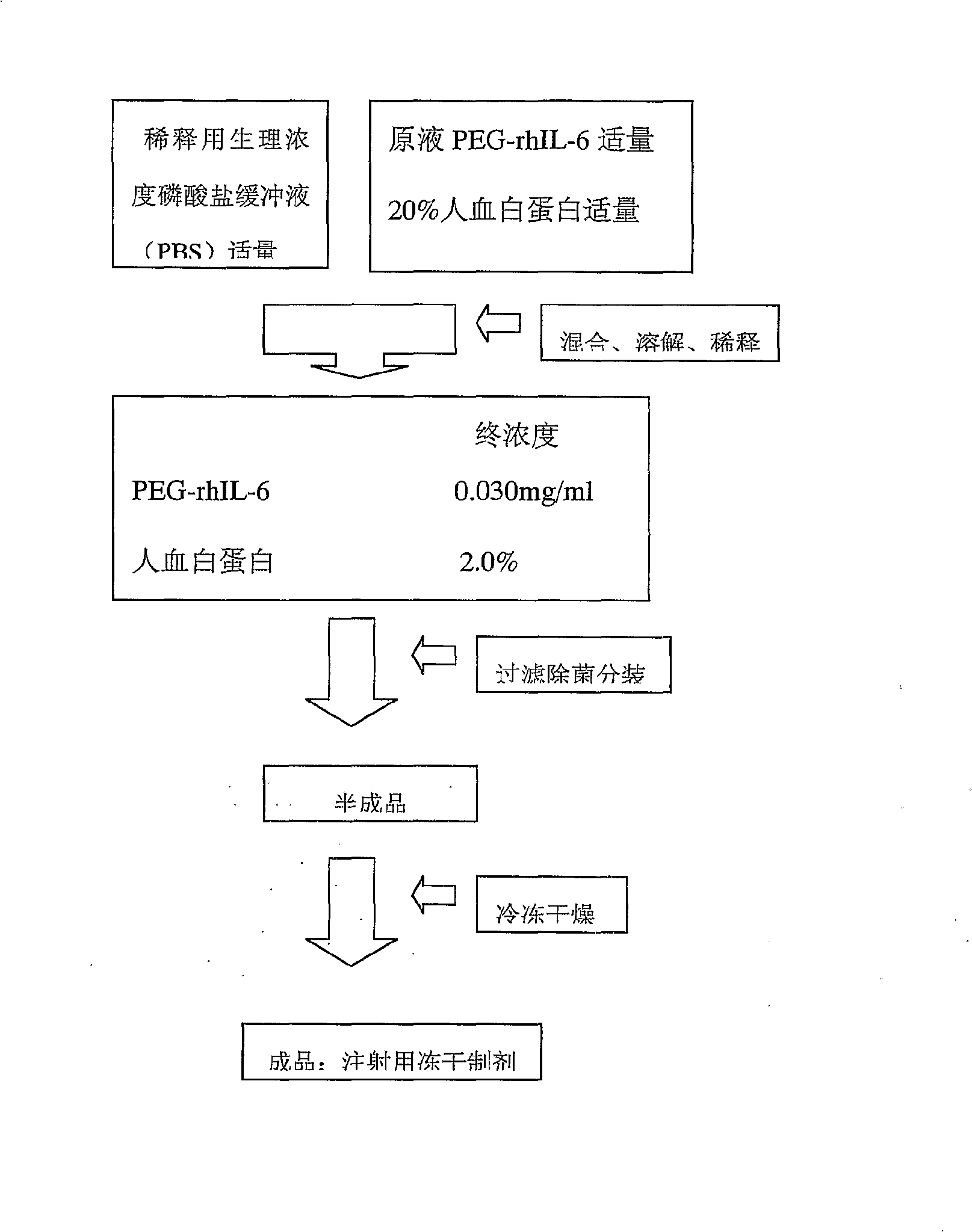
Interleukin-6 polyethylene glycol conjugate and its preparing method and use
InactiveCN101163716BBiologically activeImprove uniformityPeptide/protein ingredientsDepsipeptidesInterleukin 6Adjuvant
A interleukin-6 polyethylene glycol conjugate and its preparing method and medical compositions comprising the conjugate and pharmaceutically acceptable excipients. The conjugate according to the invention is used for producing medicines treating thrombocytopenia, chemotherapy adjuvants or medicines enhancing immunity. The mono-PEG-modified IL-6 enhances markedly biostability, has longer in vivo half life and lower plasma clearance compared with unmodified IL-6, resulting in a great decrease in frequency and dose of administration as well as side effects. The mono-PEG-modified IL-6 according to the present invention can reach medicinal standards due to its good uniformity.
