Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Liver cell cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibodies for treatment of cancer
InactiveUS8946388B2Strong formationStrong proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
Monoclonal antibodies for treatment of cancer
InactiveUS20110223182A1Minimize adverse effectsStrong formationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMelanoma
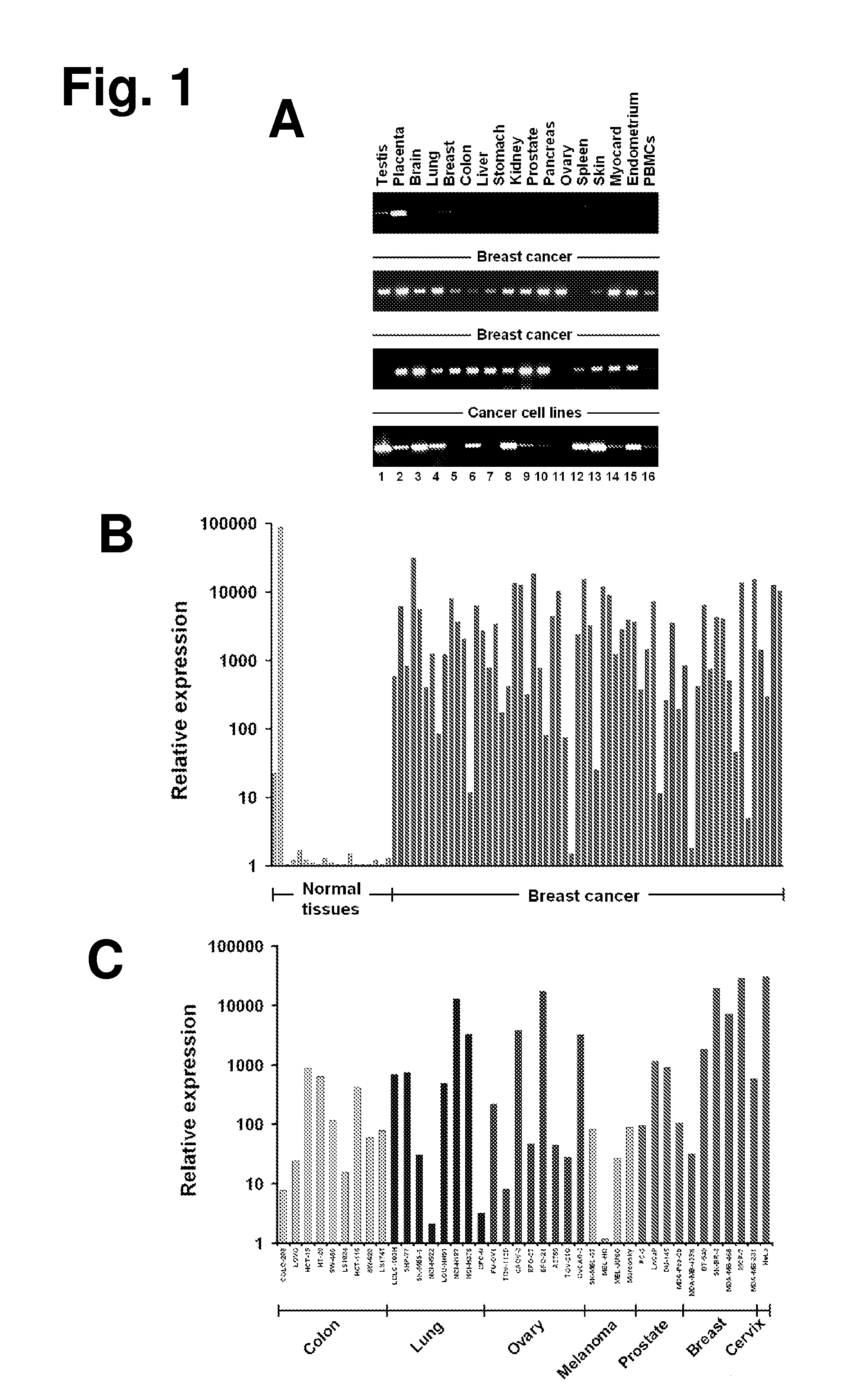
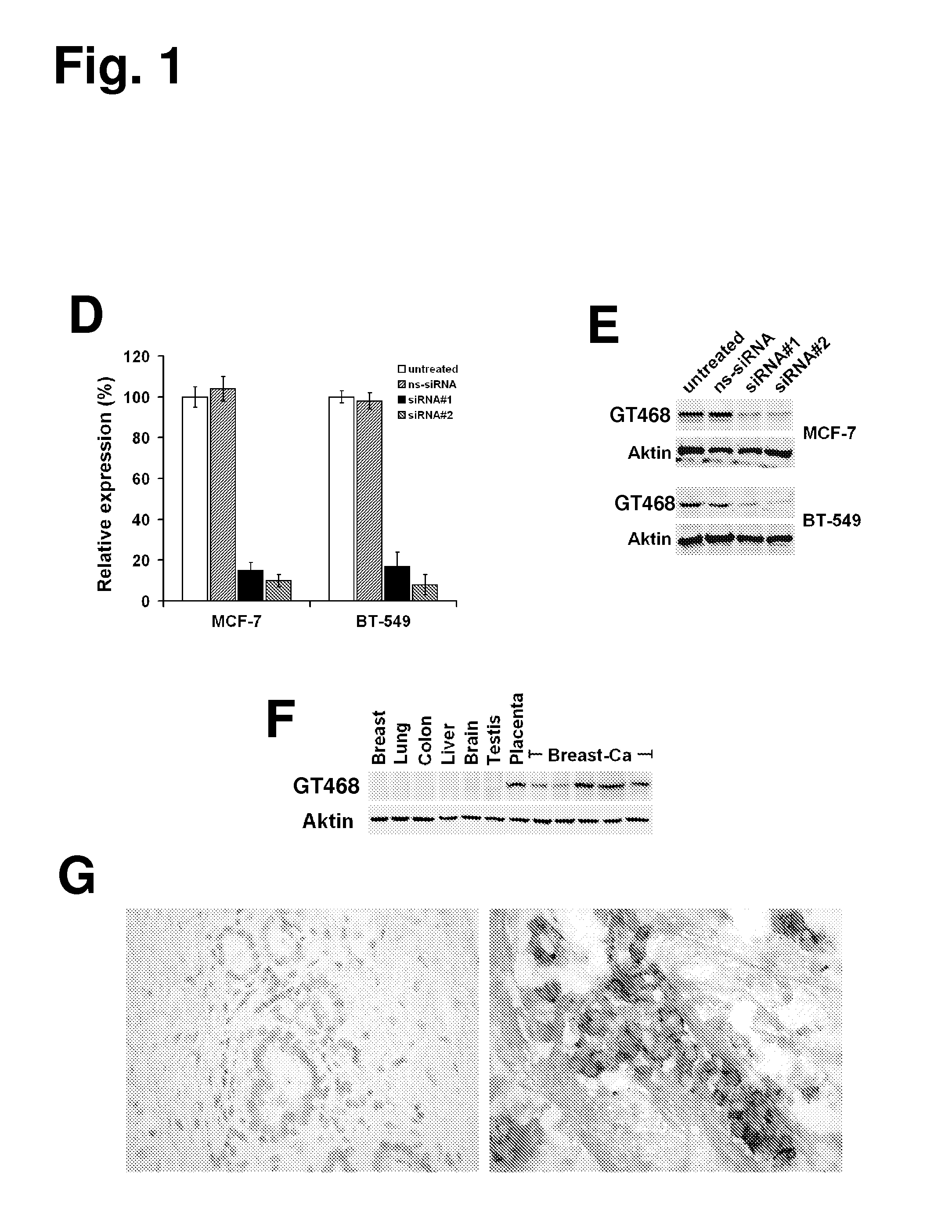
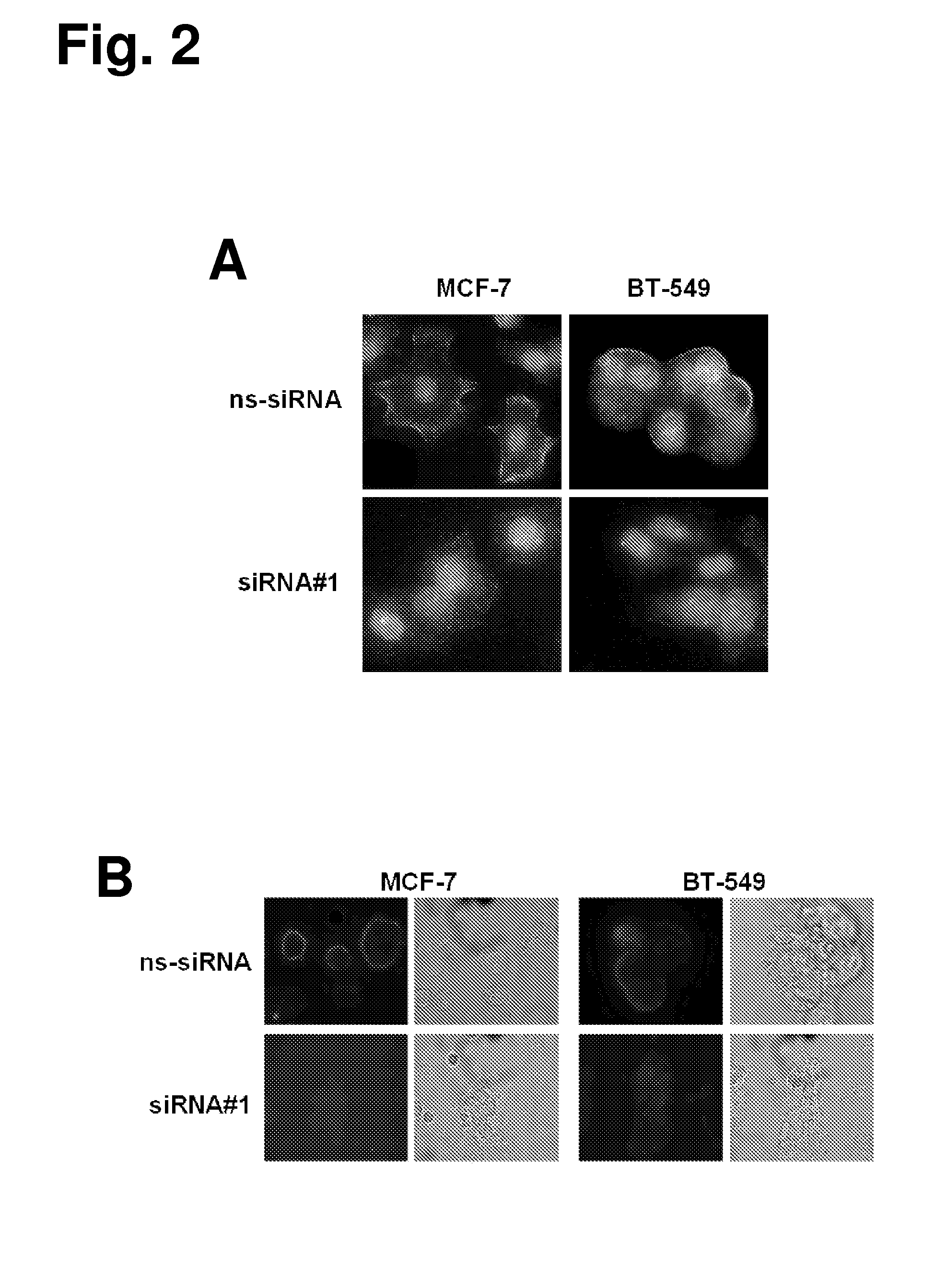
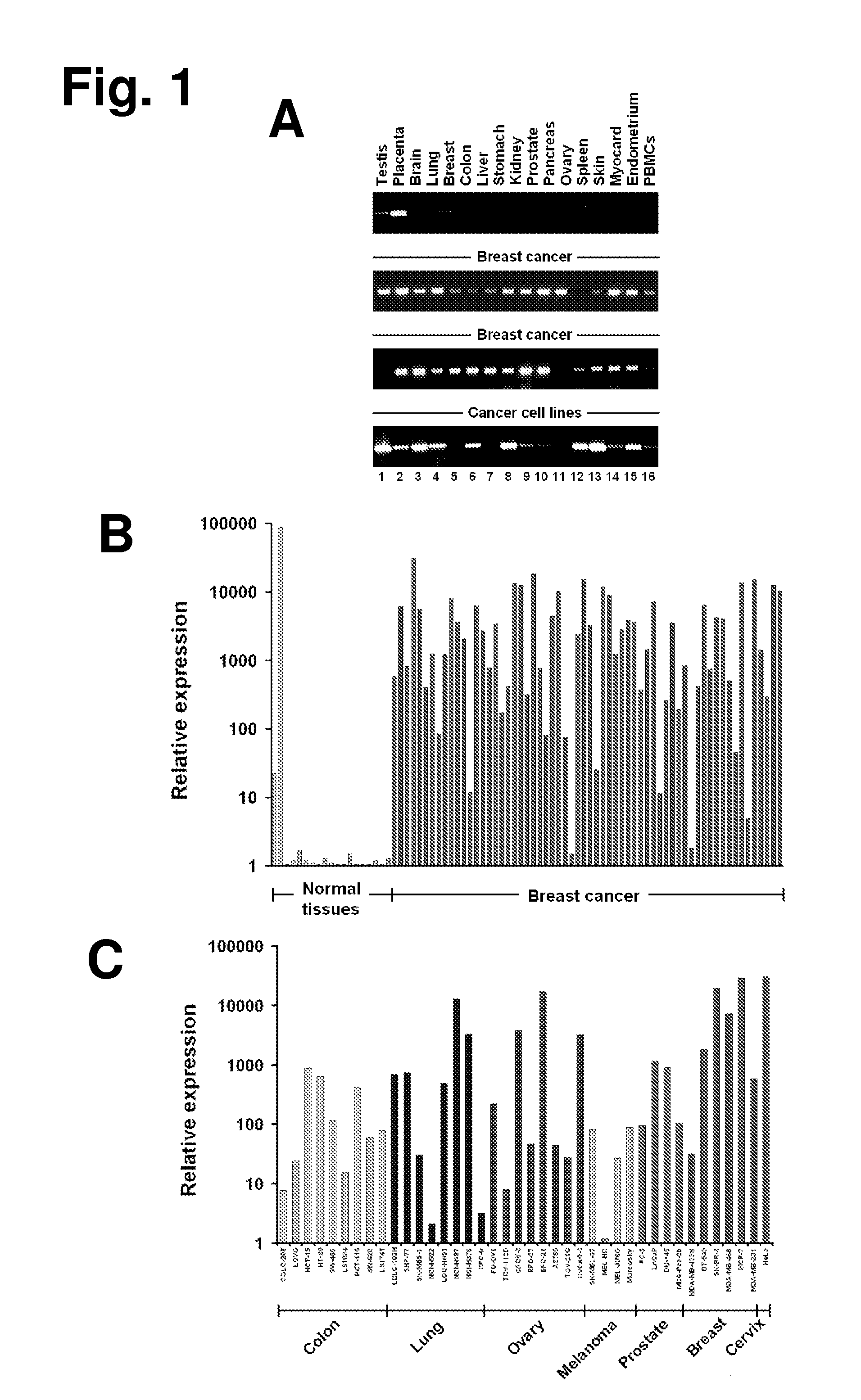
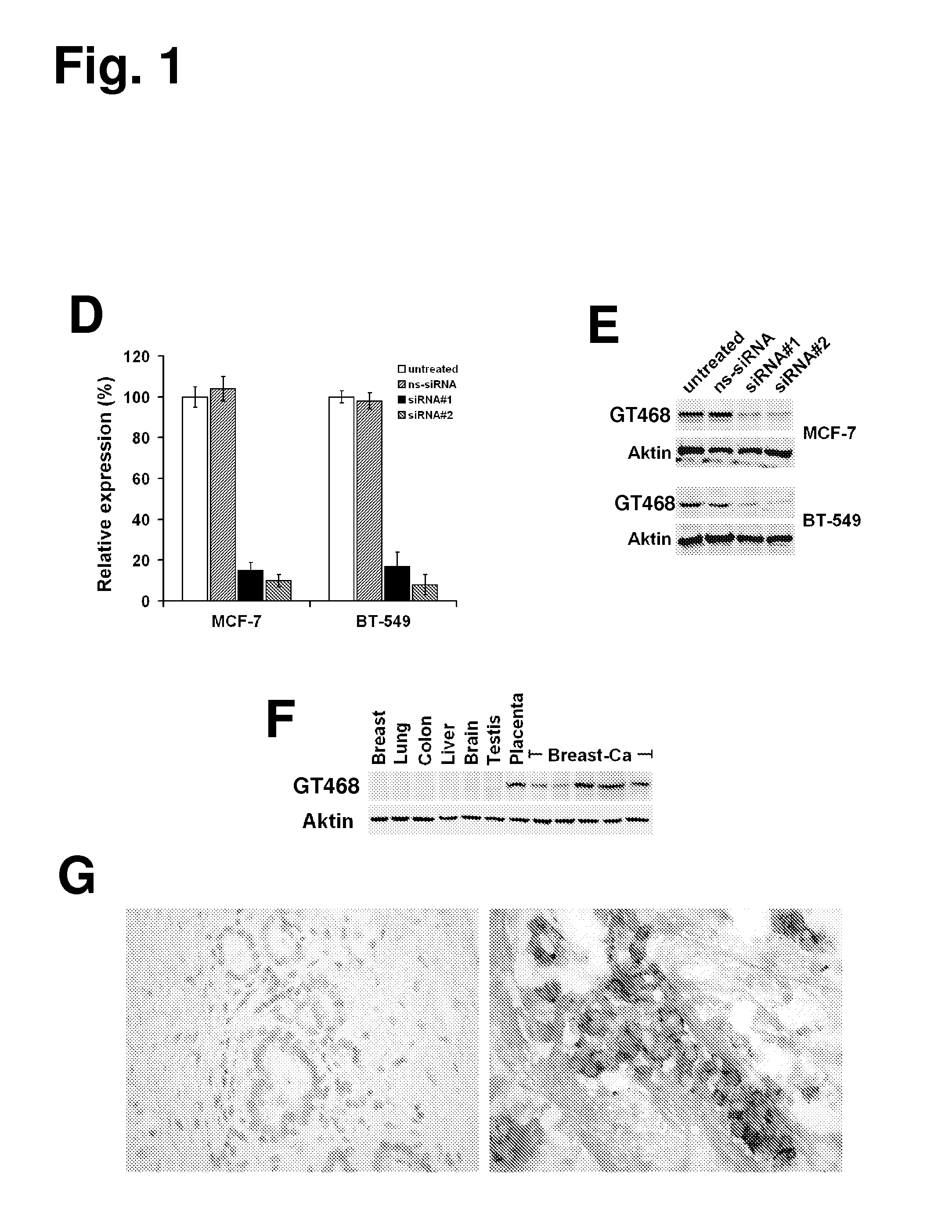
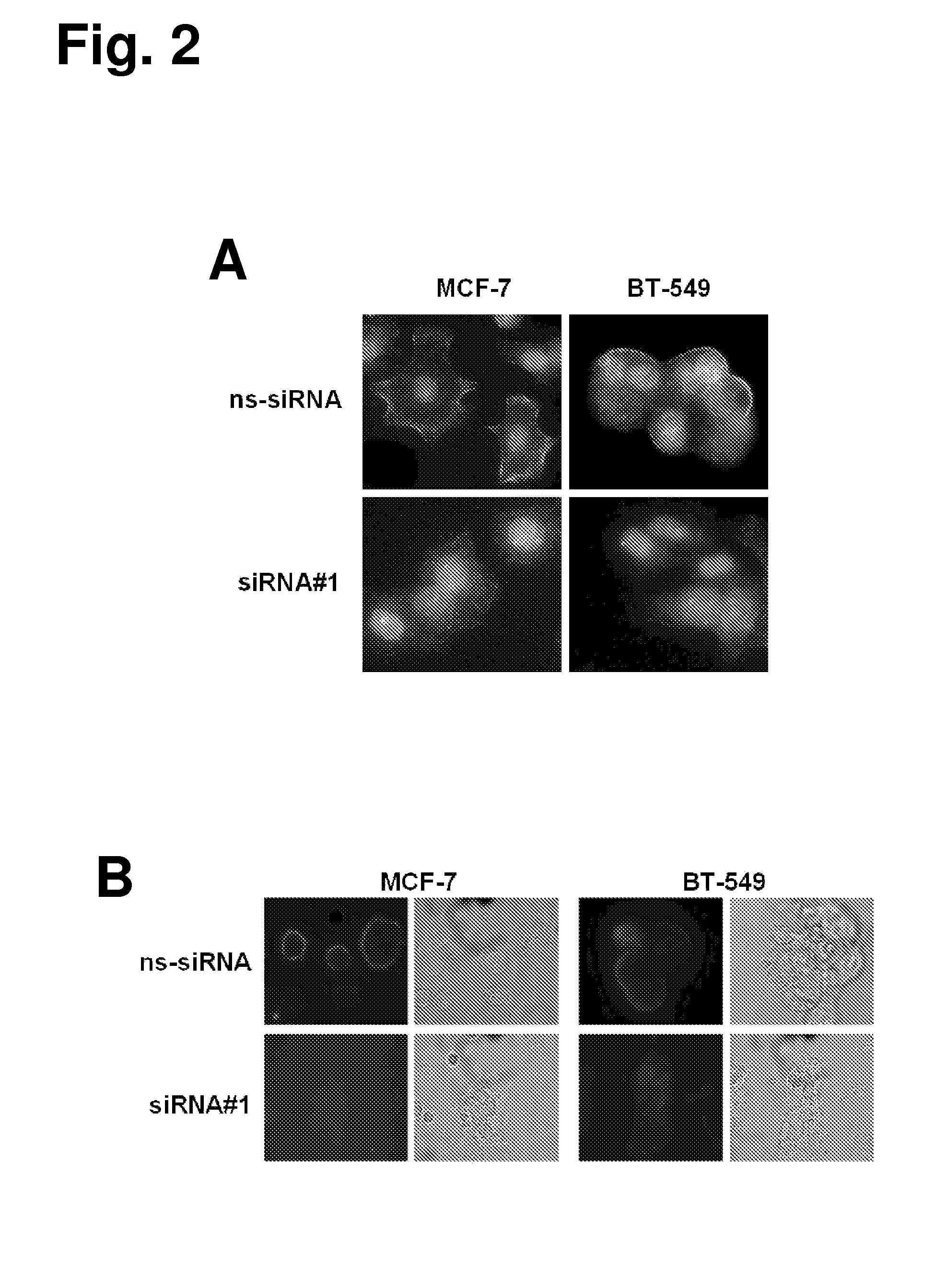
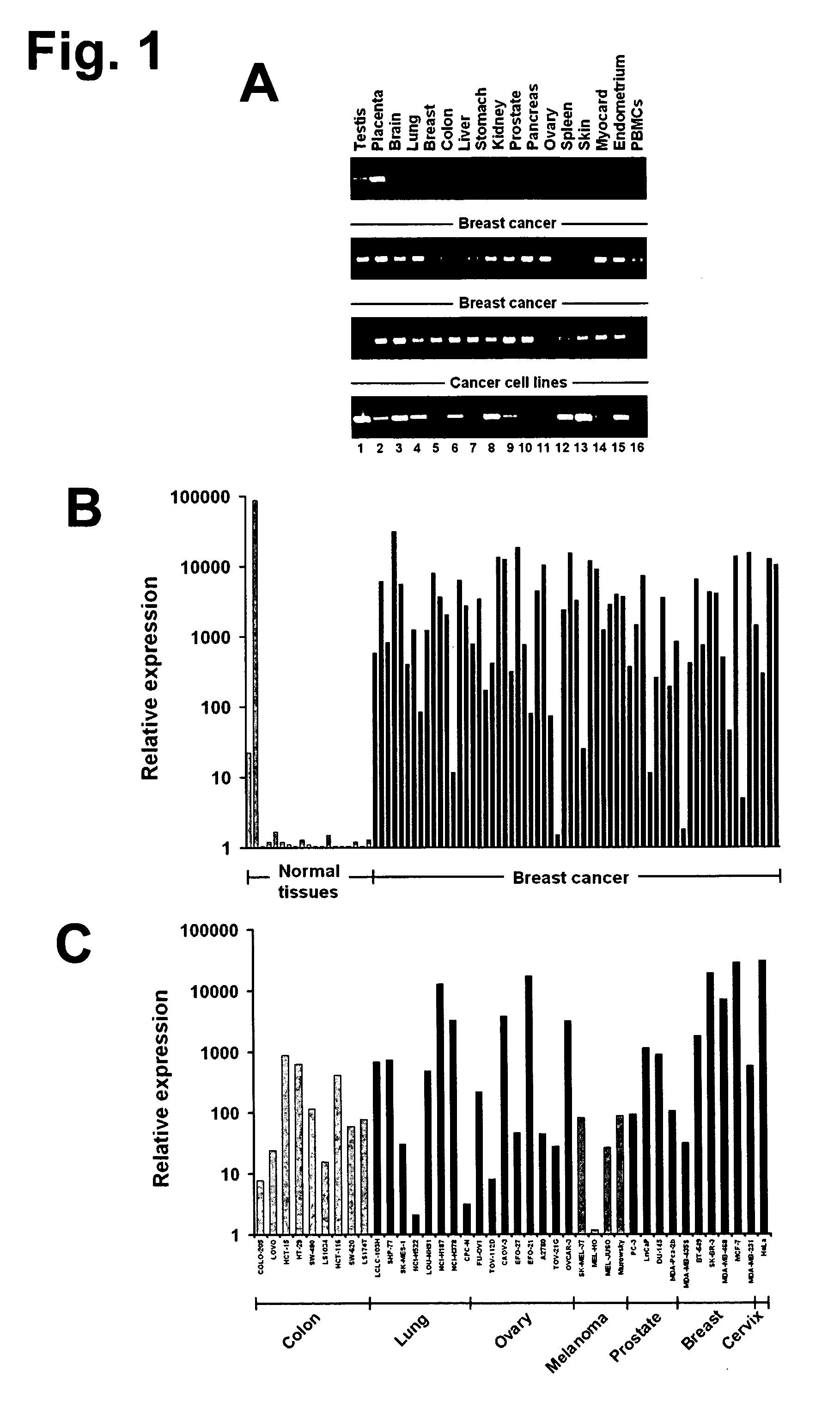
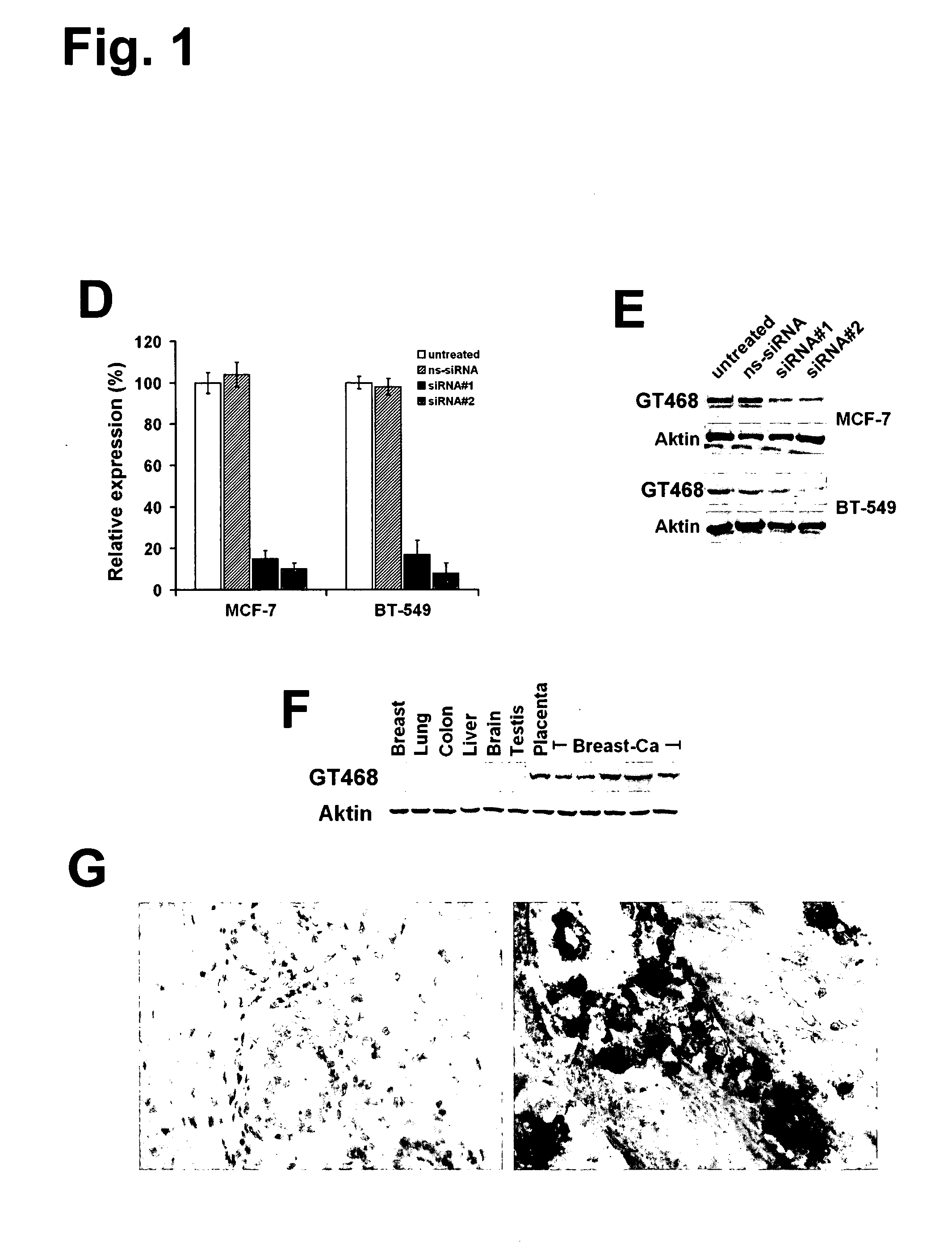
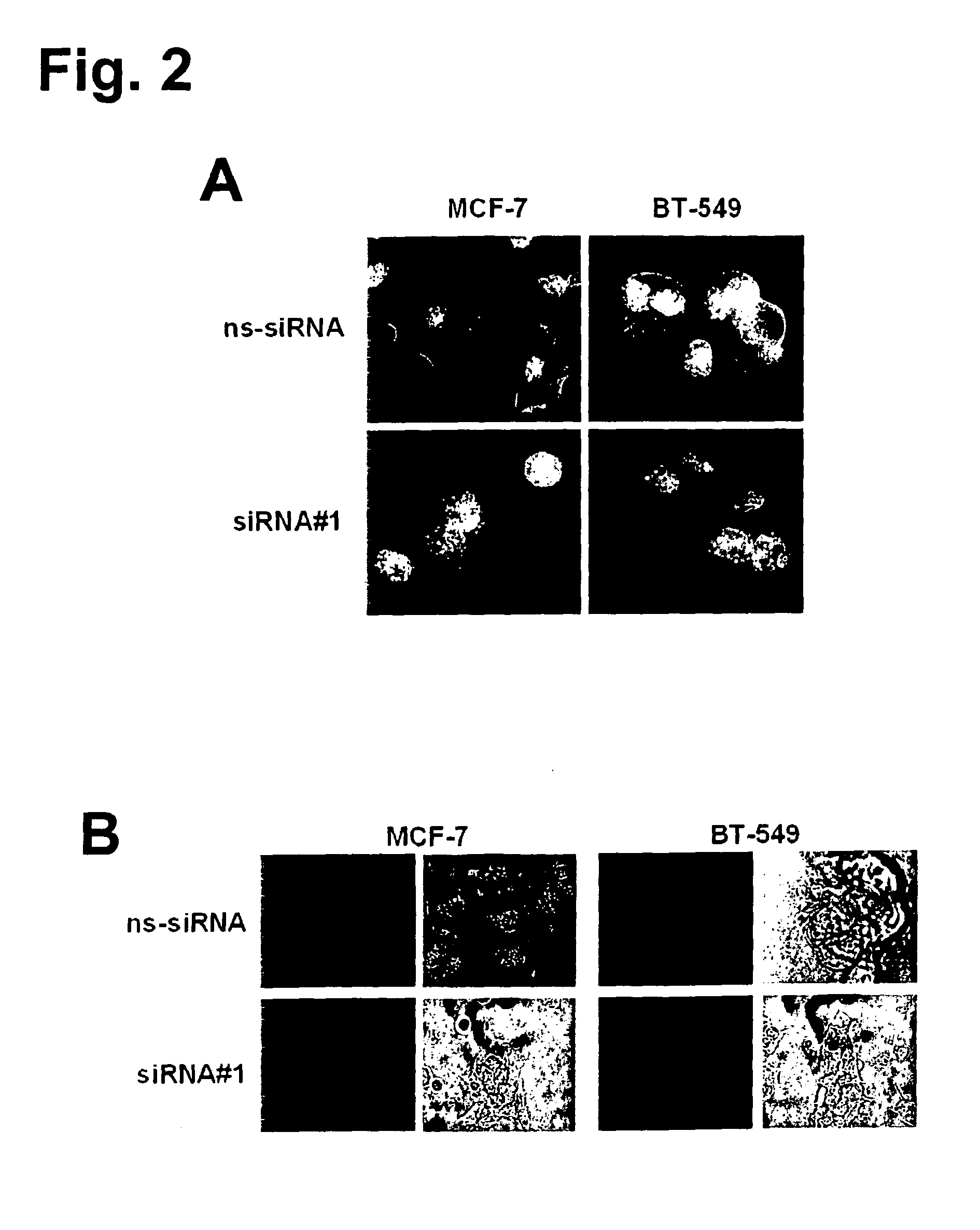
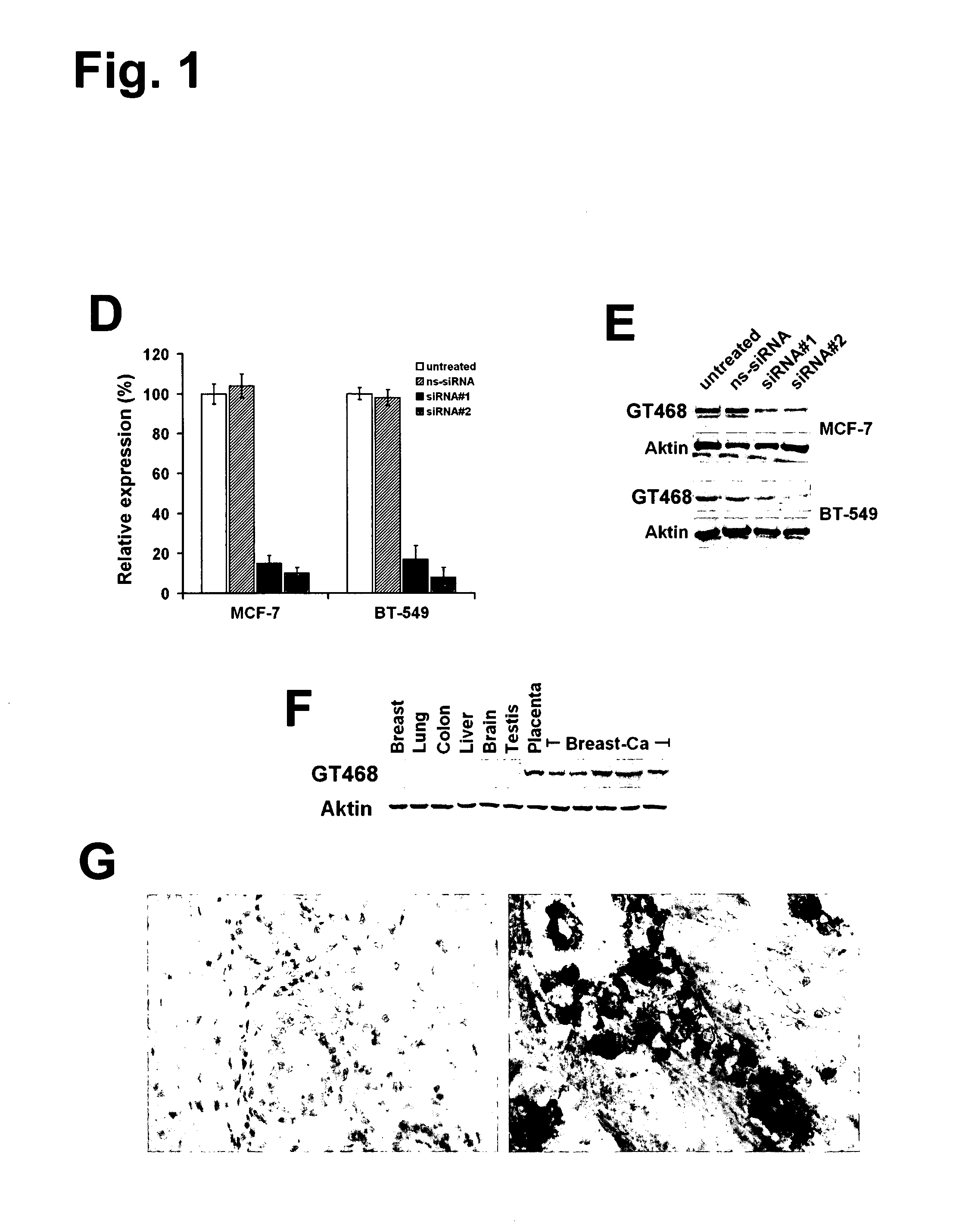
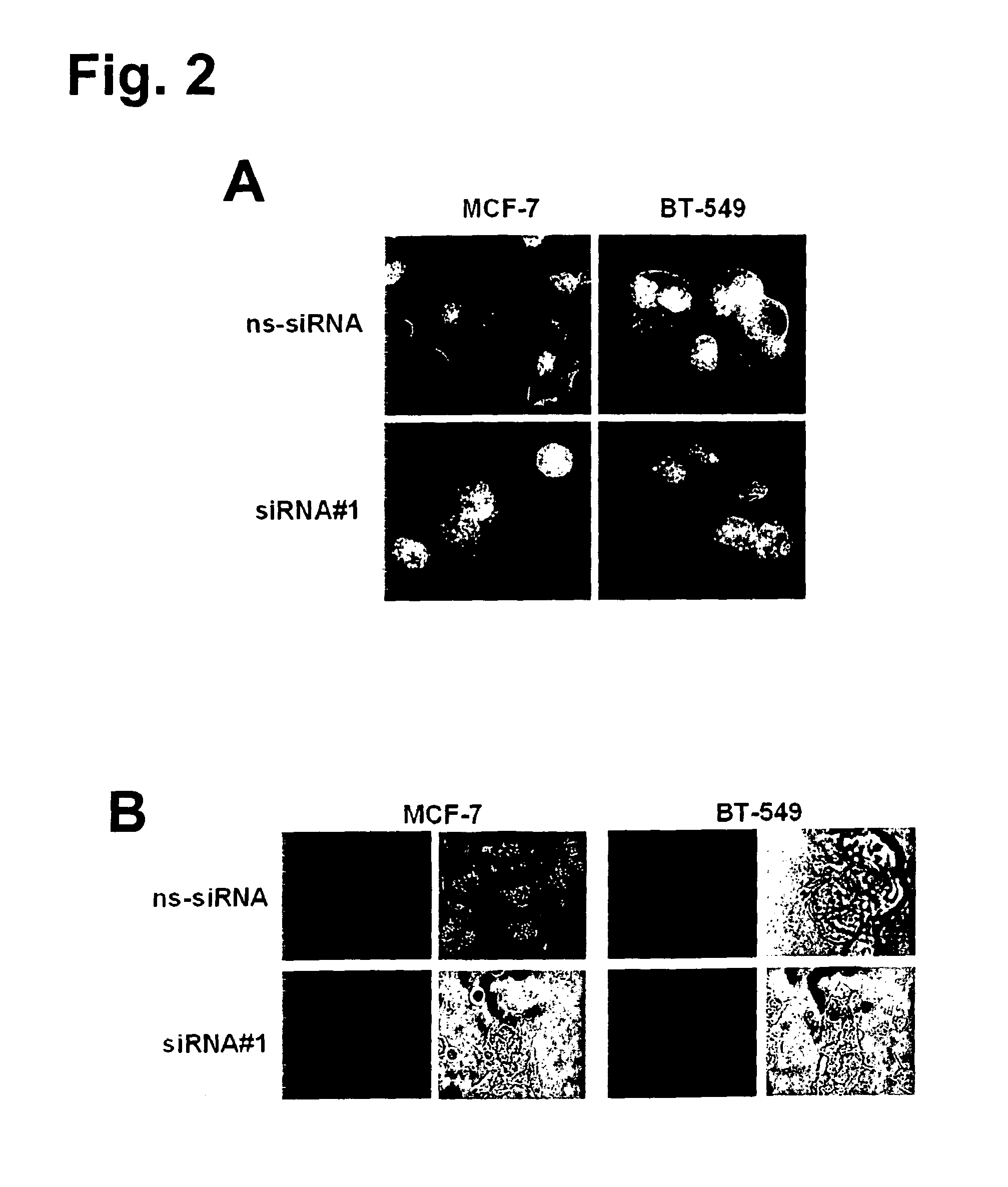
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast Cancer, lung Cancer, gastric Cancer, ovarian Cancer, hepatocellular Cancer, colon Cancer, pancreatic Cancer, esophageal Cancer, head & neck Cancer, kidney Cancer, in particular renal cell Carcinoma, prostate Cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid Cancer, and the metastatic forms thereof. In one embodiment, the rumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
Monoclonal antibodies for treatment of cancer
