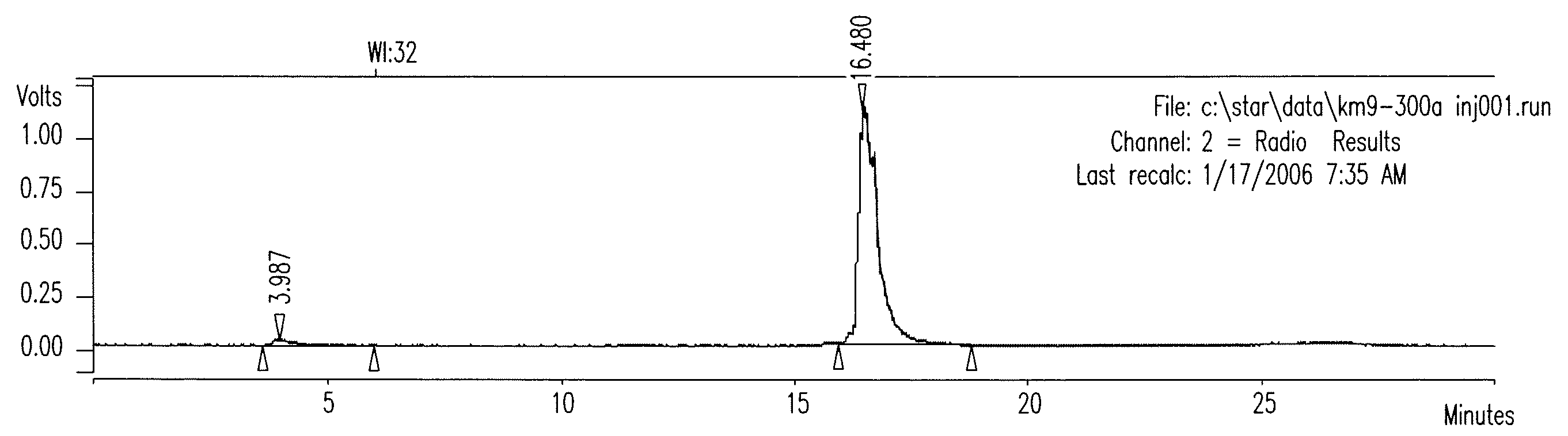
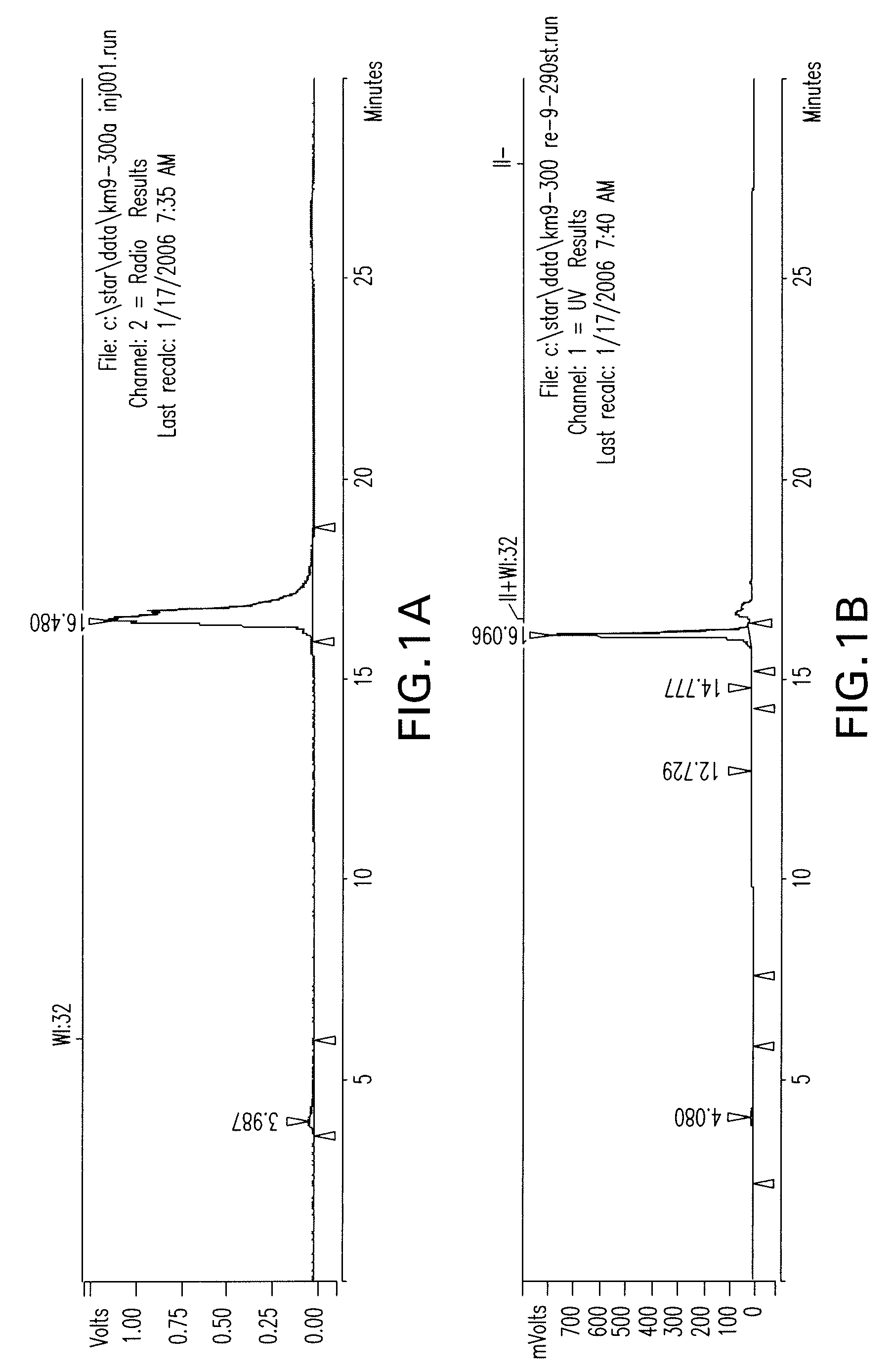
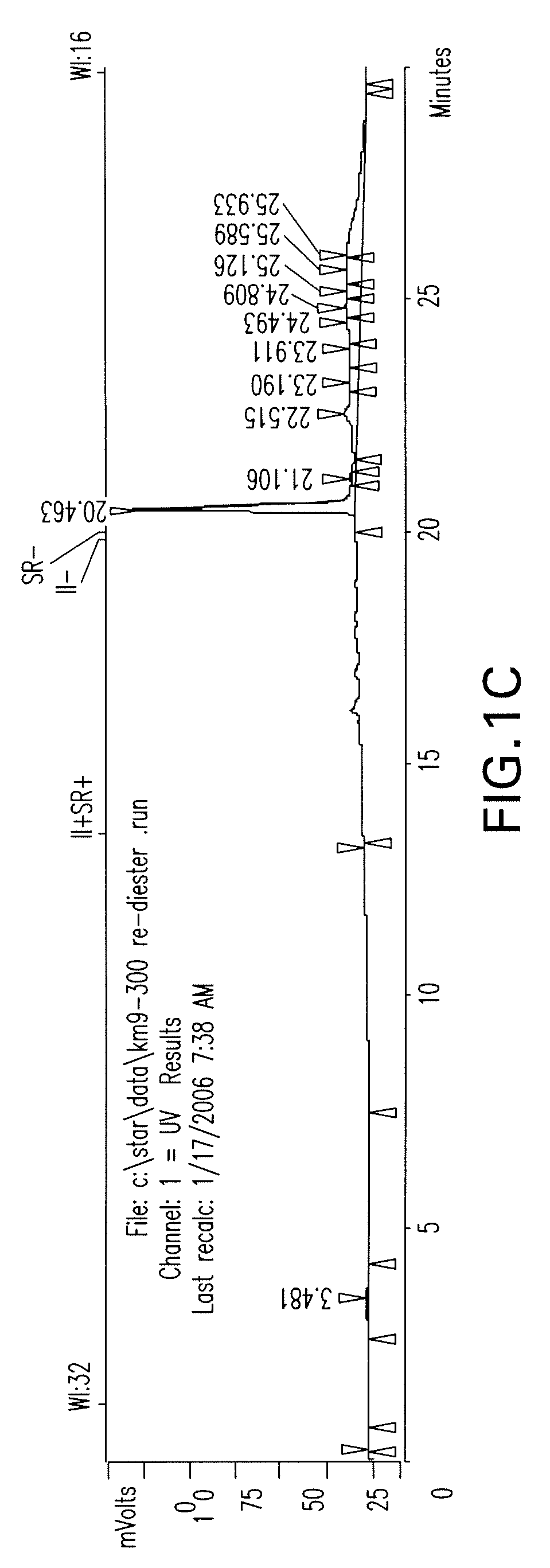
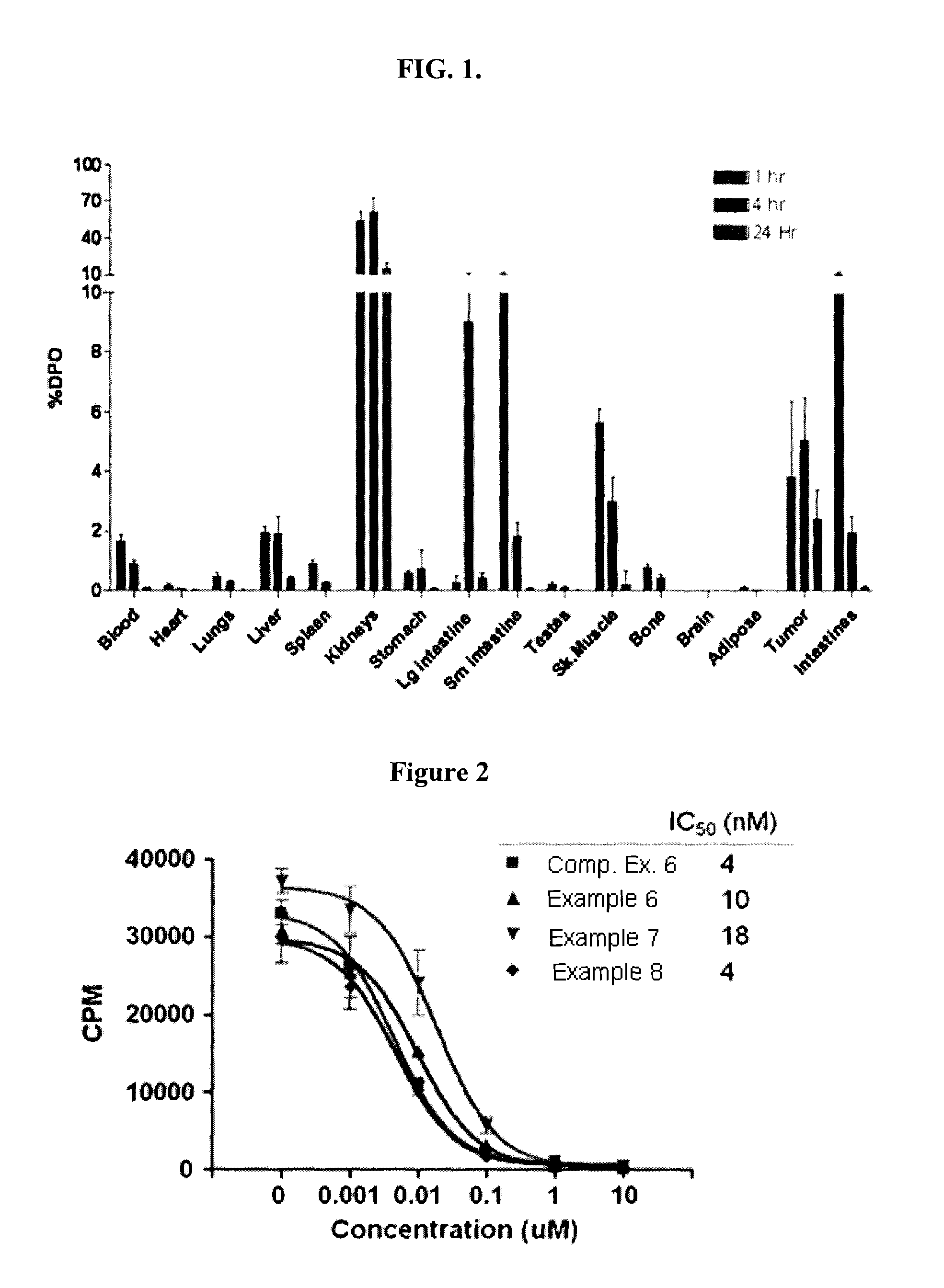
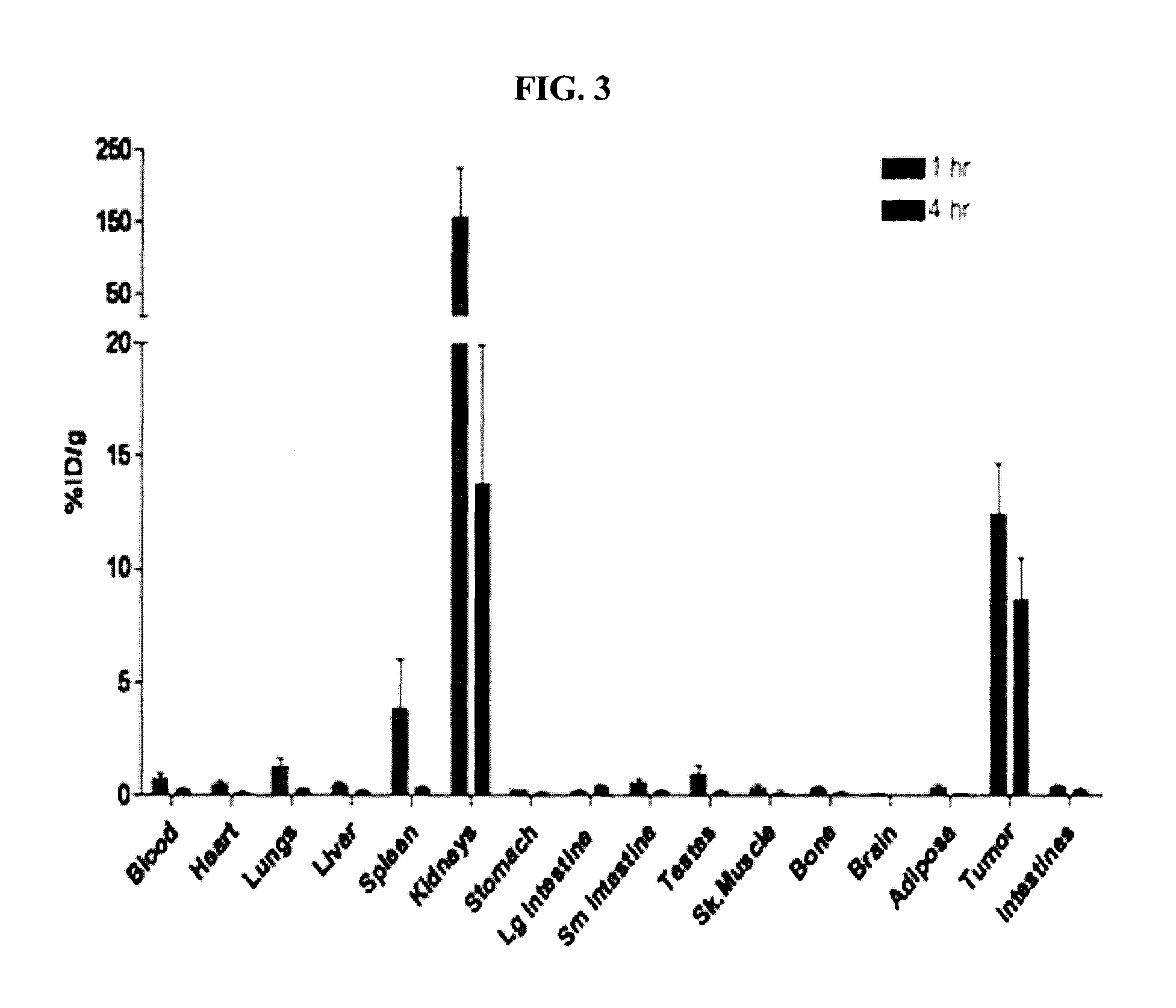
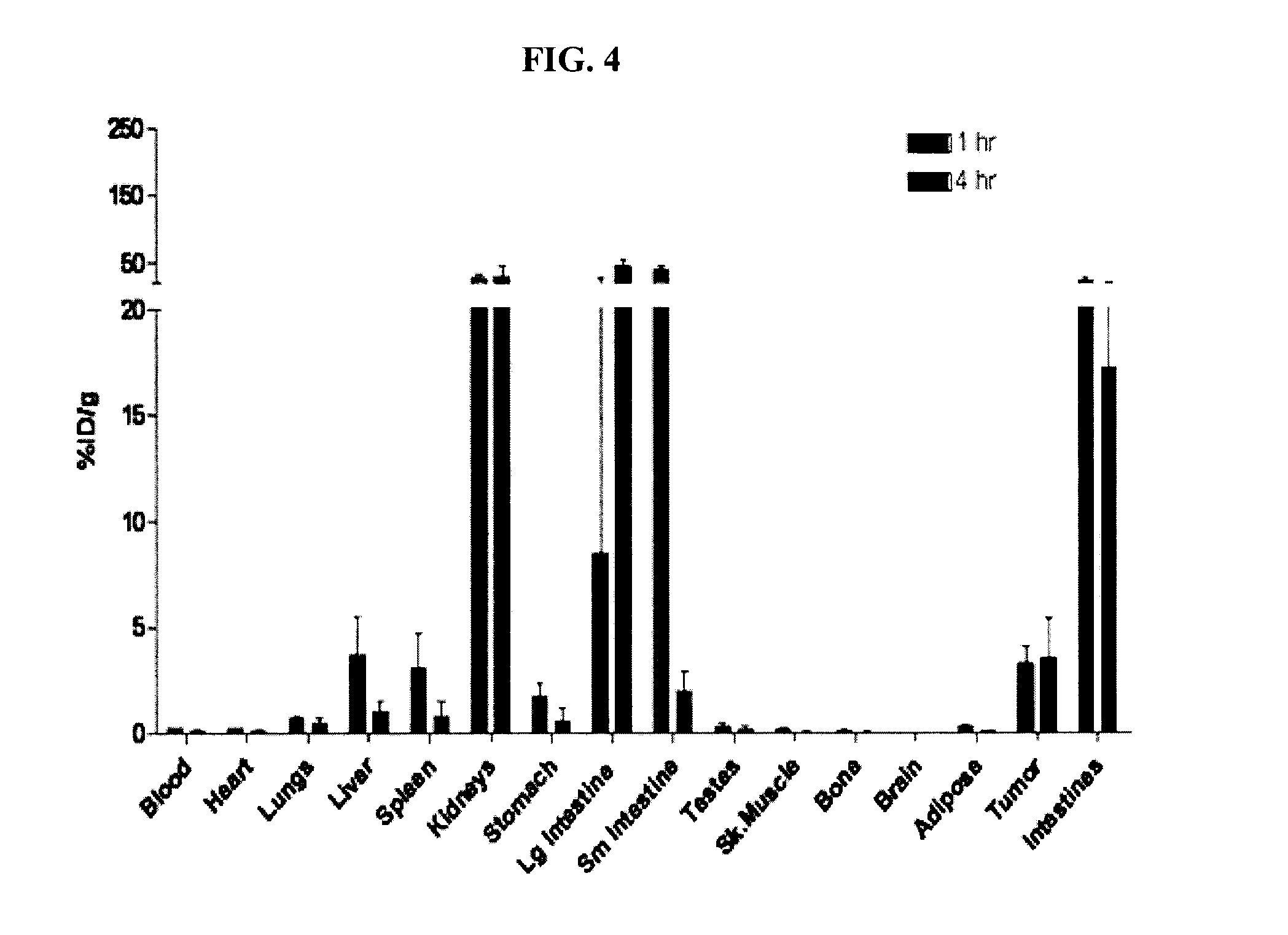
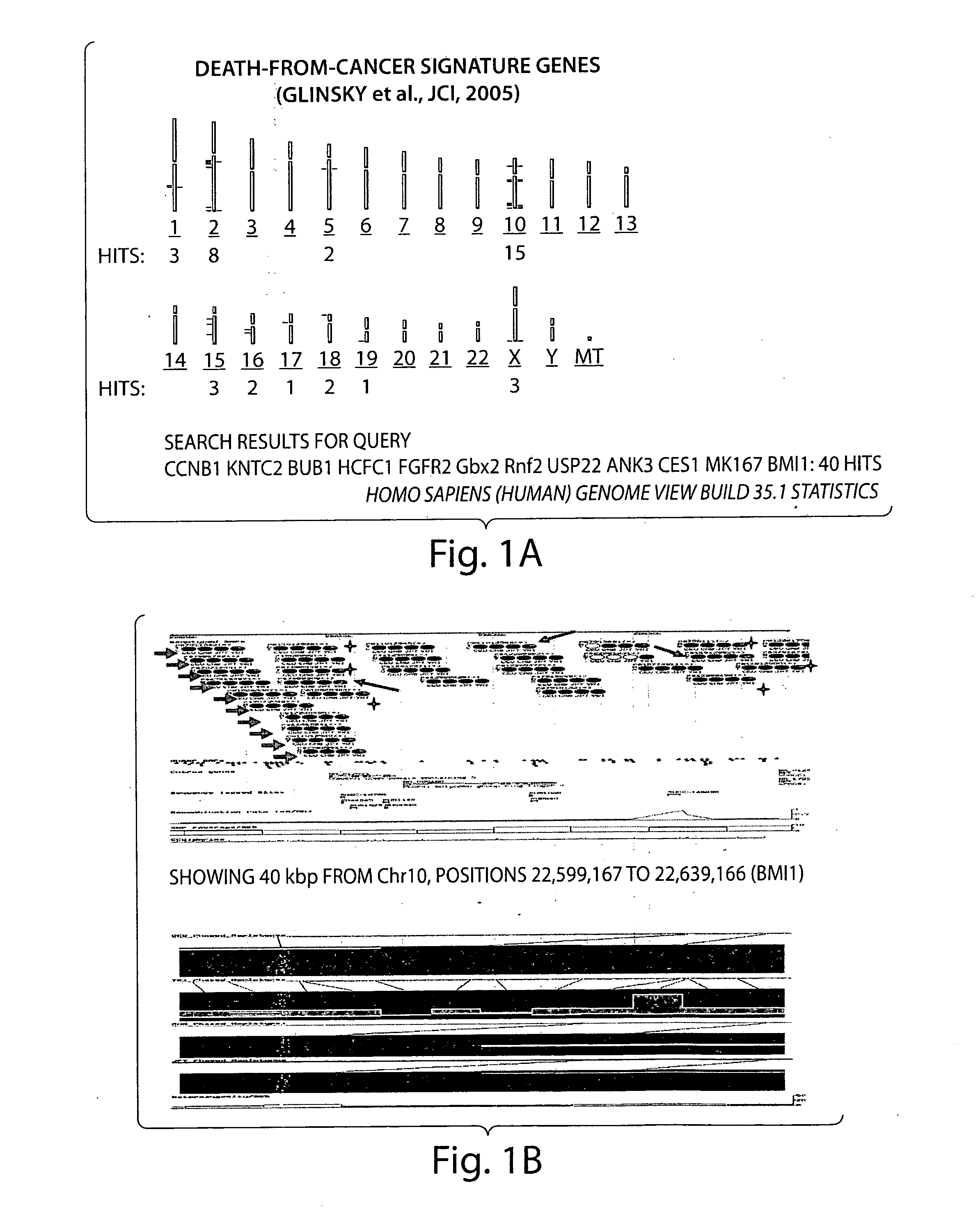
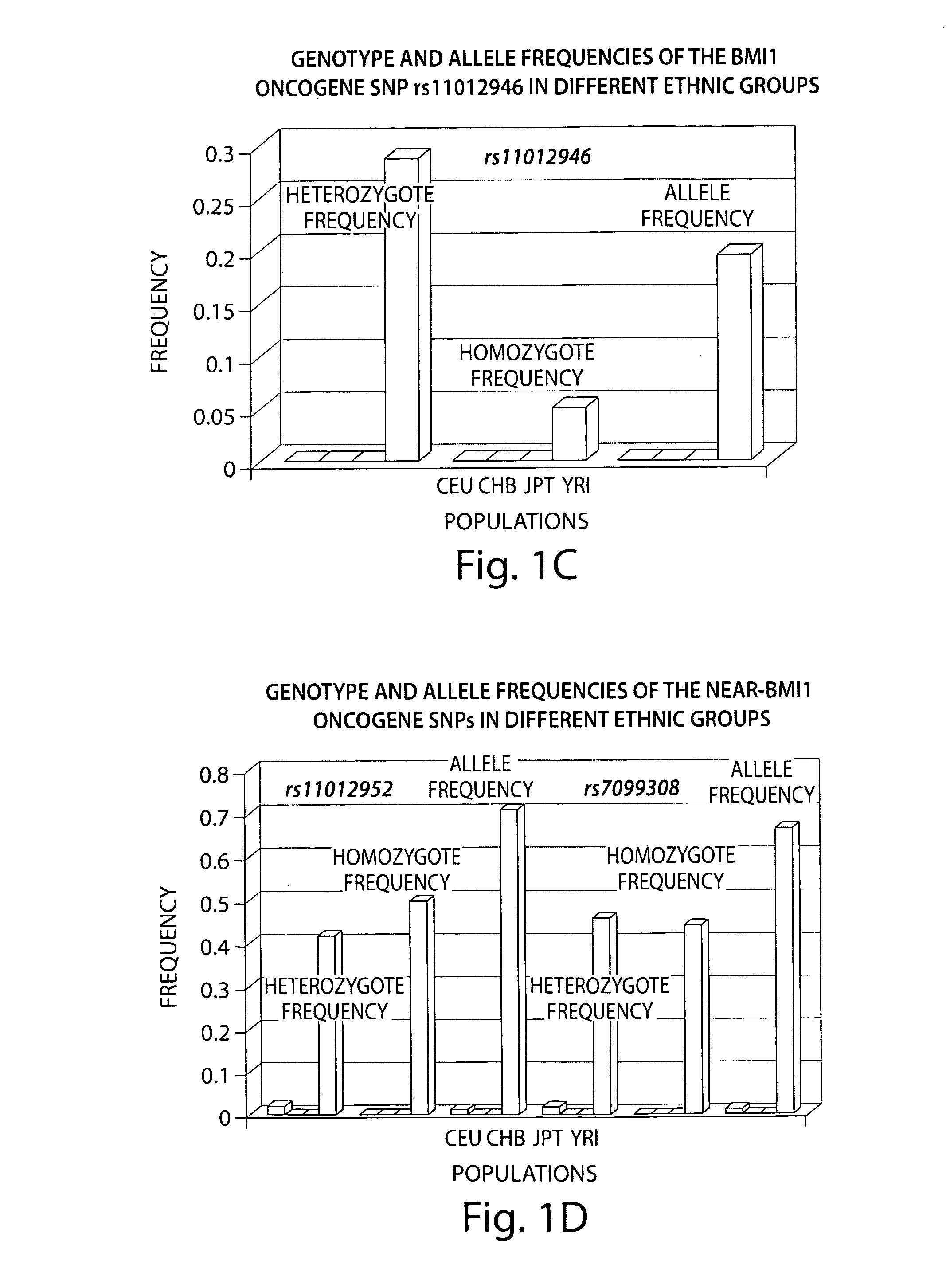
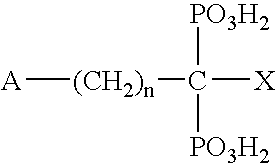Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Prostate carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carcinoma of the prostate is the most common internal malignancy among men in the United States and 10th common malignancy in India. This patient has a non-PSA-producing carcinoma of the prostate, called neuroendocrine prostate cancer (NEPC), that develops from neuroendocrine cells of the prostate.
Heterodimers of glutamic acid
InactiveUS20080193381A1Group 1/11 organic compounds without C-metal linkagesUrea derivatives preparationHalogenStereochemistry
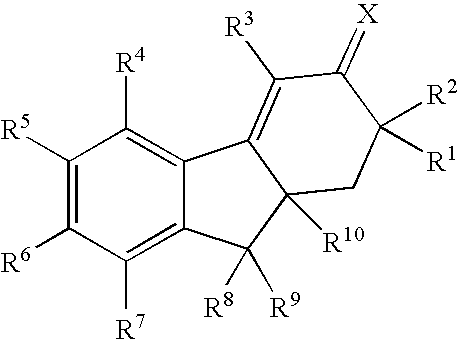
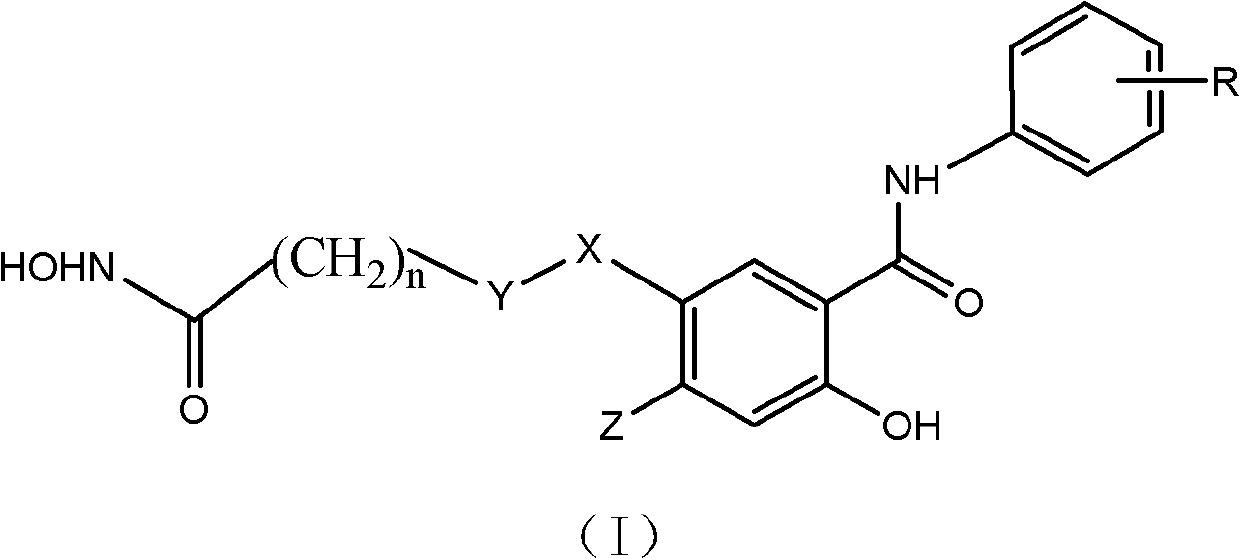
Compounds of Formula (Ia)wherein R is a C6-C12 substituted or unsubstituted aryl, a C6-C12 substituted or unsubstituted heteroaryl, a C1-C6 substituted or unsubstituted alkyl or —NR′R′,Q is C(O), O, NR′, S, S(O)2, C(O)2 (CH2)pY is C(O), O, NR′, S, S(O)2, C(O)2 (CH2)p Z is H or C1-C4 alkyl,R′ is H, C(O), S(O)2, C(O)2, a C6-C12 substituted or unsubstituted aryl, a C6-C12 substituted or unsubstituted heteroaryl or a C1-C6 substituted or unsubstituted alkyl, when substituted, aryl, heteroaryl and alkyl are substituted with halogen, C6-C12 heteroaryl, —NR′R′ or COOZ, which have diagnostic and therapeutic properties, such as the treatment and management of prostate cancer and other diseases related to NAALADase inhibition. Radiolabels can be incorporated into the structure through a variety of prosthetic groups attached at the X amino acid side chain via a carbon or hetero atom linkage.
Owner:MOLECULAR INSIGHT PHARMA
Technetium- and rhenium-bis(heteroaryl) complexes and methods of use thereof for inhibiting PSMA
Owner:MOLECULAR INSIGHT PHARMA
Treatments of therapy resistant diseases and drug combinations for treating the same
InactiveUS20080312199A1Accurately predict resistanceEasy to carryAntibacterial agentsBiocideCancers diagnosisMesothelioma
