Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87 results about "Management of prostate cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
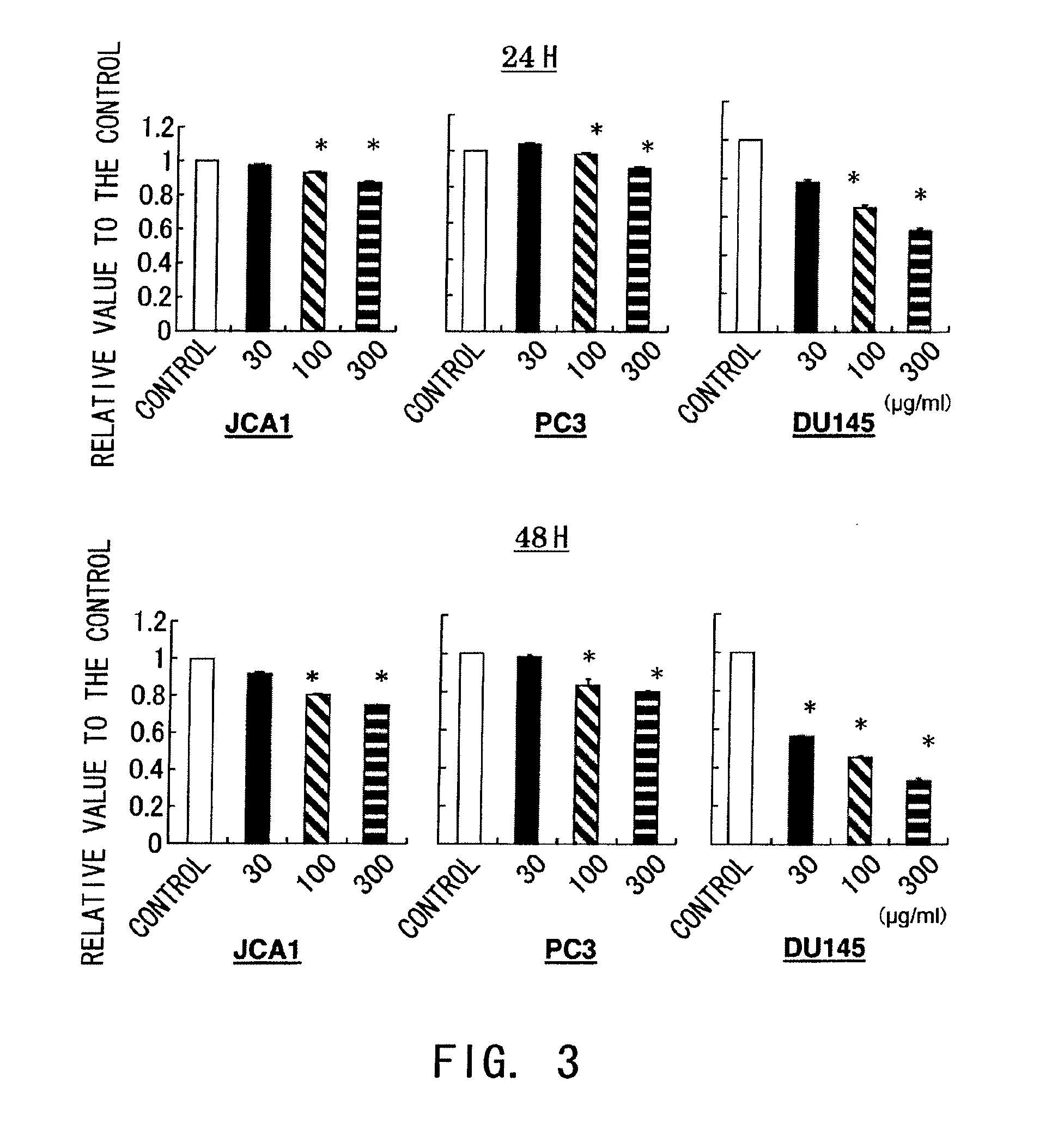
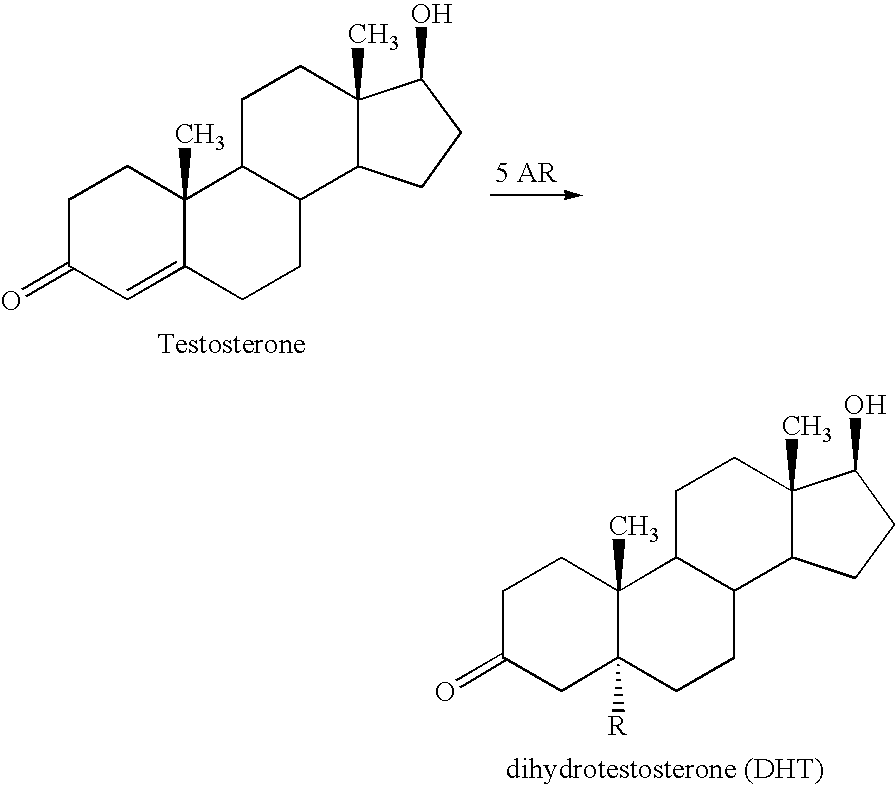
Treatment for prostate cancer may involve active surveillance, surgery, radiation therapy - including brachytherapy (prostate brachytherapy) and external-beam radiation therapy, proton therapy, high-intensity focused ultrasound (HIFU), cryosurgery, hormonal therapy, chemotherapy, or some combination. Treatments also extend to survivorship based interventions. These interventions are focused on five domains including: physical symptoms, psychological symptoms, surveillance, health promotion and care coordination.. However, a published review has found only high levels of evidence for interventions that target physical and psychological symptom management and health promotion, with no reviews of interventions for either care coordination or surveillance.. The favored treatment option depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors include the man's age, his general health, and his feelings about potential treatments and their possible side-effects. Because all treatments can have significant side-effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
Treatment and diagnosis of prostate cancer
InactiveUS7666425B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Therapeutic agent for prostate cancer
InactiveUS20090269335A1Confirm in antitumor effectTumor growth suppressedAntibody ingredientsImmunoglobulinsIL-2 receptorIn vivo
The present inventors investigated the antitumor effects of anti-IL-6 receptor antibodies against prostate cancer. The result showed that the anti-IL-6 receptor antibodies had both in vivo and in vitro antitumor effects against prostate cancer. It was also revealed that the hPM1 antitumor effect is via IL-6 receptor.
Owner:CHUGAI PHARMA CO LTD
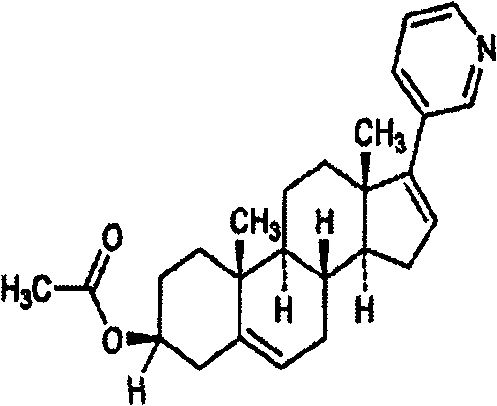
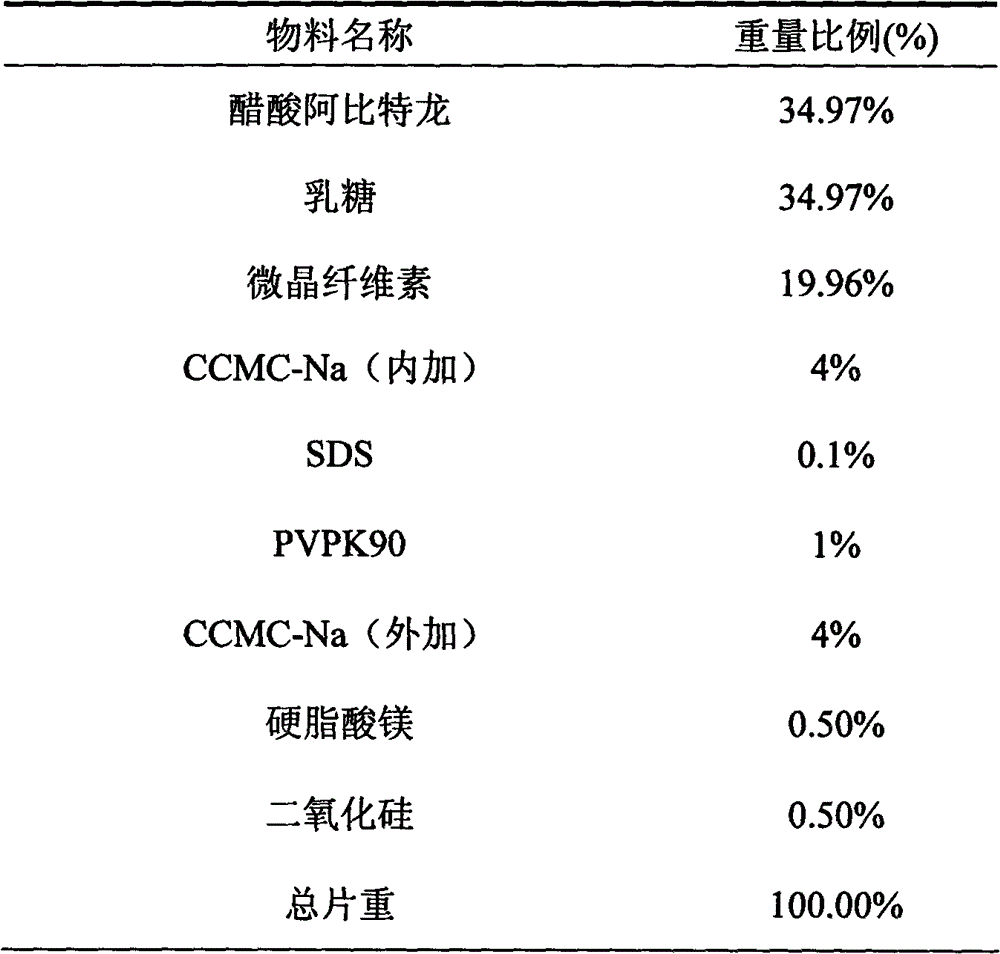
Medicinal composition containing abiraterone acetate and preparation technology thereof
The invention belongs to the technical field of medicine, and particularly relates to a medicinal composition containing abiraterone acetate and a preparation technology of the medicinal composition. The medicinal composition comprises abiraterone acetate, a solubilizer, a binder and a disintegrating agent and can be prepared into tablets, granules or capsules. The invention is characterized in that the abiraterone acetate and hydrophilic auxiliary materials are crushed based on a proportion, and the dissolution rate and bioavailability of the medicine are effectively improved, the medicinal composition can be used for treating the prostate cancer.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Treatment and diagnosis of prostate cancer
InactiveUS7112412B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Prostatic hormonal implants treatment of prostate cancer
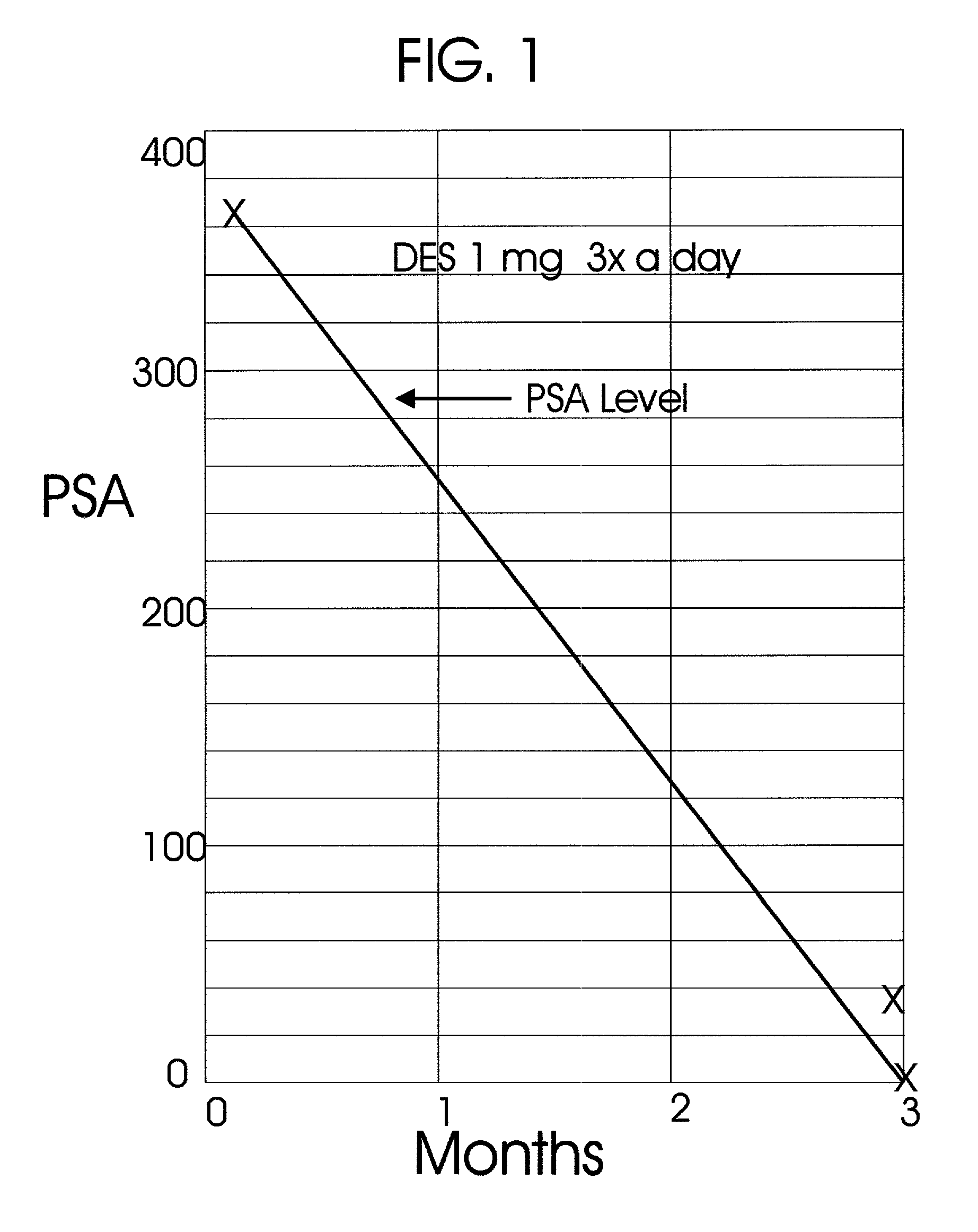
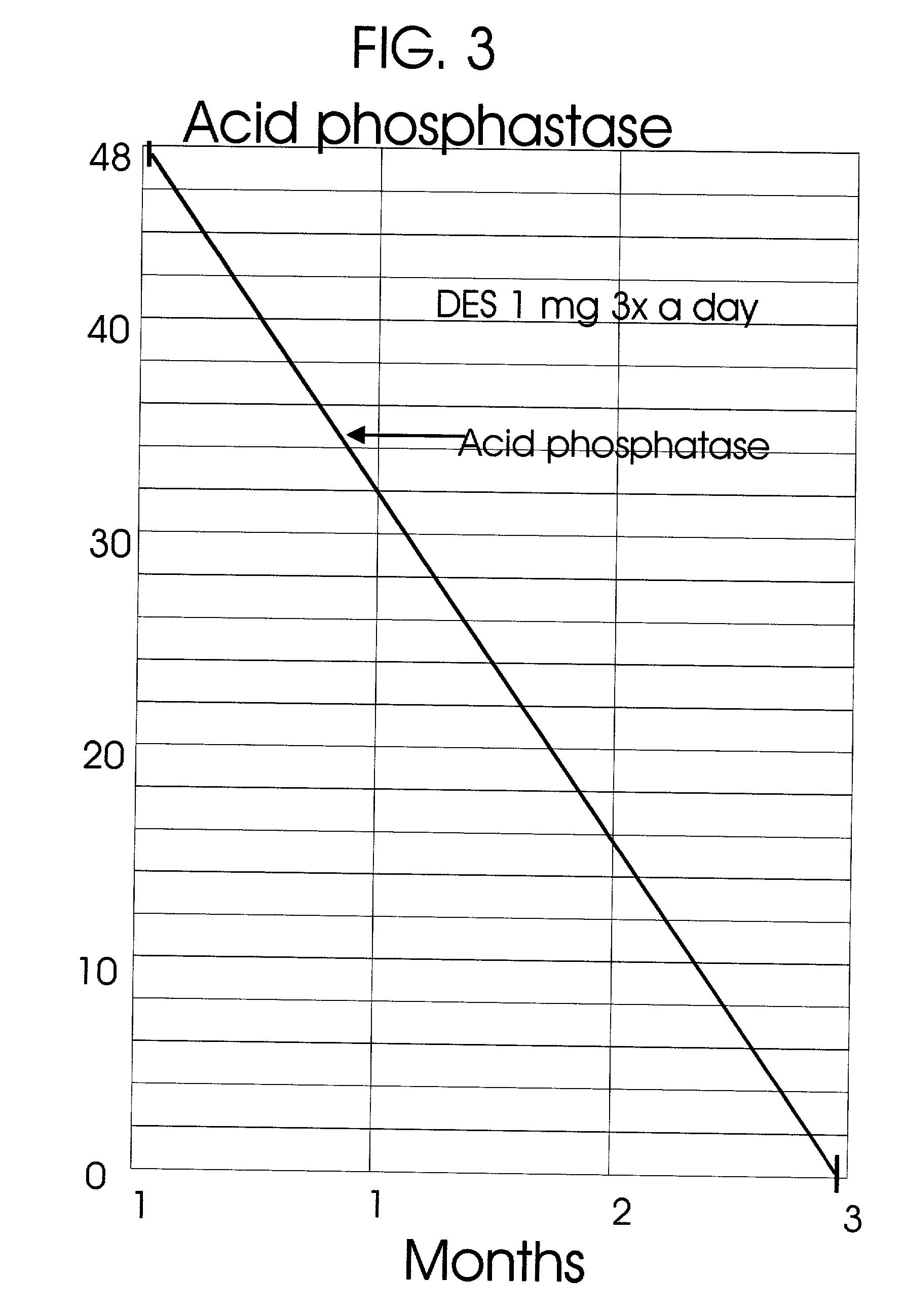
An improved method and products for the primary hormonal treatment of early stage, low and intermediate risk prostate cancers by prostatic implants of androgen suppressive drugs formulated as fused with a lipoid carrier or encapsulated in microcapsules or in Silastic capsules is provided. Such prostatic implants renders a constant slow-release of their contents to the prostate for extended periods by biodegradation and diffusion. It facilitates higher prostatic and lower systemic concentrations of androgen suppressive hormones. Because of their high prostatic and lower systemic concentrations, tumor control is much improved and the their systemic toxicity is minimized. Tumor control after such primary hormonal