ActiveUS20130071325A1Minimize adverse effectsStrong formationAnimal cellsIn-vivo radioactive preparationsDiseaseMelanoma
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
Monoclonal antibodies for treatment of cancer
ActiveUS9216218B2Strong formationStrong proliferationAnimal cellsHybrid immunoglobulinsDiseaseAntiendomysial antibodies
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
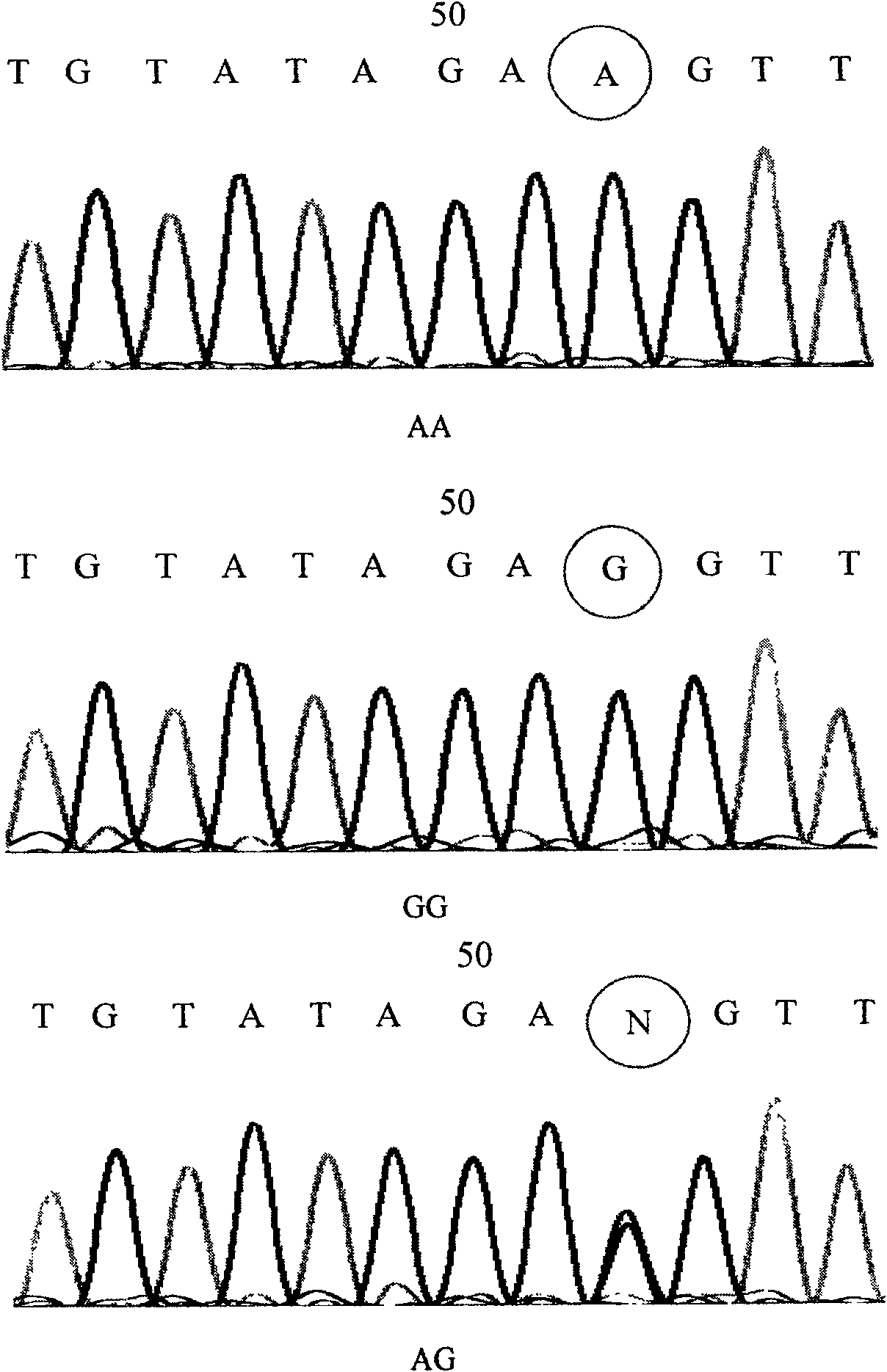
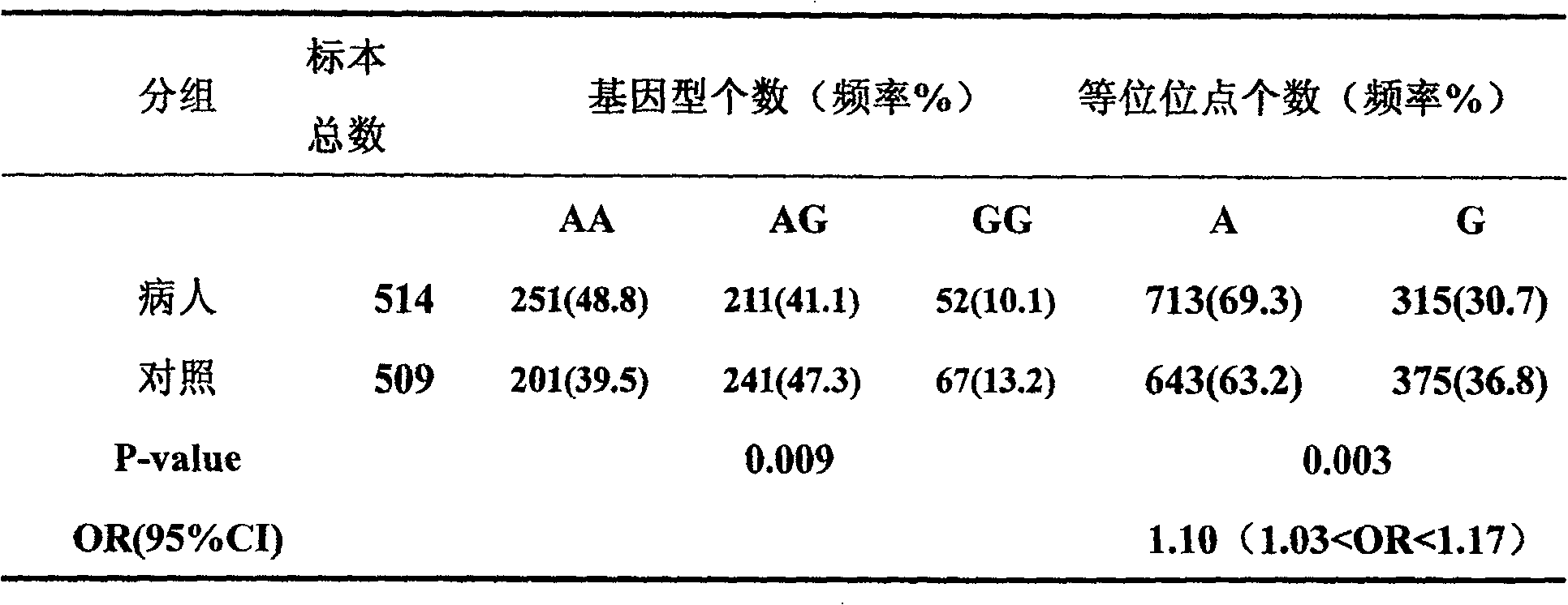
Mononucleotide polymorphic site of CDC6 gene correlated to primary liver cancer and application thereof
InactiveCN101033489AEasy to findMicrobiological testing/measurementDNA/RNA fragmentationNucleotideNucleic acid sequencing
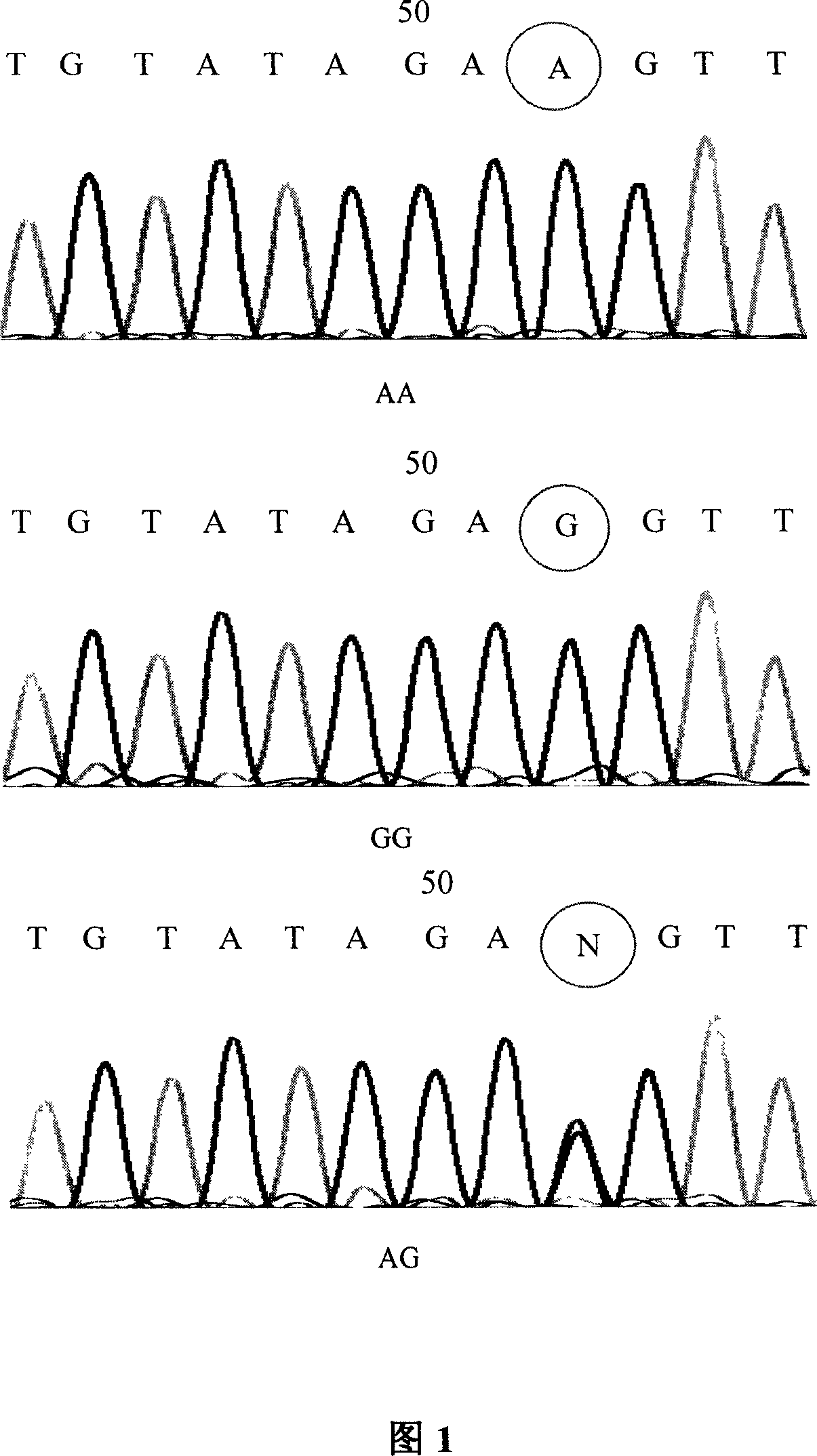
The invention discloses the polymorphic site (rs4134994) in CDC6 gene associated with primary liver cell cancer and its application. The invention also provides a method for detecting the polymorphic site and susceptibility of the primary liver cell cancer. By use of the nucleic acid sequence of the polymorphic loci and its detection method, it can produce kit for preventing primary liver cell cancer and early diagnosis and treatment.
Owner:SUN YAT SEN UNIV
Treatment using oncolytic virus
