Interleukin-6 polyethylene glycol conjugate and its preparing method and use
A technology of interleukin and polyethylene glycol, which is applied in the field of preparation of interleukin-6 polyethylene glycol conjugates, can solve the problems of short half-life, difficult selection of reagents, difficult modification, etc., and achieves the dosage and frequency of use. Reduce and improve the effect of safety in use and clinical drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] [Example 1] Preparation of interleukin-6 polyethylene glycol conjugate (PEG-IL-6)
[0088] The chemical modification reaction formula for preparing IL-6 polyethylene glycol conjugate is as follows:
[0089]
[0090] Wherein i and j are integers of 100-1000, the sum of i and j makes the molecular weight of the mPEG part of the conjugate be 15000-30000, preferably 20000, and the amino group of -NH-IL-6 in the reaction formula structure is a Lys residue side chain amino group.
[0091] The modification process is as follows:
[0092] Sample: pure human IL-6 (Sigma company), SDS-PAGE purity greater than 95%, protein concentration between 0.5-1mg / ml, pH 9.0, buffer solution is PB, and cannot contain other amino-containing compounds
[0093] Modification reagent: mPEG2-NHS MW20kDa, stored in a dry place at -20°C Modification process:
[0094] a. Heat IL-6 in a water bath to 25°C, weigh mPEG2-NHS 1 to 2 times the total amount of IL-6, and put it into a dry, clean, steril...
Embodiment 2
[0098] [Example 2] Purification of PEG-IL-6
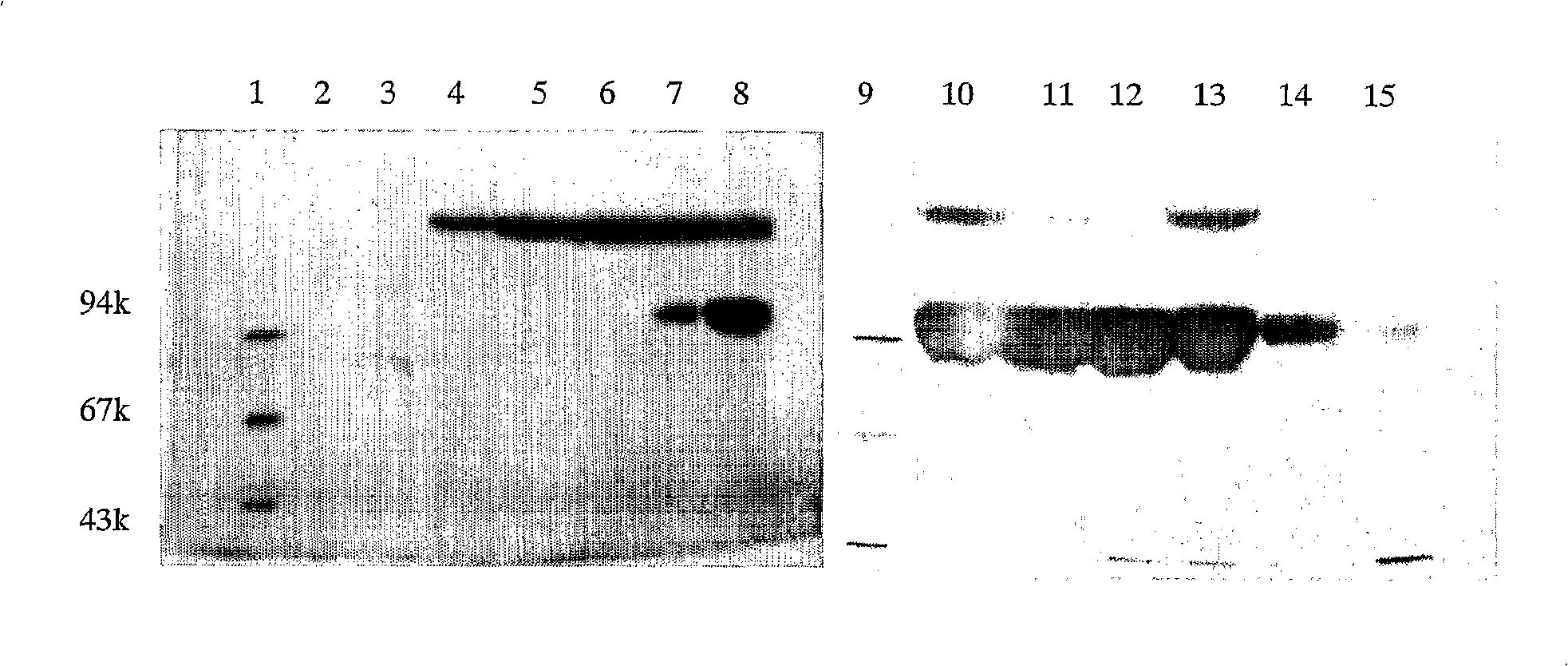
[0099] First, the three batches of products obtained by implementing the method 1 are uniformly mixed, and the G-25 gel column that is balanced to pH 5.0 through 10mM acetate buffer is used for desalting and changing the liquid, and the sample buffer (buffer formula: Na2HPO 12H2O, 15.04 g / L; NaH2PO4 2H2O, 1.25g / L; NaCl 8.77g / L. pH 9.0) the pH was adjusted from 9.0 to 5.0; the mixture of the modification reaction was separated by one-step SP Sepharose High Performance cation exchange chromatography (eluent Formula: Sodium acetate 0.82g / L in liquid A, adjust the pH to 5.0 with acetic acid; 0.82g / L sodium acetate in liquid B, 29.25g / L sodium chloride, adjust the pH to 5.0 with acetic acid). Under this condition, the hydrolyzed mPEG molecules cannot be adsorbed on the column because they are uncharged or negatively charged, and then the multi-modified mPEG-IL-6 is eluted first under the gradually increasing salt ion gradient. down, fo...
