Dihydroxy isoquinoline and preparation method and application thereof from centipedes
A technology of dihydroxyisoquinoline and centipede, which is applied in the application fields of antimalarial, antibacterial and antitumor active drugs, and can solve the problems of complex components and limitations in the application of centipede
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
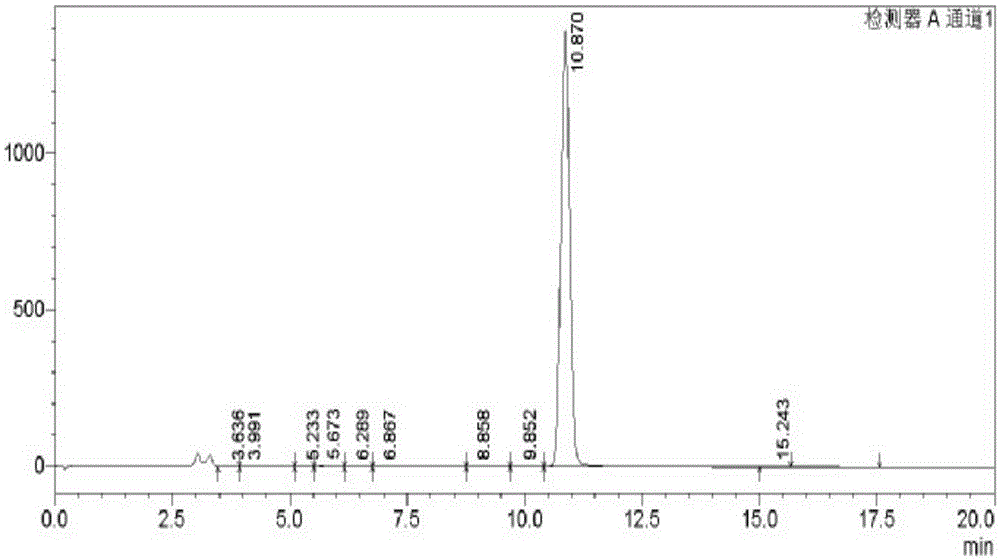
[0054] Preparation and Structure Identification of 3,8-Dihydroxyisoquinoline from Centipede
[0055] A. water extraction method and alcohol extraction method prepare centipede extract:
[0056] (1) Grinding the dry body of the centipede to obtain centipede powder, adding 10 times the volume of the centipede powder (5-10 times the volume is acceptable, preferably 10 times in this embodiment, and the same reasoning behind), and then performing ultrasonic crushing;
[0057] (2) Carry out refrigerated centrifugation to the homogenate, get the supernatant, freeze-dry to obtain centipede aqueous extract
[0058] (3) Take the precipitate obtained in step (2), add 55% (volume fraction, the same reason behind) ethanol homogenization of 10 times the volume of the precipitate, then leaching at 4°C, and freeze and centrifuge the leaching solution again , take the supernatant, freeze-dry to obtain centipede 55% ethanol extract;
[0059] (4) Take the precipitate obtained in step (2), add ...
Embodiment 2
[0085] The step of extracting active component in the present embodiment is basically the same as embodiment 1, and difference is: 1) during water extraction, the PBS solution volume that adds is 5 times of centipede powder volume, during alcohol extraction, the volume fraction of alcoholic solution is 45%; 2) When adopting Sephadex gel chromatography to separate and purify centipede ethanol extract, centipede ethanol extract is configured to 20mg / mL, mobile phase is 50% ethanol, loading volume is 8mL, and flow rate is 0.6mL / min.