An oncolytic virus is for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelial carcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patientswith no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / orcancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panelof three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
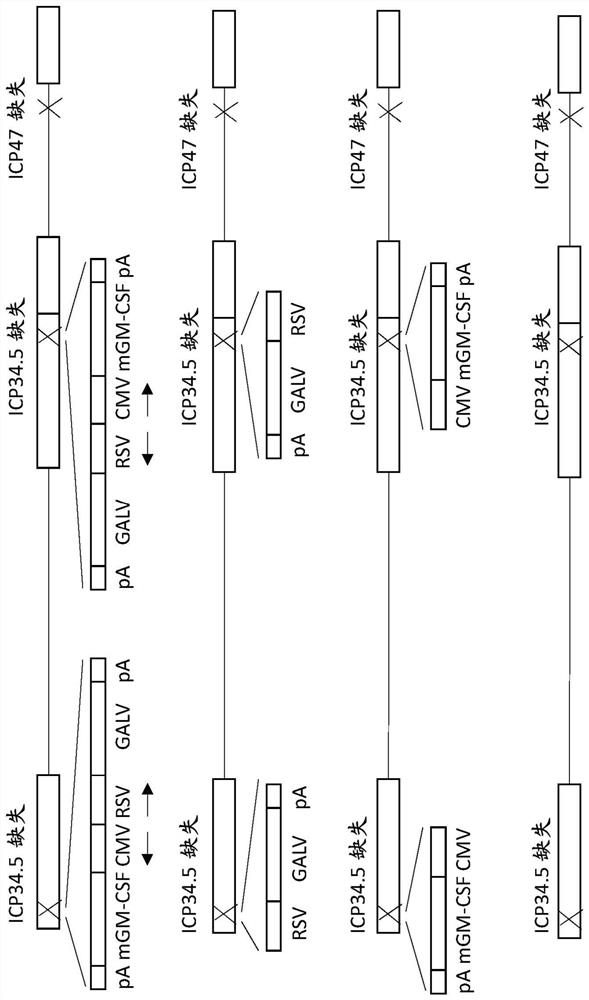
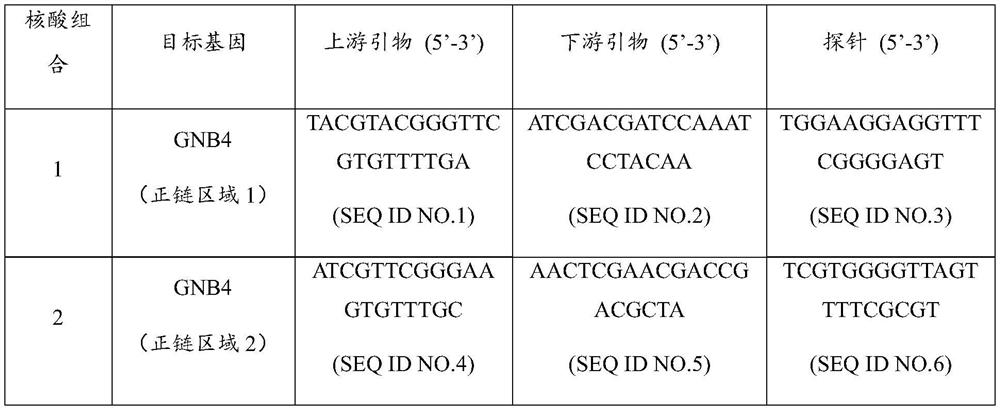
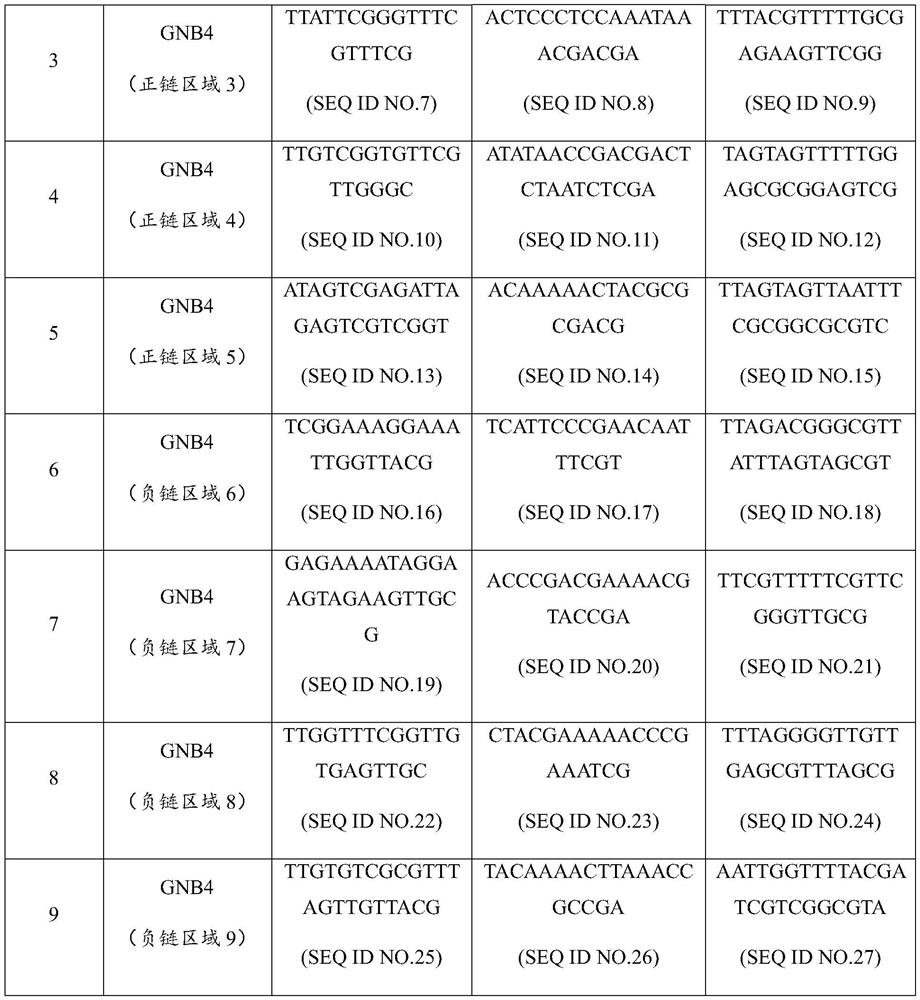
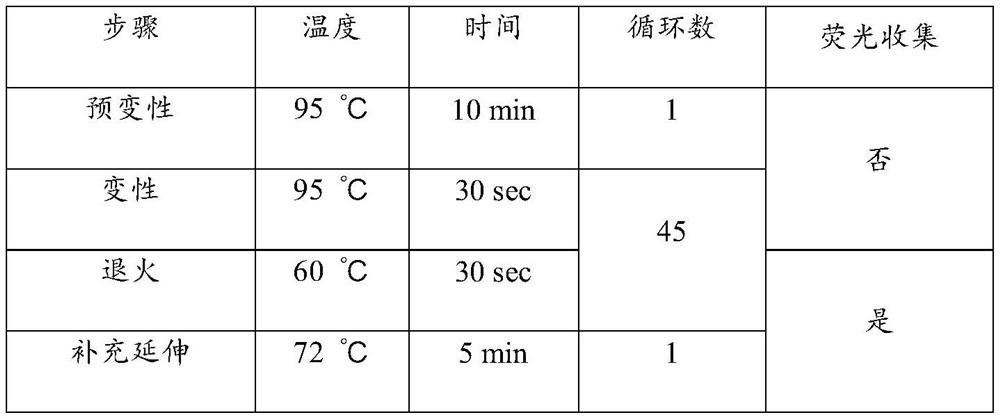
Nucleic acid composition for diagnosis or auxiliary diagnosis of liver cancer, detection kit and application thereof
ActiveCN113604563AImprove the detection rateMeet the clinical needs of early screening and early diagnosisMicrobiological testing/measurementDNA/RNA fragmentationGNB4AIDS diagnosis
The invention discloses a nucleic acid composition for diagnosis or auxiliary diagnosis of liver cancer, a detection kit and application thereof, and relates to the technical field of gene detection. The methylation of the CpG island of the human GNB4 gene is taken as a marker, the liver cancer can be diagnosed or diagnosed in an auxiliary manner by detecting the increase of the methylation, and the sensitivity and the specificity are relatively high. By adopting the detection reagent and the kit provided by the invention, the detection rate of the liver cancer can be effectively improved, so that the clinical requirements of early screening and early diagnosis of the liver cancer are met.