implant treatment is followed by clinical examinations and the biochemical tumor control is followed by periodic estimations of serum levels of PSA and acid phosphatase. More complex and expensive surgery or radiation therapy for this group of good prognostic early stage prostate cancer is reserved for those patients failing to this primary hormonal treatment. It will preserve potency more than by surgery or radiation therapy. Furthermore, it would reduce the cost of treatment for early stage prostate cancer significantly. Androgen suppressive hormonal implants to the prostate before, during or after lower dose conventional radiation therapy would also facilitate equal or better cure rates of localized prostate cancer as compared to the more complex and toxic higher dose radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
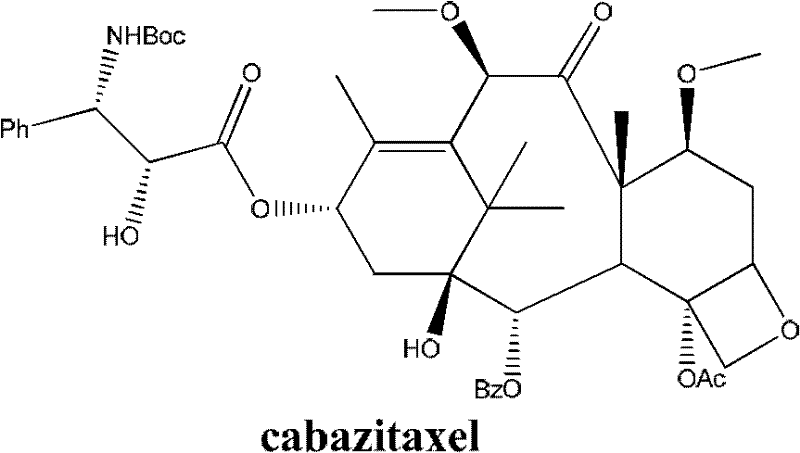
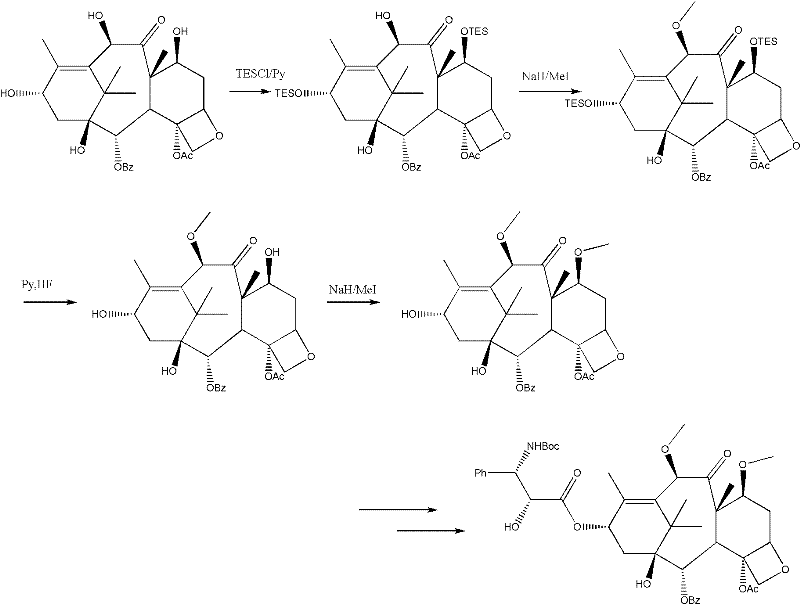
Method for preparing cabazitaxel by taking 10-deacetylate-baccatin III as raw material
ActiveCN102417491AIncrease preparation meteringEasy to operateOrganic chemistryUrinary disorderAcetic acidCabazitaxel
The invention relates to a method for preparing cabazitaxel, particularly relates to a method for preparing the cabazitaxel by taking 10-deacetylate-baccatin III as a raw material and belongs to the technical field of drug synthesis. The method comprises the following steps: firstly, reacting 10-DAB with chlorocarbonate-2,2,2-trichloro ethyl ester, thereby obtaining a product; reacting the product with DMAP (dimethylamino pyridine), DCC (dicyclohexylcarbodiimide) and (4S, 5R)-2,2-dimethyl-4-phenyl-3-tert-butoxycarbonyl-3.5-oxazolidine formic acid, thereby obtaining a product; reacting the product with acetic acid and zinc powder, and then methylating the product; and lastly, adding a p-methyl benzenesulfonic acid, thereby reacting and obtaining a cabazitaxel product. The cabazitaxel prepared according to the method can be widely applied to the treatment of prostate cancer. Compared with a traditional technique for preparing the cabazitaxel, the method has the advantages that the preparation quantity is greatly increased, the operation steps are simplified, the manpower is saved and the method has industrial application value and prospect.
Owner:无锡紫杉药业股份有限公司
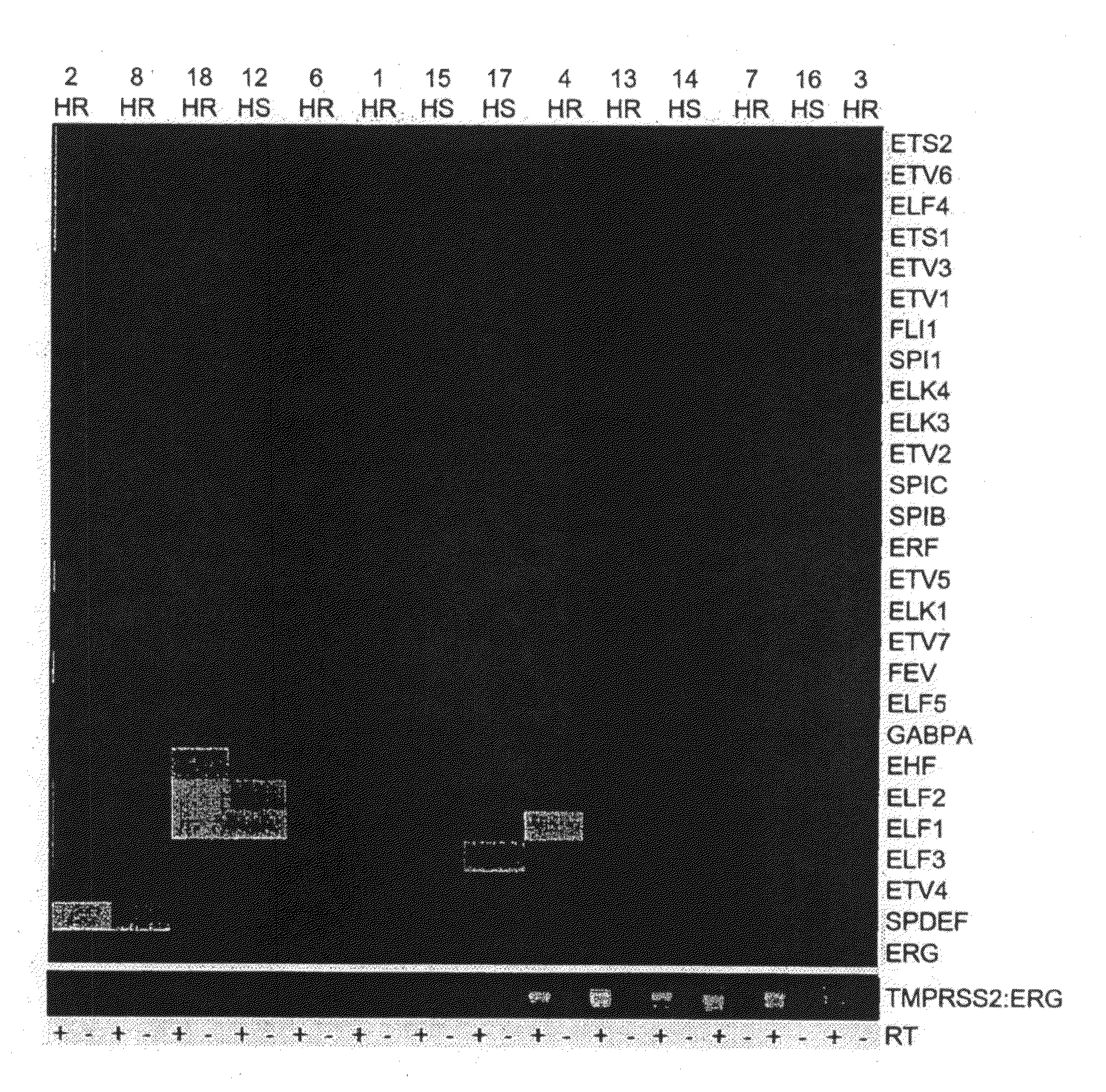
Method for treatment of prostate cancer and screening of patients benefiting from said method
InactiveUS20100215638A1Lower Level RequirementsOrganic active ingredientsPeptide/protein ingredientsAndrogenCurative effect
The invention relates to a method for treating ERG-positive prostate cancer patients with an agent counteracting one or more ERG-associated genes and / or manipulating of one or more ERG-related pathways, optionally in combination with an androgen deprivation therapy. Furthermore, the invention concerns methods for screening prostate cancer patients which may benefit from said treatment, assessing the efficacy of a therapy for treating prostate cancer in a patient, assessing progression of prostate cancer in a patient, selecting an agent to be tested for usefulness in the treatment of prostate cancer, and for assessing prostate carcinogenic potential of an agent.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS
Use of neurotoxin therapy for treatment of urological-neurological disorders associated with prostate cancer
InactiveUS20040180065A1Safe, inexpensive, out-patient methodsSimple methodBacterial antigen ingredientsPeptide/protein ingredientsNeurexinNeurological disorder
The present invention related to methods for treating neurological-urological conditions associated with prostate cancer or methods of treatment of prostate cancer, which is accomplished by administration of botulinum toxin to the patient.