The present invention provides novel methods and kits for diagnosing the presence of cancer within a patient, and for determining whether a subject who has cancer is susceptible to different types of treatment regimens. The cancers to be tested include, but are not limited to, prostate, breast, lung, gastric, ovarian, bladder, lymphoma, mesothelioma, medullablastoma, glioma, and AML. Identification of therapy-resistant patients early in their treatment regimen can lead to a change in therapy in order to achieve a more successful outcome. One embodiment of the present invention is directed to a method for diagnosing cancer or predicting cancer-therapy outcome by detecting the expression levels of multiple markers in the same cell at the same time, and scoring their expression as being above a certain threshold, wherein the markers are from a particular pathway related to cancer, with the score being indicative or a cancer diagnosis or a prognosis for cancer-therapy failure. This method can be used to diagnose cancer or predict cancer-therapy outcomes for a variety of cancers. The markers can come from any pathway involved in the regulation of cancer, including specifically the PcG pathway and the “stemness” pathway. The markers can be mRNA, microRNA, DNA, or protein.
Owner:ORDWAY RES INST
Composition and method of treating diseases and disorders of the prostate
InactiveUS6277391B1Decreased libidoPrevent relapseMicrocapsulesProsthesisEccentric hypertrophyDisease
This invention relates to a composition and method for treating diseases and disorders of the prostate such as prostatitis, benign prostatic hypertrophy, and prostate carcinoma. The prostate is treated by intraprostatic injection of a biodegradable sustained release formulation. By injecting the treatment substance directly into the prostate, improved treatment results are obtained with a much lower treatment substance dosage. Additionally, by incorporating the treatment substance into a biodegradable sustained release formulation, the need for frequent repetition of injections is eliminated.
Owner:SAMYANG BIOPHARMLS CORP
Selective estrogen receptor modulators
InactiveUS7157604B2Urea derivatives preparationBiocideHormone Receptor ModulatorsPercent Diameter Stenosis
The present invention relates to compounds and derivatives thereof, their synthesis, and their use as estrogen receptor modulators. The compounds of the instant invention are ligands for estrogen receptors and as such may be useful for treatment or prevention of a variety of conditions related to estrogen functioning including: bone loss, bone fractures, osteoporosis, cartilage degeneration, endometriosis, uterine fibroid disease, hot flashes, increased levels of LDL cholesterol, cardiovascular disease, impairment of cognitive functioning, cerebral degenerative disorders, restenosis, gynecomastia, vascular smooth muscle cell proliferation, obesity, incontinence, and cancer, in particular of the breast, uterus and prostate.