Owner:WUHAN AIMISEN LIFE TECH CO LTD
Method and reagent kit for detecting two proteins expressed in human liver organization
The invention relates to a detection method for testing a protein HOP and a heterogeneous ribosome protein C1 / C2 which are both expressed in human liver tissue, as well as reagent box used in the method. The method is characterized in that: by means of the fluorescence differential two-dimensional electrophoresis method -MALDI-TOF / TOF, the inventor manages to identify the overexpression of the two proteins on human liver cell carcinoma tumor tissue, and carries out immune histochemistry validations of Western bolt and large amount of samples over the expression levels in the human liver cell carcinoma tissue. The detection method for proteins and the reagent box can be used in the detections for clinical or research purposes.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
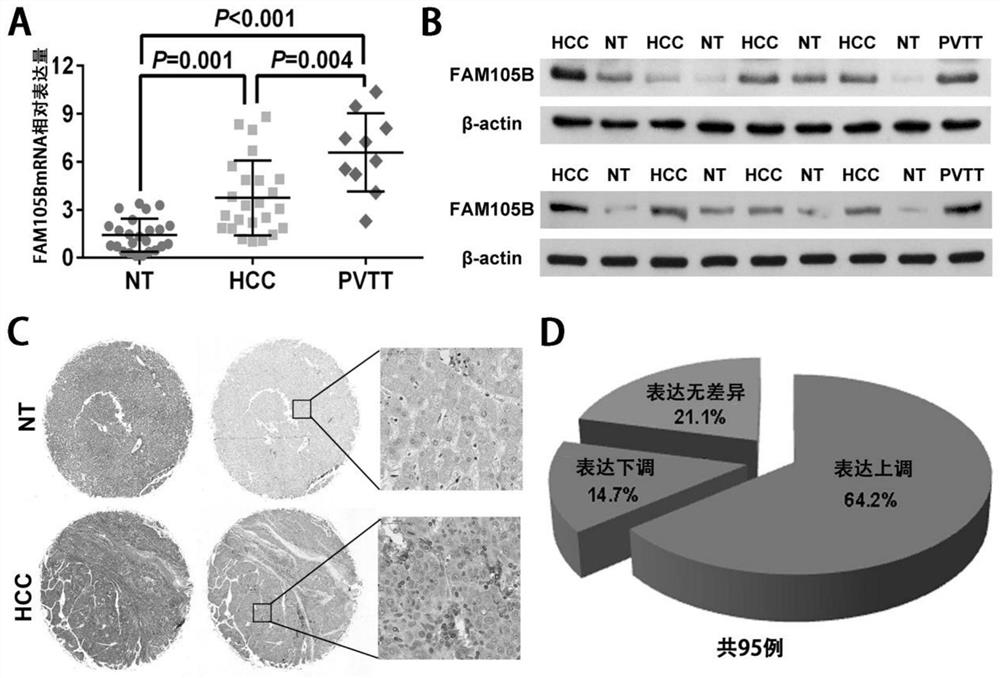
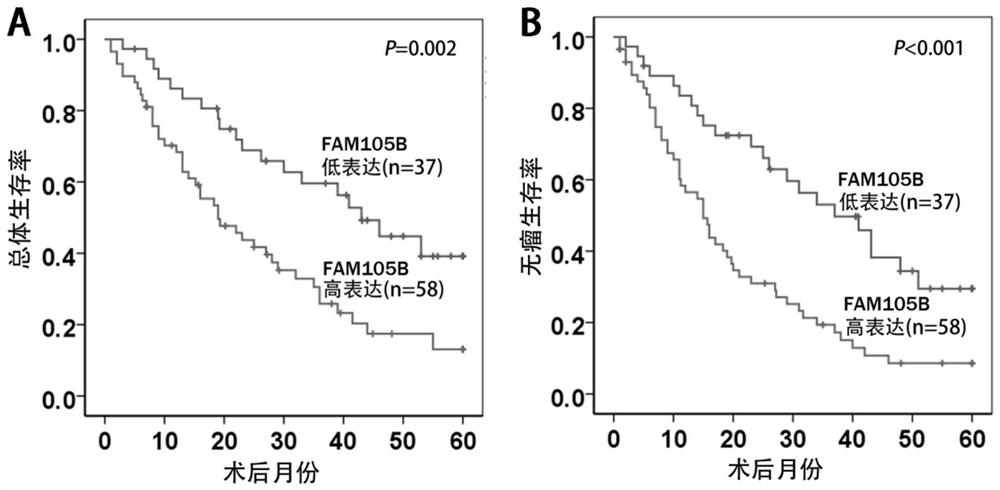
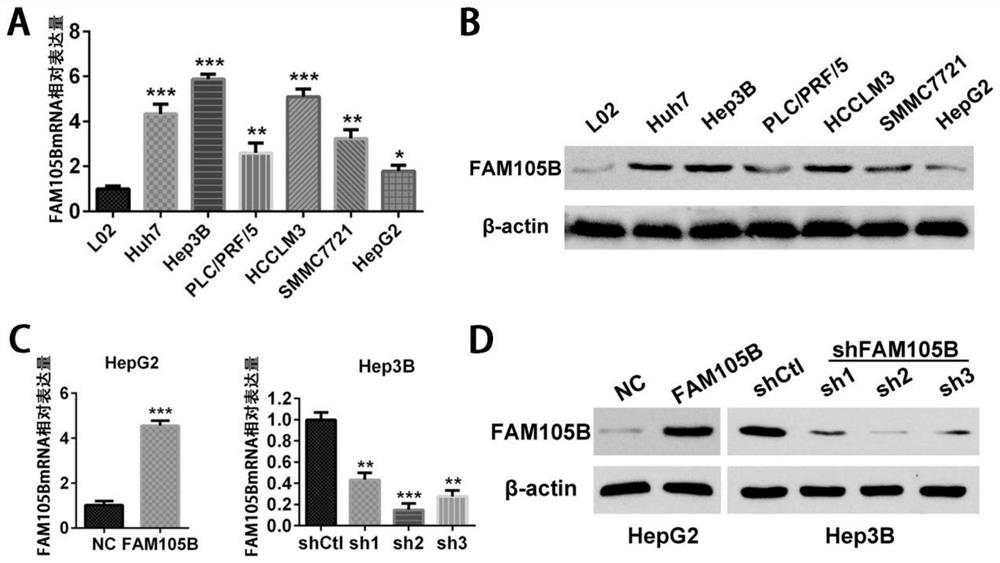
Application of delinearized ubiquitin enzyme family with sequence similarity 105, member B (FAM105B) to liver cancer diagnosis, treatment and prognosis judgment
ActiveCN111690742APromote proliferationIncrease aggressivenessOrganic active ingredientsMicrobiological testing/measurementOncologyClinicopathologic feature
The invention provides application of delinearized ubiquitin enzyme family with sequence similarity 105, member B (FAM105B) in liver cancer diagnosis, treatment and prognosis judgment. Various technical detection finds that the FAM105B is highly expressed in a liver cancer cell line and liver cancer tissue, and high expression of the FAM105B is positively correlated with adverse clinical pathological characteristics of a hepatocellular carcinoma (HCC) patient, a poor postoperative overall survival rate and a poor tumor-free survival rate; it is proved that high expression of the FAM105B in theliver cancer tissue is an independent risk factor influencing long-term survival of liver cancer patients; an in-vitro hepatoma cell model constructed by gene knockout and overexpression techniques finds that the FAM105B can promote the proliferation, migration and invasion abilities and epithelial-mesenchymal transition of hepatoma cells. The research findings indicate that the delinearized ubiquitin enzyme FAM105B can be used as a marker for developing a new liver cancer diagnosis and prognosis judgment kit, and an effective target is provided for preparing liver cancer treatment medicines.
Owner:王继龙 +2
miRNA (micro ribonucleic acid) for diagnosis and treatment on hepatocellular carcinoma
ActiveCN111172290AAchieve early diagnosisReduce mortalityOrganic active ingredientsMicrobiological testing/measurementOncologyCancer research
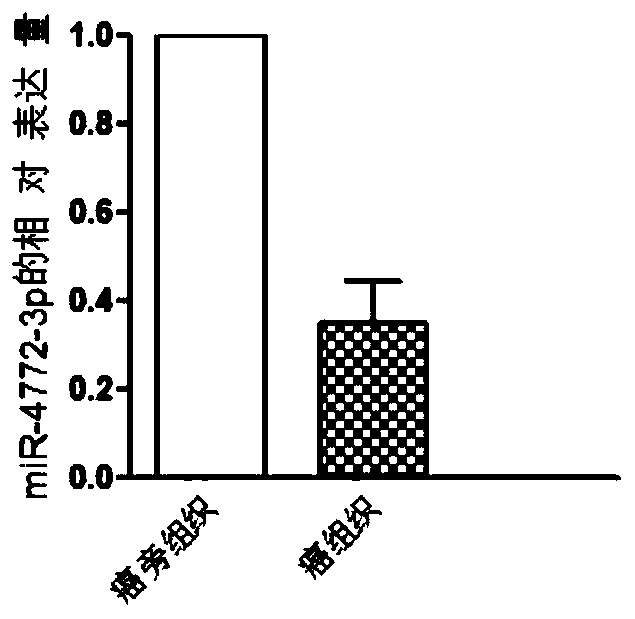
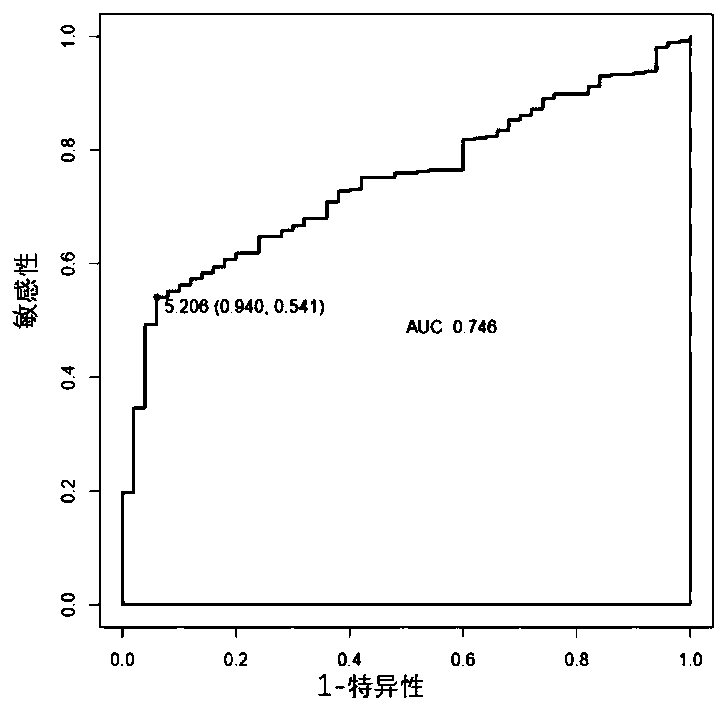
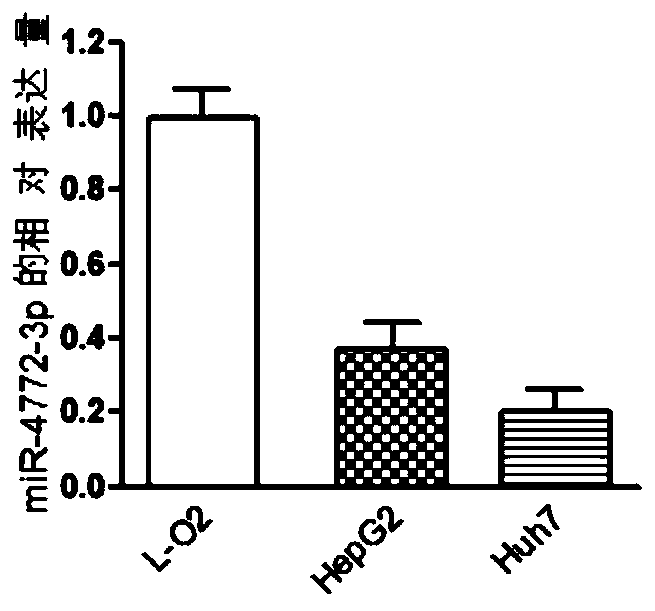
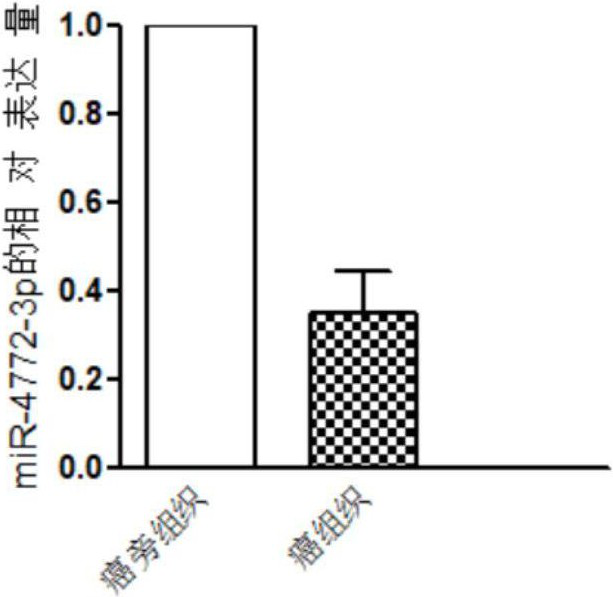
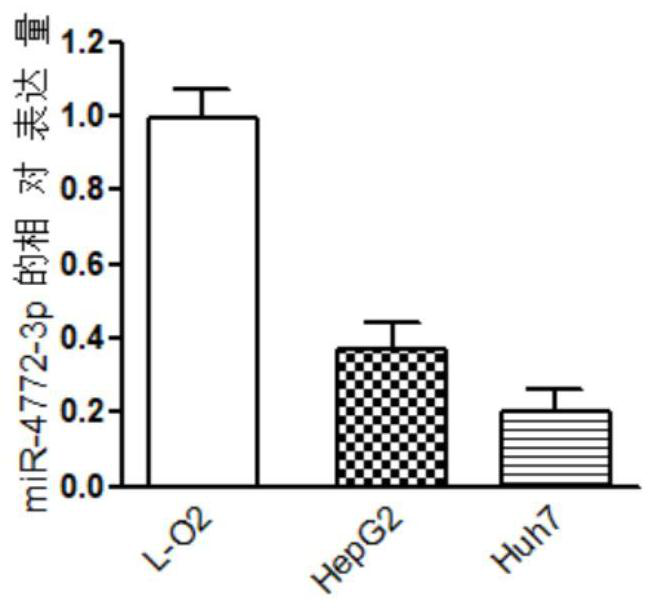
The invention discloses a miRNA (micro ribonucleic acid) for diagnosis and treatment on hepatocellular carcinoma. The invention finds for a first time that expression of miR-4772-3p is decreased in liver cancer, and further verifies that miR-4772-3p has high diagnosis efficiency in liver cancer. The invention further discloses that miR-4772-3p plays significant roles in proliferation, migration and invasion of hepatoma carcinoma cells, and reminds that miR-4772-3p can be applied to treatment on liver cancer.
Owner:THE THIRD HOSPITAL OF HEBEI MEDICAL UNIV
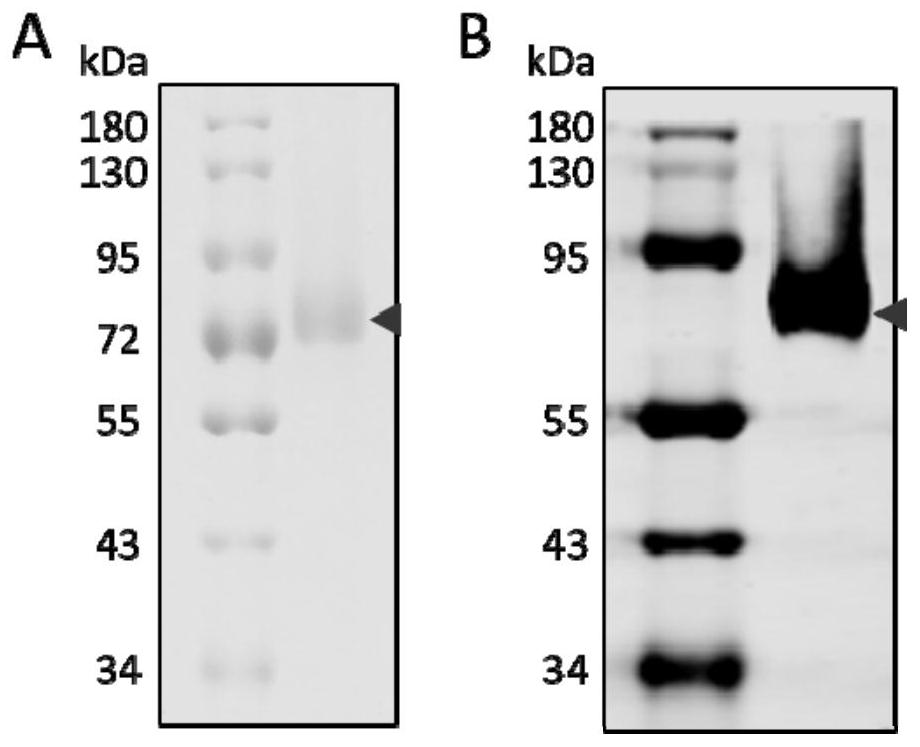
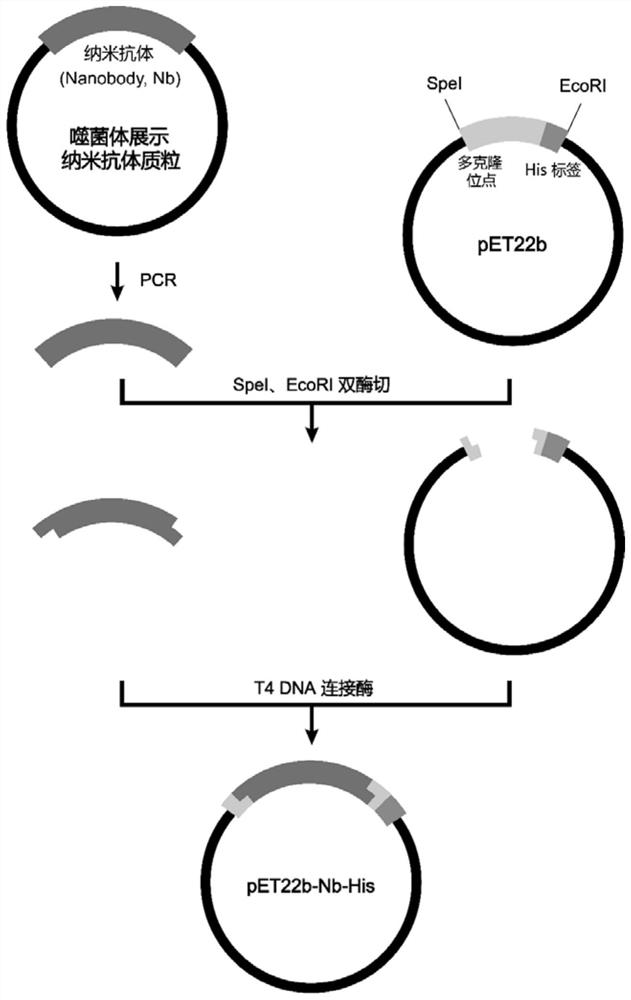
Phosphatidylinositol proteoglycan 3 nanometer antibody as well as preparation method and application thereof
ActiveCN111732659AImprove stabilityEasy to operateBacteriaMicroorganism based processesComplementarity determining regionAntiendomysial antibodies
The present invention discloses a phosphatidylinositol proteoglycan 3 (Glypican-3, GPC3) nanometer antibody and a preparation method thereof. The nanometer antibody has three complementarity determining regions: CDR1, CDR2 and CDR3, wherein the CDR1 comprises an amino acid sequence as shown in SEQ ID NO.1, and the CDR2 comprises an amino acid sequence as shown in SEQ ID NO.2, and the CDR3 comprises an amino acid sequence as shown in SEQ ID NO.3. The nanometer antibody disclosed by the invention can be used for specifically identifying liver cancer cells with high GPC3 expression by combining with GPC3 expressed by a cell membrane. The antibody has high stability in serum, can be used for function research of the GPC3, and can also be used for development of diagnosis reagents and treatmentdrugs of the hepatocellular carcinoma.