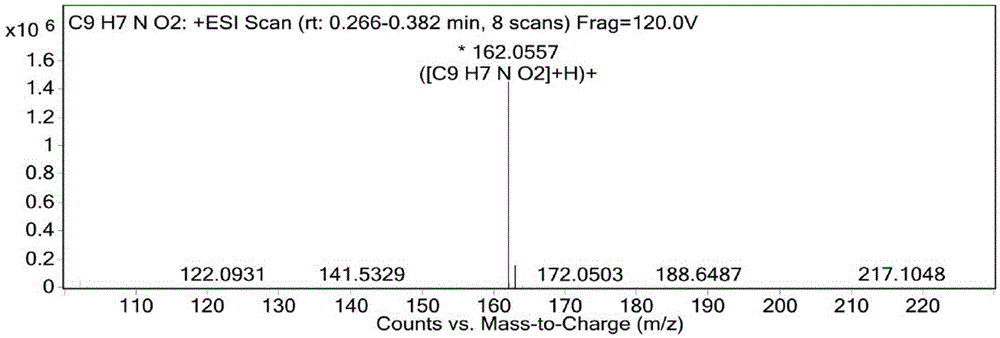
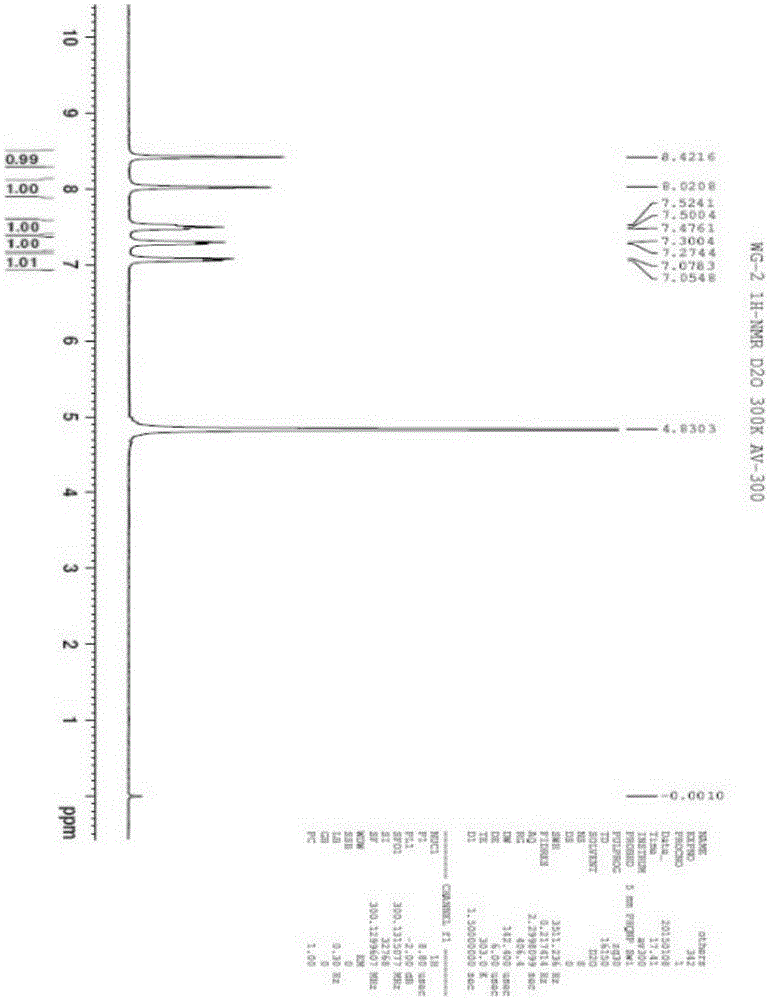
[0086] The result of this example is the same as Example 1, and the active substance finally obtained is identified as 3,8-dihydroxyisoquinoline (isoquinoline-3,8-diol) by structure, and the molecular formula is C 9 h 7 NO 2 , the molecular weight is 161.1, and the structural formula is:
[0087]
Embodiment 3
[0089] The step of extracting active component in the present embodiment is basically the same as embodiment 1, and difference is: 1) during water extraction, the PBS solution volume that adds is 8 times of centipede powder volume, during alcohol extraction, the volume fraction of alcoholic solution is 65%; 2) When adopting Sephadex gel chromatography to separate and purify centipede ethanol extract, centipede ethanol extract is configured to 35mg / mL, mobile phase is 30% ethanol, sample volume is 6mL, and flow rate is 1.5mL / min.
[0090] The result of this example is the same as Example 1, and the active substance finally obtained is identified as 3,8-dihydroxyisoquinoline (isoquinoline-3,8-diol) by structure, and the molecular formula is C 9 h 7 NO 2 , the molecular weight is 161.1, and the structural formula is:
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com