Owner:MERCK & CO INC
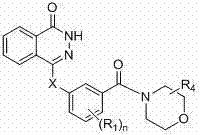
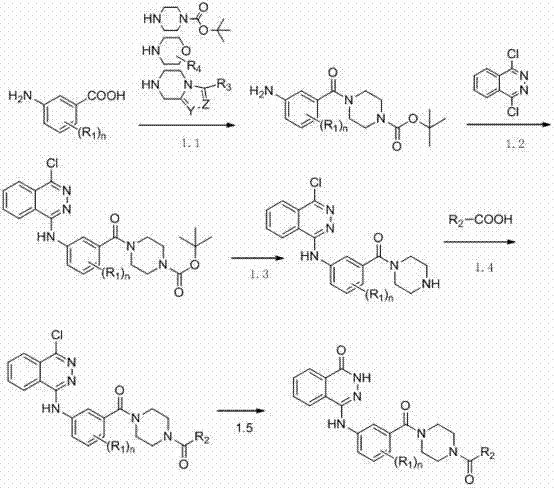
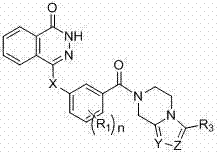
Novel phthalazinone derivatives and uses thereof
The present invention provides novel phthalazinone compounds and isomer thereof, pharmaceutically acceptable salts, solvates, chemically protected forms, and prodrugs; which can be used as PARP inhibitor and pharmaceutical compositions containing the novel phthalazinone compounds; wherein A, R1 and X are defined as shown. The medicine is used for the treatment of: vascular diseases, neurotoxicity, or diseases improved through the inhibition of PARP activity; or used as adjuvants for the treatment of cancers, or used for enhancing the therapeutic effect of radiation or chemotherapeutic agents on tumor cells, wherein the cancers includes breast cancer, ovarian cancer, colon cancer, melanoma, lung cancer, gastrointestinal stromal tumor, brain cancer, cervical cancer, pancreatic cancer, prostate cancer, gastric cancer, chronic myeloid leukocytes hypercytosis, liver canser, lymphoma, peritoneal cancer, soft tissue sarcoma, neuroendocrine tumors, advanced solid tumors, and glioblastoma.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Monoclonal antibodies for treatment of cancer
InactiveUS8946388B2Strong formationStrong proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
Methods and compositions for the treatment of diseases characterized by pathological calcification
InactiveUS20060069068A1Good curative effectBiocidePeptide/protein ingredientsGrowth retardantCalcification
Methods and compositions are provided which contains preparations of calcium chelators, bisphosphonates, antibiotics, antimicrobial agents, cytostatic agents, calcium ATPase and pyrophosphatase pump inhibitors, calcium phosphate-crystal dissolving agents, agents effective against calcium phosphate-crystal nucleation and crystal growth, and / or a combination of supportive agents and which may be used for treating and or reducing pathological calcifications, the growth of Nanobacterium and calcification-induced diseases including, but not limited to, Arteriosclerosis, Atherosclerosis, Coronary Heart Disease, Chronic Heart Failure, Valve Calcifications, Arterial Aneurysms, Calcific Aortic Stenosis, Transient Cerebral Ischemia, Stroke, Peripheral Vascular