Owner:珠海中科先进技术研究院有限公司
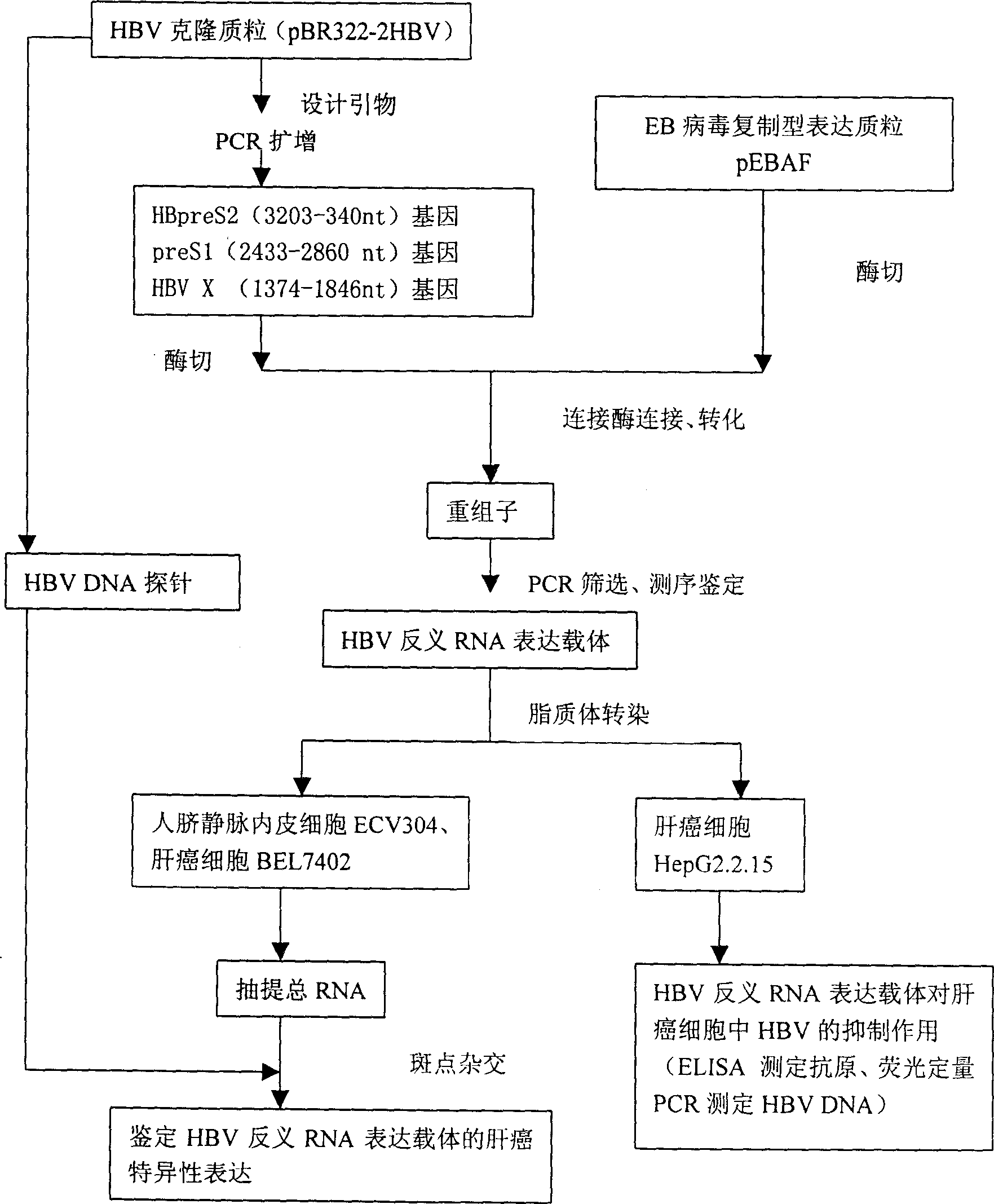
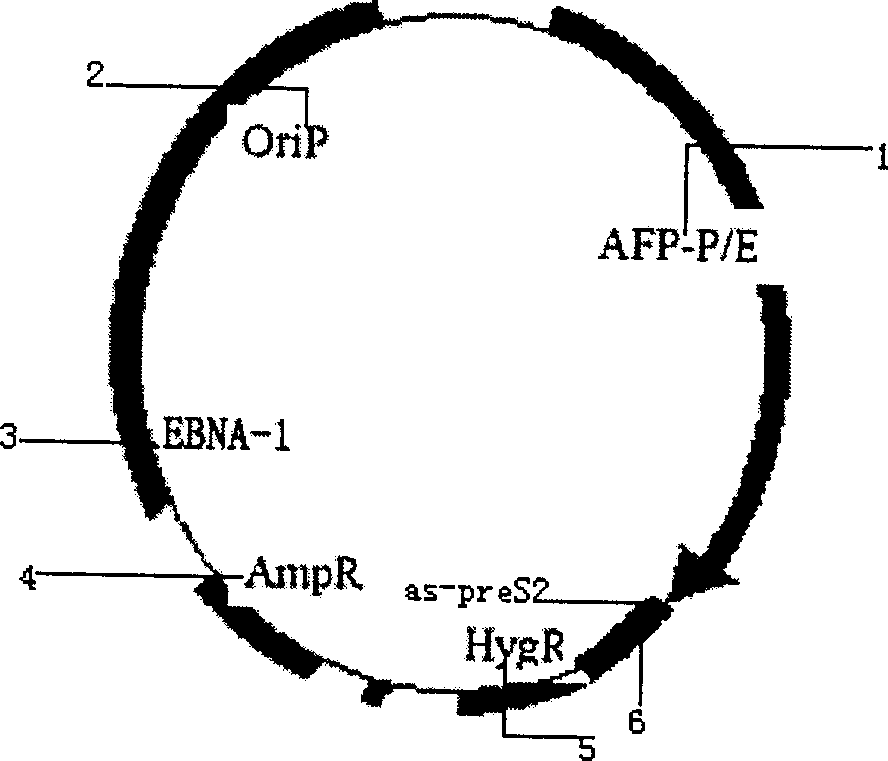
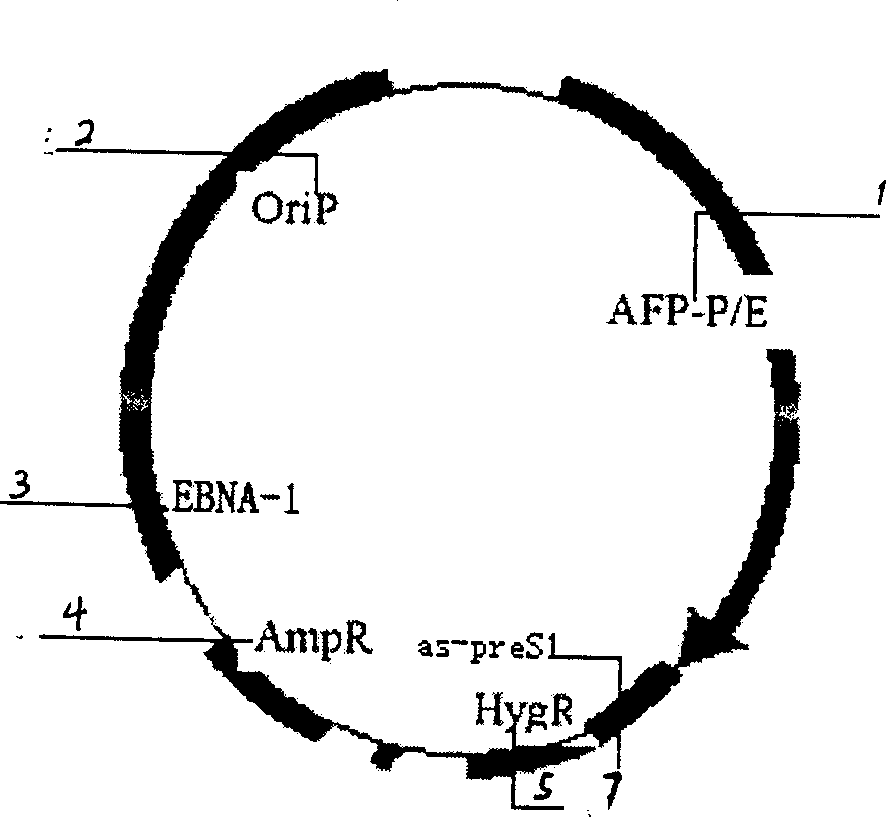
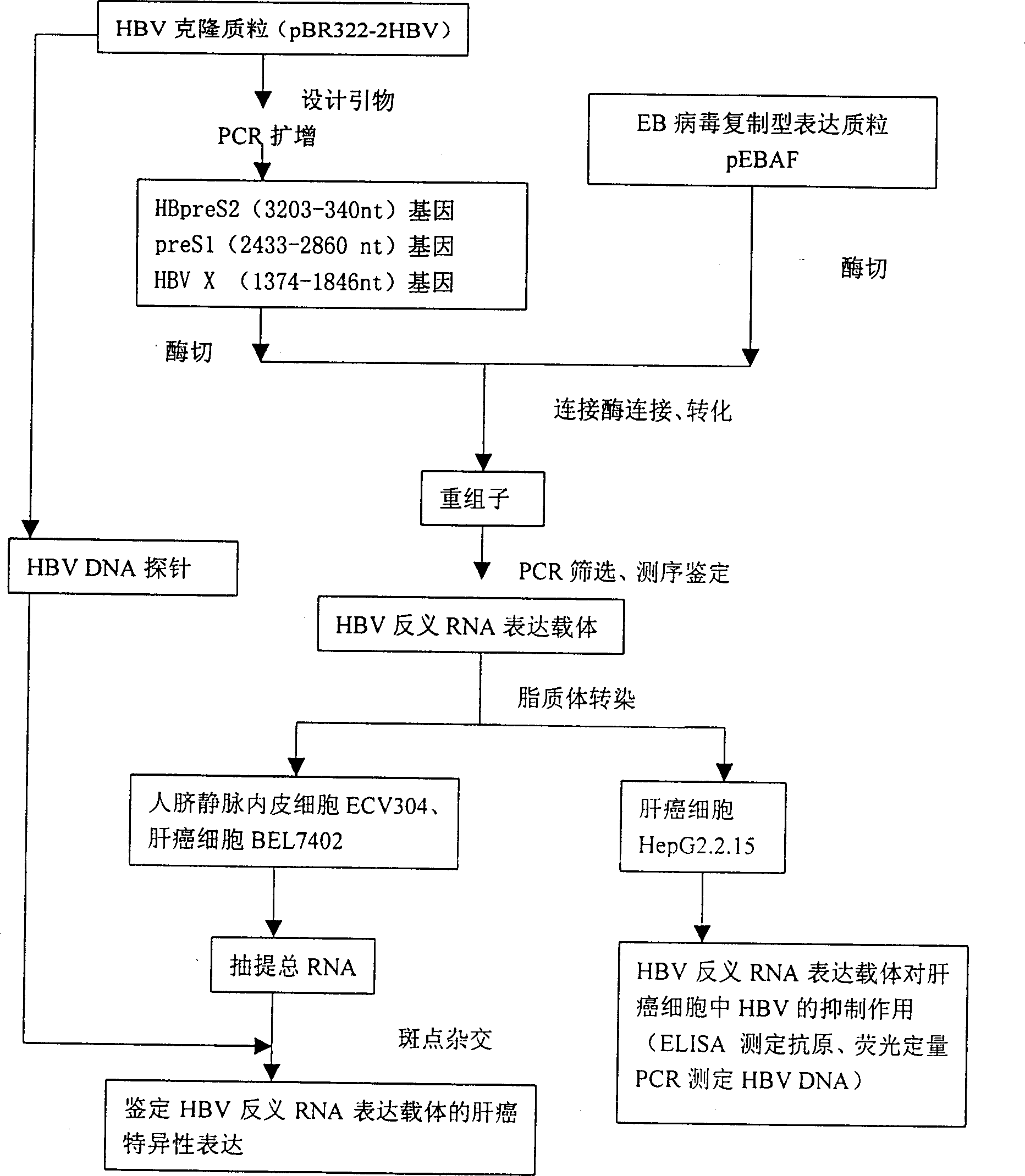
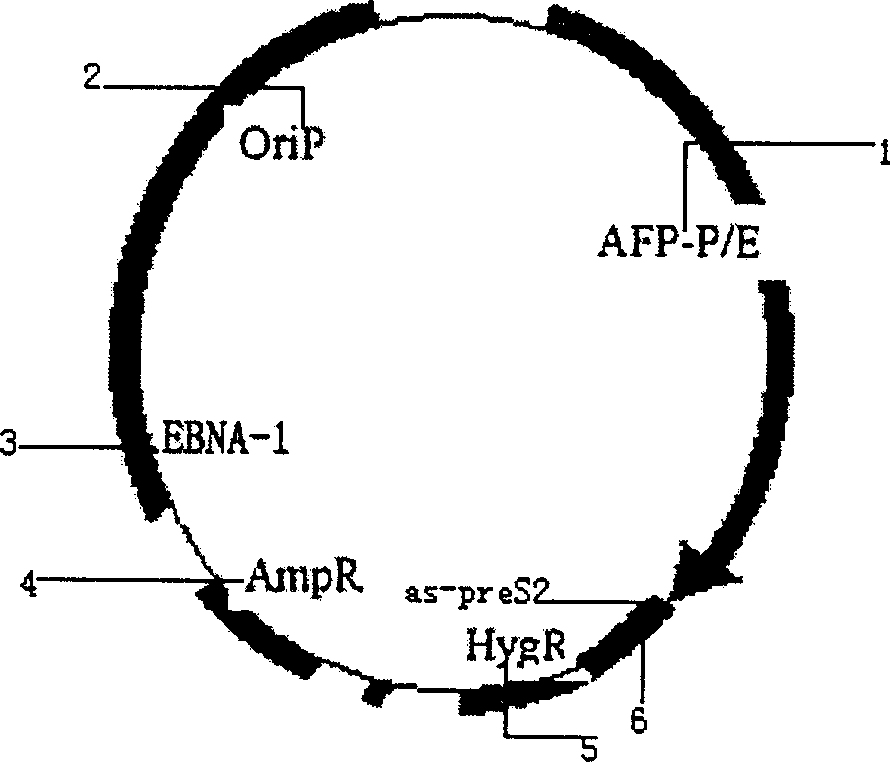
Liver cancer targeting hepatitis B antisense RNA expression vector and its construction process and use
InactiveCN1398973AGood inhibitory effectGood ability to inhibit colony formationMicrobiological testing/measurementGenetic material ingredientsHBxAntisense RNA
A hepatitis B virus antisense RNA expression vector targeted toward liver cancer is a gene therapeutic matter for hepatitis B virus related liver cell cancer. The present invention adopts EB virus expressing plasmid pEBAF as vector and positive liver cancer tissue expressing vector controlled by AFP promotor controlling and specifically expressed in AFP only to construct three kinds of HBV antisence RNA expression vectors pEBAF-as-preS2, pEBAF-as-preS1 and pEBAF-as-X, which contain HBV preS2, preS1 and X segment genes separately. Antisense RNA expression vector is prepared through amplifying a destination gene, designing, a primer, and a PCR amplification of HBpreS2, HBpreS1 and HBX genes. The antisense RNA expression vector is used for treating hepatitis B virus related liver cell cancer and hepatitis B virus infection.
Owner:SHANDONG UNIV
Hepatitis B virus antisense RNA expression vector targeting liver cancer and its construction process and use
InactiveCN1162547CGood Anti-HBVGood anticancer effectGenetic material ingredientsMicrobiological testing/measurementAntisense RNAHBx
A hepatitis B virus antisense RNA expression vector targeted toward liver cancer is a gene therapeutic matter for hepatitis B virus related liver cell cancer. The present invention adopts EB virus expressing plasmid pEBAF as vector and positive liver cancer tissue expressing vector controlled by AFP promotor controlling and specifically expressed in AFP only to construct three kinds of HBV antisence RNA expression vectors pEBAF-as-preS2, pEBAF-as-preS1 and pEBAF-as-X, which contain HBV preS2, preS1 and X segment genes separately. Antisense RNA expression vector is prepared through amplifying a destination gene, designing, a primer, and a PCR amplification of HBpreS2, HBpreS1 and HBX genes. The antisense RNA expression vector is used for treating hepatitis B virus related liver cell cancer and hepatitis B virus infection.
Owner:SHANDONG UNIV
Medicine for treating liver cancer and application
InactiveCN108815214AGood inhibitory effectHigh swelling inhibition rateAntiviralsFungi medical ingredientsPharmaceutical drugCell strain
The invention discloses a medicine for treating liver cancer. The medicine comprises adhatoda ventricosa extracts. A preparation method of the adhatoda ventricosa extracts comprises the following steps that S1, adhatoda ventricosa is put into boiled water for extraction to obtain an aqueous extract solution; S2, after the aqueous extract solution obtained in S1 is separated and purified, the adhatoda ventricosa extracts are obtained. The medicine for treating liver cancer comprises the adhatoda ventricosa extracts, and experiments show that the adhatoda ventricosa extracts have a remarkable inhibition effect on growth of BEL-7402 and Hep-B3 liver cancer cell strains.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
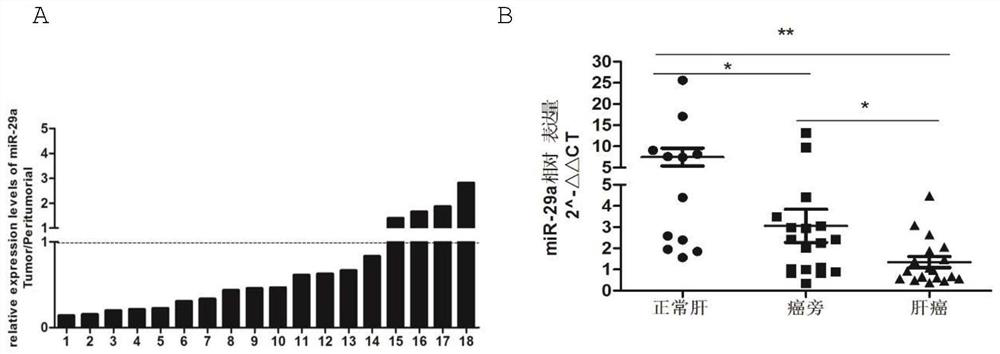
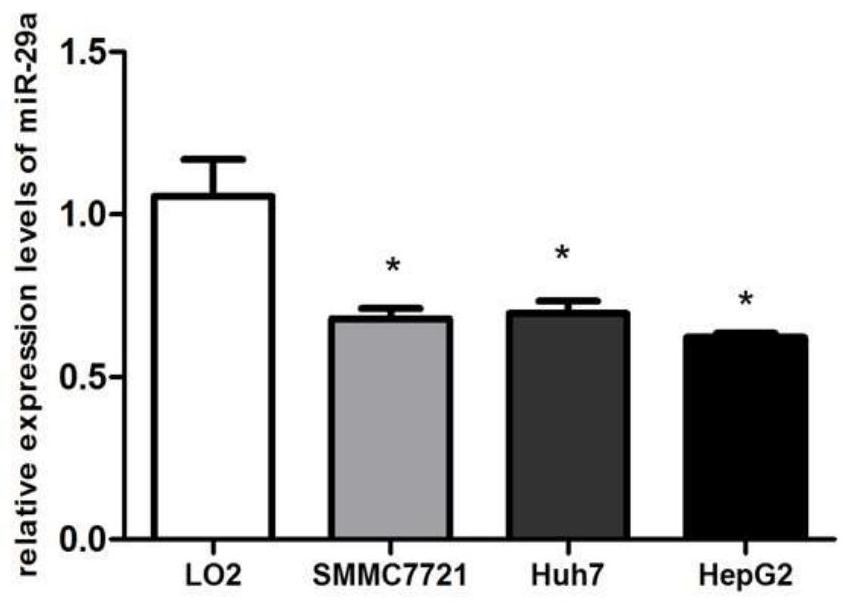
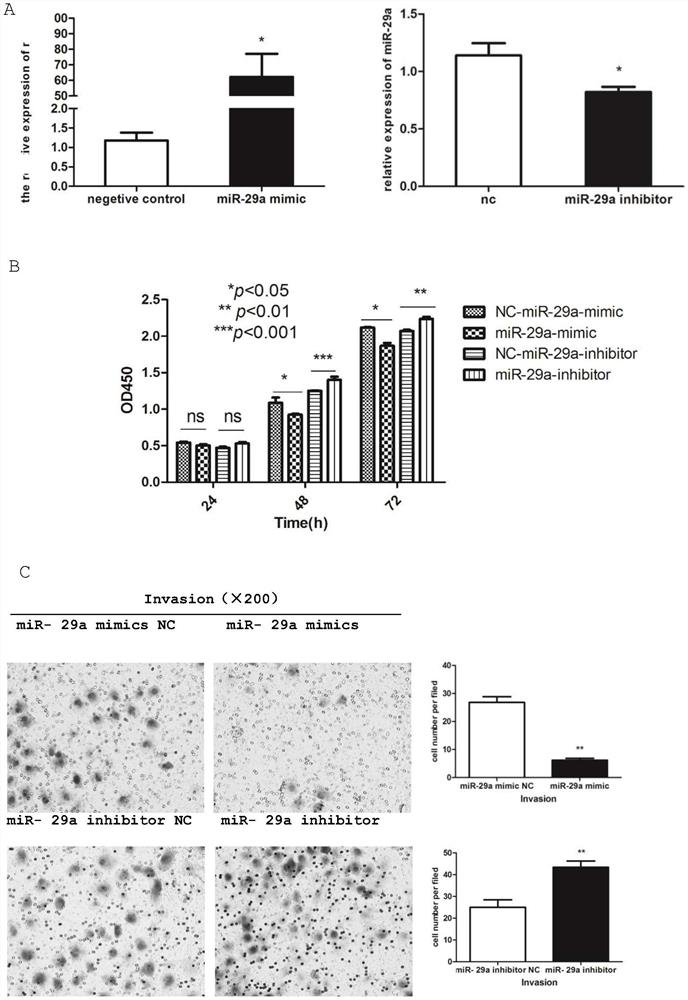
Application of mir-29a gene in detecting liver cancer and liver fibrosis, and construction method of gene conditional knock-in mice
PendingCN111647656AMicrobiological testing/measurementStable introduction of DNASchistosomiasesCarcinoma cell line
The invention provides an application of a mir-29a gene in detecting primary hepatocellular carcinoma, and an application of the mir-29a gene in detecting Schistosoma japonicum liver fibrosis. The invention also provides an application of mir-29a gene conditional knock-in mice in the study of primary hepatocellular carcinoma and / or Schistosoma japonicum liver fibrosis. The invention confirms thatthe expression of mir-29a is significantly reduced in liver cancer, paracancerous and liver cancer cell lines, and the result suggests that the low-level expression of mir-29a may be related to the occurrence and development of liver cancer cells. In addition, the mir-29a conditional knock-in mice can be used as animal models for the study of liver fibrosis caused by primary hepatocellular carcinoma and chronic schistosomiasis infection or advanced schistosomiasis, and thereby the evidence and basis are provided for the follow-up study of drugs for treating liver fibrosis caused by hepatocellular carcinoma and schistosomiasis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Application of SLC7A11 gene in hepatocellular carcinoma interventional embolization post operation
PendingCN113679735AInterfering with proliferation and apoptosisPrevent proliferationOrganic active ingredientsAntineoplastic agentsSignalling moleculesApoptosis
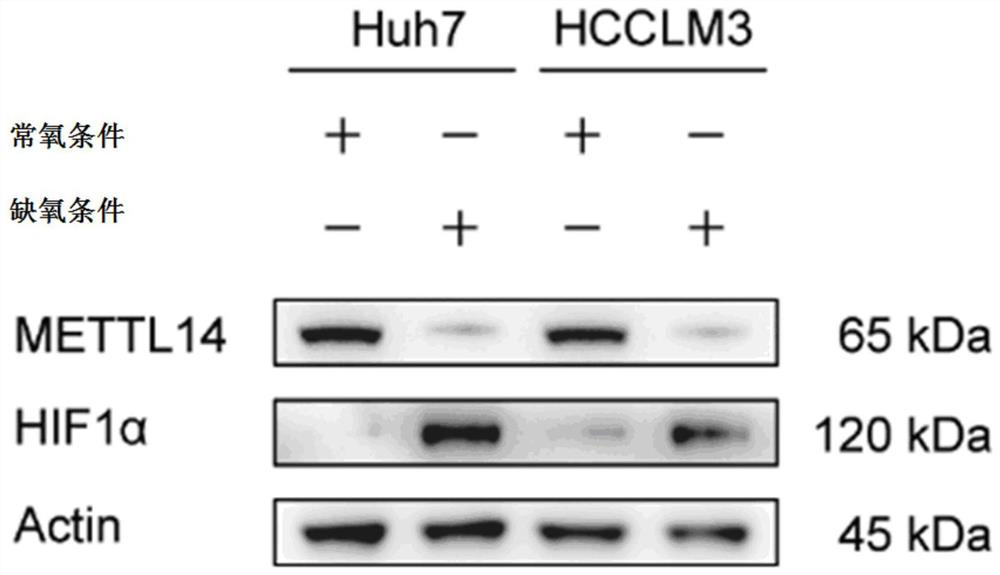
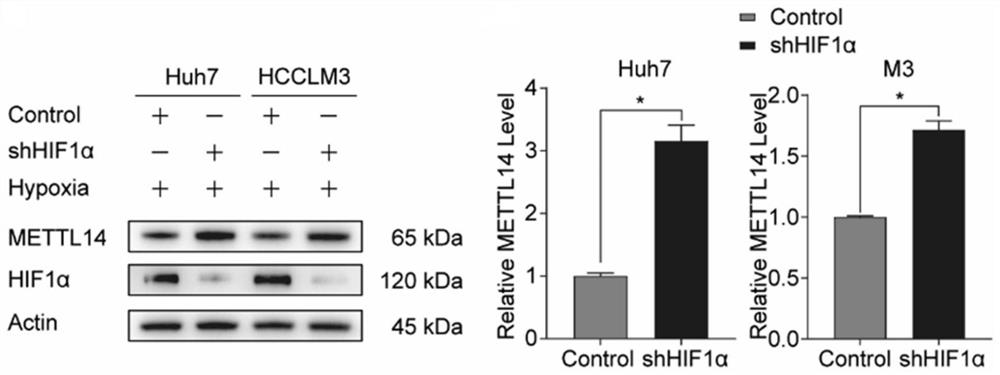
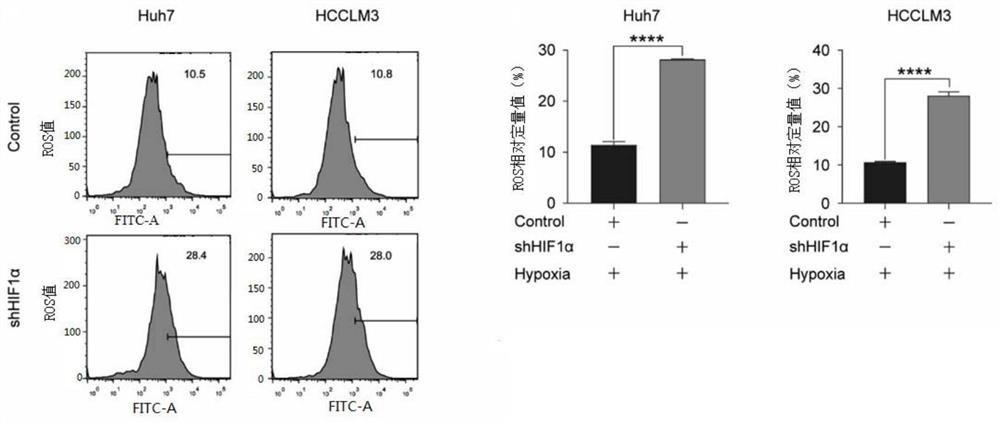
The invention discloses an application of an SLC7A11 gene in a hepatocellular carcinoma interventional embolization post operation. On the basis of molecular biology experiments, it is verified that SLC7A11 is a key member of a ferroptosis signal channel, and related small-molecule inhibitors are found and designed for SLC7A11 and related signal molecule channels to intervene in proliferation and apoptosis of liver cancer cells; and animal model experiments prove that the proliferation and migration of HCC cells can be inhibited by knocking down the SLC7A11 through a small-molecule inhibitor (shRNA of the SLC7A11), the tumor inhibition effect of METTL14 induced under the HCC hypoxia condition can be remarkably saved through overexpression of the SLC7A11, and a new molecular target is provided for inhibiting recurrence and metastasis of HCC after liver cancer interventional embolization clinically.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Mononucleotide polymorphic site of CDC6 gene correlated to primary liver cancer and application thereof
InactiveCN100547083CEasy to findMicrobiological testing/measurementDNA/RNA fragmentationNucleotideNucleic acid sequencing
The invention discloses the polymorphic site (rs4134994) in CDC6 gene associated with primary liver cell cancer and its application. The invention also provides a method for detecting the polymorphic site and susceptibility of the primary liver cell cancer. By use of the nucleic acid sequence of the polymorphic loci and its detection method, it can produce kit for preventing primary liver cell cancer and early diagnosis and treatment.
