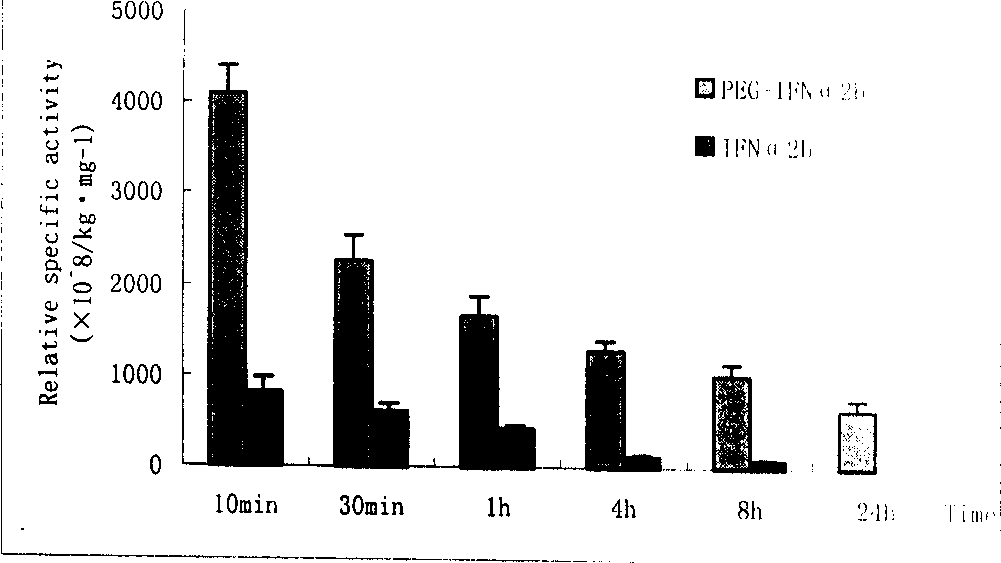
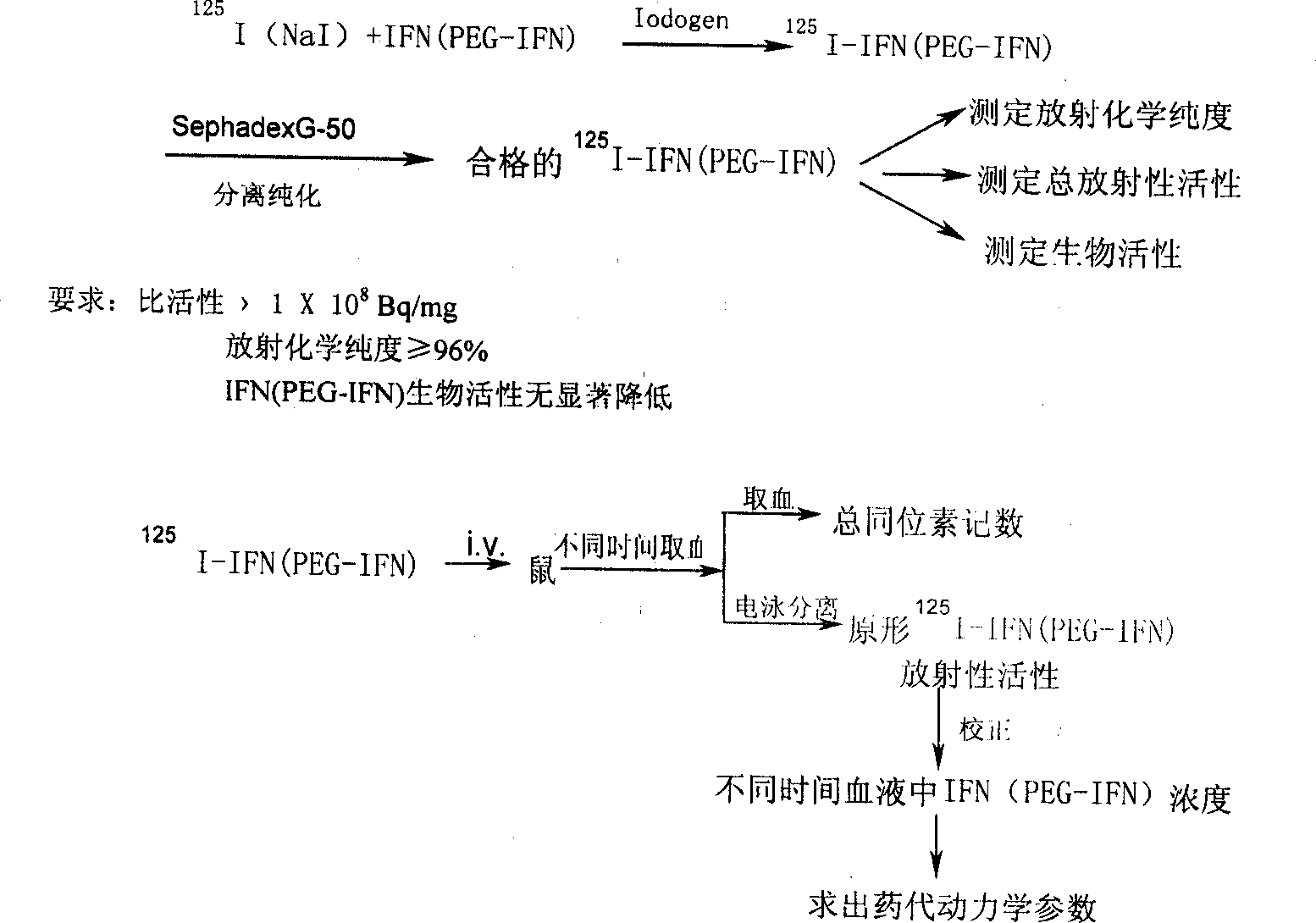
Polyglycol modified recombinant human interferon
A technology of recombinant human interferon and polyethylene glycol, applied in the field of interferon, can solve the problems of short half-life in vivo and obvious side effects, and achieve long half-life in vivo, good thermal stability and enzyme stability, and low plasma clearance rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1 carbonic acid monomethoxy polyethylene glycol succinimide ester (SC-mpEG)
[0015] Weigh 6g of monomethoxypolyethylene glycol 5000, 0.56g of triphosgene, dissolve in 20ml of toluene and 12ml of dichloromethane, add 1ml of anhydrous triethylamine dropwise, and stir overnight. The reaction solution was evacuated, most of the solvent was evaporated, and the obtained residue was redissolved in 8 ml of toluene and 10 ml of dichloromethane. Add 0.21g of hydroxysuccinimide, take 0.3ml of triethylamine, dilute with 3ml of dichloromethane, add dropwise to the reaction solution, and continue to complete the reaction, filter the reaction solution and vacuumize, and dissolve the obtained residue in acetic acid at 50°C In 60ml of ethyl ester, filter, and obtain crystals after cooling, which is crude SC-PEG. The resulting product was repeatedly recrystallized from ethyl acetate. Measure the infrared spectrum of product, following characteristic peak ...
Embodiment 2
[0016] Example 2 SC-PEG selection of modification conditions for interferon
[0017] Choice of reaction time: Take 1ml (0.9mg / ml) of interferon concentrate, add 0.2M disodium hydrogen phosphate solution to adjust the pH value to 7.5-8.5, take 0.2ml and put them in 4 test tubes with stoppers respectively. Add 0.1mg of SC-PEG to each test tube to react for 15min, 30min, 1h, 2h, then terminate the reaction, and perform SDS-PAGE electrophoresis. Compare the modification rate and determine the modification condition. The results show that the modification rate is low after 15 minutes of reaction, and most of the interferon has not been modified. The modification rate increases after 30 minutes of reaction, and the product is mainly interferon modified with one or two chains. After 1-2 hours of reaction, There was no significant difference in the spectral bands.