Disease, Vascular Thrombosis, Dental Plaque, Gum Disease (dental pulp stones), Salivary Gland Stones, Chronic Infection Syndromes such as Chronic Fatigue Syndrome, Kidney and Bladder Stones, Gall Stones, Pancreas and Bowel Diseases (such as Pancreatic Duct Stones, Crohn's Disease, Colitis Ulcerosa), Liver Diseases (such as Liver Cirrhosis, Liver Cysts), Testicular Microliths, Chronic Calculous Prostatitis, Prostate Calcification, Calcification in Hemodialysis Patients, Malacoplakia, Autoimmune Diseases. Erythematosus, Scleroderma, Dermatomyositis, Antiphospholipid Syndrome, Arteritis Nodosa, Thrombocytopenia, Hemolytic Anemia, Myelitis, Livedo Reticularis, Chorea, Migraine, Juvenile Dermatomyositis, Grave's Disease, Hypothyreoidism, Type 1 Diabetes Mellitus, Addison's Disease, Hypopituitarism, Placental and Fetal Disorders, Polycystic Kidney Disease, Glomerulopathies, Eye Diseases (such as Corneal Calcifications, Cataracts, Macular Degeneration and Retinal Vasculature-derived Processes and other Retinal Degenerations, Retinal Nerve Degeneration, Retinitis, and Iritis), Ear Diseases (such as Otosclerosis, Degeneration of Otoliths and Symptoms from the Vestibular Organ and Inner Ear (Vertigo and Tinnitus)), Thyroglossal Cysts, Thyroid Cysts, Ovarian Cysts, Cancer (such as Meningiomas, Breast Cancer, Prostate Cancer, Thyroid Cancer, Serous Ovarian Adenocarcinoma), Skin Diseases (such as Calcinosis Cutis, Calciphylaxis, Psoriasis, Eczema, Lichen Ruber Planus), Rheumatoid Arthritis, Calcific Tenditis, Osteoarthritis, Fibromyalgia, Bone Spurs, Diffuse Interstitial Skeletal Hyperostosis, Intracranial Calcifications (such as Degenerative Disease Processes and Dementia), Erythrocyte-Related Diseases involving Anemia, Intraerythrocytic Nanobacterial Infection and Splenic Calcifications, Chronic Obstructive Pulmonary Disease, Broncholiths, Bronchial Stones, Neuropathy, Calcification and Encrustations of Implants, Mixed Calcified Biofilms, and Myelodegenerative Disorders (such as Multiple Sclerosis, Lou Gehrig's and Alzheimer's Disease) in humans and animals. The method comprises administering the various classes of compositions of the present invention, which together effectively inhibit or treat the development of calcifications in vivo.
Owner:CIFTCIOGLU NEVA