Owner:SUN YAT SEN UNIV
ELISA method of transcriptomics and proteomics in biological process of liver cancer
The invention discloses an ELISA method of transcriptomics and proteomics in a biological process of liver cancer. The method comprises the steps: (1) diluting an antibody with a buffer solution; (2)allowing the diluted antibody to stay overnight in each reaction hole of a polystyrene board; (3) next day, discarding the solution in the holes, and washing and buffering; (4) adding a certain diluted to-be-tested sample into the reaction holes, and then washing; (5) adding a fresh diluted enzyme labeled antibody in each reaction hole; (6) incubating and washing; (7) adding temporarily prepared TMB into each reaction hole; (8) adding a stop solution in each reaction hole; and (9) judging a result. A clinical sample is adopted for positioning quantitative expression verification, an evidence of the clinical sample with clinical relevance is searched, clinical value is evaluated, and a new clue is provided for research of liver cancer pathogenesis and a liver cancer mechanism. Screened keymolecules of liver cancer can lay the research basis for exploration of early detection, classification, evaluation of prognosis relevant liver cancer markers, and choice of more effective and accurate liver cancer treatment target sites.
Owner:南宁科城汇信息科技有限公司
Kit for early screening of liver cell cancer and preparation method and use thereof
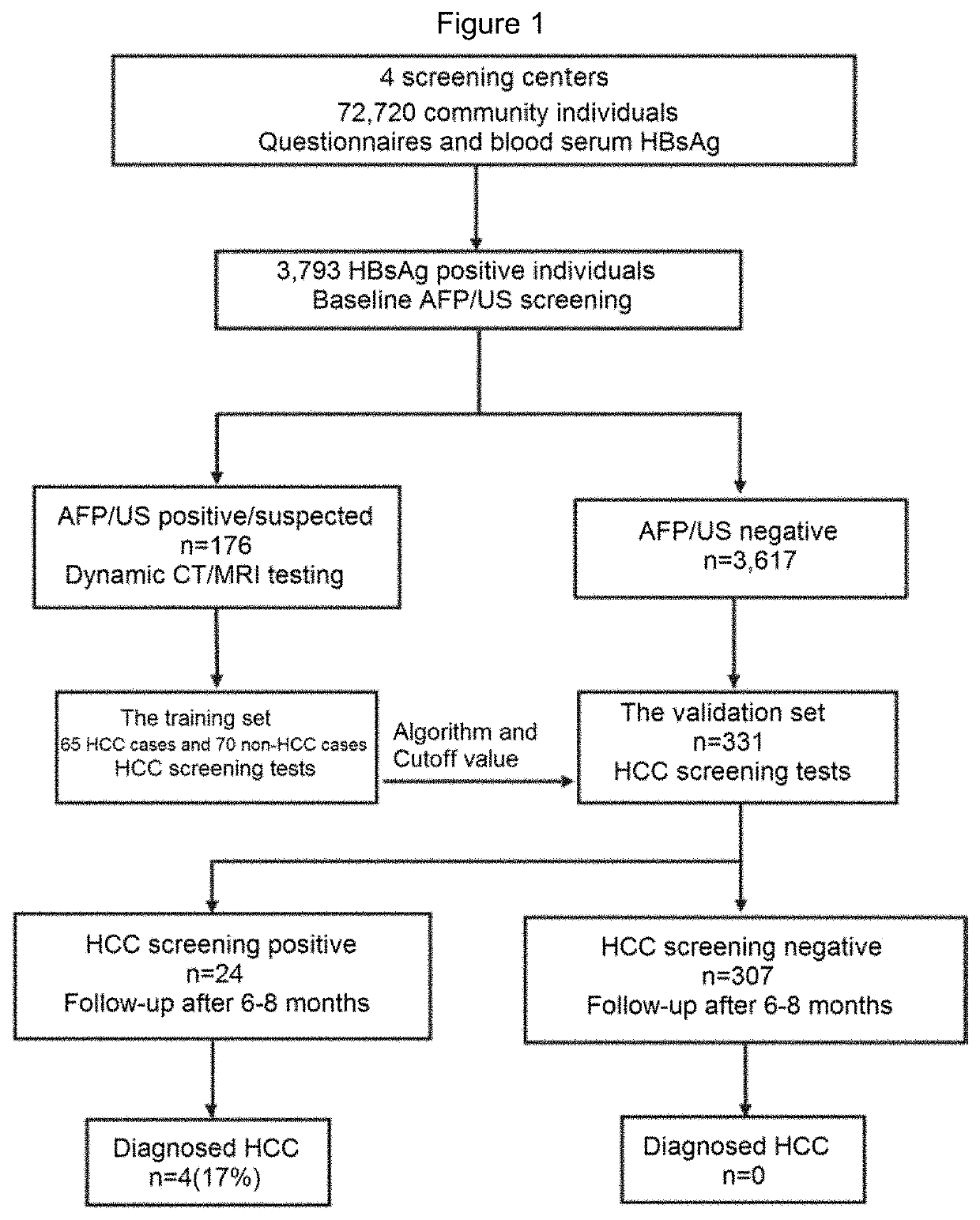
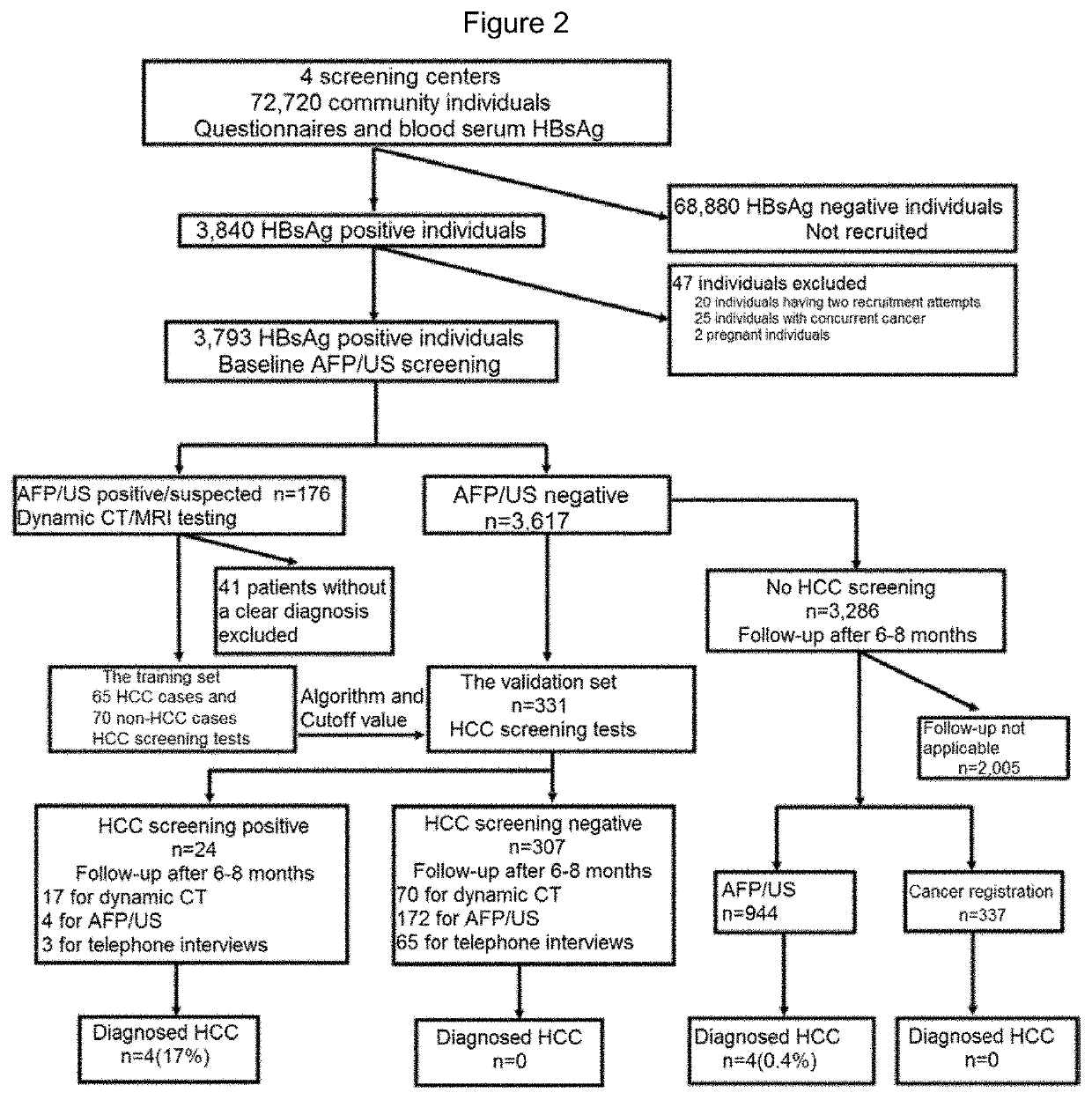
PendingUS20220145399A1Early screening of HCCEfficient implementationHealth-index calculationMicrobiological testing/measurementProtein markersCancer research
The present invention provides a kit for early screening of hepatocellular carcinoma, comprising a gene marker detection reagent and a protein marker detection reagent. The invention also provides a preparation method and application of the kit. The kit comprising specific gene markers and protein markers of the present invention has been demonstrated to be effective in achieving early screening of HCC in community populations, particularly in prospective studies.
Owner:GENETRON HEALTH (BEIJING) CO LTD +1
MiR-221/222 and application of its inhibitor in preparation of drugs to regulate liver fat deposition, liver fibrosis and liver cell carcinoma
ActiveCN109136224AOrganic active ingredientsMetabolism disorderLIVER FATTY DEPOSITIONLIVER FATTY DEPOSIT
The invention discloses miR-221 / 222 and application of its inhibitor in preparation of drugs and detection targets to regulate liver fat deposition, liver fibrosis and liver cell carcinoma; by inhibiting activity of miR-221 and / or miR-222, liver fat infiltration level, liver collagen fiber deposition, plasma cholesterol and serum transaminase are lowered, insulin resistance is improved, and malignant proliferation of liver cells is inhibited.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +1
Preparing method of lipid soluble platinum coordination compound with antitumor activities
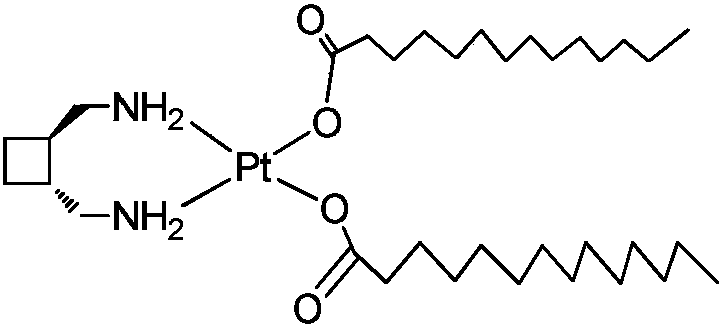
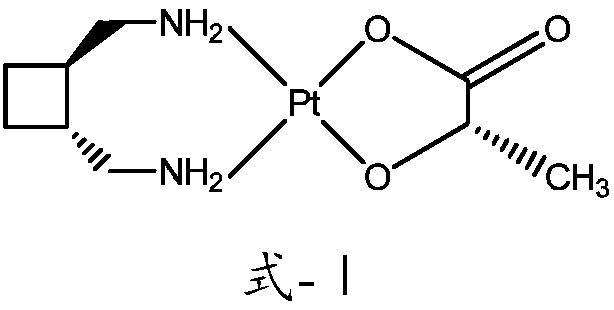
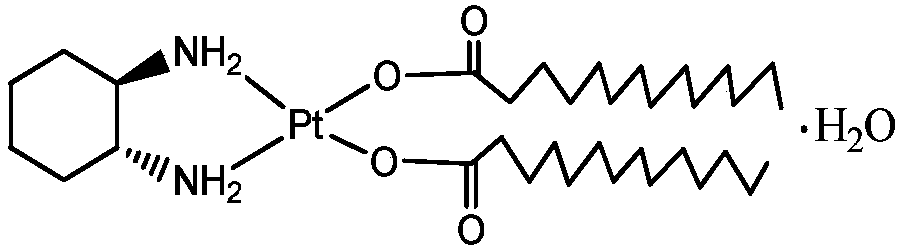
InactiveCN108047277AHas antitumor activityIncrease fat solubilityPlatinum organic compoundsAntineoplastic agentsSolubilityLipid formation
The invention provides a preparing method of a lipid soluble platinum coordination compound with antitumor activities. The prepared platinum coordination compound is called cis-[bis-myristic acid-trans-dimethylamino-cyclobutane chloride (II)], and the molecular formula is C34H68N2O4Pt. The preparing method comprises the specific steps of firstly, preparing trans-diiodine-cyclobutane chloride, thendissolving silver nitrate and prepared trans-dimethylamino-cyclobutane chloride into a reaction solvent to be subjected to a hydrolysis reaction to obtain hydrolysate and filtering the hydrolysate; adding the filtered hydrolysate into myristic acid or myristic acid salt, so that the white substance generated in the reaction is the coarse product of the platinum coordination compound, and after the coarse product is recrystallized, the fine product can be obtained. The lipid soluble platinum coordination compound prepared according to the method is high in lipid solubility and stable and has agood inhibiting effect on liver cancer cells, can be prepared into freeze-dried powder and lipiodol turbid liquid and is conveniently used for clinically preparing liver cell cancer.
