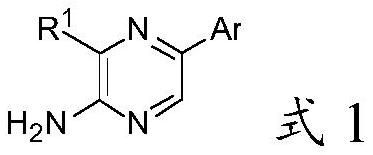
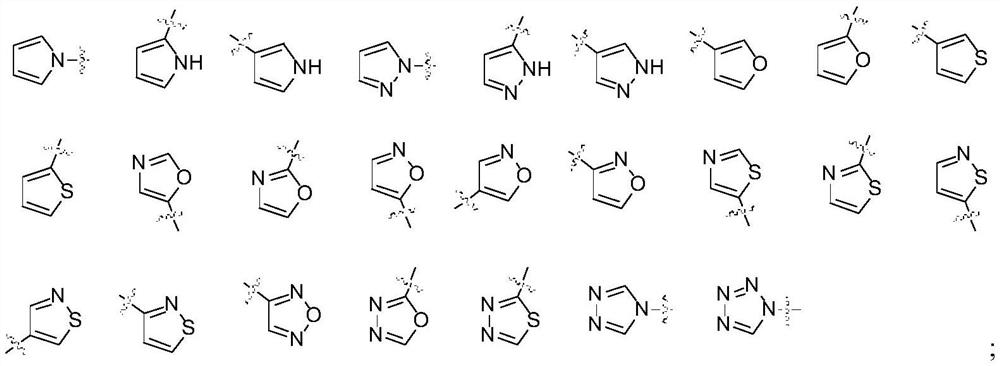
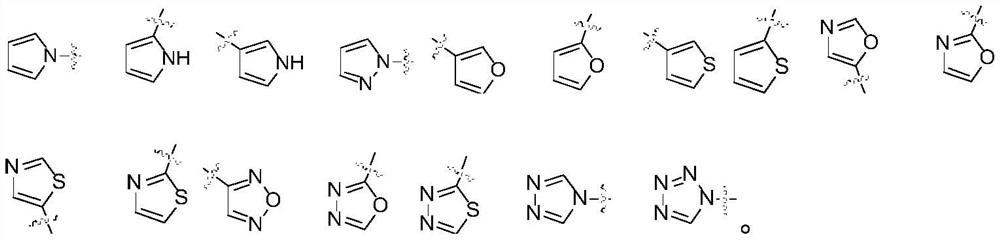
2-amino-5-heteroaryl substituted pyrazine derivative and application thereof
A technology of pyrazine derivatives and heteroaryl groups, applied in the field of pyrazine derivatives, can solve problems such as toxic side effects, expensive drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: The synthetic route of compound 6 is as follows:
[0065]
[0066] Step 1): Make 6-bromopyrazole into Grignard reagent, add trimethyl borate dropwise to it, control the molar ratio of trimethyl borate to 6-bromopyrazole to be (1.1~1.2):1, during the reaction Cool and control the reaction temperature at 10-20°C. After the dropwise addition, continue to cool and react for 30 minutes to obtain a 6-substituted pyrazole boronic acid compound;
[0067] Step 2): dissolving the pyrazole boronic acid compound obtained in step 1) in tetrahydrofuran, adding 2-amino-3-bromopyrazine to it, and controlling the molar ratio of the pyrazole boronic acid compound to 2-amino-3-bromopyrazine The ratio is (1.05~1.10):1, the reaction temperature is 45~55°C, and after 1~2h of reaction, compound 6, the product of Suzuki coupling reaction, is obtained with a yield of 66%.
Embodiment 2
[0068] Embodiment 2: The synthetic route of compound 18 is as follows:
[0069]
[0070] Step 1): Make 2-bromooxazole into Grignard reagent, add trimethyl borate dropwise to it, control the molar ratio of trimethyl borate to 2-bromooxazole to be (1.1~1.2):1, react Cool during the process, control the reaction temperature at 10-20°C, continue to cool and react for 30 minutes after the dropwise addition, and obtain the 2-substituted oxazole boronic acid compound;
[0071] Step 2): Dissolving the oxazole boronic acid compound obtained in step 1) in toluene, adding 2-amino-3-ethylpyrazine to it to control the interaction between the oxazole boronic acid compound and 2-amino-3-ethylpyrazine The molar ratio was (1.05-1.10):1, the reaction temperature was 45-55°C, and after 1-2 hours of reaction, compound 18, the product of Suzuki coupling reaction, was obtained with a yield of 73%.
Embodiment 3
[0072] Embodiment 3: The synthetic route of compound 38 is as follows:
[0073]
[0074] Step 1): Use 2-amino-5-(5-pyrazolyl)-pyrazine as a raw material, dissolve it in tetrahydrofuran to prepare a solution, add liquid bromine dropwise to it under ventilated conditions, and control the liquid bromine and 2-amino- The molar ratio of 5-(5-pyrazolyl)-pyrazine is (0.9~0.95):1, add catalyst iron filings to it, and react at room temperature to obtain 2-amino-3-bromo-5-(5-pyridine Azolyl)-pyrazine, the yield is 85%;
[0075] Step 2): Add trimethyl borate dropwise to propionamide, control the molar ratio of trimethyl borate to propionamide to be (1.1-1.2):1, cool during the reaction, control the reaction temperature at 10-20°C, drop After the addition was completed, the cooling reaction was continued for 30 minutes to obtain the propionamide boric acid compound;
[0076] Step 3): Dissolve the 2-amino-3-bromo-5-(5-pyrazolyl)-pyrazine obtained in step 1) in tetrahydrofuran, and add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com