Therapeutics And Methods For Treating Neoplastic Diseases Comprising Determining The Level Of Caveolin-1 And/Or Caveolin-2 In A Stromal Cell Sample
InactiveUS20120039805A1Modulate activityOrganic active ingredientsPeptide/protein ingredientsDiseaseProstate carcinoma
The invention provides diagnostic and therapeutic methods for neoplastic disease patients with neoplasms of for example, the breast, skin, kidney, lung, pancreas, rectum and colon, prostate, bladder, epithelial, non-epithelial; lymphomas, sarcomas, melanomas, and the like, comprising determining the level of caveolin-1 and / or caveolin-2 in stromal cells adjacent to a neoplasm.
Owner:THOMAS JEFFERSON UNIV
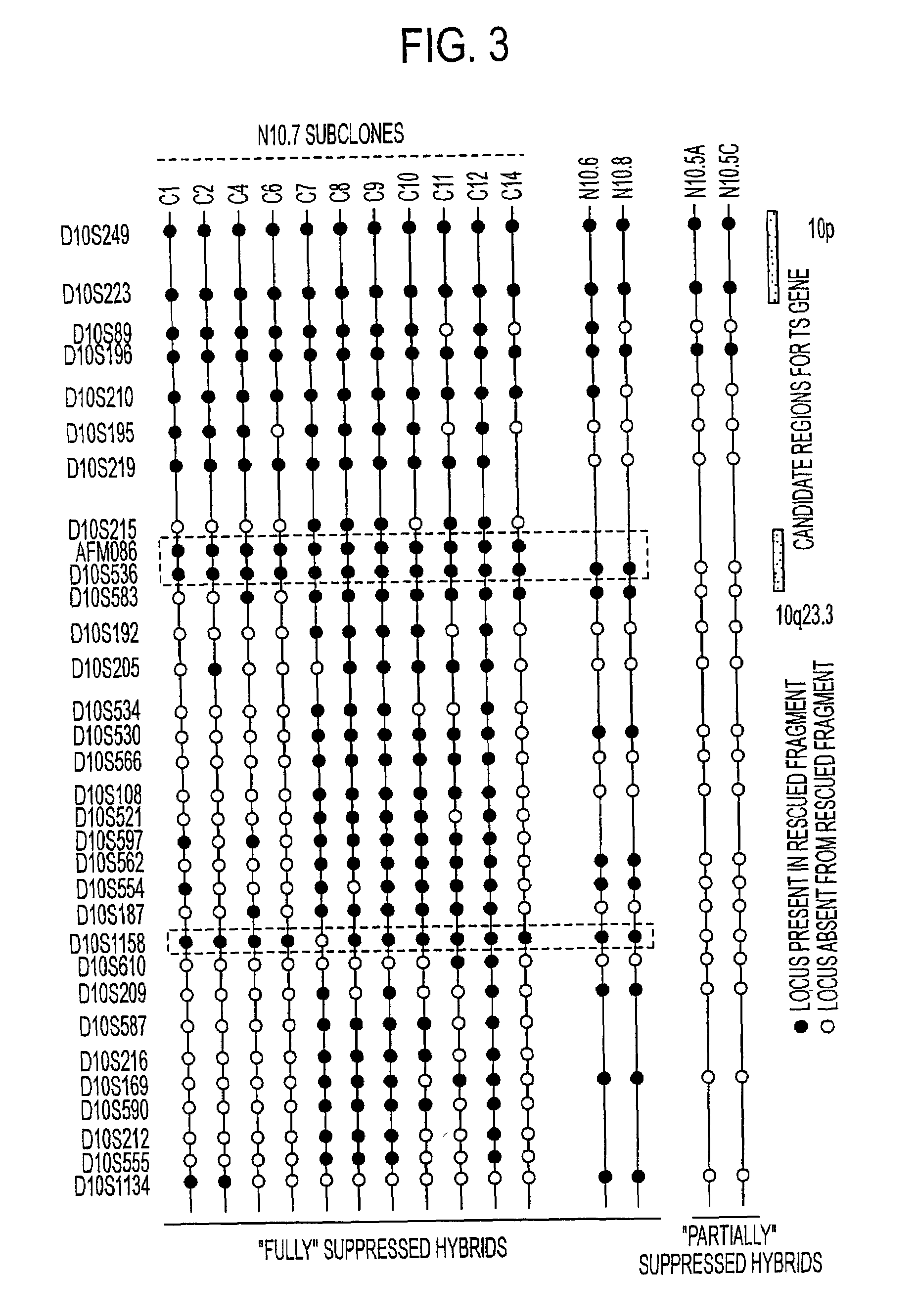
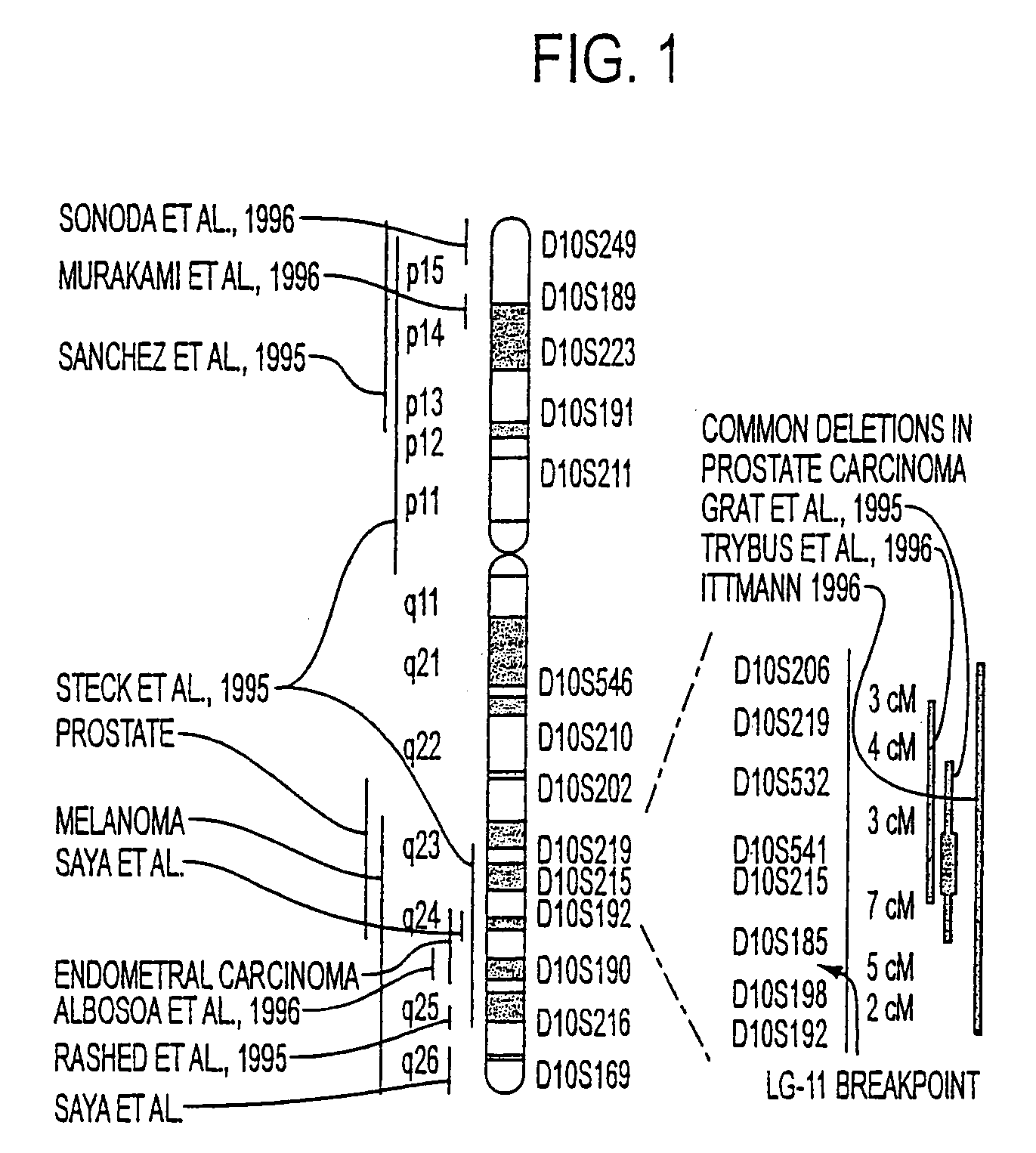
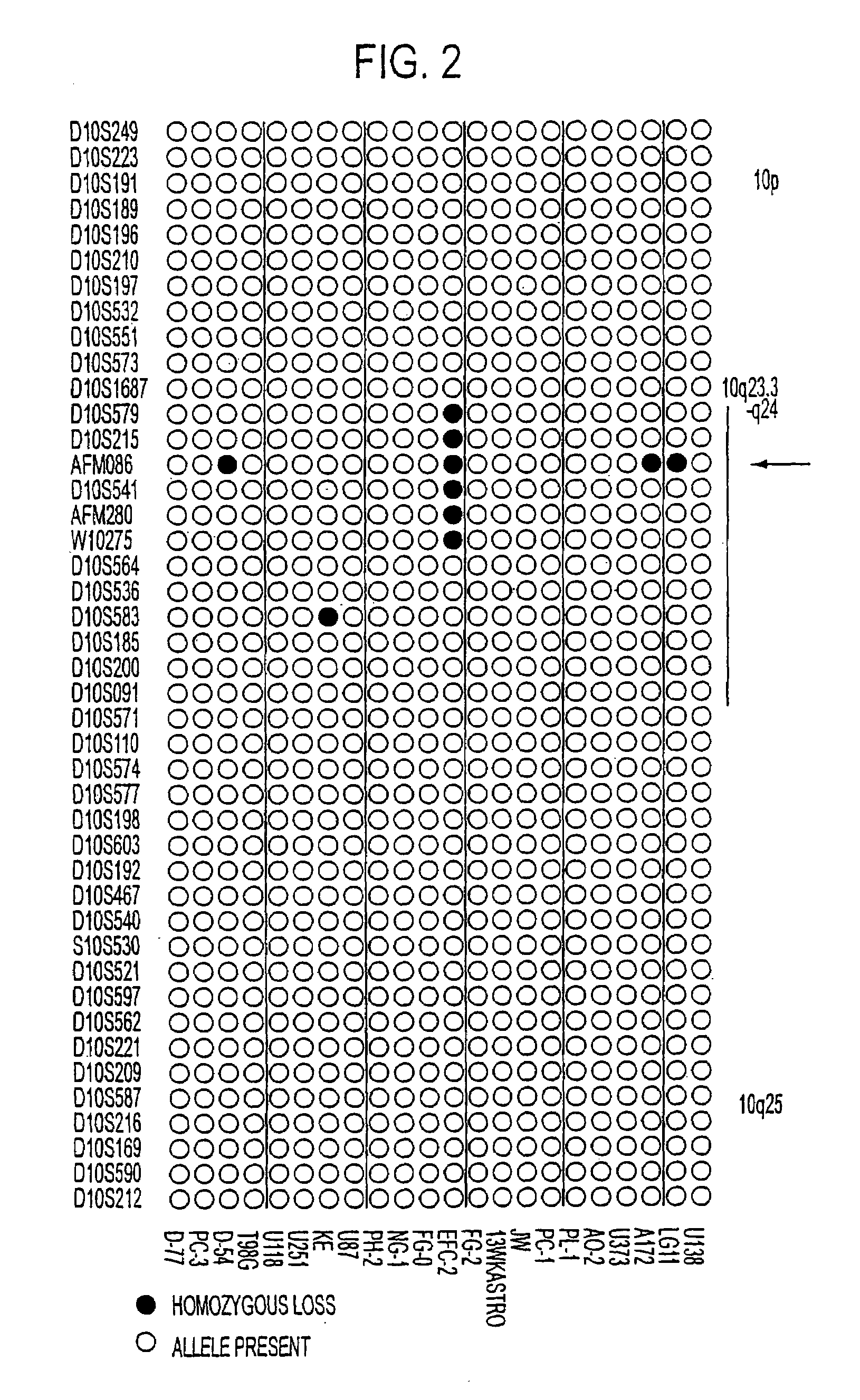
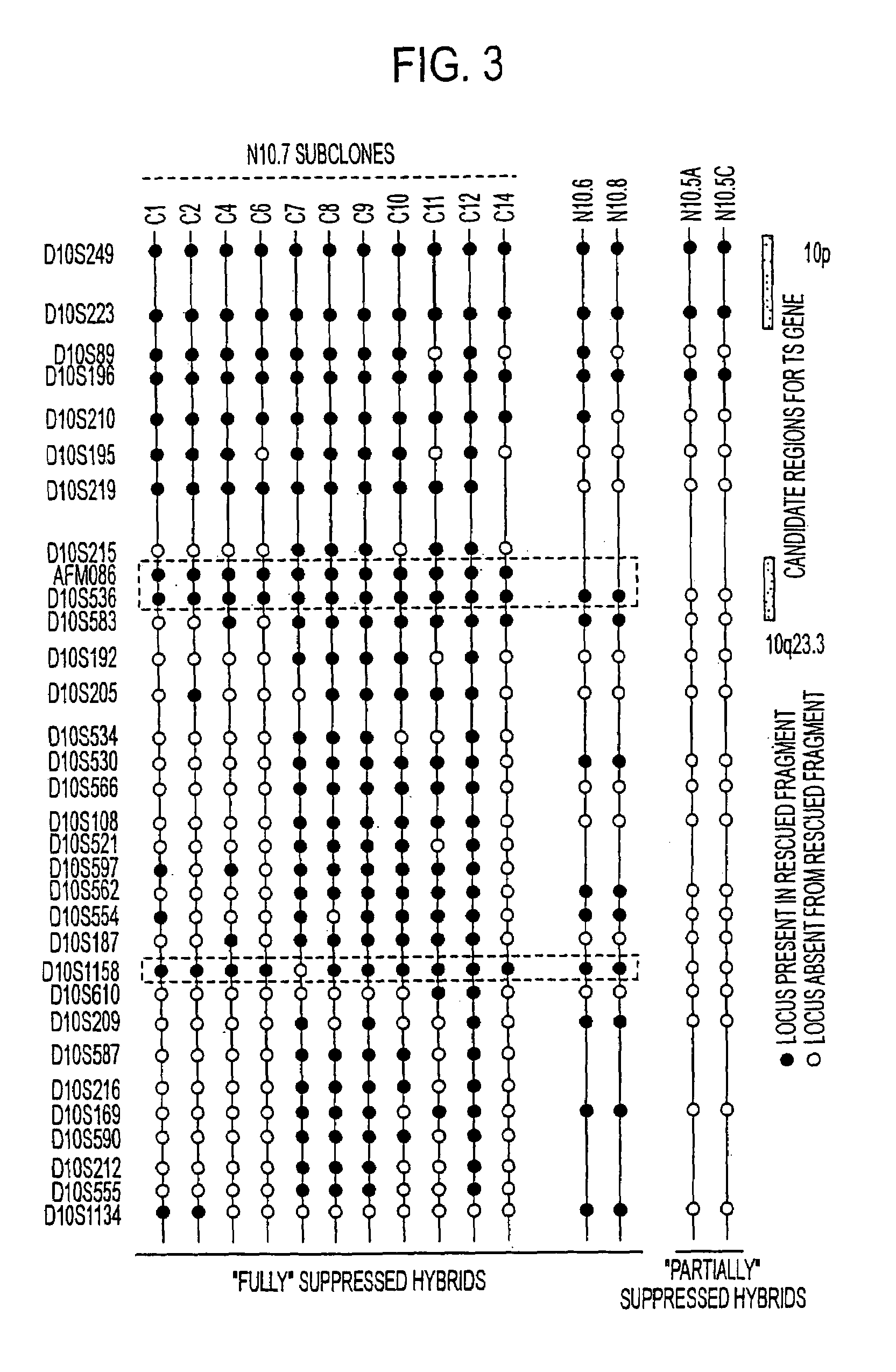
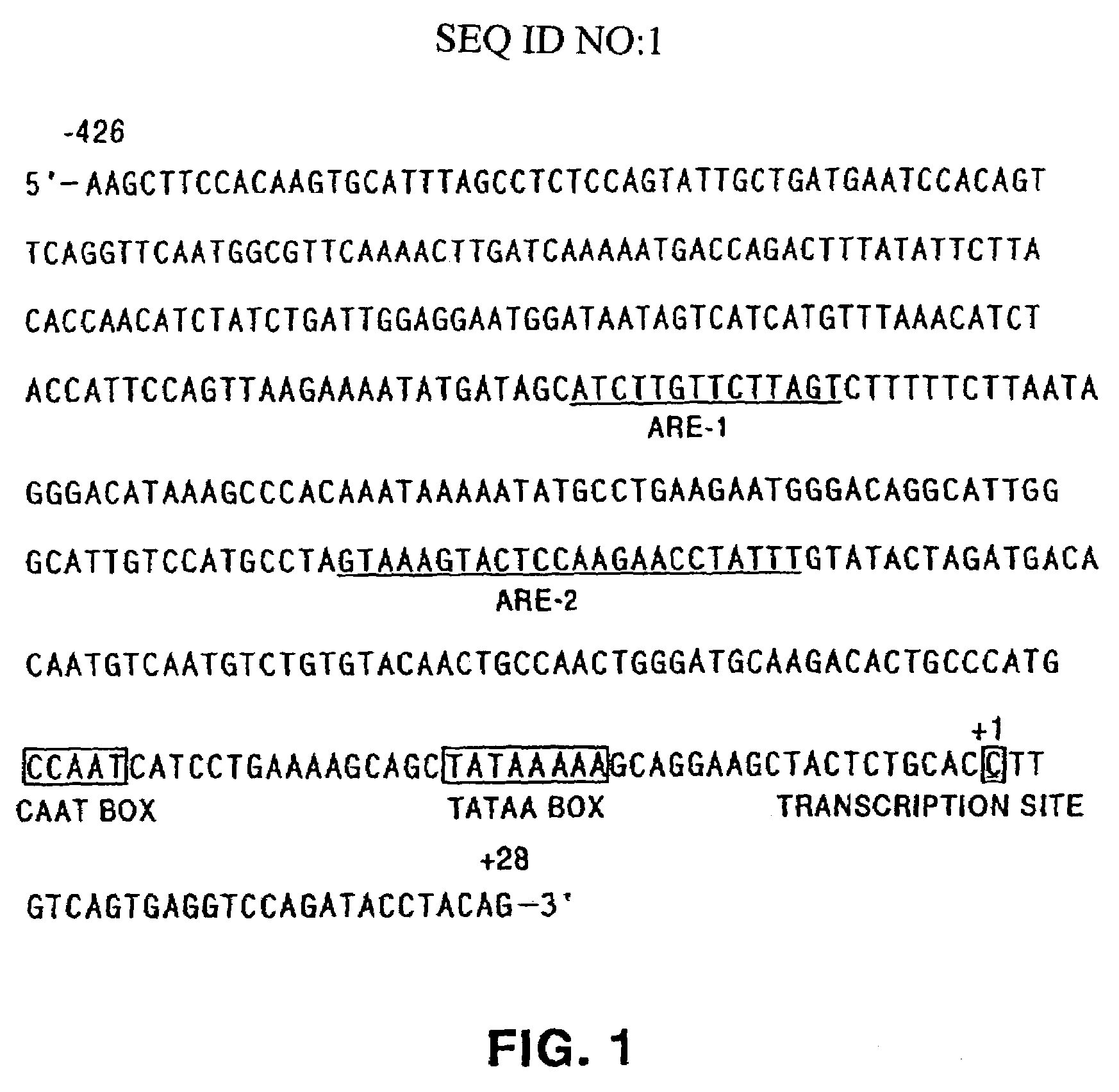
Tumor suppressor designated TS10q23.3
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
Gene upregulated in cancers of the prostate
InactiveUS6893818B1Improve the level ofVirusesSugar derivativesRibonucleoprotein complexOpen reading frame
The present invention relates to a novel protein designated 20P2H8 which shares homology with several heterogenous nuclear ribonucleoproteins (hnRNPs). A full length approximately 3600 bp 20P2H8 cDNA (SEQ ID NO: 10, encoding a 517 amino acid open reading frame (SEQ ID NO: 2), is provided herein.
Owner:AGENSYS INC
Tumor suppressor designated TS10q23.3
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Adenovirus vectors specific for cells expressing androgen receptor and methods of use thereof
Replication-competent adenovirus vectors specific for cells which allow a probasin transcriptional response element (PB-TRE) to function, such as cells which express the androgen receptor (AR), and methods of use of such viruses are provided. These viruses comprise an adenoviral gene under control of a transcriptional regulatory portion of a PB-TRE, which is in turn dependent upon AR expression. The gene can be, for example, a gene required for viral replication or the adenovirus death protein gene (ADP). The viruses can also comprise at least one additional adenoviral gene under control of at least one additional prostate-specific transcriptional response element, such as that controlling prostate-specific antigen expression (PSA-TRE). Thus, virus replication can be restricted to target cells exhibiting prostate-specific gene expression, particularly prostate carcinoma cells.