Owner:ALLERGAN INC
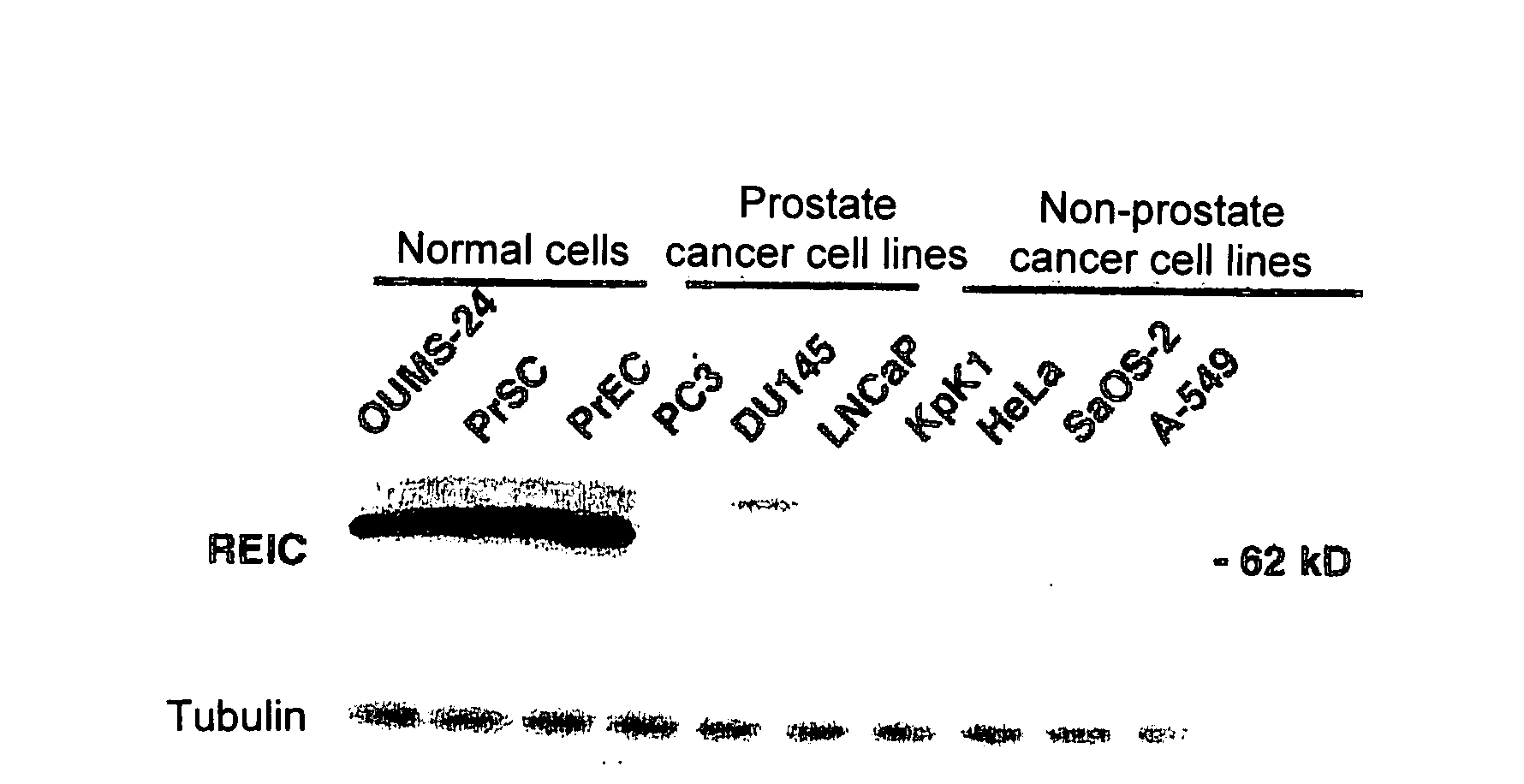
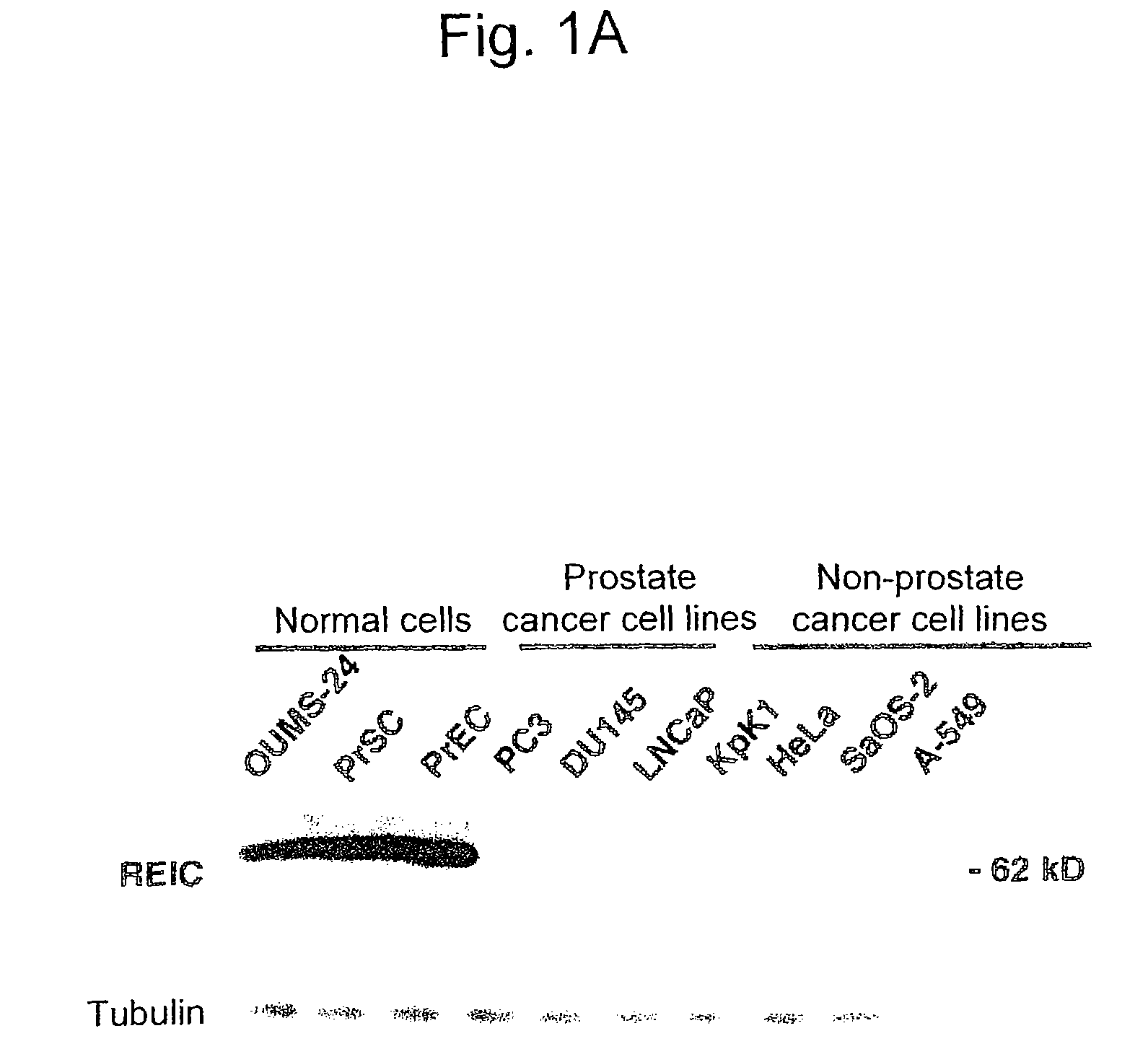
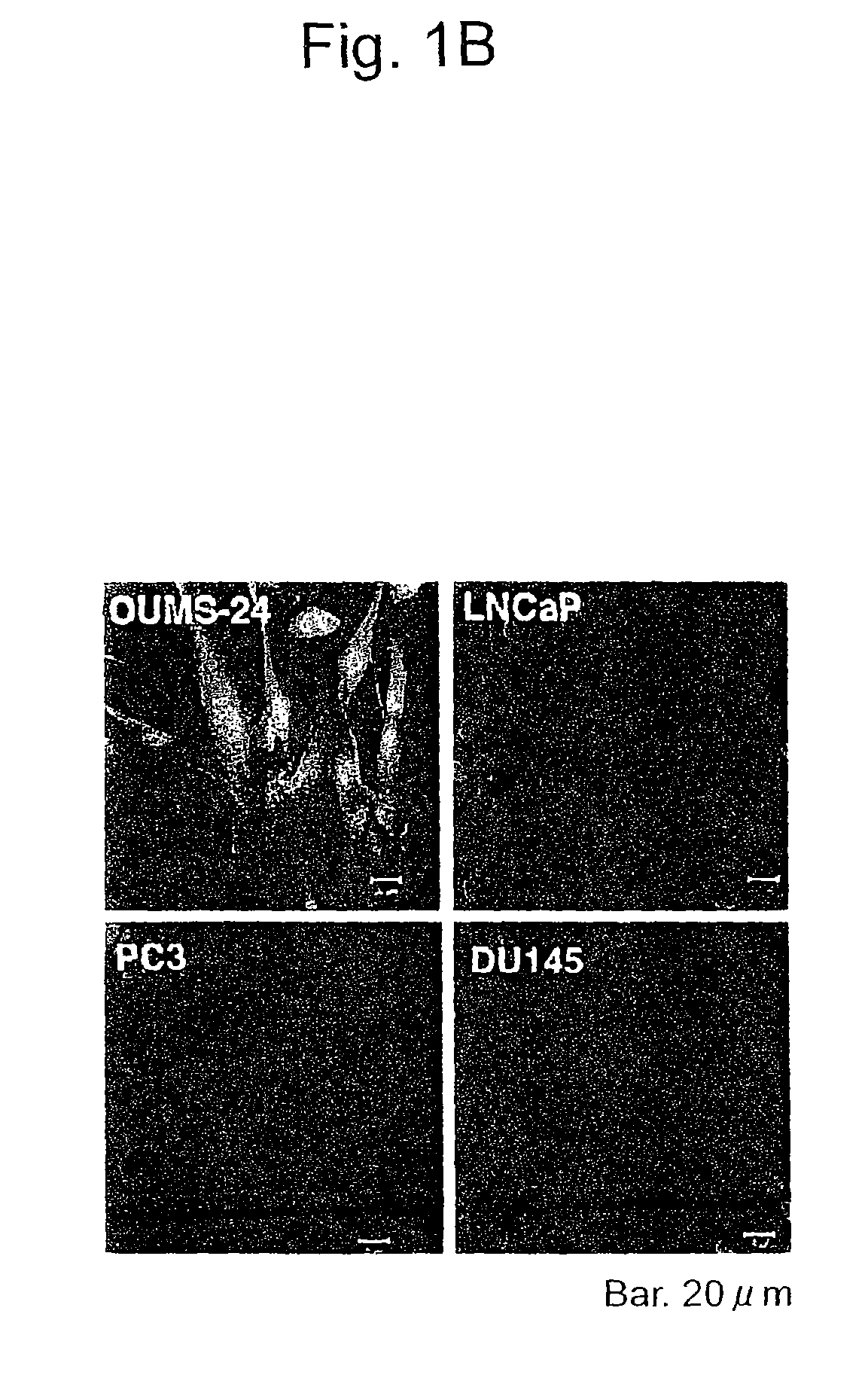
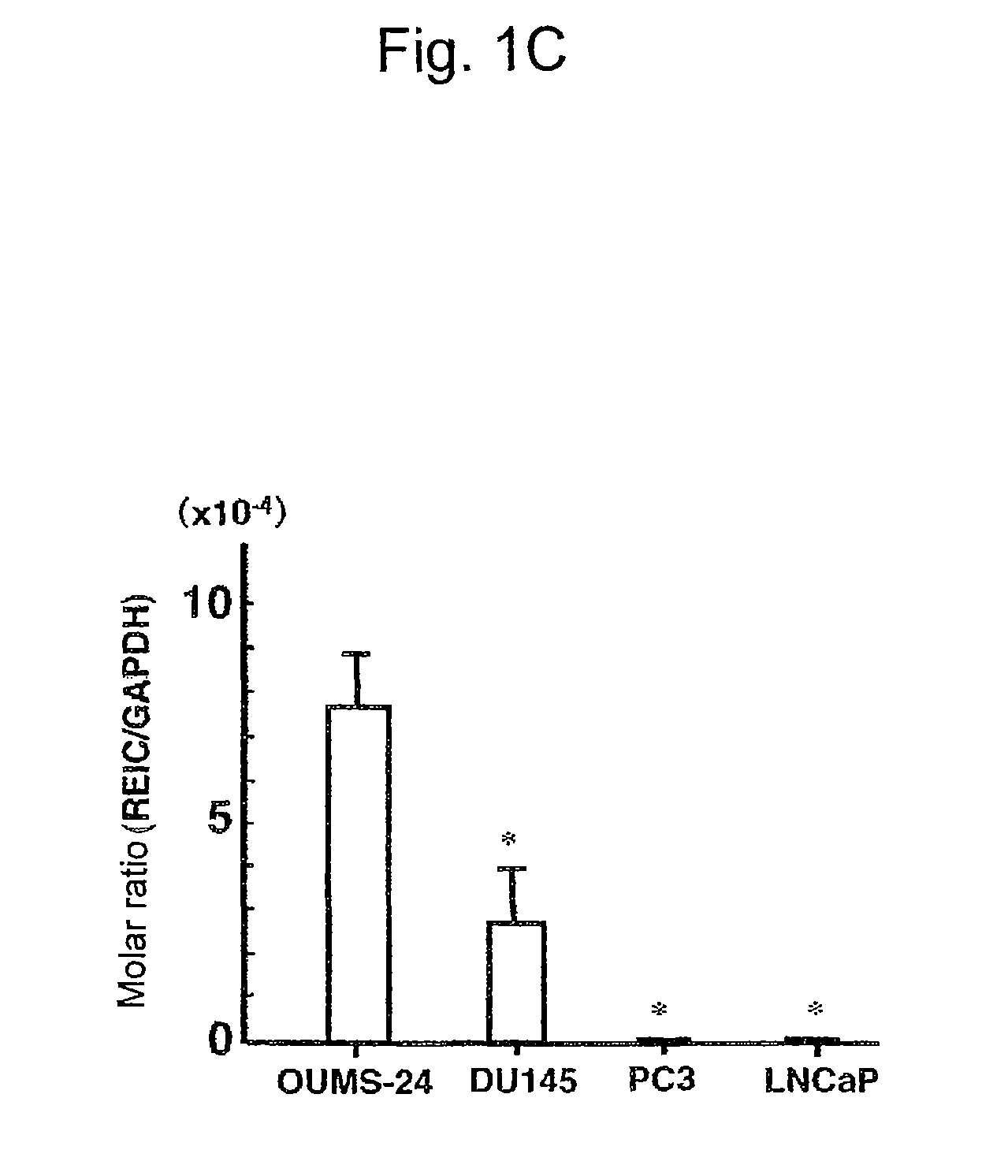
Apoptosis-Inducing Agent for Prostate Cancer Cells
InactiveUS20090005538A1Reduced expression levelInhibit prostate cancerPeptide/protein ingredientsDepsipeptidesProstate cancer cellLymphatic Spread
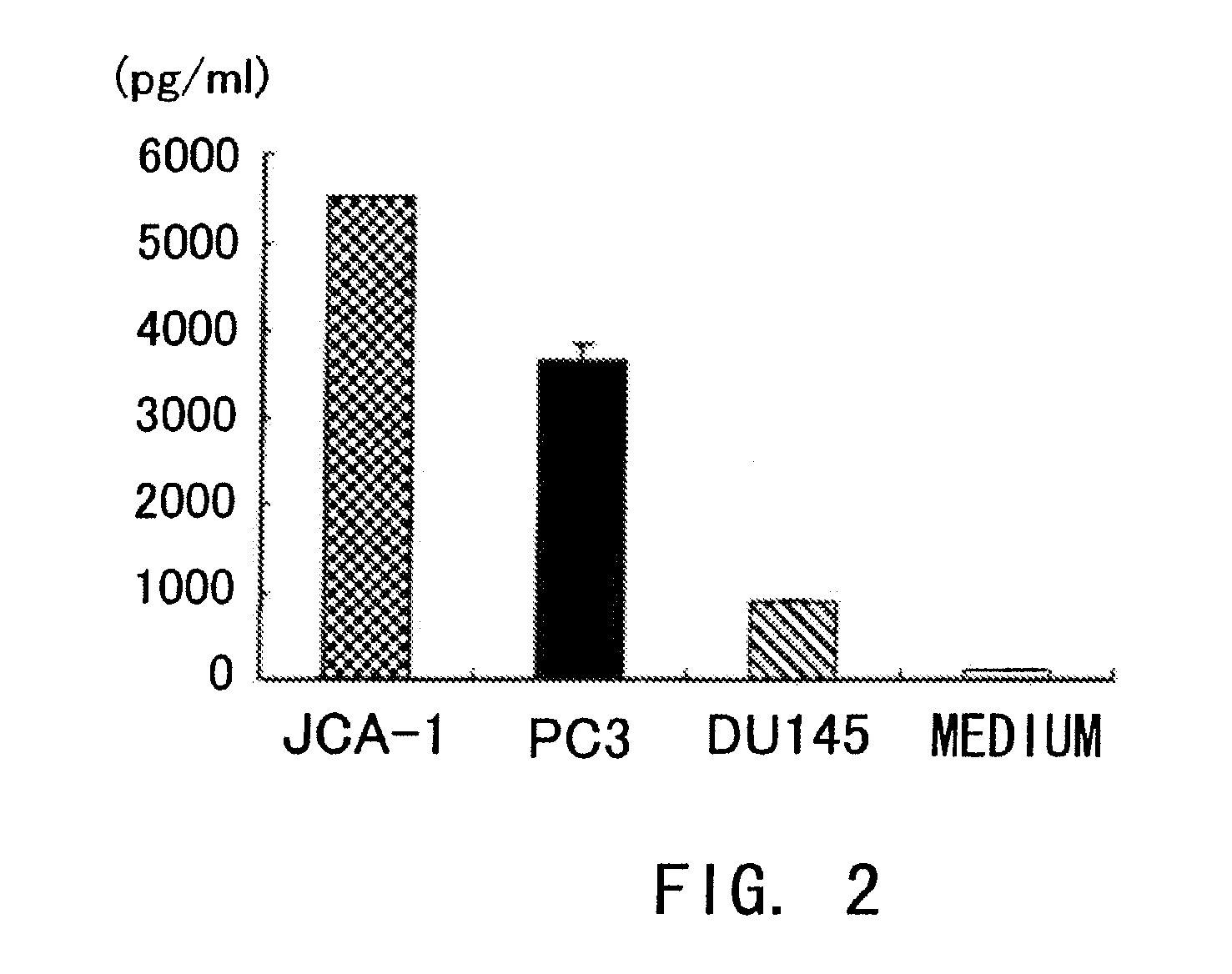
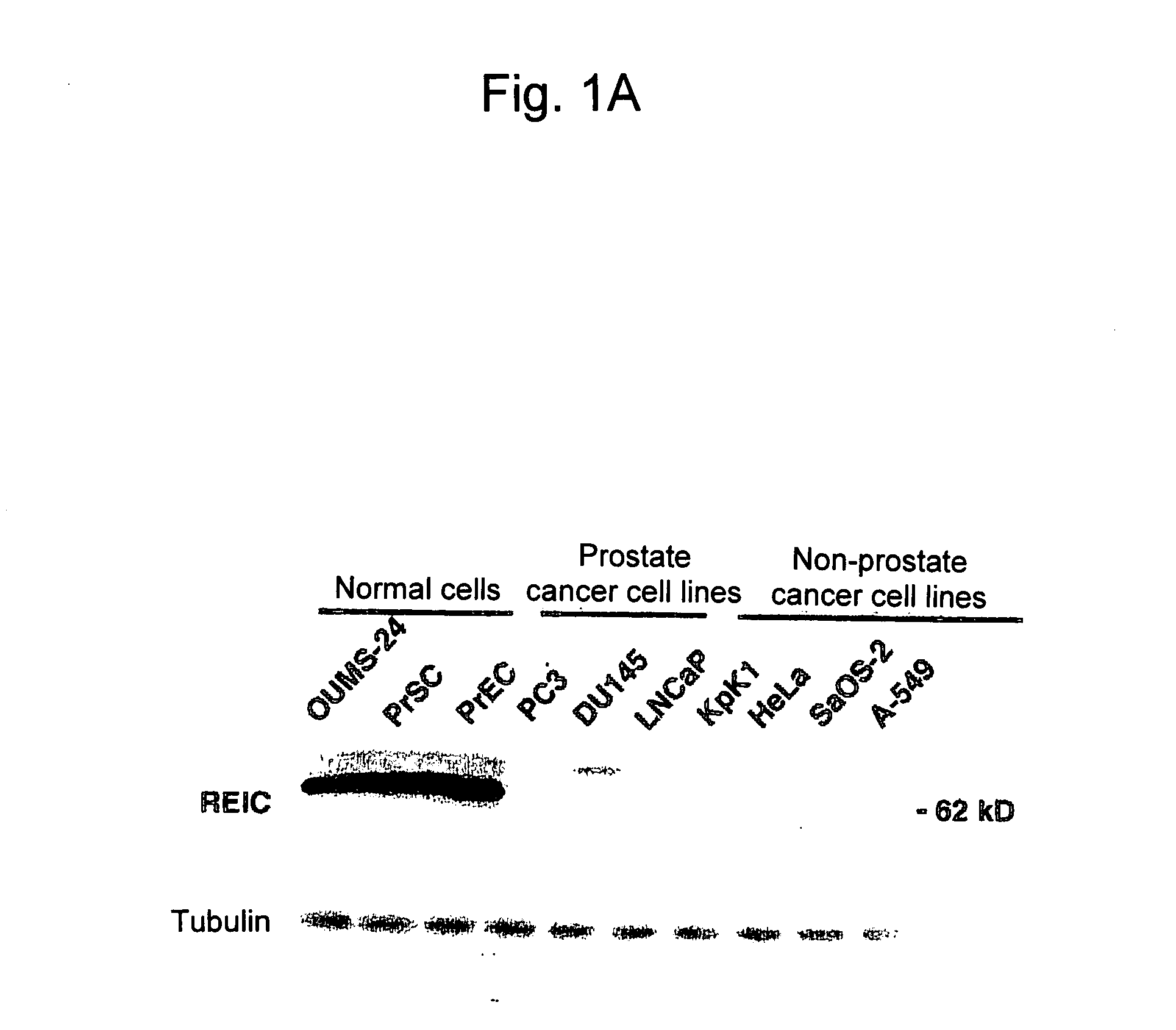
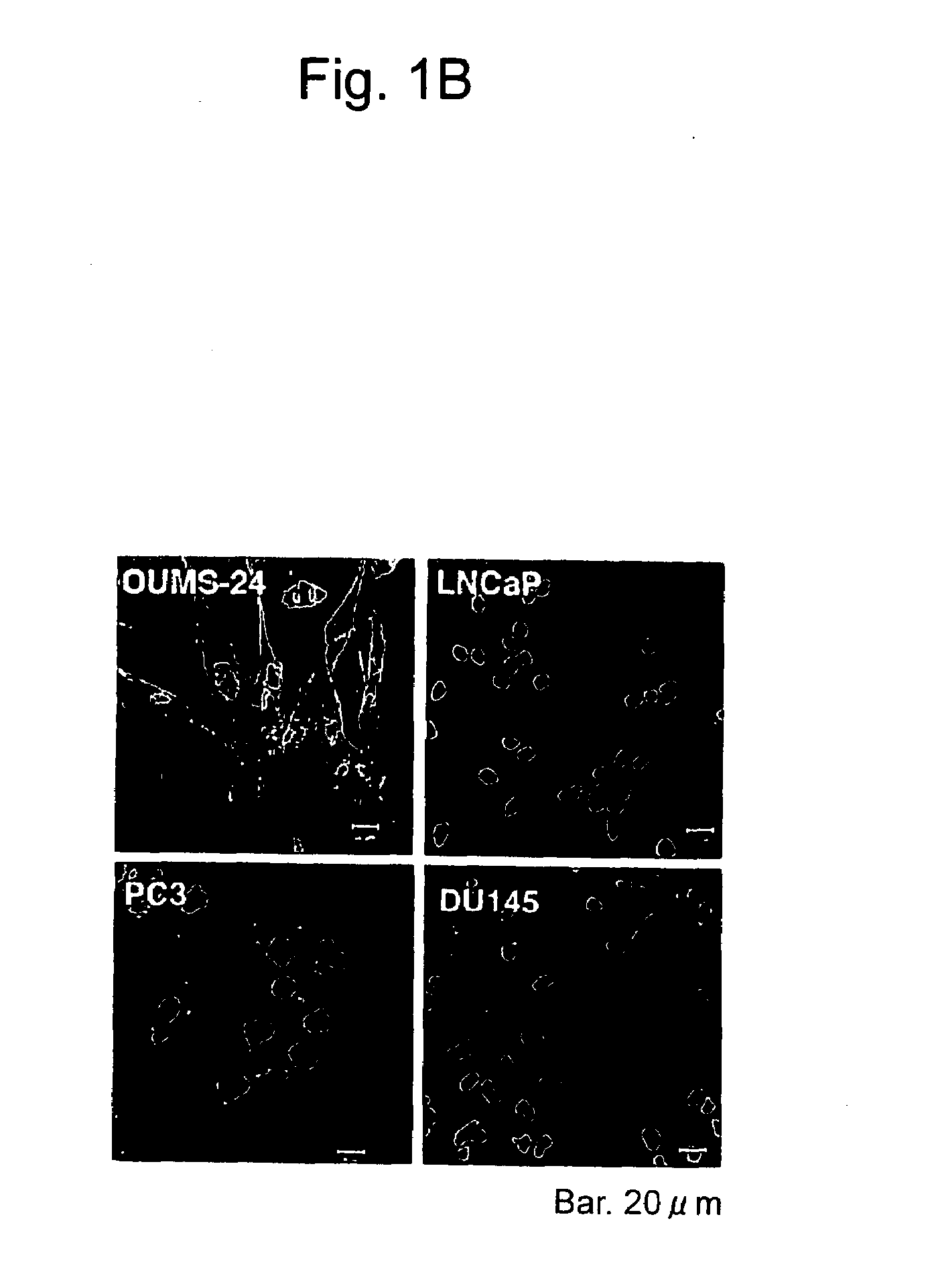
According to the present invention, an apoptosis-inducing agent for prostate cancer comprising REIC / Dkk-3 DNA or an REIC / Dkk-3 protein, and a therapeutic agent for prostate cancer and an agent for inhibiting prostate cancer metastasis that comprise such apoptosis-inducing agent are provided.An apoptosis-inducing agent for prostate cancer, comprising, as an active ingredient, an REIC / Dkk-3 protein (a) or (b) or REIC / Dkk-3 DNA (c) or (d):(a) a protein consisting of the amino acid sequence represented by SEQ ID NO: 2;(b) a protein consisting of an amino acid sequence derived from the amino acid sequence represented by SEQ ID NO: 2 by substitution or deletion of 1 or more amino acids;(c) DNA consisting of the nucleotide sequence represented by SEQ ID NO: 1; or(d) DNA that hybridizes under stringent conditions to DNA consisting of a nucleotide sequence complementary to the nucleotide sequence represented by SEQ ID NO: 1 and encodes a protein having apoptosis activity; anda therapeutic agent for prostate cancer comprising such apoptosis-inducing agent are provided
Owner:KUMON HIROMI +3
Use of neurotoxin therapy for treatment of urological-neurological disorders associated with prostate cancer
InactiveUS7470431B2Inexpensive and safeSimple methodBacterial antigen ingredientsPeptide/protein ingredientsNeurological disorderBotulinum toxin
The present invention related to methods for treating neurological-urological conditions associated with prostate cancer or methods of treatment of prostate cancer, which is accomplished by administration of botulinum toxin to the patient.
Owner:ALLERGAN INC
Treatment of Prostate Cancer