Owner:KUNMING GUIYAN PHARMA
Use of polyphosphoinositide phosphatase 1 as a target molecule
ActiveCN112245584BGrowth inhibitionPrevent invasionDigestive systemAntineoplastic agentsPolyphosphoinositide PhosphataseOncology
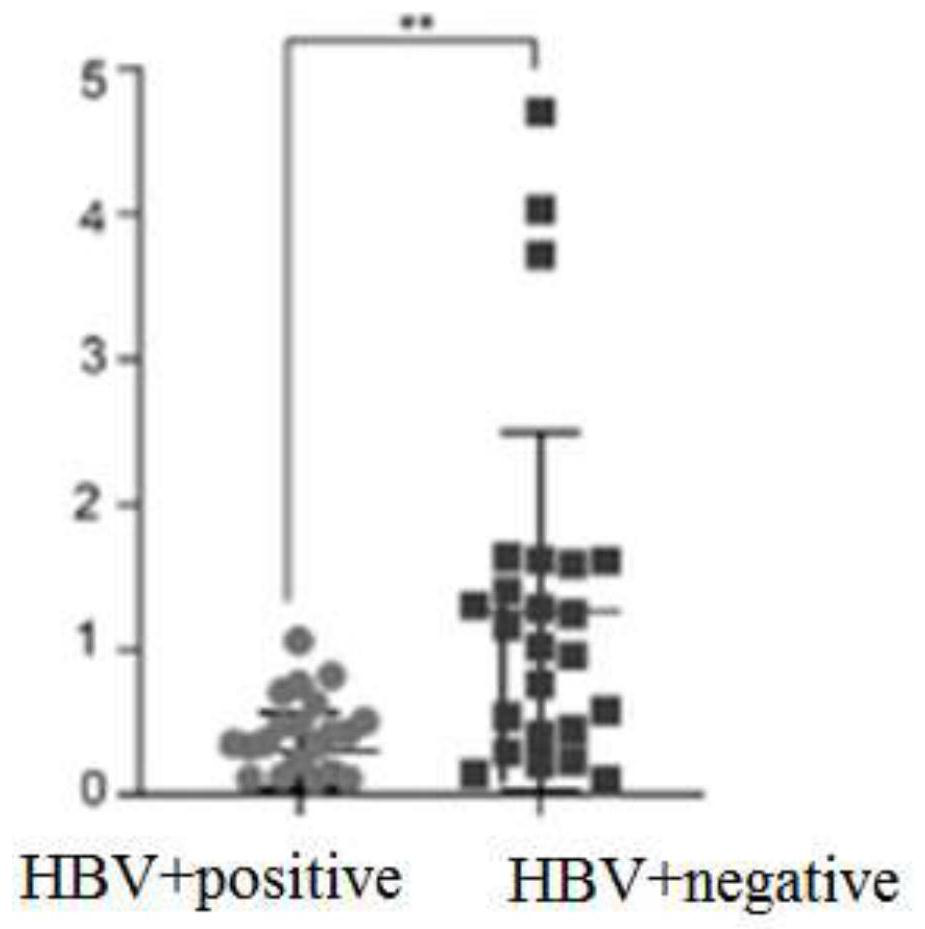
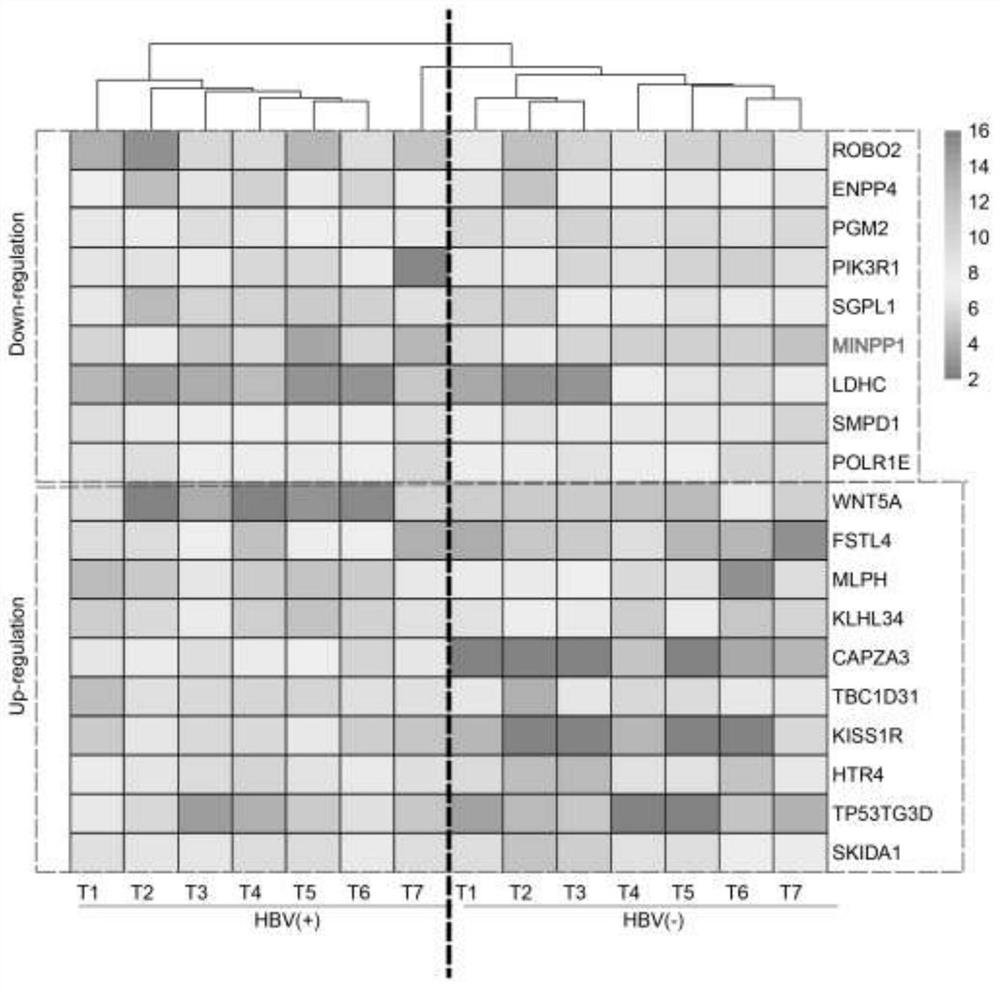
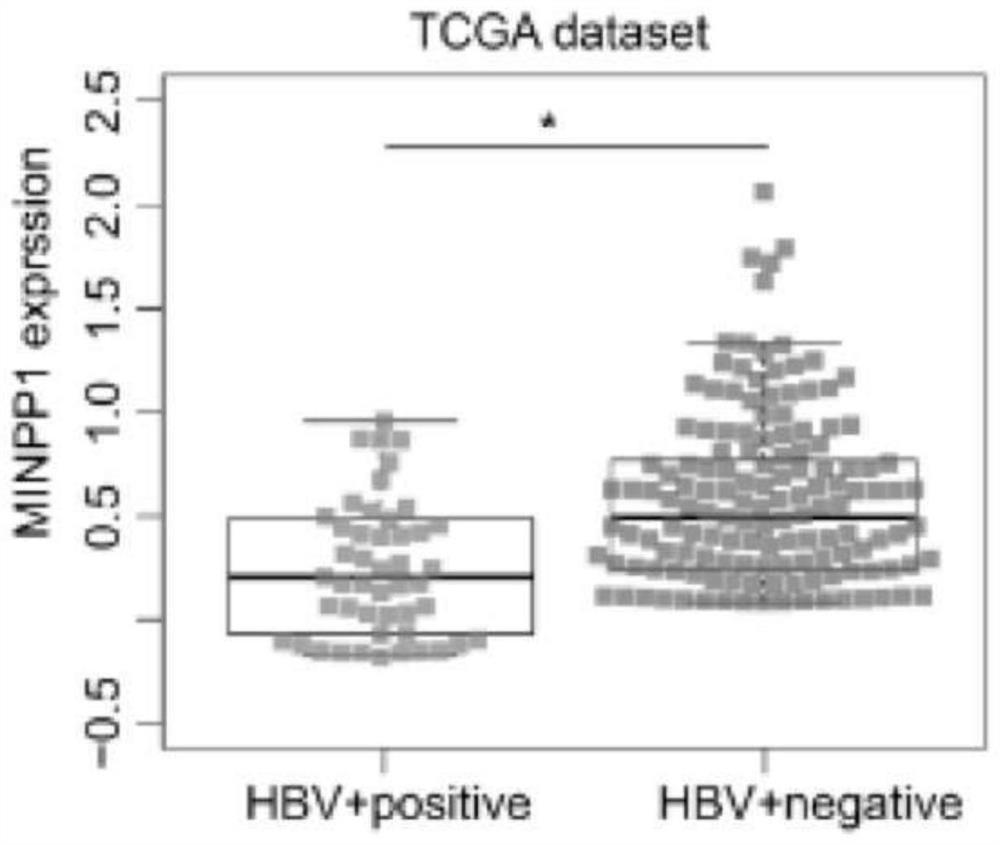
The invention discloses the application of MINPP1 as a target molecule in the preparation of anti-HBV-related hepatocellular carcinoma drugs, and inhibits the growth and invasion of HBV-related hepatocellular carcinoma cells by overexpressing or activating the MINPP1 gene. The present invention confirms that the MINPP1 gene can be used as an important target for effectively inhibiting the proliferation of HBV-related liver cancer cells.
Owner:ZHEJIANG UNIV
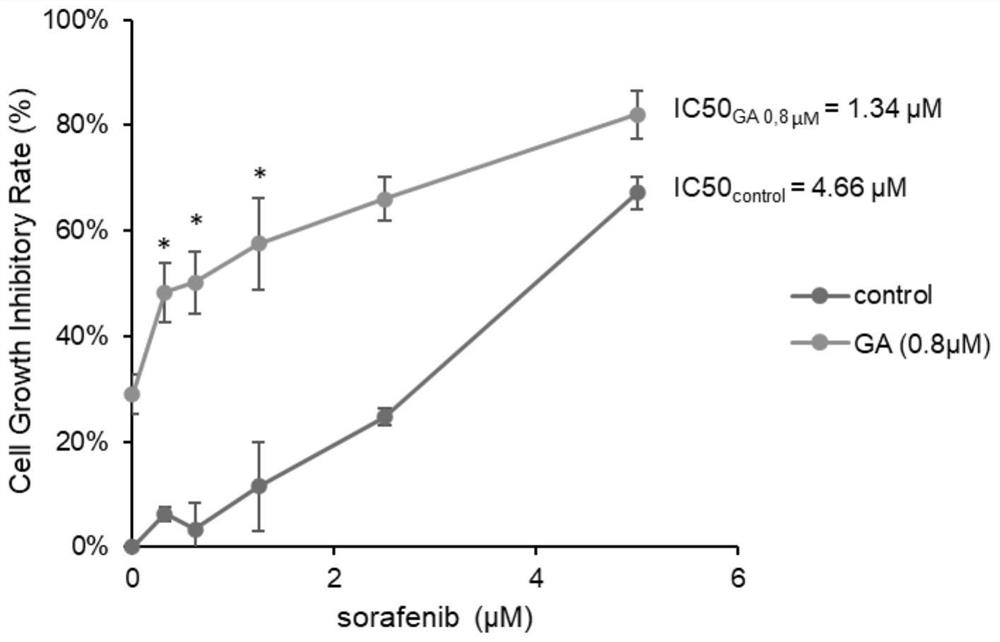
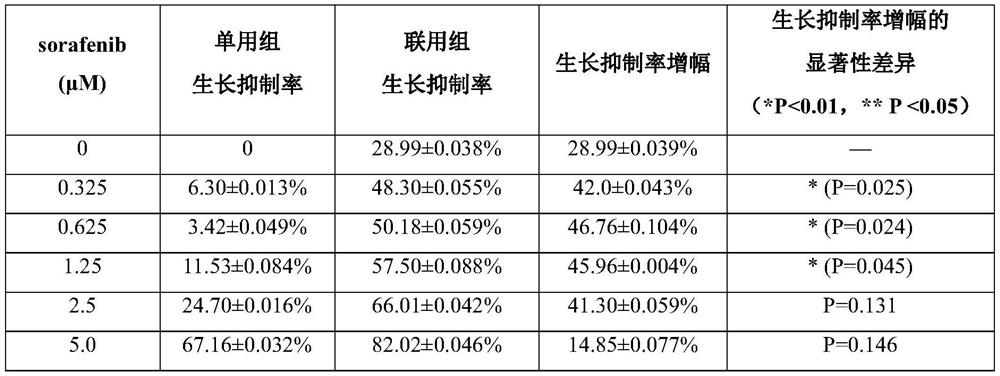
Application of combination of gambogic acid and sorafenib in preparation of liver cancer treatment medicine
InactiveCN114522162AEnhanced liver cancer growth inhibitory effectGrowth inhibitionAntineoplastic agentsHeterocyclic compound active ingredientsGambogic acidOncology
The invention discloses application of gambogic acid and sorafenib in preparation of a medicine for treating liver cancer, and belongs to the technical field of biological medicine. The invention finds that the growth of human hepatocellular carcinoma HepG2 cells can be obviously inhibited by combining gambogic acid (GA) extracted from a natural product gamboge with a first-line targeted therapeutic drug sorafenib for liver cancer. The MTT experiment verifies that the gambogic acid with lower concentration can enhance the liver cancer growth inhibition effect of sorafenib, and the application potential of the combined administration mode in treatment of hepatocellular carcinoma is prompted.
Owner:南京芩康医药科技有限公司
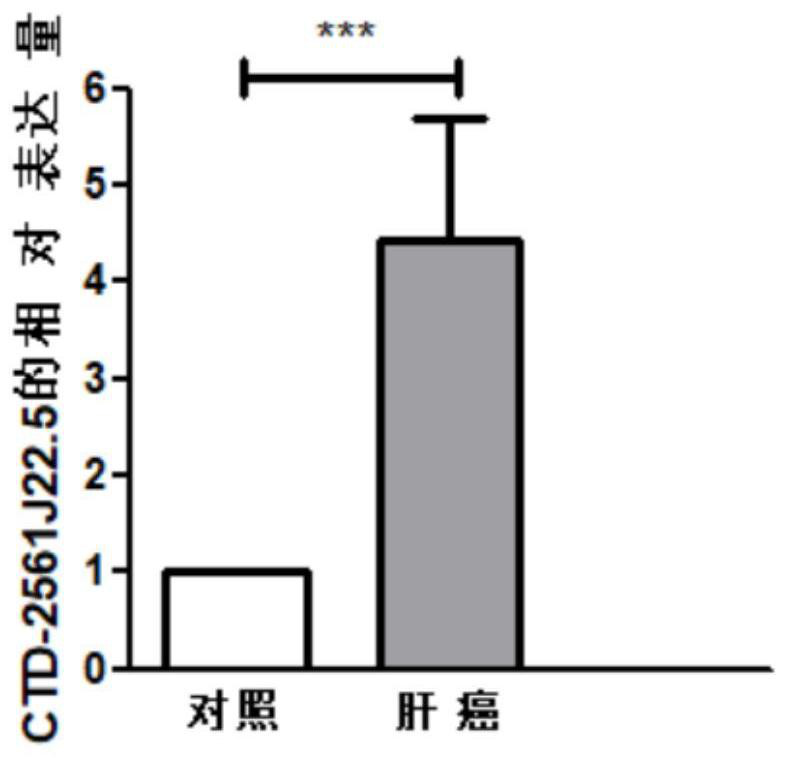
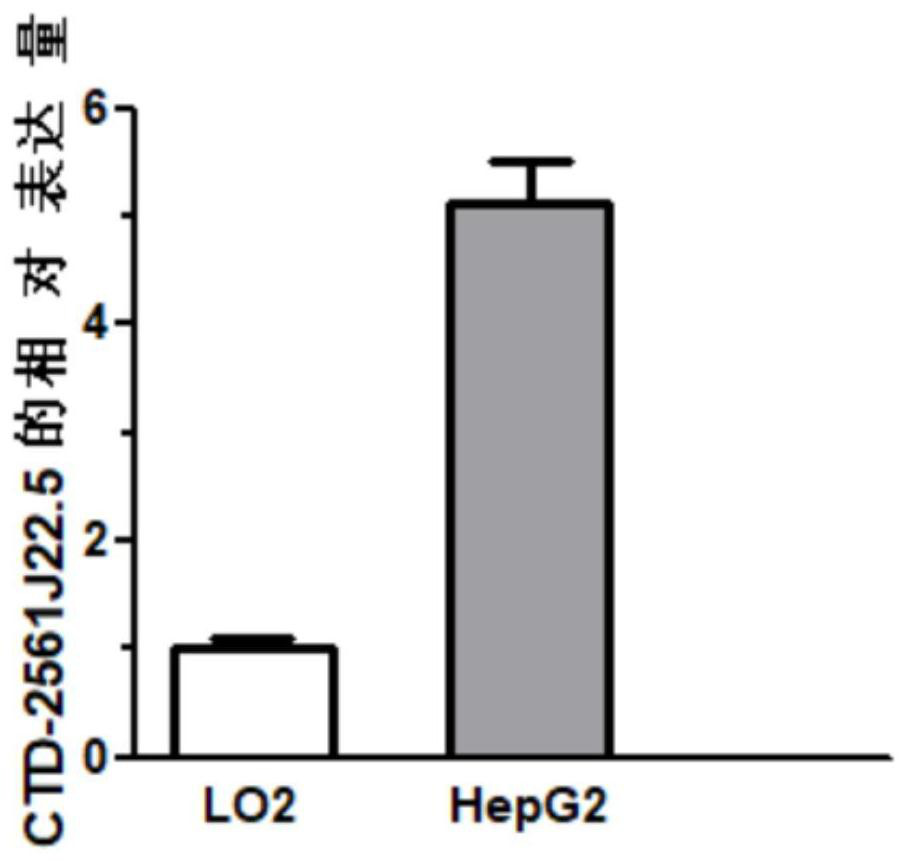
Reagents for detecting and targeting lncRNA biomarkers and their application in hepatocellular carcinoma
ActiveCN111304326BDetermining your risk of developing hepatocellular carcinomaAlter proliferative activityOrganic active ingredientsMicrobiological testing/measurementBiologic markerProliferation activity
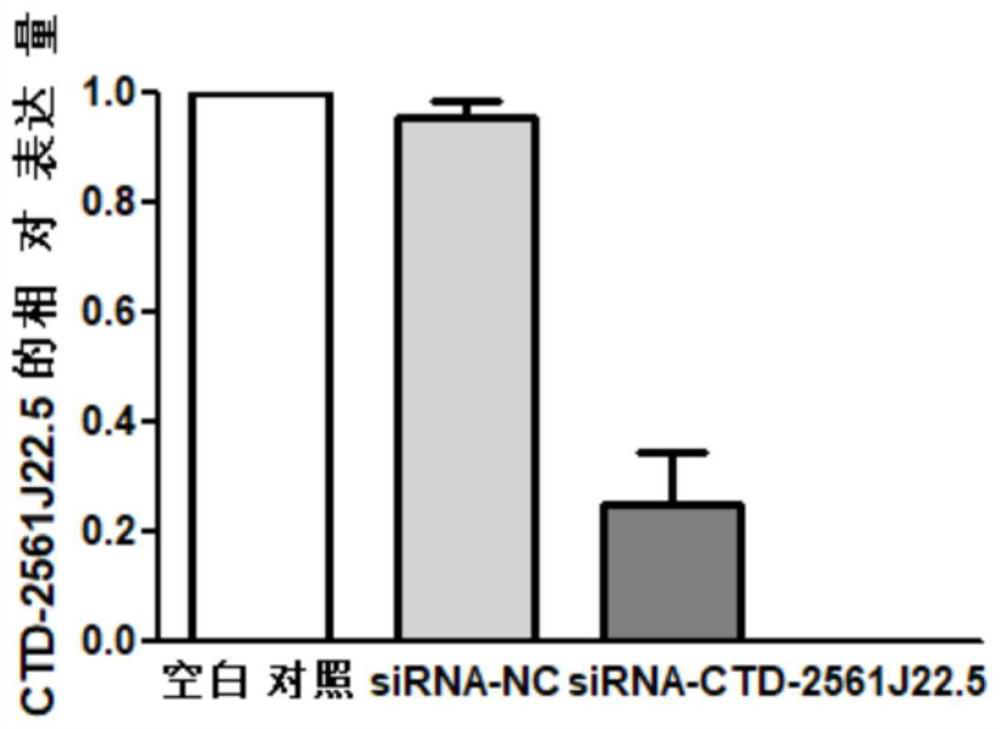
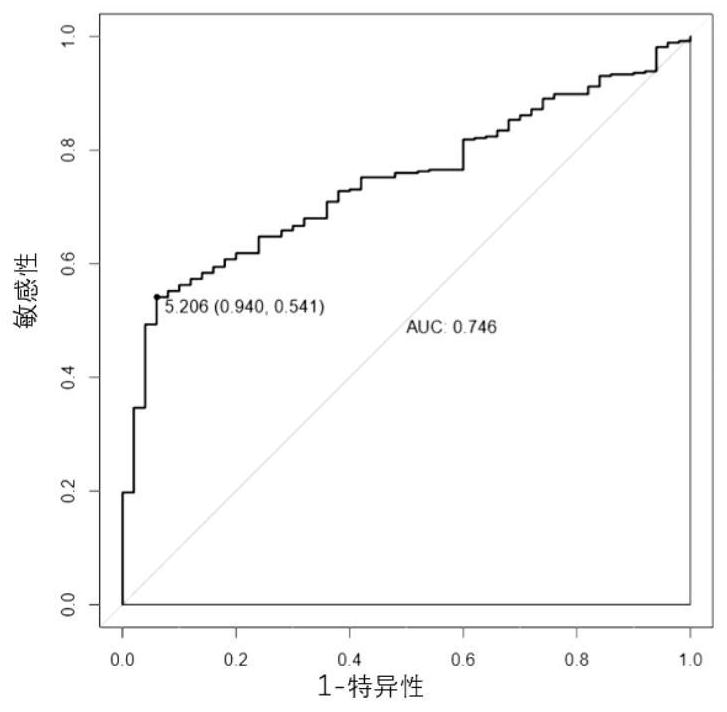
The invention discloses a reagent for detecting and targeting a lncRNA biomarker and application of the reagent to hepatocellular carcinoma. The up-regulated expression of CTD-2561J22.5 in a patient with liver cancer is discovered for the first time; and through ROC curve analysis on relevant data, the result that the CTD-2561J22.5 has the higher diagnosis efficiency is discovered. Cell experiments prove that through down-regulating of the expression level of the CTD-2561J22.5, the proliferation activity of liver cancer cells can be reduced; and the result shows that the CTD-2561J22.5 can be used as a molecular target to be applied to the treatment of the hepatocellular carcinoma.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
A nanobody of Glypican 3 with outstanding acid-base stability and preparation method thereof
ActiveCN111848803BAntibody stability is highEasy to operateBacteriaMicroorganism based processesAntiendomysial antibodiesNucleotide
The present invention relates to a nanobody of Glypican 3 with outstanding acid-base stability and a preparation method thereof, and also relates to the amino acid sequence of the nanobody, a gene coding sequence and an expression vector capable of expressing the nanobody and host cells. The amino acid sequence of the nanobody to GPC3 provided by the present invention is shown in SEQ ID NO:7, and the nucleotide sequence of the encoding gene is shown in SEQ ID NO:8. The GPC3 nanobody involved in the present invention can specifically recognize liver cancer cells with high GPC3 expression by binding to the GPC3 expressed on the cell membrane. The antibody has outstanding acid-base stability, and can be used in the functional research of GPC3, and can also be used in the development of diagnostic reagents and therapeutic drugs for hepatocellular carcinoma.
