PEG-leuprolide conjugate and preparation method thereof
A technology for leuprolide and conjugates, applied in the field of PEG-leuprolide conjugates and their synthesis, can solve the problems of large losses, many synthesis steps, etc., and achieves small losses, easy commercialization, and synthetic routes short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a new PEG-leuprolide conjugate and its synthesis method, through the direct reaction of monomethoxypolyethylene glycol succinimide active ester with unprotected leuprolide to obtain leuprolide PEG-leuprolide conjugate (I or II) modified by the imidazole group on the 2-position histidine of propylidene, the reaction formula is shown in the figure below:
[0029]
[0030] In the present invention, the leuprolide includes leuprolide and leuprolide acetate. The structure of the active ester of monomethoxy polyethylene glycol succinimide is CH 3 O-(CH 2 CH 2 O)x-(CH 2 CH 2 )y-NHS; x is an integer value of 10 to 1300, the structure of polyethylene glycol includes linear structure and branched structure, and the molecular weight is 400-60KDa; y is an integer value of 0, 0.5, 1 to 5, including monomethyl Carbonate, acetate, propionate, succinate, valerate, etc. of oxypolyethylene glycol succinimide. In the reaction, the molar ratio of leu...
Embodiment 1
[0035] Preparation of PEG-leuprolide conjugate (PEG2K-LEU):
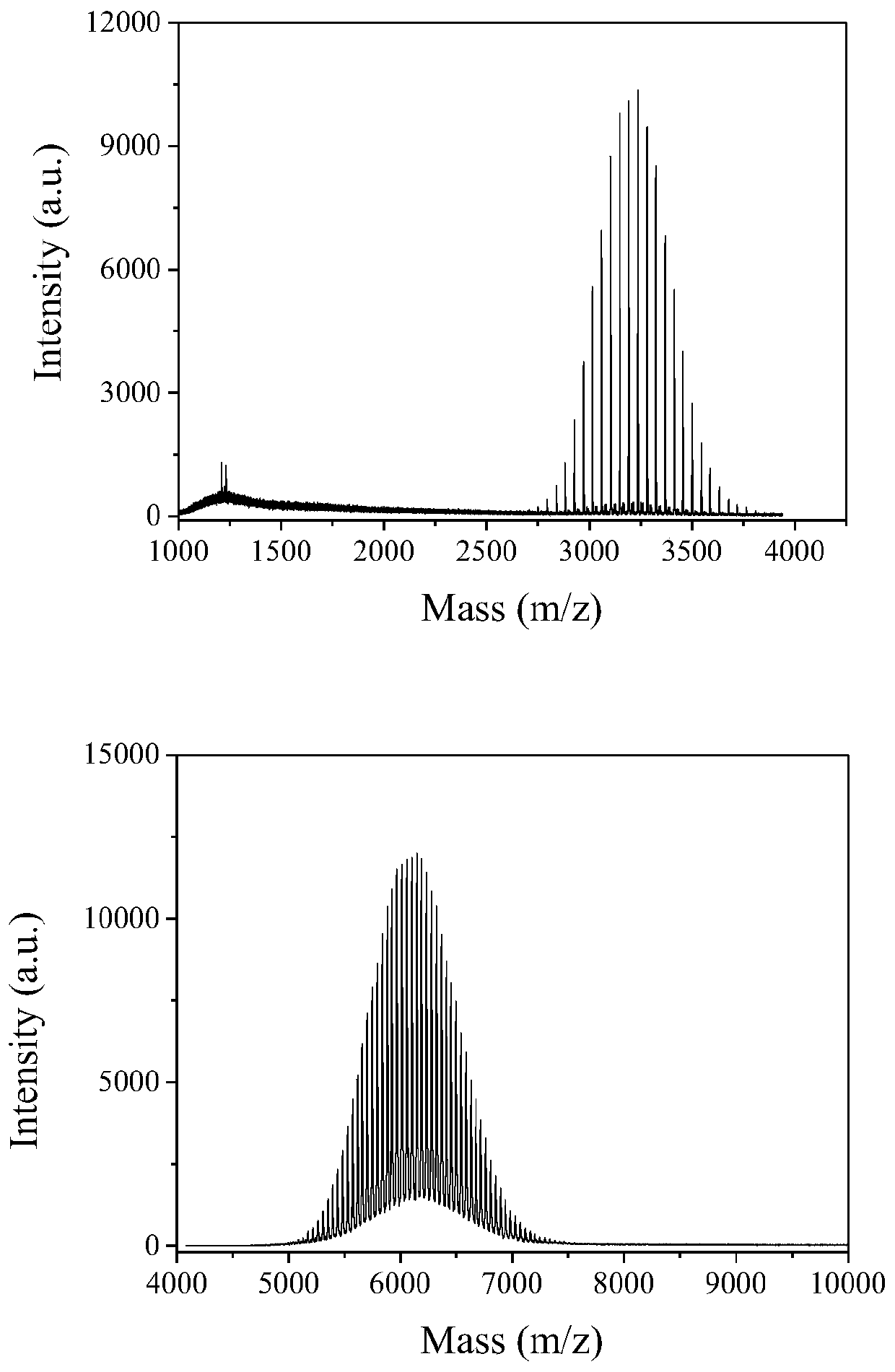
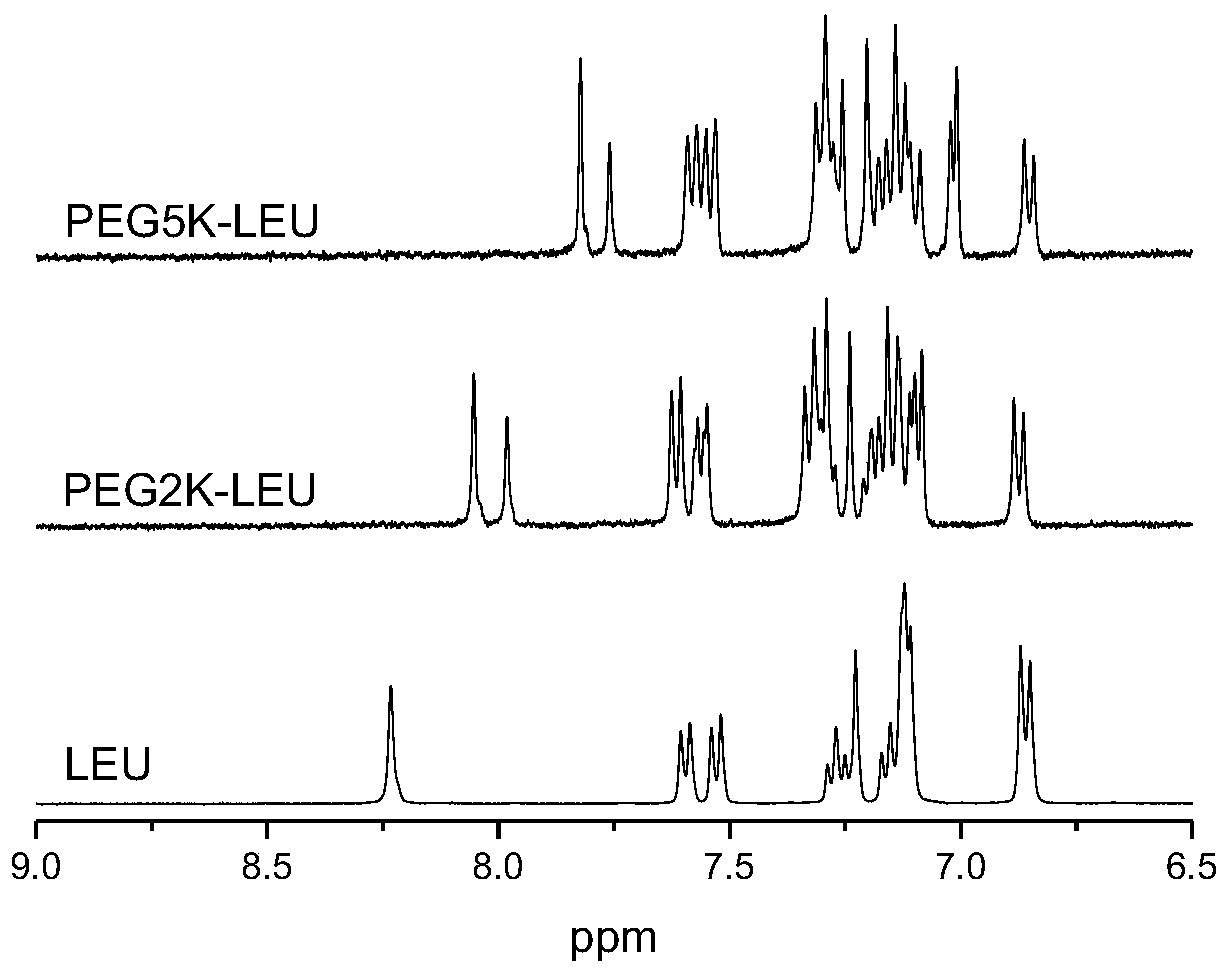
