Polyethylene glycol modified and recombined human interleukin and its preparation method
A technology of human interleukin and polyethylene glycol, applied in the field of interleukin, can solve the problems of easily causing immune response, short half-life, etc., and achieve the effects of simple and easy preparation method, reduced immunogenicity, and long in vivo half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Monomethoxy Polyethylene Glycol Succinimide Carbonate (SC-mPEG)
[0024] Weigh 14.4g of monomethoxypolyethylene glycol 12,000 and 0.56g of triphosgene, dissolve in 20ml of toluene and 12ml of dichloromethane, add 1ml of anhydrous triethylamine dropwise, and stir overnight.
[0025] The reaction solution was evacuated, most of the solvent was evaporated, and the obtained residue was redissolved in 8 ml of toluene and 10 ml of dichloromethane. Add 0.21 g of N-hydroxysuccinimide, take 0.3 ml of triethylamine, dilute with 3 ml of dichloromethane, add dropwise to the reaction solution, and continue the reaction to complete, filter the reaction solution and vacuumize, and dissolve the obtained residue in 50 °C in 60 ml of ethyl acetate, filtered, and the crystals obtained after cooling were the crude SC-PEG. The resulting product was repeatedly recrystallized from ethyl acetate. Measure the infrared spectrum of product, following characteristic peak is arrang...
Embodiment 2
[0027] Selection of Modification Conditions of Interleukin-2 by SC-PEG
[0028] Choice of reaction time:
[0029] Take 4 1.5ml Eppendorf tubes, add 0.5ml of interleukin-2 concentrate (2.0mg / ml) to each tube, then add an equal volume of 0.05M borax buffer solution with a pH value of 8.0, mix well, and then add 6.0mg of SC -PEG, stop the reaction after reacting for 15min, 30min, 1h, and 2h, and perform SDS-PAGE electrophoresis. The results showed that the modification rate was low when reacting for 15 minutes, most of the interleukin-2 was not modified, and the modification rate increased when reacting for 30 minutes, and the product was mainly modified interleukin-2. After reacting for 1 hour, a highly modified band The highly modified bands increased slightly after 2 hours of reaction compared with 1 hour of reaction.
[0030] Choice of reaction pH:
[0031] Take five 1.5ml Eppendorf tubes, add 0.5ml (2.0mg / ml) of interleukin-2 concentrated solution to each tube, and then a...
Embodiment 3
[0033] Separation, purification and identification of modified products
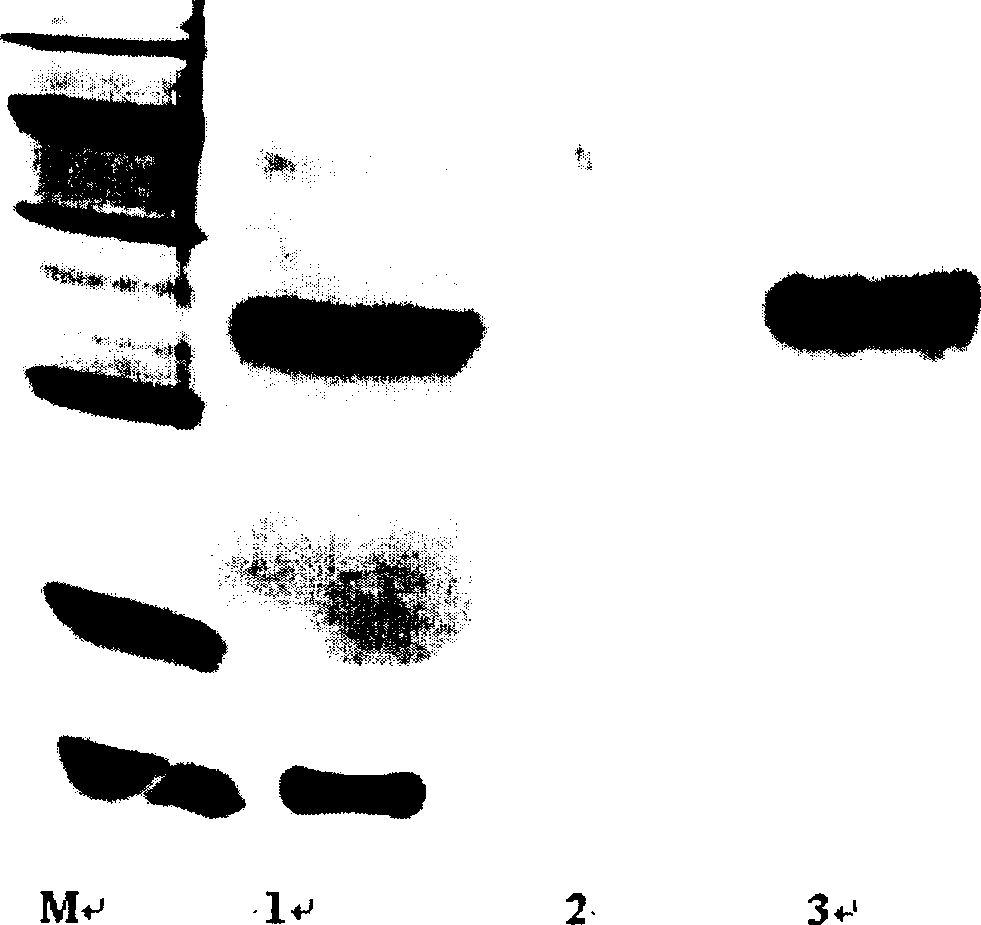
[0034] Take two 1.5ml Eppendorf tubes, add 0.5ml of interleukin-2 concentrate (2.0mg / ml) to each tube, then add an equal volume of 0.05M borax buffer solution with a pH of 7.5, then add 6.0mg of SC-mPEG solid, mix Mix well, react at 4°C for 30 min, and terminate the reaction.
[0035] Take the above reaction solution and put it on the column for separation respectively. Chromatographic conditions: Sephacryl S-200 (1cm×50cm), mobile phase is 50mM acetate buffer (pH6.0), flow rate: 0.3ml / min. Collect each elution peak.
[0036] The results showed that there were two elution peaks with a molecular weight greater than that of the standard interleukin-2, which were single-modified interleukin-2 and multi-modified interleukin-2.
[0037] The eluted peaks were appropriately concentrated and detected by SDS-PAGE electrophoresis, the separation gel was 15%, and the stacking gel was 5%. see results figure 1 , e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com