[0018] Selection of reaction pH value: Take 1ml (0.9mg / ml) of interferon concentrate, take 0.2ml each and put them in ...
Embodiment 3
[0019] Example 3 Separation, purification and identification of modified products
[0020] Take 50ml of interferon α2b stock solution (0.09mg / ml), adjust its pH value to 8.5, then add 80mg of SC-mPEG solid, dissolve, mix well, react at 4°C for 1h, add 2g of glycine solid to terminate the reaction.
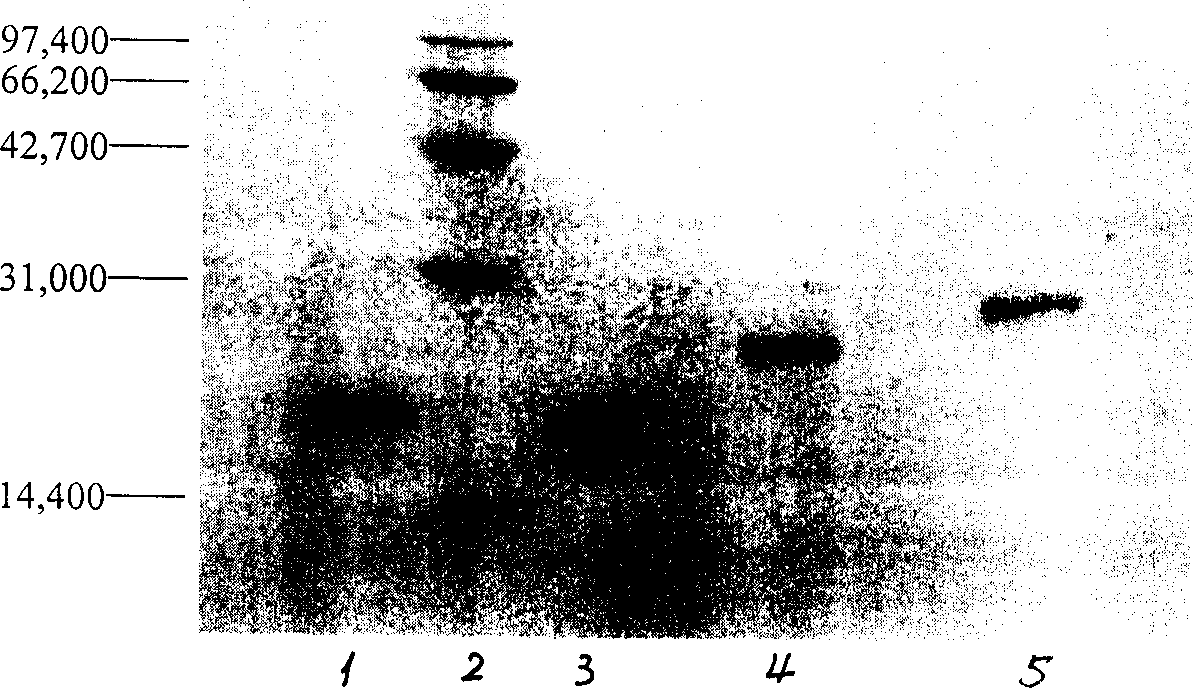
[0021] Take 10ml of the above reaction solution and put it on the column for separation. Chromatographic conditions: Superdex 75 Highload preparative gel chromatography column (26×600mm, 22-24μm), column volume 320ml, mobile phase is 0.01M phosphate buffer (pH7.18) containing 0.15M NaCl, flow rate 1.5ml / min, detection wavelength 215nm. Collect the individual peaks. The results showed that there were two elution peaks with a molecular weight greater than that of the standard interferon, which were monomodified interferon and polymodified interferon.
[0022] The eluted peaks were appropriately concentrated and detected by SDS-PAGE electrophoresis, the separation gel was 15%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com