Owner:CELLS GENESYS INC
Monoclonal antibodies for treatment of cancer
InactiveUS20110223182A1Minimize adverse effectsStrong formationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMelanoma
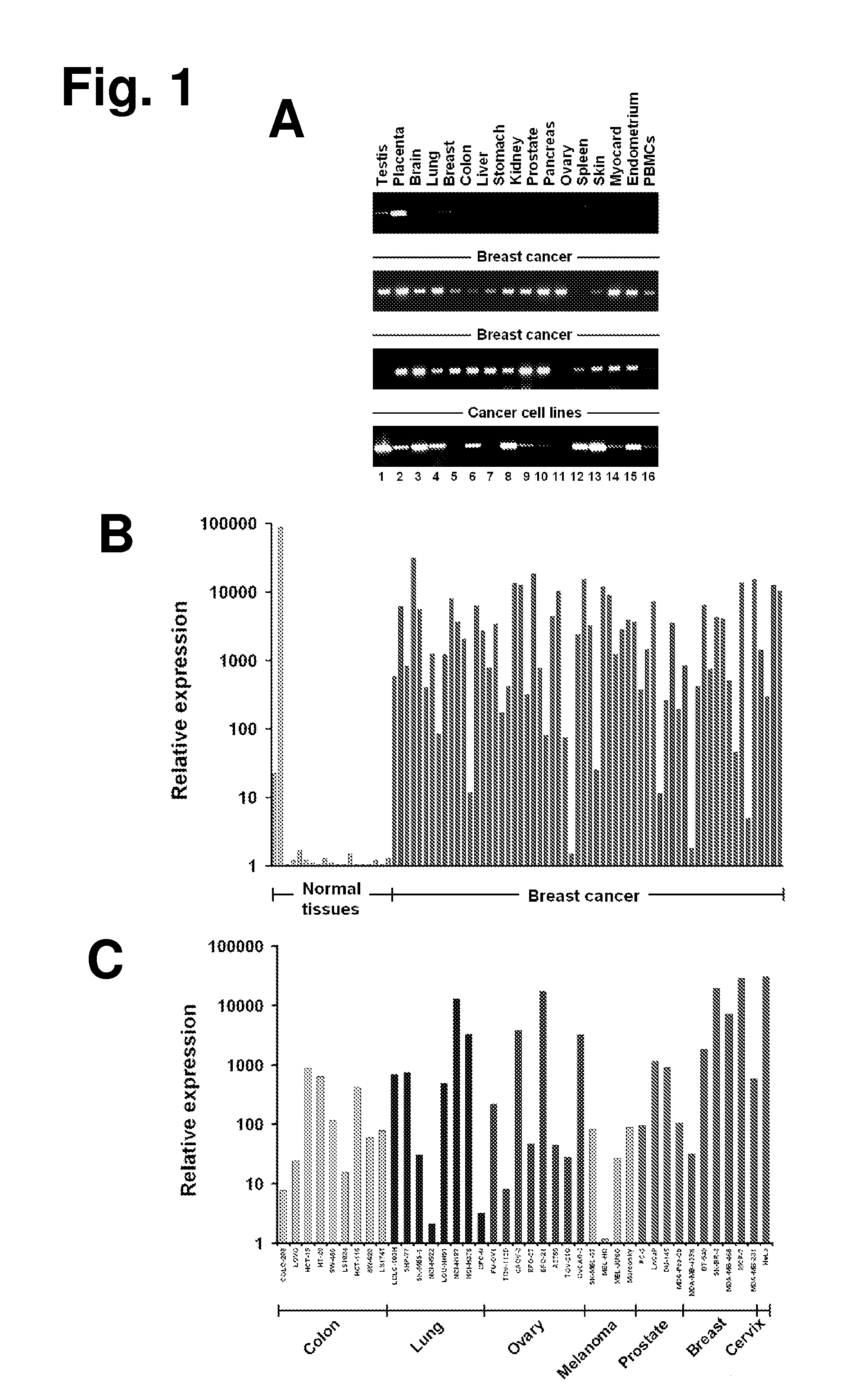
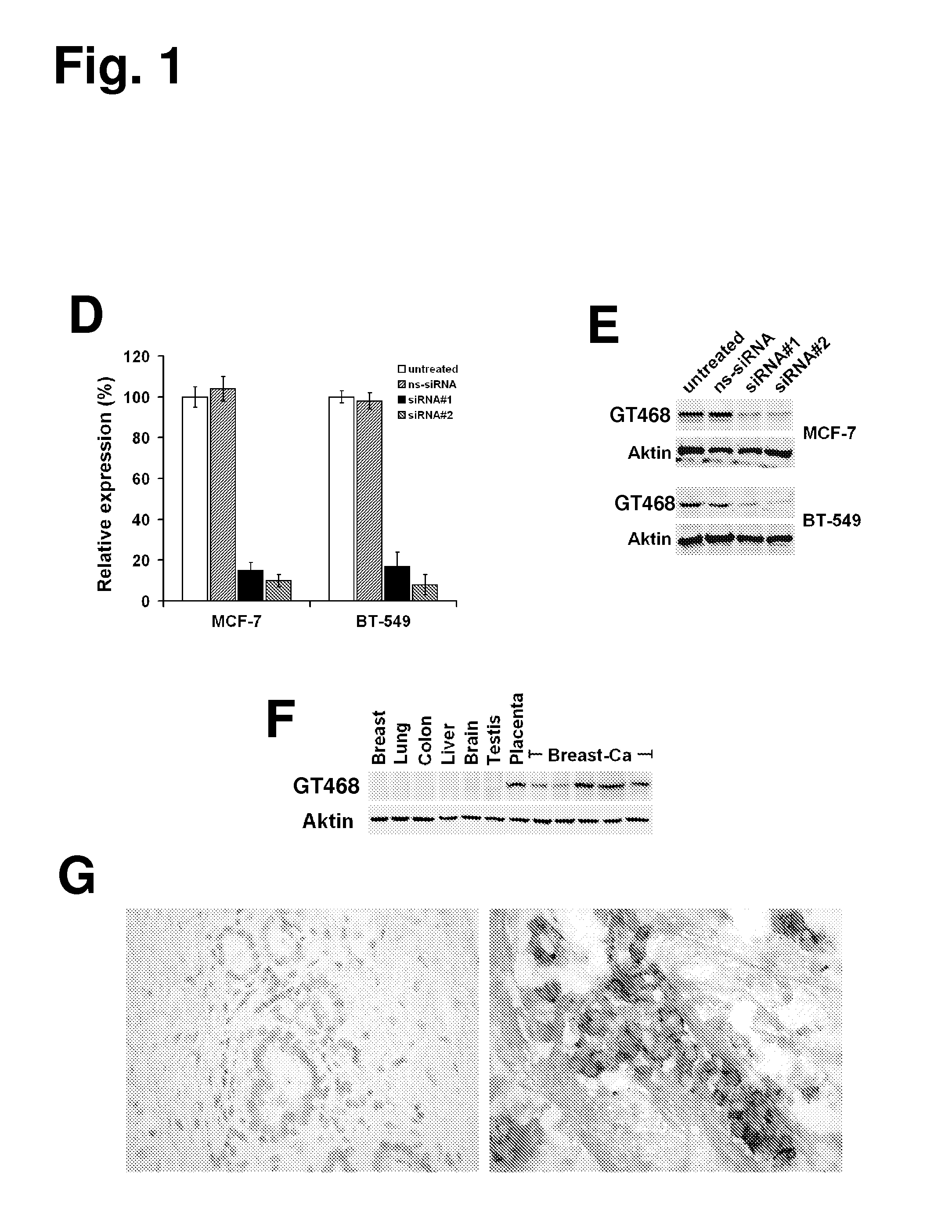
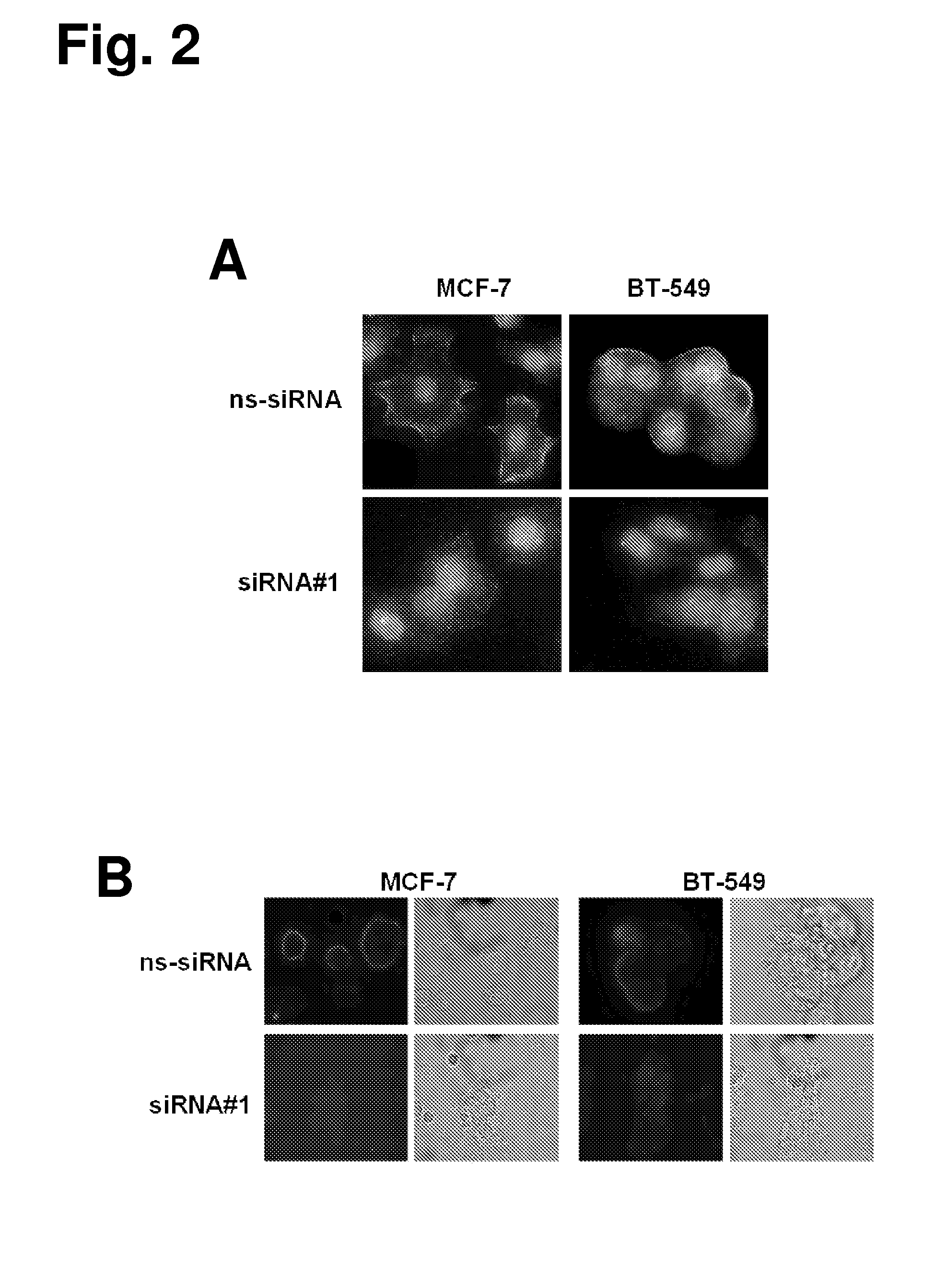
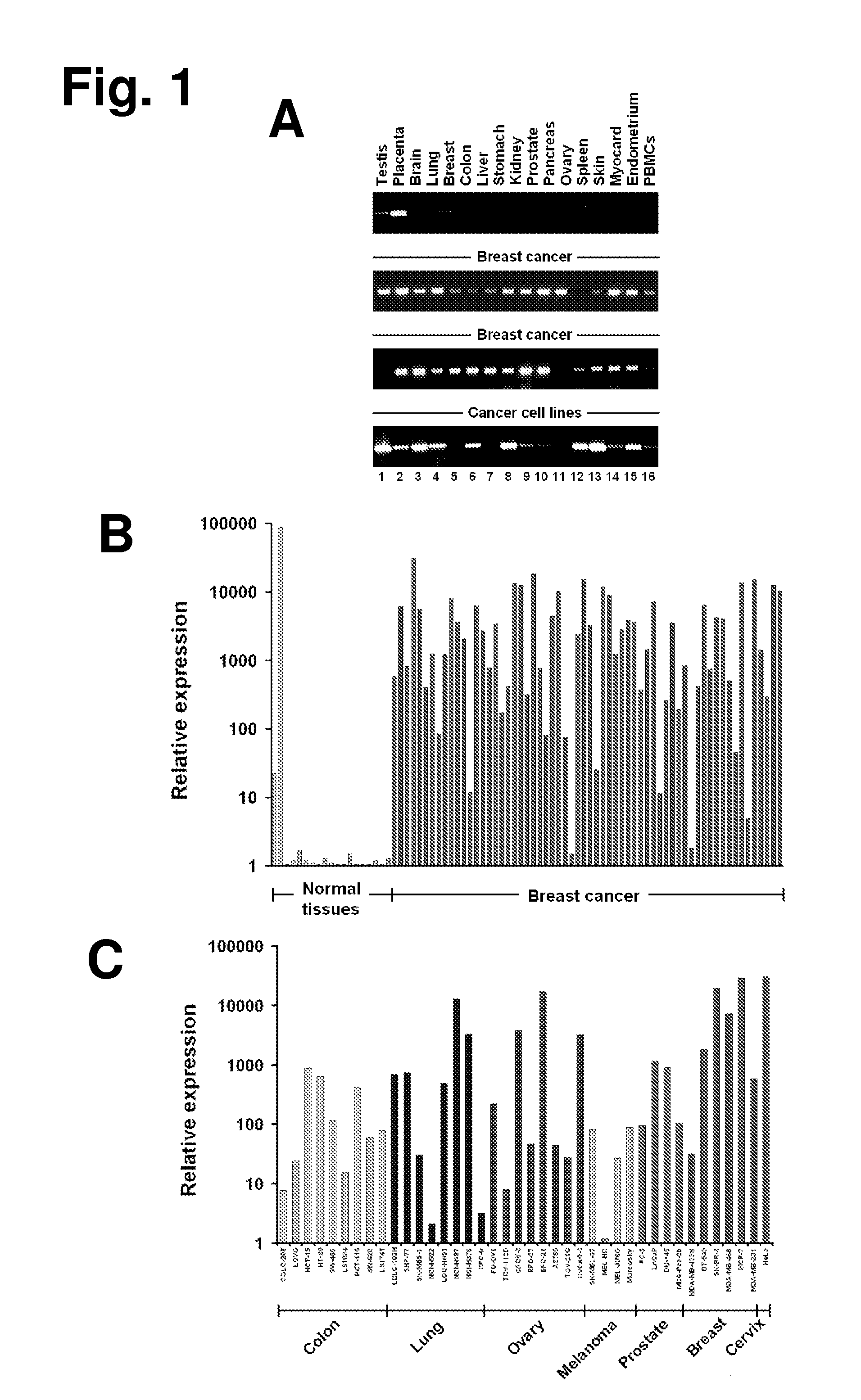
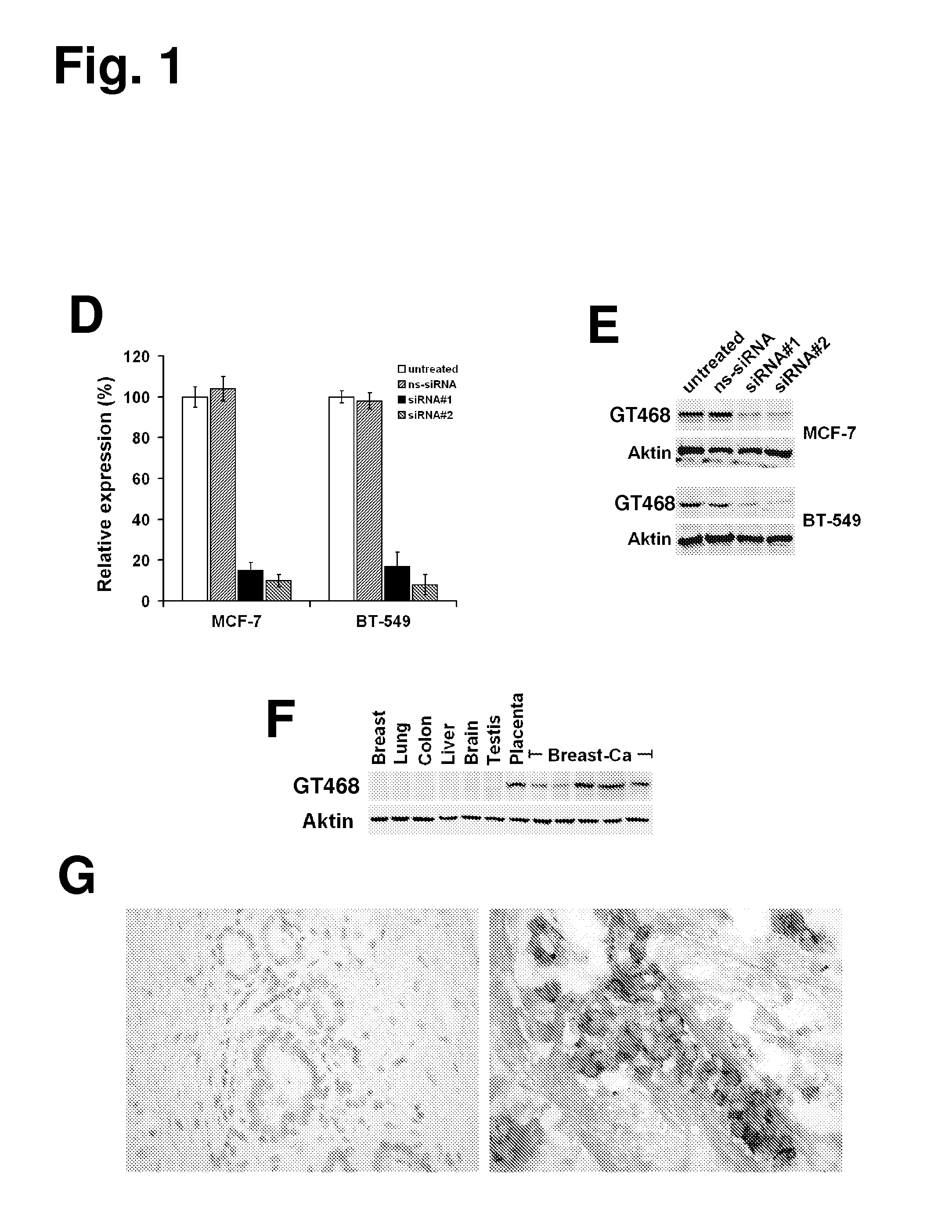
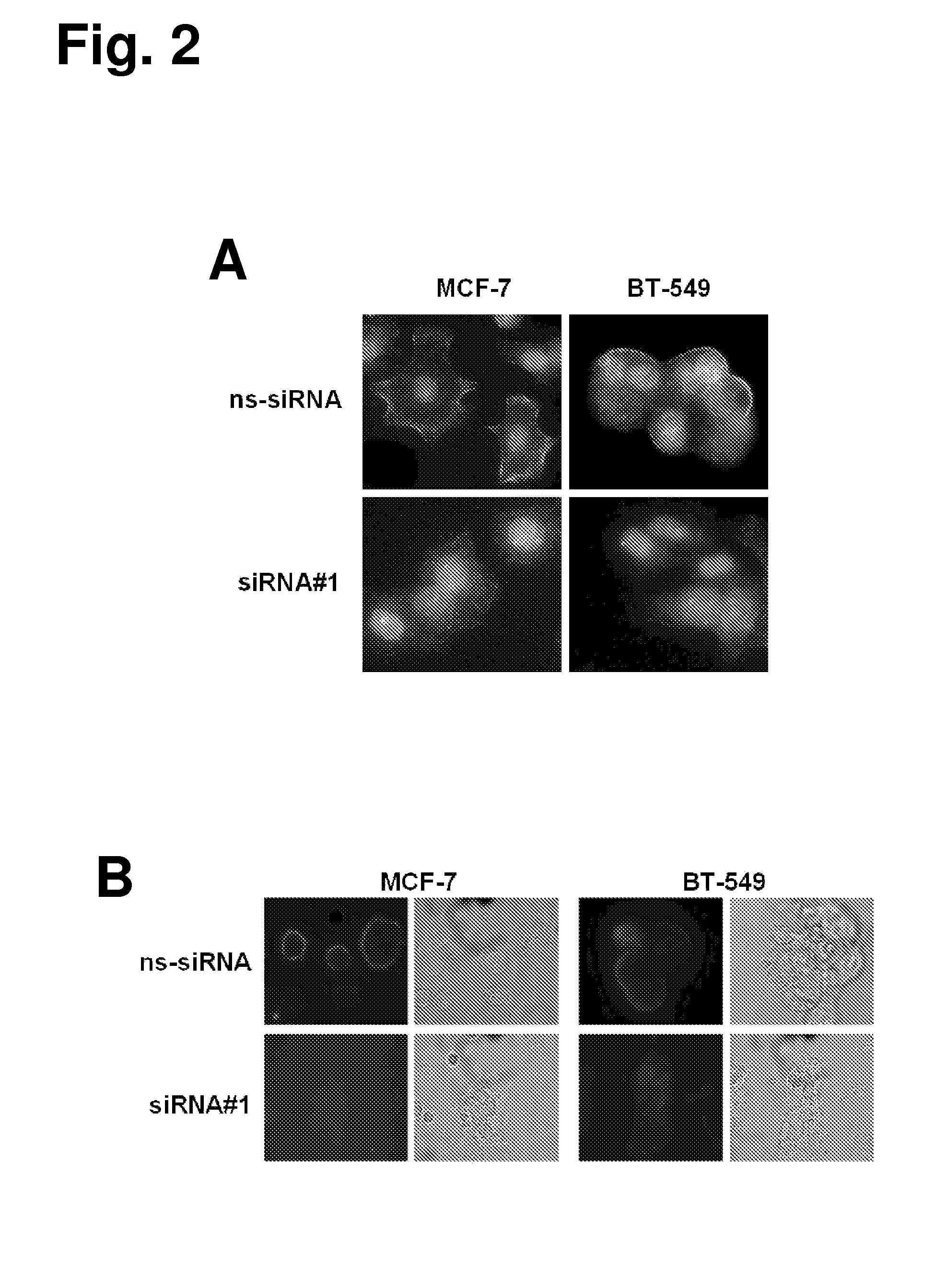
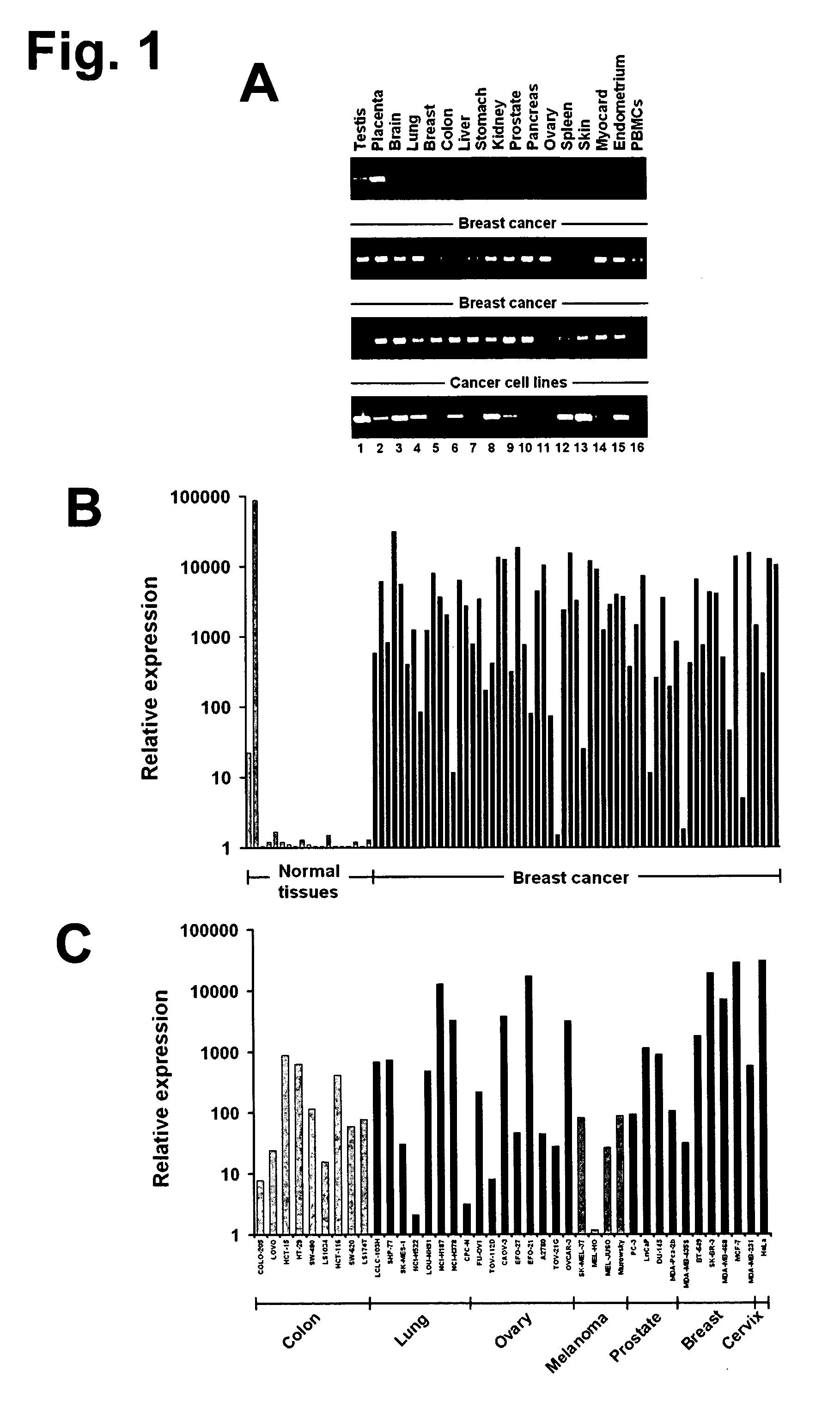
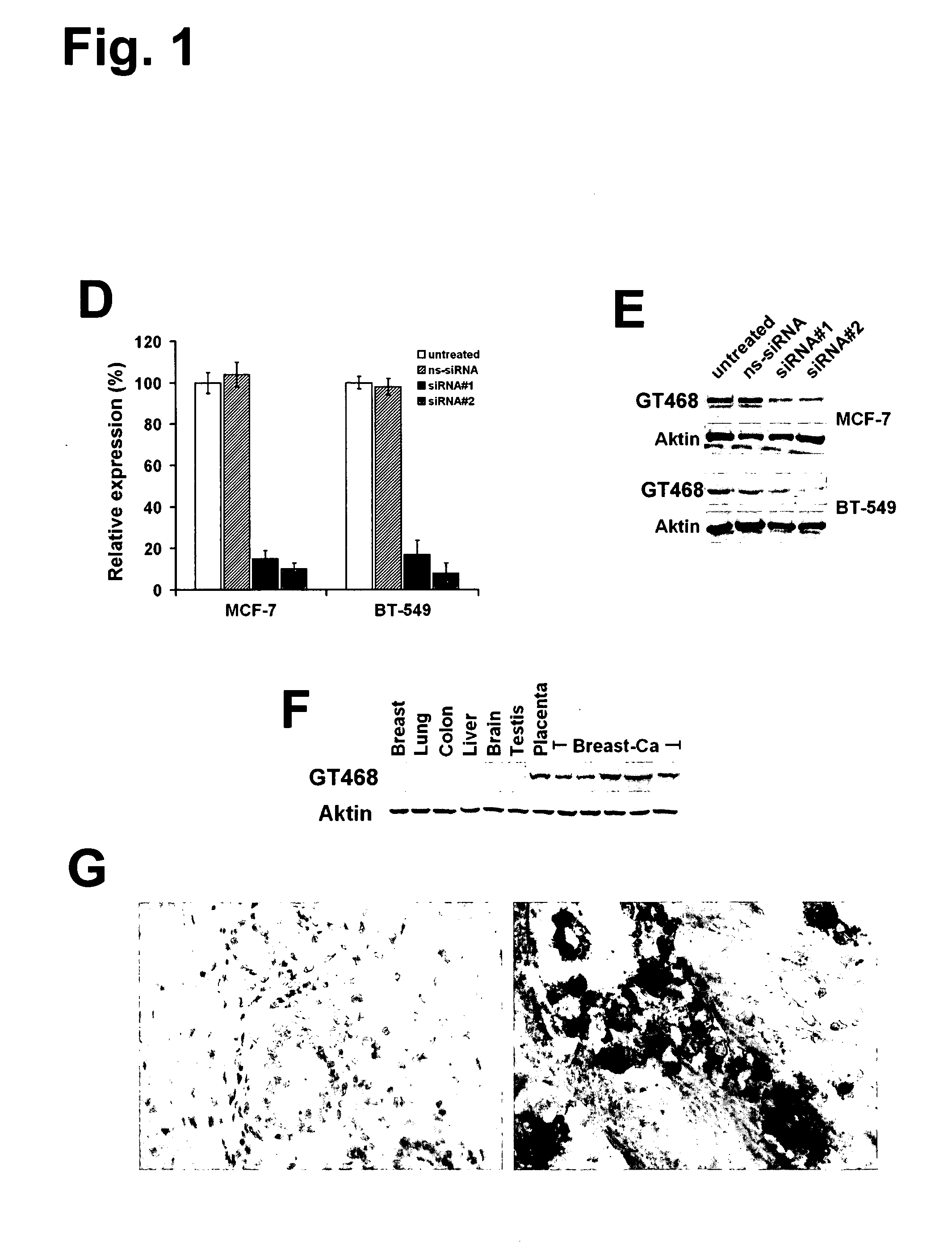
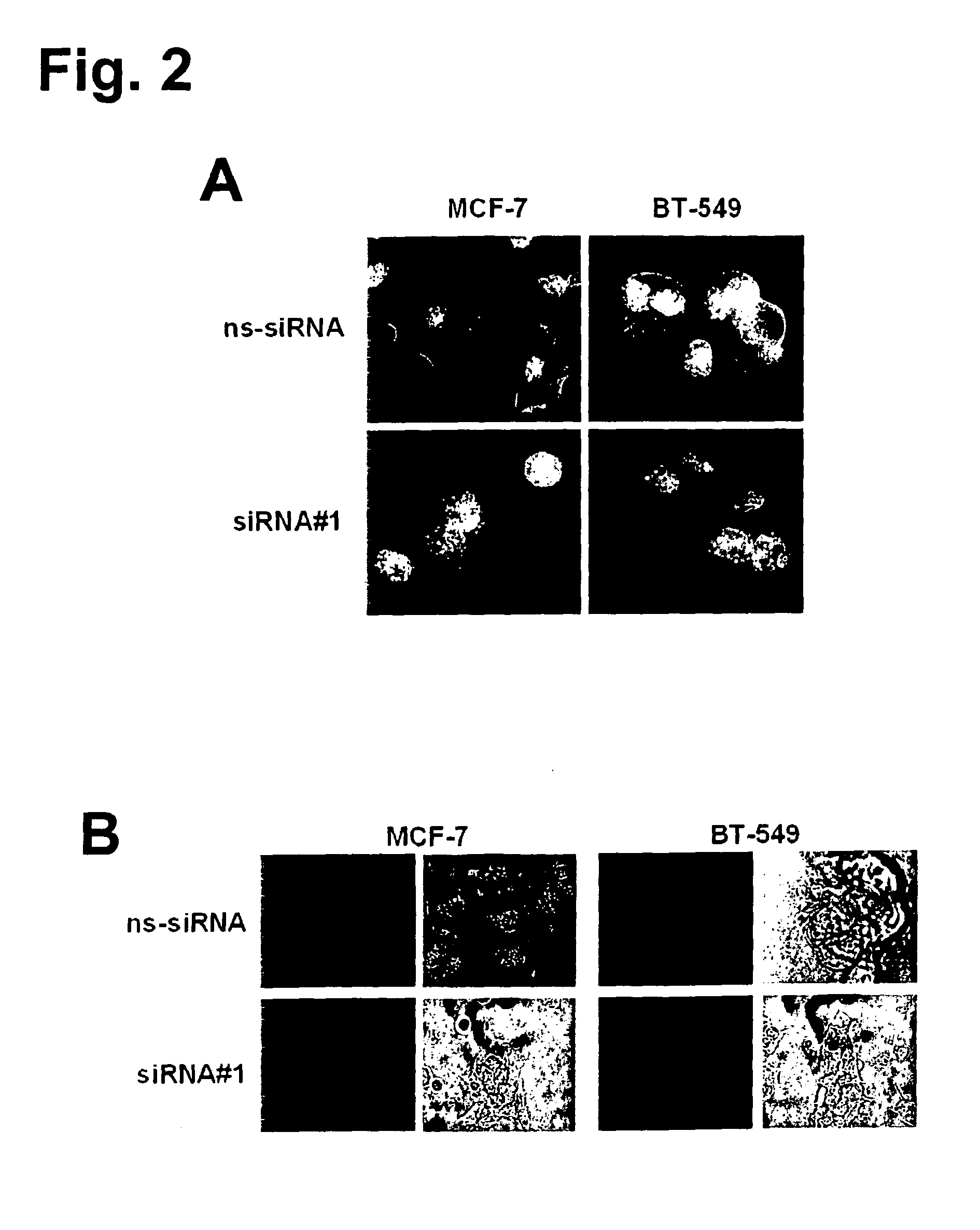
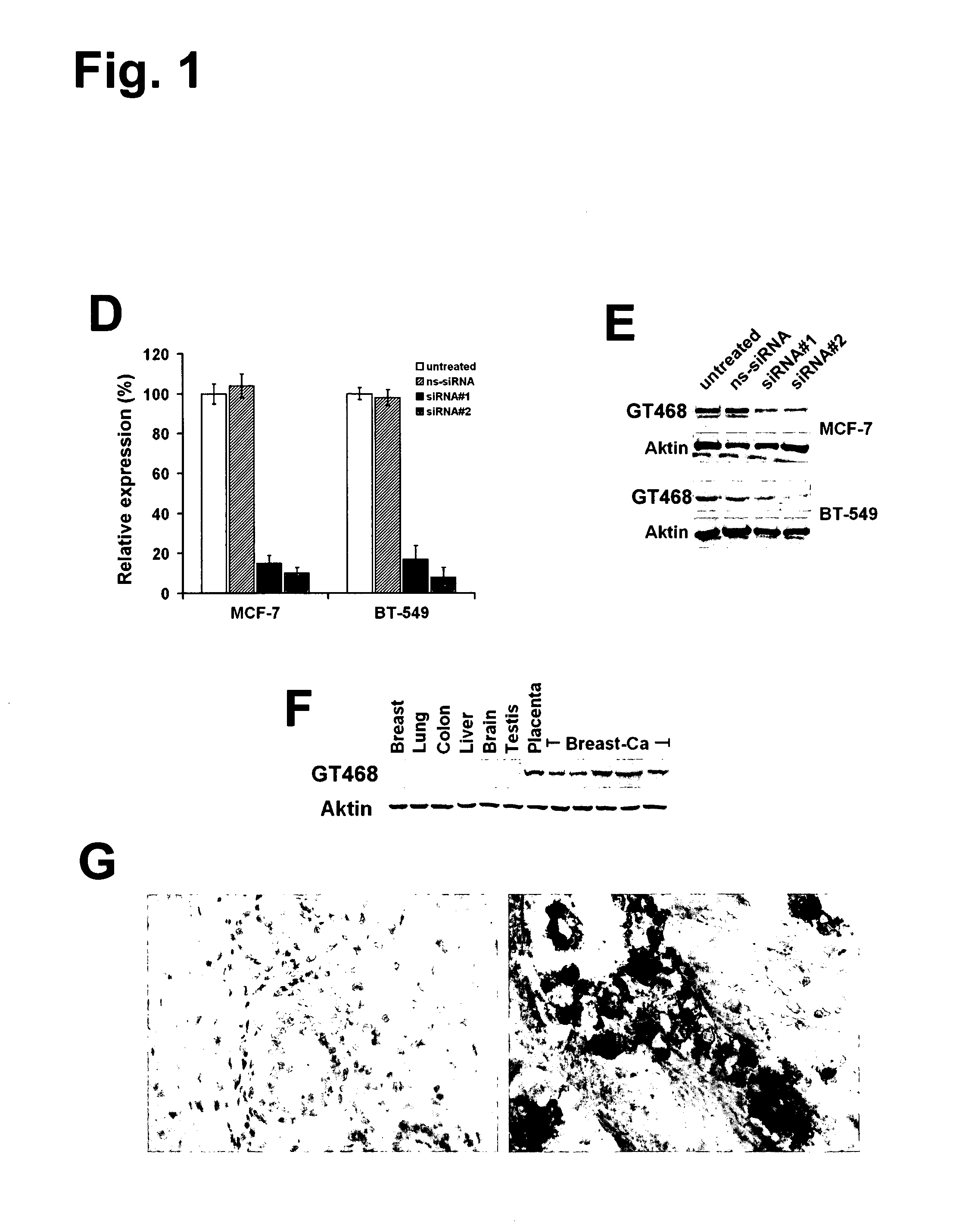
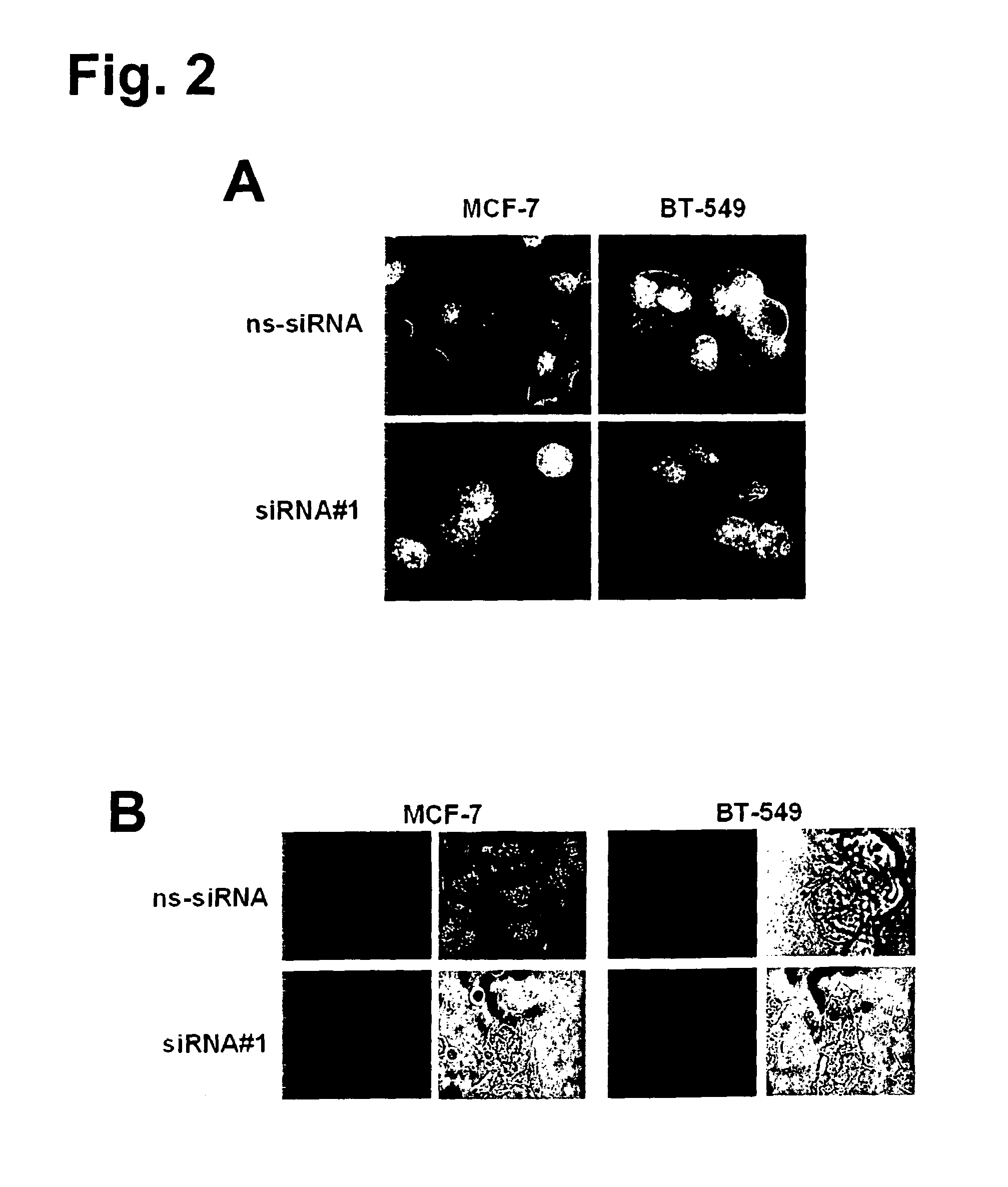
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast Cancer, lung Cancer, gastric Cancer, ovarian Cancer, hepatocellular Cancer, colon Cancer, pancreatic Cancer, esophageal Cancer, head & neck Cancer, kidney Cancer, in particular renal cell Carcinoma, prostate Cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid Cancer, and the metastatic forms thereof. In one embodiment, the rumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
Salicylamide antitumor compound and its synthesis method and application
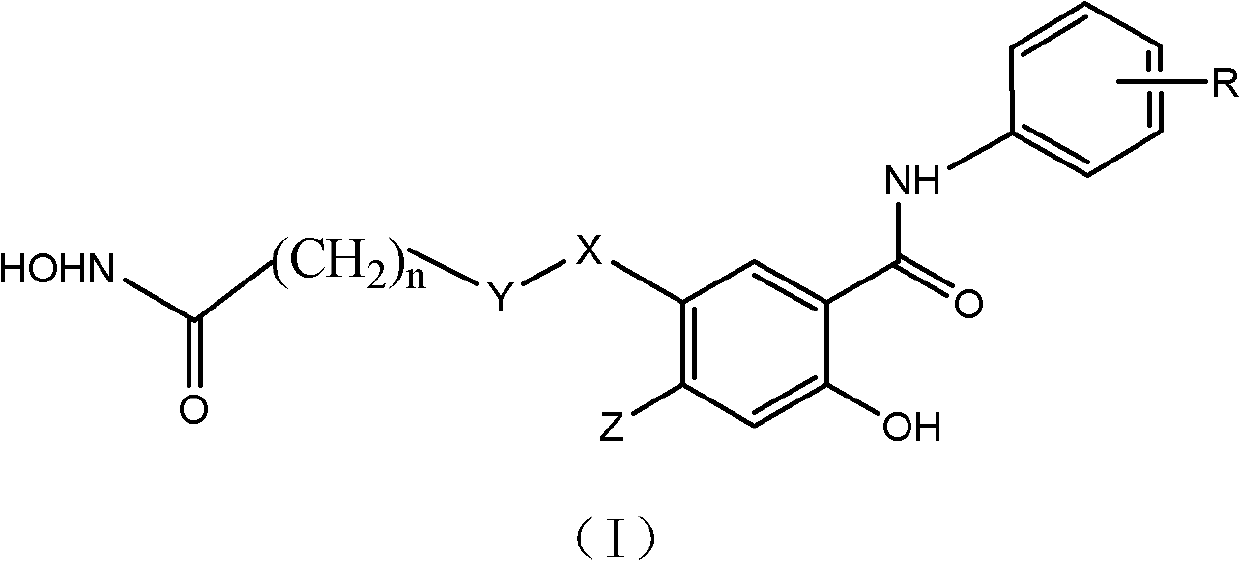
InactiveCN102276500ASimple structureThe synthesis method is simpleOrganic active ingredientsOrganic chemistryTrifluoromethylCarbonyl group
The invention discloses a class of compounds with antitumor activity. The structural formula is as follows: In the formula, R is one or two substituents of ethynyl, chlorine, fluorine, trifluoromethyl and 3-fluorobenzyloxy; X is O or NH; Y is CH2 or CO (carbonyl); Z is H or methoxy; n=5 or 6. The salicylic amide compounds in the structural formula are synthesized from 5-nitrosalicylic acid or 4-methoxysalicylic acid and aniline derivatives through amidation, reduction, and reaction with monoethyl pimelic acid chloride. . The compound has a novel structure and the synthesis method is easy to realize. The anti-tumor activity test showed that it has inhibitory effect on the growth of human liver cancer cells (HepG-2), human colon cancer (SW 480) and human prostate cancer cells (PC3), and the activity of most compounds is stronger than that of gefitinib. The use of preparing anti-tumor drug preparations.