Embodiment 3
[0102] [Example 3] Preparation prescription and technical process
[0103] 1 preparation prescription
[0104] 1.1 Preparation prescription (based on 1000 bottles)
[0105] PEG-rhIL-6 was prepared according to the aforementioned method
[0106] Main drug PEG-rhIL-6 15mg
[0107] Human albumin 10g
[0108] Glycine 25g
[0109] Na2HPO412H2O 0.619g
[0110] KH2PO4 0.103g
[0111] NaCl 3.440g
[0112] KCl 0.086g
[0113] 1.2 Types of preparations
[0114] It belongs to the lyophilized preparation for injection stipulated in Appendix I "General Rules of Preparations" of the 2005 edition of "Pharmacopoeia of the People's Republic of China".
[0115] 1.3 Preparation specifications
[0116] 15μg / 0.5ml / bottle (200,000 active units)
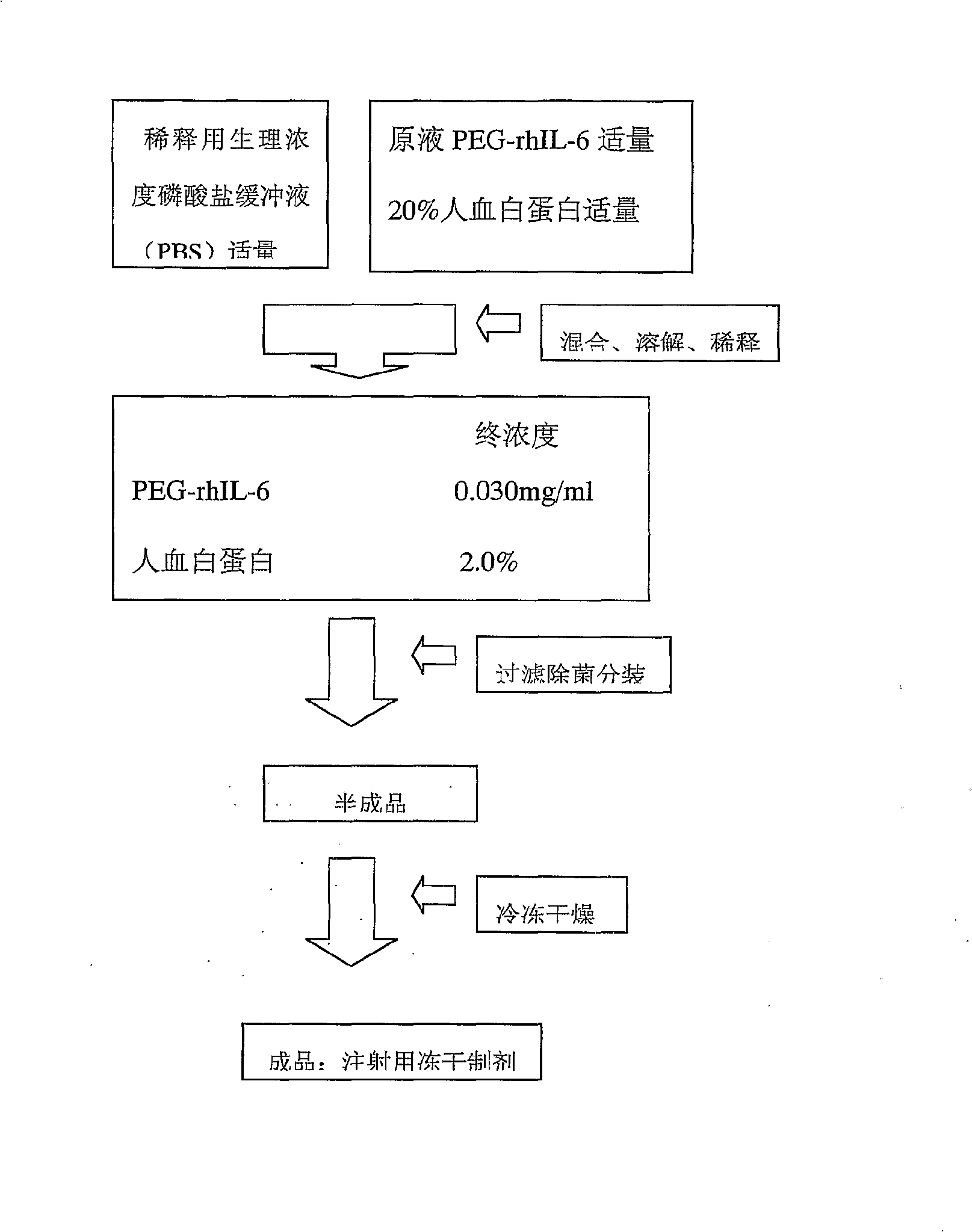
[0117] 2 For preparation process, see image 3 .