Described herein are compounds, methods of making such compounds, pharmaceutical compositions, and medicaments comprising such compounds, and methods of using such compounds to treat androgen receptor mediated diseases or conditions. The present invention provides therapies and therapeutic regimens for the treatment of prostate cancer.
Owner:TOKAI PHARMA
Treatment and diagnosis of prostate cancer
InactiveUS20040105865A1Quick upgradeImprove abilitiesIn-vivo radioactive preparationsPeptide/protein ingredientsProstatic epitheliumAntigen
The present invention is directed to the use of antibodies or binding portions thereof or probes which recognize an antigen of normal, benign, hyperplastic, and cancerous prostate epithelial cells or portions thereof. These antibodies or binding portions thereof or probes can be labeled and used for detection of such cells. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed is a hybridoma cell line which produces a monoclonal antibody recognizing antigens of normal, benign, hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
Halogenated compounds for cancer imaging and treatment and methods for their use
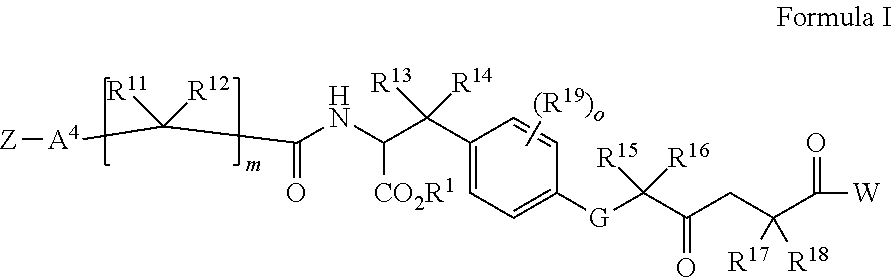
Compounds having a structure of Formula I:or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, wherein R1, R2, R3, R4, R5, X1, X2, X3 and X4 are as defined herein, and wherein the compound comprises at least one F, Cl, Br, I or 123I moiety, are provided. Uses of such compounds for imaging diagnostics in cancer and therapeutics methods for treatment of subjects in need thereof, including prostate cancer as well as methods and intermediates for preparing such compounds are also provided.
Owner:THE UNIV OF BRITISH COLUMBIA +1
Prostate-specific gene for diagnosis, prognosis and management of prostate cancer
InactiveUS6369195B1Organic active ingredientsTumor rejection antigen precursorsGene productHuman prostate
Disclosed are nucleic acid and amino acid sequences encoded by a novel, prostate specific gene (UC41) and diagnostic techniques for the detection of human prostate cancer utilizing such nucleic acid and amino acid sequences. Genetic probes and methods useful in monitoring the progression and diagnosis of prostate cancer are described. Methods of treatment for prostate cancer utilizing antisense constructs or antibodies specific for UC41 gene products are also described.