Owner:XI AN JIAOTONG UNIV
Monoclonal antibodies for treatment of cancer
ActiveUS20130071325A1Minimize adverse effectsStrong formationAnimal cellsIn-vivo radioactive preparationsDiseaseMelanoma
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
Targeted Osmotic Lysis of Cancer Cells
ActiveUS20130184218A1Effective treatmentHigh expressionBiocideElectrotherapyNon cancerSmall-cell carcinoma
A targeted osmotic lysis (TOL) of tumor cells that over-express voltage-gated sodium channels (VGSCs) has been developed that uses a combined therapy of a drug that blocks sodium, potassium-adenosine triphosphatase (Na+, K+-ATPase) that is then followed by an activation of VGSCs, for example, by electrical or pharmacological stimulation. Activation of VGSCs conducts sodium into the cancer cells in much greater amounts than non-cancer cells. Water follows this sodium gradient into the cancer cells, causing swelling and lysis. Because non-cancerous cells do not over-express VGSCs, less sodium and less water will enter the cells, and the non-cancerous cells will not lyse. This method is applicable to all cells that over-express VGSCs, including, but not limited to, highly invasive breast cancer, prostate cancer, small cell lung cancer, non-small cell lung carcinoma, lymphoma, mesothelioma, neuroblastoma, and cervical cancer.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Compounds and compositions for treating dysproliferative diseases, and methods of use thereof
Compounds are disclosed with activity towards killing dysproliferative cells in vitro and treating cancer in vivo. Cancers such as cancer of the colon, pancreas, prostate, lung, breast, urinary bladder, skin and liver are exemplary. Compounds, pharmaceutical compositions and methods of use are described.
Owner:CHESTERFORD ENTERPRISES
Monoclonal antibodies for treatment of cancer
ActiveUS9216218B2Strong formationStrong proliferationAnimal cellsHybrid immunoglobulinsDiseaseAntiendomysial antibodies
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
Treatment and diagnosis of cancer
InactiveCN1244921APeptide/protein ingredientsMicrobiological testing/measurementAntigenAntiendomysial antibodies
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of cancerous tissues, particularly cancerous tissues proximate to or containing vascular endothelial cells, which express an extracellular domain of prostate specific membrane antigen. The labeled biological agents can also be used to detect normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate or other cancers. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Pharmaceutical composition
A pharmaceutical composition for intraperitoneal delivery of an anti-neoplastic agent is provided for treating cancers associated with aberrant mucin expression, preferably ovarian cancer and pancreatic, prostate, metastatic breast, bladder and lung cancers. The composition comprises nanomicelles loaded with the anti-neoplastic agent, and antibodies such as anti-MUC16, anti-MUC1 or anti-MUC4 are conjugated to these nanomicelles. The antibody-bound nanomicelles are optionally embedded in a biodegradable pH- and thermo-responsive hydrogel capable of sol-gel transition at body temperature. The pharmaceutical composition is implantable in the peritoneum, where it transforms into a semi-solid gel at the body's core temperature. In response to pH, the hydrogel swells and releases the antibody-bound nanomicelles. The nanomicelles specifically target mucin antigens on cancer cells. The anti-mucin antibodies can be internalized by the tumour cells, enabling the drug-loaded nanomicelles to gain entry and deliver the chemotherapeutic drugs inside the tumour cell.
Owner:UNIVERSITY OF THE WITWATERSRAND
Isoflavonoid analogs and their metal complexes as anti-cancer agents
InactiveUS20070122843A1Lipophilic natureCellular internalization is improvedBiocideOrganic compound librariesAnticarcinogenPharmacophore
A pharmacologic agent for treating and / or preventing cancer, among other diseases and conditions, and particularly breast, prostate, and pancreatic cancer, in humans and animals. The novel pharmacologic agent is an isoflavonoid or isoflavonoid mimetic covalently attached to a cytotoxic pharmacophore that, preferably has the ability to conjugate with a metal salt to form a more potent metal complex, particularly a Cu(II) complex. The isoflavonoid or isoflavonoid mimetic may be non-fragmented steroidal hormone, such as progesterone which is structurally related to the isoflavone genistein, or a small molecule hormone mimetic, such as chromone. An illustrative non-fragmented steroidal embodiment is 17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,13,14,15,16,17-tetradecahydrocyclopenta[a]phenantnren-3-thiosemicarbazone and its Cu(II) complex. Effective chromone analogs include the thiosemicarbazone and hydrazone analogs of 4-oxo-4H-chromene-3-carboxaldehyde and their Cu(II) complexes.
Owner:SARKAR FAZLUL +1
Application of disubstituted phenyl biguanide to inducing malignant tumor cell for anoikis
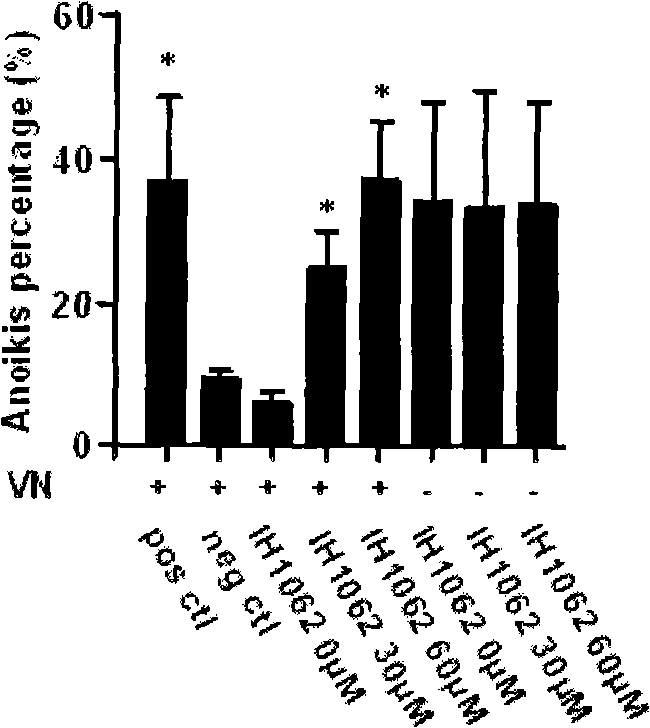
InactiveCN101862315AOvert anoikisGrowth inhibitionOrganic active ingredientsAntineoplastic agentsLymphatic SpreadProstate cancer
The invention relates to disubstituted phenyl biguanide compounds and application containing the same to inducing malignant tumor cell for anoikis and inhibiting tumor growth and transfer. Proved by in vitro-in vivo experiments, the disubstituted phenyl biguanide compounds show the remarkable functions of inducing the malignant tumor cell for anoikis, starting caspase cascade and inhibiting tumor growth and transfer and also have the characteristics of high efficiency and low toxicity, so the disubstituted phenyl biguanide compounds as pharmaceutical compounds are expected to be applied to a plurality of malignant tumor medicaments for inhibiting the tumor growth, invasion and metastasis and treating human melanoma, neurospongioma, lung cancer, gastric cancer, prostate cancer, ovarian cancer and the like.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
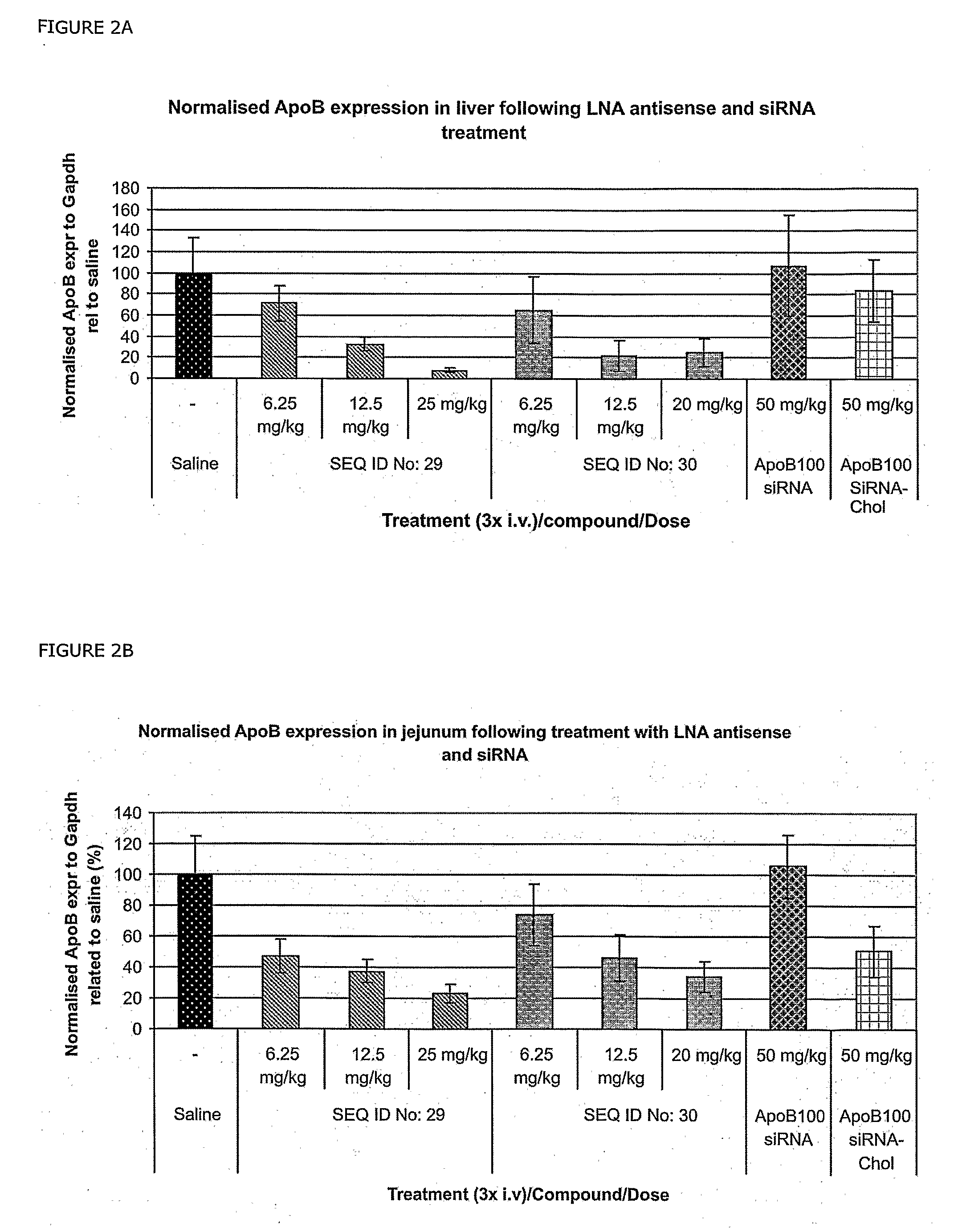
RNA antagonist compounds for the inhibition of apo-b100 expression
InactiveUS20090118213A1Lower Level RequirementsOrganic active ingredientsSugar derivativesIntestinal structureSpinal cord
Oligonucleotides directed against the Apo-B100 gene are provided for modulating the expression of Apo-B100. The compositions comprise oligonucleotides, particularly antisense oligonucleotides, targeted to nucleic acids encoding the Apo-B100. Methods of using these compounds for modulation of Apo-B100 expression and for the treatment of diseases associated with either overexpression of Apo-B100, expression of mutated Apo-B100 or both are provided. Examples of diseases are cancer such as lung, breast, colon, prostate, pancreas, lung, liver, thyroid, kidney, brain, testes, stomach, intestine, bowel, spinal cord, sinuses, bladder, urinary tract or ovaries cancers. The oligonucleotides may be composed of deoxyribonucleosides or a nucleic acid analogue such as for example locked nucleic acid or a combination thereof.
Owner:SANTARIS PHARMA AS
Isolation of N-butylbenzenesulfonamide, synthesis of benzenesulfonamide derivatives, and use of N-butylbenzenesulfonamide and benzenesulfonamide derivatives for treating benign prostatic hyperplasia and/or prostate carcinoma
A process for isolating N-butylbenzenesulfonamide (NBBS) from biological material, the chemical synthesis of benzenesulfonamide derivatives, the use of NBBS and benzenesulfonamide derivatives for treating benign prostatic hyperplasia and / or prostate carcinoma, the production of medicaments for the treatment thereof, and the use of NBBS and benzenesulfonamide derivatives as a lead substance in the development of active substances for treating benign prostatic hyperplasia and / or prostate carcinoma are provided.