[0036] 12.6 mg (0.01 mmol) of leuprolide acetate was dissolved in 5 mL of distilled water, and an equimolar amount of CH was added 3 O-(CH 2 CH 2 O) 45 -CH 2 CH 2 -NHS, reacted at 37°C for 4 hours, purified by cation exchange chromatography, purification conditions: 20mM pH 6.5PBS, sodium chloride gradient: 0-0.5M, collected target peaks for dialysis, and freeze-dried to obtain PEG2K-LEU. MALDI-TOF mass spectrometry detection shows that the molecular weight is 3235.6, which is exactly equal to the sum of the molecular weights of leuprolide and PEG, indicating that only one PEG chain is attached to each leuprolide molecule ( figure 1) . Dissolve PEG2K-LEU in deuterated water, do 1 H NMR spectrum ( figure 2); Compared with leuprolide, the chemical shift of the hydrogen on the imidazole group moves to the high field, indicating that the PEG segment is connected to the imidazole group of the 2-position histidi...
Embodiment 2
[0038] Preparation of PEG-leuprolide conjugate (PEG5K-LEU):
[0039] 12.6 mg (0.01 mmol) of leuprolide acetate was dissolved in 5 mL of distilled water, and an equimolar amount of CH was added 3 O-(CH 2 CH 2 O) 110 -CH 2 CH 2 -NHS, reacted at 37°C for 6 hours, purified by cation exchange chromatography, purification conditions: 20mM pH6.5PBS, sodium chloride gradient: 0-0.5M, collected target peaks for dialysis, and freeze-dried to obtain PEG5K-LEU. MALDI-TOF mass spectrometry detection shows that the molecular weight is 6144.2, which is exactly equal to the sum of the molecular weights of leuprolide and PEG, indicating that only one PEG chain is attached to each leuprolide molecule ( figure 1) . Dissolve PEG5K-LEU in deuterated water, do 1 H NMR spectrum ( figure 2) ; Compared with leuprolide, the chemical shift of the hydrogen on the imidazole group moves to the high field, indicating that the PEG segment is connected to the imidazole group of the 2-position histid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com