Owner:LAB OF AMERICA HLDG
Purification method for two active monomer compounds in saxifrage and application of product of the same
InactiveCN102766180AEnhanced inhibitory effectOrganic active ingredientsSugar derivativesProstate cancer cellPurification methods
The invention discloses a purification method for two active monomer compounds in saxifrage and application of product of the same. The method is as below: extracting saxifrage with ethanol; extracting the extract successively with petroleum ether and ethyl acetate; and separating the ethyl acetate extract by silica gel column chromatography, medium pressure column chromatography and gel column chromatography, so as to separate out the two monomer compounds (quercetin 3-O-beta-L-rhamnoside, quercetin 5-O-beta-D glucoside) from saxifrage. Biological activity determination results indicate that the saxifrage extract and the two monomer compounds have good inhibition effect on growth and proliferation of prostate cancer cells, can be used in preparation of medicine for treating prostate cancer, and have good application prospects in drugs for treating prostate cancer.
Owner:GUIZHOU NORMAL UNIVERSITY
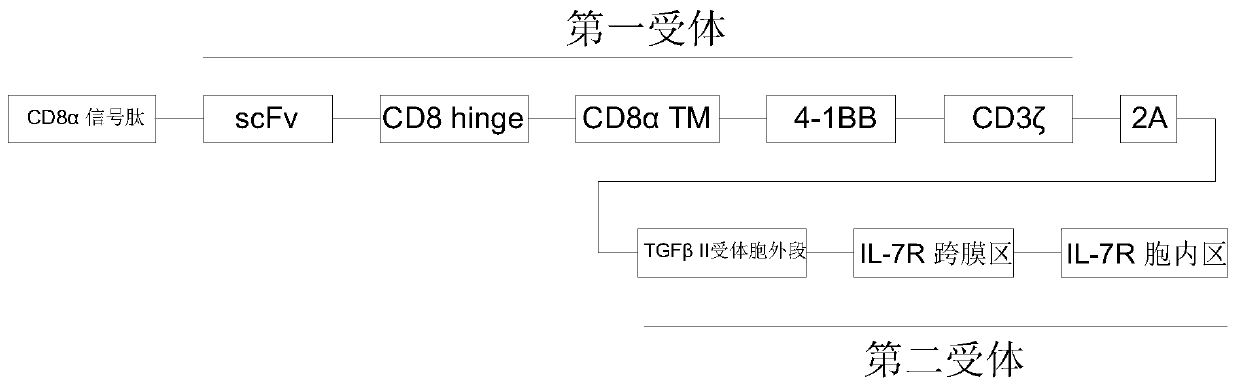
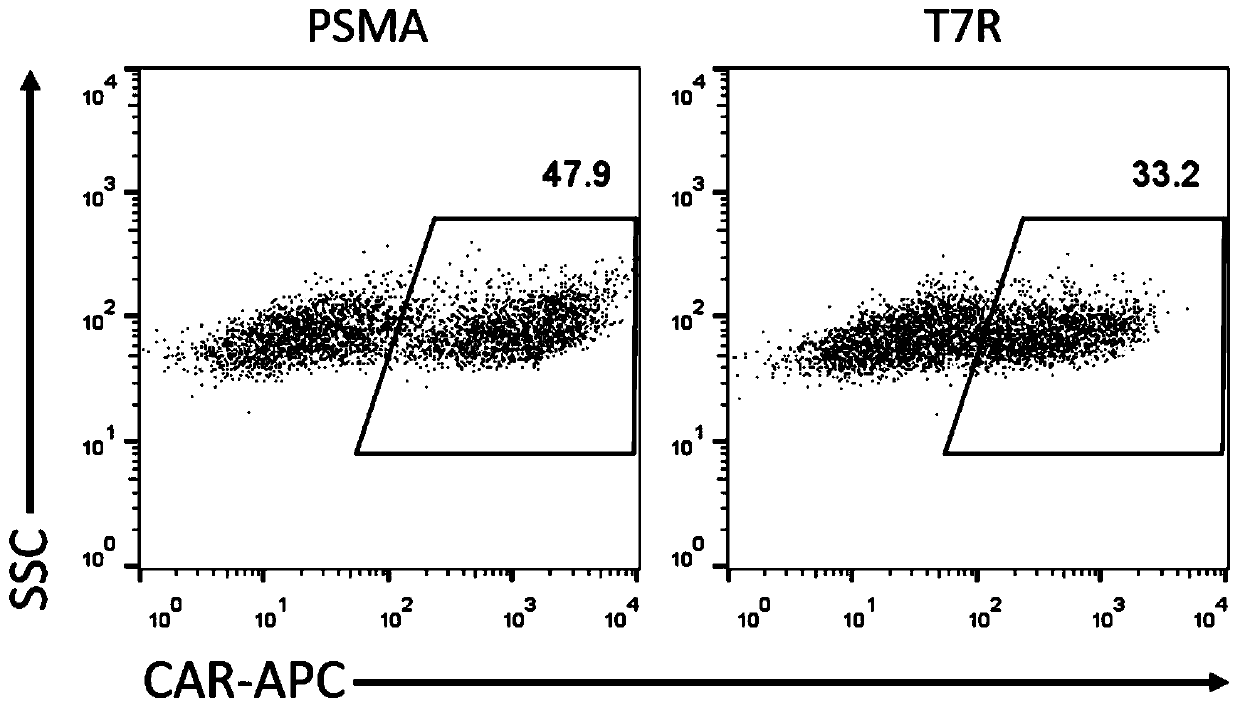
Enhanced CAR-T cell targeting prostate cancer, and preparation method and drug thereof
The invention discloses an enhanced CAR-T cell targeting prostate cancer, and a preparation method and a drug thereof, and relates to the technical field of immunotherapy. A first receptor and a second receptor are expressed on a cell membrane of the CAR-T cell. The first receptor is a chimeric antigen receptor, and the chimeric antigen receptor has an antigen binding domain for binding a prostatecancer antigen; the second receptor includes an extracellular TGF beta binding domain that specifically binds to TGF beta and an intracellular IL-7 activation signal transduction domain that persistently activates an IL-7 signaling pathway. The CAR-T cell provided by the invention can target prostate cancer, neutralize the inhibiting effect of TGF beta on the T cell in a tumor microenvironment and activate an IL-7-STAT 5 signaling pathway to promote proliferation and survival of the T cell. The CAR-T cell has enhanced anti-tumor activity and can be used for treating the prostate cancer.
Owner:EAST CHINA NORMAL UNIV +1
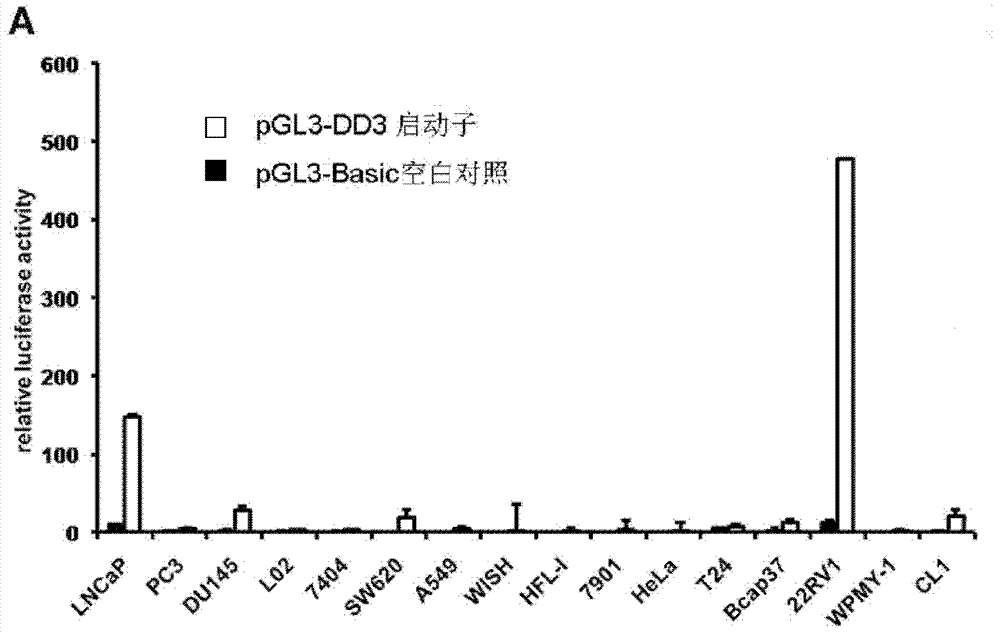
Gene-viro-therapy medicine specific for prostate cancer
InactiveCN102813939AGood treatment effectGenetic material ingredientsViral/bacteriophage medical ingredientsTreatment effectOncolytic adenovirus
The invention discloses a targeting gene-viro-therapy medicine specific for prostate cancer. An adenovirus is used, a natural promoter of an early gene EIA of the adenovirus is replaced by a promoter DD3 specific for the prostate cancer, and an oncolytic adenovirus is formed and carries anti-oncogene PTEN specific for the prostate cancer. If the anti-cancer effect of the anti-oncogene PTEN is not strong enough and is insufficient to completely kill the cancer, genes such as TRAIL, IL-24, MNSOD, CD, Smac, GM-CSF, IFN, IL-12, p53, RNAi, microRNA, Caspase 3 and Bax can added to the same carrier to be commonly used with the anti-oncogene PTEN, or two genes are linked to used through various schemes. The gene-viro-therapy medicine specific for the prostate cancer has high targeting treatment effect on the prostate cancer and basically has no influence on normal cells.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Apoptosis-inducing agent for prostate cancer cells
ActiveUS8658611B2Reduced expression levelInhibit prostate cancerPeptide/protein ingredientsGenetic material ingredientsProstate cancer cellLymphatic Spread
According to the present invention, an apoptosis-inducing agent for prostate cancer comprising REIC / Dkk-3 DNA or an REIC / Dkk-3 protein, and a therapeutic agent for prostate cancer and an agent for inhibiting prostate cancer metastasis that comprise such apoptosis-inducing agent are provided.An apoptosis-inducing agent for prostate cancer, comprising, as an active ingredient, an REIC / Dkk-3 protein (a) or (b) or REIC / Dkk-3 DNA (c) or (d):(a) a protein consisting of the amino acid sequence represented by SEQ ID NO: 2;(b) a protein consisting of an amino acid sequence derived from the amino acid sequence represented by SEQ ID NO: 2 by substitution or deletion of 1 or more amino acids;(c) DNA consisting of the nucleotide sequence represented by SEQ ID NO: 1; or(d) DNA that hybridizes under stringent conditions to DNA consisting of a nucleotide sequence complementary to the nucleotide sequence represented by SEQ ID NO: 1 and encodes a protein having apoptosis activity; anda therapeutic agent for prostate cancer comprising such apoptosis-inducing agent are provided.
Owner:MOMOTARO GENE INC (JP)
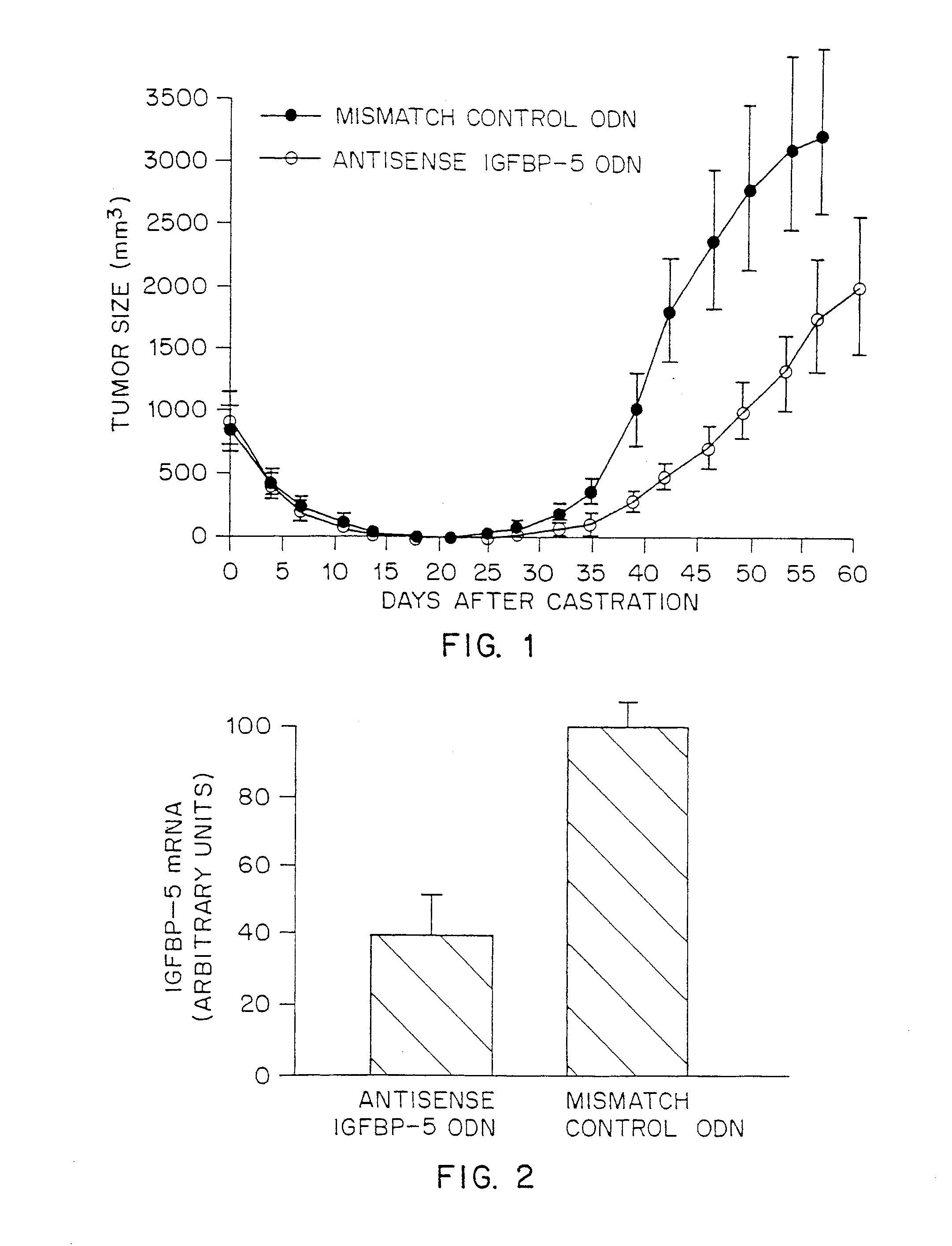
Antisense therapy for hormone-regulated tumors
InactiveUS7297684B1Reduce spreadInhibit expressionBiocidePeptide/protein ingredientsMammalAntisense oligodeoxynucleotides
A method is provided for treating hormone-regulated tumors (for example, breast and prostatic tumors) in mammals, including humans, by administration of an antisense ODN which is complementary to a portion of the gene encoding IGFBP-5. Using the Shionogi tumor model in vitro and in vivo, the administration of such an ODN was shown to reduce proliferation of tumor cells, and also to delay the progression to androgen independence. Thus, treatment of prostate cancer in mammals, including humans, and delay of the progression of prostate tumors to androgen independence is accomplished by administering to the mammal a therapeutically effective amount of an antisense oligodeoxynucleotide which is complementary to a portion of the nucleic acid sequence encoding IGFBP-5 and which hybridizes with such a sequence to inhibit expression of IGFBP-5. Specific antisense ODN's which are suitable for use in the method are GACCACGCTGATCACCAT (Seq. ID. No. 1), which is derived from the murine gene sequence, and CGCGGTGAGCAACACCAT (Seq. ID. No. 3) and AGGTCATGCAGCAGCCGC (Seq. ID No 4), which are derived from the human gene sequence.