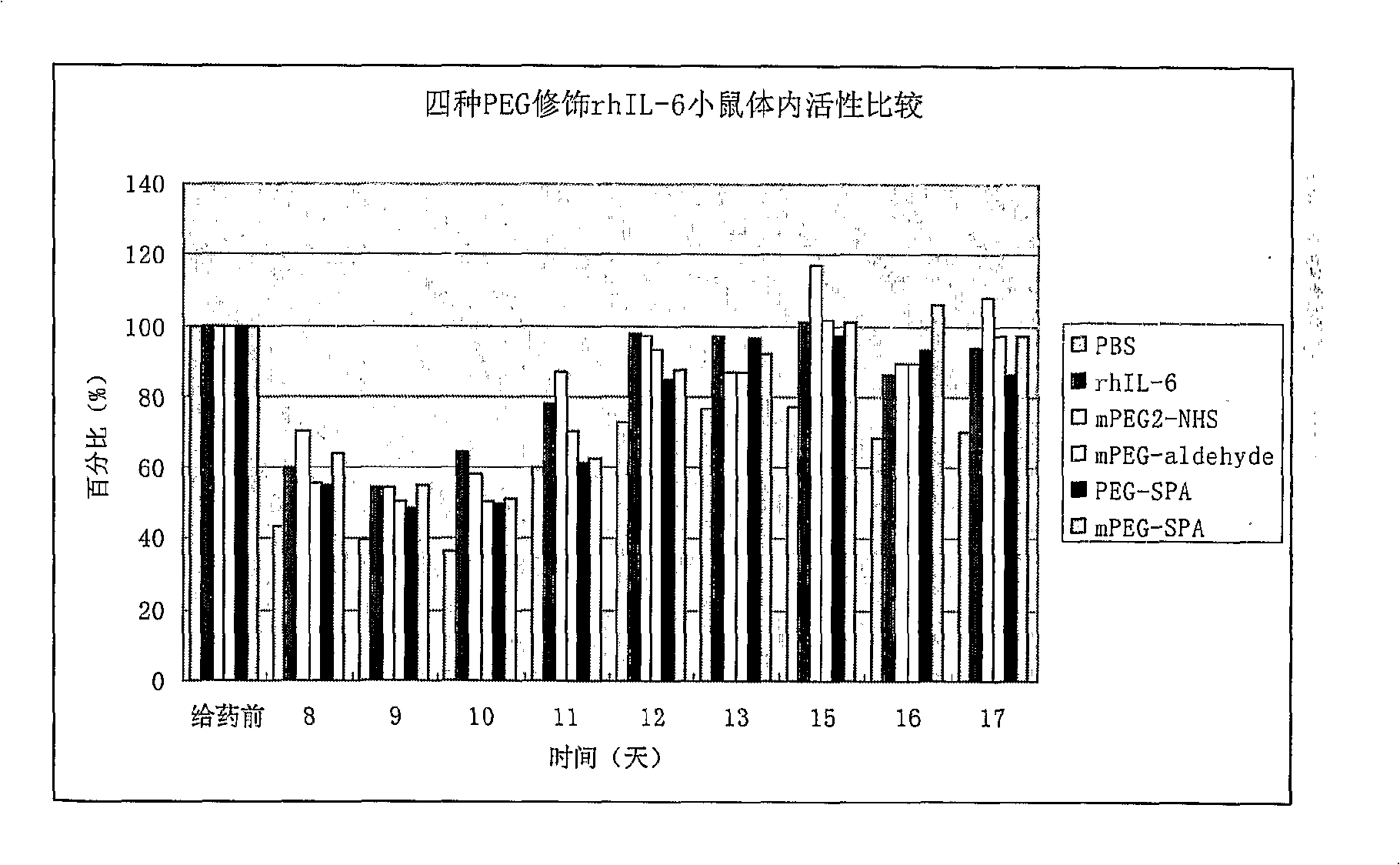
[0118] Pharmacodynamics test, safety test and pharmacokinetic test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com