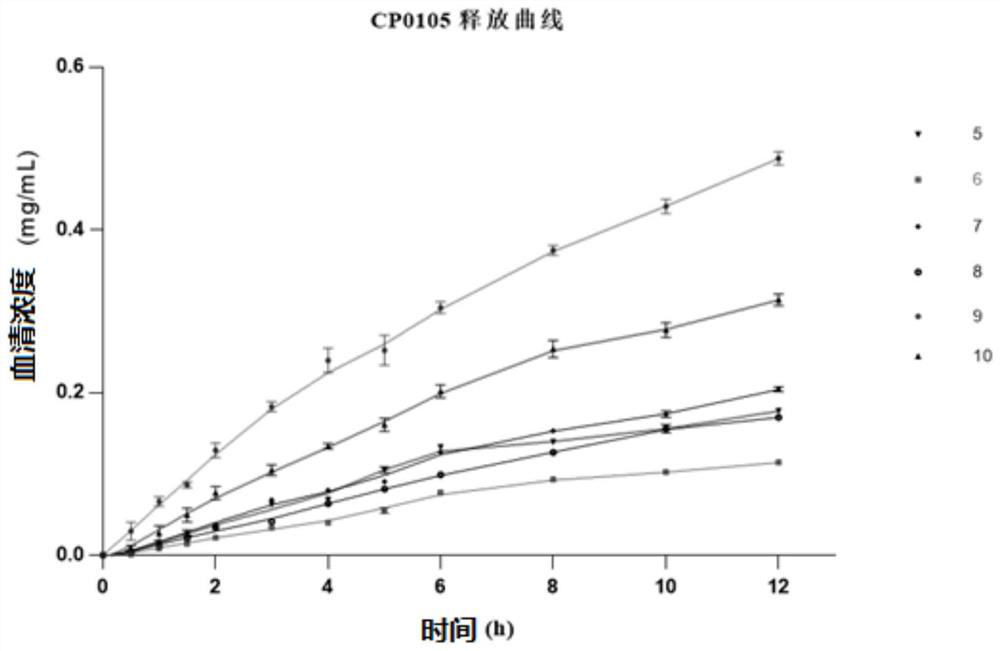
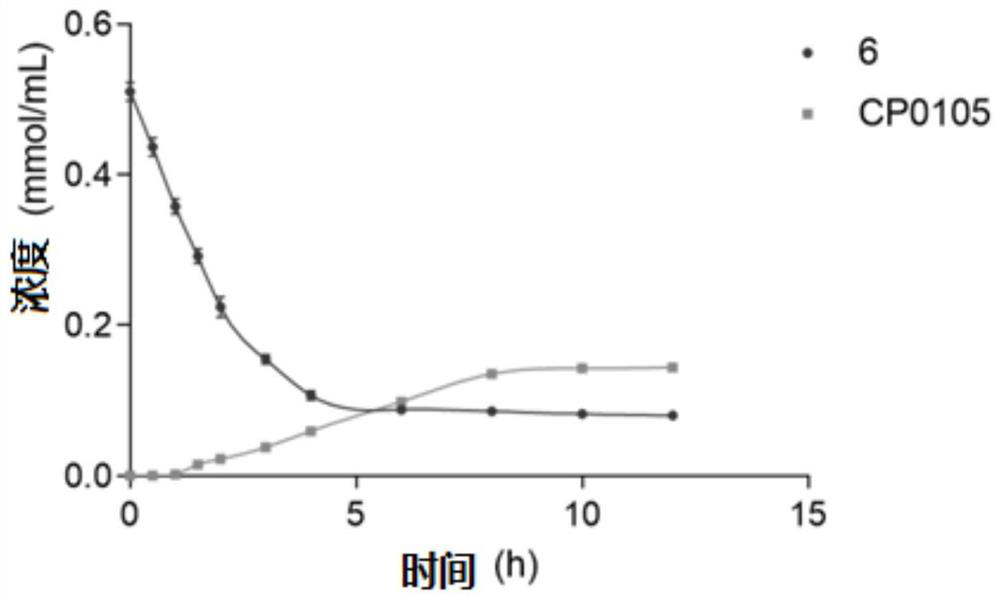
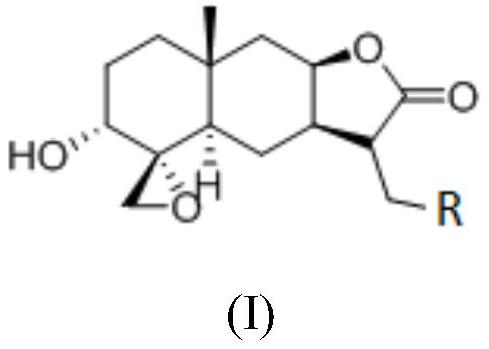
Sesquiterpene derivative as well as pharmaceutical composition, preparation method and application thereof
A derivative and composition technology, applied in the field of medicinal chemistry, can solve the problems of difficult to maintain long-term drug concentration, fast prodrug release, short half-life, etc., and achieve the effects of stable drug release time, long half-life and stable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Compound 1
[0042] The structure of compound 1 is as follows:
[0043]
[0044] Its preparation process is as follows:
[0045] At 0°C, selenium dioxide (2.86 g, 25.8 mmol) was dissolved in dichloromethane (250 mL), tert-butanol peroxide (15.5 mL) was added, and after stirring for 30 minutes, isoinulin (30 g) was added. , 0.129mol) dichloromethane (250mL) solution was slowly added to the above system, stirred at room temperature for 8 hours, and then quenched the reaction with saturated aqueous sodium thiosulfate solution (500mL), after separation, the aqueous phase was mixed with dichloromethane Methane extraction (300 mL×3), the organic phases were combined, dried, concentrated, and recrystallized with petroleum ether / ethyl acetate mixed solvent to obtain Intermediate 1 (white solid, 19.5 g, yield 61%), which was then directly used with in the next step.
[0046] At 0 °C, compound intermediate 1 (19.5 g, 78.5 mmol) was dissolved in dic...
Embodiment 2
[0051] Example 2: Fumarate salt of compound 1 - preparation of compound 5
[0052] The structure of compound 5 is as follows:
[0053]