Owner:THE UNIV OF BRITISH COLUMBIA
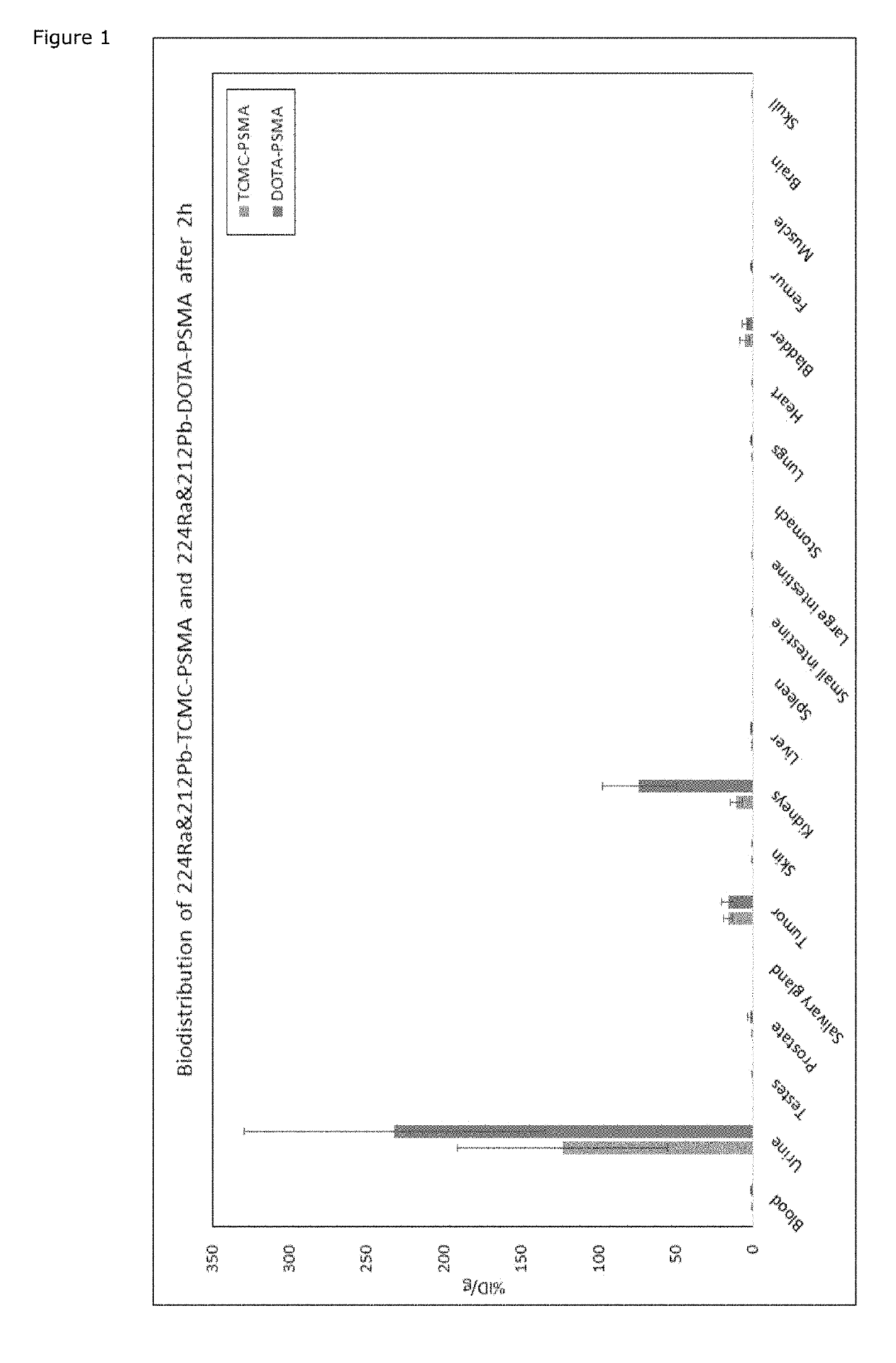
Novel lead and thorium compounds
ActiveUS20190177345A1Isotope introduction to heterocyclic compoundsIn-vivo radioactive preparationsMedicineDual targeting
The present invention relates complexes comprising a PSMA targeting compound linked to a radionuclide, such as 212Pb or 227Th. These compounds, and pharmaceutical compositions comprising them, can be used for medical applications. These applications include the treatment of prostate cancer, and the complexes allow for dual targeting of cancers.
Owner:SCIENCONS AS
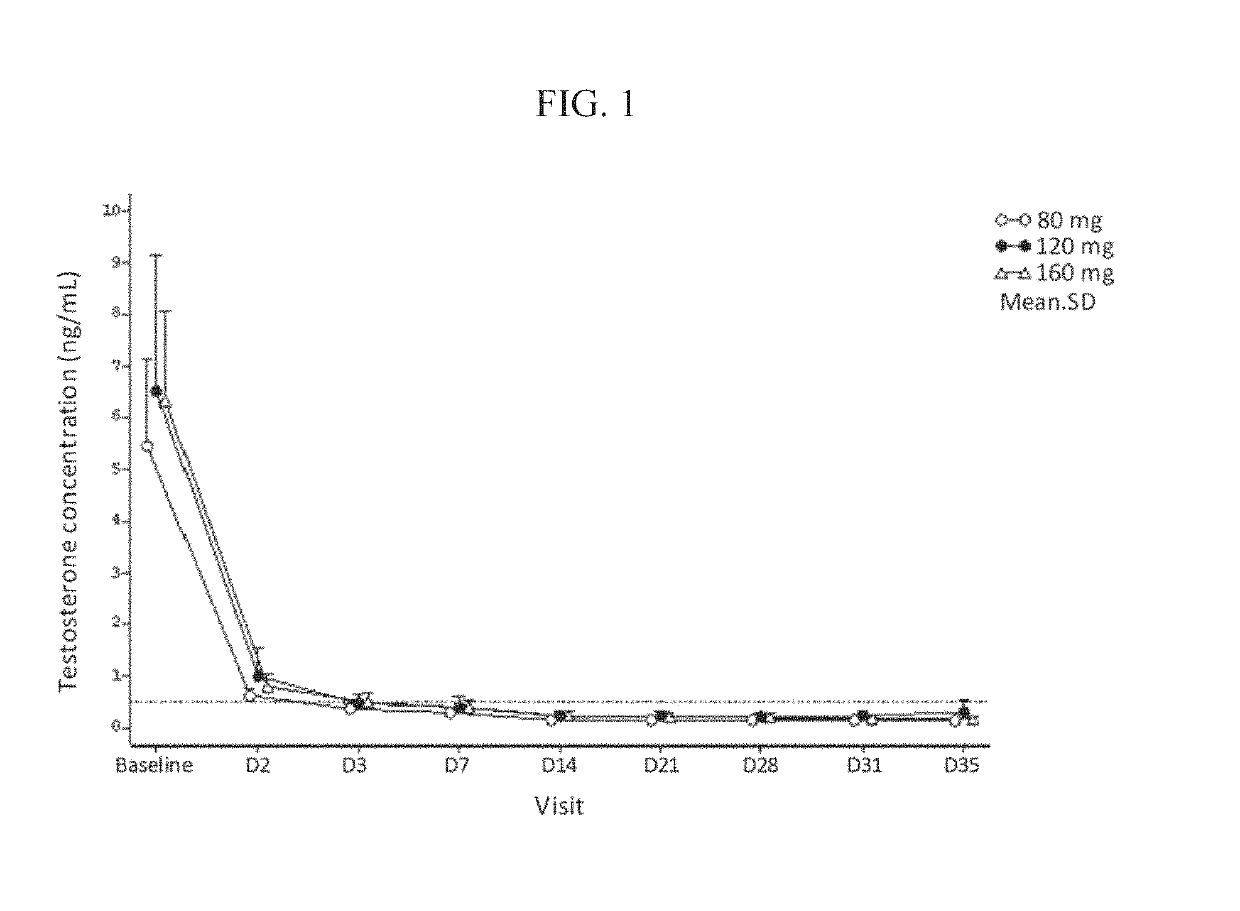
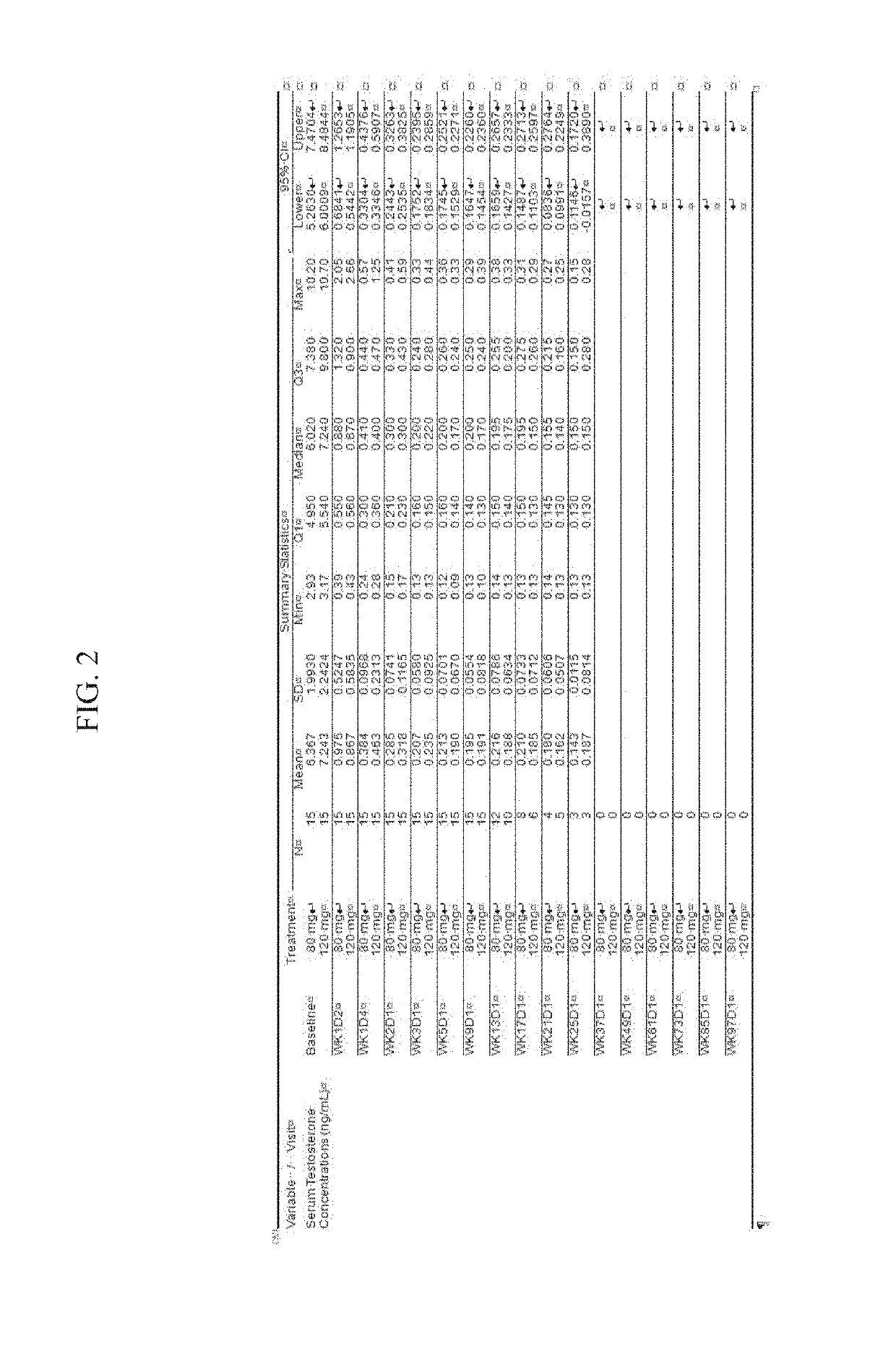
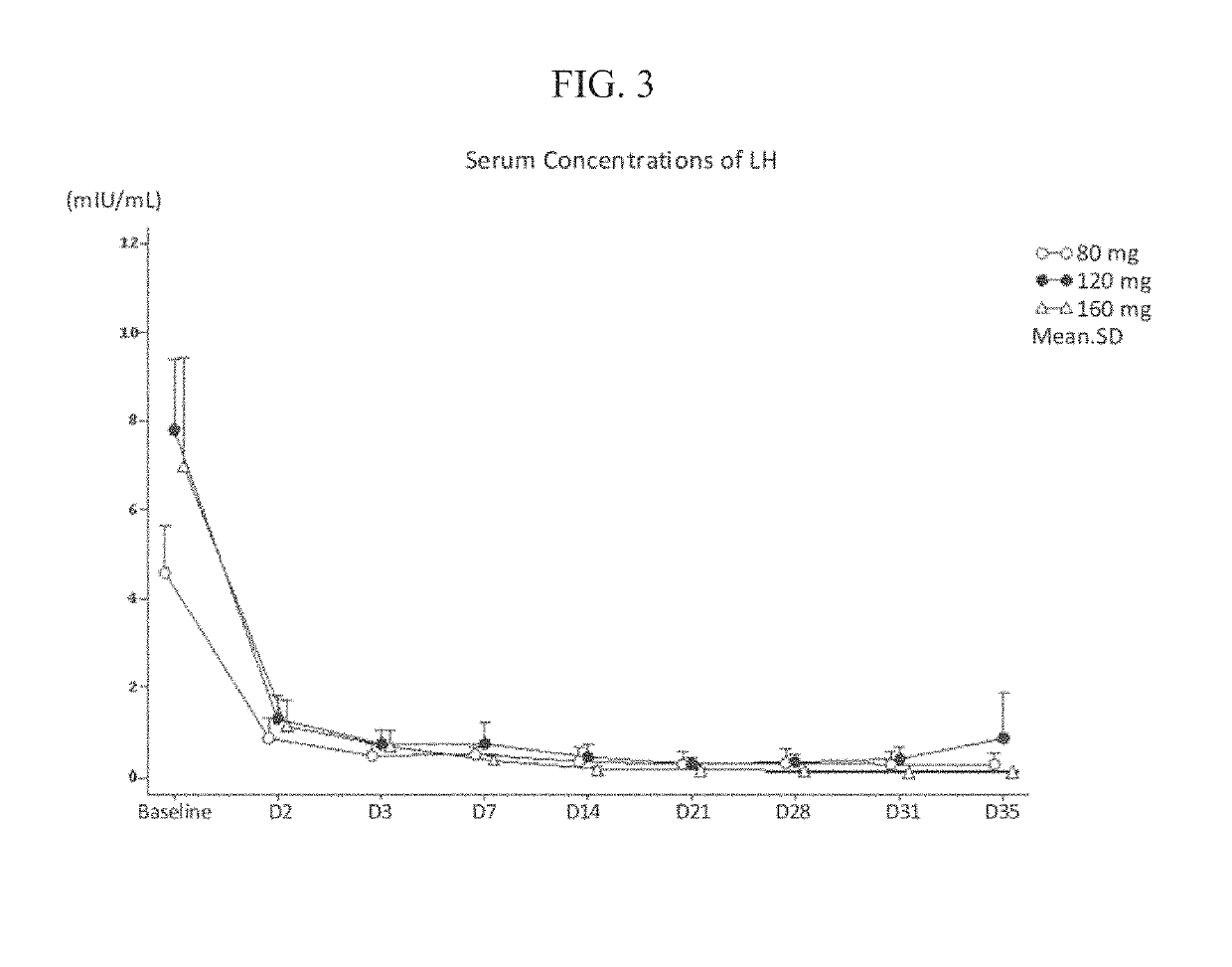
Treatment of prostate cancer
Methods for treating prostate cancer, including advanced prostate cancer, in a subject in need thereof, include administering once-daily to the subject, at least 80 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4- tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof. Another method includes: administering once-daily to the subject in need thereof, an oral load dose formulation having from 240 mg to 480 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof; and thereafter administering once-daily to the subject, an oral maintenance dose formulation having 80 mg to 160 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof.