Owner:ZHUHAI INST OF ADVANCED TECH
miRNAs for the diagnosis and treatment of hepatocellular carcinoma
ActiveCN111172290BAchieve early diagnosisReduce mortalityOrganic active ingredientsMicrobiological testing/measurementOncologyCancer research
The invention discloses a miRNA (micro ribonucleic acid) for diagnosis and treatment on hepatocellular carcinoma. The invention finds for a first time that expression of miR-4772-3p is decreased in liver cancer, and further verifies that miR-4772-3p has high diagnosis efficiency in liver cancer. The invention further discloses that miR-4772-3p plays significant roles in proliferation, migration and invasion of hepatoma carcinoma cells, and reminds that miR-4772-3p can be applied to treatment on liver cancer.
Owner:THE THIRD HOSPITAL OF HEBEI MEDICAL UNIV
Medicament for treating cancer
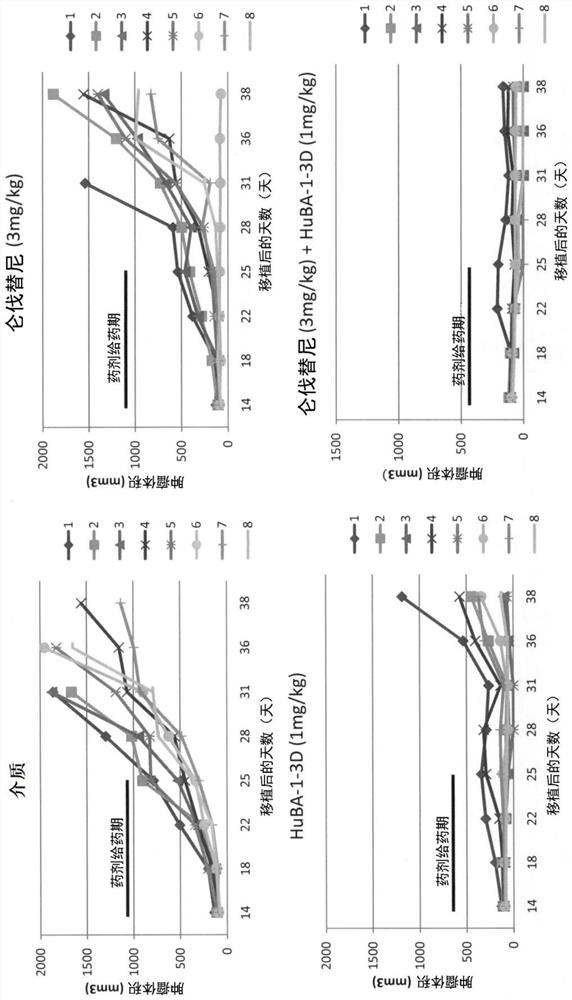
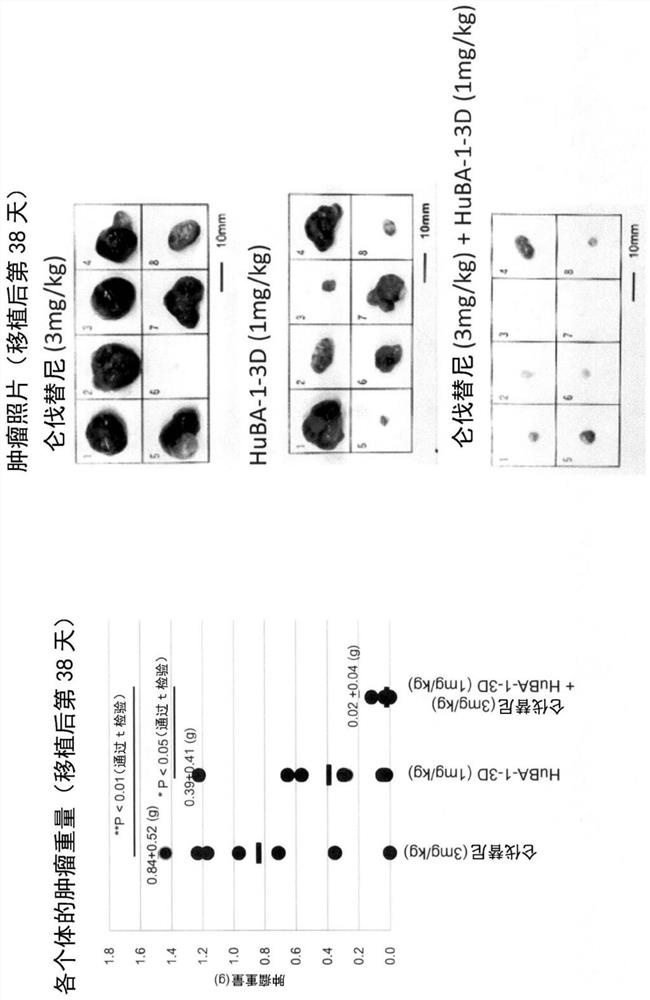
PendingCN113660954AStrong and long-lasting anti-tumor effectDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsLenvatinibAntiendomysial antibodies
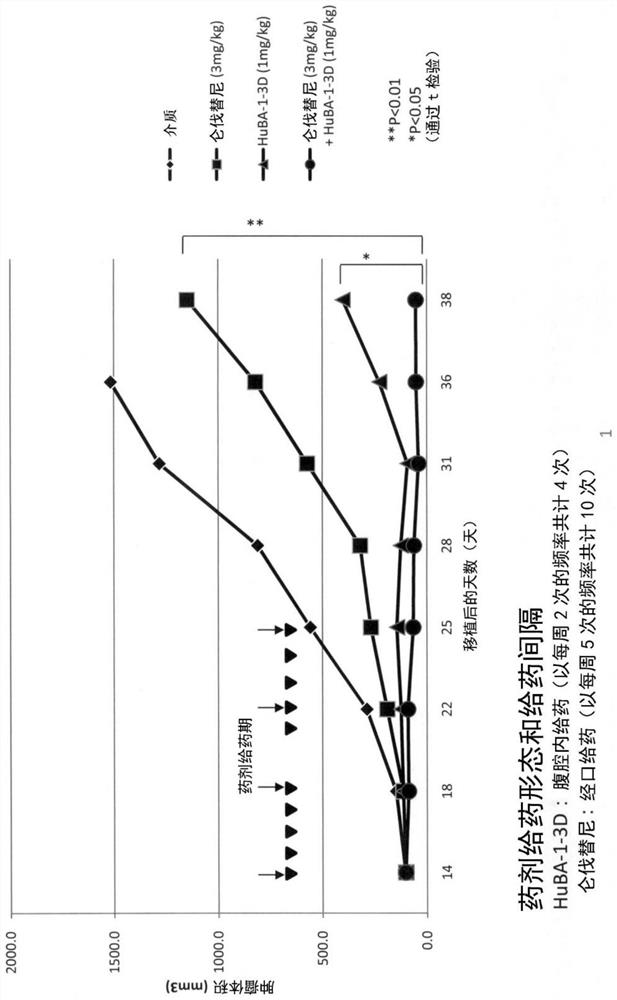
Provided are a liver cell cancer therapeutic agent, a therapeutic method, etc., which can exhibit more potent and sustained antitumor effects than a conventional multi-kinase inhibitor. The medicament is characterized by including, as a combination medicament for treating liver cell cancer: lenvatinib or a prodrug thereof, or a pharmaceutically acceptable salt thereof, or a hydrate or solvate thereof; and an antibody against human DLK-1 having an in-vivo anti-tumor activity or antibody fragments derived from said antibody.
Owner:CHIOME BIOSCIENCE INC
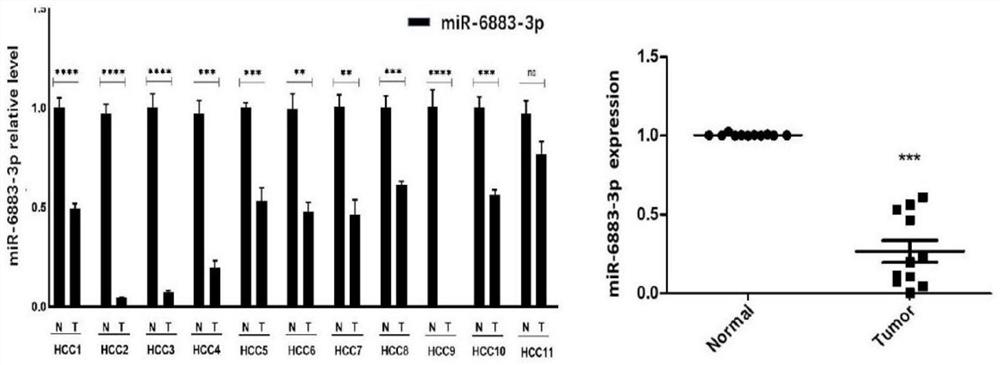
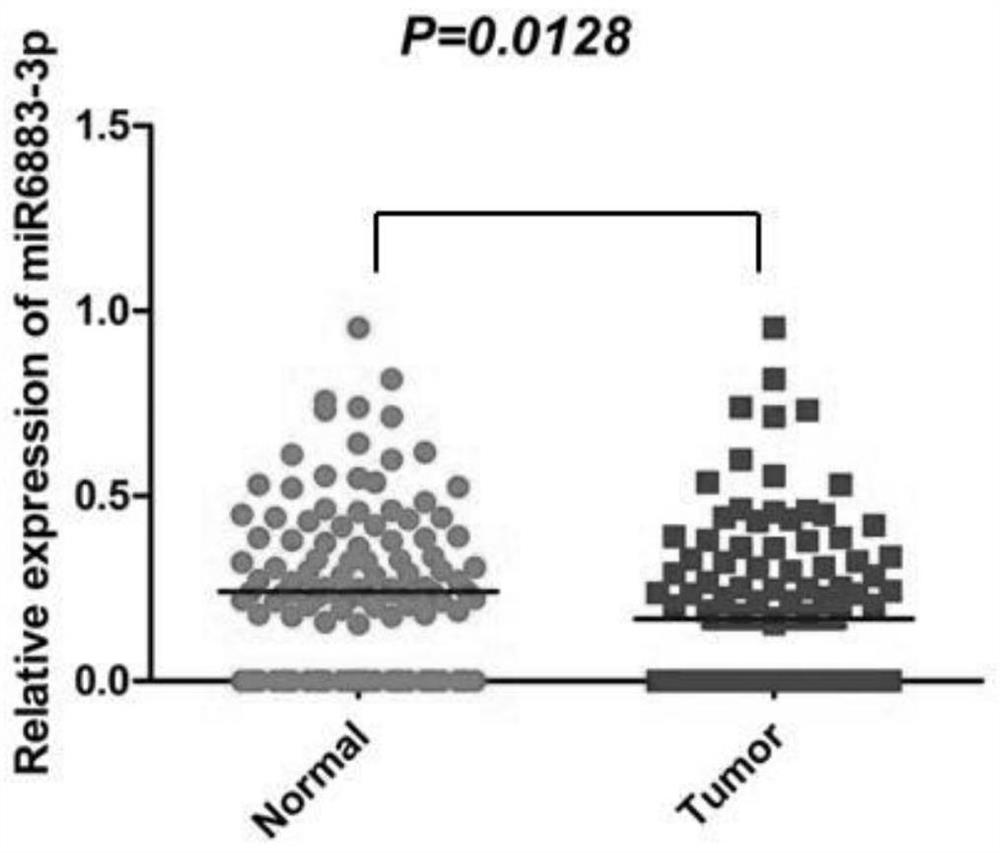
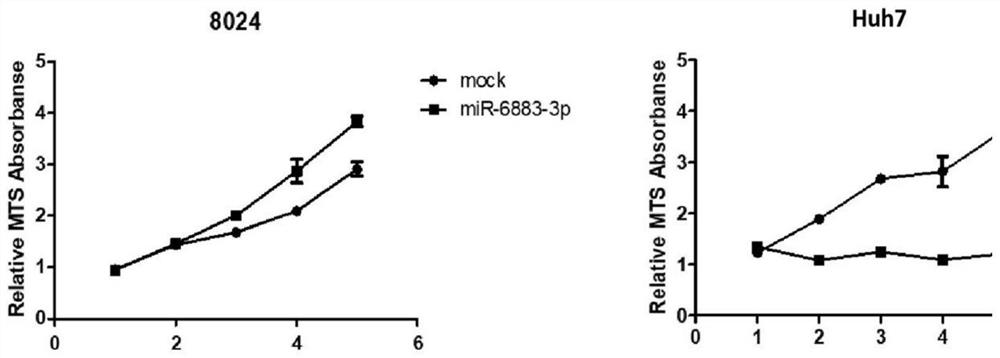
Application of miR-6883-3p in preparation of anti-liver cancer or liver cancer prognosis evaluation product
PendingCN113604569APrevent proliferationInhibit migrationOrganic active ingredientsMicrobiological testing/measurementParanasal Sinus CarcinomaMalignant phenotype
The invention relates to the technical field of biomedicine, discloses an application of miR-6883-3p in preparation of an anti-liver cancer product or a liver cancer prognosis evaluation product, and particularly relates to an application of miR-6883-3p in preparation of an anti-liver cancer drug or a liver cancer prognosis evaluation kit. The miR-6883-3p targeted gene regulation network provided by the invention is closely related to malignant phenotype and poor prognosis of tumors, overexpression of miR-6883-3p can effectively inhibit proliferation and migration of liver cancer cells, promote apoptosis of the liver cancer cells and reverse the differentiation state of the liver cancer cells, and the miR-6883-3p is low in expression in liver cancer tissues, so that the miR-6883-3p can be used for preparing antitumor drugs or detection kits and has good application prospects.The method has potential application value in treatment or prognosis evaluation of hepatocellular carcinoma.
Owner:GUANGZHOU MEDICAL UNIV
A kind of serum cenpf antibody quantitative detection kit
ActiveCN104678110BSensitiveDisease diagnosisBiological testingAntiendomysial antibodiesSerum autoantibodies
The invention relates to a serum CENPF antibody quantitative detection kit which can be used for specifically and quantitatively detecting the level of a CENPF antibody in a serum specimen. According to the serum CENPF antibody quantitative detection kit, recombinant CENPF antigen protein is coated in a 96-pore enzyme label board, the level of the CENPF antibody in the serum specimen can be quantitatively tested by using an enzyme linked immunosorbent assay, and the disease state of a liver cell cancer patient or high risk groups can be evaluated according to the level of the CENPF antibody in the serum. By adopting the serum CENPF antibody quantitative detection kit, a novel liver cell cancer screening and early diagnosis method is provided, and the sensitivity of the detected serum CENPF self-antibody is remarkably superior to that of a conventional clinical serum marker AFP when being used for diagnosing liver cell cancer.
Owner:北京博清科创生物技术有限公司
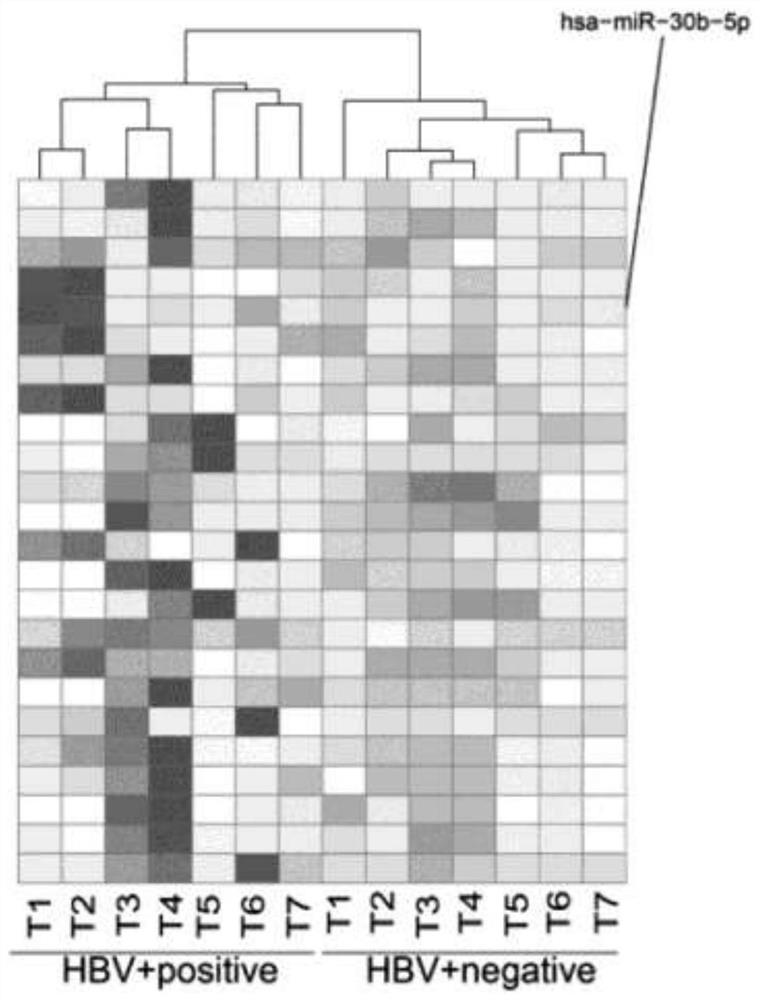
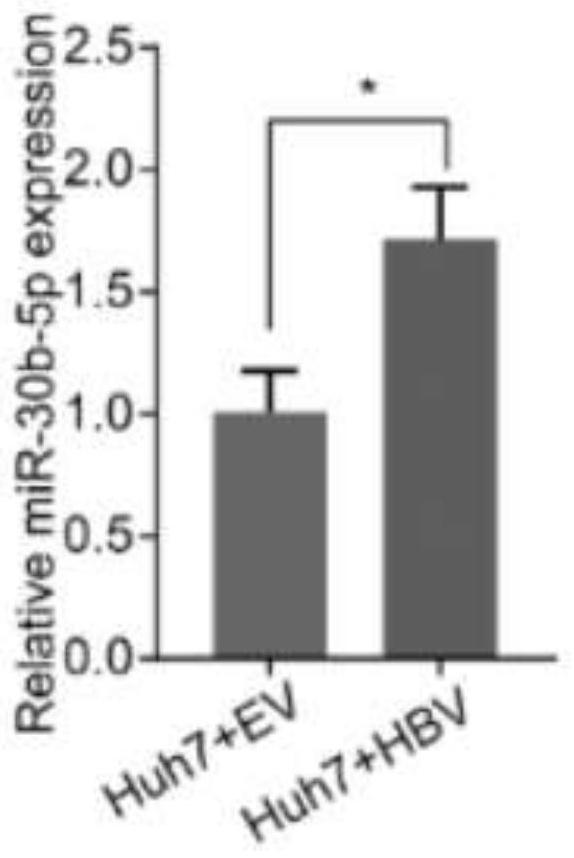
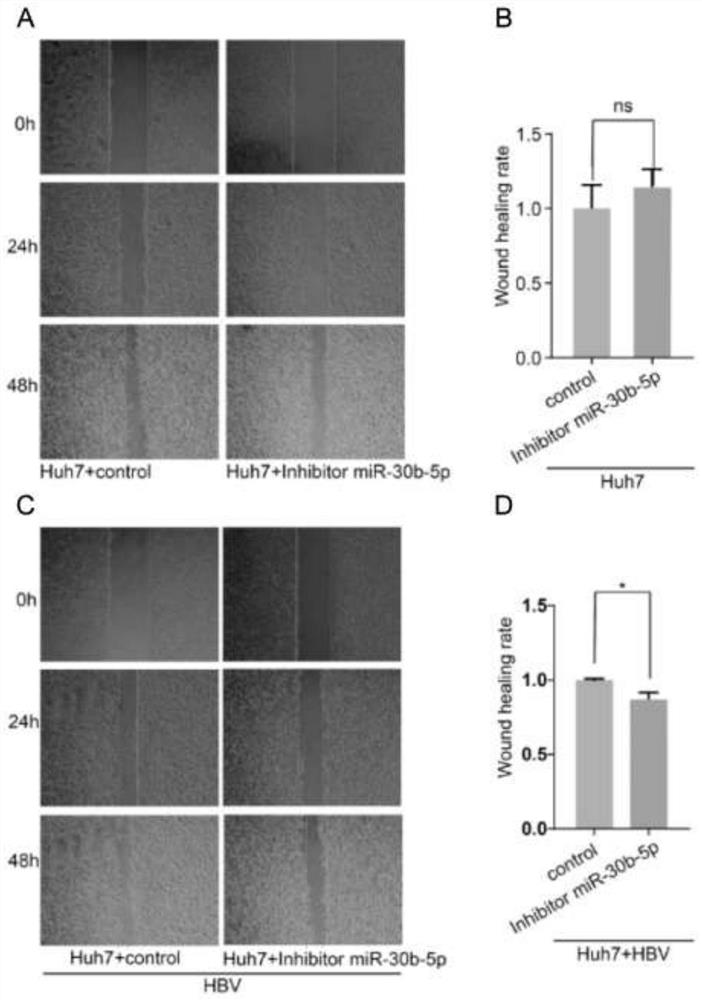
Use of micromolecule RNA-30b-5p as target molecule
ActiveCN112294960BPromote growthPromote invasionDigestive systemAntiviralsHepatitis B immunizationHepatitis B virus
The present invention relates to the use of a micromolecule RNA-30b-5p as a molecular target for resisting hepatitis B virus-related hepatocellular carcinoma. The invention discloses an application of miR-30b-5p as a target molecule in the preparation of anti-HBV-related hepatocellular carcinoma drugs. By inhibiting the transcription of miR-30b-5p or making it lose the function of binding to target genes, the growth and invasion of HBV-related liver cancer cells can be inhibited.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com