Treatment and prevention of malaria
一种疟疾、序列的技术,应用在治疗和预防疟疾领域,能够解决抗体诱导弱效力等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0356] Example 1. Identification of pfRip as a Rh5 complex partner
[0357] Purification of processed 45 kDa pfRh5 from parasite culture supernatants by ion exchange chromatography. Analysis of pfRH5 by size exclusion chromatography on a Superdex 200 analytical column confirmed that pfRH5 elutes as a ~150-200 kDa species ( figure 1 A). Blue native gel electrophoresis confirms that pfRh5 migrates on the gel as a ~150-200kDa species ( figure 1 B).
[0358] To determine whether pfRh5 complexes with other molecules or forms homo-oligomers, proteins were incubated with pfRh5 antibodies and analyzed by size exclusion chromatography. The 300 μl pfRh5-containing fraction separated from the culture supernatant was loaded onto a Superdex200 analytical column and eluted with PBS ( figure 2 A). The same 300 μl sample was pre-incubated with 25 μg monoclonal pfRh5 antibody for 15 minutes at room temperature and then 2 hours on ice before loading onto a Superdex200 analytical column ...
Embodiment 2
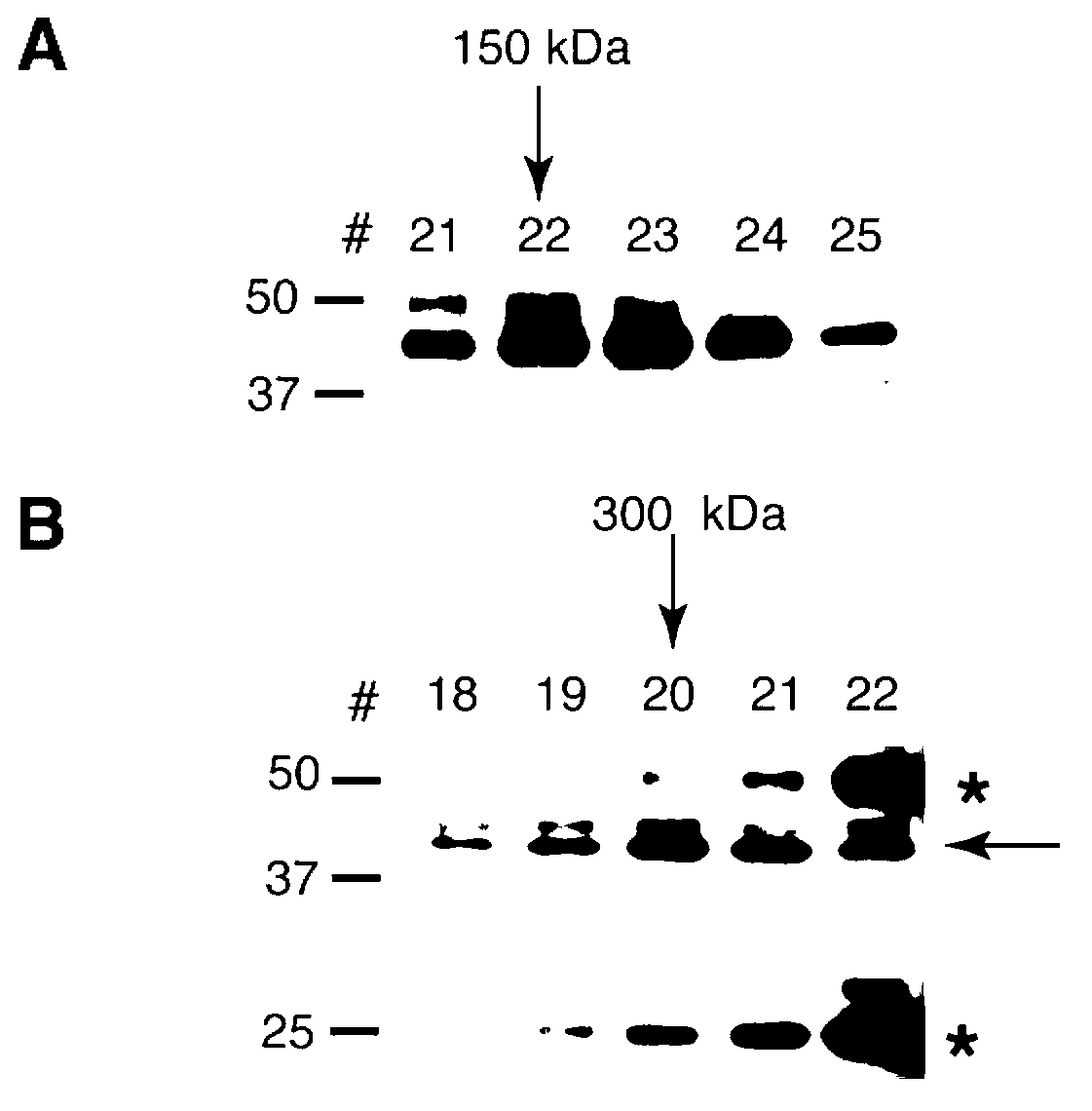
[0362] Example 2.pfRip falls off in the culture supernatant
[0363]A single Strep tag and a triple hemagglutinin (HA) tag were added to the C-terminus of pfRip by 3′-single homologous exchange recombination ( image 3 A). Western blotting of saponin clumps and HA-tagged proteins purified from the culture supernatant of the pfRip HA line with an anti-HA antibody confirmed that PfRip was processed and shed into the culture supernatant ( image 3 B). PfRipHA was analyzed by SDS-PAGE under reducing and non-reducing conditions and transferred to nitrocellulose membranes. Immunoblot with anti-HA antibody showed that the processed C-terminal fragment migrated similarly under reducing and non-reducing conditions, implying that the N-terminus and C-terminus of pfRip are not linked by any disulfide bond after processing ( image 3 C).
Embodiment 3
[0364] Example 3. Immunoprecipitation of pfRip
[0365] Culture supernatants from wt3D7 and 3D7-pfRipHA parasite lines were immunoprecipitated with anti-HA-Sepharose beads. Bound material was separated by SDS-PAGE and transferred to nitrocellulose membranes to probe for pfRh5 (clone 2F1 ) using a monoclonal anti-pfRh5 antibody. Detection of pfRh5 in bound material from only the 3D7-pfRipHA line indicated that pfRh5 specifically co-immunoprecipitated with pfRipHA ( Figure 4 A).
[0366] Culture supernatants from wt3D7 and 3D7-pfRipHA parasite lines were immunoprecipitated with monoclonal anti-pfRh5 antibody coupled to microbeads, and culture supernatants from 3D7-pfRipHA parasites were incubated with microbeads alone as additional controls . Bound material was separated by SDS-PAGE and transferred to nitrocellulose membranes to probe pfRipHA using an anti-HA antibody ( Figure 4 B). Detection of pfRipHA in bound material from only the 3D7-pfRipHA parasite line immunopre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com