Owner:TAKEDA PHARMA CO LTD +1
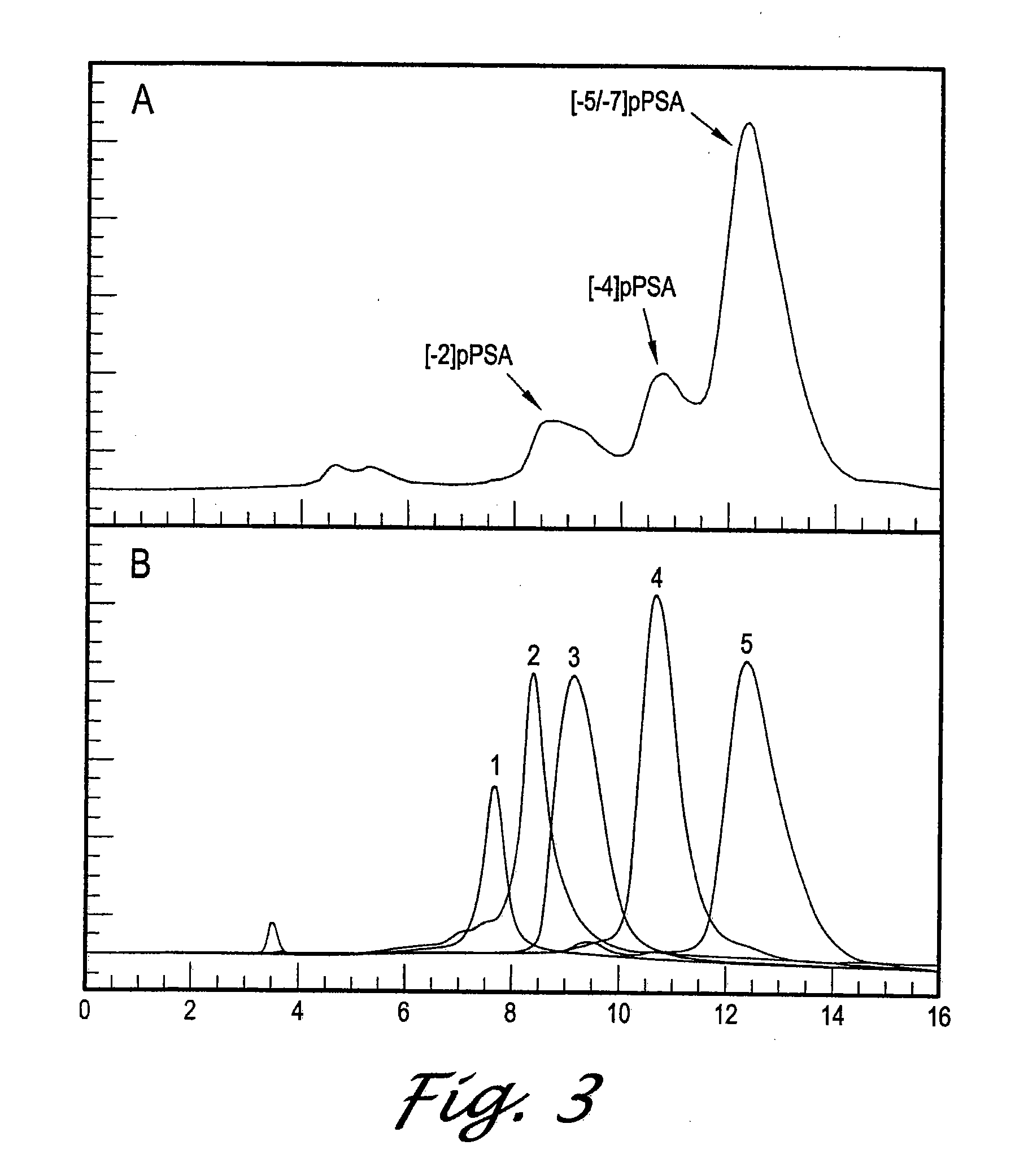
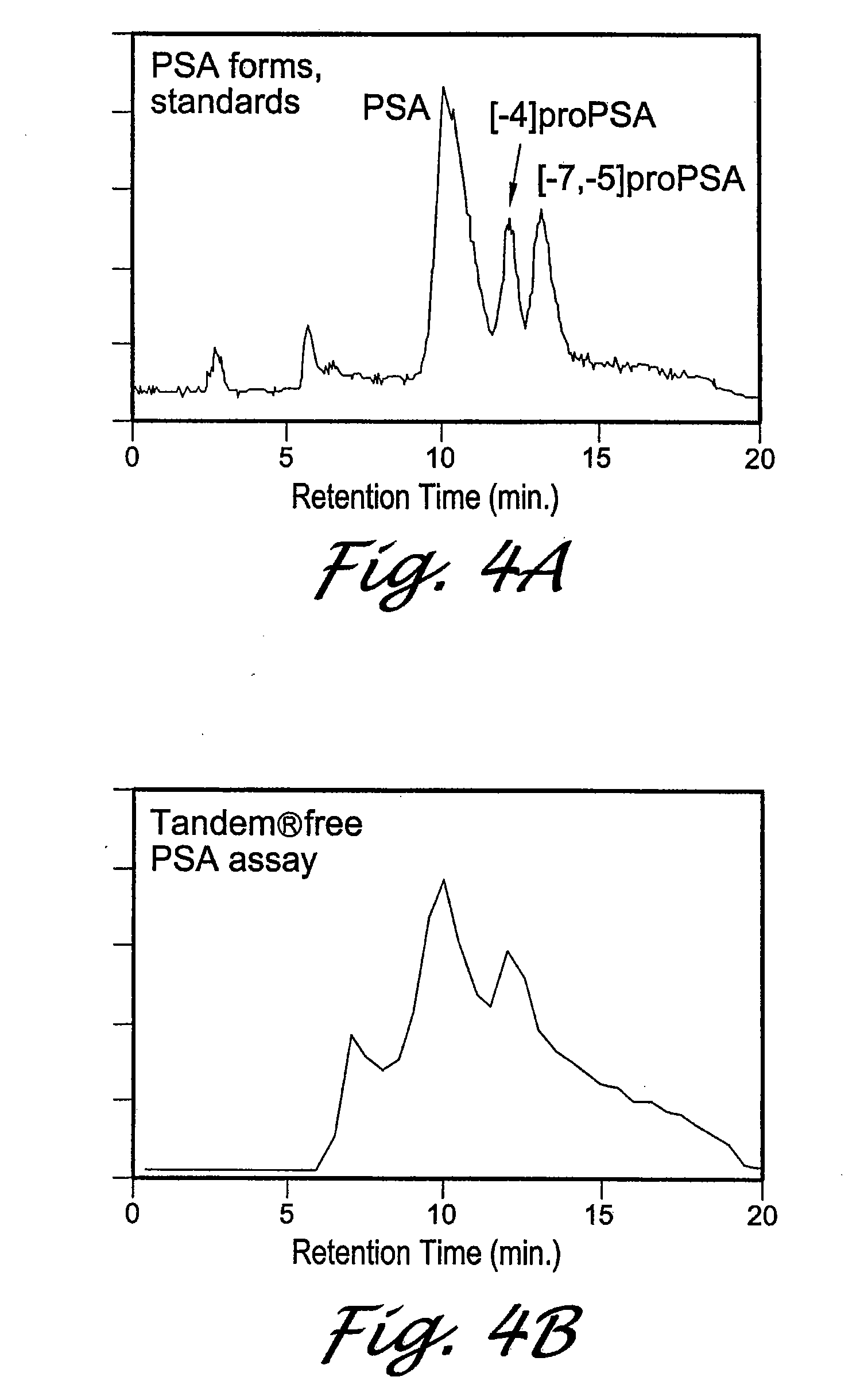
Forms of prostate specific antigens and methods for their detection
InactiveUS7288636B2Marker can be usedAnimal cellsFused cellsPhysiological fluidProstate-specific antigen
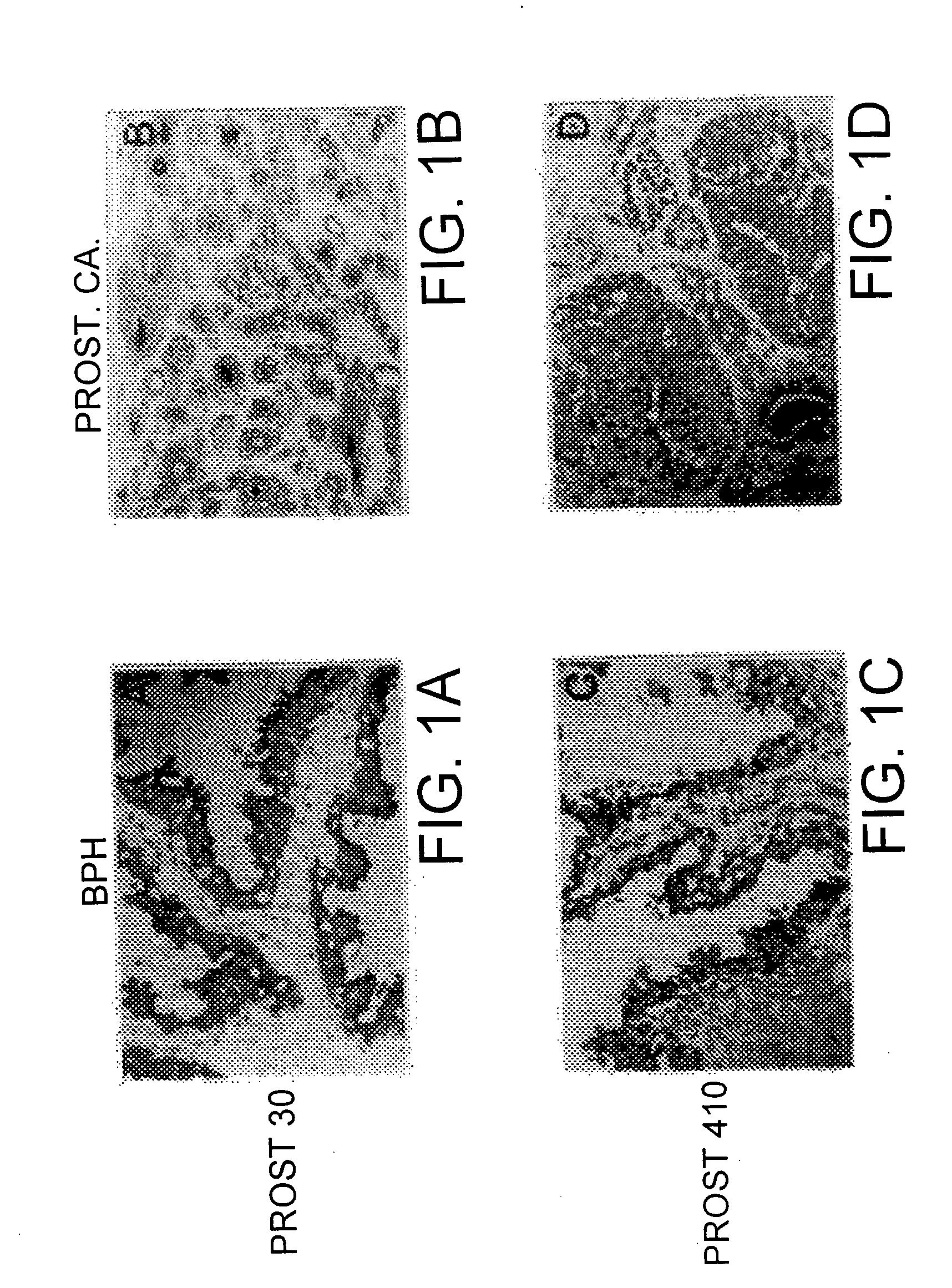
Inactive precursor forms of PSA (pPSA) have been identified to exist in serum and tissues of patients with prostate cancer. Antibodies specific for pPSA are provided. Methods for detecting inactive precursors of PSA in human physiological fluid and tissues are also provided, as well as diagnostic kits and methods useful in the diagnosis and management of prostate cancer.
Owner:HYBRITECH
Forms of Prostate Specific Antigens and Methods for their Detection
InactiveUS20100151501A1Marker can be usedMaterial analysisPhysiological fluidProstate-specific antigen
Inactive precursor forms of PSA (pPSA) have been identified to exist in serum and tissues of patients with prostate cancer. Antibodies specific for pPSA are provided. Methods for detecting inactive precursors of PSA in human physiological fluid and tissues are also provided, as well as diagnostic kits and methods useful in the diagnosis and management of prostate cancer.
Owner:HYBRITECH
Treatment of prostate carcinoma
ActiveUS9132105B2Inhibiting and delaying growthReduce the amount requiredBiocideHydroxy compound active ingredientsMedicineProstate carcinoma
Owner:PELLFICURE PHARMA
Treatment of metastatic prostate cancer
InactiveUS20170315127A1Good treatment effectPeptide/protein ingredientsMicrobiological testing/measurementNiclosamideAnti-Androgen
The present invention provides new compositions and methods for treating prostate cancer, e.g., drug-resistant prostate cancer, such as anti-androgen drug (e.g., enzalutamide) resistant and / or castration resistant prostate cancer (CRPC). These new compositions include, but are not limited to, pharmaceutical compositions that include an AR-V7 inhibitor, such as niclosamide. Alternatively, these new compositions can include, but are not limited to, pharmaceutical compositions that include an AKR1C3 inhibitor, such as indomethacin. These new methods include, but are not limited to, methods of administering an AR-V7 inhibitor, such as niclosamide, and / or an AKR1C3 inhibitor, such as indomethacin, to treat patients having prostate cancer. The present invention also provides methods of inhibiting androgen receptor variant expression, e.g. AR-V7, and methods of killing cells expressing AR-V7. The present invention further provides methods of inhibiting AKR1C3 expression or activity, and methods of killing cells that express AKR1C3.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Optical Imaging Contrast Agents for Imaging Lung Cancer
The invention provides contrast agents for optical imaging of prostate cancer in patients. The contrast agents may be used in diagnosis of prostate cancer, for follow up of progress in disease development, for follow up of treatment of prostate cancer and for surgical guidance. Further, the invention provides methods for optical imaging of prostate cancer in patients.