Owner:LTS LOHMANN THERAPIE-SYST AG
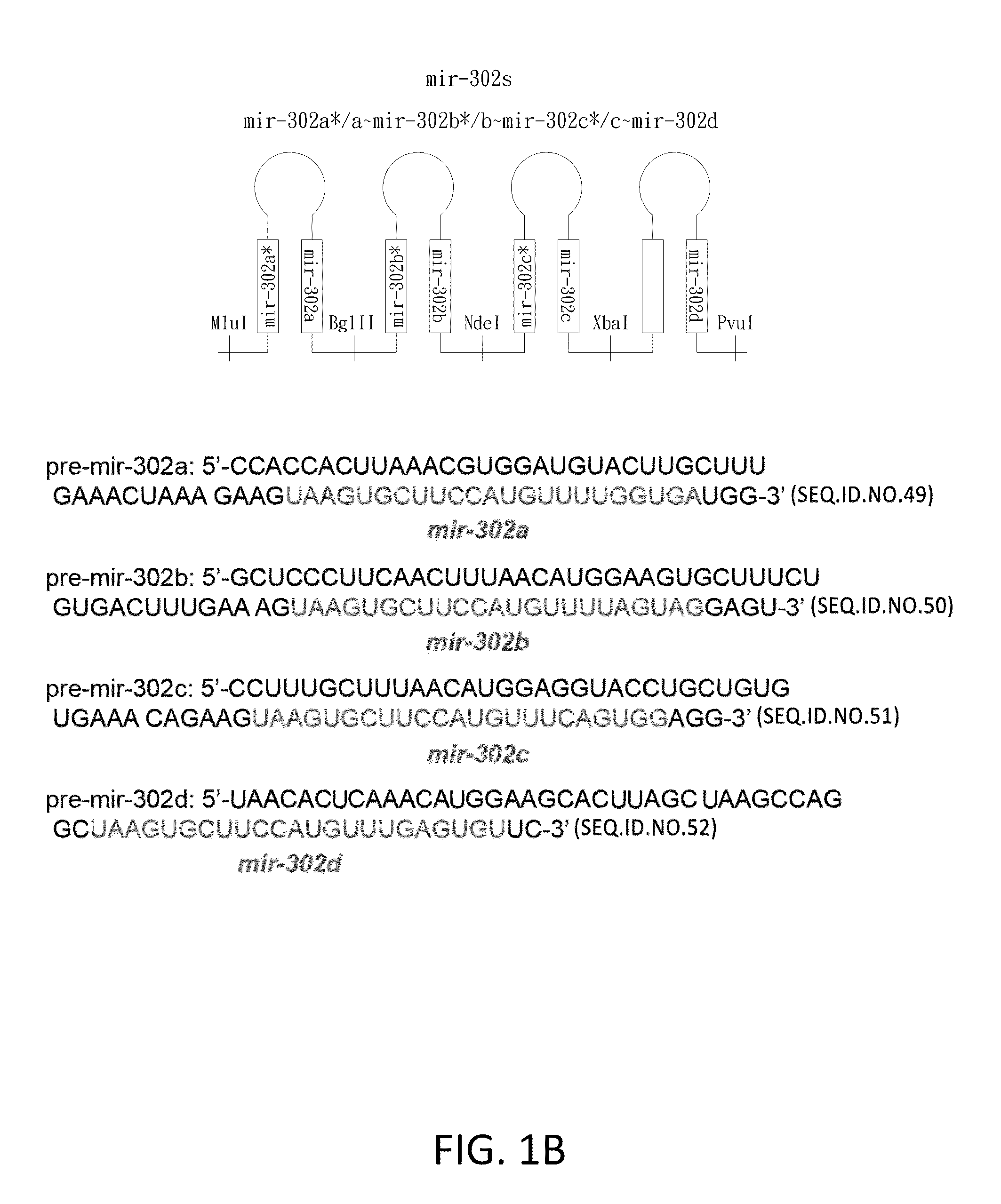
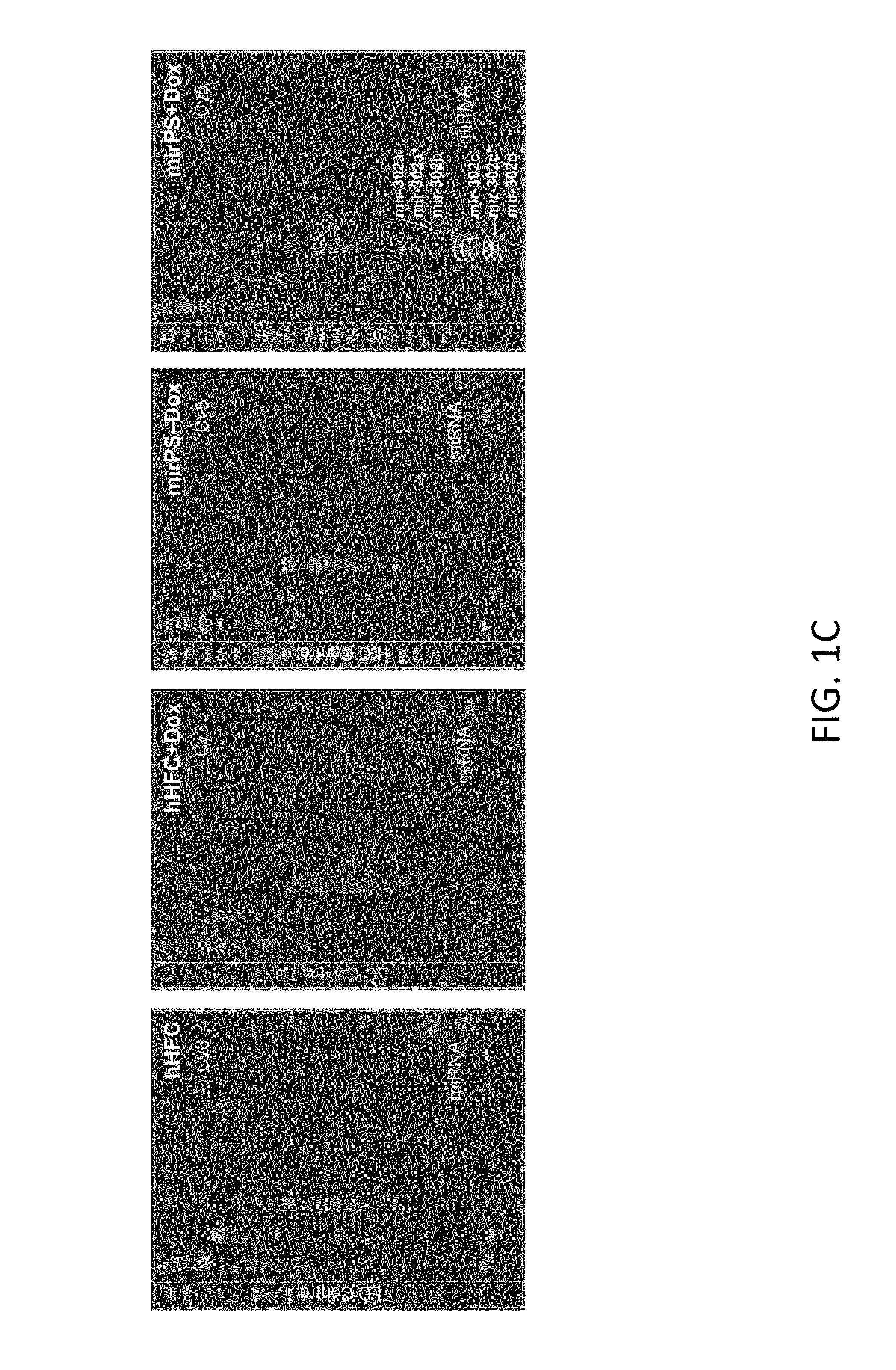
Development of universal cancer drugs and vaccines
ActiveUS9394538B2Reduce the number of copiesStrong specificitySugar derivativesMicrobiological testing/measurementLymphatic SpreadEmbryo
This invention generally relates to a design and method for developing novel anti-tumor / cancer drugs, vaccines and therapies, using microRNA (miRNA) and its shRNA homologues / derivatives. More particularly, the present invention relates to the use of a nucleic acid composition capable of expressing mir-302-like gene silencing effectors upon delivery into human cells and then silencing mir-302-targeted cell cycle regulators and oncogenes, resulting in an inhibitory effect on tumor / cancer cell growth and metastasis. Mir-302 is the most predominant miRNA found in human embryonic stem (hES) and induced pluripotent stem (iPS) cells, yet its function is unclear. The present invention establishes that in humans mir-302 concurrently suppressed both cyclin-E-CDK2 and cyclin-D-CDK4 / 6 pathways and eventually blocked over 70% of the G1-S transition. Simultaneously, mir-302 also silences BMI-1, a cancer stem cell marker, and subsequently promotes the tumor suppressor functions of p16Ink4a and p14 / p19Arf in inhibiting CDK4 / 6-mediated cell proliferation. Therefore, the present invention for the first time reveals the tumor suppressor function of mir-302 in humans. This novel finding advances the design and method for developing new cancer drugs, vaccines and therapies directed against multiple kinds of human tumors and cancers, in particular including, but not limited, malignant skin, prostate, breast and liver cancers as well as various tumors.
Owner:LIN SHI LUNG +1
Application of yew amylose in pharmacy
InactiveCN1957944AEnhance immune functionImprove enduranceOrganic active ingredientsImmunological disordersCurative effectProstate carcinoma
Owner:宁波泰康红豆杉生物工程有限公司
Treatment of prostate carcinoma
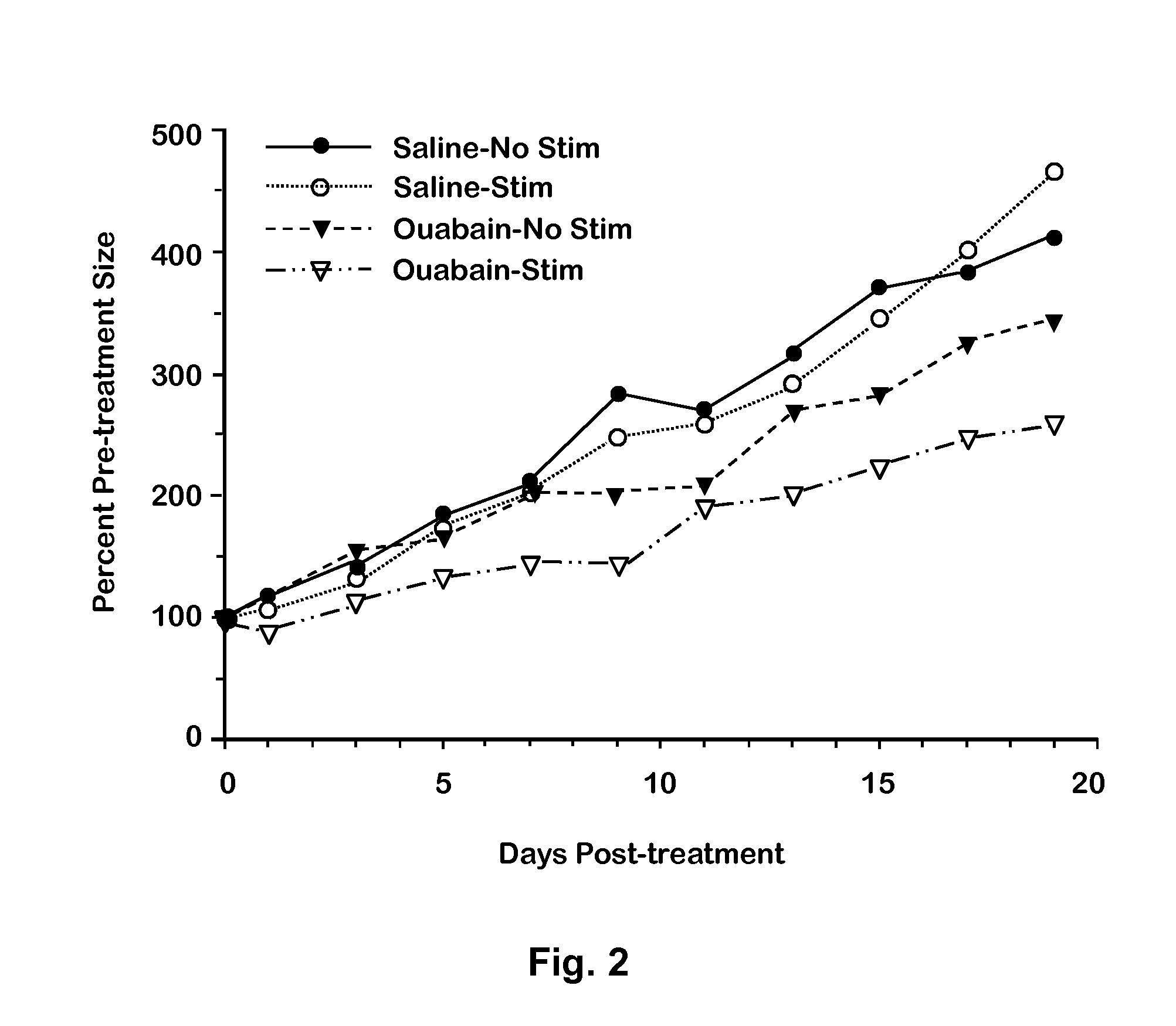
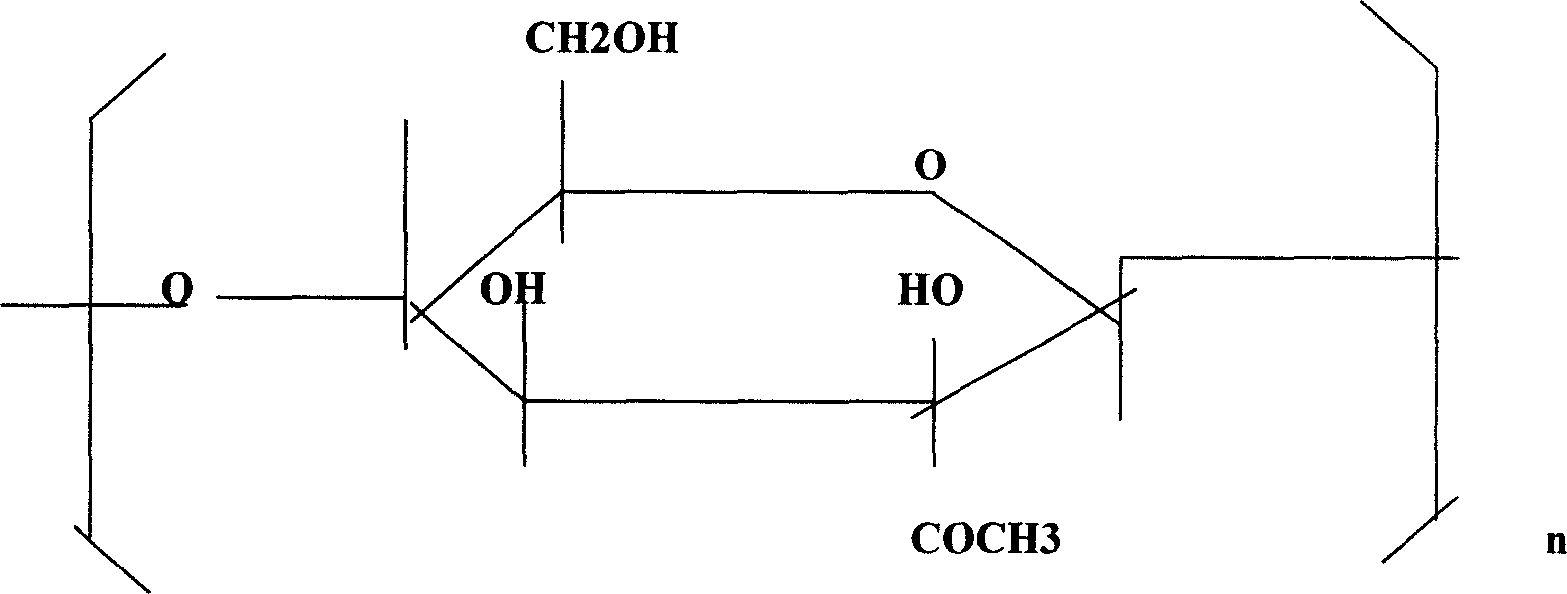
ActiveUS9132105B2Inhibiting and delaying growthReduce the amount requiredBiocideHydroxy compound active ingredientsMedicineProstate carcinoma
Owner:PELLFICURE PHARMA
Method and kit for distinguishing between prostate carcinoma and benign prostatic hyperplasia
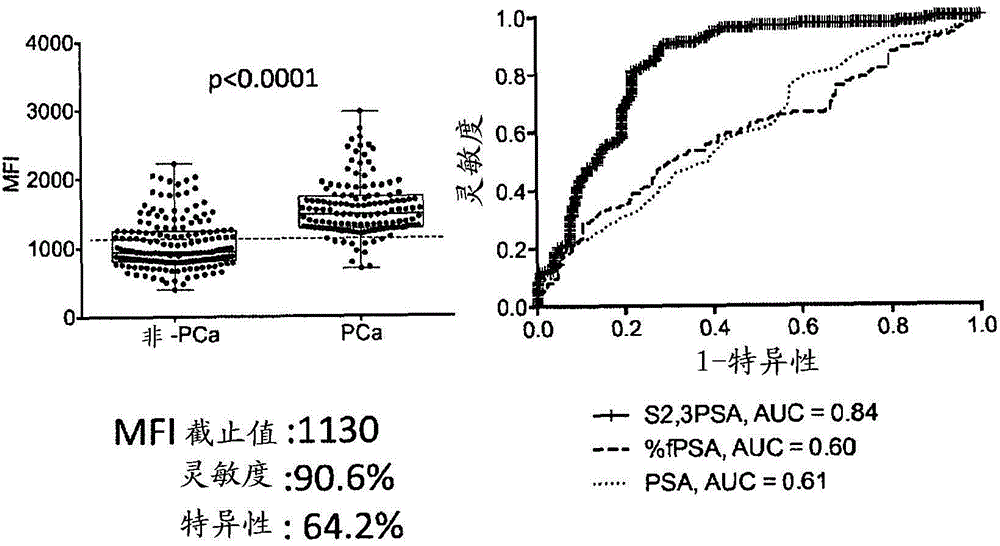
The present invention addresses the problem of providing a method for distinguishing between prostate carcinoma and benign prostatic hyperplasia with high sensitivity and good reproducibility using an analyte sample in a small amount. The method for distinguishing between prostate carcinoma and benign prostatic hyperplasia according to the present invention, which is a solution for the problem, is as follows: an analyte sample containing a prostate-specific antigen (PSA) is brought into contact with a carrier having an anti-free PSA antibody immobilized thereon, thereby causing the binding of a free PSA to the anti-free PSA antibody immobilized on the carrier; the carrier in which the free PSA has been bound to the immobilized anti-free PSA antibody is brought into contact with a monoclonal antibody capable of specifically recognizing a sugar chain in which a terminal sialic acid residue is bound to galactose via an α(2,3) bond, thereby causing the binding of the monoclonal antibody capable of specifically recognizing the sugar chain in which the terminal sialic acid residue has been bound to galactose via an α(2,3) bond to the free PSA that has been bound to the anti-free PSA antibody immobilized on the carrier; the amount of the free PSA that has an N-type sugar chain in which the terminal sialic acid residue has been bound to galactose via an α(2,3) bond is measured; and subsequently the amount measured in the proceeding step is compared with a preset cut-off value for prostate carcinoma and benign prostatic hyperplasia. When the measured amount is larger than the cut-off value, it is determined that prostate carcinoma is developed or the probability of prostate carcinoma is high. When the measured amount is smaller than the cut-off value, it is determined that benign prostatic hyperplasia is developed or the probability of benign prostatic hyperplasia is high.
Owner:HIROSAKI UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com