[0054] Compound 1 (998 mg, 2.84 mmol) prepared in Example 1 was dissolved in tetrahydrofuran (20 mL), and after stirring uniformly, fumaric acid (313 mg, 2.70 mmol) was added to the system, and the reaction was stirred at room temperature for 3 hours. After completion, the tetrahydrofuran was removed by concentration under reduced pressure, then ethyl acetate (100 mL) was added to the reaction system to obtain a suspension, which was filtered with suction to obtain compound 5 (white solid, 815 mg, yield 61%).
[0055] Compound 5 was detected, and its NMR data were as follows:
[0056] 1 H NMR(400MHz, DMSO)δ6.62(s,2H),4.48(d,J=4.2Hz,1H),3.56(t,J=4.7Hz,4H),3.16(dd,J=10.7,4.7Hz ,2H),2.75(d,J=4.5Hz,1H),2.47–2.35(m,6H),2.28(dt,J=10.7,4.5Hz,2H),2.09(dd,J=13.0,2.4Hz, 1H), 1.96 (d, J=15.3Hz, 1H), 1.80–1.65 (m, 1H), 1.63–1.42 (m, 3H), 1.35–1.24...
Embodiment 3
[0057] Example 3: Preparation of Compound 2
[0058] The structure of compound 2 is as follows:
[0059]
[0060] Its preparation process is as follows:
[0061] Using N-methylpiperazine (1.89 g, 18.9 mmol), following the synthetic procedure of compound 1 in Example 1, the target compound 2 (white solid, 993 mg, yield 72%) was obtained.
[0062] Compound 2 was detected, and its NMR data were as follows:
[0063] 1 H NMR(400MHz, DMSO)δ4.47(s,1H),3.17(d,J=2.7Hz,1H),3.13(td,J=6.2,3.1Hz,1H),2.75(d,J=4.5Hz) ,1H),2.60(s,4H),2.57–2.51(m,2H),2.51(d,J=1.8Hz,1H),2.49–2.45(m,2H),2.41(dd,J=12.9,4.5 Hz, 2H), 2.36(s, 1H), 2.34(s, 4H), 2.07(dd, J=12.9, 2.3Hz, 1H), 1.95(dd, J=15.5, 1.9Hz, 1H), 1.71(dt , J=15.0, 3.8Hz, 1H), 1.60–1.52 (m, 2H), 1.48 (dd, J=15.5, 4.3Hz, 1H), 1.32–1.24 (m, 1H), 1.19 (dd, J=12.4 ,3.2Hz,1H),0.83(s,3H),0.69–0.59(m,1H). 13 C NMR(100MHz, DMSO)δ177.5,77.5,71.1,60.9,53.7,52.5,48.0,44.3,44.1,41.3,38.4,36.8,34.7,34.3,27.7,18.0,15.5.HRMS(ESI):m / zcalcd for C 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com