Owner:CHENGDU INST OF BIOLOGICAL PROD
A kind of recombinant porcine interferon beta 1-fc fusion protein and its coding gene and expression method
ActiveCN103570836BMaintain biological activityExtended half-lifeBacteriaMicroorganism based processesEscherichia coliInclusion bodies
The invention provides a recombinant porcine interferon IFN beta1-Fc fusion protein as well as an encoding gene and expression, purification and inclusion body renaturation methods thereof, belonging to the biological genetic engineering field. The IFNbeta can enhance the immunity of pig and has a good application prospect in the veterinary medicine industry. However, natural porcine IFNbeta1 is less in expression quantity and is insufficient for research and development and application and has the deficiency of quick plasma clearance rate. The invention provides the recombinant porcine interferon IFN beta1-Fc fusion protein applicable to a coliform bacteria prokaryotic expression system. Part of the porcine IFNbeta1 is all sequences in an ectoenzyme area of the porcine IFNbeta1. The Fc section comprises a hinge region, a CH2 region and a CH3 region of an antibody. The porcine IFNbeta1 and the Fc section are directly fused. The fusion protein provided by the invention not only maintains the biological activity of the original protein IFNbeta1 to a great extent, but also extremely prolongs the half-life period of the original protein IFNbeta1, thereby providing the opportunity of industrialized development of the fusion protein.
Owner:GENSUN INST OF BIOMEDICINE
Hydroxylsafflor yellow A vesica and preparation method thereof
InactiveCN105596315AExtend cycle timeHigh drug loadingOrganic active ingredientsAntipyreticWater bathsDrugs solution
The invention discloses a hydroxylsafflor yellow A vesica and a preparation method thereof. According to the vesica, a hydroxylsafflor yellow A solution serves as a core material, and a nonionic surfactant and cholesterol serve as wall materials. The preparation method includes the steps that firstly, the nonionic surfactant and cholesterol are added into an ethanol solution, and the mixture is stirred in a magnetic stirrer at a maintained stirring speed to be completely dissolved; then, the ethanol solution is rapidly injected into a drug solution in a constant-temperature water bath, the mixture is stirred at a constant speed till ethanol volatilizes completely, the mixture is filtered with a 0.45 micrometer microporous filtration membrane, and a hydroxylsafflor yellow A vesica solution is obtained. The preparation method is good in reproducibility, easy to implement and convenient to control, and the prepared hydroxylsafflor yellow A vesica is large in drug loading capacity and low in plasma clearance and has the advantages of controlled release, lasting and stable plasma-drug concentration, safety, high efficiency and the like.
Owner:QINGDAO UNIV OF SCI & TECH +1
Methods of treating depression with vortioxetine
InactiveUS20190216803A1Extended half-lifeReduce eliminateOrganic active ingredientsNervous disorderDiseasePsychiatry
The present disclosure relates to methods of transitioning patients or obese patients being treated with vortioxetine to treatment with a monoamine oxidase inhibitor (MAOI). The methods provided include delaying administration of the MAOI for certain time periods after stopping administration of vortioxetine. The patients or obese patients possess various capabilities of metabolizing vortioxetine. The current disclosure also includes methods of switching patients to a MAOI intended to treat psychiatric disorders while being treated with vortioxetine. The methods disclosed further comprise determining vortioxetine plasma clearance and washout time for patients with different body fat status and / or different CYP2D6 metabolizer status.
Owner:RUNDLE RES LLC
Method for evaluating renal function
ActiveUS8738345B2Error minimizationEasy to useSamplingAnalogue computers for chemical processesMedicineErrors and residuals
Plasma concentration of a compound of interest is measured in two or ideally 4 or more blood samples taken from a patient over a period of time following bolus injection. The measured values are input into a computer processor programmed to execute a computer program comprising an algorithm that uses the gamma variate (GV) function to model drug plasma concentration, then uses Tikhonov regularization to perform the fit, selecting a regularization constant so that the relative error in the plasma clearance is minimized. One or more output values representative of renal function are generated.