Owner:GE HEALTHCARE AS
Diagnosis and treatment integrated PSMA inhibitor, compound and preparation method and application thereof
ActiveCN112898270AAccelerated excretion of radioactivityReduce non-specific uptakeIron organic compoundsRadioactive preparation carriersAntigenNeoplasm diagnosis
The invention discloses a diagnosis and treatment integrated PSMA inhibitor, a compound and a preparation method and application thereof. The structure of the compound is shown as a formula A in the specification, wherein the R1 is DOTA or NODA used for complexing (radioactive) metal ions, radioactive metal nuclide is used for diagnosis or treatment, non-radioactive metal nuclide is used for being combined with radionuclide, and R2 is a coupling agent which is benzylamine, cyclohexylmethylamine or butylamine. The invention also provides a preparation method and application of the compound. The compound has very high affinity with prostate cancer specific membrane antigen (PSMA), and compared with existing PSMA treatment and imaging agents, the compound has the advantages that high swelling uptake is maintained, radioactive excretion of in-vivo non-target regions is accelerated, non-specific uptake of glands is reduced, and treatment and imaging of tumors are facilitated; and meanwhile, when the developing agent is marked by nuclide, the effect of integrating tumor diagnosis and treatment can be achieved, and the PSMA inhibitor is particularly suitable for treatment, diagnosis, staging and the like of prostate cancer.
Owner:北京瑞达福明科技有限公司
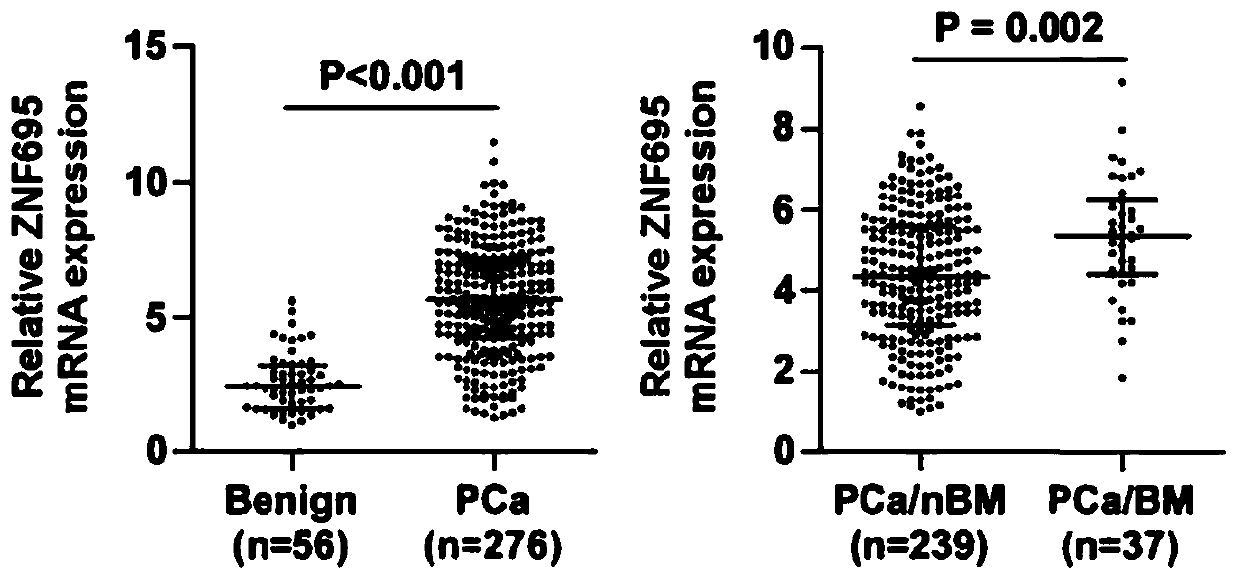
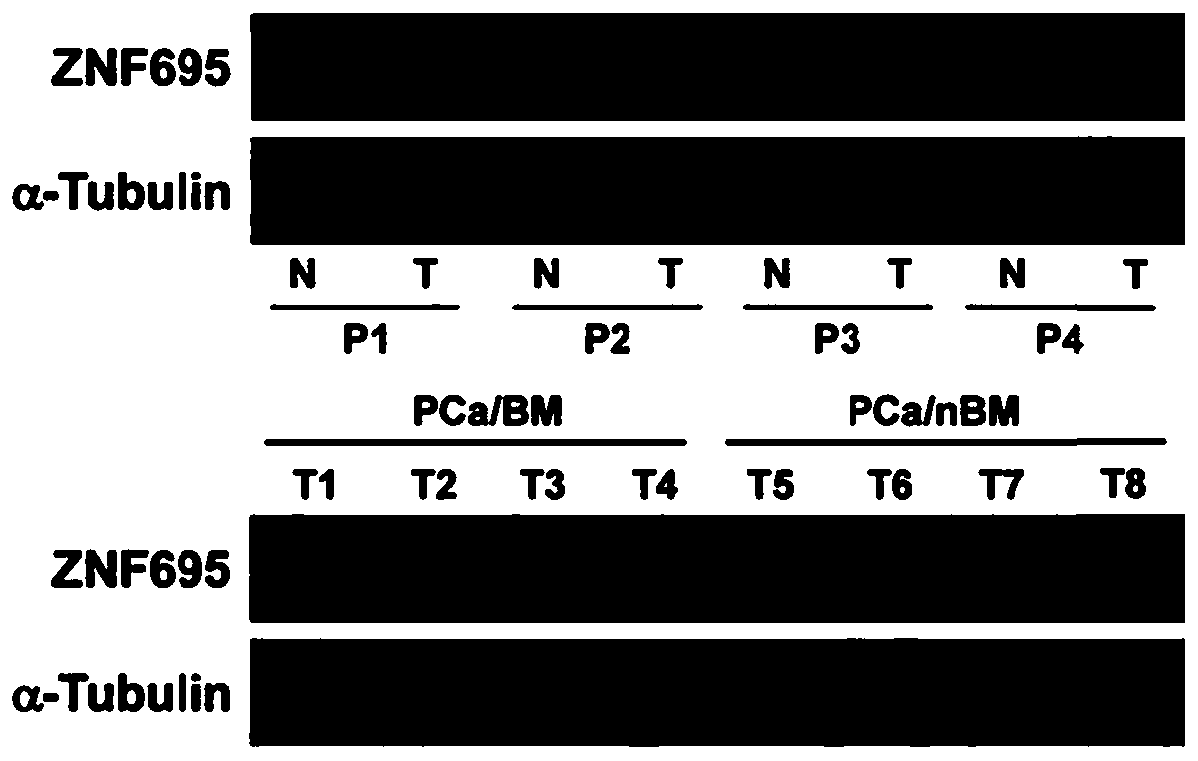
ZNF695 as marker and treatment target of prostate cancer bone metastasis
PendingCN111549139AInhibit bone metastasisInhibit transferMicrobiological testing/measurementMaterial analysisTreatment targetsOncology
The invention provides ZNF695 as a marker and a treatment target of prostate cancer bone metastasis. It is determined that the ZNF695 is highly expressed in a prostate cancer tissue and is particularly specific in the prostate cancer tissue accompanied by bone metastasis, and in-vivo prostate cancer bone metastasis can be remarkably inhibited by down-regulation of the ZNF695. Therefore, the ZNF695can be used as an early diagnosis marker of prostate cancer and bone metastasis thereof and a treatment target of prostate cancer.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
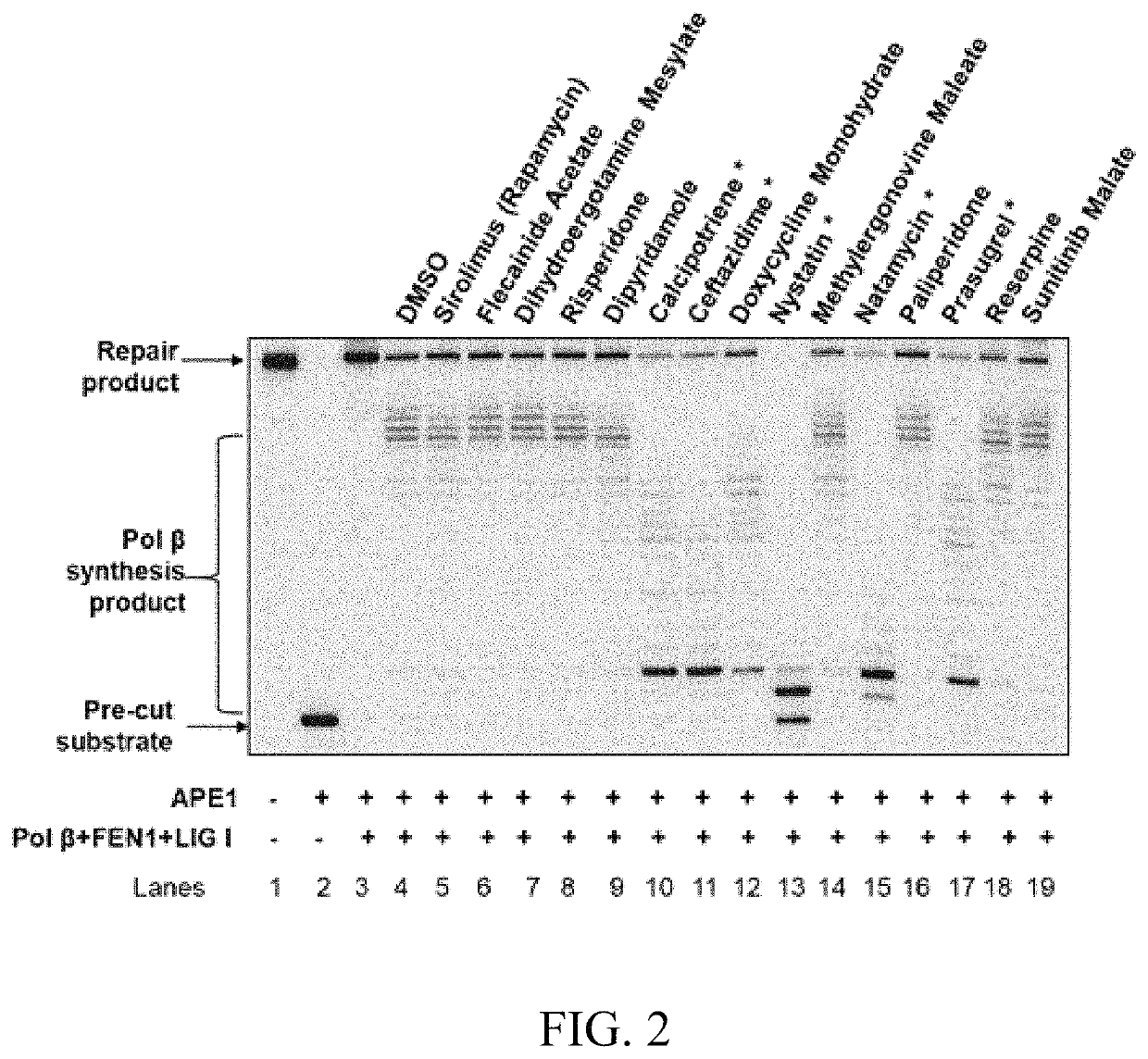
Treatments of prostate cancer
ActiveUS11304969B2Preventing/slowing down/reducing progress and proliferationReduce recurrenceOrganic active ingredientsAntineoplastic agentsProstate cancer cellCancer cell
The present invention provides compounds, compositions and methods for the treatment of prostate cancers, preferably, advanced prostate cancers. The subject invention also provides compounds, compositions and methods for preventing / slowing down / reducing the progress and proliferation of prostate cancer cells. The subject invention further provides compounds, compositions and methods for inhibiting DNA repair within cancer cells to slow tumor growth, preferably, by inhibiting BER capacity, including pol β and LIG I.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
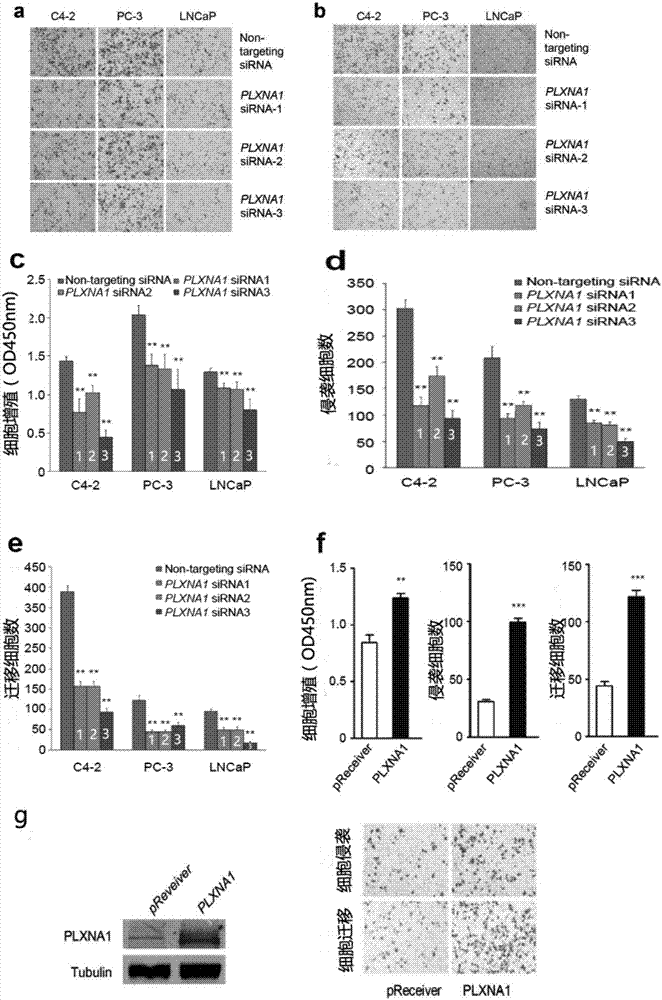
Prostatic cancer marker PLXNA1 and application thereof
InactiveCN106967789APeptide/protein ingredientsMicrobiological testing/measurementOncologyMalignancy
The invention discloses application of PLXNA1 (PlexinA1) as a marker for prediction of prostatic cancer generation, development and prognosis and distinguishment of prostatic cancer with high progressive malignant degree. By detecting the PLXNA1 DNA copy number change, the expression level of mRNA and its encoding protein, diagnosis and prognosis can be carried out on prostatic cancer, the PLXNA1 has the characteristics of high accuracy, high specificity and high sensitivity. The invention also discloses application of the PLXNA1 as a therapeutic target of prostatic cancer. The invention also discloses a kit for diagnosing and predicting prostatic cancer, the kit is convenient for use, and can be used for diagnosis and prediction of prostatic cancer.
Owner:SHANGHAI CHANGHAI HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com