Anti -malarial agents
A solvate, aminopyrazine technology, applied in the direction of anti-infective drugs, resistance to vector-borne diseases, drug combinations, etc., can solve the problems of high price, human toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
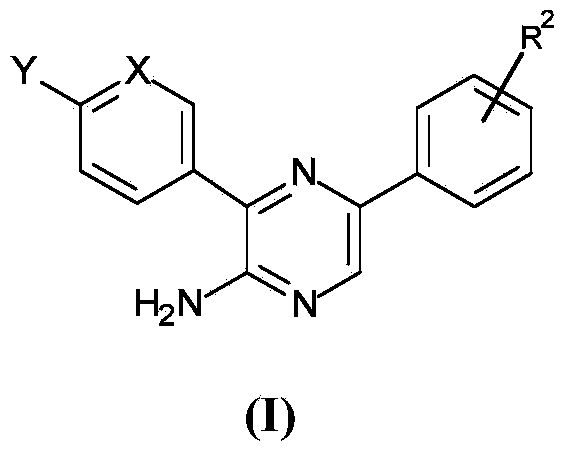
[0193] In one embodiment, the present invention provides aminopyrazine derivatives according to formula (I), and pharmaceutically acceptable salts, complexes, hydrates, solvates thereof, or polymorphs and tautomers thereof Use of isomers, geometric isomers, optically active forms and pharmaceutically active derivatives for the preparation of pharmaceutical compositions for the treatment or prevention of malaria:
[0194]
[0195] Among them, X is CR 1 or N; Y is selected from CF 3 , -C(O)-NR 3 R 4 , O-R 6 , SO 2 -R 6 ; 1 selected from H and halogen; R 2 from SO 2 -R 5 and -C(O)-R 10 ; 3 and R 4 independently selected from H and optionally substituted C 1 -C 6 the alkyl group; R 5 from-NR 7 R 8 and R 9 ; 6 is optionally substituted C1 -C 6 the alkyl group; R 7 and R 8 independently selected from H and optionally substituted C 1 -C 6 the alkyl group; R 9 is optionally substituted C 1 -C 6 Alkyl and optionally substituted C 3 -C 8 Cycloalkyl; R 10...
Embodiment 1
[0237] Example 1: Synthesis of compounds according to the invention
[0238] The aminopyrazine derivatives can be prepared from readily available starting materials using methods and procedures well known to those skilled in the art. It is to be understood that where typical or preferred experimental conditions (ie, reaction temperatures, times, moles of reagents, solvents, etc.) have been given, other experimental conditions can also be used unless otherwise stated. Preferred reaction conditions may vary with the particular reactants or solvent employed, but such conditions can be determined by one skilled in the art using routine optimization procedures.
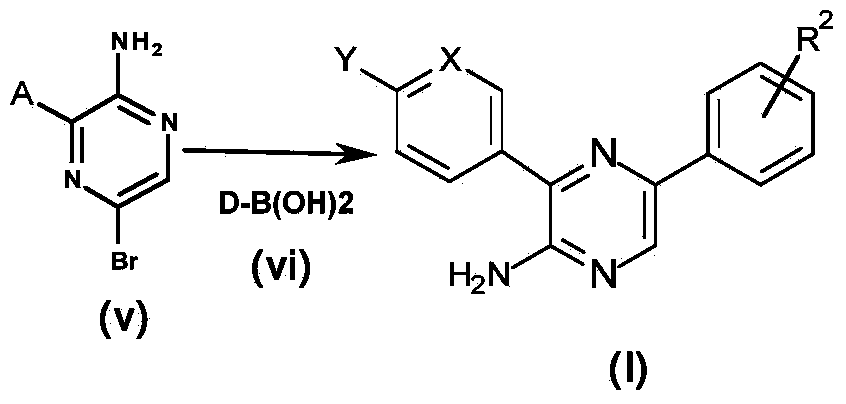
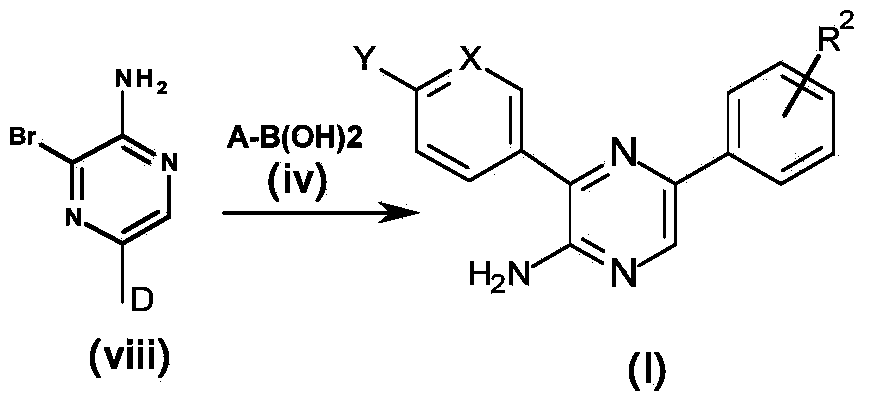
[0239] A general synthetic method for obtaining compounds of formula (I) is shown in Scheme 1 below. Aminopyrazine derivatives according to formula (I), wherein the substituents are as defined above, can be prepared using four steps: from custom-made or commercially available aminopyrazines according to formula (i), acco...
Embodiment 2
[0298] Example 2: Synthesis of additional compounds of the invention
[0299] The following compounds listed in Table 1 below were prepared using a procedure similar to that described in Example 1.
[0300] Table 1
[0301]
[0302]
[0303]
[0304]
[0305] Compounds 13-19 and compounds 20-32 were synthesized according to Scheme 1 and Scheme 5, respectively. Compound 33 and compound 34 were synthesized according to Scheme 1. Chiral HPLC of individual enantiomers Compound 35 and Compound 36 in a mixture [using Chiral Pak IA (250X4.6) mm5u; 0.1% DEA in hexane: ethanol (40:60) as mobile phase, flow rate 1.0 mL / min] obtained after isolation, and compound 21 was synthesized according to scheme 3.
[0306] Starting materials generally described in the reaction schemes used to synthesize the compounds of the examples are listed in Table 2 below.
[0307] Table 2
[0308]
[0309]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com