Owner:WESOLOWSKI CARL A
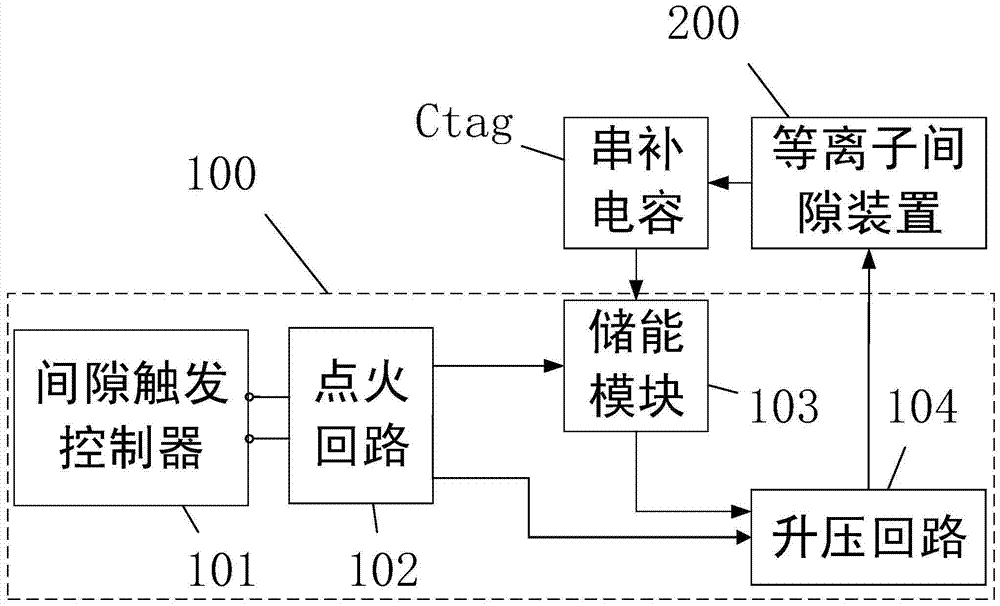
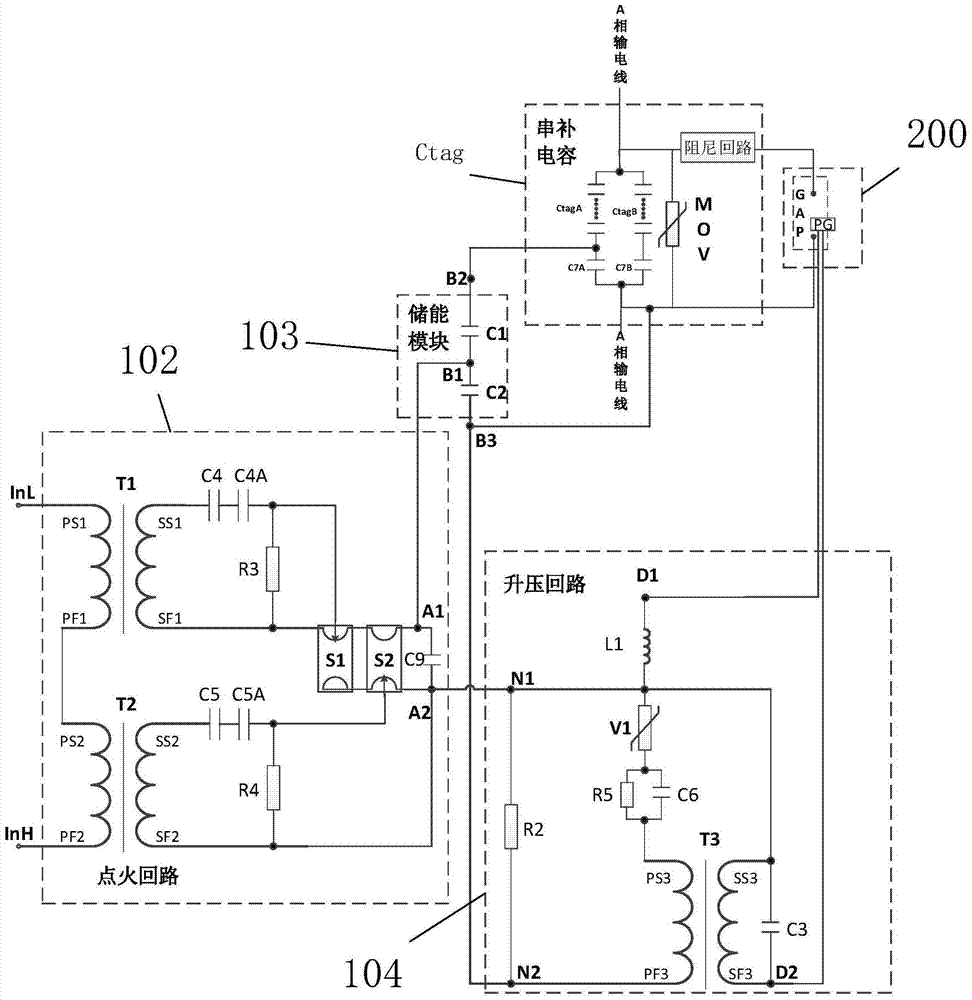
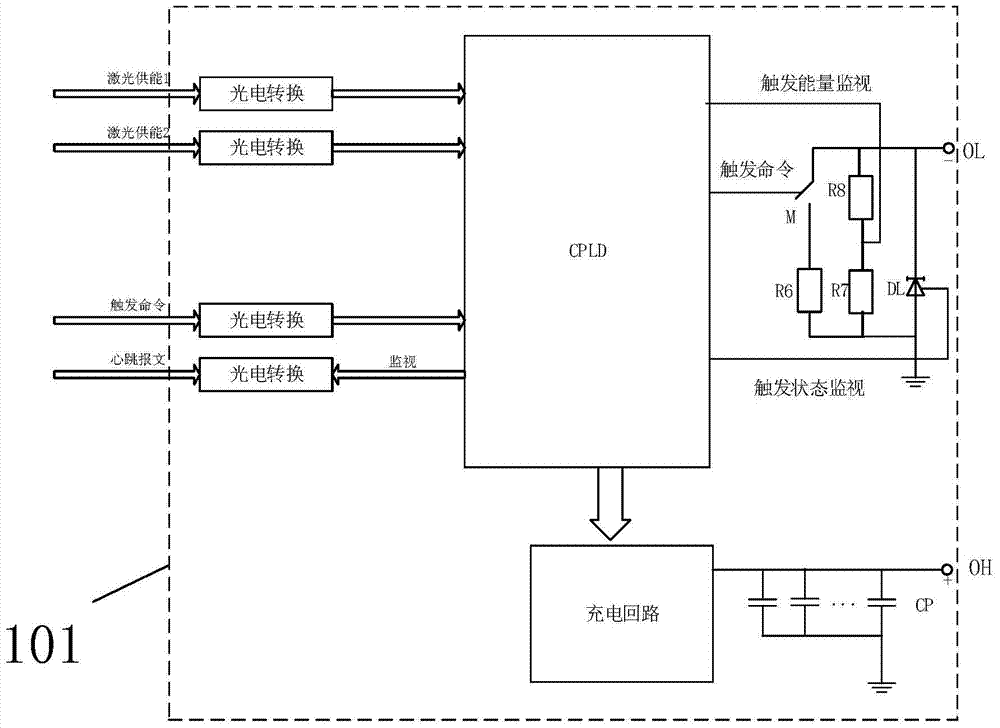
A Plasma Series Compensation Gap System
ActiveCN104917167BRealize self-oscillationAccurateEmergency protective arrangements for limiting excess voltage/currentCapacitancePlasma generator
A plasma series compensation gap system of the present invention is used to protect the series compensation capacitor connected in series on the high-voltage transmission line and the metal oxide voltage limiter connected in parallel at both ends, and the plasma gap device includes a gap connected in parallel with the series compensation capacitor And a plasma generator for triggering the gap action; a gap trigger device for receiving external energy supply and gap triggering instructions, and outputting pulse voltage to the plasma generator to trigger the gap action. The gap action can be triggered by the plasma generator, which can realize the wide gap setting and effectively reduce the false triggering of the gap; and the use of multiple ignition circuits can enhance the reliability of the gap trigger; the laser energy supply method can reduce the influence of electromagnetic shielding.
Owner:CHINA SOUTHERN POWER GRID EHV POWER TRANSMISSION COMPANY WUZHOU BUREAU +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com